Hal Broxmeyer highlights the multitude of erythropoietin’s biological functions, including a novel EPO–EPOR signaling cascade involving a serpin–lysosome–cathepsin axis.
Abstract
Erythropoietin (EPO), a humoral regulator of erythropoiesis and replacement therapy for selected red blood cell disorders in EPO-deficient patients, has been implicated in a wide range of activities on diverse cell, tissue, and organ types. EPO signals via two receptors, one comprising EPO receptor (EPOR) homodimers and the other a heterodimer of EPOR and CD131—the common β chain component of the GM-CSF, interleukin (IL)-3, and IL-5 receptors. Ligation of EPORs triggers various signaling pathways, including the JAK2–STAT5 and MAPK–NF-κB pathways, depending both on the receptor and the target cell type. A new study in this issue reveals a novel EPO-triggered pathway involving a Spi2A serpin–lysosome–cathepsin cascade that is initiated through the homodimeric EPOR complex and is required for the survival of erythroid progenitors. A full understanding of EPO’s effects on various cell types and their potential clinical relevance requires more work on the signaling events initiated through both EPORs, the effects of other cytokines and growth factors that modulate EPO’s actions, and a comparison of the effects of full-length versus truncated forms of EPO.
Biologically active molecules and their receptors regulate growth and development of different cells, tissues, and organ systems (Shaheen and Broxmeyer, 2009, 2011, 2012). Identifying the functions of these cytokines, their range of actions, and the underlying mechanisms is an ongoing endeavor. Such knowledge has helped elucidate the normal roles of these factors—either alone or as part of multifactorial networks—as well as their involvement in abnormal responses associated with initiation and progression of malignant and nonmalignant diseases. This information also offers hope for the potential modulation of these molecules and their receptors for clinical benefit.
The many effects of EPO
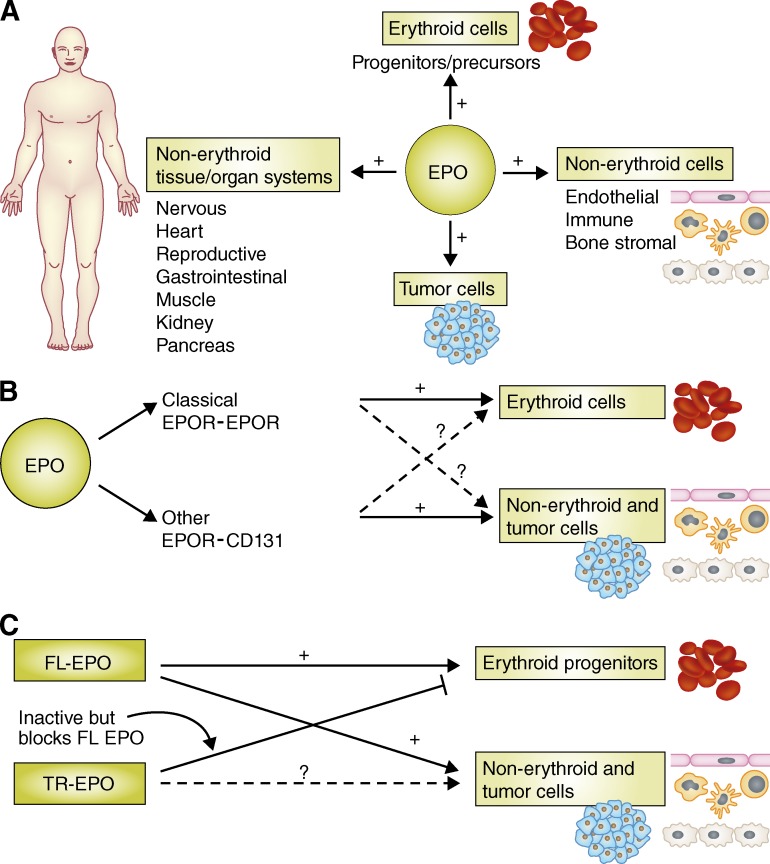
EPO was the first hematopoietically active humoral factor to be identified, purified, and have its gene cloned and expressed (Papayannopoulou et al., 2009). Now that it is clear that the actions of EPO extend well beyond erythropoiesis (Brines and Cerami, 2006; Hand and Brines, 2011), it’s difficult to believe that to be considered physiologically relevant in the 1970s, it was necessary to demonstrate that a factor had one activity only. In fact, it is now evident that many cytokines and growth factors have multiple targets and actions (Shaheen and Broxmeyer, 2009, 2011). The identification of EPOR expression on different cell types kicked off a search for nonerythropoietic effects of EPO. As a result, we now know that EPO has direct effects on immune cells (Broxmeyer 2011; Nairz et al., 2011, 2012), endothelial cells, and bone marrow stromal cells, as well as cells of the heart, reproductive system, gastrointestinal tract, muscle, kidney, pancreas, and nervous systems (Brines and Cerami, 2006; Choi et al., 2010; Hand and Brines, 2011; McGee et al., 2012; Sytkowski 2011; Fig. 1 A). The deletion of EPO or EPOR has identified and clarified several nonerythropoietic functions of EPO, as far ranging as promoting cardiac and CNS development, blocking cell death in stroke models, and improving learning and memory (Vogel and Gassmann, 2011). EPO is also involved in regulating angiogenesis (Kertesz et al., 2004), tumor angiogenesis (Ribatti 2010), and, perhaps directly, in the survival and growth of tumor cells (Szenajch et al., 2010; Hand and Brines, 2011; Oster et al., 2012).
Figure 1.
Multifaceted effects and targets of EPO. (A) EPO targets many cell types and tissues, including erythroid cells and their progenitors, tumor cells, and a variety of other nonerythroid cells and tissues. (B) EPO signals in erythroid cells via EPOR-EPOR homodimers and in nonerythroid cells via EPOR-CD131 heterodimers. (C) The effects of full-length EPO (FL-EPO) on both erythroid and nonerythroid cells may be blocked by DPP4-truncated EPO (TR-EPO), which itself may lack biological activity depending on which EPOR it targets. +, stimulating effect; ?, action/function not yet known.
EPO was first used to treat patients with end-stage renal disease and anemia based on their deficiency in production of EPO (Papayannopoulou et al., 2009; Shaheen and Broxmeyer, 2009). These treatments were successful in increasing erythrocyte numbers and hemoglobin and hematocrit levels, leading to a decreased need for red cell transfusions and, in many cases, to transfusion independence (Rizzo et al., 2010). EPO has also been used to treat patients with cancer-associated anemia. However, side effects of EPO treatment quickly emerged, including potentially life-threatening cardiac complications in patients with kidney disease, caused in part by off-target effects on nonerythroid cells (Szenajch et al., 2010; Hedley et al., 2011; Oster et al., 2012). This led to updated practice guidelines for clinical use of EPO and erythropoiesis-stimulating agents (Rizzo et al., 2010). Given its known off-target effects, it is essential to better understand the range of cell targets responding to EPO and how EPO manifests its effects at the cellular, biochemical, and molecular level.
EPO-induced signaling pathways
EPO-induced intracellular signaling in erythroid progenitor and precursor cells is mediated via EPOR homodimerization triggered by picomolar concentrations of EPO (Wojchowski et al., 2010; Nairz et al., 2012). This initiates activation of Janus kinase (JAK) 2 and signal transducer and activator of transcription (STAT) 5, as well as mitogen-activated protein kinase (MAPK) and NF-κB. Activation of NF-κB itself initiates a set of downstream events, including the release of multiple cytokines, which has a plethora of effects on many cell types, including erythroid cells themselves (Broxmeyer 2011; Nairz et al., 2011). The effects of EPO can vary in different cell types. For example, although EPO activates NF-κB in erythroid cells, it inhibits this pathway in macrophages, resulting in decreased production of TNF and expression of nitric oxide synthetase (Nairz et al., 2011). As a result, EPO protects mice against disease in a colitis model but results in reduced pathogen clearance and survival in mice infected with Salmonella. Notably, either neutralization of endogenous Epo or knockout of the epoR gene enhanced elimination of Salmonella (Nairz et al., 2011).
EPO also protects against both type 1 (streptozotocin model) and type 2 (db/db mouse model) diabetes. Protection in these models was mediated by JAK2 signaling directly in pancreatic β cells, resulting in β cell survival and proliferation, reduced inflammation, and increased angiogenesis in the islets (Choi et al., 2010). In this issue of the JEM, Dev et al. delineate a novel EPO–EPOR signaling cascade involving a serpin–lysosome–cathespin axis that is required for the cytoprotective effects of EPO on maturing populations of erythroblasts. The same group originally identified Serpina3g (Spi2A) as an EPO-responsive gene that is activated to a level similar as other major EPO responsive genes, such as Oncostatin-M (Sathyanarayana et al., 2008; Wojchowski et al., 2010). Oncostatin M is a homeostasis factor for the proliferation of different myeloid progenitor cells, including the erythroid progenitor cell (Broxmeyer et al., 2002). Thus, several EPO target genes are factors in erythroid progenitor cell maintenance Activation of Spi2A, which is downstream of JAK2, inhibited cathepsins B and L, as well as lysosome-derived proteases, thus protecting the cell from death (Dev et al., 2012).
EPO can also signal via a heterodimeric receptor composed of an EPOR monomer chain and CD131 (Brines and Cerami, 2006; Zhang et al., 2009). This heterodimeric complex, the activation of which requires much higher concentrations of EPO compared with that of the homodimeric EPOR (Hand and Brines, 2011), is found in nonerythroid cells (Fig. 1 B). Less is known about this complex compared with the homodimeric EPOR, and more studies are warranted to address unanswered questions regarding the expression of the different EPOR complexes and their activation in different cell types. It’s unclear, for example, whether the EPO-triggered signaling cascades downstream of the two EPORs differ, and if so how. Also unknown is whether one cell type can express both EPORs and how this might affect EPO-induced cellular and intracellular effects. In addition, whether other cytokines such as GM-CSF, IL-3, or IL-5 can signal through, interfere with, or modify EPO signaling through the EPOR–CD131 complex remains to be determined.
Multiple influences
Cytokines often work in combination with each other, creating events that may be more physiologically meaningful than the actions of a single cytokine (Shaheen and Broxmeyer, 2009, 2011, 2012). Although EPO alone can stimulate mature subsets of erythroid progenitors, combining EPO with the potent co-stimulating cytokine stem cell factor (SCF) induces proliferation of more immature erythroid progenitors (Broxmeyer et al., 1991). Similarly, IL-3 and GM-CSF can also team up with EPO to act on more immature erythroid progenitors (Shaheen and Broxmeyer, 2009, 2011). The cytokine synergy noted in vitro has held up in vivo (Broxmeyer et al., 1987). This brings up the question of how modifying, enhancing, or suppressing cytokines may influence the effects of EPO on nonerythroid cells that express one or both EPORs, as well as receptors for GM-CSF, IL-3, and SCF.
Several investigators have undertaken efforts to modify the EPO molecule from its physiological form such that it interacts with only the heterodimeric EPOR–CD131 complex (Hand and Brines, 2011). The goal of these studies is to harness the tissue-protective effects of EPO without activating hematopoietic and coagulation pathways, which might limit the clinical use of EPO for settings other than promoting erythropoiesis. In addition to developing a more clinically useful EPO molecule, it is necessary to consider potential effects of endogenous or exogenous EPO molecules that may be modified in vivo through normal physiological processes.
Beyond the influences of other cytokines on EPO effects, new and surprising information on EPO should also be taken into consideration. For example, the enzyme DPP4 (CD26), which is present on the surface of many cell types and in soluble form in the circulation, truncates chemokines such as stromal cell–derived factor-1 (SDF-1/CXCL12), and changes their biological activity (Christopherson et al., 2002, 2004). DPP4 has similar effects on several CSFs, including EPO, truncating the protein at the N terminus–penultimate alanine or proline. Unlike full-length SDF-1, DPP4-truncated SDF-1 is inactive as a chemotactic molecule and survival factor in vitro (Christopherson et al., 2002; Broxmeyer et al., 2012) and as a homing molecule in vivo (Christopherson et al., 2004), and can block the activity of full-length SDF-1. These effects are counteracted by inhibition of DPP4 by specific peptides (Diprotin A [ILE-PRO-ILE] or Val-Pyr) or a small molecule (sitagliptin). Similarly, DPP4 truncates EPO into a molecule incapable of inducing erythropoiesis in vitro and in vivo (Broxmeyer et al., 2012), and truncated EPO blocks the erythropoietic activity of full-length EPO. Inhibiting DPP4 on human or mouse cells, or functionally deleting dpp4 in mice, greatly enhances EPO-driven proliferation of erythroid progenitor cells (Broxmeyer et al., 2012).
Although the mechanisms underlying the actions of truncated EPO have not been worked out yet, they may mimic that of DPP4-truncated GM-CSF, which fails to bind and/or activate the dodecameric GM-CSFR complex required for downstream signaling (Broxmeyer et al., 2012). DPP4-truncated GM-CSF also binds to the GM-CSFR with greater affinity than full-length GM-CSF, thus blocking the binding of the full-length cytokine and acting as a dominant–negative inhibitor (Broxmeyer et al., 2012). It would be of interest and potential clinical relevance to determine whether truncated EPO affects nonerythroid cell types, and if so, whether it acts as a dominant–negative molecule (Fig. 1 C). It also remains to be seen whether nonerythroid EPO target cells, or other cells in proximity to EPO targets, express DPP4, and whether inhibition or deletion of DPP4 might enhance EPO responses in these cells. Finally, it would be interesting to test whether DPP4-truncated EPO could specifically block unwanted EPO effects.
Future efforts to better understand the range of EPO target cells, the EPORs they express, and the intracellular signaling cascades they activate should be enlightening and of potential clinical utility. It will also be critical to elucidate the modifying effects of other cytokines and growth factors, as well as enzymes such as DPP4 (and perhaps others), on the structure and actions of EPO.
Acknowledgments
H.E. Broxmeyer is supported by Public Health Service Grants from the National Institutes of Health: R01 HL056416, R01 HL67384, R01 HL112669, and P01 DK090948.
References
- Brines M., Cerami A. 2006. Discovering erythropoietin’s extra-hematopoietic functions: biology and clinical promise. Kidney Int. 70:246–250 10.1038/sj.ki.5001546 [DOI] [PubMed] [Google Scholar]
- Broxmeyer H.E. 2011. Erythropoietin surprises: an immune saga. Immunity. 34:6–7 10.1016/j.immuni.2011.01.004 [DOI] [PubMed] [Google Scholar]
- Broxmeyer H.E., Williams D.E., Hangoc G., Cooper S., Gillis S., Shadduck R.K., Bicknell D.C. 1987. Synergistic myelopoietic actions in vivo after administration to mice of combinations of purified natural murine colony-stimulating factor 1, recombinant murine interleukin 3, and recombinant murine granulocyte/macrophage colony-stimulating factor. Proc. Natl. Acad. Sci. USA. 84:3871–3875 10.1073/pnas.84.11.3871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broxmeyer H.E., Cooper S., Lu L., Hangoc G., Anderson D., Cosman D., Lyman S.D., Williams D.E. 1991. Effect of murine mast cell growth factor (c-kit proto-oncogene ligand) on colony formation by human marrow hematopoietic progenitor cells. Blood. 77:2142–2149 [PubMed] [Google Scholar]
- Broxmeyer H.E., Bruns H.A., Zhang S., Cooper S., Hangoc G., McKenzie A.N., Dent A.L., Schindler U., Naeger L.K., Hoey T., Kaplan M.H. 2002. Th1 cells regulate hematopoietic progenitor cell homeostasis by production of oncostatin M. Immunity. 16:815–825 10.1016/S1074-7613(02)00319-9 [DOI] [PubMed] [Google Scholar]
- Broxmeyer H.E., Hoggatt J., O’Leary H.A., Mantel C., Chitteti B.R., Cooper S., Messina-Graham S., Hangoc G., Farag S., Rohrabaugh S.L., et al. 2012. Dipeptidylpeptidase 4 negatively regulates colony-stimulating factor activity and stress hematopoiesis. Nat. Med. 18:1786–1796 10.1038/nm.2991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi D., Schroer S.A., Lu S.Y., Wang L., Wu X., Liu Y., Zhang Y., Gaisano H.Y., Wagner K.U., Wu H., et al. 2010. Erythropoietin protects against diabetes through direct effects on pancreatic beta cells. J. Exp. Med. 207:2831–2842 10.1084/jem.20100665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopherson K.W., II, Hangoc G., Broxmeyer H.E. 2002. Cell surface peptidase CD26/dipeptidylpeptidase IV regulates CXCL12/stromal cell-derived factor-1 alpha-mediated chemotaxis of human cord blood CD34+ progenitor cells. J. Immunol. 169:7000–7008 [DOI] [PubMed] [Google Scholar]
- Christopherson K.W., II, Hangoc G., Mantel C.R., Broxmeyer H.E. 2004. Modulation of hematopoietic stem cell homing and engraftment by CD26. Science. 305:1000–1003 10.1126/science.1097071 [DOI] [PubMed] [Google Scholar]
- Dev A., Byrne S.M., Verma R., Ashton-Rickardt P.G., Wojchowski D.M. 2012. Erythropoietin-directed erythropoiesis depends upon serpin inhibition of erythroblast lysosomal cathepsins. J. Exp. Med. 210:225–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand C.C., Brines M. 2011. Promises and pitfalls in erythopoietin-mediated tissue protection: are nonerythropoietic derivatives a way forward? J. Investig. Med. 59:1073–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedley B.D., Allan A.L., Xenocostas A. 2011. The role of erythropoietin and erythropoiesis-stimulating agents in tumor progression. Clin. Cancer Res. 17:6373–6380 10.1158/1078-0432.CCR-10-2577 [DOI] [PubMed] [Google Scholar]
- Kertesz N., Wu J., Chen T.H., Sucov H.M., Wu H. 2004. The role of erythropoietin in regulating angiogenesis. Dev. Biol. 276:101–110 10.1016/j.ydbio.2004.08.025 [DOI] [PubMed] [Google Scholar]
- McGee S.J., Havens A.M., Shiozawa Y., Jung Y., Taichman R.S. 2012. Effects of erythropoietin on the bone microenvironment. Growth Factors. 30:22–28 10.3109/08977194.2011.637034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nairz M., Schroll A., Moschen A.R., Sonnweber T., Theurl M., Theurl I., Taub N., Jamnig C., Neurauter D., Huber L.A., et al. 2011. Erythropoietin contrastingly affects bacterial infection and experimental colitis by inhibiting nuclear factor-κB-inducible immune pathways. Immunity. 34:61–74 10.1016/j.immuni.2011.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nairz M., Sonnweber T., Schroll A., Theurl I., Weiss G. 2012. The pleiotropic effects of erythropoietin in infection and inflammation. Microbes Infect. 14:238–246 10.1016/j.micinf.2011.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oster H.S., Neumann D., Hoffman M., Mittelman M. 2012. Erythropoietin: the swinging pendulum. Leuk. Res. 36:939–944 10.1016/j.leukres.2012.04.017 [DOI] [PubMed] [Google Scholar]
- Papayannopoulou T., Migliaccio A.R., Abkowitz J.L., D’Andrea A.D. 2009. Biology of erythropoiesis, erythroid differentiation, and maturation. Hematology: Basic Principles and Practice. Fifth edition. Hoffman R., Benz E.J., Jr, Shattil S.J., Furie B., Silberstein L.E., McGlave P., Heslop H., Anastasi J., Elsevier Churchill Livingston, Philadelphia: 276–294 [Google Scholar]
- Ribatti D. 2010. Erythropoietin and tumor angiogenesis. Stem Cells Dev. 19:1–4 10.1089/scd.2009.0402 [DOI] [PubMed] [Google Scholar]
- Rizzo J.D., Brouwers M., Hurley P., Seidenfeld J., Arcasoy M.O., Spivak J.L., Bennett C.L., Bohlius J., Evanchuk D., Goode M.J., et al. ; American Society of Hematology and the American Society of Clinical Oncology Practice Guideline Update Committee 2010. American Society of Hematology/American Society of Clinical Oncology clinical practice guideline update on the use of epoetin and darbepoetin in adult patients with cancer. Blood. 116:4045–4059 10.1182/blood-2010-08-300541 [DOI] [PubMed] [Google Scholar]
- Sathyanarayana P., Dev A., Fang J., Houde E., Bogacheva O., Bogachev O., Menon M., Browne S., Pradeep A., Emerson C., Wojchowski D.M. 2008. EPO receptor circuits for primary erythroblast survival. Blood. 111:5390–5399 10.1182/blood-2007-10-119743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaheen M., Broxmeyer H.E. 2009. The humoral regulation of hematopoiesis. Hematology: Basic Principles and Practice. Fifth edition. Hoffman R., Benz EJ E.J., Jr, Shattil S.J., Furie B., Silberstein L.E., McGlave P., Heslop H., Anastasi J., Elsevier Churchill Livingston, Philadelphia: 253–275 [Google Scholar]
- Shaheen M., Broxmeyer H.E. 2011. Hematopoietic Cytokines and Growth Factors. Cord Blood Biology, Transplantation, Banking, and Regulation. Broxmeyer H.E., editor AABB Press, Bethesda, MD: 35−74 [Google Scholar]
- Shaheen M., Broxmeyer H.E. 2012. Principles of Cytokine Signaling. Hematology: Basic Principles and Practice. Sixth edition. Hoffman R., Benz EJ E.J., Jr, Shattil S.J., Furie B., Silberstein L.E., McGlave P., Heslop H., Anastasi J., Elsevier Churchill Livingston, Philadelphia: In Press [Google Scholar]
- Sytkowski A.J. 2011. The neurobiology of erythropoietin. Cell. Mol. Neurobiol. 31:931–937 10.1007/s10571-011-9695-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szenajch J., Wcislo G., Jeong J.Y., Szczylik C., Feldman L. 2010. The role of erythropoietin and its receptor in growth, survival and therapeutic response of human tumor cells From clinic to bench - a critical review. Biochim. Biophys. Acta. 1806:82–95 [DOI] [PubMed] [Google Scholar]
- Vogel J., Gassmann M. 2011. Erythropoietic and non-erythropoietic functions of erythropoietin in mouse models. J. Physiol. 589:1259–1264 10.1113/jphysiol.2010.196147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojchowski D.M., Sathyanarayana P., Dev A. 2010. Erythropoietin receptor response circuits. Curr. Opin. Hematol. 17:169–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.L., Radhakrishnan M.L., Lu X., Gross A.W., Tidor B., Lodish H.F. 2009. Symmetric signaling by an asymmetric 1 erythropoietin: 2 erythropoietin receptor complex. Mol. Cell. 33:266–274 10.1016/j.molcel.2008.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]