Abstract
Objectives
Test the hypotheses that there are equivalent wear rates for enamel-versus-enamel and ceramic-versus-enamel, analyzing the in vivo wear of crown ceramics, their natural enamel antagonists, and the corresponding two contralateral teeth; and, that bite force does not correlate with the wear.
Methods
A controlled, clinical trial was conducted involving patients needing full coverage crowns opposing enamel antagonists. Bite forces were measured using a bilateral gnathodynamometer. Single-unit restorations of metal/ceramic (Argedent 62, Argen Corp/IPS d.SIGN veneer); or, core-ceramic/veneer from either, Empress2/Eris, or e.maxPress core/e.maxCeram glaze (ceramics: Ivoclar Vivadent, USA) were randomly assigned, fabricated and cemented. Impressions were made of the ceramic crowns, as well as each maxillary and mandibular quadrant at one week (baseline) and one, two and three years. Resulting models were scanned (3D laser scanner). Maximum wear was calculated by superimposing baseline with annual images.
Results
There were a total of thirty-six crowns required for thirty-one patients. Each restoration had three associated enamel teeth; 1) crown, 2) antagonist, 3) contralateral, and 4) contralateral-antagonist. SAS PROC MIXED (α=0.05) indicated no statistical significance for mean maximum wear among crown ceramics, enamel antagonists and contralaterals. However, enamel wear was statistically significant in relation to intraoral location (p=0.04) and among years (p<0.02). Analyzed alone, the enamel contralateral-antagonist exhibited significantly greater wear (p<0.001). Considering all wear sites, there was no correlation with bite force (p=0.15).
Significance
The ceramics and their antagonists exhibited in vivo wear rates within the range of normal enamel. Future studies should examine the wear implications of the contralateral-antagonist enamel.
Keywords: ceramic wear, enamel wear, core ceramic, clinical, in vivo, antagonists
1. Introduction
Recent demand for esthetic improvement has led to the introduction of ceramic products for crowns and fixed dental prostheses (FDPs) before the clinical performances and limitations of these products have been fully explored and reported. One limitation of restorative ceramics is the abrasiveness to natural tooth structure that may accelerate the wear of natural enamel [1, 2]. Unfortunately, very little is understood of the mechanisms, occurrence o f wear and patterns among individuals [3], especially since more clinical in vivo studies analyze survival rates and fracture rates, not wear [4, 5]. Wear occurs based on complex masticatory movements, as the jaw moves in different directions, and the patterns vary depending upon joint pathology, occlusion and muscle tone.
Given the complexity of our masticatory system, bite force, long considered a contributing factor to prostheses wear and survival, has been a point of interest since Borelli’s work "De Motu Animalium” in biomechanics in 1680 [6]. Few measurement techniques represent the multiple directional forces and muscles used while elevating or depressing the mandible and maxilla during mastication [7]. However, some gnathodynamometers do test molar mastication and maximum bite force bilaterally [8], such as in this study. Until recently, the vast majority of wear studies concentrated on composite wear conducted in vitro [1, 9–14] without involving bite force. Testing mechanical properties in vitro may be a preliminary indicator for events such as subcritical crack formation and failure over time [15, 16]. However, while this yields important material property information, there is essentially no scientific correlation for in vitro findings with clinical occurrences.
An in vitro method for measuring one of the seven types of wear as defined in the International Organization for Standardization (ISO) [17] is a pin-on-disk system in which an experimental stylus (pin) of restorative material is placed in contact with a flat, rotating, enamel disk [18]. The resulting decrease in stylus length is then quantified based upon specified loads and frictional forces. However, teeth have asperities and do not rotate on a 360-degree plane. Furthermore, the effects of solid and liquid dietary components cannot be simulated effectively. The in vivo wear process is multifactorial.
Given these considerations, three-dimensional laser scanners are used to measure intraoral wear using non-contact optical sensors on attentively fabricated replicate dental models [19]. The scanners [20–22] project a laserbeam through an optical system onto the target surface, scanning without physical contact within an estimated precision from 5 to 8 µm [12]. Surface differences are quantified using reference-free digital subtraction analysis [23]. While few in vivo studies have used this technology to date, two were relatively comprehensive [24, 25]. However, they have provided no correlation with the ceramic characteristics that may affect the materials’ abrasiveness.
This in vivo study was conducted to quantify the wear of natural enamel opposing three ceramic materials as influenced by the ceramic, observation time and bite force testing the following hypotheses: 1) wear for enamel-versus-enamel and ceramic-versus enamel is commensurate; 2) wear loss for enamel antagonists opposing each veneer and core ceramics is similar; and, 3) biting force does not correlate with the amount of wear.
2. Materials and Methods
2.1 Study Design
To analyze in vivo wear of both enamel and ceramic, a randomized, controlled, clinical trial was conducted. Prior to the single-blind pilot study initiation, three different commercially available ceramic systems were selected for fabricating full coverage restorative crowns as needed on a second premolar, first molar or second molar that opposed natural antagonist teeth in any arch. A random number table was formulated by the study’s statistician for assignment of the clinical restorative materials. Restorable teeth required a crown to root ratio of at least 1:1, with a full complement of opposing non-restored or minimally restored natural teeth. “Minimally restored” indicated that nothing more extensive than a Class II amalgam restoration was present in the opposing arch, and that there was a natural contralateral tooth.
Prior to the study, two investigators (dentists) were identified to prepare the restoration sites and place the crowns that would be fabricated by one professional commercial lab. Two different investigators (dentists), not involved in the crown preparations, were selected to evaluate the crowns post cementation.
2.2 Study Population Criteria
The clinical protocol for treating patients was approved by the Institutional Review Board of University of Texas Health Science Center at San Antonio (UTHSCSA). Flyers and e-mail advertisements were used to recruit patients meeting the following criteria: minimum age of 18 years; good overall general health with no contraindications to dental treatment; good overall dental health, (i.e., no caries, periodontal disease or pocket depths in excess of 4 mm); no evidence of temporomandibular disorders (e.g. clicking, popping, or pain on opening); no parafunctional habits (e.g., bruxism or clenching); good oral hygiene (e.g., compliant with instructions as determined by plaque present); normal saliva flow (e.g., no medical pathologies or chronic medication intake that limited salivary volume or flow); capable of paying the crown laboratory cost of two hundred dollars (USD); and, agreeable to three consecutive yearly appointments. The informed consent for each participant included the following baseline data: general medical history with physical examination, primary dental models, maximum bite force measurement, periodontal pocket depths and, periapical radiographs of abutment teeth.
2.3 Crown Materials
The three restorative material combinations included: one metal/ceramic: 1) Argedent 62 alloy, (Argen Corp., USA)/IPSd.SIGN glass-veneer; and, two all-ceramics: 2) IPSEmpress 2 core ceramic/ IPSEris veneer; or 3) IPSe.max Press core ceramic/e.max Ceram glaze (ceramics, Ivoclar Vivadent AG, Schaan, Liechtenstein).
The gold-based, high noble metal alloy (Argedent 62) has a reported composition of 61.7% Au, 24.2% Pd, 8.75% Ag. The glass-ceramic veneer used in conjunction with it was sintered at 890 °C and is composed of both needle-like apatite crystals and leucite crystals [26]. One core ceramic, lithium disilicate and lithium orthophosphate ceramic (Empress 2) [27], was pressed at 920 °C. A glass-matrix ceramic containing fluorapatite crystals sintered at 755 °C, was designed specifically for application on Empress 2 ceramic core [28]. The lithium-disilicate-based core (e.max Press) was pressed at 920 °C, with the glaze paste and stain (e.max Ceram) applied directly to the core and fired at 770 °C. The ceramics used in the study met the criteria for ISO 6872 [29].
2.4 Impressions and Models
Impressions for crown fabrication and the wear measurement were made using both light and medium body vinylpolysiloxane (Affinis, Coltene/Whaledent, Ohio, USA) to capture all possible detail. After cleaning, rinsing and drying the patient’s teeth, a first impression was made to remove plaque and salivary residue and discarded. A second impression was made immediately, maximizing the marginal quality. After 24 h at ambient temperature, the impressions were cleaned with alcohol and misted with a silicone relaxation liquid (Smoothex, Whip Mix Corp, Kentucky, USA). The impressions were poured with white Type IV gypsum material (GC Fuji Super Hardrock, Leuven, Belgium), vibrated, set under pressure (2 bar) for 30 min, then stored in ambient conditions for a minimum of 24 h. The baseline impressions were made after crown cementation.
2.5 Study Intervention
Prior to restorative preparation procedures, bite force measurements were recorded with each patient. The bilateral gnathodynamometer bite fork was positioned along the posterior teeth, extending back with the second molar as the forward contact point. Each patient was instructed to “bite down” exerting pressure until there was an initial sense of pain. The resulting maximum bilateral bite force was recorded.
Teeth identified for restoration were prepared for full crowns and provisional restorations were fabricated using a bis-acryl resin (Integrity, Dentsply, USA). Impressions were made and poured. The master models were mounted in centric relation, representing the most posterior relation of the lower to the upper jaw from which lateral movements can be made [30]. Once a final crown was fabricated and tried-in place, occlusal adjustments were made using a high-speed dental handpiece (Kavo Dental GmbH, Germany) and a fine diamond bur (Brasseler, USA). Prior to cementation, all adjusted surfaces were polished (Shofu porcelain polishing kit, Shofu Dental Corporation, JP) or glazed depending on the adjustment areas. Areas involving more than two cusps were glazed, while smaller areas were polished to the same smoothness as the non-adjusted surfaces. Final crowns were cemented with a dual-cure resin luting agent (Variolink II, Ivoclar Vivadent). All crown fabrication was conducted according to the manufacturer’s recommendations at one professional commercial lab (Creative Smiles Inc., Dental Laboratory, San Antonio, TX, USA).
2.6 Wear Determination Methods
Models were produced from all of the post cementation impressions. A three-dimensional laser scanner (es 1 Scanner, etkon, Germany) was used to record the anatomical surfaces of the white model replicates (Scan 3D software). White stone maximizes the diffuse reflection areas using the high resolution charge-coupled device (CCD) with 10 mobile axes under a light angle of 45° establishing 28500 measuring points per second.
The baseline scan images were superimposed over each of the successive annual images. Wear amounts (µm) were calculated (Match 3D software) as the maximum loss in height of the occlusal surface. Reported wear data were determined from baseline for each of years one, two and three. Statistical analyses were performed using SAS PROC MIXED.
2.7 Micrographs
In order to examine surface aspects of in vivo wear, representative models of crowns and enamel antagonists with measured wear values were fixed on aluminum mounts using conductive carbon paint (SPI-Chem, PA, USA) and sputter coated with 250Å gold-palladium (AuPd; Hummer II, Technics, CA, USA). Micrographs, secondary electron images (SEI) and backscattered images (BSI), were recorded with a scanning electron microscope (SEM, JSM 6400, Joel, Ltd., Tokyo, JP)
3. RESULTS
The clinical study conducted over a five year period involved 36 crowns. No patient received more than two study crowns. The population of patients (23 females, 8 males) ranged from 24 – 62 years of age. After Year 1, one patient with a single crown withdrew from the study. A second patient experienced a crown fracture after 1.5 years, attributable to recent bruxing due to numerous personal stressors. A full gold crown replacement was advised, and the patient w a s released from the study. Consequently, there were 29 patients with 34 crowns analyzed in years 2 and 3 of this pilot study.
Crown placements involved: ten mandibular right first molars; nine mandibular left first molars; five maxillary right first molars; five mandibular left second molars; four mandibular right second molars; one maxillary left first molar; one maxillary right first premolar; one mandibular right second premolar.
3.1 Wear Results
This study analyzed three years of in vivo ceramic and enamel wear for 560 sites measured at baseline, and years 1, 2 and 3. Wear measurements for each patient involved making new impressions for each quadrant at each time interval, the gypsum models, and laserscans for each location. Consequently, the calculations of wear (µm) were performed for all the restorative crowns, as well as, for the three related natural enamel sites. The natural enamel teeth were identified as; antagonists (opposes and contacts the ceramic crown); contralaterals (opposite side of the mouth from the crown); and, contralateral-antagonists (opposes and contacts the contralateral). Due to the large variance for wear, the mean wear of the contralateral-antagonist was compared with that of the ceramic crown, enamel antagonist crown, and enamel contralateral based on an unequal variance assumption.
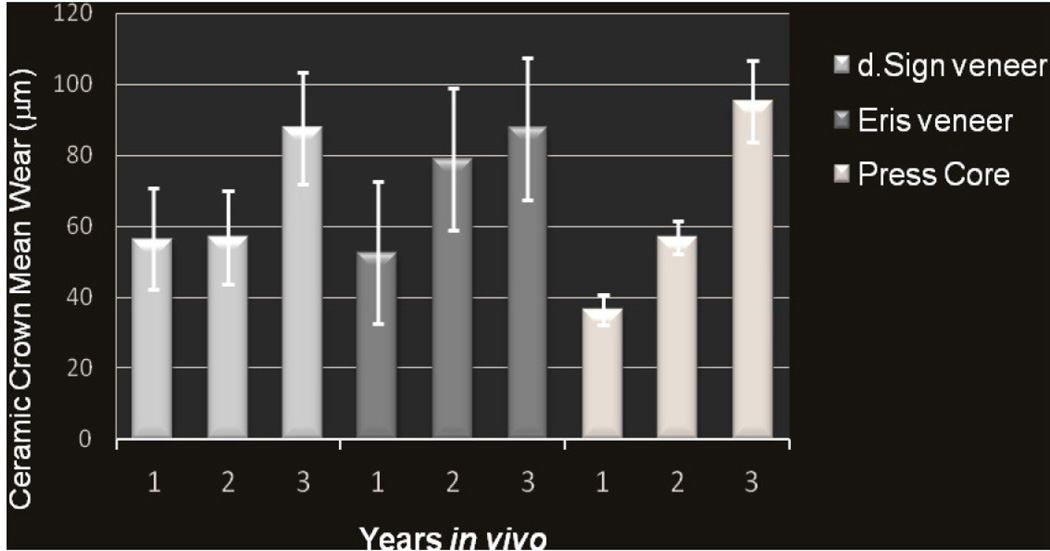
When analyzing all sites in the first two years, no statistically significant correlation was indicated among the amount of wear and the ceramic (p=0.09), or bite force (p=0.15). However, when considering only natural enamel wear in relation to tooth location there were significant differences (p=0.04) with regard to time between years 1 and 2 (p<0.02). Further analysis indicated that the contralateral enamel wore less than the ceramics and the enamel antagonist (p=0.05). The overall mean ceramic wear in year 2 increased relative to year 1 (Figure 1).
Figure 1.
Mean ceramic crown wear for each of three ceramic surfaces measured annually for three consecutive years.
Relative to the wear of only the natural contralateral-antagonist enamel, the time of service and ceramic were not significant factors (p>0.25). However, bite force did demonstrate an effect with regard only to the contralateral-antagonist enamel wear (p<0.0001). It also exhibited significantly more wear than the natural enamel crown contralateral (p=0.0006). The enamel wear for the three enamel antagonists over three years is shown in Figure 2. Any decrease in wear values was associated with patients released from the study.
Figure 2.
Natural enamel mean wear for the three antagonists associated with each restorative crown in relation to intra-oral location, year, and ceramic. The decrease in Year 2 core antagonist mean wear data is due to patient attrition.
3.2 Bite Force Results
The maximum bite forces recorded within this study population ranged from 1272 N to 125 N (range = 1147 N). The overall mean bite force was 360 N and the median was 285 N. There was no correlation between bite force, considered as a covariate in the analysis, and wear through year 1 (Figure 3). In this study population, 77% demonstrated bite forces between 100 and 400 N despite the wide range of values. The highest ceramic wear in year 1 was for d.Sign veneer (166 µm) and corresponded to a bite force of 267 N. However, the highest bite force (1272 N) was associated with only 30 µm wear for the same porcelain, also in year 1. The bite forces for this study population, as they relate to wear amounts for each ceramic, are presented in Table 1.
Figure 3.
Note the lack of correlation between the measured bite force and the amount of in vivo single-tooth ceramic crown wear in Year 1.
Table 1.
The bite force values (N) for the clinical patient population are shown in relation to the respective restorative ceramics.
| Bite Forces (N) in relation to Ceramic for Year 1 | |||
|---|---|---|---|
| Core | Veneer (d.Sign) | Veneer (Eris) | |
| Mean | 291.7 | 469.8 | 308.1 |
| Median | 285 | 325 | 329 |
| Range | 498.2 | 1138.8 | 551.6 |
3.3 Images: 3D Laserscans and SEM Micrographs
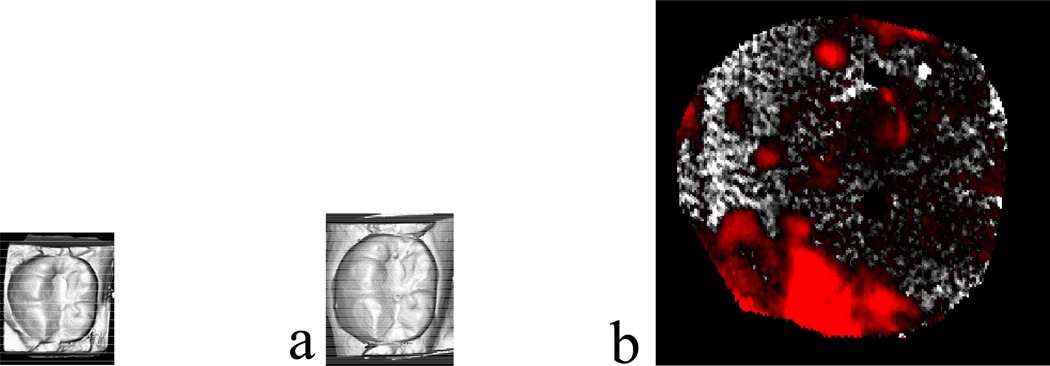
The wear values were based on subtractive analysis calculations of the laser 3D surface scans. Micrographs of these same wear effects, such as roughness and directional patterns were captured using replicate models of ceramic crowns and enamel antagonist teeth that exhibited wear in excess of 100 µm. The veneered surface of an all-ceramic crown specimen from a lower left, first molar restoration exhibited rougheness on the buccal cusps after one year in vivo. This wear (Figures 4 and 5) was considered clinically significant because the patient complained of surface roughness on the crown.
Figure 4.
Laserscan 3D images of a glass-ceramic veneer restoration for one lower left, first molar: a) baseline; b) after one year; c) superimposed subtractive image. The red areas indicate less surface material, i.e., the most wear.
Figure 5.
Micrographs (SEM) for glass-ceramic veneer surface on a mandibular molar restoration after one year: a) mesiobuccal cusp area wear; b) arrows indicating surface area wear; c) left-to-right directional indications; d) ridges within the wear area. (25× magnification).
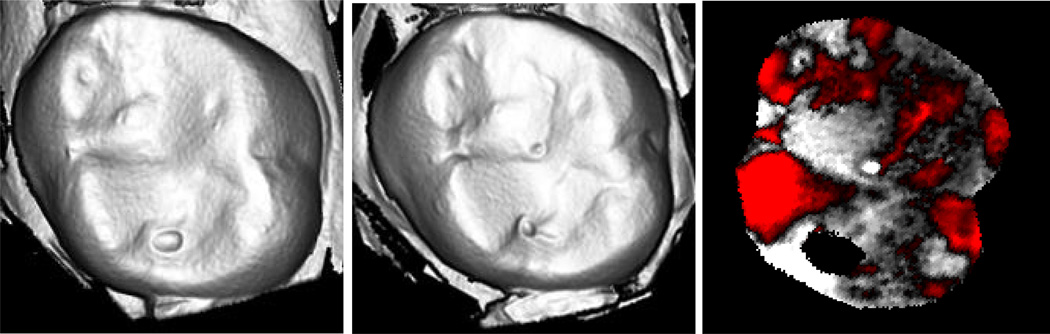
The mean wear of enamel antagonists (141 µm) is represented in laser scans (Figure 6) for a maxillary right first molar that opposed a veneered surface. The micrographs of the same specimen (Figure 7) highlight the surface roughening at the wear facets. However, the wear rates on the crown and tooth opposing these representative samples were not significant.
Figure 6.
Laserscan 3D images for one natural enamel, maxillary, right first molar antagonist (opposed ceramic crown): a) baseline; b) after one year; c) superimposed subtractive image. The red areas represent enamel wear on buccal and lingual cusps after year 1.
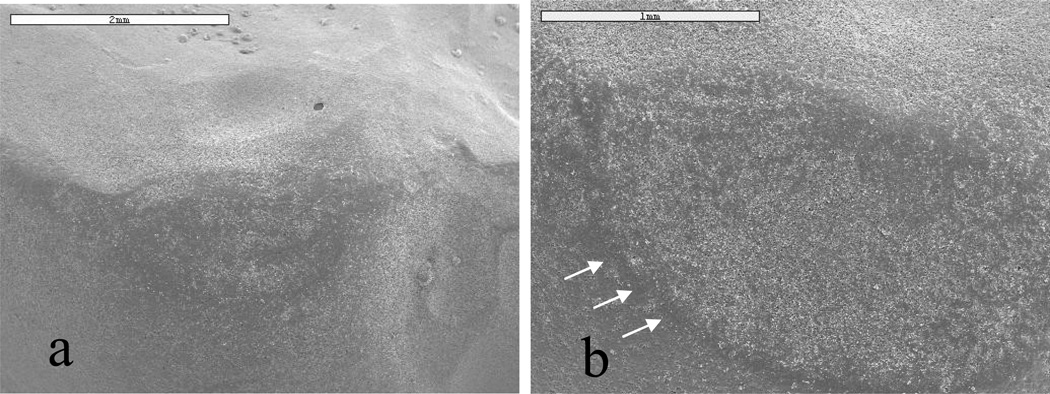
Figure 7.
Micrographs (SEM) for natural enamel, maxillary molar that opposed glass-ceramic veneer: a) baseline; and, b) after year 1. The increased area concentration of white (b) depicts the surface change in the natural enamel. Note the definition on the wear facet (b) after year 1.
4. Discussion
The clinically relevant significance of this in vivo pilot study was that core ceramic crowns fabricated with a glaze, not a traditional veneer, did not cause excessive wear of the opposing enamel antagonists. These results demonstrated that the overall mean in vivo wear for the three ceramics over three years was comparable to that of the enamel antagonist.
Dental literature has relatively few reports of in vivo enamel wear. Using multiple analytical methods, reported enamel wear has ranged from 18 to 261 µm. One study reported mean enamel/enamel wear for 21 patients over a 2 year period as 38 µm for molars and 18 µm for premolars [31]. Kramer et al. reported 4 years of wear for ceramic inlays (78 µm) against enamel (120 µm) [25]. In another study, Etman et al. [32] determined that wear of enamel antagonists ranged from 156 to 261 µm when opposed by three ceramics (176 to 321 µm wear) after 2 years. The use of different methods in obtaining wear data may account for such a broad range among the results.
In our in vivo study, enamel wear on the contralateral side (opposite side, not opposing the restorative crown) was analyzed for two natural teeth as an enamel/enamel control. This is referred to as a “cross-over” study, whereby the patient provides their own enamel wear “control”. This protocol was implemented given that enamel and intraoral conditions can vary considerably among individuals. Thus, while most studies include only the restoration antagonist wear, this study included two natural enamel antagonistic controls. Interestingly enough, the enamel on the contralateral-antagonist teeth exhibited the most wear.
The significant effect of maximum bite force on the analyses for a separate wear site indicates that the measured maximum bite force is most likely not distributed uniformly over the occlusal surface. In general, reported isometric maximum bite forces exceed the variability of recorded masticatory forces. The primary focus in this study was wear analysis, therefore, it was determined that the one-time measurement per patient for maximum isometric bite force would be used. One may also suggest that masticatory posturing could exist, whereby the patient either consciously or unconsciously favors chewing on the opposite side of the crown. However, this theory fails to explain why the contralateral enamel demonstrated the least overall wear. While crossover studies are practical, the results should be viewed very cautiously as the experimental side may affect the non-restored control side indirectly through a n immeasurable means. Other clinical factors such as bite force and individual patient behavior may well play a heretofore minimized role in wear analyses. Statistical significance cannot be judged solely on the basis of wear. As excessive wear of teeth may cause detrimental effects on the stomatognathic system, the mechanism of wear should be examined in vivo in relation to the microstructure of ceramic materials to more fully assess the effects of ceramics and wear on human health.
We accept the hypotheses that equivalent wear occurs between ceramic/enamel and enamel/enamel pairs. The results of this clinical research indicate that the ceramics studied offer a restorative option with low abrasive wear potential. The fact that the contralateral-antagonist enamel demonstrated the greatest variability of wear among the four intraoral sites in this study was not anticipated. Future in vivo clinical research should address this phenomenon.
Acknowledgments
The authors acknowledge Ivoclar Vivadent for their support of this project; as well as, Robert B Lee, a n d Dr. Chuchai Anumana (University of Florida); and Javier Luna of Creative Smiles Labs for their research support efforts.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Clelland NL, Agarwala V, Knobloch LA, Seghi RR. Wear of enamel opposing low-fusing and conventional ceramic restorative materials. J Prosthodont. 2001;10:8–15. doi: 10.1111/j.1532-849x.2001.00008.x. [DOI] [PubMed] [Google Scholar]
- 2.Okeson JP. Etiology and treatment of occlusal pathosis and associated facial pain. J Prosthet Dent. 1981;45:199–204. doi: 10.1016/0022-3913(81)90340-1. [DOI] [PubMed] [Google Scholar]
- 3.Heintze SD, Cavalleri A, Forjanic M, Zellweger G, Rousson V. Wear of ceramic and antagonist-a systematic evaluation of influencing factors in vitro. Dent Mater. 2008;24:433–449. doi: 10.1016/j.dental.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 4.Della Bona A, Kelly JR. The clinical success of all-ceramic restorations. J Am Dent Assoc. 2008;139:8S–13S. doi: 10.14219/jada.archive.2008.0361. [DOI] [PubMed] [Google Scholar]
- 5.Taskonak B, Sertgöz A. Two-year clinical evaluation of lithia-disilicate-based all-ceramic crowns and fixed partial dentures. Dent Mater. 2006;22:1008–1013. doi: 10.1016/j.dental.2005.11.028. [DOI] [PubMed] [Google Scholar]
- 6.Borelli GA. Excudit Petrus Vander. Rome: A. Bernabo; 1680–1681. De Motu Anumalium. [Google Scholar]
- 7.Rowlett AE. The gnathodynamometer and its use in dentistry. Proc R Soc Med. 1933;26:463–471. doi: 10.1177/003591573302600456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gibbs CH, Mahan PE, Mauderli A, Lundeen HC, Walsh EK. Limits of human bite strength. J Prosthet Dent. 1986;2:226–229. doi: 10.1016/0022-3913(86)90480-4. [DOI] [PubMed] [Google Scholar]
- 9.Abe Y, Sato Y, Taji T, Akagawa Y, Lambrechts P, Vanherle G. An in vitro wear study of posterior denture tooth materials on human enamel. J Oral Rehabil. 2001;28:407–412. doi: 10.1046/j.1365-2842.2001.00670.x. [DOI] [PubMed] [Google Scholar]
- 10.Clelland NL, Agarwala V, Knobloch LA, Seghi RR. Relative wear of enamel opposing low-fusing dental porcelain. J Prosthodont. 2003;12:168–175. doi: 10.1016/S1059-941X(03)00051-2. [DOI] [PubMed] [Google Scholar]
- 11.Elmaria A, Goldstein G, Vijayaraghavan T, Legeros RZ, Hittelman EL. An evaluation of wear when enamel is opposed by various ceramic materials and gold. J Prosthet Dent. 2006;96:345–353. doi: 10.1016/j.prosdent.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Heintze SD, Cavalleri A, Forjanic M, Zellweger G, Rousson V. A comparison of three different methods for the quantification of the in vitro wear of dental materials. Dent Mater. 2006;22:1051–1062. doi: 10.1016/j.dental.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 13.Kadokawa A, Suzuki S, Tanaka T. Wear evaluation of porcelain opposing gold, composite resin, and enamel. J Prosthet Dent. 2006;96:258–265. doi: 10.1016/j.prosdent.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 14.Olivera AB, Marques MM. Esthetic restorative materials and opposing enamel wear. Oper Dent. 2008;33:332–337. doi: 10.2341/07-95. [DOI] [PubMed] [Google Scholar]
- 15.DeHoff PH, Barrett AA, Lee RB, Anusavice KJ. Thermal compatibility of dental ceramic systems using cylindrical and spherical geometries. Dent Mater. 2008;24:744–752. doi: 10.1016/j.dental.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cesar PF, Soki FN, Yoshimura HN, Gonzaga CC, Styopkin V. Influence of leucite content on slow crack growth of dental porcelains. Dent Mater. 2008;24:1114–1122. doi: 10.1016/j.dental.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Dental Materials – Guidance on testing of wear. Part 2: Wear by two- and/or three body contact. First edition. Geneva, Switzerland: 2001. ISO Standard/Technical Report 14569-2. [Google Scholar]
- 18.Suzuki S, Suzuki SH, Cox CF. Evaluating the antagonistic wear of restorative materials when placed against human enamel. J Am Dent Assoc. 1996;127:74–80. doi: 10.14219/jada.archive.1996.0033. [DOI] [PubMed] [Google Scholar]
- 19.Heintze SD. Memorandum: Guidelines for the fabrication of plaster models. Ivoclar Vivadent, Liechtenstein: Research and Development; [Google Scholar]
- 20.Mehl A, Gloger W, Kunzelmann KH, Hickel R. A new optical 3-D device for the detection of wear. J Dent Res. 1997;76:1799–1807. doi: 10.1177/00220345970760111201. [DOI] [PubMed] [Google Scholar]
- 21.Folwaczny M, Mehl A, Kunzelmann KH, Hickel R. Determination of changes on tooth-colored cervical restorations in vivo using a three-dimensional laser scanning device. Eur J Oral Sci. 2000;108:233–238. doi: 10.1034/j.1600-0722.2000.108003233.x. [DOI] [PubMed] [Google Scholar]
- 22.Perry R, Kugel G, Kunzelmann KH, Flessa HP, Estafan D. Composite restoration wear analysis: conventional methods vs. three-dimensional laser digitizer. J Am Dent Assoc. 2000;131:1472–1477. doi: 10.14219/jada.archive.2000.0060. [DOI] [PubMed] [Google Scholar]
- 23.User’s Manual, Laserscan 3D. Special Edition. etkon, Gräfeling Germany: es 1 Scanner; 2001. [Google Scholar]
- 24.Suputtamongkol K, Anusavice KJ, Suchatlampong C, Sithiamnuai P, Tulapornchai C. Clinical performance and wear characteristics of veneered lithia-disilicate-based ceramic crowns. Dent Mater. 2008;24:667–673. doi: 10.1016/j.dental.2007.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kramer N, Kunzelmann KH, Taschner M, Mehl A, Garcia-Godoy F, Frankenberger R. Antagonist enamel wears more than ceramic inlays. J Dent Res. 2006;85:1097–1100. doi: 10.1177/154405910608501206. [DOI] [PubMed] [Google Scholar]
- 26.Holand W, Schweiger M, Frank M, Rheinberger V. A comparison of the microstructure and properties of the IPS Empress 2 and the IPS Empress glass-ceramics. J Biomed Mater Res. 2000;53:297–303. doi: 10.1002/1097-4636(2000)53:4<297::aid-jbm3>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 27.Höland W, Beall G. Glass Ceramic Technology. Westerville, OH, USA: Amercian Ceramic Society; 2002. [Google Scholar]
- 28.Oh SC, Dong JK, Luthy H, Scharer P. Strength and microstructure of IPS Empress 2 glass-ceramic after different treatments. Int J Prosthodont. 2000;13:468–472. [PubMed] [Google Scholar]
- 29.International Standards Organization. ISO 6872: Dentistry-Ceramic Materials. Geneva Switzerland: 2008. [Google Scholar]
- 30.Boucher CO. Occlusion in prosthodontics. J Prosthet Dent. 1953;3:633–656. [Google Scholar]
- 31.Lambrechts P, Braem M, Vuylsteke-Wauters M, Vanherle G. Quantitative in vivo wear of human enamel. J Dent Res. 1989;68:1752–1754. doi: 10.1177/00220345890680120601. [DOI] [PubMed] [Google Scholar]
- 32.Etman MK, Woolford M, Dunne S. Quantitative measurement of tooth and ceramic wear: in vivo study. Int J Prosthodont. 2008;21:245–252. [PubMed] [Google Scholar]