Abstract
In clinical trials mesenchymal stem cells (MSCs) are transplanted into cardiac ischemic regions to decrease infarct size and improve contractility. However, the mechanism and time course of MSC-mediated cardioprotection are incompletely understood. We tested the hypothesis that paracrine signaling by MSCs promotes changes in cardiac excitation-contraction (EC) coupling that protects myocytes from cell death and enhances contractility. Isolated mouse ventricular myocytes (VMs) were treated with control tyrode, MSC conditioned-tyrode (ConT) or co-cultured with MSCs. The Ca handling properties of VMs were monitored by laser scanning confocal microscopy and whole cell voltage clamp. ConT superfusion of VMs resulted in a time dependent increase of the Ca transient amplitude (ConT15min: ΔF/F0=3.52±0.38, n=14; Ctrl15min: ΔF/F0=2.41±0.35, n=14) and acceleration of the Ca transient decay (τ: ConT: 269±18 ms n=14; vs. Ctrl: 315±57 ms, n=14). Voltage clamp recordings confirmed a ConT induced increase in ICa,L (ConT: −5.9±0.5 pA/pF n=11; vs. Ctrl: −4.04±0.3 pA/pF, n=12). The change of τ resulted from increased SERCA activity. Changes in the Ca transient amplitude and τ were prevented by the PI3K inhibitors Wortmannin (100 nmol/L) and LY294002 (10 μmol/L) and the Akt inhibitor V (20 μmol/L) indicating regulation through PI3K signal transduction and Akt activation which was confirmed by western blotting. A change in τ was also prevented in eNOS−/− myocytes or by inhibition of eNOS suggesting an NO mediated regulation of SERCA activity. Since paracrine signaling further resulted in increased survival of VMs we propose that the Akt induced change in Ca signaling is also a mechanism by which MSCs mediate an anti-apoptotic effect.
Keywords: Mesenchymal stem cells, Excitation contraction coupling, Akt, Ca imaging, Paracrine signaling, Nitric oxide synthase
1. Introduction
The systematic review and meta-analysis of clinical studies indicate that transplantation of bone marrow derived stem cells after myocardial infarction or ischemic cardiomyopathy is beneficial [1,2]. Patients exhibit decreased infarct size, increased left ventricular ejection fraction, and decreased left ventricular end systolic volume, leading to an overall improved outcome [2–4]. However, even after direct injection into the ventricular wall the retention or homing of cells to the cardiac muscle is low [5]. Differentiation of bone marrow derived stem cells into cardiomyocytes was demonstrated in vitro [6,7], but transplanted stem cell derived cardiomyocytes can be identified only in low numbers [8–12]. The low retention rate together with the low potential for cardiac differentiation makes it likely that MSCs improve cardiac contractile function through the secretion of paracrine factors rather than the addition of functional cardiomyocytes. A direct effect on cardiac contractility has been proposed but the mechanism has not been elucidated [13,14].
Experimental evidence that paracrine signaling of MSCs plays a significant role in cardiac protection is derived from co-culture experiments with cardiomyocytes [9,15–17] and from injection of stem cell conditioned media into the heart [14,17]. In addition, changes in the MSC secretion profile by IGF [18], SDF [19], or Akt [20,21] overexpression also changed their effect on cardiomyocytes or cardiac tissue. However, isolated application of a single growth factor (e.g. IGF) did not replicate the benefit of MSC conditioned media [22].
The overall cardioprotective effect of MSCs after cardiac ischemic injury was attributed to activation of pro-survival signaling including the Akt, JAK-STAT, and ERK signaling pathways [23]. It has been speculated that an early improvement of cardiac contractility was not due to the increased number of surviving cells but due to a direct effect of MSC released inotropic factors onto the cardiomyocytes [24]. So far the mechanism by which these changes in EC coupling are induced is not yet identified.
Presently we have tested the hypothesis that MSCs can induce an immediate change in cardiac EC coupling through the release of paracrine factors producing an Akt mediated activation of nitric oxide synthase (NOS) that can promote cell survival.
2. Methods
2.1. Cell culture
Mouse bone marrow was isolated from tibia and femur bones [7,12] and mesenchymal stem cells were enriched for CD5, CD45R, CD11b, Gr1, TER119 (Murine Progenitor Enrichment Cocktail, Stemcell Technologies) and found to be Sca-1+, CD34+, ckit+, CD105+, CD90.1+ positive and negative for CD45−. Cells were cultured in Mesencult medium (Stemcell Technologies) supplemented with penicillin and streptomycin. Human MSCs were obtained from Dr. DJ. Prockop (Texas A&M, Institute for Regenerative Medicine) and cultured as previously described [25].
Ventricular myocytes were isolated from 3 to 5 month old C57BL/6, phospholamban knockout (PLN−/−) and endothelial nitric oxide synthase knockout (eNOS−/−) mice by Langendorff perfusion [26]. All animal procedures were performed with the approval of IACUC at the University of Illinois at Chicago and in accordance with the National Institute of Health's Guide for the Care and Use of Laboratory Animals. Cells were plated on laminin (Sigma Aldrich: 1 mg/mL) coated 25 mm coverslips in standard tyrode solution (in mmol/L: NaCl 130, KCl 5.4, CaCl2 1, MgCl2 1.5, NaHCO3 10, Glucose 10, Hepes 25; pH 7.4). A co-culture (CoC) of VMs and MSCs was established by plating the cells together on the same coverslip at 1:1 ratio. To establish conditioned cultures (ConC), cell culture inserts (Millipore: pore size: 1 μm) containing MSCs were added to the previously plated VMs. Conditioned tyrode (ConT) was obtained by overnight incubation (15 h) of 80% confluent MSC culture dishes (10 cm) with tyrode solution (10 mL) at 37 °C (in mmol/L: NaCl 130, KCl 5.4, CaCl2 1, MgCl2 1.5, NaHCO3 10, glucose 10, HEPES 25, L-glutamine 4, nonessential amino acids 0.1; pH 7.4). CoC and ConC cultures were used for measurements of [Ca]i between 3 h and 7 h after plating. All experiments were performed at room temperature.
2.2. Intracellular Ca2+ measurements
To visualize changes of the intracellular Ca2+ concentration, VMs were incubated (15 min) at room temperature with fluo-4 acetoxymethyl ester (5 μmol/L; fluo-4/AM; Invitrogen). After washout, 15 min was allowed for de-esterification of the dye. Ca2+ measurements were performed as previously described [27,28]. Whole-cell Ca2+-transients were obtained from confocal linescan images through single VMs by averaging the signal of an individual cell. Ca2+-transients are presented as background-subtracted normalized fluorescence (F/F0). VMs were stimulated at a frequency of 0.5 Hz for the duration of the experiment.
2.3. Calibration of [Ca]i
The calibration of Fluo-4/AM was performed based on the method published by Ward et al. [29]. Briefly, F/F0=min (Fmin/F0) and F/F0=max (Fmax/F0) were determined for every VM at the end of an experiment. For this purpose VMs were exposed to a solution containing (mmol/L: NaCl 140, KCl 5.4, MgCl2 1.5, HEPES 5, glucose 10, EGTA 10, caffeine 10, 2,3-butanedione monoxime 10, ouabain 1, ionomycin 0.005; pH=7.2) to obtain Fmin/F0. Additionally cells were exposed to a solution where EGTA was replaced by CaCl2 (2 mmol/L) and NaCl replaced by LiCl (140 mmol/L). The resulting fluorescence was designated Fmax/F0. The following equation was used to convert Fluo-4 F/F0 fluorescence signals into [Ca2+]i concentrations:
where Kd=770 nmol/L was used as the apparent Fluo-4 dissociation constant [30].
2.4. SDS-PAGE and immunoblotting
Plated ventricular myocytes were recovered following experimental treatment with the addition of hot 1-X Laemmli sample buffer without β-mercaptoethanol (β-ME) or bromophenol blue dye and heated to 95 °C for 5 min. Sample protein determinations were made with a BCA protein assay kit (Pierce) and then β-ME and dye were added to the final concentrations for 1-X sample buffer and heated as before. Cell lysates were separated using pre-cast 4–20% Novex tris-glycine gels (Invitrogen) and following standard electrophoresis protocols for SDS-PAGE and immunoblotting as previously described [31]. Typically 25–45 μg of protein was loaded per well. Primary antibodies used for western blotting were against phospho-Akt (Thr308) and (Ser473), pan-Akt and phospho-GSK-3 α/β (Ser21/9) from Cell Signaling Technology and p-PLN(Thr17) from Santa Cruz Biotech. Species-specific horseradish peroxidase-conjugated secondary antibodies were used and visualization was accomplished using Western Lighting chemiluminescence reagents (PerkinElmer) and Kodak BioMax film.
2.5. Patch clamp recordings
Ventricular myocytes incubated (3 h) in either Ctrl tyrode or ConT were used for patch clamp studies of the L-type Ca current (Ica,L). Experiments were performed in the ruptured patch configuration. The microelectrodes had a resistance of 1–3 MΩ when filled with pipette solution containing (mmol/L: CsCl 125, TEA-Cl 20, EGTA 10, HEPES 10, phosphocreatine 5, Mg2ATP 5, GTP 0.3; pH=7.2). To isolate ICa,LVMs were voltage clamped to a holding potential of −50 mV and voltage pulses of 300 ms duration were applied from −50 to +50 mV in 10 mV increments.
2.6. Cell shortening
Cell length was recorded with a CCD camera and offline cell shortening was analyzed by Metamorph software (Molecular Devices, Sunnyvale, CA) tracking the cell ends to determine diastolic and systolic cell lengths.
2.7. Chemicals
Akt inhibitor V Tricirbine was purchased from EMD/Calbiochem, all other chemicals were purchased from Sigma-Aldrich. Data sets were statistically evaluated using paired t-test. All averaged data are presented as means ±S.E.M.
3. Results
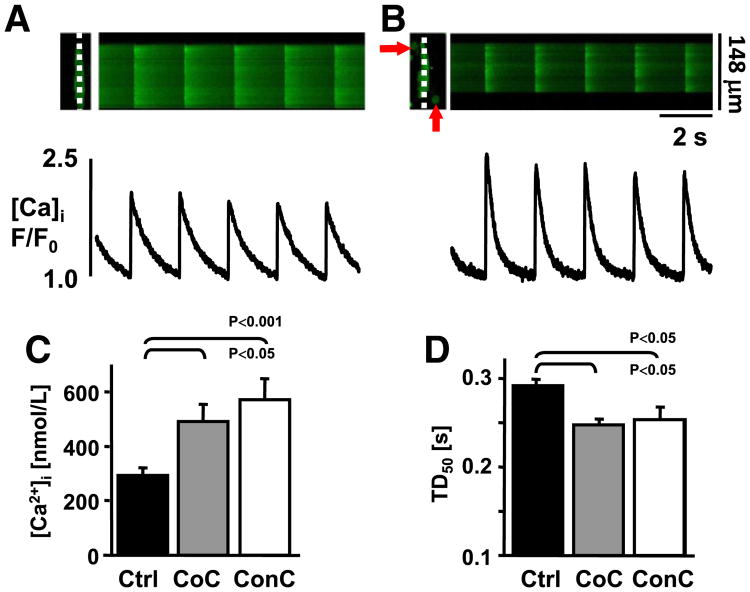
To determine if MSCs influence the EC coupling of VMs we compared Ca transient properties of isolated VMs under control conditions with those that were co-cultured with MSCs (CoC) for 3 h at room temperature. During field stimulation (0.5 Hz) ventricular myocytes in CoC conditions (Figs. 1A,B) exhibited increased Ca transient amplitude ([Ca]i: Ctrl: 293±29 nmol/L, (n=8); CoC: 492±63 nmol/L, n=8; p<0.05; Fig. 1C), and decreased Ca transient duration at 50% decay (TD50: Ctrl: 291±6 ms, n=151 vs. CoC: 247±4 ms, n=161; Fig. 1D).
Fig. 1.
MSCs modulate cardiac excitation–contraction coupling. Confocal linescan images (top) and F/F0 plots of field stimulated isolated VMs that were maintained (3 h) alone (A) or in the presence of MSCs (B; arrows). (C) Ca transient amplitudes are increased in CoC (gray) and ConC (white) cultures, [Ca]i is presented after calibration. (D) Transient duration measured at 50% decay is shortened in CoC and ConC conditions.
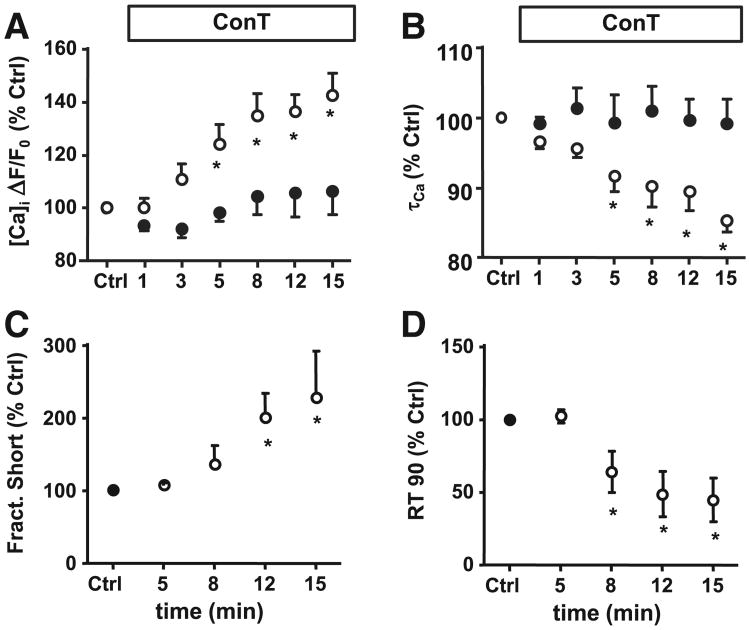
It was demonstrated in vivo as well as in vitro that MSCs can establish intercellular coupling with adult myocytes [32–34]. To test the hypothesis that cell–cell contact or coupling between MSCs and VMs is not necessary for the MSC induced changes in Ca signaling we used an MSC conditioned culture model (ConC) and MSC conditioned tyrode (ConT). As shown in Figs. 1C,D, after 3 h ConC VMs exhibited increased Ca transient amplitudes (Fig. 1C) and their TD50 (Fig. 1D) was significantly decreased in comparison to Ctrl ([Ca]i: ConC: 572±75 nmol/L, n=7; TD50: 253±13 ms, n=46). Superfusion of VMs with ConT resulted in a progressive change of the Ca transient (Fig. 2A). The TA increased from F/F0 t0: 2.5±0.23 to t15′: 3.52±0.38 (n=14; p<0.05) after 15 min of ConT superfusion which correlated with an increase in fractional shortening (Fig. 2C). Over the same time period τ decreased from t0′: 315±21 ms to t15′: 269±18 ms (p<0.05; Fig. 2B) and the time to 90% relaxation (RT90) decreased (Fig. 2D). For VMs superfused with Ctrl solution TA and τ did not change (ΔF/F0: t0: 2.28±0.3, n=6; t15′: 2.41±0.35; τ: t0′: 315±57 to t15′: 304±49 ms; n=6). The results support the hypothesis that MSCs promote rapid changes in the EC coupling of VMs independent of cell–cell contact.
Fig. 2.
MSCs modulate E-CC through paracrine signaling. Time dependent changes of (A) Ca transient amplitude and (B) time constant of inactivation (τCa) during superfusion of VMs with Ctrl (●) or ConT (o) solution. (C) Fractional shortening was enhanced and (D) time to 90% relaxation was decreased upon superfusion with ConT (*=p<0.05).
3.1. Mechanism of Ca transient increase
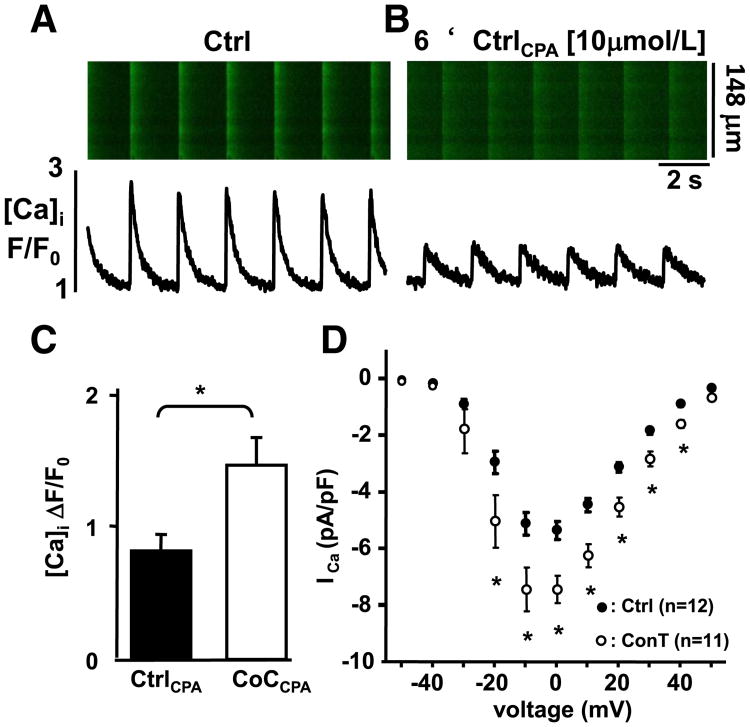
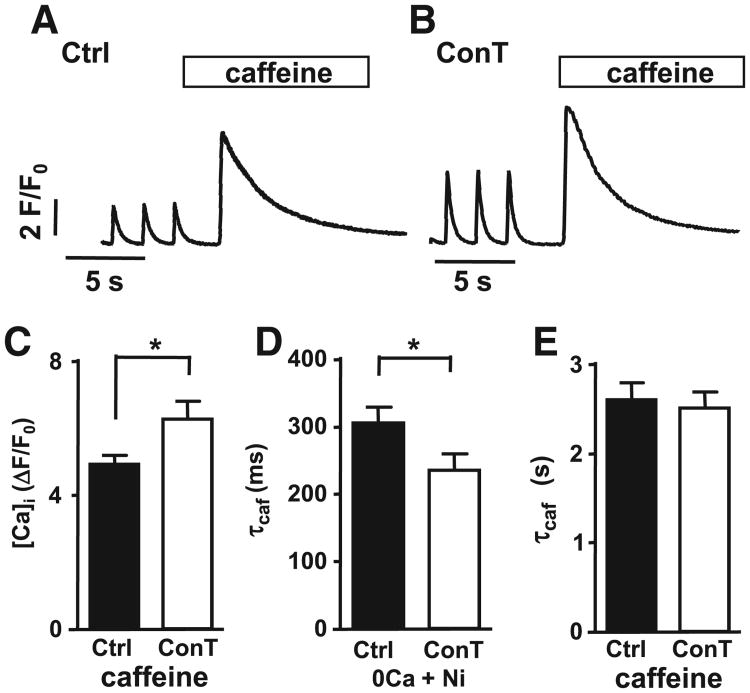
The Ca transient amplitude depends on the voltage-dependent influx of Ca through ICa,L and the subsequent Ca-induced Ca release (CICR) from ryanodine receptors (RyRs) that are located in the sarcoplasmic reticulum (SR) [35]. To determine the contribution of ICa,Land SR Ca release to the MSC-induced increase in Ca transient amplitude we measured Ca influx transients after inhibition of SR Ca-uptake with CPA [10 μmol/L]. The overall Ca transient amplitude was significantly reduced in the presence of CPA (Figs. 3AB; ΔF/F0: Ctrl: 2.48±0.34 vs. CtrlCPA: 0.8±0.09; n=4) however, in CoCCPA conditions the TAs remained significantly increased in comparison to CtrlCPA (ΔF/F0: CoCCPA: 1.49±0.19; n=5; Fig. 3C). To confirm that the increased influx transient can be induced by ConT alone, we measured ICa,L in the whole cell voltage clamp configuration in VMs maintained in Ctrl tyrode or ConT (3 h). As shown in Fig. 3D VMs in ConT (3 h) exhibited a significant increase in ICa,L density (at − 10mV: Ctrl: 5.15±0.4 pA/pF, n=12 vs ConT: 7.43±0.7 SE pA/pF, n=11; unpaired t-test p<0.01). To determine the SR load, Ctrl or ConT treated cells were superfused with caffeine [10 mmol/L] (Figs. 4AB). The experiments revealed a significant increase in the amplitude of the caffeine transient in the presence of ConT (Ctrl: TAcaf=4.9±0.27, n=14; ConT: TAcaf 6.27±0.5, n=11, p<0.05; Fig. 4C) and support the hypothesis that MSCs can enhance the VMs Ca transient by increasing Ca influx and SR load.
Fig. 3.
MSC paracrine signaling induces an increase in ICa,L. (A) Representative confocal line scan images and F/F0 plots from a cardiomyocyte in control conditions (Ctrl) and (B) during superfusion with CPA. (C) Representation of the mean Ca transient amplitude in Ctrl (black) and CoC (white) conditions in the presence of CPA. (D) Current voltage relationship of ICa,L measured by whole cell voltage clamp in Ctrl (●, n=12) and ConT treated (3h; o, n=11) VMs (*=p<0.05).
Fig. 4.
MSC paracrine signaling increases SERCA mediated Ca uptake. Representative F/F0 plots from field stimulated VMs incubated in (A) Ctrl and (B) ConT where the SR was depleted by application of caffeine (10 mM). (C) Summary data of caffeine transient amplitude in Ctrl (black) and ConT (white) treated cells. Summary data of the time constant of caffeine transient decay (τcaf) in the presence of (E) caffeine and (D) 0 Ca + Nickel. (*=p<0.05).
3.2. Mechanism of accelerated Ca transient decay time
After the excitation induced rise in [Ca]i, Ca is predominantly removed from the cytoplasm by the sarcoplasmic reticulum Ca ATPase (SERCA) and the sodium calcium-exchanger (NCX). We aimed to determine if the accelerated decay constant in the presence of MSCs or ConT results from a change in SERCA and/or NCX dependent Ca removal. To quantify SERCA mediated Ca removal we blocked NCX by superfusion of VMs with nickel (5 mmol/L) in the absence of [Ca]o while SR Ca release was induced by caffeine [28]. The decay time of the caffeine induced transient (Fig. 4D) was shorter in ConT (235±25 ms, n=11) than in Ctrl (306±24 ms, n=14, p<0.05) conditions, indicating that SERCA activity is increased by ConT. In contrast NCX activity which was monitored in the continued presence of caffeine (Fig. 4E) [28], was not significantly different between Ctrl (A) and ConT (B) treated cells (Ctrl: 2.60±0.19s, n=14; ConT: 2.51±0.18 s, n=11).
3.3. Akt signaling pathway
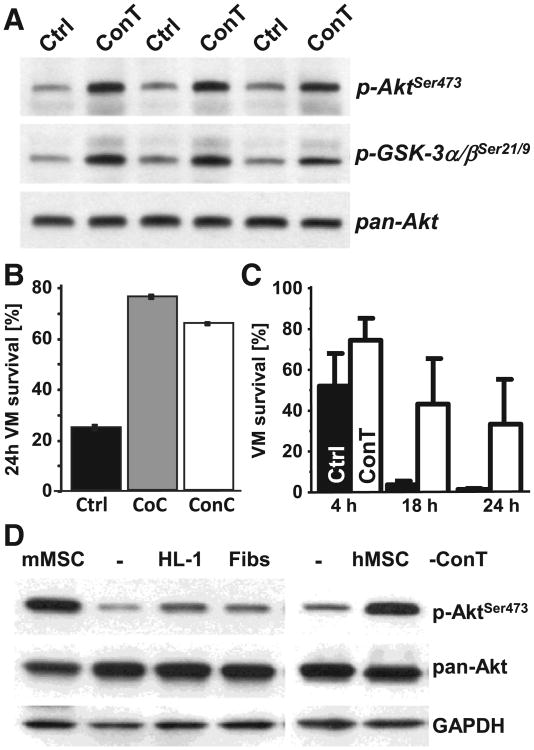
MSCs secrete an array of factors that have auto- and paracrine functions [24] many of them are growth factors whose effect is mediated by the PI3K/Akt pathway. To determine if ConT can induce Akt phosphorylation in cardiomyocytes we blotted whole cell lysates from VMs (Fig. 5A) treated with ConT or Ctrl tyrode (15 min). In contrast to Ctrl, ConT treated cells exhibited increased phosphorylation of Akt serine 473 (p-AktSer473). Also GSK-3α/β a downstream target of Akt exhibited phosphorylation indicating its inactivation. Akt promotes anti-apoptotic signaling therefore, we analyzed VM cell survival (24 h) by trypan blue exclusion. In CoC, ConC and ConT conditions the percentage of trypan blue negative VMs was significantly increased compared to Ctrl cultures (Figs. 5B,C) clearly indicating that MSCs enhance cell survival. The secretion of paracrine factors is not unique to MSCs; however, comparison of the effect of ConT derived from HL-1 cells and fibroblasts and with that from human and mouse MSCs (ConTh, ConTm) revealed that Akt phosphorylation was much more activated by MSC derived ConT relative to other cell types (Fig. 5D). The ConT induced Akt phosphorylation can be directly linked to changes in EC coupling. When VMs were superfused with ConTh a significant increase in Ca transient amplitude and acceleration of τCa could be determined (Figs. 6A,B) while fibroblast derived ConT remained without effect (Supplementary Figs. 3A,B).
Fig. 5.
MSC derived paracrine factors induce pro-survival Akt signaling in VMs. (A) Western blotting shows 3 representative experiments of cell lysate from VMs that were treated with Ctrl or ConT (15 min). Immunoblotting for p-Akt(Ser473), p-GSK-3α/β(21/9), and pan-Akt as a loading control are shown. (B) The percentage of VMs surviving after isolation is significantly increased in CoC (gray) and ConC (white) and ConT (C) conditions in comparison to Ctrl (black). (D) Western blot of Akt phosphorylation for HL-1 cells treated with ConT (30 min) derived from mMSCs, HL-1 cells, mouse fibroblasts or hMSCs show that increased Akt phosphorylation is mediated primarily by MSCs. Total Akt (pan-AKT) and GAPDH are shown as loading controls.
Fig. 6.
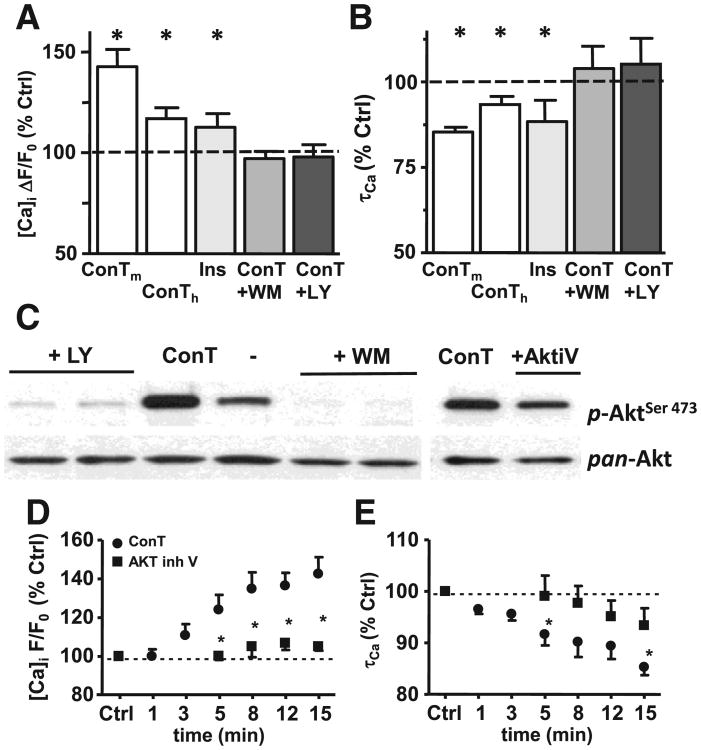
Changes in EC coupling are induced by ConT mediated activation of PI3K and Akt. (A) Change in Ca transient amplitude and τCa (B) as % of Ctrl after 15 min superfusion with ConTm, ConTh (white), insulin (light gray), or ConT in the presence of the PI3K inhibitors wortmannin [WM: 100 nmol/L] (gray) or LY294002 [LY: 10 μmol/L] (black). (C) Western blotting shows that LY, WM and Akt iV [25 μM] suppress ConT (30 min) mediated phosphorylation of Akt. Total Akt (pan-Akt) is shown as a loading control. (D) ConT (●) induced changes in [Ca]i or (E) τCa are suppressed by pretreatment with the Akt inhibitor V [20 μmol/L] (■).
To test the hypothesis that an activator of the Akt pathway can mimic the ConT induced modulation of Ca signaling we superfused VMs with insulin [10 μmol/L] [36]. As described for ConT, insulin induced Akt phosphorylation in cardiomyocytes (Supplementary Fig. 1), an increase in the Ca transient amplitude, and an acceleration of τ (ΔF/F0: t0: 2.88±0.28, n=11; t15′: 3.4±0.44; τ: t0′: 332.8±27 ms to t15′: 301.5±33 ms; n=11; Figs. 6AB).
To establish the interdependence between the increase in Akt phosphorylation and the enhanced EC coupling we determined the effect of ConT (30 min) in cells where PI3K was blocked by wortmannin [WM: 100 nmol/L] and LY294002 [LY: 10 μmol/L] or Akt was inhibited by the Akt inhibitor V [20 μmol/L]. The experimental results presented in Fig. 6 show that WM, LY and Akt inhibitor V attenuate ConT induced Akt phosphorylation (C) and changes in Ca transient amplitude (A) and τCa (B). Superfusion of VMs with the Akt inhibitor V also prevented ConT induced changes in EC coupling (D, E) confirming Akt as the mediator of ConT induced signaling. The Akt inhibitor V by itself did not induce a significant change in EC coupling (Supplementary Figs. 2A,B).
3.4. Mechanism of Akt-induced changes in EC coupling
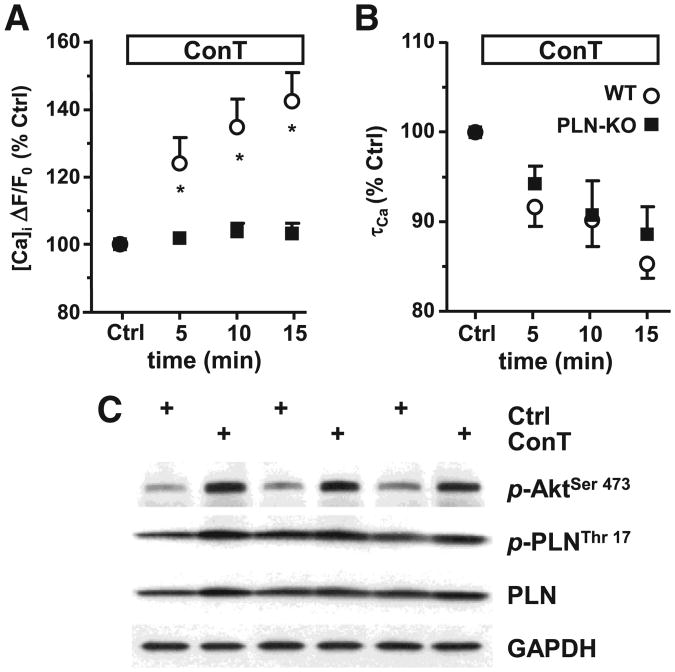
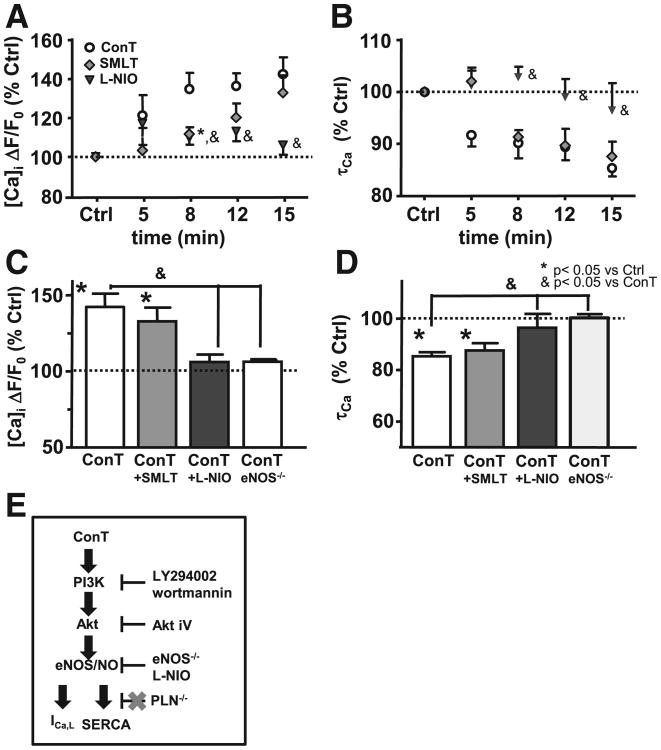
A multitude of effector proteins are regulated by Akt. Aktmediated phosphorylation of phospholamban (PLN) has been reported which would induce its dissociation from SERCA and relieve its inhibitory function [37–39]. We used ventricular myocytes from mice deficient for the expression of PLN (PLN−/−) to determine its role in MSC mediated changes in EC coupling [37]. As shown in Figs. 7A,B while no change in Catransient amplitude was determined, τCa was still shortened after 15 min ConT superfusion (Ctrl: 94±5 ms, n=7; ConT: 82±5 ms, n=7) indicating that Akt-mediated PLN phosphorylation is not predominantly involved in SERCA regulation. The pro-survival benefit of ConT was maintained in PLN−/− VMs (Supplementary Fig. 4). Superfusion of PLN−/− VMs with Ctrl solution induced no change in EC coupling (Supplementary Figs. 3C,D). PLN phosphorylation was not significantly changed after 15 min ConT treatment of VMs (Fig. 7C). PLN independent regulation of SERCA has been described through PKA and CaMK dependent phosphorylations as well as through nitric oxide synthase (NOS) dependent snitrosylation. Since NOS is a downstream target of Akt we tested if ConT induced changes in EC coupling could be induced in the presence of inhibitors against neuronal (n) NOS (SMLT: 10 μmol/L) or endothelial (e) NOS (L-NIO: 1 μmol/L). While the nNOS inhibitor did not prevent ConT induced changes in the Ca transient the eNOS inhibitor significantly attenuated the ConT induced increase in Ca transient amplitude (ΔF/F0: t0: 2.27±0.35; t15′<: 2.43±0.42; n=6) and decrease in τ (τCa: t0′: 454±49ms to t15′>: 433.46±46ms; n=6). SMLT and L-NIO by itself did not induce a significant change in EC coupling (Supplementary Figs. 2A,B). In VMs isolated from eNOS−/− mice no ConT induced change in F/F0 (ΔF/F0: t0: 1.67±0.2; t15′>: 1.73±0.2; n=8) or τCa (τCa: t0: 403±32; t15′: 400±32; n=8) could be determined (Figs. 8C,D) supporting a ConT-mediated PLN-independent regulation of SERCA by PI3K/Akt signaling (for control experiments see Supplementary Figs. 3E,F). A schematic representation summarizing the experimental results and the proposed signal transduction pathway is shown in Fig. 8E.
Fig. 7.
Lack of PLN does not prevent ConT induced shortening of Ca transient. (A) Ca transient amplitude and (B) τCa in PLN−/− VMs recorded during superfusion with Ctrl (O) or ConT (■). (C) Western blotting analysis of 3 representative experiments shows that while ConT (15 min) activates Akt phosphorylation (p-AktSer473) PLN phosphorylation (p-PLNThr17) in WT myocytes remains almost unchanged. Total PLN and GAPDH are shown as loading controls.
Fig. 8.
Changes in EC coupling depend on the activation of eNOS. Changes in Ca transient amplitude (A) and τCa (B) upon superfusion with ConT (o), ConT plus nNOS inhibitor SMLT (10 μmol/L; gray ◊), or eNOS inhibitor L-NIO (1 μmol/L; ▲). Summary of the experimental results for Ca transient amplitude (C) or τCa (D) after 15 min of superfusion (*=p<0.05). (E) Pictoral summarizing the signal transduction pathway proposed based on the experimental results.
4. Discussion
We demonstrate that bone marrow-derived MSCs when cocultured with ventricular myocytes have an immediate impact on the myocyte physiological properties of EC coupling. While MSCs do establish intercellular coupling by gap junction channels, the changes in EC coupling characterized here are solely mediated by paracrine signals released by the MSCs. We further demonstrate that the mechanism of EC coupling change depends on a PI3K/Akt mediated increase of ICa,L and enhanced Ca extrusion from the cytoplasm depends on an eNOS mediated increase in SERCA activity.
4.1. Enhanced EC coupling is independent of cell–cell contact
When cells are co-cultured they can influence each other through cell adhesion, intercellular communication through gap junction channels, and the secretion of paracrine factors. The role of MSC induced paracrine signaling is supported by the injection of conditioned media or tyrode into cardiac infarct regions where the thickness of the ventricular wall is maintained [40] and the capillary density is increased [15,17]. A modulation of the MSCs secretome by i. hypoxia, ii. over-expression of Akt [14], VEGF, or HGF [5] and iii. down-regulation of IGF [18] concomitantly resulted in an alteration of the MSCs cardioprotective effect.
MSCs express connexin (Cx) 43, the major cardiac gap junction channel isoform [32,41,42] and can establish intercellular coupling with ventricular myocytes within 1–2 h of co-culture [32]. Intercellular coupling of cardiomyocytes with unexcitable cells can result in a change of the VMs membrane potential and consequently its electro-physiological properties [43,44]. While we see that cell–cell coupling can be established between VMs and MSCs the changes in cardiomyocyte EC coupling can be reproduced in conditioned cultures or with ConT. In addition, the increase in ICa,L supports the idea that observed changes do not solely depend on a change of the VMs passive electrical properties like membrane potential and capacitance; nevertheless, we would like to acknowledge that cell–cell contact could change parameters in VMs that have not been determined with our method of detection. While the changes we observe in EC coupling are comparable under ConT and ConC conditions we cannot rule out that the secretome varies. MSCs could influence the secretory properties of the ventricular myocytes and vice versa [45]. However, when we superfuse the myocytes with ConT, factors released by the myocytes should be of minor importance since they are immediately washed out.
4.2. Paracrine signaling of ConT
Stem cells secrete cytokines, chemokines and growth factors known to have autocrine as well as paracrine functions. Among these are adrenomedullin, bone morphogenetic protein-2 and -6 (BMP), endothelin (ET-1), and growth factors (GF) like fibroblast-GF (-2 and -7; FGF), transforming-GF (TGF), insulin like-GF (IGF), platelet derived-GF (PDGF), and vascular endothelial-GF (VEGF) (for review see [24]). Many of these factors have been demonstrated by themselves to be cardioprotective. The protective mechanism has been linked to the PI3K→Akt→eNOS (IGF, insulin), the PI3K→Akt→BAD (insulin), or the RAS→MEK→ERK (FGF, TGF, VEGF) signaling cascades [46–48]. Some of the secreted factors were reported to modulate cardiomyocyte EC coupling through the activation of signal transduction cascades (e.g. IGF-1 [49]) or even through genetic reprogramming [45]. We have not yet identified the primary active components in ConT that are required for the changes seen in EC coupling and it is likely that not one but a combination of active components are involved. This is supported by experiments with MSCs deficient for IGF expression where the cardioprotective effect was reduced but not eliminated [50] and by the fact that insulin despite significant Akt activation has a more attenuated effect on EC coupling than ConT. Therefore, this could also point to i. the inhibition of signaling pathways (e.g. phosphatases) that counteract Akt phosphorylation under control conditions or ii. to a differential activation of Akt isoforms by ConT and insulin. Recent evidence suggests that mircoparticles or exosomes from MSCs mediate pro-survival signaling and transfer microRNAs [51,52]. While we cannot rule out the role of microparticles, the reported changes in EC coupling are independent of transcription events given the rapid induction time and more extended periods of ConT treatment were not evaluated. We obtained comparable results with human and mouse MSCs that were selected under different constraints. However, that does not exclude that factors secreted by these cells are part of both secretomes, where analysis of conditioned media by mass spectroscopy shows significant overlap [53–55]. Stem cell specificity of the effects is suggested, given that the effect is not simply reproduced by other mesenchyme derived cell types. However, this may also result from quantitative differences in their secretome.
4.3. MSC mediated changes in EC coupling
We demonstrate that mouse MSCs and mouse and human MSC-derived ConTs induce an immediate time-dependent increase in the Ca transient amplitude and a decrease in its time constant of decay. A positive inotropic and lusitropic effect is often related to a beta adrenergic stimulation of myocytes where a G-protein mediated increase in cAMP enhances ICa,L open probability and SERCA activity through PKA mediated phosphorylation of Cavα1 and PLN. However, recent literature suggests a role of the PI3K/Akt signaling pathway in the regulation of cardiac EC coupling [56–58]. The regulatory potentials of this pathway are many fold. They can include direct protein phosphorylation through Akt (e.g. PLN, CAvα1, eNOS), NO and cGMP productions, and changes in protein expression (NCX, SERCA [59]). All of these mechanisms are known to have a profound impact on cardiac EC coupling. For example insulin mediated Akt activation has been described to phosphorylate the Cavα1 subunit, prevent its degradation and consequently increase ICa,L [56,58]. This result could be reproduced by over-expression of PI3Kα [60] or Akt [61] and could explain the ConT-induced increase in the Ca transient amplitude and ICa,L that was attenuated by Akt inhibitors (Figs. 6A–E).
Akt has further been proposed to phosphorylate PLN thereby enhancing Ca removal from the cytoplasm by SERCA [39,61]; however, the MSC induced acceleration of τCa in PLN−/− mice suggests that PLN is not the dominant mechanism of Akt-mediated regulation of the Ca transient decay constant. SERCA activity can further be enhanced in a PLN-independent manner by direct phosphorylation through calmodulin kinase [62], or S-nitrosylation [63,64]. If Akt mediated NO production is induced, also a nitrosylation of the α1-subunit of ICa,L which was reported to result in reduced current could be expected. An overall increase in ICa,L could be due to the minor role of caveolar ICa,L in EC coupling in mouse ventricular myocytes. The reported decrease in Ca transient amplitude by eNOS inhibition does not contradict this interpretation. In mouse VMs 90% of the Ca that contributes to the twitch transient derives from the SR which exhibits a decreased load when eNOS dependent SERCA nitrosylation is suppressed. In contrast to eNOS, nNOS was shown to localize to the SR and to regulate SERCA in a PLN dependent manner [65]. While we do not exclude a SERCA regulation by nNOS, our experiments using the nNOS inhibitor SMLT, and the fact that ConT mediated SERCA regulation was independent of PLN support the hypothesis that the regulation described is mediated primarily by eNOS.
4.4. ConT mediated cell survival
We demonstrate an Akt-mediated change in EC coupling and enhanced cell survival 24 h after VM isolation. While Akt-mediated pro-survival signaling is mostly referred to in context with proteins like GSK-3β or Bcl-2β that prevent the opening of the mitochondrial permeability transition pore (MPTP), the ConT-mediated decrease in [Ca]i could also produce a cardioprotective effect. ConT could i. reduce mitochondrial Ca uptake and prevent the Ca-induced opening of MPTP [66–68], or ii. it could attenuate the risk of spontaneous Ca release from the SR and the generation of arrhythmic activity. Given the involved mechanism proposed and demonstrated here it appears likely that the changes in EC coupling induced by PI3K/Akt signaling can also contribute to the pro-survival signaling of ConT.
5. Conclusion
We demonstrate for the first time that MSCs can induce immediate changes in cardiac EC coupling upon co-culture. The effect is mediated through paracrine factors in the conditioned tyrode which induce a PI3K/Akt mediated increase in ICa,L and an eNOS mediated increase in SERCA activity. While Akt signaling is cardioprotective by preventing the opening of MPTP, its positive inotropic and lusitropic signaling promotes cardiac contractile function and can be seen as an additional mechanism of cardioprotection by preventing mitochondrial Ca overload or the induction of arrhythmic activity.
Supplementary Material
Acknowledgments
We would like to thank the laboratory of Prof. Dr. L. Kranias for the gift of the PLN-KO mouse. This work was supported by Grants from the National Institutes of Health HL089617 and HL089617-03S1 (to KB), HL79032 and HL064035 (to BMW) and HL071626 (to RDM). Some of the materials employed in this work were provided by the Texas A&M Health Science Center College of Medicine, Institute for Regenerative Medicine at Scott & White through a grant from NCRR of the NIH (P40RR017447).
Footnotes
Disclosure statement: The authors declare that there are no conflicts of interest.
Appendix A. Supplementary data: Supplementary data to this article can be found online at doi:10. 1016/j.yjmcc.2012.03.008.
Contributor Information
J. DeSantiago, Email: jdesant@uic.edu.
D.J. Bare, Email: dbare@uic.edu.
D.L. Geenen, Email: geenen@uic.edu.
B.M. Wolska, Email: bwolska@uic.edu.
K. Banach, Email: kbanach@uic.edu.
References
- 1.Fukuda K. Application of mesenchymal stem cells for the regeneration of cardiomyocyte and its use for cell transplantation therapy. Hum Cell. 2003;16:83–94. doi: 10.1111/j.1749-0774.2003.tb00138.x. [DOI] [PubMed] [Google Scholar]
- 2.Psaltis PJ, Zannettino AC, Worthley SG, Gronthos S. Concise review: mesenchymal stromal cells: potential for cardiovascular repair. Stem Cells. 2008;26:2201–10. doi: 10.1634/stemcells.2008-0428. [DOI] [PubMed] [Google Scholar]
- 3.Abdel-Latif A, Bolli R, Tleyjeh IM, Montori VM, Perin EC, Hornung CA, et al. Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Arch Intern Med. 2007;167:989–97. doi: 10.1001/archinte.167.10.989. [DOI] [PubMed] [Google Scholar]
- 4.Lipinski MJ, Biondi-Zoccai GG, Abbate A, Khianey R, Sheiban I, Bartunek J, et al. Impact of intracoronary cell therapy on left ventricular function in the setting of acute myocardial infarction: a collaborative systematic review and meta-analysis of controlled clinical trials. J Am Coll Cardiol. 2007;50:1761–7. doi: 10.1016/j.jacc.2007.07.041. [DOI] [PubMed] [Google Scholar]
- 5.Deuse T, Peter C, Fedak PW, Doyle T, Reichenspurner H, Zimmermann WH, et al. Hepatocyte growth factor or vascular endothelial growth factor gene transfer maximizes mesenchymal stem cell-based myocardial salvage after acute myocardial infarction. Circulation. 2009;120:S247–54. doi: 10.1161/CIRCULATIONAHA.108.843680. [DOI] [PubMed] [Google Scholar]
- 6.Shim WS, Jiang S, Wong P, Tan J, Chua YL, Tan YS, et al. Ex vivo differentiation of human adult bone marrow stem cells into cardiomyocyte-like cells. Biochem Biophys Res Commun. 2004;324:481–8. doi: 10.1016/j.bbrc.2004.09.087. [DOI] [PubMed] [Google Scholar]
- 7.Grajales L, Garcia J, Banach K, Geenen DL. Delayed enrichment of mesenchymal cells promotes cardiac lineage and calcium transient development. J Mol Cell Cardiol. 2010;48:735–45. doi: 10.1016/j.yjmcc.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rota M, Kajstura J, Hosoda T, Bearzi C, Vitale S, Esposito G, et al. Bone marrow cells adopt the cardiomyogenic fate in vivo. Proc Natl Acad Sci U S A. 2007;104:17783–8. doi: 10.1073/pnas.0706406104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dai Y, Ashraf M, Zuo S, Uemura R, Dai YS, Wang Y, et al. Mobilized bone marrow progenitor cells serve as donors of cytoprotective genes for cardiac repair. J Mol Cell Cardiol. 2008;44:607–17. doi: 10.1016/j.yjmcc.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schuleri KH, Amado LC, Boyle AJ, Centola M, Saliaris AP, Gutman MR, et al. Early improvement in cardiac tissue perfusion due to mesenchymal stem cells. Am J Physiol Heart Circ Physiol. 2008;294:H2002–11. doi: 10.1152/ajpheart.00762.2007. [DOI] [PubMed] [Google Scholar]
- 11.Schuleri KH, Feigenbaum GS, Centola M, Weiss ES, Zimmet JM, Turney J, et al. Autologous mesenchymal stem cells produce reverse remodelling in chronic ischaemic cardiomyopathy. Eur Heart J. 2009;30:2722–32. doi: 10.1093/eurheartj/ehp265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boomsma RA, Swaminathan PD, Geenen DL. Intravenously injected mesenchymal stem cells home to viable myocardium after coronary occlusion and preserve systolic function without altering infarct size. Int J Cardiol. 2007;122:17–28. doi: 10.1016/j.ijcard.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 13.Dhein S, Garbade J, Rouabah D, Abraham G, Ungemach FR, Schneider K, et al. Effects of autologous bone marrow stem cell transplantation on beta-adrenoceptor density and electrical activation pattern in a rabbit model of non-ischemic heart failure. J Cardiothorac Surg. 2006;1:17. doi: 10.1186/1749-8090-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gnecchi M, He H, Noiseux N, Liang OD, Zhang L, Morello F, et al. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 2006;20:661–9. doi: 10.1096/fj.05-5211com. [DOI] [PubMed] [Google Scholar]
- 15.Uemura R, Xu M, Ahmad N, Ashraf M. Bone marrow stem cells prevent left ventricular remodeling of ischemic heart through paracrine signaling. Circ Res. 2006;98:1414–21. doi: 10.1161/01.RES.0000225952.61196.39. [DOI] [PubMed] [Google Scholar]
- 16.Xu M, Uemura R, Dai Y, Wang Y, Pasha Z, Ashraf M. In vitro and in vivo effects of bone marrow stem cells on cardiac structure and function. J Mol Cell Cardiol. 2007;42:441–8. doi: 10.1016/j.yjmcc.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takahashi M, Li TS, Suzuki R, Kobayashi T, Ito H, Ikeda Y, et al. Cytokines produced by bone marrow cells can contribute to functional improvement of the infarcted heart by protecting cardiomyocytes from ischemic injury. Am J Physiol Heart Circ Physiol. 2006;291:H886–93. doi: 10.1152/ajpheart.00142.2006. [DOI] [PubMed] [Google Scholar]
- 18.Haider H, Jiang S, Idris NM, Ashraf M. IGF-1-overexpressing mesenchymal stem cells accelerate bone marrow stem cell mobilization via paracrine activation of SDF-1alpha/CXCR4 signaling to promote myocardial repair. Circ Res. 2008;103:1300–8. doi: 10.1161/CIRCRESAHA.108.186742. [DOI] [PubMed] [Google Scholar]
- 19.Unzek S, Zhang M, Mal N, Mills WR, Laurita KR, Penn MS. SDF-1 recruits cardiac stem cell-like cells that depolarize in vivo. Cell Transplant. 2007;16:879–86. doi: 10.3727/096368907783338271. [DOI] [PubMed] [Google Scholar]
- 20.Mirotsou M, Zhang Z, Deb A, Zhang L, Gnecchi M, Noiseux N, et al. Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proc Natl Acad Sci U S A. 2007;104:1643–8. doi: 10.1073/pnas.0610024104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Z, Deb A, Zhang Z, Pachori A, He W, Guo J, et al. Secreted frizzled related protein 2 protects cells from apoptosis by blocking the effect of canonical Wnt3a. J Mol Cell Cardiol. 2009;46:370–7. doi: 10.1016/j.yjmcc.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lai VK, Linares-Palomino J, Nadal-Ginard B, Galinanes M. Bone marrow cell-induced protection of the human myocardium: characterization and mechanism of action. J Thorac Cardiovasc Surg. 2009;138:1400–8 e1. doi: 10.1016/j.jtcvs.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 23.Mirotsou M, Jayawardena TM, Schmeckpeper J, Gnecchi M, Dzau VJ. Paracrine mechanisms of stem cell reparative and regenerative actions in the heart. J Mol Cell Cardiol. 2011;50:280–9. doi: 10.1016/j.yjmcc.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–19. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prockop DJ, Sekiya I, Colter DC. Isolation and characterization of rapidly self-renewing stem cells from cultures of human marrow stromal cells. Cytotherapy. 2001;3:393–6. doi: 10.1080/146532401753277229. [DOI] [PubMed] [Google Scholar]
- 26.Wolska BM, Solaro RJ. Method for isolation of adult mouse cardiac myocytes for studies of contraction and microfluorimetry. Am J Physiol. 1996;271:H1250–5. doi: 10.1152/ajpheart.1996.271.3.H1250. [DOI] [PubMed] [Google Scholar]
- 27.Sheehan KA, Blatter LA. Regulation of junctional and non-junctional sarcoplasmic reticulum calcium release in excitation–contraction coupling in cat atrial myocytes. J Physiol. 2003;546:119–32. doi: 10.1113/jphysiol.2002.026963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kapur N, Banach K. Inositol-1,4,5-trisphosphate-mediated spontaneous activity in mouse embryonic stem cell-derived cardiomyocytes. J Physiol. 2007;581:1113–27. doi: 10.1113/jphysiol.2006.125955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ward ML, Pope AJ, Loiselle DS, Cannell MB. Reduced contraction strength with increased intracellular [Ca2+] in left ventricular trabeculae from failing rat hearts. J Physiol. 2003;546:537–50. doi: 10.1113/jphysiol.2002.029132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woodruff ML, Sampath AP, Matthews HR, Krasnoperova NV, Lem J, Fain GL. Measurement of cytoplasmic calcium concentration in the rods of wild-type and transducin knock-out mice. J Physiol. 2002;542:843–54. doi: 10.1113/jphysiol.2001.013987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramos-Franco J, Bare D, Caenepeel S, Nani A, Fill M, Mignery G. Single-channel function of recombinant type 2 inositol 1,4, 5-trisphosphate receptor. Biophys J. 2000;79:1388. doi: 10.1016/S0006-3495(00)76391-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang MG, Tung L, Sekar RB, Chang CY, Cysyk J, Dong P, et al. Proarrhythmic potential of mesenchymal stem cell transplantation revealed in an in vitro coculture model. Circulation. 2006;113:1832–41. doi: 10.1161/CIRCULATIONAHA.105.593038. [DOI] [PubMed] [Google Scholar]
- 33.Quevedo HC, Hatzistergos KE, Oskouei BN, Feigenbaum GS, Rodriguez JE, Valdes D, et al. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proc Natl Acad Sci U S A. 2009;106:14022–7. doi: 10.1073/pnas.0903201106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang T, Xu Z, Jiang W, Ma A. Cell-to-cell contact induces mesenchymal stem cell to differentiate into cardiomyocyte and smooth muscle cell. Int J Cardiol. 2005;109:74–81. doi: 10.1016/j.ijcard.2005.05.072. [DOI] [PubMed] [Google Scholar]
- 35.Bers DM. Excitation–contraction coupling and cardiac contractile force. Dordrecht: Kluwer Academic Publisher; 2001. [Google Scholar]
- 36.Matsui T, Tao J, del Monte F, Lee KH, Li L, Picard M, et al. Akt activation preserves cardiac function and prevents injury after transient cardiac ischemia in vivo. Circulation. 2001;104:330–5. doi: 10.1161/01.cir.104.3.330. [DOI] [PubMed] [Google Scholar]
- 37.Wolska BM, Stojanovic MO, Luo W, Kranias EG, Solaro RJ. Effect of ablation of phospholamban on dynamics of cardiac myocyte contraction and intracellular Ca2+ Am J Physiol. 1996;271:C391–7. doi: 10.1152/ajpcell.1996.271.1.C391. [DOI] [PubMed] [Google Scholar]
- 38.Cittadini A, Monti MG, Iaccarino G, Di Rella F, Tsichlis PN, Di Gianni A, et al. Adenoviral gene transfer of Akt enhances myocardial contractility and intracellular calcium handling. Gene Ther. 2006;13:8–19. doi: 10.1038/sj.gt.3302589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Catalucci D, Latronico MV, Ceci M, Rusconi F, Young HS, Gallo P, et al. Akt increases sarcoplasmic reticulum Ca2+ cycling by direct phosphorylation of phospholamban at Thr17. J Biol Chem. 2009;284:28180–7. doi: 10.1074/jbc.M109.036566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mazhari R, Hare JM. Mechanisms of action of mesenchymal stem cells in cardiac repair: potential influences on the cardiac stem cell niche. Nat Clin Pract Cardiovasc Med. 2007;4(Suppl. 1):S21–6. doi: 10.1038/ncpcardio0770. [DOI] [PubMed] [Google Scholar]
- 41.Valiunas V, Doronin S, Valiuniene L, Potapova I, Zuckerman J, Walcott B, et al. Human mesenchymal stem cells make cardiac connexins and form functional gap junctions. J Physiol. 2004;555:617. doi: 10.1113/jphysiol.2003.058719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hahn JY, Cho HJ, Kang HJ, Kim TS, Kim MH, Chung JH, et al. Pre-treatment of mesenchymal stem cells with a combination of growth factors enhances gap junction formation, cytoprotective effect on cardiomyocytes, and therapeutic efficacy for myocardial infarction. J Am Coll Cardiol. 2008;51:933–43. doi: 10.1016/j.jacc.2007.11.040. [DOI] [PubMed] [Google Scholar]
- 43.Fahrenbach JP, Mejia-Alvarez R, Banach K. The relevance of non-excitable cells for cardiac pacemaker function. J Physiol. 2007;585:565–78. doi: 10.1113/jphysiol.2007.144121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gaudesius G, Miragoli M, Thomas SP, Rohr S. Coupling of cardiac electrical activity over extended distances by fibroblasts of cardiac origin. Circ Res. 2003;93:421. doi: 10.1161/01.RES.0000089258.40661.0C. [DOI] [PubMed] [Google Scholar]
- 45.Rogers TB, Pati S, Gaa S, Riley D, Khakoo AY, Patel S, et al. Mesenchymal stem cells stimulate protective genetic reprogramming of injured cardiac ventricular myocytes. J Mol Cell Cardiol. 2011;50:346–56. doi: 10.1016/j.yjmcc.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 46.Nishida S, Nagamine H, Tanaka Y, Watanabe G. Protective effect of basic fibroblast growth factor against myocyte death and arrhythmias in acute myocardial infarction in rats. Circ J. 2003;67:334–9. doi: 10.1253/circj.67.334. [DOI] [PubMed] [Google Scholar]
- 47.Zhou L, Ma W, Yang Z, Zhang F, Lu L, Ding Z, et al. VEGF165 and angiopoietin-1 decreased myocardium infarct size through phosphatidylinositol-3 kinase and Bcl-2 pathways. Gene Ther. 2005;12:196–202. doi: 10.1038/sj.gt.3302416. [DOI] [PubMed] [Google Scholar]
- 48.Bertrand L, Horman S, Beauloye C, Vanoverschelde JL. Insulin signalling in the heart. Cardiovasc Res. 2008;79:238–48. doi: 10.1093/cvr/cvn093. [DOI] [PubMed] [Google Scholar]
- 49.Kinugawa S, Tsutsui H, Ide T, Nakamura R, Arimura K, Egashira K, et al. Positive inotropic effect of insulin-like growth factor-1 on normal and failing cardiac myocytes. Cardiovasc Res. 1999;43:157–64. doi: 10.1016/s0008-6363(99)00058-9. [DOI] [PubMed] [Google Scholar]
- 50.Enoki C, Otani H, Sato D, Okada T, Hattori R, Imamura H. Enhanced mesenchymal cell engraftment by IGF-1 improves left ventricular function in rats undergoing myocardial infarction. Int J Cardiol. 2010;138:9–18. doi: 10.1016/j.ijcard.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 51.Chen TS, Lai RC, Lee MM, Choo AB, Lee CN, Lim SK. Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs. Nucleic Acids Res. 2010;38:215–24. doi: 10.1093/nar/gkp857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lai RC, Arslan F, Tan SS, Tan B, Choo A, Lee MM, et al. Derivation and characterization of human fetal MSCs: an alternative cell source for large-scale production of cardioprotective microparticles. J Mol Cell Cardiol. 2010;48:1215–24. doi: 10.1016/j.yjmcc.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 53.Luecke N, Templin C, Muetzelburg MV, Neumann D, Just I, Pich A. Secreted proteome of the murine multipotent hematopoietic progenitor cell line DKmix. Rapid Commun Mass Spectrom. 2010;24:561–70. doi: 10.1002/rcm.4412. [DOI] [PubMed] [Google Scholar]
- 54.Schinkothe T, Bloch W, Schmidt A. In vitro secreting profile of human mesenchymal stem cells. Stem Cells Dev. 2008;17:199–206. doi: 10.1089/scd.2007.0175. [DOI] [PubMed] [Google Scholar]
- 55.Sze SK, de Kleijn DP, Lai RC, Khia Way Tan E, Zhao H, et al. Elucidating the secretion proteome of human embryonic stem cell-derived mesenchymal stem cells. Mol Cell Proteomics. 2007;6:1680–9. doi: 10.1074/mcp.M600393-MCP200. [DOI] [PubMed] [Google Scholar]
- 56.Sun H, Kerfant BG, Zhao D, Trivieri MG, Oudit GY, Penninger JM, et al. Insulin-like growth factor-1 and PTEN deletion enhance cardiac L-type Ca2+ currents via increased PI3Kalpha/PKB signaling. Circ Res. 2006;98:1390–7. doi: 10.1161/01.RES.0000223321.34482.8c. [DOI] [PubMed] [Google Scholar]
- 57.Yano N, Tseng A, Zhao TC, Robbins J, Padbury JF, Tseng YT. Temporally controlled overexpression of cardiac-specific PI3Kalpha induces enhanced myocardial contractility—a new transgenic model. Am J Physiol Heart Circ Physiol. 2008;295:H1690–4. doi: 10.1152/ajpheart.00531.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Catalucci D, Zhang DH, DeSantiago J, Aimond F, Barbara G, Chemin J, et al. Akt regulates L-type Ca2+ channel activity by modulating Cavalpha1 protein stability. J Cell Biol. 2009;184:923–33. doi: 10.1083/jcb.200805063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim SJ, Abdellatif M, Koul S, Crystal GJ. Chronic treatment with insulin-like growth factor I enhances myocyte contraction by upregulation of Akt-SERCA2a signaling pathway. Am J Physiol Heart Circ Physiol. 2008;295:H130–5. doi: 10.1152/ajpheart.00298.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lu Z, Jiang YP, Wang W, Xu XH, Mathias RT, Entcheva E, et al. Loss of cardiac phosphoinositide 3-kinase p110 alpha results in contractile dysfunction. Circulation. 2009;120:318–25. doi: 10.1161/CIRCULATIONAHA.109.873380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rota M, Boni A, Urbanek K, Padin-Iruegas ME, Kajstura TJ, Fiore G, et al. Nuclear targeting of Akt enhances ventricular function and myocyte contractility. Circ Res. 2005;97:1332–41. doi: 10.1161/01.RES.0000196568.11624.ae. [DOI] [PubMed] [Google Scholar]
- 62.Picht E, DeSantiago J, Huke S, Kaetzel MA, Dedman JR, Bers DM. CaMKII inhibition targeted to the sarcoplasmic reticulum inhibits frequency-dependent acceleration of relaxation and Ca2+ current facilitation. J Mol Cell Cardiol. 2007;42:196–205. doi: 10.1016/j.yjmcc.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sun J, Morgan M, Shen RF, Steenbergen C, Murphy E. Preconditioning results in S-nitrosylation of proteins involved in regulation of mitochondrial energetics and calcium transport. Circ Res. 2007;101:1155–63. doi: 10.1161/CIRCRESAHA.107.155879. [DOI] [PubMed] [Google Scholar]
- 64.Sun J, Murphy E. Protein S-nitrosylation and cardioprotection. Circ Res. 2010;106:285–96. doi: 10.1161/CIRCRESAHA.109.209452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang H, Kohr MJ, Traynham CJ, Wheeler DG, Janssen PM, Ziolo MT. Neuronal nitric oxide synthase signaling within cardiac myocytes targets phospholamban. Am J Physiol Cell Physiol. 2008;294:C1566–75. doi: 10.1152/ajpcell.00367.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Argaud L, Gateau-Roesch O, Augeul L, Couture-Lepetit E, Loufouat J, Gomez L, et al. Increased mitochondrial calcium coexists with decreased reperfusion injury in postconditioned (but not preconditioned) hearts. Am J Physiol Heart Circ Physiol. 2008;294:H386–91. doi: 10.1152/ajpheart.01035.2007. [DOI] [PubMed] [Google Scholar]
- 67.Delcamp TJ, Dales C, Ralenkotter L, Cole PS, Hadley RW. Intramitochondrial [Ca2+] and membrane potential in ventricular myocytes exposed to anoxia-reoxygenation. Am J Physiol. 1998;275:H484–94. doi: 10.1152/ajpheart.1998.275.2.H484. [DOI] [PubMed] [Google Scholar]
- 68.Miyamoto S, Howes AL, Adams JW, Dorn GW, II, Brown JH. Ca2+ dysregulation induces mitochondrial depolarization and apoptosis: role of Na+/Ca2+ exchanger and AKT. J Biol Chem. 2005;280:38505–12. doi: 10.1074/jbc.M505223200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.