Abstract
Rheumatoid arthritis (RA) is a chronic, systemic autoimmune disease and may lead to joint damage, synovial membrane destruction and cartilage and bone damage. RA is closely associated with increased expression of interleukin (IL)-6, IL-8, tumor necrosis factor-α (TNF-α) and nuclear transcription factor-κB (NF-κB). Therefore, inhibition of the expression of IL-6, IL-8, TNF-α and NF-κB is a promising strategy for the development of novel anti-RA therapies. The aim of this study was to investigate the effect of shuangtengbitong tincture (STBT) on the expression of IL-6, IL-8, TNF-α and NF-κB in synovial tissues of rats with collagen-induced arthritis (CIA). STBT as a clinical prescription created at Fujian University of Traditional Chinese Medicines (TCM) Affiliated People’s Hospital has been shown to be clinically effective in the treatment of RA. The model of Wistar rats with CIA was created using bovine type II collagen. The two treatment groups with CIA were administered STBT (1 ml per time) or Votalin (∼1 cm per time) for ∼1 month continuously. Following treatment, STBT suppressed paw swelling significantly (P<0.05) compared with the model group. STBT also improved pathological changes, STBT-treated rats showed a significant improvement in synovial hyperplasia, inflammatory infiltration, cartilage and bone destruction and other symptoms. The protein expression levels of IL-6, IL-8, TNF-α and NF-κB were markedly suppressed in synovial tissues of STBT-treated and Votalin-treated rats. Our findings demonstrate for the first time that STBT markedly reduces paw swelling, improves pathological changes and increases the expression of IL-6, IL-8, TNF-α and NF-κB in synovial tissues of CIA rats, which may partially explain the anti-RA activity of STBT.
Keywords: shuangtengbitong tincture, rheumatoid arthritis, interleukin-6, interleukin-8, tumor necrosis factor-α, nuclear transcription factor-κB
Introduction
Rheumatoid arthritis (RA) is a chronic, systemic autoimmune disease that may lead to joint damage, synovial membrane destruction, and cartilage and bone damage (1,2). Its prevalence varies between 0.5 and 1% worldwide (3). RA is an incurable disease that has a serious impact on human physical and mental health (4). Despite the prevalence of RA, its etiology and pathogenesis remain controversial. RA may be associated with immune regulation, infection, genetics, environment, neuro-psychological status and other factors (5). There are various mechanisms for the occurrence of RA in which joint inflammation and the autoimmune reaction play an important role.
Pro-inflammatory cytokines, including interleukin (IL)-6, IL-8 and tumor necrosis factor (TNF)-α, are important cytokines in inflammation and are considered to be the most important mediators involved in the pathogenesis of RA (6). Previous studies have also discussed the role of cytokines in RA (6,7). TNF-α has been shown to play an important role in RA (8–10) and is known to mediate a variety of effector functions relevant to the pathogenesis of RA. In addition, therapies targeting TNF-α are also recognized as effective treatments for patients with RA. Proinflammatory cytokine IL-6 is an abundantly expressed cytokine in rheumatoid synovium which is considered to lead to joint damage (11) and chemotactic activity (12). The chemokine IL-8 may activate neutrophils and enhance the phagocytosis of neutrophils and the activity of their lysosomal enzyme. IL-8 has a chemotactic effect on basophils and T cells. A number of of these cytokines are not randomly present, but their expression is controlled by a network or hierarchy which induces nuclear transcription factor (NF)-κB expression. Therefore downregulation of pro-inflammatory cytokines may be an effective and rational approach for the treatment of RA.
Shuangtengbitong tincture (STBT) is a clinical prescription of Fujian University of Traditional Chinese Medicines (TCM) Affiliated People’s Hospital and is officially recorded in the Min drug system (Z20111008) for therapy of RA. STBT consists of four components, namely Tripterygium wilfordii Hook f., Caulis sinomenii, Dioscorea nipponica Makino and Glycyrrhiza uralensis Fisch. These components have the ability to dispel wind, eliminate dampness, dredge meridians, reduce swelling and relieve pain. It has been shown that STBT is effective against RA. However, no studies on the anti-inflammatory activity of STBT have been performed. In the present study, a CIA model was used to investigate the effects of STBT on acute arthritis, chronic joint damage and anti-inflammatory activity.
Materials and methods
Materials
A Dionex Ultimate 3000 liquid chromatograph equipped with DAD detector was bought from Dionex Ltd. [Thermo Fisher Scientific (Shanghai) Co. Ltd, China]; a KQ-500DE ultrasonic clearing machine was purchased from Kunshan Ultrasonic Instrument Co., Ltd. (Kunshan, China); a XS105 electronic analytical balance was supplied by Mettler-Toledo Instruments (Shanghai) Co., Ltd., (Shanghai, China); a YLS-7B paw plethysmometer was obtained from Yi Yan Technology Development Co., Ltd. (Jinan, China). Complete Freund’s adjuvant (CFA) and type II collagen were purchased from Sigma Chemical (St. Louis, MO, USA). Antibodies were bought from Cell Signaling (Beverly, MA, USA). All other chemicals used, unless otherwise stated, were obtained from commercial sources.
Animals
A total of 40 SPF Wistar male rats provided by the Animal Care and Use Committee of Fujian University of TCM, weighing 180–220 g, were purchased from Shanghai Slac Laboratory Animal Co. Ltd. (Shanghai, China, license number 200700518360). The animals were housed under controlled temperatures (21–23°C), relative humidity 55±5%, a 12-h light/dark cycle and had free access to a standard rat diet and water. All animal experiments were conducted in accordance with international ethical guidelines and the National Institutes of Health Guide concerning the Care and Use of Laboratory Animals.
Preparation of STBT
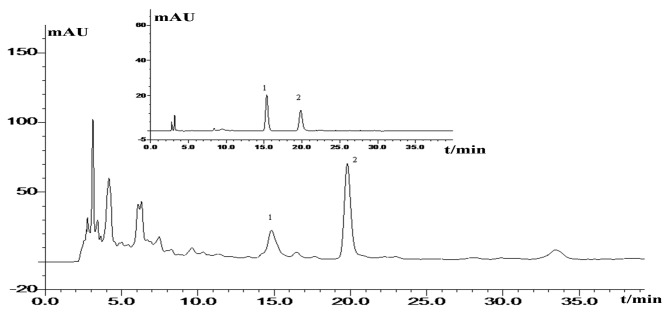
STBT is a mixture of four herbs that were bought from Fujian University of TCM Affiliated People’s Hospital. The herbs were verified by Professor Wei Lu from the pharmacy college of Fujian University of TCM. The herb voucher specimens were deposited in the pharmacy college of Fujian University of TCM. STBT was prepared as the preparation of STBT hospital prescription of Fujian University of TCM Affiliated People’s Hospital. Tripterygium wilfordii Hook f., Caulis sinomenii, Dioscorea nipponica Makino and Glycyrrhiza uralensis Fisch. were crushed into coarse powder, mixed and then steeped 10 times in 80% ethanol. After 48 h, extraction was percolated slowly, collected and filtrated. Finally the solution was adjusted to 10 g/ml (original medicinal materials/volume). The prepared STBT was processed according to our preliminary study (13) and then was filtrated through 0.45-μm microporous membranes prior to analysis by liquid chromatography. The major peaks were identified as the marker compounds (Fig. 1).
Figure 1.
HPLC of target analytes in the STBT sample. Inset: HPLC of target analytes standard mixture. In liquid chromatograms 1 is triptolide and 2 is sinomenine. STBT, shuangtengbitong tincture; HPLC, high-performance liquid chromatography.
Induction of CIA model and treatments
A total of 40 Wistar rats were randomly assigned to five experimental groups with ten animals in each: normal, model, Votalin ointment and STBT groups. With the exception of the normal group, the other groups were used to create the CIA model. First, 2 mg/ml collagen was emulsified with an equal volume of CFA to a final concentration of 0.1 mg/ml. Second, 2 ml collagen emulsion was injected into the base of the tail of each rat via intradermal injection. After 7 days, a provocation test was performed; 1 ml collagen emulsion was injected into the base of the tail of each rat via intradermal injection. Drugs were administered intragastrically twice a day from the first day after primary immunization. In the STBT group, STBT at a dose of 1 ml per time was sprayed onto the back of shaved rats. The area of back treated was 3×3 cm, according to the manufacturer’s instructions. The Votalin ointment group were administered Votalin ointment at a dose of ∼1 cm per time as described above. The normal group and model group were not treated. Animals were medicated for 35 days continuously.
Swelling dimension observation
A YLS-7B plethysmometer was employed to measure the volume of the hind paws of rats (14). The paw volumes before and after modeling were recorded. The volume of each rat paw was measured every 3 days from the 7th day following the primary immunization.
Histopathological assessment
On the final day of treatment, all rats were anesthetized and the right hind knee was removed and fixed in 4% neutral-buffered formalin for 24 h. After decalcifying with 12.5% EDTA (pH 7.0) for ∼20 days, the right hind knee was paraffin-embedded, sectioned at a 5-mm thickness and stained with hematoxylin and eosin.
Immunohistochemistry (IHC) analysis
The above paraffin sections were used for IL-6, IL-8, NF-κB and TNF-α immunohistochemical analysis (15) according to the manufacturer’s instructions (USCN Life Science Inc., Houston, TX, USA). Five high-power fields (×400) were selected in each slide randomly and the average proportion of positive cells in each field were counted by the true color multi-functional cell image analysis management system (Image-Pro Plus, Media Cybernetics, Inc., Rockville, MD, USA). The average proportion of positive cells in each field was counted and classified into 5 grades: no staining was scored as 0, 1–10% of cells stained scored as 1, 11–50% as 2, 51–80% as 3 and 81–100% as 4. Staining intensity was rated on a scale of 0 to 3, with 0, negative; 1, weak; 2, moderate; and 3, strong. The raw data were converted into the IHS by multiplying the quantity and staining intensity scores (16).
RNA extraction and RT-PCR analysis
Total RNA was extracted from synovial tissue with TRIzol reagent. Oligo(dT)-primed RNA was reverse transcribed to cDNA. The obtained cDNA was used to determine the mRNA levels of TNF-α and NF-κB by PCR with Taq DNA polymerase (Fermentas Life Science, Beijing, China) according to the manufacturer’s instructions. The sequences of NF-κB and TNF-α used for amplification were as follows: NF-κB forward, 5′-GCG CAT CCA GAC CAA CAA TAA C-3′ and reverse, 5′-GCC GAA GCT GCA TGG ACA CT-3′; TNF-α forward, 5′-CTCCCA GGT TCT CTT CAA GG-3′ and reverse, 5′-TGG AAG ACT CCT CCC AGG TA-3′. The conditions for PCR were set at the following: initial denaturation at 95°C for 3 min, 30 sec to denature at 95°C, 72°C for 45 sec to extend and 35 cycles for amplification. The annealing temperatures were 60°C. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was employed as a normalization control. Finally, PCR products were analyzed by gel electrophoresis (1.5% agarose) and the DNA bands were examined in a Gel Documentation System (Model Gel Doc 2000; Bio-Rad, Hercules, CA, USA).
Statistical methods
Data are expressed as mean ± standard deviation (SD). SPSS 16.0 statistical software (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. The comparisons between groups were performed using the Student’s t-test. P<0.05 was considered to indicate a statistically significant result.
Results
Swelling dimension
From the 7th to the 31st day, the changes in paw swelling are displayed in Table I. On approximately the 10th day, the knee joints of the rats began to swell. With time, joint and paw swelling became more severe. Paw and knee joint swelling volume reached a maximum on approximately the 18th day in the model group. The groups treated with Votalin ointment and STBT demonstrated inhibition and were significantly different (P<0.05 and P<0.01, respectively) to the model group. STBT suppressed paw swelling at the start and produced a greater inhibition effect than Votalin ointment.
Table I.
Changes in paw swelling.
| Group | Paw volume before in ammation (ml) | Paw swelling after in ammation at different times (ml) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| 7 days | 10 days | 13 days | 16 days | 19 days | 22 days | 25 days | 28 days | 31 days | ||
| Control | 1.58±0.07 | 1.59±0.07 | 1.59±0.05 | 1.61±0.08 | 1.60±0.06 | 1.63±0.07 | 1.63±0.08 | 1.64±0.05 | 1.66±0.04 | 1.66±0.07 |
| Model | 1.59±0.01 | 1.62±0.10 | 1.72±0.09b | 1.80±0.13b | 2.31±0.22b | 2.96±0.25b | 3.01±0.17b | 2.99±0.14b | 2.99±0.14b | 2.87±0.23b |
| Votalin ointment | 1.60±0.05 | 1.62±0.06 | 1.69±0.07c | 1.75±0.11c | 2.22±0.13d | 2.60±0.13d | 2.63±0.16d | 2.15±0.41d | 2.00±0.16d | 1.98±0.14d |
| STBT | 1.58±0.06 | 1.62±0.03 | 1.67±0.08c | 1.72±0.03d | 2.02±0.21d | 2.39±0.17d | 2.57±0.04d | 2.02±0.14d | 1.92±0.14d | 1.84±0.24d |
Values are presented as mean ± SD.
P <0.05,
P <0.01 compared with the control group;
P <0.05,
P <0.01 compared with the model group. STBT, shuangtengbitong tincture.
Histopathological changes
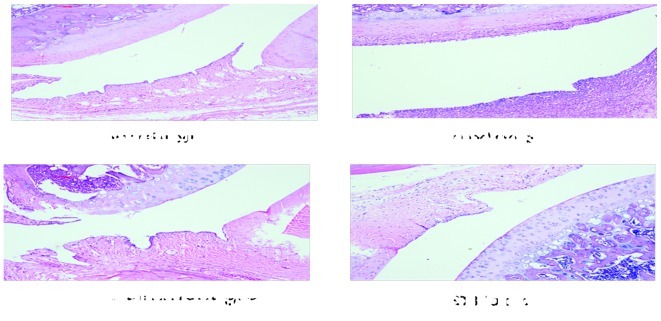
Representative histopathological lesions in the hind knee joint from normal, model, Votalin ointment and STBT groups are shown in Fig. 2. Synovial hyperplasia, disorganized arrangement, infiltration of inflammatory cells, cartilage destruction and bone destruction were observed in the model group. Histopathological lesions were ameliorated in the treated groups to a different extent. The histopathological lesions of the STBT group improved well. It is shown that only moderate proliferation of the synovium occurred, cell morphology was regularly arranged, a small number of inflammatory cells infiltrated, the surface of the cartilage was smooth and there were no clear signs of bone erosion in the CIA rats of the STBT group.
Figure 2.
Histological changes in CIA rats. Representative lesions using HE stain of hind knee joint from rats are shown (magnification, ×100). CIA, collagen-induced arthritis; HE, hematoxylin and eosin; STBT, shuangtengbitong tincture.
Protein and mRNA expression of IL-6, IL-8, TNF-α and NF-κB
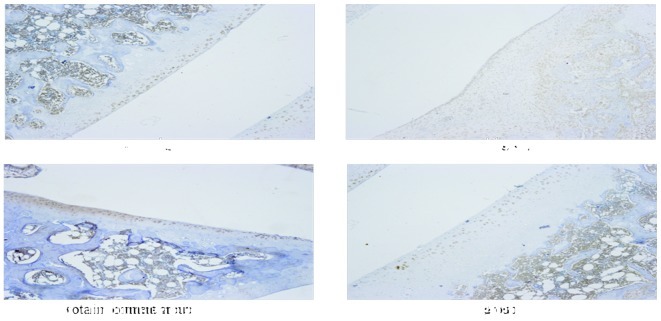
The protein and mRNA expression were determined by IHC analysis and RT-PCR. A semi-quantitative score was calculated according to positive cell ratio and staining intensity. IHS is shown in Table II. The cytoplasm of positive cells were brown, representative IHC pictures of TNF-α are shown in Fig. 3. Positive areas of IL-6, IL-8, NF-κB and TNF-α were significantly enlarged in the model group compared with the normal group, the difference was statistically significant (P<0.01). In the STBT group, expression of IL-6, IL-8, NF-κB, TNF-α was weaker compared with the model group (P<0.05). The results of the RT-PCR (Fig. 4) showed that the mRNA expression of TNF-α and NF-κB in the model group was significantly increased, compared with that in the normal group (P<0.05), however, NF-κB and TNF-α were downregulated by the treatment with Votalin ointment or STBT. Data from RT-PCR showed that the pattern of mRNA expression of TNF-α and NF-κB was similar to their respective protein levels.
Table II.
Comparison of immunohistochemical scores of IL-6, IL-8, TNF-α and NF-κB in synovial tissue.
| Group | IL-6 | IL-8 | TNF-α | NF-κB |
|---|---|---|---|---|
| Normal | 0 | 0 | 0 | 0 |
| Model | 6.87±2.02b | 6.03±1.07b | 6.35±2.58b | 6.60±2.60b |
| Votalin ointment | 5.83±0.95c | 4.80±2.03c | 5.65±1.64c | 5.93±2.53 |
| STBT | 5.80±1.30c | 5.07±1.56c | 5.51±2.03c | 5.71±0.96c |
Values are presented as mean ± SD.
P<0.05,
P<0.01 compared with the normal group;
P<0.05,
P<0.01 compared with the model group. STBT, shuangtengbitong tincture; IL, interleukin; TNF-α, tumor necrosis factor-α; NF-κB, nuclear factor-κB.
Figure 3.
Immunohistochemical determination of protein expression. Representative images of staining for TNF-α are shown (magnification, ×10). TNF, tumor necrosis factor; STBT, shuangtengbitong tincture.
Figure 4.
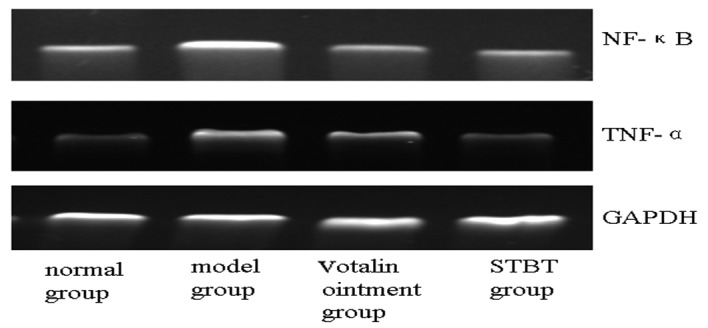
Determination of mRNA expression of TNF-α and NF-κB. The levels of TNF-α and NF-κB in synovial tissues were determined by RT-PCR analysis. GAPDH was used as the internal control. TNF-α, tumor necrosis factor-α; NF-κB, nuclear transcription factor-κB; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Discussion
RA is a chronic and systemic autoimmune disease which is characterized by arthrosynovitis; its main clinical manifestations are multi-joint synovitis and articular damage (17,18). CIA has been proved to share various immunological and pathological features with human RA (18,19). CIA has become one of the most widely used models for screening new drugs for the treatment of rheumatoid diseases. CIA rats were used to demonstrate the anti-inflammatory effect of STBT in the current study.
The herbs in STBT have been used for hundreds of years and their efficacy is documented. The STBT formula is chemically complex with hundreds of components. Certain bioactive chemicals have been acknowledged, including: triptolide and tripterine in Tripterygium wilfordii Hook f. (20–22), sinomenine in Caulis sinomenii(23,24), dioscin in Dioscorea nipponica Makino (25) and glycyrrhizic acid and Liquiritin in Glycyrrhiza uralensis Fisch. (26). Each component of STBT may play an important role in the treatment of RA. However, numerous herbs are often used together under the theories of TCM. It is believed that the interactions among these herbs contribute more to the anti-RA effect of STBT than their effects alone.
In the present study, it was observed that the swelling dimensions of the group treated with STBT decreased as compared with the model group. In addition, according to the histopathological change of ankle joint analysis, it was suggested that STBT was able to alleviate the hyperplasia of the synovial membrane, bone destruction and other symptoms. These results are consistent with those of a previous study (27). STBT possesses certain anti-inflammatory effects.
Pro-inflammatory cytokines located in synovium, including IL-1, IL-6, IL-8 and TNF-α, have been known to play a major role in the progression of joint destruction (6,19). IL-1 and TNF-α promote the induction of adhesion molecules and proteinase gene expression, and play a major role in the progression of joint destruction and proliferation of synoviocytes, and also increase the production of IL-6, IL-8 and other chemokines (28). A number of these cytokines were not randomly present but formed a network or hierarchy which controlled their expression. The molecular basis of destruction in RA or CIA remains to be elucidated. The present study showed that STBT has an inhibitory action on expression levels of IL-6, IL-8, TNF-α and NF-κB, as revealed by IHC analysis and RT-PCR. It has been reported that the efficacy of IL-6, IL-8 and TNF-α blockade has been shown by their specific inhibitors (29).
The transcription regulator NF-κB is an important nuclear transcription factor in RA. NF-κB plays a pivotal role at the stages of initiation and perpetuation of inflammation in disease. NF-κB regulates the expression of numerous types of cytokines and proinflammatory mediators (30). Our results for the expression level of NF-κB were in agreement with those reported in the literature (23,2). The suppression of NF-κB may be responsible for the decrease of levels of IL-6, IL-8 and TNF-α in synovial tissue.
We observed that STBT has a therapeutic effect on CIA and the effect may correlate with the suppressive effect on the production of pro-inflammatory cytokines and NF-κB.
Acknowledgments
This study was supported by the National Technology Support Project of China (2011BAI01B05), the Major Projects of Science and Technology Bureau of Fujian Province (2009YZ0001-1-1) and Fujian Province Science-Technology Plan Projects (2010Y2004).
Abbreviations:
- RA
rheumatoid arthritis
- STBT
shuangtengbitong tincture
- CIA
collagen-induced arthritis
- IL-6
interleukin-6
- IL-8
interleukin-8
- TN F-α
tumor necrosis factor-α
- NF-κB
nuclear transcription factor κB
- IHC
immunohistochemstry
- IHS
immunohistochemical score
References
- 1.Helliwell P, Woodburn J, Redmond A, Turner D, Davys H. Current concepts in rheumatoid arthritis. In: Helliwell P, Woodburn J, Redmond A, Turner D, Davys H, editors. The Foot and Ankle in Rheumatoid Arthritis. Churchill Livingstone; Edinburgh: 2007. pp. 1–16. [Google Scholar]
- 2.Yang M, Xiao C, Wu Q, et al. Anti-inflammatory effect of Sanshuibaihu decoction may be associated with nuclear factor-kappa B and p38 MAPK alpha in collagen-induced arthritis in rat. J Ethnopharmacol. 2010;127:264–273. doi: 10.1016/j.jep.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 3.Brenner M, Meng HC, Yarlett NC, et al. The non-MHC quantitative trait locus Cia5 contains three major arthritis genes that differentially regulate disease severity, pannus formation, and joint damage in collagen- and pristane-induced arthritis. J Immunol. 2005;174:7894–7903. doi: 10.4049/jimmunol.174.12.7894. [DOI] [PubMed] [Google Scholar]
- 4.Wolfe F, Michaud K, Gefeller O, Choi HK. Predicting mortality in patients with rheumatoldarthritis. Arthritis Rheum. 2003;48:1530–1542. doi: 10.1002/art.11024. [DOI] [PubMed] [Google Scholar]
- 5.Choi EM, Kim YH. Hesperetin attenuates the highly reducing sugar-triggered inhibition of osteoblast differentiation. Cell Biol Toxicol. 2008;24:225–231. doi: 10.1007/s10565-007-9031-0. [DOI] [PubMed] [Google Scholar]
- 6.Brennan FM, Mclnnes IB. Evidence that cytokines play a role in rheumatoid arthritis. J Clin Invest. 2008;118:3537–3545. doi: 10.1172/JCI36389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mclnnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 2007;7:429–442. doi: 10.1038/nri2094. [DOI] [PubMed] [Google Scholar]
- 8.Deleuran BW, Chu CQ, Field M, Brennan FM, Mitchell T, Feldmann M, Maini RN. Localization of tumor necrosis factor receptors in the synovial tissue and cartilage-pannus junction in patients with rheumatoid arthritis. Implications for local actions of tumor necrosis factor alpha. Arthritis Rheum. 1992;35:1170–1178. doi: 10.1002/art.1780351009. [DOI] [PubMed] [Google Scholar]
- 9.Dayer JM, Beutler B, Cerami A. Cachectin/tumor necrosis factor stimulates collagenase and prostaglandin E2 production by human synovial cells and dermal fibroblasts. J Exp Med. 1985;162:2163–2168. doi: 10.1084/jem.162.6.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bertolini DR, Nedwin GE, Bringman TS, Smith DD, Mundy GR. Stimulation of bone resorption and inhibition of bone formation in vitro by human tumour necrosis factors. Nature. 1986;319:516–518. doi: 10.1038/319516a0. [DOI] [PubMed] [Google Scholar]
- 11.Jazayeri JA, Carroll GJ, Vernallis AB. Interleukin-6 subfamily cytokines and rheumatoid arthritis: role of antagonists. Int Immunopharmacol. 2010;10:1–8. doi: 10.1016/j.intimp.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 12.van Leeuwen MA, Westra J, Limburg PC, van Riel PL, van Rijswijk MH. Interleukin-6 in relation to other proinflammatory cytokines, chemotactic activity and neutrophil activation in rheumatoid synovial fluid. Ann Rheum Dis. 1995;54:33–38. doi: 10.1136/ard.54.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li H, Xu W, Chu K, Chen L, Zhang X. Determination of Triptolide in Bitongling Tincture by HPLC. Chinese Journal of Information on Traditional Chinese Medicine. 2012;19:51–52. (In Chinese). [Google Scholar]
- 14.Ganesan K, Tiwari M, Balachandran C, Manohar B, Puvanakrishnan R. Estrogen and testosterone attenuate extra-cellular matrix loss in collagen induced arthritis in rats. Calcif Tissue Int. 2008;83:354–364. doi: 10.1007/s00223-008-9183-9. [DOI] [PubMed] [Google Scholar]
- 15.Keyszer G, Redlich A, Häupl T, Zacher J, Sparmann M, Engethüm U, Gay S, Burmester GR. Differential expression of cathepsins B and L compared with matrix metalloproteinases and their respective inhibitors in rheumatoidarthritis and osteoarthritis: a parallel investigation by semiquantitative reverse transcriptase-polymerase chain reaction and immunohistochemistry. Arthritis Rheum. 1998;41:1378–1387. doi: 10.1002/1529-0131(199808)41:8<1378::AID-ART6>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 16.Soslow RA, Dannenberg AJ, Rush D, Woerner BM, Khan KN, Masferrer J, Koki AT. Cox-2 is expressed in human pulmonary, colonic, and mammary tumors. Cancer. 2000;89:2637–2645. doi: 10.1002/1097-0142(20001215)89:12<2637::aid-cncr17>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 17.Weyand CM. New insights into the pathogenesis of rheumatoid arthritis. Rheumatology (Oxford) 2000;39(Suppl 1):3–8. doi: 10.1093/oxfordjournals.rheumatology.a031491. [DOI] [PubMed] [Google Scholar]
- 18.Brand DD, Kang AH, Rosloniec EF. Immunopathogenesis of collagen arthritis. Springer Semin Immunopathol. 2003;25:3–18. doi: 10.1007/s00281-003-0127-1. [DOI] [PubMed] [Google Scholar]
- 19.Buckwalter JA, Einhorn TA, Simon SR, editors. People’s Health Publishing House. 2001. Orthopaedic Basic Science; pp. 987–1204. [Google Scholar]
- 20.Tu S, Hu Y, Zeng K, Zhang M, Lai X, Weichen Z. Effects of triptolide on the expression and activity of NF-КB in synovium of collagen induced arthritis rats. J Huazhong Univ Sci Technolog Med Sci. 2005;25:543–545. doi: 10.1007/BF02896012. [DOI] [PubMed] [Google Scholar]
- 21.Xiao C, Zhao L, Liu Z, Lu C, Zhao N, Yang D, Chen S, Tang JC, Chan A, Lu AP. The effect of triptolide on CD4+ and CD8+ cells in the Peyer’s patch of DA rats with collagen induced arthritis. Nat Prod Res. 2009;23:1699–1706. doi: 10.1080/14786410802187783. [DOI] [PubMed] [Google Scholar]
- 22.Li H, Zhang YY, Tan HW, Jia YF, Li D. Therapeutic effect of tripterine on adjuvant arthritis in rats. J Ethnopharmacol. 2008;118:479–484. doi: 10.1016/j.jep.2008.05.028. [DOI] [PubMed] [Google Scholar]
- 23.Fang Y, Wang Y, Zhou X, Zhong B, Bai G, Zhang C. Effect and mechanism of sinomenine on the signal transduction of the synovial cell nuclear factor-КB in rats with adjuvant arthritis. Chinese Journal of Clinical Rehabilitation. 2005;9:204–205. (In Chinese). [Google Scholar]
- 24.Fang Y, Zhang Y, Wang Y. Effects of sinomenine on svnovioctve apoptosis, cell cycle and change of bcl-2 protein in patients with rheumatoid arthritis. Chinese Remedies & Clinics. 2008;8:261–264. [Google Scholar]
- 25.Zhang N, Kang TG, Yi HB. Research progress of chemical composition and pharmacological effects of Dioscorea nipponica Maknio. Chin Med J Res Prac. 2010;24:87–90. [Google Scholar]
- 26.Li W, Song XB, Zhang LJ, Li W, Yu HS, An J. Advances in Research on Chemical Constituents of Radix Glycyrrhizae. Journal of Gansu College of Traditional Chinese Medicine. 2012;7:40–44. [Google Scholar]
- 27.Pope RM. Apoptosis as a therapeutic tool in rheumatoid arthritis. Nat Rev Immunol. 2002;2:527–535. doi: 10.1038/nri846. [DOI] [PubMed] [Google Scholar]
- 28.Sweeney SE, Firestein GS. Rheumatoid arthritis: regulation of synovial inflammation. Int J Biochem Cell Biol. 2004;36:372–378. doi: 10.1016/s1357-2725(03)00259-0. [DOI] [PubMed] [Google Scholar]
- 29.Maini RN, Taylor PC. Anti-cytokines therapy for rheumatoid arthritis. Annu Rev Med. 2000;51:207–229. doi: 10.1146/annurev.med.51.1.207. [DOI] [PubMed] [Google Scholar]
- 30.Gilmore TD. Introduction to NF-kappaB: players, pathways, perspectives. Oncogene. 2006;25:6680–6684. doi: 10.1038/sj.onc.1209954. [DOI] [PubMed] [Google Scholar]