Abstract
Plant phenolics are generally thought to play significant roles in plant defense against herbivores and pathogens. Many plant taxa, including Solanaceae, are rich in phenolic compounds and some insect herbivores have been shown to acquire phenolics from their hosts to use them as protection against their natural enemies. Here we demonstrate that larvae of an insect specialist on Solanaceae, the tobacco hornworm, Manduca sexta L. (Lepidoptera: Sphingidae), acquire the plant phenolic chlorogenic acid (CA), and other caffeic acid derivatives as they feed on one of their hosts, Nicotiana attenuata L. (Solanaceae), and on artificial diet supplemented with CA. We test the hypothesis that larvae fed on CA-supplemented diet would have better resistance against bacterial infection than larvae fed on a standard CA-free diet by injecting bacteria into the hemocoel of fourth instars. Larvae fed CA-supplemented diet show significantly higher survival of infection with Enterococcus faecalis (Andrewes & Horder) Schleifer & Kilpper-Bälz, but not of infection with the more virulent Pseudomonas aeruginosa (Schroeter) Migula. Larvae fed on CA-supplemented diet possess a constitutively higher number of circulating hemocytes than larvae fed on the standard diet, but we found no other evidence of increased immune system activity, nor were larvae fed on CA-supplemented diet better able to suppress bacterial proliferation early in the infection. Thus, our data suggest an additional defensive function of CA to the direct toxic inhibition of pathogen proliferation in the gut.
Keywords: chemical defense, acquired plant metabolite, immune defense, Lepidoptera, Solanaceae, Sphingidae, Nicotiana attenuata, tobacco hornworm, Enterococcus faecalis, Pseudomonas aeruginosa, chlorogenic acid
Introduction
Herbivorous insects have evolved multiple ways of coping with the arsenal of plant chemical defenses, and in some instances acquire plant compounds for defense against their own enemies (Rosenthal & Berenbaum, 1992; Nishida, 2002; Opitz & Muller, 2009). Phenolic compounds comprise one of the largest classes of defensive secondary metabolites against herbivores and pathogens in vascular plants (Harborne, 1964; Rosenthal & Berenbaum, 1992; Walling, 2000; Odjakova & Hadjiivanova, 2001; Shahidi & Ho, 2005; Cory & Hoover, 2006; Vermerris & Nicholson, 2008; Bidel et al., 2010). These may be acquired and subsequently utilized by herbivores (Opitz & Muller, 2009). Many phenolics are efficient scavengers of oxidative free radicals, protecting cell membrane fatty acids, proteins, and DNA in plants (Shahidi & Ho, 2005; Vermerris & Nicholson, 2008; Bidel et al., 2010). This antioxidant activity has renewed nutritional and pharmaceutical interest in phenolics under the premise that they may have the capacity to prevent degenerative diseases such as cancers (Shahidi & Ho, 2005; Ferguson & Philpott, 2007; Hadi et al., 2007; Laranjinha, 2010) and cardiovascular diseases (Riemersma et al., 2001; Vermerris & Nicholson, 2008; Laranjinha, 2010), and they may also enhance immunity to and/or tolerance of infection in humans and other animals ingesting them.
Several studies have demonstrated correlations between ingested plant phenolics and attenuated virulence of viruses, bacteria, and fungi in herbivorous insects. However, the observed effects have primarily been attributed to a direct effect of the dietary phenolics on the pathogen in the insect’s gut or on the surface of foliage prior to ingestion (Koike et al., 1979; Felton et al., 1987, 1989; Felton & Duffey, 1990; Schultz et al., 1992; Young et al., 1995; Hoover et al., 1998; Cory & Hoover, 2006; Sandre et al., 2011). It remains unknown whether acquired plant phenolics can enhance the immune response of herbivorous insects and mediate protection from infectious disease.
The plant phenolic chlorogenic acid (CA, Figure 1) is an ester of caffeic acid and quinic acid and is widespread among vascular plants. Chlorogenic acid is found primarily in three isomer forms, neo-CA, CA, and crypto-CA in plants. Chlorogenic acid is a potent antioxidant (Shahidi & Chandrasekara, 2010) and antimicrobial agent (Almeida et al., 2006), as well as a deterrent to many insect herbivores (Rosenthal & Berenbaum, 1992; Vermerris & Nicholson, 2008). Larvae of the moth Manduca sexta L. (Lepidoptera: Sphingidae) are facultative specialists on Solanaceae (Yamamoto, 1974; del Campo et al., 2001), a plant family comprising more than a thousand species including tomato, tobacco, and potato. Chlorogenic acid and other caffeic acid derivatives are abundant in solanaceous plant tissues (Eich, 2008), and concentrations can range from few micrograms to several milligrams per gram of fresh foliage (Keinanen et al., 2001). Using M. sexta caterpillars, we test the hypothesis that plant phenolics acquired from the food may increase defense against pathogenic infection.
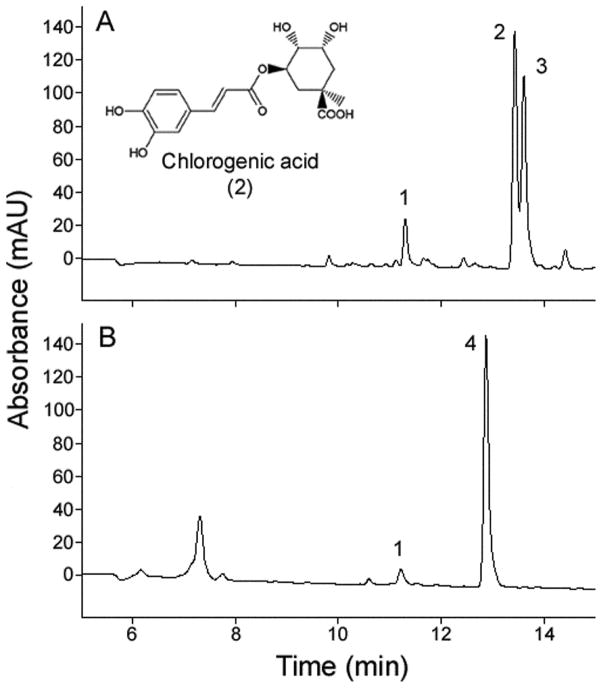
Figure 1.
Larvae of Manduca sexta acquired plant phenolics from solanaceous foliage. The plant phenolics neo-CA (1), CA (2), and crypto-CA (3), and an unknown CA-derivative compound (4), were identified in HPLC chromatograms (UV absorption at 320 nm) of (A) Nicotiana attenuata leaf sample, and (B) M. sexta larval hemolymph fed on N. attenuata (quantitative results are presented in Table 1).
Materials and methods
Insects, plants, artificial diets, and bacteria
Eggs of M. sexta were obtained from a laboratory colony maintained on a wheat germ-based diet (Yamamoto, 1969) at Liddell Laboratories at Cornell University. For larvae reared on plants, neonates were fed ad libitum on wild tobacco, Nicotiana attenuata L. (Solanaceae), in a greenhouse. For larvae reared on wheat germ-based diet, neonates were fed ad libitum in individual cups, and received fresh pieces of diet every 5 days. All larvae were grown at ca. 25 °C under a L16:D8 photoperiod during the complete duration of the experiments.
CA+ and CA− diets were prepared based on a recipe by Yamamoto (1969) for M. sexta larvae. Streptomycin was excluded from the mix. The CA+ diet was identical to the CA− diet, but was supplemented with CA (MP Biomedicals, Solon, OH, USA) at 200 μg g−1, a concentration commonly found in host plants of M. sexta. During the preparation of the artificial diet, all basic ingredients were mixed in liquid form at about 65 °C, and then split into two batches. One of the batches (the CA+ diet) received CA dissolved in ethanol, resulting in a concentration of CA of 200 μg g−1 of artificial diet, whereas the other batch of artificial diet (the CA− diet) received ethanol alone. Diets were allowed to cool to room temperature and were stored at 4 °C until use. Each batch of diet was used within 1 week of preparation.
Two bacteria, Enterococcus faecalis (Andrewes & Horder) Schleifer & Kilpper-Bälz and Pseudomonas aeruginosa (Schroeter) Migula, were used in the experimental infections. The strain of E. faecalis was originally isolated from the hemolymph and thoracic muscle of a wild-caught Drosophila melanogaster Meigen by BP Lazzaro in State College, PA, USA. The strain of P. aeruginosa used was the type strain PAO1. Bacterial cultures used for infection were each grown overnight in Luria broth (LB) at 37 °C prior to infection. On the day of infection, E. faecalis cultures were diluted with additional LB to A600 = 0.5, and then further diluted 1:100 in sterile LB. Each M. sexta larva was injected with 5 μl of this final dilution, delivering approximately 5 000 bacteria per injection. Cultures of P. aeruginosa were diluted on the day of infection to A600 = 1.0, then further diluted 1:100 000, yielding a 5-μl injection of 50 bacteria into each larva.
Phytochemical analyses of plants, insects, and artificial diet samples
Fresh samples from leaves and artificial diets from which larvae have been feeding were weighed and flash-frozen in liquid nitrogen. Hemolymph was sampled from larvae reared on N. attenuata, CA+ diet, or CA− diets on the 2nd or 3rd day after molting into the fourth instar. Hemolymph was bled from a cut on the tip of the dorsal horn and a 100 μl aliquot was taken for analysis. After hemolymph collection, larvae reared on CA+ and CA− diets were dorsally opened lengthwise with fine scissors, and the body cavity was flushed with insect saline. The complete intact gut and fat body were removed from each larva and extra fluids were quickly absorbed with a Kimwipe® from the remaining carcass. Gut tissue samples, fat bodies, and remaining carcasses were separately weighed and flash frozen in liquid nitrogen. Plant and insect samples were stored at −80 °C until used. Plant, insect, and artificial diet samples were extracted with 1 ml of 80% MeOH by shaking at 4 °C for 2 h. Similarly, 100 μl of hemolymph was diluted in 100 μl 80% MeOH and vortexed. After centrifugation (19,700 g at 4°C for 30 min), samples were analyzed for CA, its isomers, and caffeic acid derivatives content by high-performance liquid chromatography (HPLC) on a reversed phase column (Gemini C18, 150 × 4.6 mm; Phenomenex, Torrance, CA, USA) as described by Keinanen et al. (2001). The identity of CA isomers was confirmed by comparison of retention times and ultraviolet (UV) spectra with synthetic standards. Additionally, we performed liquid chromatography-mass spectrometry (LC-MS) analyses with similar chromatographic conditions replacing the 0.25% phosphoric acid in the aqueous HPLC solvent by a 0.1% formic acid solution. The identity of CA isomers was further confirmed by molecular ions of 355 [M+H]+ and 353 [M−H]− under positive and negative electrospray ionization.
Infection of larvae
All bacterial infections were performed on day 1 or 2 of the M. sexta fourth instars reared on CA+ or CA− diet. Immediately prior to injection, each larva was weighed and randomly assigned to one of three treatments: injection of bacteria (test), LB media (sham), or not injected at all (intact). Each larva was placed on ice for 5–10 min before injection. Injections of 5 μl were performed with a micro-syringe, dorsally at 1–2 mm from the base of the distal dorsal horn on the abdomen of each larva, taking care not to hurt the underlying hindgut. Larvae were then placed in their individual cups with a piece of their respective diets, CA+ or CA−, and positioned head down to prevent excessive bleeding and to speed wound healing. Early to mid fourth instars were selected for infections because they could recover well from cooling and the injury caused by injection alone. The survival of larvae was followed over the last two instars, i.e., the fourth and fifth.
Analysis of larval survival and bacterial load
Hemolymph samples were taken from infected and control (sham and intact) larvae at 24 ±1 h after infection by cutting about half of the dorsal horn with fine scissors. Immediately after the cut, a few droplets of hemolymph were collected in a plastic microtube and vortexed; 10 μl hemolymph aliquots were diluted 1:10 and 1:1 000 to cover a wide range in potential bacterial load. A 50-μl aliquot of each diluted hemolymph sample was plated on LB agar medium using a WASP II spiral plater (Microbiology International, Bethesda, MD, USA), and incubated at 37°C overnight. Bacterial colony counts were obtained for each hemolymph sample using the ProtoCOL plate counting system (Microbiology International). In the cases in which both dilutions yielded countable colonies, the average hemolymph bacterial density estimated from the two dilutions was used for the calculations. Natural log transformation of the bacterial load estimates provided an adequate fit to normality for ANOVA. Analysis of variance models included larval diet as an independent variable and larval mass as a covariant in the model. ANOVAs were performed using SPSS 16.0.
After the larvae were sampled for hemolymph, they were placed back in their individual cups with a fresh piece of diet, CA+ or CA−, and positioned head down to prevent excessive bleeding and to speed healing. Larval survival was monitored daily until all larvae had died or completed the final (= fifth) instar. Larvae surviving until the end of the fifth instar were allowed to pupate and emergence of adults was recorded. Survivorship data were collected daily until day 19 post-infection with E. faecalis, when the last surviving larvae began to wander and subsequently pupated, and until day 17 post-infection with P. aeruginosa, when the only surviving larva completed its last larval instar. All larvae completing the larval stage eventually emerged as healthy adults. Survivorship was analyzed using Cox regression analysis in SAS (SAS Institute, Cary, NC, USA), with larval diet and mass of the larvae at the time of infection included as independent variables in the model.
Analysis of hemocyte concentration in larval hemolymph
Hemocytes are central to the cellular immune system in insects, and, among other functions, phagocytose and eliminate pathogenic bacteria (Strand, 2008; Marmaras & Lampropoulou, 2009). Moreover, hemocytes are known to aggregate around infected tissues in E. faecalis-infected M. sexta larvae (Mason et al., 2011). To test whether dietary CA affects circulating hemocytes, hemolymph samples from uninfected larvae reared on CA+ or CA− diets were taken at day 1 or 2 of their fourth instar. The weight of each larva was recorded at the time of hemolymph sample. Larvae were returned to their individual cups with a piece of diet, CA+ or CA−, and positioned head down to prevent excessive bleeding and to speed healing. Larvae were given 24 h to recover from hemolymph sampling prior to infection. At this time, larvae were weighed and immediately injected with E. faecalis (1:100 dilution), as described above. Note that the animals infected in this experiment are distinct from those described above; the initial data on survivorship and bacterial load were collected from M. sexta that were not injured prior to infection.
After a 24-h period post-injection, surviving larvae were weighed, and hemolymph was sampled again. Aliquots of 10 μl hemolymph from each sample were pipetted onto a Bright-Line hemocytometer (Hausser Scientific) immediately after bleeding without dilution. The hemocytometer was viewed using an Olympus 1X71 inverted microscope, and the total number of hemocytes in a volume of hemolymph equal to 0.02 mm3 was counted. This value was then used to estimate the concentration of hemocytes in the hemolymph (number of hemocytes per ml of hemolymph). Because it is known that the concentration of circulating hemocytes in the hemolymph increases as M. sexta larvae grow within an instar, but then drastically drops just before and during a molt (Beetz et al., 2008), the concentration of hemocytes in the hemolymph per larva was adjusted by the corresponding body mass of the larva by dividing the hemocyte concentration by body mass of the larva at the time hemolymph was sampled. Hemocyte concentration per body mass of larvae did not follow a normal distribution, so data were log-transformed for statistical analysis. A repeated measures ANOVA was used to analyze the concentration of hemocytes in hemolymph per body mass in larvae reared on CA+ or CA− diets at 24 h before and 24 h after infection. Control larvae injected with LB media or left intact until the last hemolymph sampling developed more quickly than infected larvae, so their hemocyte counts were not directly comparable to those of treated animals because treated and control animals were at different instars, and the linear relationship between hemocyte concentration and body mass of the larvae only holds true within an instar. We therefore only contrasted the hemocyte counts from infected larvae, 24 h before and 24 h after infection. The independent variable in the model was larval diet. The independent repeated variable for each larva was time of hemolymph sampling, 24 h before and 24 h after bacterial infection. Larval mass at the time of infection was included as a covariate. Repeated measures ANOVA was conducted using SPSS 16.0.
Analysis of hemolymph phenoloxidase activity
Phenoloxidase (PO) is an important component of the humoral immune system of arthropods, and is essential for wound healing and defense against infection (Cerenius et al., 2008; González-Santoyo & Córdoba-Aguilar, 2012). To test whether dietary CA affects PO activity, hemolymph samples were taken from larvae reared on CA+ and CA− diets on the 2nd or 3rd day after they had molted into the fourth instar. Larvae were injected with E. faecalis, injected with LB media, or not injected at all and kept as an intact control group approximately 24 h before collection of hemolymph samples for PO activity analysis. Handling of the larvae and experimental procedures for injections were as described above. All larvae were weighed immediately prior to hemolymph collection. Hemolymph samples were collected from the dorsal horn. From each larva, two aliquots of 10 μl each were immediately diluted individually in 500 μl PBS (pH 7.0) in plastic microcentrifuge tubes, briefly vortexed, and immediately submerged in liquid nitrogen. Samples were stored at −80°C for no longer than 3 weeks before being assayed for PO activity. Samples were thawed on ice while they were covered with aluminum foil to prevent photo-degradation as much as possible. After thawing, each sample was briefly vortexed, and three 100 μl aliquots were transferred to wells in a microplate. To each well 100 μl of 4 mM dopamine was added and the plate was incubated at 30°C in a microplate reader (Synergy HT; Bio-Tek, Winooski, VT, USA). Absorbance at 492 nm was recorded every 10 min for 1 h, and then every hour for four more hours. Three replicate aliquots were measured per sample, and the absorbances were averaged. The linear phase of the PO reaction was analyzed. In addition, protein content per sample was measured in triplicate by adding 200 μl of Bradford’s solution (Sigma, St. Louis, MO, USA) to 10 μl of sample, incubating for 10 min at 25°C in the microplate reader and recording the absorbance at 595 nm. The protein content per sample was calculated from the average of the three replicates of each sample from a calibration curve constructed using bovine serum immunoglobulin (Sigma) as a protein standard. Phenoloxidase activity was then expressed as PO units per mg of total soluble protein in the hemolymph. Repeated measures ANOVAs were performed (using SPSS 16.0) to analyze the PO activity data, in which larval diet and type of injection were the independent variables, and larval mass at the time of the hemolymph sample collection was a covariate in the general linear model (GLM).
Results
Larvae of Manduca sexta can acquire phenolics from Nicotiana attenuata leaves
We detected neo-CA (compound 1 in Figure 1A), CA (compound 2), and crypto-CA (compound 3) in N. attenuata foliage on which M. sexta larvae had been feeding. The most abundant CA isomers in the foliage were CA and crypto-CA (Table 1). These CA isomers were also present in hemolymph of M. sexta larvae feeding on N. attenuata foliage (Figure 1B; Table 1), but not in the hemolymph of M. sexta fed on artificial diet lacking CA (Table 1). Chlorogenic acid derivatives occurred at lower concentrations in M. sexta hemolymph than in the plant foliage. The concentration of neo-CA in the hemolymph was almost 10-fold lower than in the foliage, and CA and crypto-CA were in trace concentrations in the hemolymph (Table 1). Interestingly, an unknown CA-derivative compound (compound 4 in Figure 1A) was found in low concentration in leaf tissue, but was found almost 10-fold higher in the hemolymph of larvae reared on N. attenuata (Table 1), suggesting that this may be a conversion product whose generation is catalyzed by the caterpillar. Liquid chromatography-MS analyses using positive and negative ionization yielded molecular ions with mass to charge ratios of m/z 296 and 294, respectively, suggesting a molecular weight of 295 for the unknown CA-derivative compound (4). Further tandem mass spectrometry (MS/MS) analyses in positive mode yielded a fragment with m/z 163, corresponding to the caffeic acid moiety indicated by the UV absorption spectra.
Table 1.
Mean (± SEM) concentration of chlorogenic acid (CA), its isomers, their sum, and unknown CA-derivative compound in plant, insect, CA+ diet, and CA− diet samples expressed in microgram per gram of fresh sample
| Plant-reared animals
|
Diet-reared animals
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Leaf tissue | Hemolymph | Diet
|
Gut
|
Hemolymph
|
Fat body
|
Carcass
|
||||
| CA− | CA+ | CA− | CA+ | CA− | CA+ | CA− | CA+ | CA− | ||
| n = 6 | n = 11 | n = 5 | n = 5 | n = 8 | n = 7 | n = 10 | n = 10 | n = 8 | n = 7 | n = 9 |
| 25.6 ± 5.4 | 2.4 ± 0.4 | n.d. | 90.4 ± 3.6 | n.d. | 6.4 ± 0.6 | n.d. | 0.1 ± 0.03 | 0.03 ± 0.03a | 0.2 ± 0.1a | n.d. |
| 176.1 ± 42.6 | 0.09 ± 0.03 | 0.2 ± 0.1a | 215.0 ± 6.2b | 0.07 ± 0.04a | 3.3 ± 0.4b | n.d. | n.d. | 0.1 ± 0.04a | 0.7 ± 0.3b | 0.02 ± 0.02a |
| 147.8 ± 34.9 | 0.3 ± 0.1 | n.d. | n.d. | 0.06 ± 0.02a | 5.3 ± 0.4b | n.d. | n.d. | n.d. | 0.1 ± 0.07 | 0.05 ± 0.05a |
| 349.5 ± 81.5 | 2.8 ± 0.5 | 0.2 ± 0.1a | 305.4 ± 8.3b | 0.1 ± 0.06a | 14.9 ± 1.1b | n.d. | 0.1 ± 0.03 | 0.1 ± 0.06a | 1.1 ± 0.3b | 0.07 ± 0.07a |
| 1.9 ± 0.6 | 17.9 ± 3.7 | 0.08 ± 0.05a | 0.1 ± 0.03a | 0.03 ± 0.02a | 1.7 ± 0.2b | n.d. | 0.1 ± 0.02 | n.d. | 0.1 ± 0.05 | 0.08 ± 0.03a |
n.d., not detected.
Different letters following means within a category indicate significant differences between CA+ and CA− diets (independent t-tests: P<0.05).
Acquisition of CA from artificial diet
We compared phytochemical profiles of M. sexta larvae reared on CA− diet to larvae reared on CA+ diet. We confirmed that CA isomers and the unknown CA-derivative compound (compound 4 in Figure 1A) were absent or present in minute concentrations in the CA− diet. Chlorogenic acids and the unknown CA-derivative compound (4) were present in the CA+ diet at concentrations comparable to those detected in N. attenuata foliage (Table 1). Crypto-CA was not detected in the CA+ diet.
We additionally performed phytochemical analyses of the gut (n = 15), hemolymph (n = 20), fat body (n = 15), and remaining carcass (n = 17) of larvae fed on CA− and CA+ diets (Table 1). The guts of larvae reared on CA+ diet had significantly higher concentrations of CA, neo-CA, crypto-CA, and the unknown CA-derivative compound than guts of larvae reared on CA− diet (independent t-tests: P<0.05). All four compounds were found at higher concentrations in the fat bodies and carcasses of larvae reared on the CA+ diets compared with larvae reared on the CA− diet (independent t-tests: P<0.05, in all cases except neo-CA in the fat body: P = 0.06). Hemolymph of larvae reared on CA+ diet contained significantly higher concentrations of neo-CA and the unknown CA-derivative compound (4) than hemolymph of larvae reared on CA− diet (independent t-tests: P<0.05; n = 20,). Thus, CA is assimilated from the diet in the gut into the body by M. sexta larvae, and it is present in most of the larval tissues in much smaller amounts than in the ingested food. Moreover, CA seems to isomerize and to conjugate in the larval guts, so that the resulting isomers, neo-CA (compound 1 in Figure 1A) and crypto-CA (3), and conjugate (4) become present at significantly elevated relative levels in most of the larval tissues analyzed.
Dietary CA promotes survival in bacterially infected Manduca sexta larvae
We tested the hypothesis that acquired CA could contribute to the ability of M. sexta larvae to survive bacterial infection. Over 30% of the M. sexta fed on CA+ diet survived infection with the Gram-positive bacterium E. faecalis for 11 days post-infection, whereas only 5% of larvae fed on CA− diet survived this long. Larvae fed on CA+ diet were significantly more likely to survive infection with E. faecalis than larvae fed on CA− diet over the 19-day experiment (Figure 2A; Cox’s regression analysis: hazard ratio = 2.053, P<0.001; n = 114). Moreover, 100% of larvae surviving to the end of the 19-day experiment were able to pupate and to emerge as healthy adults weeks later. Larval size was also a significant predictor of survival after infection with E. faecalis irrespective of diet, with larger larvae having a higher probability of survival (hazard ratio = 0.237, P = 0.001; n = 114). No increase in survival was seen when M. sexta larvae fed on CA+ diet were infected with the much more virulent Gram-negative bacterium P. aeruginosa (Figure 2B; hazard ratio = 0.751, P>0.05; n = 44). In this treatment, >90% of caterpillars on both diets died within 4 days, with the severity of infection presumably overwhelming any protective effect of the chlorogenic acid. The vast majority of control larvae successfully completed larval development and emerged as healthy adults (Figure 2C). The probability of survival among control larvae did not significantly differ across CA+ or CA− diet (P>0.05), regardless of whether they were non-injected (n = 15) or injected with only media (n = 18).
Figure 2.
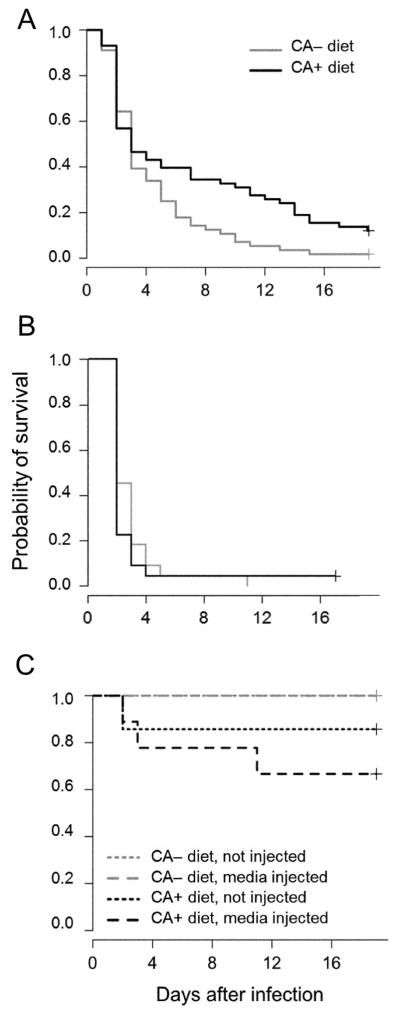
Dietary chlorogenic acid (CA) increased survivorship of Manduca sexta larvae. Survival of M. sexta larvae when raised on CA+ or CA− diet and subsequently infected with (A) Enterococcus faecalis (n = 114 larvae), or with (B) Pseudomonas aeruginosa (n = 44 larvae). Control larvae were either not injected (n = 15) or injected with Luria broth (LB) media (n = 18 larvae).
Dietary CA does not restrict bacterial proliferation in infected Manduca sexta larvae
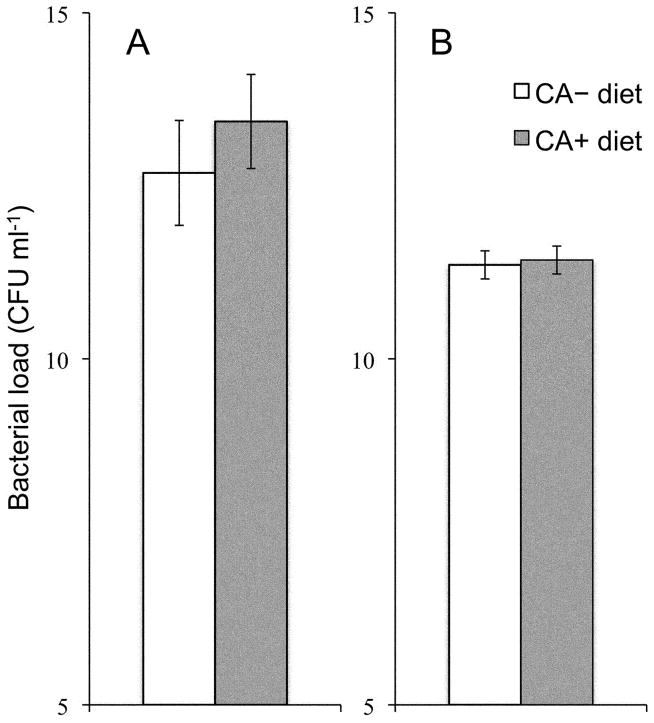
Increased ability of M. sexta larvae to survive bacterial infection could in principle be due to increased ability to eliminate infecting bacteria (e.g., through enhanced immune activity). We measured the number of bacteria in the hemolymph of infected larvae 24 h after infection. Supplementation of the diet with CA had no effect on proliferation of either E. faecalis (ANOVA: F1,37 = 0.080, P>0.05; n = 40) or P. aeruginosa (F1,41 = 1.194, P>0.05; n = 44), with caterpillars in both dietary treatments carrying bacterial loads of approximately 5×105 E. faecalis or 6×104 P. aeruginosa per ml of hemolymph (Figure 3). We detected no bacteria in hemolymph of larvae sham-infected with sterile media. Larval mass at the time of infection was a significant predictor of bacterial density in the hemolymph 24 h after infection, with smaller larvae carrying higher bacterial loads (ANOVA; E. faecalis: F1,37 = 10.659, P<0.005; n = 40; P. aeruginosa: F1,41 = 4.848, P<0.05; n = 44).
Figure 3.
Dietary chlorogenic acid (CA) did not affect bacterial load in hemolymph of Manduca sexta larvae 24 h after being infected with pathogens. Bacterial load [mean number of the natural logarithm of colony forming units (CFU) per ml hemolymph ± SEM) in hemolymph of M. sexta larvae reared on CA+ or CA− diet, and subsequently infected with (A) Enterococcus faecalis (n = 40 larvae), or (B) Pseudomonas aeruginosa (n = 44 larvae).
Effects of dietary CA on hemocytes in larval hemolymph
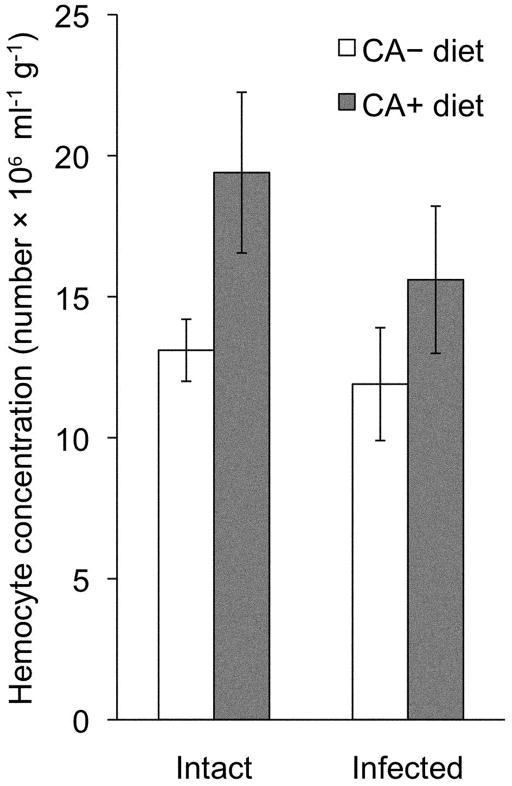
Larvae fed CA+ diet had a higher number of circulating hemocytes in their hemolymph than larvae reared on CA− diet, both 24 h before and 24 h after infection (Figure 4; repeated measures ANOVA; diet: F1,25 = 4.702, P<0.05; n = 27 larvae). Injection of bacteria did not itself induce a significant change in hemocyte number during a 24-h period post infection (repeated measures ANOVA; time of sampling: F1,25 = 0.815, P<0.05; n = 27 larvae, and two sampling times per larva). Thus, CA supplementation increased constitutive hemocyte number in M. sexta larvae, although, as reported above, this does not translate into increased control of bacterial proliferation at an early stage of infection.
Figure 4.
Dietary chlorogenic acid (CA) increased the constitutive number of circulating hemocytes in Manduca sexta larvae. Hemocyte concentration [mean (± SEM) number per ml hemolymph] per g body mass of M. sexta larvae reared on CA+ or CA− diet, in intact larvae (A) 24 h before infection and (B) 24 h after infection with Enterococcus faecalis. Larvae are the same sampled animals, before and after infection (n = 27 larvae).
Effects of dietary CA on hemolymph phenoloxidase (PO) activity
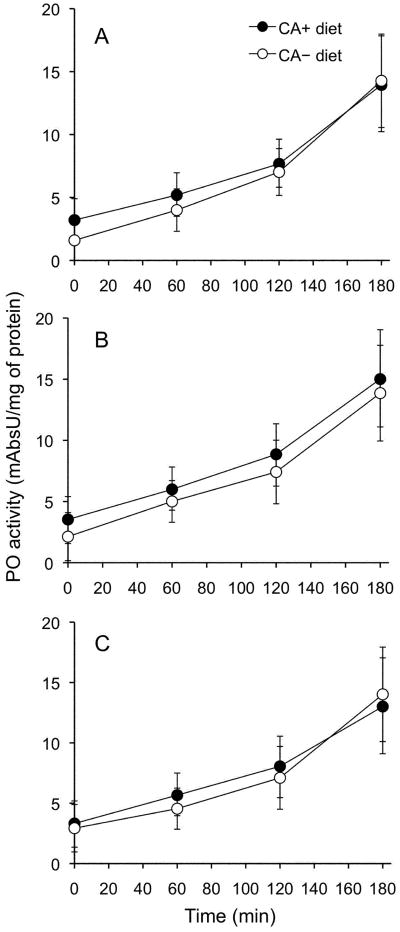
Dietary CA did not affect the hemolymph PO activity of M. sexta larvae injected with either sterile media (n = 30) or E. faecalis (n = 44), or of non-manipulated control larvae (n = 30) (Figure 5; repeated measures ANOVA, between and within analyses; diet: F1,101 = 0.065, P>0.05). Phenoloxidase activity was not induced in caterpillars from either diet 24 h after injection with E. faecalis (Figure 5A) or sterile media (Figure 5B) compared to non-manipulated controls (Figure 5C) (repeated measure ANOVA, between and within analyses; type of injection: F2,101 = 0.224, P>0.05). Thus, we conclude that dietary CA does not affect PO levels in the hemolymph of M. sexta larvae.
Figure 5.
Dietary chlorogenic acid (CA) did not affect the Manduca sexta larvae hemolymph’s phenoloxidase (PO) activity on dopamine. Mean (± SEM) PO activity of hemolymph samples from M. sexta larvae reared on CA+ or CA− diet, and subsequently infected with (A) Enterococcus faecalis (n = 44 larvae), (B) injected with the control, Luria broth (LB) media (n = 30 larvae), or (C) non-manipulated controls (n = 30 larvae).
Discussion
Our results demonstrate that an herbivorous caterpillar, M. sexta, acquires the plant defensive phenolic compound chlorogenic acid from host plant leaves or artificial diet, and that acquired CA and/or its chemical derivatives may confer increased defense against bacterial infection. Manduca sexta provided with CA are better able to survive E. faecalis bacterial infection than M. sexta deprived of CA. At 24 h post-infection, M. sexta provided with CA show no change in the ability to suppress bacterial growth, nor do they demonstrate increases in standard measures of immune system activity. At 4 days post-infection, however, M. sexta larvae fed on CA begin to survive significantly better than control larvae. Thus, larvae acquire and assimilate CA and its derivatives in small amounts, and CA exposure results in a constitutive higher number of circulating hemocytes, which could contribute to either resistance or tolerance to pathogens.
Previous reports indicate that dietary phenolics may undergo intensive isomerization, conjugation, and break down in the alkaline environment of caterpillar’s guts (Barbehenn et al., 2003, 2005; Cory & Hoover, 2006). These restructured chemical derivatives have been speculated to react and inhibit proliferation of ingested pathogens such as viruses (Felton et al., 1987, 1989; Felton & Duffey, 1990; Schultz et al., 1992; Young et al., 1995; Hoover et al., 1998; Cory & Hoover, 2006), fungi (Cory & Hoover, 2006; Sandre et al., 2011), and bacteria (Koike et al., 1979; Cory & Hoover, 2006) in the insect gut, inhibiting invasive pathogens before they can establish an infection. We infected larvae by direct injection of bacteria into their body cavities, thus preventing any direct contact of the infectious pathogen with ingested CA and its derivatives in the insect’s gut or diet. We found that CA and caffeic acid derivatives can be extracted from food and stored in larval tissues. Because infected M. sexta provided with dietary CA show increased survivorship, it appears that the role of plant phenolics in insect defense against infection may be more complex than simple toxicity to the pathogen that derives from the leaf substrate or characteristics of the insect gut milieu (Koike et al., 1979; Felton et al., 1987, 1989; Felton & Duffey, 1990; Schultz et al., 1992; Young et al., 1995; Hoover et al., 1998; Cory & Hoover, 2006; Sandre et al., 2011).
Acquisition of trace amounts of dietary phenolics has previously been correlated with improved defense against infection in humans and other mammals (Scalbert et al., 2002; Laranjinha, 2010; Nichols & Katiyar, 2010). In one well-studied example, the phenolic curcumin, present in the herb turmeric (Curcuma longa L.), is poorly absorbed by the intestine and undergoes severe reduction and conjugation prior to elimination via the intestine, liver, and kidneys (Anand et al., 2007). Curcumin is therefore present only transiently and in trace amounts in the animal’s tissues after consumption, yet dietary curcumin exerts pleiotropic cytoprotective and anti-inflammatory effects (Anand et al., 2007; Shapiro et al., 2009). Laboratory trials in mammalian experimental models suggest that these effects likely confer protection against microbial sepsis and other diseases (Shapiro et al., 2009). Thus, absorption of minute amounts of CA, its isomers, and the unknown CA-derivative compound from food by M. sexta larvae may fall into a more common phenomenon where compounds of low bioavailability have important immunological functions. Moreover, phenolics could also be toxic at higher concentrations (Rosenthal & Berenbaum, 1992), so that there may be a trade-off between the immune system-enhancing and toxic effects that necessitates maintaining relatively low concentrations of the compounds in the body.
Although CA and other caffeic acids are known to be effective antibacterial agents at higher concentrations (Almeida et al., 2006), the low bioavailability of the acquired phenolics from CA-supplemented diet by M. sexta larvae is in agreement with our findings that bacterial proliferation is not controlled by their antibiotic properties. Dietary CA and other secondary plant metabolites ingested by Helicoverpa zea (Boddie) caterpillars regulate expression of detoxification cytochrome P450 genes in the gut, fat body, and ovary (Li et al., 2002a, b). Thus, it is possible that phenolics’ acquired from food up- or down-regulate expression of molecular and/or cellular effectors, which mediate survival to bacterial infection in M. sexta larvae. We found that even before infection, larvae fed on CA-supplemented diet had constitutively higher numbers of circulating hemocytes than larvae deprived from dietary CA. Hemocytes can be phagocytically active and produce and release most of the humoral components of the insect’s immune system (Lavine & Strand, 2002; Hillyer et al., 2003; Strand, 2008; Marmaras & Lampropoulou, 2009) such as phenoloxidases, which are essential enzymes in melanization and wound healing, and immunopeptides, which are involved in immune responses’ signaling cascades, pathogen recognition, and killing (Jiang et al., 1998; Ji et al., 2004; Kanost et al., 2004; Nappi & Christensen, 2005; Jiang, 2008; Franssens et al., 2008; Kim et al., 2008; An et al., 2009). Several of these mechanisms of defense against pathogens, especially those involving signaling cascades and production of peptides and proteins, may take time and not be obvious at the early stages of infection. Thus, increased hemocyte number as a consequence of dietary CA may facilitate the fight against infection at time points beyond those we measured. As alternative mechanisms, dietary CA may increase survivorship through an effect on general health, or other unknown mechanism.
It is well established that mammals (Victor et al., 2000; Bhaskaram, 2002; Rivera et al., 2003; Cunningham-Rundles et al., 2005; Heyland et al., 2005; Wintergerst et al., 2007; Mizock, 2010) and insects (Lee et al., 2006; Raymond & Hails, 2007) derive increased tolerance to diseases from a diet containing a balanced complement of macronutrients, vitamins, and minerals. Interestingly, this perspective has recently been expanded to include other dietary compounds, including dietary phenolics for tolerance and/or prevention of diseases in humans and other vertebrates (Riemersma et al., 2001; Scalbert et al., 2002; Plat & Mensink, 2005; Shahidi & Ho, 2005; Ferguson & Philpott, 2007; Hadi et al., 2007; Romeo et al., 2007; Clarke & Mullin, 2008; Magrone et al., 2008; Vermerris & Nicholson, 2008; Shapiro et al., 2009; Laranjinha, 2010). Plant secondary metabolites such as phenolics (Rosenthal & Berenbaum, 1992) have a function in protection against insect herbivory, but we have shown that insects may also acquire phenolics from their food and use them to enhance defense against pathogen infection. This dual function of plant secondary metabolites can theoretically be predicted as a direct result of a coevolutionary arms race between plants and their herbivores (Ehrlich & Raven, 1964), but as a concept it has yet to be incorporated into a more integrative functional analysis of organismal interactions mediated by phytochemicals.
Acknowledgments
We thank Angela Douglas and Georg Jander for their critical reading of the manuscript. For help in statistics and data interpretation, we thank Simona Despa. For advice in designing PO analyses we thank Courtney Couch. We also thank the Department of Neurobiology and Behavior at Cornell University for allowing us to grow insects and plants at Liddell laboratory facilities, and the Cornell Chemical Ecology Group Core facility for phytochemical analysis. This work was supported in part by the National Institutes of Health grant R01-AI083932 to BPL, the National Science Foundation grant IOB-0950225 to AK, and a Ford Foundation Diversity Fellowship to SS.
References
- Almeida AAP, Farah A, Silva DAM, Nunan EA, Gloria MBA. Antibacterial activity of coffee extracts and selected coffee chemical compounds against enterobacteria. Journal of Agricultural and Food Chemistry. 2006;54:8738–8743. doi: 10.1021/jf0617317. [DOI] [PubMed] [Google Scholar]
- An CJ, Ishibashi J, Ragan EJ, Jiang HB, Kanost MR. Functions of Manduca sexta hemolymph proteinases HP6 and HP8 in two innate immune pathways. Journal of Biological Chemistry. 2009;284:19716–19726. doi: 10.1074/jbc.M109.007112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: Problems and promises. Molecular Pharmaceutics. 2007;4:807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- Barbehenn R, Cheek S, Gasperut A, Lister E, Maben R. Phenolic compounds in red oak and sugar maple leaves have prooxidant activities in the midgut fluids of Malacosoma disstria and Orgyia leucostigma caterpillars. Journal of Chemical Ecology. 2005;31:969–988. doi: 10.1007/s10886-005-4242-4. [DOI] [PubMed] [Google Scholar]
- Barbehenn RV, Walker AC, Uddin F. Antioxidants in the midgut fluids of a tannin-tolerant and a tannin-sensitive caterpillar: Effects of seasonal changes in tree leaves. Journal of Chemical Ecology. 2003;29:1099–1116. doi: 10.1023/a:1023873321494. [DOI] [PubMed] [Google Scholar]
- Beetz S, Holthusen TK, Koolman J, Trenczek T. Correlation of hemocyte counts with different developmental parameters during the last larval instar of the tobacco hornworm, Manduca sexta. Archives of Insect Biochemistry and Physiology. 2008;67:63–75. doi: 10.1002/arch.20221. [DOI] [PubMed] [Google Scholar]
- Bhaskaram P. Micronutrient malnutrition, infection, and immunity: An overview. Nutrition Reviews. 2002;60:S40–S45. doi: 10.1301/00296640260130722. [DOI] [PubMed] [Google Scholar]
- Bidel LPR, Coumans M, Baissac Y, Doumas P, Jay-Allemand C. Biological activity of phenolics in plant cells. In: Santos-Buelga C, Escribano-Bailon MT, Lattanzio V, editors. Recent Advances in Polyphenol Research. Vol. 2. Wiley-Blackwell; Oxford, UK: 2010. pp. 163–205. [Google Scholar]
- Cerenius L, Lee BL, Soderhall K. The proPO-system: pros and cons for its role in invertebrate immunity. Trends in Immunology. 2008;29:263–271. doi: 10.1016/j.it.2008.02.009. [DOI] [PubMed] [Google Scholar]
- Clarke JO, Mullin GE. A review of complementary and alternative approaches to immunomodulation. Nutrition in Clinical Practice. 2008;23:49–62. doi: 10.1177/011542650802300149. [DOI] [PubMed] [Google Scholar]
- Cory JS, Hoover K. Plant-mediated effects in insect-pathogen interactions. Trends in Ecology & Evolution. 2006;21:278–286. doi: 10.1016/j.tree.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Cunningham-Rundles S, McNeeley DF, Moon A. Mechanisms of nutrient modulation of the immune response. Journal of Allergy and Clinical Immunology. 2005;115:1119–1128. doi: 10.1016/j.jaci.2005.04.036. [DOI] [PubMed] [Google Scholar]
- del Campo ML, Miles CI, Schroeder FC, Mueller C, Booker R, Renwick JA. Host recognition by the tobacco hornworm is mediated by a host plant compound. Nature. 2001;411:186–189. doi: 10.1038/35075559. [DOI] [PubMed] [Google Scholar]
- Ehrlich PR, Raven PH. Butterflies and plants - a study in coevolution. Evolution. 1964;18:586–608. [Google Scholar]
- Eich E. Solanaceae and Convolvulaceae: Secondary Metabolites: Biosynthesis, Chemotaxonomy, Biological and Economic Significance (A Handbook) Springer; Berlin, Germany: 2008. [Google Scholar]
- Felton GW, Donato K, Del Vecchio RJ, Duffey SS. Activation of plant foliar oxidases by insect feeding reduces nutritive quality of foliage for noctuid herbivores. Journal of Chemical Ecology. 1989;15:2667–2694. doi: 10.1007/BF01014725. [DOI] [PubMed] [Google Scholar]
- Felton GW, Duffey SS. Inactivation of baculovirus by quinones formed in insect-damaged plant tissues. Journal of Chemical Ecology. 1990;16:1221–1236. doi: 10.1007/BF01021021. [DOI] [PubMed] [Google Scholar]
- Felton GW, Duffey SS, Vail PV, Kaya HK, Manning J. Interaction of nuclear polyhedrosis virus with catechols - potential incompatibility for host plant resistance against noctuid larvae. Journal of Chemical Ecology. 1987;13:947–957. doi: 10.1007/BF01020174. [DOI] [PubMed] [Google Scholar]
- Ferguson LR, Philpott M. Cancer prevention by dietary bioactive components that target the immune response. Current Cancer Drug Targets. 2007;7:459–464. doi: 10.2174/156800907781386605. [DOI] [PubMed] [Google Scholar]
- Franssens V, Simonet G, Breugelmans B, van Soest S, van Hoef V, Broeck JV. The role of hemocytes, serine protease inhibitors and pathogen-associated patterns in prophenoloxidase activation in the desert locust, Schistocerca gregaria. Peptides. 2008;29:235–241. doi: 10.1016/j.peptides.2007.07.032. [DOI] [PubMed] [Google Scholar]
- González-Santoyo I, Córdoba-Aguilar A. Phenoloxidase: a key component of the insect immune system. Entomologia Experimentalis et Applicata. 2012;142:1–16. [Google Scholar]
- Hadi SM, Bhat SH, Azmi AS, Hanif S, Shamim U, Ullah MF. Oxidative breakage of cellular DNA by plant polyphenols: A putative mechanism for anticancer properties. Seminars in Cancer Biology. 2007;17:370–376. doi: 10.1016/j.semcancer.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Harborne JB. Biochemistry of Phenolic Compounds. Academic Press; New York, NY, USA: 1964. [Google Scholar]
- Heyland DK, Dhaliwal R, Suchner U, Berger MM. Antioxidant nutrients: a systematic review of trace elements and vitamins in the critically ill patient. Intensive Care Medicine. 2005;31:327–337. doi: 10.1007/s00134-004-2522-z. [DOI] [PubMed] [Google Scholar]
- Hillyer JF, Schmidt SL, Christensen BM. Hemocyte-mediated phagocytosis and melanization in the mosquito Armigeres subalbatus following immune challenge by bacteria. Cell and Tissue Research. 2003;313:117–127. doi: 10.1007/s00441-003-0744-y. [DOI] [PubMed] [Google Scholar]
- Hoover K, Kishida KT, DiGiorgio LA, Workman J, Alaniz SA, et al. Inhibition of baculoviral disease by plant-mediated peroxidase activity and free radical generation. Journal of Chemical Ecology. 1998;24:1949–2001. [Google Scholar]
- Ji CY, Wang Y, Guo XP, Hartson S, Jiang HB. A pattern recognition serine proteinase triggers the prophenoloxidase activation cascade in the tobacco hornworm, Manduca sexta. Journal of Biological Chemistry. 2004;279:34101–34106. doi: 10.1074/jbc.M404584200. [DOI] [PubMed] [Google Scholar]
- Jiang HB. The biochemical basis of antimicrobial responses in Manduca sexta. Insect Science. 2008;15:53–66. [Google Scholar]
- Jiang HB, Wang Y, Kanost MR. Pro-phenol oxidase activating proteinase from an insect, Manduca sexta: A bacteria-inducible protein similar to Drosophila easter. Proceedings of the National Academy of Sciences of the USA. 1998;95:12220–12225. doi: 10.1073/pnas.95.21.12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanost MR, Jiang HB, Yu XQ. Innate immune responses of a lepidopteran insect, Manduca sexta. Immunological Reviews. 2004;198:97–105. doi: 10.1111/j.0105-2896.2004.0121.x. [DOI] [PubMed] [Google Scholar]
- Keinanen M, Oldham NJ, Baldwin IT. Rapid HPLC screening of jasmonate-induced increases in tobacco alkaloids, phenolics, and diterpene glycosides in Nicotiana attenuata. Journal of Agricultural and Food Chemistry. 2001;49:3553–3558. doi: 10.1021/jf010200+. [DOI] [PubMed] [Google Scholar]
- Kim CH, Park JW, Ha NC, Kang HJ, Lee BL. Innate immune response in insects: recognition of bacterial peptidoglycan and amplification of its recognition signal. BMB Reports. 2008;41:93–101. doi: 10.5483/bmbrep.2008.41.2.093. [DOI] [PubMed] [Google Scholar]
- Koike S, Iizuka T, Mizutani J. Determination of caffeic acid in the digestive juice of silkworm larvae and its antibacterial activity against the pathogenic Streptococcus faecalis AD-4. Agricultural and Biological Chemistry. 1979;43:1727–1731. [Google Scholar]
- Laranjinha J. Translation of chemical properties of polyphenols into biological activity with impact on human health. In: Santos-Buelga C, Escribano-Bailon MT, Lattanzio V, editors. Recent Advances in Polyphenol Research. Vol. 2. Wiley-Blackwell; Oxford, UK: 2010. pp. 163–205. [Google Scholar]
- Lavine MD, Strand MR. Insect hemocytes and their role in immunity. Insect Biochemistry and Molecular Biology. 2002;32:1295–1309. doi: 10.1016/s0965-1748(02)00092-9. [DOI] [PubMed] [Google Scholar]
- Lee KP, Cory JS, Wilson K, Raubenheimer D, Simpson SJ. Flexible diet choice offsets protein costs of pathogen resistance in a caterpillar. Proceedings of the Royal Society of London B. 2006;273:823–829. doi: 10.1098/rspb.2005.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XC, Berenbaum MR, Schuler MA. Plant allelochemicals differentially regulate Helicoverpa zea cytochrome P450 genes. Insect Molecular Biology. 2002a;11:343–351. doi: 10.1046/j.1365-2583.2002.00341.x. [DOI] [PubMed] [Google Scholar]
- Li XC, Schuler MA, Berenbaum MR. Jasmonate and salicylate induce expression of herbivore cytochrome P450 genes. Nature. 2002b;419:712–715. doi: 10.1038/nature01003. [DOI] [PubMed] [Google Scholar]
- Magrone T, Candore G, Caruso C, Jirillo E, Covelli V. Polyphenols from red wine modulate immune responsiveness: Biological and clinical significance. Current Pharmaceutical Design. 2008;14:2733–2748. doi: 10.2174/138161208786264098. [DOI] [PubMed] [Google Scholar]
- Marmaras VJ, Lampropoulou M. Regulators and signalling in insect haemocyte immunity. Cellular Signalling. 2009;21:186–195. doi: 10.1016/j.cellsig.2008.08.014. [DOI] [PubMed] [Google Scholar]
- Mason KL, Stepien TA, Blum JE, Holt JF, Labbe NH, et al. From commensal to pathogen: Translocation of Enterococcus faecalis from the midgut to the hemocoel of Manduca sexta. mBio. 2011;2(3):e00065–11. doi: 10.1128/mBio.00065-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizock BA. Immunonutrition and critical illness: An update. Nutrition. 2010;26:701–707. doi: 10.1016/j.nut.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Nappi AJ, Christensen BM. Melanogenesis and associated cytotoxic reactions: Applications to insect innate immunity. Insect Biochemistry and Molecular Biology. 2005;35:443–459. doi: 10.1016/j.ibmb.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Nichols JA, Katiyar SK. Skin photoprotection by natural polyphenols: anti-inflammatory, antioxidant and DNA repair mechanisms. Archives of Dermatological Research. 2010;302:71–83. doi: 10.1007/s00403-009-1001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida R. Sequestration of defensive substances from plants by Lepidoptera. Annual Review of Entomology. 2002;47:57–92. doi: 10.1146/annurev.ento.47.091201.145121. [DOI] [PubMed] [Google Scholar]
- Odjakova M, Hadjiivanova C. Bulgarian Journal of Plant Physiology. 2001;27:101–109. [Google Scholar]
- Opitz SEW, Muller C. Plant chemistry and insect sequestration. Chemoecology. 2009;19:117–154. [Google Scholar]
- Plat J, Mensink RP. Food components and immune function. Current Opinion in Lipidology. 2005;16:31–37. doi: 10.1097/00041433-200502000-00007. [DOI] [PubMed] [Google Scholar]
- Raymond B, Hails RS. Variation in plant resource quality and the transmission and fitness of the winter moth, Operophtera brumata nucleopolyhedrovirus. Biological Control. 2007;41:237–245. [Google Scholar]
- Riemersma RA, Rice-Evans CA, Tyrrell RM, Clifford MN, Lean MEJ. Tea flavonoids and cardiovascular health. QJM-an International Journal of Medicine. 2001;94:277–282. doi: 10.1093/qjmed/94.5.277. [DOI] [PubMed] [Google Scholar]
- Rivera MT, De Souza AP, Araujo-Jorge TC, De Castro SL, Vanderpas J. Trace elements, innate immune response and parasites. Clinical Chemistry and Laboratory Medicine. 2003;41:1020–1025. doi: 10.1515/CCLM.2003.156. [DOI] [PubMed] [Google Scholar]
- Romeo J, Waernberg J, Nova E, Diaz LE, Gomez-Martinez S, Marcos A. Moderate alcohol consumption and the immune system: A review. British Journal of Nutrition. 2007;98:S111–S115. doi: 10.1017/S0007114507838049. [DOI] [PubMed] [Google Scholar]
- Rosenthal GA, Berenbaum MR. Herbivores: Their Interactions with Secondary Plant Metabolites. Academic Press; San Diego, CA, USA: 1992. [Google Scholar]
- Sandre SL, Tammaru T, Hokkanen HMT. Pathogen resistance in the moth Orgyia antiqua: direct influence of host plant dominates over the effects of individual condition. Bulletin of Entomological Research. 2011;101:107–114. doi: 10.1017/S0007485310000258. [DOI] [PubMed] [Google Scholar]
- Scalbert A, Morand C, Manach C, Remesy C. Absorption and metabolism of polyphenols in the gut and impact on health. Biomedicine & Pharmacotherapy. 2002;56:276–282. doi: 10.1016/s0753-3322(02)00205-6. [DOI] [PubMed] [Google Scholar]
- Schultz JC, Hunter MD, Appel HM. Antimicrobial activity of polyphenols mediates plant-herbivore interactions. In: Hemingway RW, Laks PE, editors. Plant Polyphenols: Biogenesis, Chemical Properties, and Significance. Plenum Press; New York, NY, USA: 1992. pp. 621–637. [Google Scholar]
- Shahidi F, Chandrasekara A. Hydroxycinnamates and their in vitro and in vivo antioxidant activities. Phytochemistry Reviews. 2010;9:147–170. [Google Scholar]
- Shahidi F, Ho CT. Phenolic Compounds in Food and Natural Health Products. American Chemical Society; Washington, DC, USA: 2005. [Google Scholar]
- Shapiro H, Lev S, Cohen J, Singer P. Polyphenols in the prevention and treatment of sepsis syndromes: rationale and pre-clinical evidence. Nutrition. 2009;25:981–997. doi: 10.1016/j.nut.2009.02.010. [DOI] [PubMed] [Google Scholar]
- Strand MR. The insect cellular immune response. Insect Science. 2008;15:1–14. [Google Scholar]
- Vermerris W, Nicholson R. Phenolic Compound Biochemistry. Springer; Dordrecht, The Netherlands: 2008. [Google Scholar]
- Victor VV, Guayerbas N, Puerto M, Medina S, De la Fuente M. Ascorbic acid modulates in vitro the function of macrophages from mice with endotoxic shock. Immunopharmacology. 2000;46:89–101. doi: 10.1016/s0162-3109(99)00162-9. [DOI] [PubMed] [Google Scholar]
- Walling LL. The myriad plant responses to herbivores. Journal of Plant Growth Regulation. 2000;19:195–216. doi: 10.1007/s003440000026. [DOI] [PubMed] [Google Scholar]
- Wintergerst ES, Maggini S, Hornig DH. Contribution of selected vitamins and trace elements to immune function. Annals of Nutrition and Metabolism. 2007;51:301–323. doi: 10.1159/000107673. [DOI] [PubMed] [Google Scholar]
- Yamamoto RT. Mass rearing of tobacco hornworm. 2. Larval rearing and pupation. Journal of Economic Entomology. 1969;62:1427–1431. [Google Scholar]
- Yamamoto RT. Induction of host plant specificity in tobacco hornworm, Manduca sexta. Journal of Insect Physiology. 1974;20:641–650. [Google Scholar]
- Young SY, Yang JG, Felton GW. Inhibitory effects of dietary tannins on the infectivity of a nuclear polyhedros virus to Helicoverpa zea (Noctuidae, Lepidoptera) Biological Control. 1995;5:145–150. [Google Scholar]