Abstract
Protein tyrosine phosphatases (PTPs) constitute a large enzyme family with important biological functions. Inhibition of PTP activity through reversible oxidation of the active-site cysteine residue is emerging as a general, yet poorly characterized, regulatory mechanism. In this study, we describe a generic antibody-based method for detection of oxidation-inactivated PTPs. Previous observations of oxidation of receptor-like PTP (RPTP) α after treatment of cells with H2O2 were confirmed. Platelet-derived growth factor (PDGF)-induced oxidation of endogenous SHP-2, sensitive to treatment with the phosphatidylinositol 3-kinase inhibitor LY294002, was demonstrated. Furthermore, oxidation of RPTPα was shown after UV-irradiation. Interestingly, the catalytically inactive second PTP domain of RPTPα demonstrated higher susceptibility to oxidation. The experiments thus demonstrate previously unrecognized intrinsic differences between PTP domains to susceptibility to oxidation and suggest mechanisms for regulation of RPTPs with tandem PTP domains. The antibody strategy for detection of reversible oxidation is likely to facilitate further studies on regulation of PTPs and might be applicable to analysis of redox regulation of other enzyme families with active-site cysteine residues.
Protein tyrosine phosphatases (PTPs) constitute a structurally diverse enzyme family with high selectivity, nonredundant biological functions, and multiple mechanisms for regulation of specific activity (reviewed in refs. 1–3). The subset of “classical” PTPs is defined by a conserved signature motif, (V/I)HCSXG, which contains the active-site cysteine residue (4). The thiolate anion of the active-site cysteine is essential for the catalytic mechanism but also makes the cysteine residue susceptible to oxidation (5).
PTPs are broadly divided into cytosolic PTPs and receptor-like PTPs (RPTPs). The large majority of RPTPs have a tandem arrangement of PTP domains. Most, if not all, of the catalytic activity of RPTPs resides in the first PTP domain. The second domain has been proposed to function predominantly as a regulatory domain. Unique properties of the second domains of RPTPs are indicated by shared structural features of this PTP domain subset (4).
Mechanisms for regulation of PTP-specific activity include serine/threonine or tyrosine phosphorylation and SH-2-domain-mediated binding to tyrosine phosphorylated proteins (6–10). In the case of RPTPs, agonistic and antagonistic extracellular ligands have been described (11, 12). Regulated dimerization has also been implicated as a control mechanism for RPTPα (13, 14). More recently, oxidation of the active-site cysteine residue has emerged as an important mechanism for regulation of PTPs (15, 16).
Inactivation of PTPs by oxidation was first indicated as a mechanism for PTP regulation by the finding of irreversible oxidation of the active-site cysteine residue of PTP-1B to the sulfonic acid form (–SO3H) after in vitro treatment with pervanadate (17). Reversible inactivation of PTPs after in vitro treatment with H2O2 was subsequently shown to occur through conversion of the active-site cysteine residue to the reversibly oxidized sulfenic acid form (–SOH) (16). Evidence has also been presented that the reversibly oxidized sulfenic acid form undergoes glutathionylation (18, 19). Additionally, recent structural studies of oxidized PTP-1B have identified a sulfenylamide species formed after oxidation of PTP-1B, which involves an S–N bond between the active-site cysteine and the mainchain nitrogen of serine 216 (20, 21). Thus, physiological oxidants appear to convert the active-site cysteine residue to reversibly oxidized forms, whereas treatment with pervanadate leads to formation of the irreversible sulfonic acid form (–SO3H).
Indications that reversible oxidation might operate also in vivo were provided by the demonstration that EGF treatment of intact cells leads to inhibition of PTP-1B activity and insensitivity of the active-site cysteine residue to alkylation by iodoacetic acid (15). Insulin or PDGF stimulation of intact cells is associated with inhibition of PTP-1B and SHP-2, respectively, through reversible oxidation of the active-site cysteine residue after transient H2O2 production (22, 23). In addition, reversible oxidation of the second PTP domain of RPTPα induces a conformational change, associated with stabilization of catalytically inactive PTPα dimers (24).
Studies of the regulation of PTPs by oxidation have been hampered by the absence of sensitive and robust methods for detection, e.g., in cell lysates, of oxidized PTPs. In the present study, we present a generic antibody-based method for assaying oxidation-induced inactivation of PTPs with which preferential oxidation of the second regulatory domain in RPTPα after UV-mediated irradiation was revealed.
Materials and Methods
Generation of oxPTP antibodies. The peptide VHCSAG was synthesized by fluorenylmethoxycarbonyl (Fmoc) chemistry on an Applied Biosystems 433A peptide synthesizer. After purification, the cysteine residue was oxidized to the sulfonic acid form of cysteine (–SO3H) by incubation in performic acid as described (25). Complete oxidation of the cysteine residue was confirmed by electrospray MS (Esquire 3000, Bruker Daltonics, Bremen, Germany).
Rabbits were immunized with the oxidized peptide conjugated to keyhole limpet hemocyanin. An IgG-fraction from the serum was obtained by following standard procedures for purification with protein A-Sepharose. To obtain affinity-purified oxPTP antibodies, serum was first passed over a column with cysteic acid (1 mg/ml of gel, Sigma) conjugated to Affigel 10 (Bio-Rad). Antibodies recognizing the oxidized peptide (oxPTP antibodies) were subsequently captured with the peptide antigen coupled to 1 ml of Affigel 10, eluted in 4.6 M MgCl2, and concentrated by ammonium sulfate precipitation as described (26).
MS Analysis of Native and Oxidized DEP-1. In-gel tryptic digestion and peptide extraction were performed as described (27). The digest was analyzed by matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) MS on a Bruker Autoflex (Bruker Daltonics, Bremen, Germany). The instrument was optimized for peptides, and the spectra were internally calibrated by using proteolytic peptide masses from a known sample.
Cell Culture and Transfections. NIH 3T3 and HEK293 cells were cultured in DMEM supplemented with 10% calf serum. Porcine aortic endothelial (PAE) cells stably transfected with PDGF β-receptor cells (PAE/PDGFRβ) were cultured in Ham's F-12 supplemented with 10% FCS. PAE/PDGFRβ cells with inducible expression of VSV-tagged PTPα (D19 cells) were cultured in 50% DMEM/50% Ham's F12/10% FCS/200 μg/ml hygromycin in the absence or presence of 100 ng/ml anhydrotetracycline. One hundred units/ml penicillin and 100 μg/ml streptomycin were added to all media. HEK293 cells were used for transient transfection with untagged or hemagglutinin (HA)-tagged RPTPα, using the calcium-phosphate method.
Validation of oxPTP Antibodies by Using in Vitro Oxidized PTPs. Recombinant His-tagged DEP-1 and a mutant in which the catalytic site cysteine residue was changed to a serine (DEP-1-CS) were purified as described (28). T cell (TC)-PTP was purchased from New England Biolabs. SHP-2 was immunoprecipitated from NIH 3T3 cells with polyclonal SHP-2 antibodies (Santa Cruz Biotechnology). RPTPα was isolated with wheat-germ agglutinin (WGA)-Sepharose from transiently transfected HEK293 cells.
DEP-1 and TC-PTP were treated with 1 mM 1,4-DTT (DTT) and dialyzed under anaerobic conditions in a degassed buffer of 20 mM Tris·HCl (pH 7.5). DEP-1 and TC-PTP, at concentrations of 28 and 1 μM, respectively, were oxidized by 100 μM pervanadate for 4 h at room temperature. Immunoprecipitated SHP-2 and WGA fractions containing precipitated RPTPα were oxidized by incubation with 100 μM pervanadate for 1 h at 4°C. Pervanadate was prepared as described (17).
Reduced DEP-1 was protected from pervanadate oxidation by alkylation in 2 mM iodoacetic acid for 30 min before pervanadate treatment. To demonstrate the absence of alkylation of PTPs oxidized by H2O2, 5 μg of recombinant DEP-1 immobilized on Ni2+-Sepharose at a concentration of 0.5 μg of protein per μl of beads was incubated in the presence or absence of 100 μM H2O2 for 20 min and subsequently treated with 2 mM iodoacetic acid for 30 min. The beads were washed three times with 1 ml of 20 mM Tris·HCl (pH 7.5), incubated with 10 mM DTT for 10 min, and further washed with 3 × 1 ml of 20 mM Tris·HCl (pH 7.5). The samples were finally incubated with or without 100 μM pervanadate for 30 min. All reactions were performed at room temperature.
For immunoblotting analyses of in vitro oxidized PTPs, recombinant DEP-1 and TC-PTP, immunoprecipitated SHP-2, and RPTPα isolated with WGA-Sepharose were subjected to SDS/PAGE, transferred to nitrocellulose membranes, and immunoblotted with affinity-purified oxPTP antibody (5 μg/ml) or an IgG-fraction from the oxPTP serum (50 μg/ml). Secondary horseradish peroxidase-conjugated anti-rabbit antibodies (Amersham Biosciences) were diluted 1:20,000–30,000, and antibody signals were visualized by ECL (Amersham Biosciences). To confirm equal loading of protein, samples were subjected to immunoblotting (DEP-1, SHP-2, and RPTPα) or Coomassie blue staining (DEP-1 and TC-PTP).
Analyses of PTP Oxidation After Stimulation of Cells with H2O2 or PDGF. D19, NIH 3T3, or transfected HEK293 cells were starved overnight in serum-free culture medium supplemented with 0.1–1 mg/ml BSA and stimulated with 3 mM H2O2 for 5 min or 100 ng/ml PDGF-BB for 10 min at 37°C. Stimulations with PDGF-BB were performed in the absence or presence of a 30-min pretreatment with 50 μM LY294002. The cells were lysed at room temperature in the dark for 20 min in lysis buffer (20 mM Tris, pH 7.5/1% Nonidet P-40/10% glycerol/1 mM benzamidine/1% Trasylol) with or without 100 mM iodoacetic acid. Before lysis, the buffer was degassed for at least 20 min. RPTPα was collected with WGA-Sepharose and SHP-2 by immunoprecipitation with SHP-2 antibody. The beads were washed three times in lysis buffer, incubated with 10 mM DTT for 10 min on ice, and washed three times in 20 mM Hepes (pH 7.5) before incubation with 100 μM pervanadate for 1 h at 4°C. oxPTP immunoblotting was performed as described above with 5 μg/ml of affinity-purified oxPTP antibodies or oxPTP IgG fraction. Filters were reprobed with VSV antibodies (Sigma) or SHP-2 antibodies. Bound antibodies were visualized as described above.
Analysis of RPTPα Oxidation After UV-Irradiation of Cells. HEK293 cells were used for transient transfection with RPTPα. The different constructs used were untagged wild-type RPTPα, HA-tagged forms of wild-type RPTPα (RPTPα-wt), RPTPα in which the conserved cysteine residues in both phosphatase domains were mutated to serines (RPTPα-C433,723S), and RPTPα in which either Cys-433 or Cys-723 were mutated to serines (RPTPα-C433S and RPTPα-C723S, respectively). Cells were washed twice in PBS and irradiated with UVC at doses of 0.05, 0.5, or 5 kJ/m2 by using a Stratalinker 1800 (Stratagene). Isolation and detection of oxidized RPTPα were performed as described above with the affinity-purified antibodies.
In Vitro H2O2 Oxidation of Recombinant PTPα. GST-fusion proteins of the PTPα intracellular domain (residues 167–793), the first PTP domain (residues 167–502), and the second PTP domain (residues 504–793), with or without cysteine-to-serine substitutions, were used for in vitro oxidation. Fifty nanograms of each of the fusion proteins were immobilized on glutathione-Sepharose beads and incubated with different concentrations of H2O2 for 5 min at room temperature. Alkylations, pervanadate treatments, and oxPTP immunoblotting were performed with affinity-purified oxPTP antibodies as described above. The antiserum against RPTPα has been described (29).
Phosphatase Activity Measurement. Transfected cells were lysed in degassed lysis buffer (20 mM Tris, pH 7.5/1% Nonidet P-40/10% glycerol/1 mM benzamidine/1% aprotinin/5 mM N-acetylcysteine). RPTPα was immunoprecipitated by using a HA antiserum. Immune complexes were washed three times in lysis buffer and one time in phosphatase assay buffer (25 mM imidazole, pH 7.4/0.1 mg/ml BSA/5 mM N-acetylcysteine). The phosphatase activity was measured by using a 32P-labeled c-Src-derived peptide as substrate (30). Total dephosphorylation did not exceed 15%. One-tenth of the immunoprecipitates were, in parallel, analyzed by immunoblotting with a monoclonal HA antibody (Roche Molecular Biochemicals).
Results
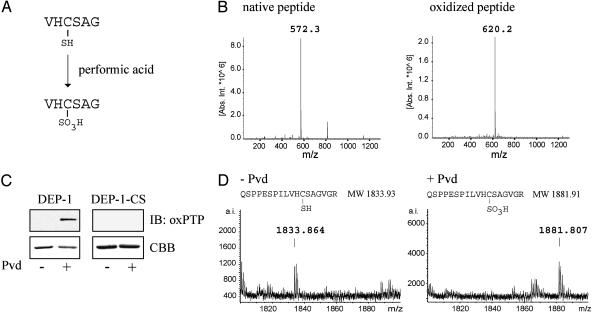
Generation of an Antibody Specific for the Oxidized Active Site of PTPs. In the present study, we set out to establish an antibody-based strategy for detection of oxidation-induced inactivation of PTPs. As a first step, a peptide corresponding to the conserved active site of classical PTPs was synthesized (Fig. 1A). The VHCSAG peptide was subsequently oxidized by incubation in performic acid. Electrospray ionization MS confirmed the complete oxidation of the cysteine residue to the sulfonic acid form (Fig. 1B). The irreversibly oxidized peptide was subsequently used for immunization.
Fig. 1.
Generation of antibodies specific for the oxidized active site of PTPs. (A) Amino acid sequence of the hexapeptide corresponding to the conserved PTP active site. (B) Electrospray ionization MS analysis of peptide before and after oxidation. Indicated masses are monoisotopic, MH+. (C) Wild-type DEP-1 and a mutant form, in which the catalytic cysteine residue has been changed to a serine residue (DEP-1-CS), were incubated with or without pervanadate (Pvd). Proteins were analyzed by oxPTP immunoblotting and Coomassie brilliant blue staining (CBB). (D) Pervanadate treated and untreated wild-type DEP-1 were excised from the gel after SDS/PAGE, and tryptic digests of the proteins were analyzed by matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) MS. The amino acid sequence and predicted masses (monoisotopic, MH+) of these peptides are shown.
After affinity purification of antibodies (oxPTP) from rabbit serum, their ability to recognize oxidized PTPs was investigated. Recombinant DEP-1 was oxidized by incubation with pervanadate. The protein was subjected to SDS/PAGE, followed by oxPTP immunoblotting analysis. The antibody recognized DEP-1, which had been treated with pervanadate, whereas untreated DEP-1 was not detected (Fig. 1C). Mass spectrometric analysis confirmed oxidation of the DEP-1 active site by identification of a peptide with a mass corresponding to the molecular weight of a tryptic peptide with the catalytic cysteine residue oxidized to a sulfonic acid form (Fig. 1D).
To confirm the specificity of the oxPTP antibody for the catalytic cysteine residue, a mutated form of DEP-1 in which this cysteine residue is replaced with a serine residue (DEP-1-CS) was included in the assay. As shown in Fig. 1C, the antibody did not recognize the pervanadate-treated mutant form, confirming its specificity for the oxidized catalytic site. The antibodies were also tested in an immunoprecipitation format. Pervanadate-dependent recovery was detected; however, DEP-1-CS was also precipitated, suggesting nonspecific recognition of epitopes outside of the catalytic domain (data not shown).
Together, these results demonstrate the successful production of antibodies (oxPTP) specific, in immunoblotting format, for the active site of pervanadate-treated PTPs.
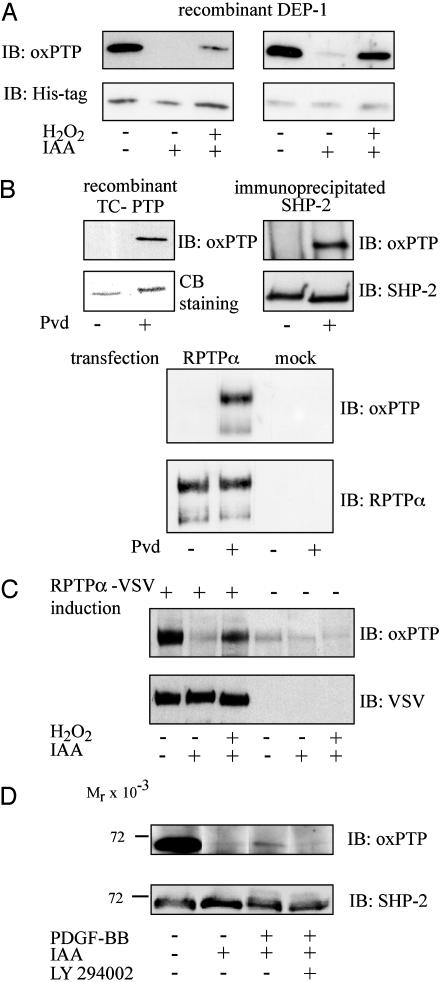
Alkylation with Iodoacetic Acid Protects Reduced but Not Reversibly Oxidized PTPs from Pervanadate Oxidation. To be able to use the antibody for detection of reversibly oxidized PTPs in the cell, efficient blocking of reduced PTPs, to prevent pervanadate-mediated oxidation, is required. To investigate whether alkylation of reduced PTPs would protect from oxidation, DEP-1 was incubated with iodoacetic acid before pervanadate treatment. After SDS/PAGE, the oxidation state of the protein was analyzed by oxPTP immunoblotting with affinity-purified oxPTP antibodies (Fig. 2A Left) or an IgG fraction from the oxPTP antiserum (Fig. 2 A Right). The analyses show that preincubation with iodoacetic acid resulted in protection from pervanadate oxidation. However, as expected, DEP-1 reversibly oxidized by incubation with H2O2 could also be converted to a sulfonic acid form, by pervanadate-treatment, after treatment with alkylating agent (Fig. 2 A). The side-by-side comparison also revealed a slightly higher sensitivity of the IgG fraction, occurring together with some background recognition of the alkylated form, as compared with the affinity-purified preparation.
Fig. 2.
In vitro and in vivo validation of oxPTP antibodies. (A) DEP-1 was incubated with or without iodoacetic acid and H2O2 as indicated and subsequently subjected to pervanadate treatment. Samples were analyzed by immunoblotting with affinity-purified oxPTP antibodies (Left Upper) or an IgG fraction from oxPTP antiserum (Right Upper). Protein loading was controlled by His-tag immunoblotting (Lower). (B) Recombinant TC-PTP (Left), immunoprecipitated SHP-2 (Right), or WGA fractions from untransfected and RPTPα-transfected HEK293 cells (lower set) were incubated with or without pervanadate and analyzed by oxPTP immunoblotting. Equal yields of PTPs with or without Pvd treatment was confirmed by Coomassie brilliant blue (CBB) staining (TC-PTP) or immunoblotting (SHP-2 and RPTPα). (C) Cells with inducible expression of VSV-tagged RPTPα were left untreated or treated with H2O2. RPTPα was captured from the lysate with WGA-Sepharose and incubated with pervanadate. After SDS/PAGE samples were subjected to consecutive immunoblotting with oxPTP and VSV antibodies. (D) NIH 3T3 cells, pretreated without or with 50 μM LY294002, were left unstimulated or stimulated with 100 ng/ml PDGF-BB for 10 min. SHP-2 was immunoprecipitated from cell lysate and incubated with pervanadate. Immunoprecipitated SHP-2 was analyzed by consecutive immunoblotting with an IgG fraction of oxPTP antiserum and with SHP-2 antibodies.
The oxPTP Antibody Detects Multiple PTPs. Because the peptide antigen that was used corresponds to the conserved PTP signature motif, the antibody was expected to recognize multiple PTPs. To assess this aspect, recombinant TC-PTP, immunoprecipitated SHP-2, and RPTPα, isolated with WGA-Sepharose, were incubated in the presence or absence of pervanadate. The proteins were subjected to SDS/PAGE analysis, followed by oxPTP immunoblotting. The antibody also recognized, in addition to DEP-1, TC-PTP, SHP-2, and RPTPα (Fig. 2B).
The oxPTP Antibody Detects Oxidized PTPs After Treatment of Cells with H2O2 or PDGF-BB. To explore the possibility of using the antibody to detect PTPs oxidized in vivo, PAE/PDGFβR cells, with inducible expression of VSV-tagged RPTPα, were treated with H2O2. The cells were lysed in the presence or absence of iodoacetic acid. RPTPα was collected with WGA-Sepharose. Precipitates were reduced with 10 mM DTT, to convert reversibly oxidized forms to species readily oxidized by pervanadate. After pervanadate treatment, the proteins were analyzed by oxPTP immunoblotting. Incubation of cells with H2O2 induced oxidation of a fraction of RPTPα as detected by the antibody (Fig. 2C).
It has previously been shown that SHP-2 is reversibly oxidized on PDGF-BB stimulation of cells (23). Furthermore, phosphatidylinositol (PI) 3-kinase has been identified as the prime mediator of PDGF-induced H2O2 production (31). Thus, the oxPTP antibodies were used to analyze whether PDGF-induced oxidation of endogenous SHP-2 could be demonstrated and to analyze the possible sensitivity of this PDGF-induced SHP-2 regulation to the PI 3-kinase inhibitor LY294002. Serum-starved NIH 3T3 cells were stimulated with PDGF-BB for 10 min, in the absence or presence of pretreatment with the PI 3-kinase inhibitor. SHP-2 was immunoprecipitated and analyzed by oxPTP immunoblotting. Oxidized SHP-2 was clearly detected after PDGF-BB stimulation (Fig. 2D). In contrast, no oxidized SHP-2 was seen after PDGF-BB stimulation of cells pretreated with the PI 3-kinase inhibitor (Fig. 2D).
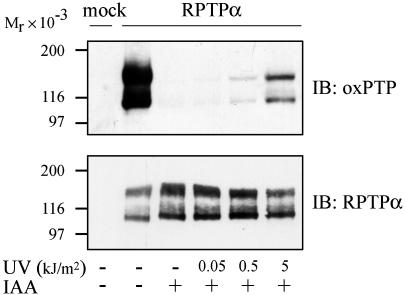
UV-Irradiation of Cells Induces Oxidation of RPTPα. UV irraditation of cells has previously been shown to cause inactivation of several PTPs including RPTPα (32). To investigate whether this effect is due to UV-induced oxidation of the catalytic site cysteine residue, HEK293 cells transiently transfected with RPTPα were irradiated with UVC at doses of 0.05, 0.5, and 5 kJ/m2. On UV-irradiation, cells were lysed with or without iodoacetic acid, and RPTPα was captured from lysate with WGA-Sepharose. As shown in Fig. 3, UV irradiation induced a dose-dependent oxidation of RPTPα.
Fig. 3.
UV-induced oxidation of RPTPα. HEK293 cells were mock transfected or transfected with RPTPα and left untreated or irradiated with 0.05, 0.5, or 5 kJ/m2 UVC. WGA fractions were isolated and subjected to pervanadate treatment. Proteins were analyzed by immunoblotting with oxPTP antibody. Ten percent of each sample was run on separate gels in parallel and immunoblotted with RPTPα antibodies.
Oxidation Occurs Preferentially in the Second Phosphatase Domain of RPTPα. To examine whether the two phosphatase domains in RPTPα display different sensitivity to oxidation, recombinant PTPα phosphatase domains were treated in vitro with H2O2. Isolated domain 2 was oxidized on treatment with 1 mM, whereas oxidation of isolated domain 1 was not observed (Fig. 4, leftmost panels). Similarly, in a protein containing both domains, mutation of the conserved D2 cysteine residue (Cys-723) resulted in a reduced oxPTP signal, whereas mutation of the conserved Cys-433 had a lesser effect (Fig. 4, right three panels).
Fig. 4.
Preferential oxidation of the second phosphatase domain in recombinant PTPα on in vitro H2O2 treatment. The effect of H2O2 treatment in vitro on oxidation of PTP domain in RPTPα was analyzed by oxPTP immunoblotting. Recombinant proteins contained the whole intracellular domain (wt), the intracellular domain with the first or second active-site cysteine substituted with serine (C433S and C732S), PTPα domain 1 (D1), domain 2 (D2), or D1 or D2 in which the conserved cysteines were mutated to serines (D1-CS and D2-CS).
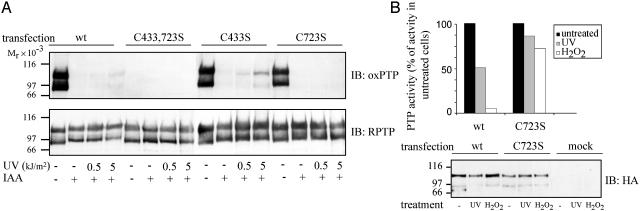
To further evaluate this finding, HEK293 cells were transfected with HA-tagged wild-type RPTPα and RPTPα with different mutations of the conserved cysteine residues (RPTPα-C433S, RPTPα-C723S, and RPTPα-C433,723S). After UV irradiation, RPTPα was captured from cell lysates with WGA-Sepharose. Consistent with the results above, oxP T P immunoblotting revealed that mutation of Cys-723 in D2 resulted in loss of the oxPTP signal, whereas mutation of Cys-433 in D1 did not (Fig. 5A). These differences between D1 and D2 were not caused by different recognition of oxidized D1 and D2 by the antibodies, because RPTPα-C433S and RPTPα-C723S were equally well recognized after pervanadate treatment of unalkylated forms (Fig. 5A).
Fig. 5.
Preferential oxidation of the second phosphatase domain of RPTPα after UV irradiation of cells. (A) HEK293 cells were transfected with wild-type RPTPα (wt) or with RPTPα with substitutions of Cys-433 (C433S), Cys-723 (C723S), or both cysteines (C4332,732S). After irradiation, WGA fractions were isolated and subjected to pervanadate treatment. The samples were analyzed by immunoblotting with oxPTP antibodies, and membranes were reprobed with RPTPα antibodies. (B) HEK293 cells were mock transfected or transfected with HA-tagged wild-type RPTPα-wt or RPTPα-C723S. The cells were left untreated or irradiated with 5 kJ/m2 UVC or treated with 1 mM H2O2 for 3 min at 37°C. RPTPα was immunoprecipitated with an HA antiserum. Ninety percent of the immunoprecipitate was used for PTP activity measurement, and 10% was analyzed by HA immunoblotting. Recovered PTP activity in untreated cells was set to 100%. The data shown are representative of three individual experiments.
The finding of preferential oxidation of D2 prompted experiments investigating the dependence of D2 for UV- and H2O2-induced inactivation of phosphatase activity. HEK293 cells were transfected with RPTPα or RPTPα-C723S and subjected to UV-irradiation or treatment with H2O2. RPTPα was immunoprecipitated by using HA antiserum, and phosphatase activity was assayed. Whereas UV or H2O2 treatment of cells expressing the D2 mutant reduced recovered PTP activity to 85% and 72% of control, a much larger inhibition was observed in cells expressing wild-type RPTPα, where UV and H2O2 reduced the activity to 50% and 4%, respectively.
Figs. 4 and 5 thus show that the conserved cysteine residue in the second phosphatase domain is more sensitive to oxidation than the cysteine residue in the first domain. The experiments also indicate that UV-induced catalytic inactivation of RPTPα is caused by preferential oxidation of the second domain.
Discussion
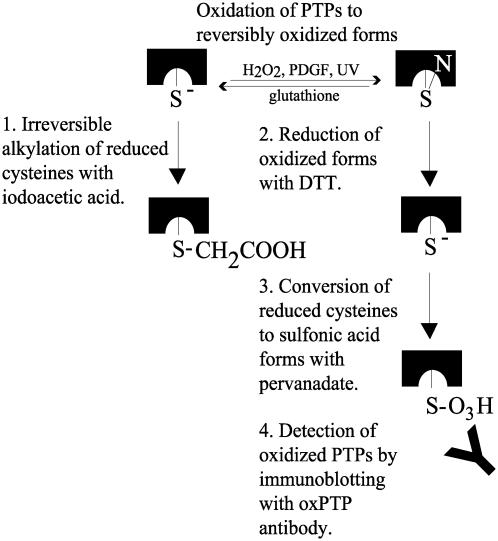
This study presents a strategy for detection of oxidation-inactivated classical PTPs. The procedure, summarized in Fig. 6, involves protection of reduced PTPs with iodoacetic acid, pervanadate-induced oxidation to sulfonic acid forms of reversibly oxidized PTPs, and detection by immunoblotting with oxPTP antibodies. Oxidized DEP-1, TC-PTP, SHP-2, and RPTPα were shown to react with the oxPTP antibodies (Fig. 2). Of 37 identified human PTPs, 28 family members contain a catalytic site sequence that perfectly match the sequence of the antigen used in this study (4). This fact, together with the finding that oxidized TC-PTP, with the active-site sequence IHCSAG, also was recognized by the antibody, predicts that the antibody can be used as part of a generic strategy for detection of oxidized forms of most classical PTPs. Whether the antibodies also can be used to monitor oxidation of dual-specificity phosphatases has not yet been determined. However, the sequence variation within the active site of this PTP subfamily is larger than among classical PTPs. It is thus predicted that the strategy should work but might require antibodies raised against different oxidized active-site peptides.
Fig. 6.
Schematic illustration of antibody-based detection of reversibly oxidized PTPs. See text for details.
Various methods have previously been used to demonstrate oxidative inactivation of PTPs in vivo. Reduced enzymatic activity in PTP immunoprecipitates was shown after insulin-induced production of H2O2 (22), and reduced susceptibility of PTPs to labeling with [3H]iodoacetic acid was demonstrated after treatment of cells with H2O2 and EGF (15). Finally, a modified in-gel PTP assay was used to show reversible oxidation of SHP-2 after PDGF stimulation (19). In contrast to the methods using immunoprecipitations and subsequent monitoring of PTP activity or incorporation of [3H]iodoacetic acid, our assay monitors the increase of oxidized PTPs rather than a decrease in the active fraction, which is likely to improve sensitivity. As compared to the modified in-gel assay, which like our assay monitors the increase in oxidized PTPs, an advantage with the oxPTP assay is that it is not dependent on the refolding of denatured proteins in SDS-gels that occurs with low efficiency for large proteins like RPTPs.
In support of the utility of this approach for monitoring of PTP oxidation, LY294002-sensitive PDGF-BB-induced oxidation of endogenous SHP-2 was detected with the oxPTP antibodies (Fig. 2D). We predict that this strategy will be of great utility for further investigations of the general, but poorly characterized, regulatory mechanism of PTP oxidation. Obviously, the usefulness will be improved if high-affinity monoclonal antibodies, raised against the same antigen, can be obtained. Efforts to obtain such antibodies have been initiated.
Using the oxPTP antibodies, we could for the first time directly demonstrate that UV-induced inactivation of RPTPα involves oxidation of the active-site cysteine residue (Fig. 3). UV-induced inhibition of the enzymatic activity of SHP-1, PTPα, PTPσ, and DEP-1 was originally demonstrated by Gross et al. (32). Subsequent studies on RPTPα have also demonstrated that UV irradiation, and other types of oxidative stress, induce a conformational change in the RPTPα second catalytic domain (24).
Most interestingly, comparison between the first and second PTP domain of RPTPα revealed a preferential oxidation of the second PTP domain (Fig. 5). This preference was shown to be context-independent, because it also appeared when the isolated first and second domain were compared side by side. There are two major implications of these findings. Firstly, they provide evidence that different PTP domains demonstrate intrinsic differences to susceptibility to oxidation. Secondly, it points to the possibility that a major function of the second domain of RPTPα is to act as redox sensors that convey inhibitory signals to the catalytically active first domain. Both of these implications suggest a series of highly warranted studies; e.g., investigations on the defining structural features of oxidation-sensitive PTP domains and on the possibly general nature of the difference between the first and second PTP domain of RPTPα.
Finally, oxidation-induced regulation has also been implicated for other biologically important enzyme families with conserved active-site cysteine residues. The strategy we have outlined appears suitable for creation of generic tools for analysis of oxidation-induced regulation also of these enzymes.
Acknowledgments
We thank Frank D. Böhmer and members of the Growth Regulation group at the Ludwig Institute for Cancer Research for constructive comments and discussions. K.K. was supported by Deutsche Forschungsgemeinschaft. A.Ö. was supported by the Swedish Cancer Foundation.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: HA, hemagglutinin; PDGF, platelet-derived growth factor; PTP, protein tyrosine phosphatase; RPTP, receptor-like PTP; TC, T cell; WGA, wheat-germ agglutinin.
References
- 1.Östman, A. & Böhmer, F.-D. (2001) Trends Cell Biol. 11, 258–266. [DOI] [PubMed] [Google Scholar]
- 2.Tonks, N. K. & Neel, B. G. (2001) Curr. Opin. Cell Biol. 13, 182–195. [DOI] [PubMed] [Google Scholar]
- 3.Li, L. & Dixon, J. E. (2000) Semin. Immunol. 12, 75–84. [DOI] [PubMed] [Google Scholar]
- 4.Andersen, J. N., Mortensen, O. H., Peters, G. H., Drake, P. G., Iversen, L. F., Olsen, O. H., Jansen, P. G., Andersen, H. S., Tonks, N. K. & Moller, N. P. (2001) Mol. Cell. Biol. 21, 7117–7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barford, D., Das, A. K. & Egloff, M. P. (1998) Annu. Rev. Biophys. Biomol. Struct. 27, 133–164. [DOI] [PubMed] [Google Scholar]
- 6.Wang, Y., Guo, W., Liang, L. & Esselman, W. J. (1999) J. Biol. Chem. 274, 7454–7461. [DOI] [PubMed] [Google Scholar]
- 7.Garton, A. J. & Tonks, N. K. (1994) EMBO J. 13, 3763–3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu, F. & Chernoff, J. (1997) Biochem. J. 327, 139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uchida, T., Matozaki, T., Noguchi, T., Yamao, T., Horita, K., Suzuki, T., Fujioka, Y., Sakamoto, C. & Kasuga, M. (1994) J. Biol. Chem. 269, 12220–12228. [PubMed] [Google Scholar]
- 10.Barford, D. & Neel, B. G. (1998) Structure (London) 6, 249–254. [DOI] [PubMed] [Google Scholar]
- 11.Sörby, M., Sandström, J. & Östman, A. (2001) Oncogene 20, 5219–5224. [DOI] [PubMed] [Google Scholar]
- 12.Meng, K., Rodriguez-Peña, A., Dimitrov, T., Chen, W., Yamin, M., Noda, M. & Deuel, T. F. (2000) Proc. Natl. Acad. Sci. USA 97, 2603–2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bilwes, A. M., den Hertog, J., Hunter, T. & Noel, J. P. (1996) Nature 382, 555–559. [DOI] [PubMed] [Google Scholar]
- 14.Majeti, R., Xu, Z., Parslow, T. G., Olson, J. L., Daikh, D. I., Killeen, N. & Weiss, A. (2000) Cell 103, 1059–1070. [DOI] [PubMed] [Google Scholar]
- 15.Lee, S. R., Kwon, K. S., Kim, S. R. & Rhee, S. G. (1998) J. Biol. Chem. 273, 15366–15372. [DOI] [PubMed] [Google Scholar]
- 16.Denu, J. M. & Tanner, K. G. (1998) Biochemistry 37, 5633–5642. [DOI] [PubMed] [Google Scholar]
- 17.Huyer, G., Liu, S., Kelly, J., Moffat, J., Payette, P., Kennedy, B., Tsaprailis, G., Gresser, M. J. & Ramachandran, C. (1997) J. Biol. Chem. 272, 843–851. [DOI] [PubMed] [Google Scholar]
- 18.Barrett, W. C., DeGnore, J. P., Keng, Y. F., Zhang, Z. Y., Yim, M. B. & Chock, P. B. (1999) J. Biol. Chem. 274, 34543–34546. [DOI] [PubMed] [Google Scholar]
- 19.Li, S. & Whorton, A. R. (2003) Arch. Biochem. Biophys. 410, 269–279. [DOI] [PubMed] [Google Scholar]
- 20.Salmeen, A., Andersen, J. N., Myers, M. P., Meng, T.-C., Hinks, J. A., Tonks, N. K. & Barford, D. (2003) Nature 423, 769–773. [DOI] [PubMed] [Google Scholar]
- 21.van Montfort, R. L. M., Congreve, M., Tisi, D., Carr, R. & Jhoti, H. (2003) Nature 423, 773–777. [DOI] [PubMed] [Google Scholar]
- 22.Mahadev, K., Zilbering, A., Zhu, L. & Goldstein, B. J. (2001) J. Biol. Chem. 276, 21938–21942. [DOI] [PubMed] [Google Scholar]
- 23.Meng, T. C., Fukada, T. & Tonks, N. K. (2002) Mol. Cell 9, 387–399. [DOI] [PubMed] [Google Scholar]
- 24.Blanchetot, C., Tertoolen, L. G. & den Hertog, J. (2002) EMBO J. 21, 493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stewart, J. M. & Young, J. D. (1984) Solid Phase Peptide Synthesis (Pierce Chemical Company, Rockford, IL).
- 26.Harlow, E. & Lane, D. (1988) Antibodies: A Laboratory Manual (Cold Spring Harbor Lab. Press, Plainview, NY).
- 27.Hellman, U. (2000) in Proteomics in Functional Genomics, eds. Jollès, P. & Jörnvall, H. (Birkhäuser, Basel), pp. 43–54.
- 28.Persson, C., Engström, U., Mowbray, S. L. & Östman, A. (2002) FEBS Lett. 517, 27–31. [DOI] [PubMed] [Google Scholar]
- 29.den Hertog, J, Tracy, S. & Hunter, T. (1994) EMBO J. 13, 3020–3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Östman, A., Yang, Q. & Tonks, N. K. (1994) Proc. Natl. Acad. Sci. USA 91, 9680–9684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bae, Y. S., Sung, J. Y., Kim, O. S., Kim, Y. J., Hur. K. C., Kazlauskas, A. & Rhee, S. G. (2000) J. Biol. Chem. 275, 10527–10531. [DOI] [PubMed] [Google Scholar]
- 32.Gross, S., Knebel, A., Tenev, T., Neininger, A., Gaestel, M., Herrlich, P. & Böhmer, F. D. (1999) J. Biol. Chem. 274, 26378–26386. [DOI] [PubMed] [Google Scholar]