Abstract
The cysteine-rich domain (CR) of the mannose receptor binds sulfated glycoprotein CR ligand (CRL) expressed by subpopulations of myeloid cells in secondary lymphoid organs (CRL+ cells). In naïve mice, these CRL+ cells, metallophilic macrophages (Mφ) in spleen and subcapsular sinus Mφ in lymph nodes, are located strategically for antigen capture and are adjacent to B cell follicles, but their role in the immune response is unknown. We have exploited the lectin activity of CR to develop a highly specific system for targeting protein to CRL+ Mφ. We demonstrate that the sulfated carbohydrates recognized by CR are exposed to the extracellular milieu and mediate highly specific targeting of CR-containing proteins. This model will allow the dissection of the role of metallophilic Mφ in an immune response in vivo.
Use of a chimeric protein-containing cysteine-rich domain (CR) of the macrophage (Mφ) mannose receptor (MR) fused to the Fc region of human IgG1 (hIgG1; CR-Fc), we discovered CR ligands (CRL) in two Mφ subpopulations adjacent to B cell follicles: marginal zone metallophilic Mφ (metMφ) in spleen and subcapsular sinus Mφ (ssMφ) in lymph nodes (LN) (1). Two of these CRL were identified as specific sulfated glycoforms of sialoadhesin (Sn) and CD45 (2). metMφ and ssMφ occupy prime positions (immediately adjacent to the marginal and subcapsular sinuses, respectively) for antigen (Ag) acquisition and cell–cell interaction within the blood and lymph. In the normal animal, the presence of CRL on metMφ is induced by the interaction of membrane lymphotoxin on B cells with its receptor on stromal cells (3). Inflammatory signals affect the distribution of CRL. During the course of an immune response, LN dendritic cells (DC), located within B cell follicles, and follicular DC (FDC) also express CRL (1, 4). Splenic CRL+ cells up-regulate the mouse homologue of the human germinal center DC marker decysin after immunization, and decysin-positive CRL+ cells can be detected in the follicles 48 h later (5). Furthermore, after LPS treatment, CRL+ cells migrate into B cell follicles (3, 6, 7). These results show that the distribution and phenotype of CRL+ cells are highly regulated during an immune response and are suggestive of a role for these cells in Ag delivery.
We have exploited CR-containing chimeric molecules to assess whether CRL on CRL+ cells are exposed to the circulation and lymph and to determine whether they can be used to target protein to these cells in vivo. In this article, we demonstrate that protein can be specifically targeted to CRL+ cells by CR. This targeting is highly specific, dependent on functional CR lectin activity and enhanced by multimerization. The development of an efficient system for the targeting of Ag to ssMφ and metMφ will allow direct probing of their contribution to an immune response.
Methods
Mice. C57BL/6, BALB/c, and osteopetrotic (op/op) mice were supplied from our own breeding colonies. C1q-deficient (C1qa–/–) mice were generated as described (8) and backcrossed to C57BL/6 for 10 generations. All mice were 8–12 weeks of age at the time of study and were matched for age, sex, and strain. Reagents. CR-Fc was generated as described (1). Three other hIgG1 Fc domain-containing proteins were used as control proteins in these studies: hIgG1 (CAMPATH-1H); CD33-Fc (containing the extracellular domains of human CD33), and EGF5–6-Fc (containing epidermal growth factor domains 5 and 6 from mouse macrophage Ag F4/80). The control proteins were kind gifts from M. Frewin (Oxford University, Oxford), P. Crocker (Dundee University, Dundee, U.K.), and M. Stacey (Oxford University), respectively.
CR-Fcmut, a version of CR-Fc that could not activate complement or bind to Fc receptors was generated by replacing the Fc domain of CR-Fc with a mutated Fc domain (a gift from Christine Ambrose, Biogen). This Fc domain was the same as the original hIgG1 Fc domain, with the substitutions L234A, L235E, and G237A to abrogate Fc-receptor binding (9, 10) and P331S to prevent activation of C1q (11). This recombinant protein was modified subsequently by introduction of the amino acid substitution W117A in the ligand-binding pocket of CR, CRW117A-Fcmut. W117 of CR shares multiple van der Waals interactions and a hydrogen bond with the galactose ring of the ligand (12), and therefore, its mutation was anticipated to largely abrogate lectin activity. Mutagenesis was performed by using the Gene-Editor kit (Promega) according to the manufacturer's instructions, and the mutagenic primer: 5′-TTCGGGATTGGCGAGCAGATGGAAG-3′. Fc-chimeric proteins were purified by protein A affinity chromatography and elution with 0.1 M glycine (pH 2.6).
Abs and Secondary Reagents. The following Abs were used in this study: anti-CR1/CD35 (8C12; Pharmingen); anti-IgD (11–26c.2a; Pharmingen); anti-IgM (11/41; Pharmingen); anti-CD19 (6D5; Serotec); anti-B220; anti-CD3 (Kt3; a gift from S. Cobbold, Oxford University); anti-CD11c (N418; Serotec); F4/80 (Serotec); anti-SR-A (2F8; Serotec); anti-SIGNR1 (ERTR9; DPC Biermann, Bad Nauheim, Germany); anti-MARCO (ED31; a gift from G. Kraal, Vrije Universiteit, Amsterdam); anti-MR [MR5D3; anti-Sn (clones 3D6 and Ser-4)] (13); anti-FDC-M1 mAb (4C11; a gift from M. Kosco-Vilbois, NovImmune, Geneva) (14); anti-FDC-M2 mAb (209) (15, 16); alkaline phosphatase-conjugated goat anti-rat IgG (H+L; Chemicon); biotinylated rabbit anti-rat IgG (H+L; Vector Laboratories); alkaline phosphatase-conjugated rabbit anti-mouse IgG (γ-chain-specific; Sigma); biotinylated goat anti-hIgG (H+L; Vector Laboratories); allophycocyanin-conjugated mouse anti-human Fc mAb (Leinco Technologies, St. Louis); mouse F(ab)2 anti-human Fc (Jackson ImmunoResearch); streptavidin-conjugated Alexa 488 (Molecular Probes); and Alexa 647-conjugated goat anti-rat IgG (Molecular Probes).
Immunohistochemistry. Immunohistochemical analysis was performed as described (17). Endogenous biotin was blocked, when appropriate, with avidin–biotin blocking kit (Vector Laboratories) according to the manufacturer's instructions. Sections were blocked with 5% normal serum from the same species in which the secondary Ab was raised for 30 min at room temperature and incubated with primary Abs diluted to 10 μg ml–1 in blocking buffer. After washing, the appropriate secondary Abs diluted in blocking buffer were added to the slides for 30 min at room temperature. If the secondary Ab was biotinylated, an avidin–biotin–alkaline phosphatase complex was used according to the manufacturer's instructions (standard alkaline phosphatase Vectastain ABC kit; Vector Laboratories). The slides were developed by using the BCIP/NBT alkaline phosphatase substrate kit IV (Vector Laboratories). For immunofluorescent detection of injected proteins, streptavidin-conjugated Alexa 488 (1:100 dilution) was used after biotinylated anti-human primary Ab. Rat primary Abs were detected with the Alexa 647-conjugated goat anti-rat IgG or Texas red-labeled goat anti-rat IgM (μ-chain) secondary Abs (1:200 dilution).
Gel Filtration. Gel filtration chromatography was carried out on a TSK G3000 SW (7.5 × 600 mm) column in PBS. The flow rate was 0.25 ml/min, and the absorbance of the eluant was monitored at 214 and 280 nm.
Complement Activation Assay. Complement activation assays were performed as described (16). In brief, enzyme immunoassay and RIA 96-well plates were coated with test Ag in PBS. Plates were then blocked with 10% (wt/vol) skimmed milk before the addition of serial dilutions of mouse serum. The plates were incubated at 37°C for 1 h to allow complement activation to proceed. Complement C4 deposited on the plates was then detected with mAb 209 (anti-FDC-M2/C4), as described (16). Equivalent coating of the recombinant proteins and hIgG1 onto the 96-well plates was confirmed by using an alkaline phosphatase-conjugated anti-human Fc polyclonal Ab (data not shown).
CRL Binding Assays. For bovine lutropin (bLH) ligand binding assays, enzyme immunoassay and RIA 96-well plates (Costar) were coated overnight at 4°C with 50 μl of 20 μg ml–1 bLH in PBS, washed twice with PBS, and blocked for 1 h at 37°C with 200 μl of 3% BSA in PBS. After washing twice with PBS/0.1% Tween 20 and once with PBS, serial dilutions of recombinant proteins (50 μl per well) were added to the plates and incubated at 37°C for 1 h to allow binding to occur. Plates were washed three times with PBS/0.1% Tween 20 and once with PBS, an alkaline phosphatase-conjugated goat anti-hIgG (Chemicon) (1:200 dilution; 50 μl per well) was added, and the plates were then incubated at room temperature for 1 h. After washing, alkaline phosphatase activity was determined, as described above.
Results
CR-Fc Targets Directly to CRL+Mφ in Vivo. To test whether the CRL identified in the spleen and LN by using ex vivo binding assays are exposed to the circulation in vivo, mice were injected i.v., s.c., and i.p. with CR-Fc or hIgG1, and the localization of the injected proteins was analyzed by immunohistochemistry.
After i.v. injection, CR-Fc showed predominant colocalization with metMφ in the spleen, whereas hIgG1 showed mostly red-pulp localization with some protein detectable in the outer marginal zone (Fig. 1 and data not shown). Injection of CR-Fc into op/op mice showed follicular localization after 30 min, consistent with the lack of metMφ and ssMφ that results from their deficiency in Mφ colony-stimulating factor (18–20) (Fig. 1 A). CR-Fc was detected predominantly in the ssMφ of the draining brachial and axillary LN 4 h after s.c. injection of 20 μg CR-Fc into the forelimb, whereas hIgG1 showed more diffuse LN staining under the same conditions (Fig. 1 A). Some CR-Fc was found also on the metMφ of the spleen after s.c. injection (data not shown). Similar results were obtained after i.p. injection of 200 μg of CR-Fc. CR-Fc was most readily detectable on the ssMφ of the draining parathymic LN 30 min after i.p. injection, but by 4 h, significant amounts were detectable also on metMφ in the spleen (data not shown).
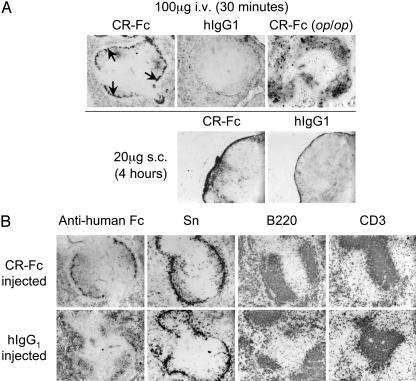
Fig. 1.
Targeting of CR-containing molecules to CRL+ Mφ in vivo.(A) Upper shows a predominantly inner marginal zone localization (arrows) 30 min after i.v. administration of CR-Fc in normal C57BL/6 mice. This localization was not evident in mice injected with the control protein hIgG1 (or CD33-Fc and EGF5– 6-Fc; data not shown) or in op/op mice, which lack metMφ. Lower shows similar targeting of CR-Fc, but not hIgG1, to the subcapsular sinus after s.c. injection. (B) Further analysis of the localization of CR-Fc after i.v. injection showed that, consistent with the distribution of the ligands, only metMφ adjacent to B cell follicles (B220) captured CR-Fc from the circulation. By this time, hIgG1 exhibited mostly red-pulp and follicular localization. The examples shown are spleens collected from BALB/c mice 4 h after i.v. injection of 200 μg of protein.
The localization of i.v.-injected CR-Fc and hIgG1 was compared with the expression of markers for metMφ (Sn), B cells (B220), and T cells (CD3) (Fig. 1B). Spleens were stained also for marginal zone Mφ (2F8, SR-A; and ED31, MARCO), FDC (FDC-M1 and FDC-M2), IgM, IgD, CD19, and red-pulp Mφ (F4/80 and MR5D3, MR) and DC (CD11c) (data not shown). CR-Fc was located predominantly on the subset of Sn+ metMφ that are located adjacent to the B-cell follicles, but not on those that are adjacent to T cell zones, 4 h after i.v. injection of BALB/c and C57BL/6 mice (Fig. 1B). hIgG1 control protein showed predominantly red pulp, outer marginal zone, and follicular localization at this time.
Role of Complement in the Targeting of CR-Fc in Vivo. A time-course study of protein localization from 30 min to 24 h after injection showed that hIgG1 exhibited predominantly red-pulp localization initially, with more marked follicular localization by 4 h after injection (remaining up to 24 h) (data not shown). In contrast, CR-Fc was retained predominantly on the metMφ for the first 8 h after injection, also with some follicular localization evident by 4 h. By 24 h after injection, detection of CR-Fc in the marginal zone was much less marked, and more follicular localization was evident (Fig. 2A and data not shown). At all times, the localization of CR-Fc was associated closely with the presence of the FDC marker FDC-M2, which was not observed on metMφ in uninjected controls or in hIgG1-injected animals. Our subsequent characterization of FDC-M2 as mouse complement component C4 (16) indicated that the binding of CR-Fc to the surface of CRL+ Mφ resulted in rapid complement deposition in situ (Fig. 2 A). During this period, no other obvious phenotypical changes were evident by using the markers mentioned above.
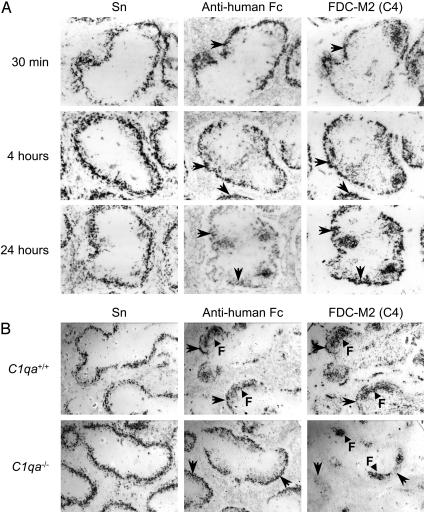
Fig. 2.
Activation of complement by CR-Fc in vivo. (A) Colocalization of i.v.-injected CR-Fc on Sn+ metMφ with complement component C4 between 30 min and 24 h after i.v. injection of 200 μg of protein into C57BL/6 mice. (B) After i.v. injection of C1q-deficient C57BL/6 mice (C1qa–/–) with 200 μg of CR-Fc, enhanced localization of the protein on metMφ was evident, and red-pulp and follicular staining (F) was reduced markedly compared with wild-type (C57BL/6) control mice. Targeting of CR-Fc in the C1q-deficient mice was not associated with fixation of complement component C4. Images were taken 4 h after i.v. administration of protein. Arrows in A and B indicate marked colocalization on serial sections of the same tissue.
To determine the contribution of complement activation by the human Fc domain in the targeting of CR-Fc, mice genetically deficient in C1q were studied (Fig. 2B). C1q-deficient (C1qa–/–) and C1q-sufficient (C1qa+/+) mice were injected i.v. with 200 μg of CR-Fc or hIgG1, as described above. The most notable impact of C1q deficiency was that the follicular localization of both molecules (which is present in both cases at 4 h afterinjection) was almost totally abrogated (Fig. 2B and data not shown). Targeting of CR-Fc to metMφ was not only unimpaired but enhanced by the loss of this alternative clearance mechanism, and no deposition of C4 was localized with the CR-Fc protein (Fig. 2B).
Generation of a Non-Complement-Activating CR-Fc Mutant. Because we had demonstrated, in vivo, that CR-Fc targeting resulted in complement deposition, we generated CR-Fcmut as described in Methods (Fig. 3) by insertion of an Fc domain with abrogated Fc-receptor binding and complement activating activity (9–11). Because the complement activating potential of this Fc domain had been tested only with human serum, we assessed its ability to fix the complement component C4 from mouse serum in a 96-well plate complement activation assay (16). Unlike the original CR-Fc recombinant protein and purified hIgG1, which were both able to fix C4, CR-Fcmut was unable to activate murine complement (Fig. 3C).
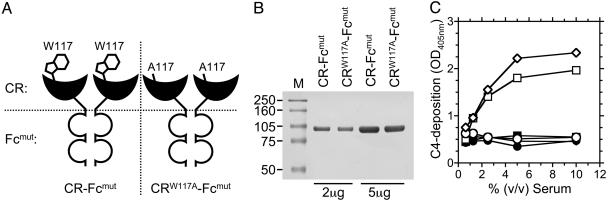
Fig. 3.
Generation of mutated CR-Fc proteins. (A) Schematic representation of CR-Fcmut showing tryptophan residue (W117), which is involved in stacking interactions with model CRL. CRW117A-Fcmut has an amino acid substitution replacing the tryptophan residue with alanine (A117). (B) SDS/PAGE analysis of protein A-purified CR-Fcmut and CRW117A-Fcmut. Coomassie blue staining of proteins resolved by SDS/6% PAGE under nonreducing conditions detected a single 100-kDa species in both protein preparations. (C) Deposition of mouse C4 on hIgG1-coated (squares), CR-Fc-coated (diamonds), or CR-Fcmut-coated (circles) 96-well plates. Normal mouse serum (open symbols) was pooled from more than five individual animals and treated with 10 mM EDTA (filled symbols) to block complement activation. Deposition of C4 was not evident when the mutated Fc domain-containing CR-Fcmut protein was used as an activator of murine complement.
Generation and Characterization of a CR-Fc Mutant Lacking Lectin Activity. To determine that the targeting of the CR-Fc protein depended on its ability to recognize sulfated sugars, a second mutated CR-Fc protein, CRW117A-Fcmut, was generated by site-directed mutagenesis of CR-Fcmut (Fig. 3 A and B). As well as containing the mutated Fc domain, which can neither bind to Fc receptors nor fix complement, CRW117A-Fcmut has a single amino acid substitution (W117A) within the carbohydrate binding pocket of the CR, which was predicted to largely abrogate its lectin activity.
The ability of these recombinant proteins to bind bLH, a known ligand of the CR, was assessed (Fig. 4A). Although CR-Fcmut, as CR-Fc, was able to recognize bLH, cross-linking of this recombinant protein with a mouse anti-human Fc F(ab′)2 preparation increased binding significantly. At a 4:1 F(ab′)2/CR-Fcmut molar ratio, the binding of CR-Fcmut to bLH was enhanced 15-fold in this assay. CRW117A-Fcmut did not show any detectable binding to bLH in all conditions tested. (Fig. 4A and data not shown). These results correlate with the binding properties of CR-Fcmut and CRW117A-Fcmut to spleen sections (data not shown). The formation of complexes after the addition of anti-human Fc F(ab′)2 to the Fc chimeric proteins was confirmed by gel filtration (Fig. 4B).
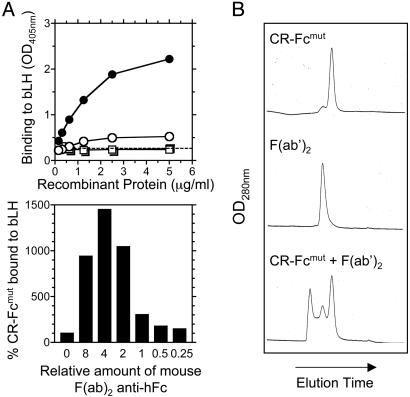
Fig. 4.
Effect of multimerization of CR on CRL binding. (A) CR-Fcmut (○) was able to bind to bLH immobilized on 96-well enzyme immunoassay and RIA plates. Cross-linking of the recombinant protein with a mouse mAb F(ab)2 fragment anti-human Fc (•) greatly enhanced the binding of CR-Fcmut to bLH. This activity was abrogated by the W117A (□) amino acid substitution, and cross-linking of CRW117A-Fcmut (▪) could not restore any detectable binding. (B) The effect of various amounts of cross-linking mouse F(ab)2 anti-human Fc Ab on the ability of CR-Fcmut to bind to bLH. Gel filtration analysis confirmed the formation of complexes of CR-Fcmut and mouse mAb F(ab)2 fragment anti-human Fc.
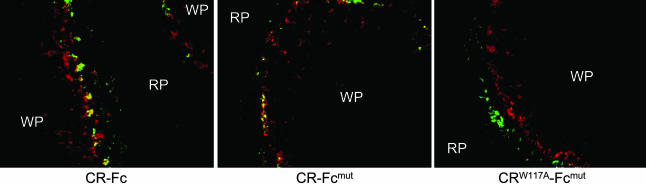
CR Lectin Activity Is Required for in Vivo Targeting. To verify that CR lectin activity was required for in vivo targeting of CR-containing molecules, 20 μg of CR-Fc, CR-Fcmut, or CRW117A-Fcmut, cross-linked with an optimal amount of mouse F(ab′)2 anti-human Fc determined to maximize ligand binding (Fig. 4), was injected i.v. into BALB/c mice (Fig. 5). CR-Fc and CR-Fcmut exhibited specific targeting to Sn+ metMφ (Fig. 5) 15 min after injection, almost identical to that seen when injecting CR-Fc into C1q-deficient mice (Fig. 2B). Localization of CRW117A-Fcmut to Sn+ metMφ was impaired dramatically. Some of these proteins colocalized with marginal zone Mφ, which stained with mAb ERTR9 (anti-SIGNR1) and stained weakly with Sn (Fig. 5 and data not shown), although this colocalization was significantly more evident in the case of CRW117A-Fcmut (Fig. 5). Small amounts of CR-Fcmut and CRW117A-Fcmut were detectable in the Kupffer cells also but only at 15 min after injection (data not shown). Uptake of these proteins by marginal zone Mφ and Kupffer cells, most likely reflects constitutive nonspecific clearance of protein from the circulation. The murine F(ab′)2 anti-human Fc Ab also had the capacity to block complement activation mediated by the hIgG1 Fc domain, both in vitro and in vivo (data not shown), and it probably enhanced the targeting efficiency of the original CR-Fc protein (which does not possess a mutated Fc domain) by blocking alternative clearance pathways.
Fig. 5.
Requirement of CR lectin activity for targeting to metMφ. Optimally cross-linked recombinant proteins (determined in Fig. 4A and indicated below the photomicrographs) were injected i.v. into BALB/c mice (20 μg of recombinant protein per mouse), and spleens were removed 15 min later. The recombinant proteins (green) were detected with a biotinylated anti-human Fc and streptavidin-conjugated Alexa 488, and Sn+ metMφ were identified with 3D6 and goat anti-rat IgG-conjugated Alexa 647 (red). The red pulp (RP) and white pulp (WP) are indicated. CR-Fc and CR-Fcmut showed predominat colocalization on the Sn+ metMφ, with some protein detectable in the outer marginal zone, possibly as a consequence of clearance of residual protein in the circulation. CRW117A-Fcmut showed enhanced localization in the outer marginal zone but no trapping on the Sn+ metMφ. The outer marginal zone staining corresponds to marginal zone Mφ.
Discussion
We described a distribution pattern for CRL, which are expressed on metMφ and ssMφ in naïve mice and also on migratory Mφ/DC populations located in B cell follicles and FDC during the germinal center reaction in immunized mice. This expression pattern suggests that CRL are restricted in secondary lymphoid organs to cells involved in Ag capture and delivery to the B cell follicles. The requirement for B cell-expressed membrane lymphotoxin β (mLTβ) for the induction of CRL expression and the close developmental association between B cell follicles and CRL+ cells (P.R.T., E. Darley, S.G., and M.-L.P., unpublished work) are indicative of a specific B cell-related function in vivo. In this article, we describe the use of CR to develop an efficient and specific targeting system that will allow the future examination of the role that these cells may play in Ag processing.
Analysis of the organs of mice injected i.v., s.c., or i.p. with CR-Fc or hIgG1 showed specific targeting of CR-Fc to the CRL+ metMφ and ssMφ, with kinetics dependent on the route of administration. Some follicular localization was observed also, and, although CR-Fc was predominantly nonaggregated (data not shown), it could not be excluded that complement or Fc receptors could influence the efficiency of CR-Fc binding to CRL+ cells by their interaction with the hIgG1 Fc region. Consistent with this finding, we observed deposition of complement component C4 on the cells targeted by the CR-Fc protein in vivo. The use of C1q-deficient mice demonstrated that follicular targeting and localization were due to CR1/2-mediated trapping of aggregated material and not, in the case of CR-Fc, CR-mediated targeting to CRL within the follicle. In these mice, targeting of CR-Fc to CRL+ metMφ seemed to be enhanced by the lack of an alternative clearance mechanism. To obtain further confirmation of the specificity of the capture of CR-Fc by CRL+ metMφ and ssMφ, we studied CR-Fc localization after i.v. and s.c. administration in op/op mice. Osteopetrotic mice are genetically deficient in Mφ colony-stimulating factor and, consequently, lack metMφ and ssMφ. In agreement with our results, these mice could not retain injected CR-Fc in the marginal zone or subcapsular sinus.
Together, these results demonstrate that CR-containing molecules are able to target specifically to CRL+ metMφ and ssMφ in vivo and, therefore, that CRL are exposed to the extracellular milieu. CRL within the follicles do not capture CR-Fc in vivo as a consequence of CR-mediated interactions either because these ligands are not exposed on the cell surface or because of the presence of a CRL+ marginal zone “barrier” separating them from the circulation and lymph. We showed that the interaction of this lectin domain with its carbohydrate ligands was required for in vivo targeting by site-directed mutagenesis within the ligand-binding pocket of CR.
As might be expected, multimerization of CR significantly influences its ability to bind to CRL in vitro and CRL+ Mφ in vivo. Cross-linking significantly enhanced the efficiency of in vivo targeting, as judged by a >10-fold increase in the sensitivity of detection in situ. Mutation of the Fc domain to abrogate complement activation and Fc-receptor interactions when combined with multimerization led to a significant increase in both the efficiency and specificity of Ag targeting in wild-type mice.
In summary, we have demonstrated that CRL are expressed on the surface of specialized Mφ adjacent to the B cell follicles and are exposed to the circulation and lymph, where they are able to capture CR-containing molecules efficiently and specifically in vivo. The anatomical location of these cells is ideal to influence the outcome of an immune response to foreign or endogenous Ag. The use of this Ag-targeting system will provide a direct approach for the characterization of the role of metMφ and ssMφ in the immune response in vivo.
Acknowledgments
We thank Mark Walport and Marina Botto for C1q-deficient mice; Pamela Bjorkman for helpful discussions regarding the site-directed mutagenesis of the CR-domain binding pocket; Simona Mozdzynski and Michael Coates for technical assistance; Gillian Griffiths, Gordon MacPherson, and Eamon McGreal for critical reading of the manuscript; and the staff of our animal facility for the care of the animals used in this study. bLH was supplied by A. F. Parlow (National Hormone and Pituitary Program, Harbor–UCLA Medical Center, Los Angeles). This work was supported by the Wellcome Trust.
Abbreviations: Ag, antigen; CR, cysteine-rich domain; CRL, CR ligand; Mφ, macrophage; MR, mannose receptor; hIgG1, human IgG1; metMφ, metallophilic Mφ; ssMφ, subcapsular sinus Mφ; LN, lymph nodes; Sn, sialoadhesin; DC, dendritic cells; FDC, follicular DC; bLH, bovine lutropin; op/op mice, osteopetrotic mice.
References
- 1.Martinez-Pomares, L., Kosco-Vilbois, M., Darley, E., Tree, P., Herren, S., Bonnefoy, J. Y. & Gordon, S. (1996) J. Exp. Med. 184, 1927–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martinez-Pomares, L., Crocker, P. R., Da Silva, R., Holmes, N., Colominas, C., Rudd, P., Dwek, R. & Gordon, S. (1999) J. Biol. Chem. 274, 35211–35218. [DOI] [PubMed] [Google Scholar]
- 3.Yu, P., Wang, Y., Chin, R. K., Martinez-Pomares, L., Gordon, S., Kosco-Vibois, M. H., Cyster, J. & Fu, Y. X. (2002) J. Immunol. 168, 5117–5123. [DOI] [PubMed] [Google Scholar]
- 4.Berney, C., Herren, S., Power, C. A., Gordon, S., Martinez-Pomares, L. & Kosco-Vilbois, M. H. (1999) J. Exp. Med. 190, 851–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mueller, C. G., Cremer, I., Paulet, P. E., Niida, S., Maeda, N., Lebeque, S., Fridman, W. H. & Sautes-Fridman, C. (2001) J. Immunol. 167, 5052–5060. [DOI] [PubMed] [Google Scholar]
- 6.Groeneveld, P. H., van Rooijen, N. & Eikelenboom, P. (1983) Cell Tissue Res. 234, 201–208. [DOI] [PubMed] [Google Scholar]
- 7.Groeneveld, P. H., Erich, T. & Kraal, G. (1986) Immunology 58, 285–290. [PMC free article] [PubMed] [Google Scholar]
- 8.Botto, M., Dell'Agnola, C., Bygrave, A. E., Thompson, E. M., Cook, H. T., Petry, F., Loos, M., Pandolfi, P. P. & Walport, M. J. (1998) Nat. Genet. 19, 56–59. [DOI] [PubMed] [Google Scholar]
- 9.Lund, J., Winter, G., Jones, P. T., Pound, J. D., Tanaka, T., Walker, M. R., Artymiuk, P. J., Arata, Y., Burton, D. R., Jefferis, R., et al. (1991) J. Immunol. 147, 2657–2662. [PubMed] [Google Scholar]
- 10.Canfield, S. M. & Morrison, S. L. (1991) J. Exp. Med. 173, 1483–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tao, M. H., Smith, R. I. & Morrison, S. L. (1993) J. Exp. Med. 178, 661–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu, Y., Chirino, A. J., Misulovin, Z., Leteux, C., Feizi, T., Nussenzweig, M. C. & Bjorkman, P. J. (2000) J. Exp. Med. 191, 1105–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinez-Pomares, L., Reid, D. M., Brown, G. D., Taylor, P. R., Stillion, R., Linehan, S. A., Zamze, S., Gordon, S. & Wong, S. Y. C. (2003) J. Leukocyte Biol. 73, 604–613. [DOI] [PubMed] [Google Scholar]
- 14.Kosco, M. H., Pflugfelder, E. & Gray, D. (1992) J. Immunol. 148, 2331–2339. [PubMed] [Google Scholar]
- 15.Kosco-Vilbois, M. H., Zentgraf, H., Gerdes, J. & Bonnefoy, J. Y. (1997) Immunol. Today 18, 225–230. [DOI] [PubMed] [Google Scholar]
- 16.Taylor, P. R., Pickering, M. C., Kosco-Vilbois, M. H., Walport, M. J., Botto, M., Gordon, S. & Martinez-Pomares, L. (2002) Eur. J. Immunol. 32, 1883–1896. [DOI] [PubMed] [Google Scholar]
- 17.Linehan, S. A., Martinez-Pomares, L., Stahl, P. D. & Gordon, S. (1999) J. Exp. Med. 189, 1961–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Witmer-Pack, M. D., Hughes, D. A., Schuler, G., Lawson, L., McWilliam, A., Inaba, K., Steinman, R. M. & Gordon, S. (1993) J. Cell Sci. 104, 1021–1029. [DOI] [PubMed] [Google Scholar]
- 19.Cecchini, M. G., Dominguez, M. G., Mocci, S., Wetterwald, A., Felix, R., Fleisch, H., Chisholm, O., Hofstetter, W., Pollard, J. W. & Stanley, E. R. (1994) Development (Cambridge, U.K.) 120, 1357–1372. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi, K., Umeda, S., Shultz, L. D., Hayashi, S. & Nishikawa, S. (1994) J. Leukoc. Biol. 55, 581–588. [DOI] [PubMed] [Google Scholar]