Abstract
This work explores the bactericidal effect of (+)-limonene, the major constituent of citrus fruits' essential oils, against E. coli. The degree of E. coli BJ4 inactivation achieved by (+)-limonene was influenced by the pH of the treatment medium, being more bactericidal at pH 4.0 than at pH 7.0. Deletion of rpoS and exposure to a sub-lethal heat or an acid shock did not modify E. coli BJ4 resistance to (+)-limonene. However, exposure to a sub-lethal cold shock decreased its resistance to (+)-limonene. Although no sub-lethal injury was detected in the cell envelopes after exposure to (+)-limonene by the selective-plating technique, the uptake of propidium iodide by inactivated E. coli BJ4 cells pointed out these structures as important targets in the mechanism of action. Attenuated Total Reflectance Infrared Microspectroscopy (ATR-IRMS) allowed identification of altered E. coli BJ4 structures after (+)-limonene treatments as a function of the treatment pH: β-sheet proteins at pH 4.0 and phosphodiester bonds at pH 7.0. The increased sensitivity to (+)-limonene observed at pH 4.0 in an E. coli MC4100 lptD4213 mutant with an increased outer membrane permeability along with the identification of altered β-sheet proteins by ATR-IRMS indicated the importance of this structure in the mechanism of action of (+)-limonene. The study of mechanism of inactivation by (+)-limonene led to the design of a synergistic combined process with heat for the inactivation of the pathogen E. coli O157:H7 in fruit juices. These results show the potential of (+)-limonene in food preservation, either acting alone or in combination with lethal heat treatments.
Introduction
The compound (+)-limonene is the major constituent of citrus fruits' essential oils (EOs) [1], [2]. Because of its citrus-like flavor, (+)-limonene is employed as a flavoring agent in perfumes, creams, soaps, household cleaning products and in some food products such as fruit beverages and ice creams [3]. In addition, (+)-limonene has been found to possess antifungal [4], [5], bacteriostatic [6], [7] and bactericidal [8] properties. Therefore, its use as a food preservative has also been proposed [9].
Antimicrobial compounds have been successfully combined with other preservation technologies in order to achieve a synergistic effect in the inactivation of the target pathogens, following the hurdle theory proposed by Leistner and Gorris [10]. For example, exposure of Escherichia coli or Cronobacter sakazakii to citral combined with high hydrostatic pressure (HHP), pulsed electric fields (PEF) or heat treatments, respectively, increased the inactivation degree achieved for each hurdle acting alone [11], [12], [13]. Similarly, plenty of other compounds present in EOs were found to be effective in combination with heat in the inactivation of E. coli and Listeria monocytogenes [14]. Combinations of (+)-limonene with heat or non-thermal technologies could likewise yield a similar synergistic effect in the inactivation of the target pathogens while preserving the organoleptic properties of the fresh food product.
The use of (+)-limonene in the design of food preservation processes requires a proper understanding of its mechanism of inactivation and of the influence of environmental factors that might affect it. (+)-Limonene belongs to the cyclic monoterpene hydrocarbon family, which are considered to accumulate in the microbial plasma membrane and thus cause a loss of membrane integrity and dissipation of the proton motive force [15]. Previous studies on the inactivation of E. coli by other terpenes and terpenoids (such as carvacrol or citral) have demonstrated the occurrence of sub-lethal injury in the outer and cytoplasmic membranes [13], [14], pointing out the membrane disruption as a mechanism of inactivation by these compounds. However, the precise targets of terpenes and terpenoids are not yet completely understood.
Description of cellular target of antimicrobial compounds could be assisted by the use of Fourier transform-infrared (FT-IR) spectroscopy [16]. FT-IR spectroscopy is a physico–chemical analytical technique based on measurement of vibration of a molecule excited by IR radiation at a specific wavelength range. Specially, attenuated total reflectance infrared microspectroscopy (ATR-IRMS) provides bands from all the cellular components of microorganisms (e.g. cell membrane and wall components, proteins and nucleic acids), giving spectral signatures or “fingerprints” that permit the classification of a microorganism at the strain and serovar level [17].
Regulation of gene expression by alternative sigma factors, which are proteins that act as transcription initiation factors through specific binding of RNA polymerase to gene promoters is key in bacterial resistance to food preservation technologies [18]. In many Gram-negative and Gram-positive genera, sigma factors, σS (encoded by rpoS gene) and σB (encoded by sigB gene), respectively, are responsible for the transcription of specific stationary-phase genes [19]. Besides, these sigma factors could also be responsible for cell protection under environmental stresses such as acid, cold, heat or osmotic shocks [19], [20], [21]. Since previous work showed that the expression of RpoS contributed to the higher resistance of E. coli to the terpene aldehyde citral [13], a similar regulation could be expected for other chemical compounds such as (+)-limonene.
The aims of this work were: (a) to study the inactivation of Escherichia coli BJ4 by (+)-limonene, describing the effect of the pH of the treatment medium, deletion of sigma factor σS and sub-lethal shocks; (b) to study the occurrence of lethal and sub-lethal injuries caused by (+)-limonene in bacterial envelopes of E. coli BJ4 and MC4100; (c) to identify the E. coli BJ4 structures affected by (+)-limonene through ATR-IRMS spectroscopy, and (d) to determine the synergistic lethal effect obtained when combining (+)-limonene with heat and PEF treatments to inactivate E. coli O157:H7.
To accomplish these objectives we used different E. coli strains. In the first part, dedicated to describing the mechanism of inactivation by (+)-limonene the strains E. coli BJ4 and its ΔrpoS mutant [22] were used to study the influence of this alternative sigma factor in the bacterial resistance to (+)-limonene, and E. coli K-12 MC4100 and its ΔlptD4213 mutant [23] to study the role of the outer membrane in this resistance. In the second part, dedicated to demonstrating that knowledge of the mechanism of inactivation by (+)-limonene may have an applied interest to develop food preservation combined processes. Thus, we evaluated the efficacy of a combined process using (+)-limonene to inactivate the foodborne bacterium E. coli O157:H7 in fruit juices in which this pathogen uses to cause food safety problems.
Materials and Methods
Micro-organisms and growth conditions
The strains used were Escherichia coli BJ4 and its ΔrpoS null mutant BJ4L1 [22], E. coli K-12 MC4100 and its ΔlptD4213 mutant [23] and E. coli O157:H7 VTEC - (Phage type 34) [24]. The cultures were maintained in cryovials at -80 °C prior to use. Broth subcultures were prepared by inoculating one single colony from a plate, a test tube containing 5 mL of sterile tryptic soy broth (Biolife, Milan, Italy) with 0.6% yeast extract added (Biolife) (TSBYE). After inoculation, the tubes were incubated overnight at 37 °C. With these subcultures, 250 mL Erlenmeyer flasks containing 50 mL of TSBYE were inoculated to a final concentration of 104 CFU/mL. These flasks were incubated with agitation (130 rpm; Selecta, mod. Rotabit, Barcelona, Spain) at 37° C until the stationary growth phase was reached.
Bacterial treatment with (+)-limonene
(+)-Limonene (97% purum) was purchased from Sigma-Aldrich (Sigma-Aldrich Chemie, Steinheim, Germany). This compound is practically immiscible in water, so a vigorous shaking method was used to prepare suspensions. (+)-Limonene was added at a final concentration of 200 µL/L to tubes containing 10 mL of citrate-phosphate buffer of pH 4.0 (23.85 g/L) and 7.0 (27.09 g/L). Before treatments, bacterial cultures were centrifuged at 6,000•g for 5 min and resuspended in the same buffer that of each treatment. Microorganisms were added at a final concentration of 3·107 CFU/mL and maintained under constant agitation (130 rpm) at 20 °C. Samples were taken at regular intervals, and survivors were enumerated. According to previous studies [13], [14], treatment time and temperature; and initial concentrations of (+)-limonene and bacteria were chosen to detect 5 log10 cycles of cell inactivation (i.e. from 3·107 to 3·102 CFU/mL). Minimal inhibitory concentration (MIC) of (+)-limonene determined using the tube dilution method [25] for E. coli BJ4 and O157:H7 was 5 µL/mL (data not shown).
Previous experiments showed that native E. coli was insensitive to incubation in citrate–phosphate buffer at pH 7.0 or pH 4.0 for 24 h at 20 °C.
Sub-lethal heat, cold and acid shock treatments
One 1-mL aliquot of bacterial suspensions was centrifuged at 10,000•g for 5 min and resuspended in 1 mL of TSBYE at 45°C or 0°C (sub-lethal heat and cold shocks, respectively) or in TSBYE acidified to pH 4.5 with HCl at 20 °C (sub-lethal acid shock). Sub-lethal heat shock was performed by immersing the bacterial suspensions in a thermostatic water bath (Bunsen, mod. BTG, Madrid, Spain) and holding at 45 °C for 2 h. Suspensions were kept on ice for 4.5 h (sub-lethal cold shock) or at 20°C (sub-lethal acid shock) for 1 h. Microbial resistance to (+)-limonene was assessed as explained above. These conditions were chosen from previous published work [26], [27].
Cell permeabilization by (+)-limonene
Permanent cell permeabilization of E. coli BJ4 was evaluated after the treatment with 200 µL/L of (+)-limonene (initial cell concentration: 3·107 CFU/mL) for different treatment times (10 min, 25 min, 1 h, 6 h, 24 h) at pH 4.0 and 7.0 at 20° C. Cells were centrifuged, supernatant was removed, and propidium iodide (PI) (Sigma – Aldrich, Madrid, Spain) was added to a final concentration of 0.08 mmol/L [28]. Cell suspensions were incubated for 15 min at 20° C, centrifuged at 10,000·g for 5 min, and washed three times until no extracellular PI remained in the buffer. Cell permeabilization was analyzed using a fluorescence microscope (Nikon, Mod. L-Kc, Nippon Kogaku KK, Japan).
Attenuated total reflectance infrared microspectroscopy (ATR-IRMS) with multivariate analysis
An aliquot of cell suspensions was centrifuged at 6,000·g for 10 min at 4° C. Pellets were washed three times with 1 mL of 0.9% NaCl and centrifuged at 6,000·g for 10 min. Pellets were placed onto grids of hydrophobic membrane (HGM; ISO-GRID, Neogen Corporation, Lansing, MI, USA) and dried out under laminar flow at room temperature for 1 h. Samples were analyzed by IR equipment (Illuminate IR, Smiths detection, The Genesis Centre Science Park South Birchwood Warrington, United Kingdom) interfaced with mercury-cadmium-telluride photoconductive detector and equipped with a microscope with a motorized x-y stage, 20x and 50x objectives, and slide-on attenuated total reflection (ATR) diamond objective (Smiths detection, United Kingdom). The hydrophobic membranes were placed on the stage of the microscope and a specific position of the microbial pellet was selected with the assistance of the microscope and live camera (Leica OM 2,500, Module FT-IR, Renishaw plc, New Mills, Wotton-under-Edge, Gloucestershire, United Kingdom). The microscope was software-controlled using Wire 3.2 version software (Renishaw plc, United Kingdom). Spectra were collected from 4,000 to 800 cm−1 with a resolution of 4 cm−1. The spectrum of each sample was obtained by taking the average of 128 scans. Spectra were displayed in terms of absorbance obtained by rationing the single beam spectrum against that of the air background. The spectrometer was completely software controlled by synchronize IR basic version 1.1 software (SensIR Technologies, Smiths detection, United Kingdom). Pirouette® multivariate analysis software (version 4.0, InfoMetrix, Inc., Woodville, WA) was used to analyze the raw spectra of bacterial cells. The IR spectral data were mean-centred, transformed to their second derivative using a 15-point Savitzky-Golay polynomial filter, and vector-length normalized; sample residuals and Mahalanobis distance were used to determine outliers [29], [30]. Soft independent modeling of class analogy (SIMCA) was used to build a predictive model based on the construction of separate principal component analysis (PCA) models for each class. SIMCA class models were interpreted based on class projections, misclassifications and discriminating power. Class projections were visible through three-dimensional graphics of clustered samples. Probability clouds (95%) are built around the clusters based on PCA scores, allowing SIMCA to be used as a predictive modeling system. Total misclassifications were analyzed and interpreted for the input data. Variable importance, also known as discriminating power, was used to define the variables (wavenumbers) that have a predominant effect on bacterial classification, minimizing the difference between samples within a cluster, and maximizing differences between samples from different clusters. SIMCA analysis assesses itself by predicting each sample included in the training set comparing that prediction to its assigned class; this assessment is referred to as misclassifications. Zero misclassifications typify a model in which all samples were correctly predicted to the pre-assigned class. Compared samples were E. coli BJ4 (initial concentration: 3·107 CFU/mL) after being incubated for 24 h at 20° C in absence or presence of 200 µL/L of (+)-limonene in citrate-phosphate buffer of pH 4.0 or 7.0.
Duration of lag phase in untreated and (+)-limonene-treated cells
E. coli BJ4 at an initial concentration of 3·107 CFU/mL were treated for 20 min with 200 µL/L of (+)-limonene in citrate – phosphate buffer of pH 4.0 so that 1 log10 cycle of inactivation was reached. At this moment, cells were centrifuged and resuspended in TSBYE without (+)-limonene. Non-treated cells were also centrifuged, resuspended in TSBYE without (+)-limonene and adjusted at the same final concentration of live cells (3·106 CFU/mL). Optical absorbance was measured at 590 nm during growth for 14 h of both samples at 37 °C with a spectrophotometer (GENios, Tecan, Austria).
Microbial inactivation by the combination of a lethal heat treatment and (+)-limonene
Tubes containing 5 mL of apple juice or orange juice in absence or presence of (+)-limonene added to a final concentration of 200 µL/L were placed in a shaking thermostatic bath at 54 °C (Bunsen, mod. BTG, Madrid, Spain). Before treatments, bacterial suspensions of E. coli O157:H7 were centrifuged at 10,000·g for 5 min and resuspended in apple or orange juice. Once the treatment temperature was reached, the microbial suspension was added to a final concentration of 3·107 CFU/mL. Samples were taken after 10 min and survivors were enumerated. These treatment conditions were chosen to make these data comparable with previously published data obtained under the same conditions [1], [12], [14], [31].
Microbial inactivation by the combination of pulsed electric fields and (+)-limonene
PEF treatments were carried out in an equipment that delivered an exponential-decay pulse previously described by García et al. [32], provided with a parallel-electrode treatment chamber with a distance of 0.25 cm between electrodes and an area of 2.01 cm2. Before treatments, bacterial suspensions of E. coli O157:H7 were centrifuged at 10,000·g for 5 min and resuspended in shelf-stable apple juice (pH 3.6) or orange juice (pH 3.8) (Don Simón, Murcia, Spain). Bacterial cultures were added to tubes containing 5 mL of each of these media with or without 200 µL/L of (+)-limonene, and 0.5 mL of these suspensions were placed into the treatment chamber with a sterile syringe. Exponential waveform pulses at an electrical field strength of 30 kV/cm and a pulse repetition rate of 1 Hz were used in this study. Experiments started at room temperature and after the application of 25 pulses the temperature of the samples was below 35° C. After treatment, samples were taken and survivors were evaluated.
Survival counts
After treatments, samples were adequately diluted in 0.1% w/v Peptone Water (Biolife), containing 1% v/v Tween 80 (Biolife) as a neutralizer. 0.1 ml aliquots from the neutralized samples were pour-plated onto Tryptic Soy Agar with 0.6% Yeast Extract added (TSAYE) as non-selective medium. Plates were incubated at 37°C for 24 h. Previous experiments showed that longer incubation times did not influence the survival counts. In order to determine bacterial cell injury, treated samples were also plated onto selective media: TSAYE with 4% (E. coli BJ4 and MC4100) or 3% (E. coli BJ4L1, O157:H7 and MC4100 ΔlptD4213) sodium chloride (Sigma-Aldrich, Madrid, Spain) added (TSAYE-SC) to evaluate cytoplasmic membrane damage; and onto TSAYE with 0.35% (E. coli O157:H7) or 0.2% (E. coli BJ4 and BJ4L1) bile salts (Biolife) added (TSAYE-BS) to evaluate outer membrane damage. These levels of sodium chloride and bile salts were determined as the maximum non-inhibitory concentrations for native cells. Plates containing selective media were incubated for 48 h at 37°C. Previous experiments showed that longer incubation times did not influence survival counts.
After incubation of plates, colonies were counted with an improved image analyzer automatic counter (Protos; Analytical Measuring Systems, Cambridge, United Kingdom) as described by Condón et al. [33]. The extent of sub-lethal injury was expressed as the difference between the log10 count (CFU) on non-selective medium (TSAYE) and the log10 count on selective media (TSAYE-SC and TSAYE-BS) after treatments. According to this representation, “2 log10 cycles of injured cells” means a 2-log10 difference in the count on selective and non-selective media or that 99% of survivors were sub-lethally injured.
Statistical analysis
Experiments were carried out in triplicate on different working days. Inactivation was expressed in terms of the extent of reduction in log10 counts after every treatment. The error bars in the figures indicate the mean ± standard error from the data obtained from at least three independent experiments. Analyses of variance (p = 0.05) were performed using SPSS software (SPSS, Chicago, IL, USA).
To characterize the growth kinetics, the absorbance values were fit using nonlinear regression with the Gompertz model [34], which in this case can be described by the equation:
where A(t) is the absorbance value in time t, C is the absorbance value in the stationary phase, B is the relative growth rate in point M, and M is the time in which the cells reach their maximum growth rate. The lag phase duration was calculated as 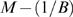 .
.
Results
Inactivation of Escherichia coli BJ4 by (+)-limonene: effect of treatment medium pH, deletion of rpoS and sub-lethal shocks
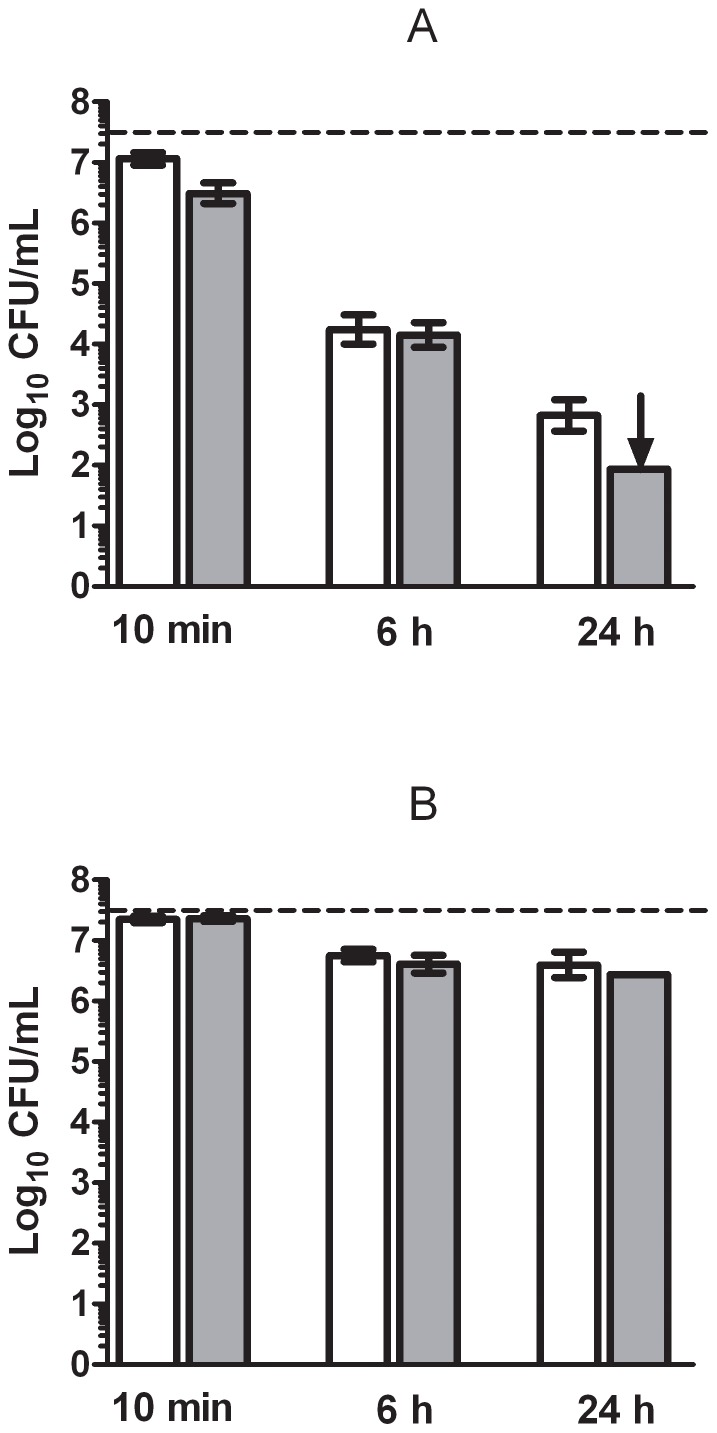
The antimicrobial activity of 200 µL/L of (+)-limonene on the survival of 3·107 CFU/ml of E. coli BJ4 and its rpoS mutant BJ4L1 was tested at pH 4.0 and 7.0 for 10 min, 6 h and 24 h (Figure 1). Both E. coli strains were less resistant at pH 4.0 than at pH 7.0: after 24 h of treatment less than 2 log10 cycles of the initial populations were inactivated at pH 7.0 (Figure 1B), while a treatment of 6 h at pH 4.0 was able to inactivate more than 3 log10 cycles of both strains, and about 5 log10 cycles of inactivation were achieved after 24 h of storage (Figure 1A).
Figure 1. Study of the effect of time, pH and rpoS deletion on Escherichia coli BJ4 inactivation by (+)-limonene.
Log10 of survival counts of Escherichia coli BJ4 (wild type: □) and BJ4L1 (ΔrpoS:  ) after 10 min, 6 h and 24 h with 200 µL/L of (+)-limonene in citrate-phosphate buffer of pH 4.0 (A) or 7.0 (B) at 20° C. Cells were recovered in TSAYE. Discontinuous line indicates initial cell concentration (3·107 CFU/mL). Arrow indicates survival counts under detection limit. Error bars indicate standard error.
) after 10 min, 6 h and 24 h with 200 µL/L of (+)-limonene in citrate-phosphate buffer of pH 4.0 (A) or 7.0 (B) at 20° C. Cells were recovered in TSAYE. Discontinuous line indicates initial cell concentration (3·107 CFU/mL). Arrow indicates survival counts under detection limit. Error bars indicate standard error.
Regarding the comparison between the wild and the mutant strain, both wild type and rpoS mutants showed a similar (+)-limonene resistance for all the treatments assayed (p>0.05).
Development of cross-resistance to (+)-limonene as a consequence of sub-lethal shocks was studied. On the one hand, exposure to a previous sub-lethal heat or acid shock did not affect the final inactivation reached by (+)-limonene in E. coli, since no statistically significant differences were found when compared to the control treatment (p>0.05). On the other hand, exposure to a sub-lethal cold shock significantly decreased (p<0.05) the resistance of both strains to (+)-limonene (Table 1).
Table 1. Influence of previous sub-lethal heat, cold and acid treatments on Escherichia coli BJ4 resistance to (+)-limonene.
| Escherichia coli BJ4 | Escherichia coli BJ4L1 | ||
| Control | 4.24a±0.24 | 4.16a±0.21 | |
| Heat-shock | 4.00a±0.18 | 3.72a±0.33 | |
| Cold-shock | 3.00b±0.41 | <2.18c | |
| Acid-shock | 4.89a±0.21 | 4.55a±0.23 | |
: same letters indicate non-significant differences among mean values; p>0.05.
Log10 of survival counts (CFU/mL) of Escherichia coli BJ4 (wild type) and BJ4L1 (ΔrpoS) (initial concentration: 3·107 CFU/mL) by a treatment with 200 µL/L of (+)-limonene in citrate-phosphate buffer of pH 4.0. Table includes data from non-stressed cells (control) and cells exposed to different sub-lethal shocks before (+)-limonene treatments (mean ± standard error). Sub-lethal heat shock: 45 °C/2 h; sub-lethal cold shock: ice/4.5 h; sub-lethal acid shock: pH 4.5/1 h.
Bacterial counts were not modified (p>0.05) by incubation in citrate–phosphate buffer at pH 7.0 or pH 4.0 without (+)-limonene for 24 h at 20 °C (data not shown).
Occurrence of sub-lethal damage after (+)-limonene treatments in E. coli BJ4
The enumeration of the survivors on the selective medium TSAYE-SC (with sodium chloride) and TSAYE-BS (with bile salts) (Figure 2) revealed that storage for 6 h with 200 µL/L of (+)-limonene caused sub-lethal damages neither to the cytoplasmic nor to the outer membrane of E. coli BJ4, since the levels of inactivation in these media were similar to those detected in TSAYE for each pH (p>0.05, Fig. 1). The same conclusion was drawn from the survival counts after 10 min and 24 h of treatment (data not shown).
Figure 2. Study of sub-lethal injury caused by (+)-limonene on Escherichia coli BJ4 determined using selective plating technique.
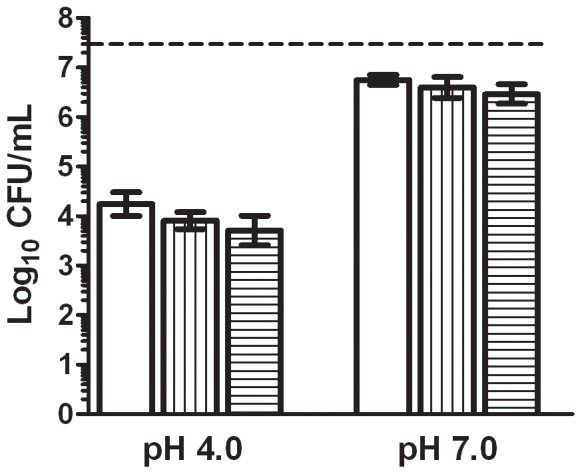
Log10 of survival counts of Escherichia coli BJ4 after 6 h with 200 µL/L of (+)-limonene in citrate-phosphate buffer of pH 4.0 or 7.0 at 20° C. Cells were recovered in TSAYE (□), TSAYE-SC (sodium chloride: vertical stripes) or TSAYE-BS (bile salts: horizontal stripes). Discontinuous line indicates initial cell concentration (3·107 CFU/mL). Error bars indicate standard error.
Bacterial counts in selective or non-selective media were not modified (p>0.05) by incubation in citrate–phosphate buffer at pH 7.0 or pH 4.0 without (+)-limonene for 24 h at 20 °C (data not shown).
Moreover, we evaluated the growth of treated and non-treated cells after exposure/non-exposure to (+)-limonene. Since exponential phase started after 2 h post-inoculation in both populations (2.19±0.19 h) no lag phase delay was detected in treated cells (p>0.05) (data not shown).
Membrane permeabilization of E. coli BJ4 by (+)-limonene
Permanent membrane permeabilization of E. coli BJ4 was demonstrated by the uptake of the fluorescent probe PI. As can be seen in Figure 3, a direct correlation (R2 = 0.96) was found between the percentage of inactivated cells and the percentage of permeabilized cells after adding 200 µL/L of (+)-limonene for different treatment times. Furthermore, as seen with cell inactivation, the percentage of permeabilized cells after 10 min of exposure to (+)-limonene was influenced by pH: after 1 h, E. coli showed maximum cell permeabilization (>90%) at pH 4.0, corresponding to more than 2 log10 cycles of cell inactivation, while at pH 7.0 only about 50% of cells were permeabilized and inactivated. After incubation in the presence of (+)-limonene for 24 h, maximum cell permeabilization (>90%) was observed at both pHs.
Figure 3. Correlation between permeabilized and inactivated Escherichia coli BJ4 cells.
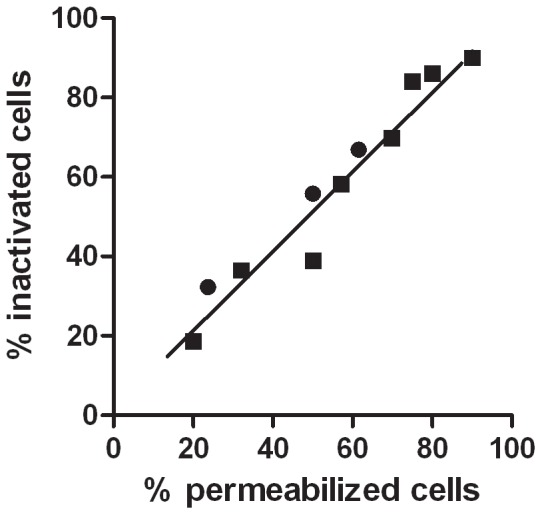
Correlation between the percentage of inactivated Escherichia coli BJ4 and the percentage of cells stained with propidium iodide (permeabilized cells) after different treatment times (10 min, 25 min, 1 h, 6 h, 24 h) with 200 µL/L of (+)-limonene (initial cell concentration: 3·107 CFU/mL) at pH 4.0 (•) or 7.0 (▪).
No membrane permeabilization (p>0.05) was detected after incubation in citrate–phosphate buffer at pH 7.0 or pH 4.0 without (+)-limonene for 24 h at 20 °C (data not shown).
Attenuated total reflectance infrared microspectroscopy of E. coli BJ4 cells after (+)-limonene treatments
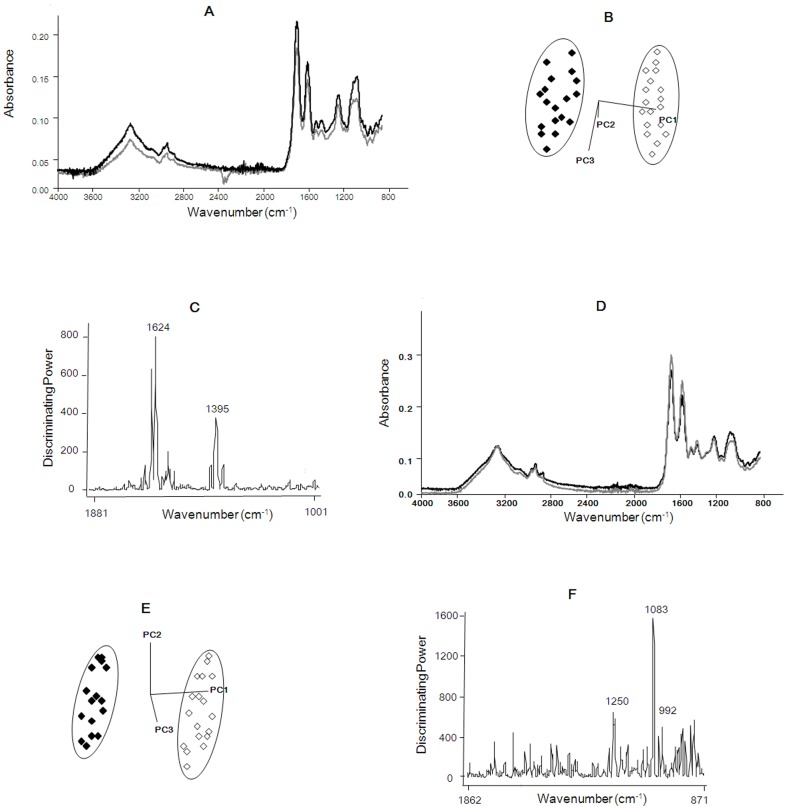
Typical spectra of E. coli BJ4 with the presence or absence of (+)-limonene at pH 4.0 and 7.0 are shown in Figures 4A and 4D, respectively. Class projections illustrate the ability of SIMCA to differentiate IR data based on the first 3 principal components. Since the range of 4000 to 2000 cm−1 was not significant to describe the biochemical differences among our samples Figures 4B, 4C, 4E and 4F only includes data obtained from the range 1,900–800 cm−1. Our classification models obtained from derivatized infrared spectra (1900–800 cm−1) of E. coli BJ4 cells (Figures 4B and 4E) allowed for the tight clustering and clear differentiation of E. coli BJ4 samples according to the presence or absence of (+)-limonene for each pH. Discriminating power of SIMCA is a measure of variable importance in infrared frequency and contributes to the development of the classification model [30]. Figures 4C and 4F show the wavenumbers that had a predominant effect on discrimination of (+)-limonene-treated and untreated cells at pH 4.0 and 7.0, respectively. As can be seen, the discriminating power of non-treated and (+)-limonene treated samples at pH 4.0 (Figure 4C) showed two spectral bands at 1,624 and 1,395 cm−1, corresponding to changes in the amide I absorption band of β-sheet proteins [35], [36]; and in the symmetric stretching of COO- groups in amino acids and/or fatty acids [37], [38], [39]. At pH 7.0 (Figure 4F), comparison of (+)-limonene treated and non-treated cells showed that the major discriminating bands were those located at 1,083, 1,250 and 992 cm−1, corresponding to the symmetric and asymmetric stretching of P = O groups in phosphodiester bonds and ring vibrations of carbohydrates [16], [37], [40]. ATR-IRMS spectra of (+)-limonene treated E. coli O157:H7 allowed us obtaining similar conclusions (data not shown).
Figure 4. ATR-IRMS spectra of (+)-limonene treated Escherichia coli BJ4 cells.
Typical raw spectra (A, D) and soft independent modeling class analogy (SIMCA) of class projections (B, E) and discriminating power (C, F) of non-treated and (+)-limonene treated (200 µL/L) Escherichia coli BJ4 (initial concentration: 3·107 CFU/mL) at pH 4.0 (A, B, C) or 7.0 (D, E, F) of transformed attenuated total reflectance infrared micro spectroscopy (ATR-IRMS) spectra. Black and gray lines and symbols represent non-treated (+)-limonene treated cells, respectively. Spectra were obtained from three independent samples.
Role of E. coli MC4100 outer membrane in (+)-limonene resistance
For this study we used an E. coli MC4100 ΔlptD4213 strain. This mutation disrupts the outer membrane permeability barrier, making E. coli sensitive to antimicrobial compounds that are not normally effective against Gram-negative bacteria [41].
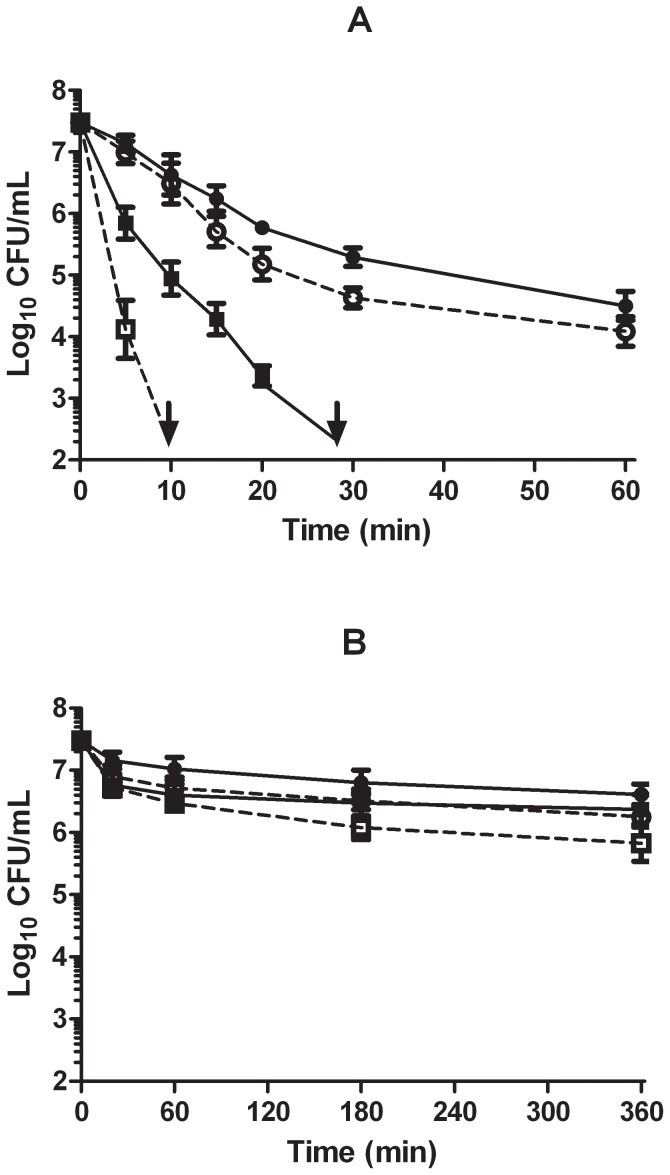
The lptD4213 mutant was less resistant to (+)-limonene at pH 4.0 than its wild type strain (Figure 5A). For example, after 30 min more than 5 log10 cycles (>99.999%) of the initial lptD4213 population were dead, whereas less than 2.5 log10 cycles (99.7%) of the wild strain population were inactivated. Surviving counts in Figure 5A indicate that (+)-limonene did not induce sub-lethal injuries in the cytoplasmic membrane of the wild type strain MC4100. However, a high proportion (>2.5 log10 cycles or 99.7% of survivors) of lptD4213 cells had sub-lethal damages in their cytoplasmic membrane after (+)-limonene treatment at pH 4.0. On the contrary, the lptD4213 mutants treated by (+)-limonene at pH 7.0 showed the same resistance as wild type cells and sub-lethal injuries in the cytoplasmic membrane were not detected (Figure 5B).
Figure 5. Effect of increased outer membrane permeability on Escherichia coli MC4100 resistance to (+)-limonene.
Survival curves of Escherichia coli MC4100 (•) and its defective mutant ΔlptD4213 (▪) after exposure to 200 µL/L of (+)-limonene in citrate-phosphate buffer of pH 4.0 (A) and pH 7.0 (B). Cells were recovered in TSAYE (•, ▪) and TSAYE-SC (sodium chloride: ○, □). Arrows indicate survival counts under detection limit. Error bars indicate standard error.
Bacterial counts in selective or non-selective media were not modified (p>0.05) by incubation in citrate–phosphate buffer at pH 7.0 or pH 4.0 without (+)-limonene for 60 min at 20 °C (data not shown).
Combined preservation processes: lethal heat treatment of E. coli O157:H7 in presence of (+)-limonene
To study a combined process of (+)-limonene with a lethal heat treatment, a pathogenic E. coli serotype, E. coli O157:H7, and acid fruit juices as treatment medium were chosen. Preliminary results showed that E. coli O157:H7 (+)-limonene resistance at 20°C was similar (data not shown).
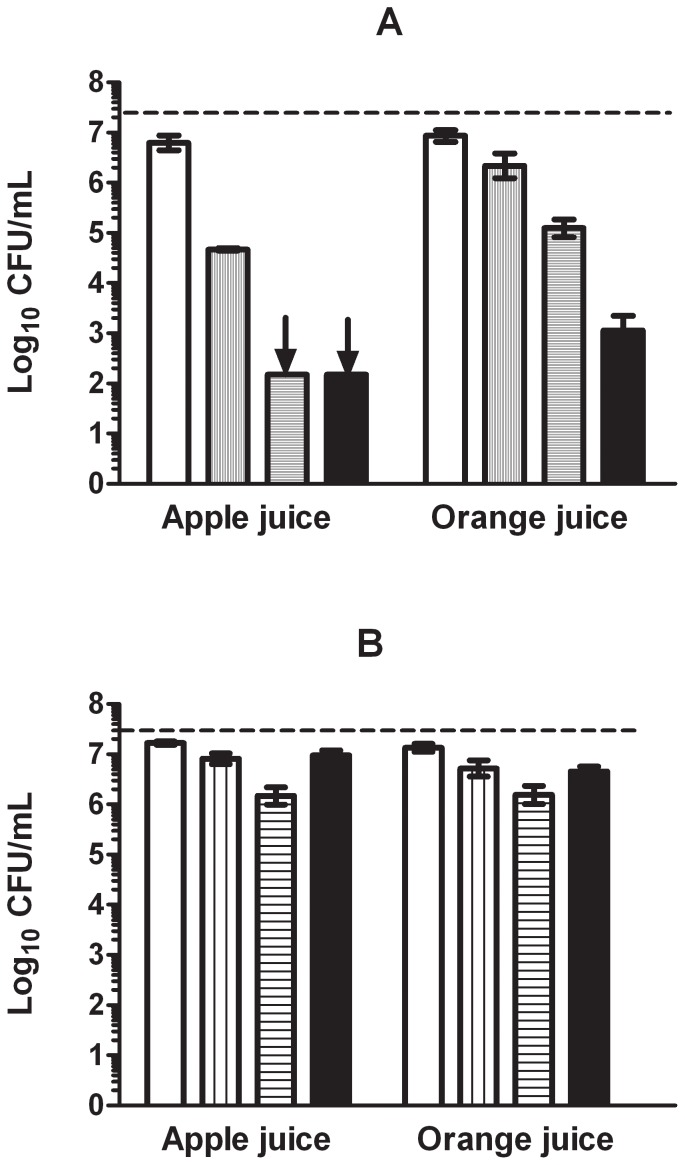
Figure 6A shows the inactivation of E. coli O157:H7 by a lethal heat treatment (54°C for 10 min) alone or in combination with 200 µL/L of (+)-limonene in apple or orange juice. A lethal heat treatment alone inactivated 0.5 log10 cycles of the initial population of E. coli O157:H7; and caused sub-lethal damages in the cytoplasmic membrane in about 2 and 0.5 log10 cycles of survivors (as seen by the difference in log10 counts between recovery in TSAYE and TSAYE with sodium chloride) when cells were treated in apple and orange juice, respectively. Moreover, 4.5 and 2 log10 cycles of survivors showed sub-lethal damages in their outer membrane (as seen by the difference in log10 counts between recovery in TSAYE and TSAYE with bile salts) after lethal heat treatments in apple or orange juice, respectively.
Figure 6. Inactivation of Escherichia coli O157:H7 by combined processes of heat and Pulsed Electric Treatments with (+)-limonene.
Log10 of survival counts of Escherichia coli O157:H7 after lethal heat treatments (54° C for 10 min) (A) or Pulsed Electric Fields treatments (30 kV/cm and 25 pulses) (B) in absence (□) or presence of 200 µL/L of (+)-limonene (▪) and recovery onto TSAYE. Cells after heat and PEF treatments without (+)-limonene were also recovered onto TSAYE-SC (sodium chloride: vertical stripes) or TSAYE-BS (bile salts: horizontal stripes). Discontinuous line indicates initial cell concentration (3·107 CFU/mL). Arrows indicate survival counts under detection limit. Error bars indicate standard error.
The combined process of lethal heat and (+)-limonene in both juices caused the inactivation of more than 4 extra log10 cycles as compared with application of separate treatments. Hence, this combination in juices resulted in a synergistic effect on the final inactivation. A synergistic effect between (+)-limonene and lethal heat treatments under the same treatment conditions was also observed in citrate-phosphate buffer at pH 7.0. As observed at pH 4.0, simultaneous application of both treatments at pH 7.0 allowed the inactivation of more than 4 extra log10 cycles (data not shown).
Bacterial counts in selective or non-selective media were not modified (p>0.05) by incubation in apple or orange juice without (+)-limonene for 60 min at 20 °C (data not shown).
Combined preservation processes: Pulsed Electric Fields treatment of E. coli O157:H7 combined with (+)-limonene
Figure 6B shows the inactivation of E. coli O157:H7 by a mild PEF treatment (25 pulses at 30 kV/cm) alone or in combination with 200 µL/L of (+)-limonene in apple or orange juice.
On the one hand, a separate treatment of 200 µL/L (+)-limonene at 20°C against 3·107 CFU/mL of E. coli O157:H7 when suspended in these juices for 10 min inactivated less than 0.5 log10 cycles of the initial population (data not shown). On the other hand, a separate PEF treatment in absence of (+)-limonene inactivated less than 0.5 log10 cycles of the initial E. coli O157:H7 population, and caused sub-lethal injury in the outer membrane in less than 1 log10 cycle of surviving cells.
The final level of inactivation resulting from the combined process (PEF with (+)-limonene) was additive, i.e. was equal to the sum of the levels of inactivation of both treatments applied separately, not observing any extra inactivation because of the simultaneous application of a lethal heat treatment in presence of (+)-limonene.
Bacterial counts in selective or non-selective media were not modified (p>0.05) by incubation in apple or orange juice without (+)-limonene for 60 min at 20 °C (data not shown).
Discussion
Previous research on the antibacterial activity of (+)-limonene has been mostly focused on its bacteriostatic activity [7], [42], but little is known about its activity as a bactericidal agent in food preservation. In this respect, an important aspect to consider is the pH of the treatment medium (or the food matrix), since the final inactivation achieved by (+)-limonene was considerably higher in acid conditions (Figure 1). It is generally considered that the bacterial resistance to essential oils (EO) and their components decreases with lowering pH values because of the increase in EO hydrophobicity at low pH. As a consequence, there is an easier EO dissolution in the lipids of the cell membrane [43].
Our research was divided into two well-differentiated parts. The first part is focused on the study of mechanism of bacterial inactivation by (+)-limonene for which two wild-type and mutant strains (BJ4 and its rpoS mutant, and MC4100 and its lptD4213 mutant) were used. The second part of our study is dedicated to a practical application in fruit juices of the knowledge obtained in the first part in order to demonstrate the key role of the outer membrane in microbial protection against (+)-limonene. For this objective, E. coli O157:H7 was used owing to its importance in food safety of fruit juices.
The expression of RpoS has been reported to cause physiological and morphological modifications that increase microbial resistance to various stresses [44]. Since deletion of rpoS did not decrease E. coli BJ4 resistance to (+)-limonene (Figure 1), probably the expression of σS-controlled genes under stationary-phase conditions, such as dps (a stress response DNA-binding protein) or uspB (universal stress protein B) [19], [20], [21] did not play a role in cell resistance to (+)-limonene. This finding would suggest different mechanisms of inactivation and microbial resistance for (+)-limonene in relation to other antimicrobial compounds such as citral [13] and food preservation technologies [44].
The application of a sub-lethal heat, cold or acid shock has been demonstrated to induce cross resistance to multiple stresses (see review [45]) in E. coli. In this study, we have shown that a previous sub-lethal heat or acid shock did not influence subsequent E. coli BJ4 resistance to (+)-limonene (Table 1). Interestingly, rpoS deletion did not modify (+)-limonene resistance of sub-lethally heat- and acid-shocked cells. However, a previous sub-lethal cold-shock decreased the resistance of both wild type and rpoS mutant cells to (+)-limonene (Table 1). Exposure to cold temperatures leads to a decrease in the membrane fluidity which triggers an increase in the ratio of unsaturated fatty acids [46], [47], that could be responsible of the decreased resistance to (+)-limonene (Table 1).
The occurrence of sub-lethal injuries after food preservation treatments can be evaluated using different techniques, such as different survival counts obtained between plating treated cells in non-selective and selective media [48], and delay in lag phase before starting growth in treated with regards non-treated cells [49], [50]. At the conditions assayed in this study no sub-lethal damage in the cell envelopes was detected after exposure of E. coli BJ4 to (+)-limonene by the selective media plating technique (Figure 2). Furthermore, the same duration of lag phase was observed for treated and untreated E. coli BJ4 cells, suggesting that neither the cell envelopes nor other cell structures were sub-lethally injured. Therefore, the action of (+)-limonene could be catalogued under the “quantal” effect, a response which can be expressed in binary terms: it is either present or absent (“all or nothing”) [51] in which bacteria are either killed or intact after the treatment. Occurrence of sub-lethal damage in E. coli BJ4 cell envelopes by other EO compounds, such as citral or carvacrol [13], [14], would suggest a different mechanism of inactivation between these compounds and (+)-limonene.
Food preservation technologies, such as heat, pulsed electric fields (PEF), high hydrostatic pressure (HHP) and essential oils (EOs) normally target cell envelopes [52], [53], [54]. Thus, we evaluated membrane permeabilization in E. coli BJ4 using propidium iodide. A direct correlation between the percentage of cell inactivation and the percentage of membrane permeabilization was obtained (Figure 3). The simultaneous occurrence of both phenomena identifies the cell envelopes as an important target in the mechanism of E. coli BJ4 inactivation by (+)-limonene.
To further study the damages caused by (+)-limonene, we included an analysis by ATR-IRMS. We used this technique that evaluates the biochemical composition of the bacterial cell constituents [16], such as water, proteins, nucleic acids, fatty acids and polysaccharides, to describe the changes caused by (+)-limonene. ATR-IRMS results allowed selecting two major discriminating bands at both pH 4.0 (Figure 4C) and pH 7.0 (Figure 4F) as the main responsible for the differences between untreated and (+)-limonene-treated E. coli BJ4 cells. At pH 4.0, the 1,624 cm−1 band corresponding to the amide I absorption band of β-sheet proteins [35], [36]; and the band at 1,395 cm−1 reflecting the symmetric stretching of COO- groups in amino acids and/or fatty acids [37], [38], [39]. Since β-barrel membrane proteins occur in the outer membranes of Gram-negative bacteria [55], the main contribution to the discrimination between untreated and (+)-limonene-treated cells at pH 4.0 could come from affected outer membrane proteins that form membrane-spanning β-barrels. However, at pH 7.0, the discriminating bands for (+)-limonene treatments were found at 1,083, 1,250 and 992 cm−1, corresponding to the symmetric (1,083 cm−1) and asymmetric (1,250 cm−1) stretching of P = O groups in phosphodiester bonds and ring vibrations of carbohydrates (992 cm−1) [16], [37], [40]. Phosphodiester bonds are present in phospholipids of the cytoplasmic membrane and of the inner leaflet of the outer membrane [56], while carbohydrates are found in the lipopolysaccharide (LPS) fraction of the cell wall [57]. In consequence, (+)-limonene would target phospholipids and LPS cell fraction at pH 7.0, and the protein fraction at pH 4.0 in E. coli BJ4.
It should be noted that these conclusions related to the mechanism of bacterial inactivation by (+)-limonene were drawn from experiments using the Gram-negative strains E. coli BJ4 and its ΔrpoS mutant. Further experiments using different microorganisms are needed to extrapolate these conclusions to other Gram-negative strains.
Once we confirmed the role of cell envelopes in the (+)-limonene antimicrobial activity, we used the mutant strain ΔlptD4213 (formerly known as Δimp4213) to evaluate the role of outer membrane in the mechanism of inactivation by (+)-limonene (Figure 5). LptD is an essential β-barrel protein of the outer membrane [58] which is implicated in lipopolysaccharide (LPS) assembly [59]. Depletion of this protein results in increased outer membrane permeability to lipophilic compounds, such as novobiocin or rifampin [24]. In effect, at pH 4.0 lptD4213 mutants showed a decreased (+)-limonene resistance, and occurrence of sub-lethal damage in the cytoplasmic membrane was demonstrated after (+)-limonene treatments. This finding, together with ATR-IRMS observations, could indicate that, at pH 4.0, (+)-limonene should damage the outer membrane in order to gain access to the periplasmic space and cytoplasmic membrane and inactivate the bacterial cell. Once outer membrane permeability to (+)-limonene is increased, there would be an enhanced interaction of (+)-limonene molecules at pH 4.0 with the components in the cytoplasmic membrane. However, the bactericidal action of (+)-limonene at pH 7.0 was not enhanced by higher outer membrane permeability in lptD4213 mutants, indicating that facilitation of (+)-limonene access to the periplasmic space and cytoplasmic membrane would not be required at pH 7.0. Furthermore, results shown by ATR-IRMS would indicate that LPS damage was related to mechanism of inactivation by (+)-limonene at pH 7.0. Therefore, mechanism of E. coli BJ4 and MC4100 inactivation by (+)-limonene was different as a function of the pH of the treatment medium. In spite of differences between E. coli BJ4 and MC4100, (+)-limonene resistance shown by both strains was similar (Figures 1 and 5). However, further research using other microorganisms is needed in order to increase the knowledge on the mechanism of bacterial inactivation by (+)-limonene and to use this compound in practical applications.
From the study of the mechanism of inactivation by (+)-limonene in E. coli BJ4 and MC4100 we could expect that application of a food preservation technology causing sub-lethal damages in outer membrane would increase the lethal effect induced by (+)-limonene, leading to an advantageous combined process. In order to prove this hypothesis and to provide a practical application of this knowledge we studied the effect of (+)-limonene in a combined process with heat or PEF in E. coli O157:H7 because of the presence of an outer membrane in this pathogenic serotype and its importance in food safety of fruit juices [60], [61]. We determined that combinations of a lethal heat treatment that damaged outer membrane with (+)-limonene also achieved a synergistic effect to inactivate E. coli O157:H7 in juice. Thus, a facilitated access of (+)-limonene to the periplasmic space and cytoplasmic membrane would cause the inactivation of these sub-lethally damaged cells (Fig. 6A). On the contrary, since PEF did not cause sub-lethal damage to the outer membrane of E. coli O157:H7 the combination of (+)-limonene and PEF did not yield any extra inactivation when compared to the inactivation by PEF alone at the assayed conditions (Figure 6B). Since E. coli O157:H7 is a virulent strain whose genome has a significant number of differences from other E. coli strains, such as the presence of more than 1,300 new genes [62], [63], transfer of the knowledge on mechanism of microbial inactivation by (+)-limonene from E. coli BJ4 and MC4100 to E. coli O157:H7 would require further studies on the influence of the factors investigated in this research.
Although preliminary results indicate that (+)-limonene concentrations used in this study were accepted by consumers, sensory analysis of apple juice with (+)-limonene should be performed to evaluate commercial viability. Previous work with citral and PEF in E. coli BJ4 reached a similar conclusion [13], as well as combined processes between PEF and different antimicrobials against E. coli O157:H7 in apple and orange juices [14].
Conclusion
The study of the mechanism of bacterial inactivation by (+)-limonene showed that the lethality of this compound was higher at pH 4.0 than at neutral pH. Contrary to other food preservation treatments, deletion of rpoS did not modify E. coli BJ4 resistance to (+)-limonene. Furthermore, a previous sub-lethal heat or acid shock did not change E. coli BJ4 resistance to (+)-limonene, independently of rpoS deletion. However, a previous sub-lethal cold shock decreased the resistance of wild-type E. coli BJ4 and even more the resistance of rpoS mutant to (+)-limonene. Assessment of E. coli BJ4 permeabilization with propidium iodide showed that this phenomenon occurred simultaneously with bacterial inactivation, identifying the cell envelopes as important (+)-limonene targets. In contrast to other essential oils compounds, (+)-limonene did not cause sub-lethal injuries in any E. coli BJ4 structure, cataloguing its lethal action under the “quantal” effect (“all or nothing”). Different resistance pattern of lptD4213 mutants and ATR-IRMS results showed the importance of outer membrane in the mechanism of inactivation by (+)-limonene at pH 4.0. At pH 7.0, increased outer membrane permeability did not lead to a decreased (+)-limonene resistance and ATR-IRMS spectra demonstrated the importance of LPS in the mechanism of E. coli BJ4 inactivation at this pH. Considering the orange-like flavor of (+)-limonene and its consideration as a GRAS (Generally Recognized As Safe) substance [64], [65], we propose the simultaneous application of (+)-limonene with other preservation technologies that damage outer membrane, such as heat treatments, in order to design combined food preservation processes with a synergistic lethal effect, as demonstrated for E. coli O157:H7 in this study. Although bacterial resistance of the studied E. coli strains was similar, further research is needed in order to increase the knowledge on the mechanism of inactivation by (+)-limonene in other bacteria and to use this compound in practical applications.
Funding Statement
This study was financially supported by the Comisón Interministerial de Ciencia y Tecnolog?a (CICYT) (Projects AGL2009-11660 and AGL2012-32165), Gobierno de Aragón, European Social Fund and Departament Quimica of Universitat Rovira i Virgili. Spanish Ministerio de Ciencia e Innovación provided LE with a grant to carry out this investigation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Espina L, Somolinos M, Lorán S, Conchello P, García D, et al. (2011) Chemical composition of commercial citrus fruit essential oils and evaluation of their antimicrobial activity acting alone or in combined processes. Food Control 22: 896–902. [Google Scholar]
- 2. Fisher K, Phillips C (2008) Potential antimicrobial uses of essential oils in food: is citrus the answer? Trends Food Sci Technol 19: 156–164. [Google Scholar]
- 3. Bakkali F, Averbeck S, Averbeck D, Idaomar M (2008) Biological effects of essential oils – A review. Food Chem Toxicol 46: 446–475. [DOI] [PubMed] [Google Scholar]
- 4. Chee HY, Kim H, Lee MH (2009) In vitro antifungal activity of limonene against Trichophyton rubrum . Mycobiology 37: 243–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dambolena JS, López AG, Cánepa MC, Theumer MG, Zygadlo JA, et al. (2008) Inhibitory effect of cyclic terpenes (limonene, menthol, menthone and thymol) on Fusarium verticillioides MRC 826 growth and fumonisin B1 biosynthesis. Toxicon 51: 37–44. [DOI] [PubMed] [Google Scholar]
- 6. Jaroenkit P, Matan N, Nisoa M (2011) In vitro and in vivo activity of citronella oil for the control of spoilage bacteria of semi dried round scad (Decapterus maruadsi). Int J Med Arom Plants 1: 234–239. [Google Scholar]
- 7. Vuuren SFv, Viljoen AM (2007) Antimicrobial activity of limonene enantiomers and 1,8-cineole alone and in combination. Flavour Fragr J 22: 540–544. [Google Scholar]
- 8. Zukerman I (1951) Effect of oxidized d-limonene on micro-organisms. Nature 168: 517–517. [DOI] [PubMed] [Google Scholar]
- 9. Dorman HJD, Deans SG (2000) Antimicrobial agents from plants : antibacterial activity of plant volatile oils. J Appl Microbiol 88: 308–316. [DOI] [PubMed] [Google Scholar]
- 10. Leistner L, Gorris LGM (1995) Food preservation by hurdle technology. Trends Food Sci Technol 6: 41–46. [Google Scholar]
- 11. Arroyo C, Somolinos M, Cebrián G, Condón S, Pagán R (2010) Pulsed electric fields cause sublethal injuries in the outer membrane of Enterobacter sakazakii facilitating the antimicrobial activity of citral. Lett Appl Microbiol 51: 525–531. [DOI] [PubMed] [Google Scholar]
- 12. Espina L, Somolinos M, Pagán R, García-Gonzalo D (2010) Effect of citral on the thermal inactivation of Escherichia coli O157:H7 in citrate phosphate buffer and apple juice. J Food Prot 73: 2189–2196. [DOI] [PubMed] [Google Scholar]
- 13. Somolinos M, García D, Condón S, Mackey B, Pagán R (2010) Inactivation of Escherichia coli by citral. J Appl Microbiol 108: 1928–1939. [DOI] [PubMed] [Google Scholar]
- 14. Ait-Ouazzou A, Cherrat L, Espina L, Lorán S, Rota C, et al. (2011) The antimicrobial activity of hydrophobic essential oil constituents acting alone or in combined processes of food preservation. Innov Food Sci Emerg 12: 320–329. [Google Scholar]
- 15. Sikkema J, de Bont J, Poolman B (1994) Interactions of cyclic hydrocarbons with biological membranes. J Biol Chem 269: 8022–8028. [PubMed] [Google Scholar]
- 16. Alvarez-Ordóñez A, Mouwen DJM, López M, Prieto M (2011) Fourier transform infrared spectroscopy as a tool to characterize molecular composition and stress response in foodborne pathogenic bacteria. J Microbiol Meth 84: 369–378. [DOI] [PubMed] [Google Scholar]
- 17. Grasso EM, Yousef AE, de Lamo Castellvi S, Rodriguez-Saona LE (2009) Rapid detection and differentiation of Alicyclobacillus species in fruit juice using hydrophobic grid membranes and attenuated total reflectance infrared microspectroscopy. J Agric Food Chem 57: 10670–10674. [DOI] [PubMed] [Google Scholar]
- 18. Abee T, Wouters JA (1999) Microbial stress response in minimal processing. Int J Food Microbiol 50: 65–91. [DOI] [PubMed] [Google Scholar]
- 19. Kazmierczak MJ, Wiedmann M, Boor KJ (2005) Alternative sigma factors and their roles in bacterial virulence. Microbiol Mol Biol Rev 69: 527–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Weber H, Polen T, Heuveling J, Wendisch VF, Hengge R (2005) Genome-wide analysis of the general stress response network in Escherichia coli: σS-dependent genes, promoters, and sigma factor selectivity. J Bacteriol 187: 1591–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ait-Ouazzou A, Mañas P, Condón S, Pagán R, García-Gonzalo D (2012) Role of general stress-response alternative sigma factors σS (RpoS) and σB (SigB) in bacterial heat resistance as a function of treatment medium pH. Int J Food Microbiol 153: 358–364. [DOI] [PubMed] [Google Scholar]
- 22. Krogfelt KA, Hjulgaard M, Sørensen K, Cohen PS, Givskov M (2000) rpoS gene function is a disadvantage for Escherichia coli BJ4 during competitive colonization of the mouse large intestine. Infect Immun 68: 2518–2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sampson BA, Misra R, Benson SA (1989) Identification and characterization of a new gene of Escherichia coli K-12 involved in outer membrane permeability. Genetics 122: 491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chapman PA, Siddons CA, Wright DJ, Norman P, Fox J, et al. (1993) Cattle as a possible source of verocytotoxin-producing Escherichia coli O157 infections in man. Epidemiol Infect 111: 439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rota C, Carraminana JJ, Burillo J, Herrera A (2004) In vitro antimicrobial activity of essential oils from aromatic plants against selected foodborne pathogens. J Food Prot 67: 1252–1256. [DOI] [PubMed] [Google Scholar]
- 26. Somolinos M, Espina L, Pagán R, Garcia D (2010) sigB absence decreased Listeria monocytogenes EGD-e heat resistance but not its Pulsed Electric Fields resistance. Int J Food Microbiol 141: 32–38. [DOI] [PubMed] [Google Scholar]
- 27. Somolinos M, García D, Mañas P, Condón S, Pagán R (2008) Effect of environmental factors and cell physiological state on Pulsed Electric Fields resistance and repair capacity of various strains of Escherichia coli . Int J Food Microbiol 124: 260–267. [DOI] [PubMed] [Google Scholar]
- 28. Pagán R, Mackey B (2000) Relationship between membrane damage and cell death in pressure-treated Escherichia coli cells: differences between exponential- and stationary-phase cells and variation among strains. Appl Environ Microbiol 66: 2829–2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hruschka WR (2001) Data analysis: wavelength selection methods. In: Williams P, Norris K, editors. Near-Infrared technology in the agricultural and food industries. Minnesota: AACC International. pp. 39–58.
- 30.Dunn WJ, Wold S (1995) SIMCA pattern recognition and classification In: van de Waterbeemd H, editor. Chemometric methods in molecular design.New York: Wiley-VCH Publishers.pp. 179–193.
- 31. Espina L, Somolinos M, Ouazzou AA, Condón S, García-Gonzalo D, et al. (2012) Inactivation of Escherichia coli O157:H7 in fruit juices by combined treatments of citrus fruit essential oils and heat. Int J Food Microbiol 159: 9–16. [DOI] [PubMed] [Google Scholar]
- 32. García D, Gómez N, Raso J, Pagán R (2005) Bacterial resistance after pulsed electric fields depending on the treatment medium pH. Innov Food Sci Emerg Technol 6: 388–395. [Google Scholar]
- 33. Condón S, Palop A, Raso J, Sala FJ (1996) Influence of the incubation temperature after heat treatment upon the estimated heat resistance values of spores of Bacillus subtilis . Lett Appl Microbiol 22: 149–152. [Google Scholar]
- 34. Gibson AM, Bratchell N, Roberts TA (1988) Predicting microbial growth: growth responses of salmonellae in a laboratory medium as affected by pH, sodium chloride and storage temperature. Int J Food Microbiol 6: 155–178. [DOI] [PubMed] [Google Scholar]
- 35. Barth A (2007) Infrared spectroscopy of proteins. BBA-Bioenergetics 1767: 1073–1101. [DOI] [PubMed] [Google Scholar]
- 36. Kong J, Yu S (2007) Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta Biochim Biophys Sin 39: 549–559. [DOI] [PubMed] [Google Scholar]
- 37. Belfer S, Gilron J, Daltrophe N, Oren Y (2005) Comparative study of biofouling of NF modified membrane at SHAFDAN. Desalination 184: 13–21. [Google Scholar]
- 38. Legal JM, Manfait M, Theophanides T (1991) Applications of FTIR spectroscopy in structural studies of cells and bacteria. J Mol Struct 242: 397–407. [Google Scholar]
- 39. Parikh SJ, Chorover J (2006) ATR-FTIR spectroscopy reveals bond formation during bacterial adhesion to iron oxide. Langmuir 22: 8492–8500. [DOI] [PubMed] [Google Scholar]
- 40. Yu C, Irudayaraj J (2005) Spectroscopic characterization of microorganisms by Fourier transform infrared microspectroscopy. Biopolymers 77: 368–377. [DOI] [PubMed] [Google Scholar]
- 41. Ruiz N, Kahne D, Silhavy TJ (2006) Advances in understanding bacterial outer-membrane biogenesis. Nat Rev Microbiol 4: 57–66. [DOI] [PubMed] [Google Scholar]
- 42. Mourey A, Canillac N (2002) Anti-Listeria monocytogenes activity of essential oils components of conifers. Food Control 13: 289–292. [Google Scholar]
- 43. Juven BJ, Kanner J, Schved F, Weisslowicz H (1994) Factors that interact with the antibacterial action of thyme essential oil and its active constituents. J Appl Microbiol 76: 626–631. [DOI] [PubMed] [Google Scholar]
- 44. Hengge-Aronis R (2002) Signal transduction and regulatory mechanisms involved in control of the σS (RpoS) subunit of RNA polymerase. Microbiol Mol Biol Rev 66: 373–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chung HJ, Bang W, Drake MA (2006) Stress response of Escherichia coli . Compr Rev Food Sci Food Saf 5: 52–64. [Google Scholar]
- 46. Fulco AJ (1974) Metabolic alterations of fatty acids. Annu Rev Biochem 43: 215–241. [DOI] [PubMed] [Google Scholar]
- 47. Yamanaka K (1999) Cold shock response in Escherichia coli . J Mol Microbiol Biotechnol 1: 193–202. [PubMed] [Google Scholar]
- 48.Mackey BM (2000) Injured bacteria. In: Lund BM, Baird-Parker TC, Gould GW, editors. The Microbiological Safety and Quality of Food. Gaithersburg: Aspen Publisher, Inc. pp. 315–341. [Google Scholar]
- 49. Mackey BM, Derrick CM (1982) The effect of sublethal injury by heating, freezing, drying and gamma-radiation on the duration of the lag phase of Salmonella typhimurium . J Appl Microbiol 53: 243–251. [DOI] [PubMed] [Google Scholar]
- 50. Shin J-K, Pyun Y-R (1997) Inactivation of Lactobacillus plantarum by pulsed-microwave irradiation. J Food Sci 62: 163–166. [Google Scholar]
- 51. Hucl T, Gallmeier E, Kern SE (2007) Distinguishing rational from irrational applications of pharmacogenetic synergies from the bench to clinical trials. Cell Cycle 6: 1336–1341. [DOI] [PubMed] [Google Scholar]
- 52. Burt S (2004) Essential oils: their antibacterial properties and potential applications in foods--a review. Int J Food Microbiol 94: 223–253. [DOI] [PubMed] [Google Scholar]
- 53.Gould GW (1989) Heat induced injury and inactivation. In: Gould GW, editor. Mechanisms of action of food preservation procedures. London: Elsevier Applied Science. pp. 11–42. [Google Scholar]
- 54. Mañas P, Pagán R (2005) Microbial inactivation by new technologies of food preservation. J Appl Microbiol 98: 1387–1399. [DOI] [PubMed] [Google Scholar]
- 55. Tamm LK, Hong H, Liang B (2004) Folding and assembly of β-barrel membrane proteins. BBA-Biomembranes 1666: 250–263. [DOI] [PubMed] [Google Scholar]
- 56. Silhavy TJ, Kahne D, Walker S (2010) The bacterial cell envelope. Cold Spring Harb Perspect Biol 2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gmeiner J, Schlecht S (1980) Molecular composition of the outer membrane of Escherichia coli and the importance of protein-lipopolysaccharide interactions. Arch Microbiol 127: 81–86. [DOI] [PubMed] [Google Scholar]
- 58. Braun M, Silhavy TJ (2002) Imp/OstA is required for cell envelope biogenesis in Escherichia coli . Mol Microbiol 45: 1289–1302. [DOI] [PubMed] [Google Scholar]
- 59. Wu T, McCandlish AC, Gronenberg LS, Chng S-S, Silhavy TJ, et al. (2006) Identification of a protein complex that assembles lipopolysaccharide in the outer membrane of Escherichia coli . Proc Natl Acad Sci U S A 103: 11754–11759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Food and Drug Administration (2001) Hazard analysis and critical control point (HACCP): procedures for the safe and sanitary processing and importing of juice: final rule (21 CFR Part 120). Fed. Regist., vol. 66 . Washington, D. C.: U.S. Food and Drug Administration.pp. 6137–6202. [Google Scholar]
- 61. Rangel JM, Sparling PH, Crowe C, Griffin PM, Swerdlow DL (2005) Epidemiology of Escherichia coli O157:H7 outbreaks, United States, 1982–2002. Emerg Infect Dis 11: 603–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hayashi T, Makino K, Ohnishi M, Kurokawa K, Ishii K, et al. (2001) Complete genome sequence of enterohemorrhagic Escherichia coli O157:H7 and genomic comparison with a laboratory strain K-12. DNA Res 8: 11–22. [DOI] [PubMed] [Google Scholar]
- 63. Perna NT, Plunkett G 3rd, Burland V, Mau B, Glasner JD, et al (2001) Genome sequence of enterohaemorrhagic Escherichia coli O157:H7. Nature 409: 529–533. [DOI] [PubMed] [Google Scholar]
- 64.Food and Drug Administration (Revised 2011) GRAS – Essential oils, oleoresins (solvent-free), and natural extractives (including distillates). 21CFR182.20.
- 65.Food and Drug Administration (Revised 2012) GRAS – Synthetic flavoring substances and adjuvants. 21CFR182.60.