Abstract
Alzheimer's disease (AD) is an age-related disorder characterized by deposition of amyloid β-peptide (Aβ) and degeneration of neurons in brain regions such as the hippocampus, resulting in progressive cognitive dysfunction. The pathogenesis of AD is tightly linked to Aβ deposition and oxidative stress, but it remains unclear as to how these factors result in neuronal dysfunction and death. We report alterations in sphingolipid and cholesterol metabolism during normal brain aging and in the brains of AD patients that result in accumulation of long-chain ceramides and cholesterol. Membrane-associated oxidative stress occurs in association with the lipid alterations, and exposure of hippocampal neurons to Aβ induces membrane oxidative stress and the accumulation of ceramide species and cholesterol. Treatment of neurons with α-tocopherol or an inhibitor of sphingomyelin synthesis prevents accumulation of ceramides and cholesterol and protects them against death induced by Aβ. Our findings suggest a sequence of events in the pathogenesis of AD in which Aβ induces membrane-associated oxidative stress, resulting in perturbed ceramide and cholesterol metabolism which, in turn, triggers a neurodegenerative cascade that leads to clinical disease.
Keywords: amyloid, apoptosis, hippocampus, lipid peroxidation, sphingomyelin
Changes that occur in the brain during aging increase the risk of Alzheimer's disease (AD), a disorder involving the progressive deposition of amyloid β-peptide (Aβ) and associated degeneration of neurons in brain regions involved in learning and memory (1). Two factors that are believed to contribute to neuronal dysfunction and degeneration in AD are increased oxidative stress and increased production of neurotoxic forms of Aβ (2). Alterations in lipid metabolism may also play roles in AD because the risk of AD is affected by inheritance of different isoforms of apolipoprotein E (3), changes in cholesterol metabolism can affect Aβ production in cell culture and in vivo (4–6), and drugs that lower cholesterol levels may reduce the risk of AD (7, 8). However, a direct link between alterations in the metabolism of cholesterol and other membrane lipids in AD has not been established, and it is not known whether and how such lipid alterations might lead to neuronal dysfunction and death.
Membrane microdomains that are rich in cholesterol and sphingolipids play important roles in various cellular signaling pathways (9, 10). Sphingomyelin is a major source of ceramides, lipid mediators that are generated when sphingomyelin is cleaved by sphingomyelinases, enzymes activated by inflammatory cytokines (11) and oxidative stress (12). Ceramides play important roles in regulating an array of physiological processes, including cell proliferation and differentiation, and a form of programmed cell death called apoptosis (13). Ceramides have been implicated in the pathological death of neurons that occurs in ischemic stroke (14) and Parkinson's disease (15). In the present study, we document significant increases in levels of membrane-associated oxidative stress, long-chain ceramides, and free cholesterol in brain cells during normal aging in mice, in AD patients, and in neurons exposed to Aβ. The intracellular accumulation of ceramides and cholesterol, and the neurotoxicity of Aβ, can be blocked by α-tocopherol and a small-molecule inhibitor of ceramide production, suggesting a potential therapeutic benefit of agents that target sphingolipid metabolism in AD.
Materials and Methods
Human and Mouse Brain Tissue Samples. Whole-tissue samples from the middle frontal gyrus and cerebellum of autopsy-confirmed AD patients and age-matched neurologically normal control patients were obtained from the Johns Hopkins Brain Bank. The average ages of the AD and control patients were 81 ± 3 years (range 68–93) and 82 ± 4 years (range 68–96), respectively (mean ± SEM, n = 7 AD and 7 control patients). The average postmortem interval for the AD and control patients were 9 ± 1 h (range 5–12) and 11 ± 2 (range 4–16), respectively (mean ± SEM, n = 7 AD and 7 control patients). Superior frontal cortex samples from autopsy-confirmed AD patients who died with mild, moderate, and severe cognitive impairment were obtained from the Rush Alzheimer's Disease Clinical Core. Clinical stages were based on the Folstein mini-mental state examination (MMSE) score (0–30) at the last yearly examination proximate to death (mean interval between MMSE and death was <1 year) The sample consisted of five mild (MMSE 23–29), eight moderate (MMSE 11–20), and 12 severe (MMSE 0–10) patients, and two neurologically normal control subjects. The average age of patients was 79.8 ± 2.0 (range 54–94). Average postmortem interval for all AD patients was 5.6 ± 0.6 h (range 2–14). No clinical stage-associated difference in average postmortem interval was present. Cerebral cortical tissue was obtained from five male C57BL/6 mice of three different ages (3, 6, and 25 months). The mice were killed by anesthetic overdose (isofluorane), brains were removed immediately, and cortical tissue was dissected, flash-frozen, and stored at –80°C. Samples from each mouse brain were prepared and analyzed separately.
Measurements of Sphingomyelin, Ceramides, Cholesterol Esters, and 4-Hydroxynonenal (HNE) Adducts. Total lipids from brain tissue samples, isolated membranes, and cultured cells were prepared by homogenizing the tissue/cells at room temperature in 10 volumes of deionized water, then in 3 volumes of 100% methanol containing 53 mM ammonium formate. Samples were vortexed, four volumes of chloroform were added, and the mixture was again vortexed and then centrifuged at 1,000 × g for 10 min. The chloroform layer was removed and analyzed by direct injection into a Sciex (Thornhill, ON, Canada) 3000 electrospray tandem mass spectrometer. Crude membranes were prepared from midfrontal cortex gray matter by dissecting away meninges and white matter, and gray matter was homogenized in ice-cold buffer (20 mM Hepes, pH 7.6/20 mM NaCl/230 mM sucrose/1 mM DTT/Complete protease inhibitor/pepstatin/soybean trypsin inhibitor/0.2% BHT for each gram of tissue) by using a Brinkman Polytron homogenizer for 30 sec at setting 6. The homogenate was centrifuged at 1,000 × g for 15 min at 4°C and the nuclear pellet was washed one time and then discarded. The combined original and wash supernatants were centrifuged at 100,000 × g for 1 h and the resulting crude membrane pellet was quick-frozen and stored at 4 C. Before lipid extraction, the membrane samples were normalized based on an average wet weight of ≈19 mg. Electrospray tandem MS analyses were performed by using methods described (16).
Cell Cultures, Experimental Treatments, and Analysis of Neuron Survival. Primary dissociated cell cultures of hippocampal neurons were prepared from 18-day-old rat embryos by using methods described (17). Cultures were maintained in 35- or 60-mm-diameter polyethyleneimine-coated dishes or polyethyleneimine-coated 22 mm2 glass coverslips in neurobasal medium with B27 supplements (GIBCO); experiments were performed in cells that had been in culture for 6–8 days. Aβ 1–42 (Bachem) was prepared as a 1-mM stock in water and was incubated overnight at room temperature before dilution into culture medium. FeSO4 was prepared as a 100× stock in water. Myriocin (ISP-1) and α-tocopherol were prepared as a 500× stocks in dimethyl sulfoxide and ethanol, respectively. The culture maintenance medium was replaced with neurobasal medium lacking B27 supplements immediately before exposure of cells to experimental treatments. Apoptosis was quantified as described (18). Briefly, cells were fixed in 4% paraformaldehyde in PBS and were stained with Hoechst 33258. Stained nuclei were observed under a fluorescence microscope and cells were scored as apoptotic if their nuclear chromatin was condensed and fragmented.
Statistical Analyses. Data were analyzed by ANOVA and pairwise comparisons were made by using Scheffé's post hoc test.
Results
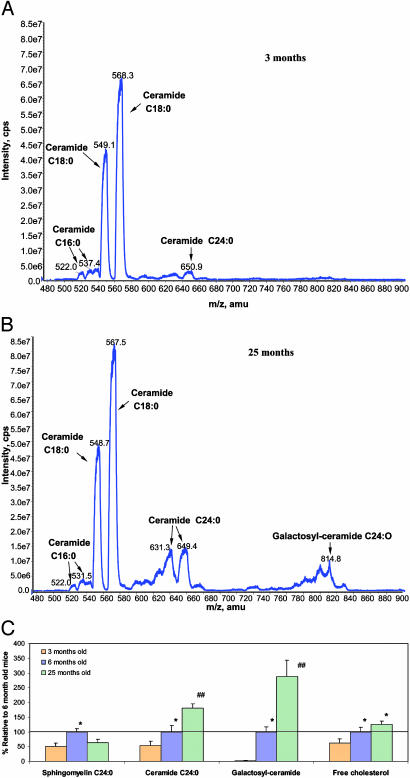
Ceramides and Cholesterol Accumulate in Brain Cells in Association with Oxidative Stress During Normal Aging. Electrospray tandem MS was used to identify and quantify sphingomyelins and ceramides of various hydrocarbon chain lengths and double-bond contents in tissue homogenates from the cerebral cortex of 3-, 6-, and 25-month-old mice. Concentrations of C24:0 ceramide in the cerebral cortex increased in an age-dependent manner such that the concentration in 25-month-old mice was >3-fold greater than in 3-month-old mice (Fig. 1 A and B). There was a dramatic age-related increase in the concentration of C24:0 galactosylceramide, with the concentration being >100-fold greater in cortical tissue from 25-month-old mice compared with 3-month-old mice (Fig. 1C). There was also a significant age-dependent increase in the concentration of free cholesterol in cortical tissue samples (Fig. 1C). In contrast to the age-related increases in levels of ceramides and free cholesterol, the concentration of C24:0 sphingomyelin was greater in 6-month-old mice than in 3- or 25-month-old mice (Fig. 1C). Oxidative damage to brain cells may increase during aging (19), and exposure of brain cells to oxidative stress increases the accumulation of cholesterol in membranes (20). To establish an association between oxidative stress and the age-related lipid alterations in the cerebral cortex of mice, we measured the amounts of HNE adducts of lysine and histidine in the same cortical tissue homogenates used for the lipid analyses. The substance HNE is a lipid peroxidation product that has been previously associated with brain aging and the pathogenesis of AD (21–23). As expected, amounts of HNE adducts were significantly increased in cortical tissue from 25-month-old mice, compared with 3- and 6-month-old mice (data not shown), suggesting an association between age-related increases in oxidative stress and increased ceramide and cholesterol levels.
Fig. 1.
Levels of ceramides and cholesterol increase in the brain during normal aging. (A and B) Representative electrospray tandem MS spectrograms showing relative levels of different ceramides in samples of cerebral cortex from a 3-month-old (A) and a 25-month-old (B) mouse. (C) Levels of sphingomyelin C24:0, ceramide C24:0, galactosyl-ceramide, and free cholesterol in samples of cerebral cortex from 3-, 6-, and 25-month-old mice. Values are the mean and SEM (five mice of each age). *, P < 0.01 compared with the value for 3-month-old mice. ##, P < 0.01 compared with the value for 6-month-old mice and P < 0.001 compared with the value for 3-month-old mice.
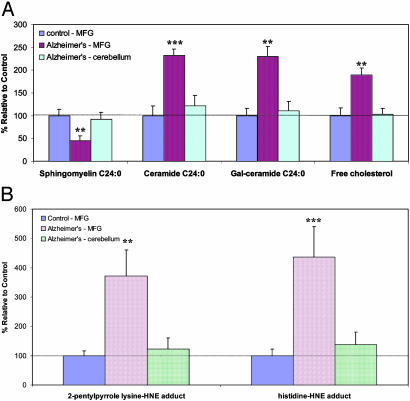
Levels of Ceramides and Cholesterol Are Increased in a Vulnerable Brain Region but Not in a Nonvulnerable Brain Region of AD Patients. We measured levels of sphingomyelins, ceramides, and cholesterol in tissue samples from two different brain regions from seven autopsy-confirmed AD patients and seven age-matched neurologically normal control subjects. The two brain regions studied were a region with extensive Aβ plaques and neurofibrillary tangles (middle frontal gyrus) and a region with only diffuse Aβ deposits and few or no neurofibrillary tangles (cerebellum). There were significant increases in the levels of ceramide 24:0 and galactosylceramide in the middle frontal gyrus, but not in the cerebellum of AD patients compared with controls (Fig. 2A). Levels of free cholesterol were also significantly increased in the middle frontal gyrus, but not in the cerebellum, of AD patients. Levels of sphingomyelin were significantly decreased in the middle frontal gyrus of AD patients compared with controls, but were not decreased in the cerebellum of AD patients (Fig. 2A). There was a highly significant increase in the amount of HNE adducts in the middle frontal gyrus, but not in the cerebellum, of AD patients compared with controls (Fig. 2B). These results indicated that specific changes in cellular cholesterol and sphingolipid metabolism are associated with membrane oxidative stress and selective vulnerability of neurons in AD.
Fig. 2.
Levels of ceramides and cholesterol increase in the brain in association with oxidative stress in AD. (A) Levels of sphingomyelin C24:0, ceramide C24:0, and free cholesterol in samples of middle frontal gyrus and cerebellum from AD- and age-matched control patients. (B) Levels of HNE adducts in samples of middle frontal gyrus and cerebellum from AD- and age-matched control patients. Values are the mean and SEM (seven control and seven AD patients). **, P < 0.01; ***, P < 0.001 compared with the value for control subjects and with the value in cerebellum of AD patients.
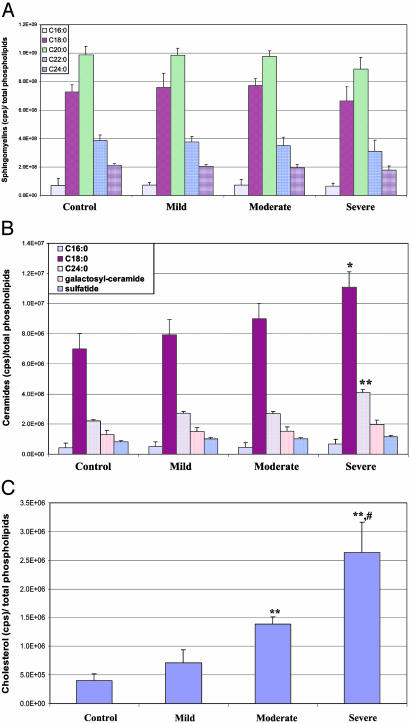
Because cholesterol and sphingolipids are enriched in membranes (24), we isolated membranes from brain tissue samples from AD patients who had died at different stages of the disease progression (mild, moderate, and severe) as defined by their cognitive functional status (see Materials and Methods) and from age-matched controls. Levels of sphingomyelins were not significantly different in samples from any of the three AD patient groups compared with controls (Fig. 3A). Levels of long-chain (C18:0 and C:24) ceramides were significantly increased in membranes from AD patients compared with controls, with the amount of the increases being positively correlated with disease severity (Fig. 3B). The concentrations of galactosylceramide and sulfatide in membranes isolated from AD patients were not significantly different from concentrations in samples from controls, although there was a trend toward increased concentrations in samples from AD patients (Fig. 3B). Levels of free cholesterol were significantly increased in membranes from AD patients, and among the AD patients, the amount of cholesterol in the membranes was greater in patients with more severe cognitive patients than in patients with mild impairment (Fig. 3C). As expected from previous studies (21), levels of HNE were significantly increased in membranes isolated from the brains of patients with mild, moderate, and severe AD, compared to membranes from controls (data not shown). Among the AD patients, levels of HNE increased in parallel with the increase in disease severity.
Fig. 3.
Levels of membrane-associated ceramides and free cholesterol are increased in association with disease severity in AD. Levels of sphingomyelins (A), ceramides and sulfatide (B), and free cholesterol (C) were quantified in isolated membrane raft preparations of frontal cortex from AD patients with mild, moderate, and severe cognitive impairment and age-matched control patients. Values are the mean and SEM. *, P < 0.05; **, P < 0.01 compared with the value for control subjects; #, P < 0.01 compared with the value for patients with moderate disease severity.
Aβ and Membrane Lipid Peroxidation Induce the Accumulation of Ceramides and Cholesterol in Neurons. Because levels of ceramides and cholesterol were increased in tissue homogenates and isolated membranes samples from vulnerable brain regions of AD patients, we tested the hypothesis that Aβ deposition was responsible for these abnormalities in membrane lipid metabolism. Previous studies (22, 25, 26) showed that cultured hippocampal neurons are vulnerable to being damaged and killed by Aβ, by a mechanism involving induction of membrane lipid peroxidation. When cultured hippocampal neurons were exposed to Aβ1–42, levels of ceramides C18:0 and C24:0, cholesterol and cholesterol esters were significantly increased in the neurons; the increases were detected within 6 h of exposure (Table 1), a time point before neuronal death which occurred after 12–24 h of exposure (data not shown). Levels of sphingomyelin 24:0 were significantly decreased in neurons exposed to Aβ1–42. The ability of Aβ1–42 to induce increased levels of ceramides and cholesterol was associated with increased levels of membrane oxidative stress, as demonstrated by increased levels of the lipid peroxidation product HNE in neurons exposed to Aβ1–42 (Table 1). Lipid peroxidation is apparently sufficient to induce the alterations in ceramide and cholesterol levels in neurons exposed to Aβ1–42, because similar increases in levels of ceramides and cholesterol occurred in neurons exposed to iron, an agent that induces hydroxyl radical production and membrane lipid peroxidation (data not shown).
Table 1. Levels of ceramides, cholesterol, and oxidative stress are increased in neurons exposed to Aβ1–42, and α-tocopherol and an inhibitor of sphingolipid synthesis attenuates these effects of Aβ1–42.
| Compound | Control | 5 μM Aβ | 100 nM ISP-1 | 100 nM ISP-1 plus 5 μM Aβ | 50 ug/ml α-tocopherol | 50 ug/ml α-tocopherol plus 5 μM Aβ |
|---|---|---|---|---|---|---|
| SM C18:0 | 182 ± 21 | 190 ± 11 | 143 ± 13 | 151 ± 20 | 176 ± 17 | 196 ± 22 |
| SM C24:0 | 69.4 ± 7.8 | 12.6 ± 2.8†† | 57.5 ± 1.2** | 66.5 ± 8.5** | 64.3 ± 4.3** | 58.2 ± 5.5** |
| Cer C18:0 | 20.0 ± 1.4 | 39.6 ± 2.1†† | 23.4 ± 4.1* | 18.7 ± 1.3** | 18.1 ± 4.7** | 22.9 ± 4.0* |
| Cer C24:0 | 0.95 ± 0.05 | 2.40 ± 0.21†† | 1.65 ± 0.20† | 0.85 ± 0.13** | 0.63 ± 0.09** | 0.90 ± 0.10** |
| Gal-cer C24:0 | 0.13 ± 0.02 | 0.20 ± 0.02† | 0.11 ± 0.01** | 0.13 ± 0.02** | 0.11 ± 0.09** | 0.15 ± 0.07* |
| Cholesterol | 50.4 ± 14.0 | 124.1 ± 13.5†† | 43.9 ± 10.0** | 71.7 ± 13.5* | 8.6 ± 4.1††** | 16.2 ± 5.5††** |
| Chol-ester C18:1 | 2.10 ± 0.31 | 3.30 ± 0.25 | 1.65 ± 0.29** | 0.90 ± 0.03††** | 2.33 ± 0.25 | 2.13 ± 0.51 |
| 4HNE | 0.19 ± 0.04 | 1.20 ± 0.28† | 0.21 ± 0.07** | 0.23 ± 0.06** | 0.03 ± 0.02††** | 0.06 ± 0.02††** |
Cultured hippocampal neurons were pretreated for 24 h with 50 ug/ml α-tocopherol or for 30 min with either 100 nM ISP-1 or vehicle, and were then exposed for 6 h to 5 uM Aβ1-42 or saline (control) and levels of sphingomyelins, ceramides, cholesterols, and HNE were quantified. The values of HNE represent the sum of free, lysine-HNE (2-pentylpyrrole), and histidine-HNE adducts. Values (cps/total phospholipids × 106) are the mean and SE of determinations made in three separate cultures. †, P < 0.05; ††, P < 0.01 compared with the control value. *, P < 0.05; **, P < 0.01 compared with the value for cultures exposed to Aβ alone. SM, sphingomyelin; Cer, ceramide.
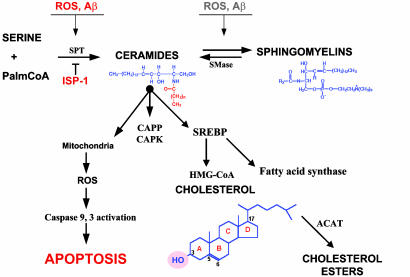
The pathways involved in sphingomyelin, ceramide, and cholesterol metabolism are shown in Fig. 4. To determine whether the increased levels of ceramides and cholesterol esters induced by Aβ and oxidative stress were the result of increased sphingolipid metabolism, we depleted hippocampal neurons of sphingomyelin by treating them with ISP-1 (an inhibitor of serine palmitoyltransferase, the rate-limiting step in sphingolipid synthesis; refs. 27 and 28). In contrast to the large increases in levels of ceramides and cholesterol esters present in control hippocampal neurons exposed to Aβ1–42, these oxidative insults caused little or no increases in levels of ceramides and cholesterol esters in hippocampal neurons pretreated with ISP-1 (Table 1).
Fig. 4.
Pathways of metabolism of sphingomyelin, ceramide, and cholesterol, their modulation by oxidative stress, and their possible roles in neuronal death in AD. The production of sphingomyelin from serine and palmitoyl CoA is catalyzed by the enzyme serine palmitoyl CoA-transferase (SPT). Ceramides are synthesized as precursors to sphingomyelin and are also generated by the hydrolysis of sphingomyelin by sphingomyelinases (SMase). Exposure of cells to reactive oxygen species and Aβ induces ceramide production. CAPK, ceramide-activated protein kinase; CAPP, ceramide-activated protein phosphatase.
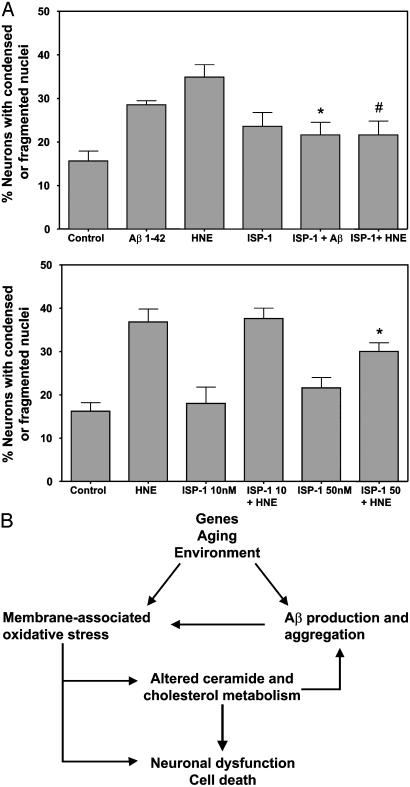
Membrane-Associated Oxidative Stress and Ceramide Production Are Required for Aβ-Induced Death of Neurons. Emerging findings suggest that many neurons may die in AD by apoptosis or a related form of programmed cell death (29). Because oxidative stress and increased production of ceramides and cholesterol have been associated with apoptosis (14, 30–32), we sought a causal role for increased sphingolipid metabolism in the death of neurons in AD. When cultured neurons were pretreated with α-tocopherol before exposure to Aβ, the amounts of HNE adducts, C:18 and C:24 ceramides and cholesterol were significantly lower than in Aβ-treated neurons that had not been pretreated with α-tocopherol (Table 1) As expected from our previous studies (17) α-tocopherol protected neurons from being killed by Aβ (data not shown). To determine whether production of ceramides played a role in the death of neurons induced by Aβ and oxidative stress, we pretreated cultured hippocampal neurons with ISP-1 to reduce levels of ceramide and then exposed the neurons to Aβ1–42 and HNE at concentrations that induce apoptosis (18). Aβ1–42 and HNE each induced the death of hippocampal neurons during a 24-h exposure period (Fig. 5A). Pretreatment of neurons with ISP-1 resulted in a significant decrease in the number of neurons killed by Aβ1–42 and HNE (Fig. 5A). When taken together with previous studies showing that ceramide can induce apoptosis of neurons (14), these findings suggest a critical and essential role for perturbed sphingolipid metabolism, and ceramide production in the neurotoxic actions of Aβ.
Fig. 5.
Inhibitors of ceramide production protect neurons against death induced by amyloid β-peptide and membrane oxidative stress. (A) Cultured hippocampal neurons were pretreated for 30 min with 10 or 50 nM ISP-1 or vehicle and were then exposed for 24 h to 5 μMAβ1–42, 1 μM HNE, or saline (control), and the numbers of neurons with apoptotic nuclei were quantified. Values are the mean and SE of determinations made in four to eight separate cultures. (B) Model for the role of altered membrane lipid metabolism in the pathogenesis of AD. The aging process, in combination with genetic and environmental factors, results in increased membrane-associated oxidative stress and increased production and accumulation of neurotoxic forms of Aβ, which itself exacerbates oxidative stress. The oxidative stress perturbs cholesterol metabolism and activates sphingomyelinases, resulting in increased ceramide production. The increased levels of ceramide and cholesterol may trigger synaptic dysfunction and neuronal death.
Discussion
The present findings document abnormalities in sphingolipid and cholesterol metabolism in the brain during normal aging, and in the brains of AD patients compared with age-matched controls. Data from studies in which neurons were exposed to Aβ suggest that this neurotoxic peptide may be responsible for the membrane lipid abnormalities in AD, and that the lipid alterations may be a pivotal event in the neurotoxic effects of Aβ. When taken together with previous findings documenting increased oxidative stress in brain aging and AD, the present findings suggest the following sequence of events in the pathogenesis of AD (Fig. 5B). Genetic and/or environmental factors, together with the aging process, result in altered proteolytic processing of the amyloid precursor protein and increased Aβ production and aggregation. Aβ induces membrane-associated oxidative stress, which alters membrane lipid metabolism, resulting in increased amounts of ceramides and cholesterols. The derangements of sphingolipid and cholesterol metabolism, in combination with oxidative stress, cause synaptic dysfunction and neuronal degeneration. The perturbed cholesterol and sphingolipid metabolism may, in turn, enhance the production of Aβ1–42 by facilitating γ-secretase cleavage of amyloid precursor protein, as suggested by recent studies (33–36). Because levels of ceramides and membrane cholesterol were increased in vulnerable brain regions but not in nonvulnerable brain regions of AD patients, and because levels of ceramides and cholesterol were increased in patients with mild to moderate symptoms, it is possible that the abnormalities in lipid metabolism occur relatively early in the course of the disease. This finding suggests an important role for altered cholesterol and sphingolipid metabolism in the early stages of the neurodegenerative process.
Our data suggest that perturbed membrane lipid metabolism in AD may result, at least in part, from increased Aβ production/deposition, and that the increased ceramide production resulting from the oxidative stress induced by Aβ may trigger the death of neurons. We found that levels of HNE were significantly increased in brain tissues of AD patients, which is consistent with previous data suggesting that oxidative stress is an early and pivotal event in the neurodegenerative process in AD (37). Oxidative stress induced by Aβ1–42 and HNE caused ceramide accumulation in hippocampal neurons, which is consistent with previous studies showing that oxidative stress increases and antioxidants decrease ceramide levels in tumor cells (38, 39). ISP-1 prevented Aβ-induced death of hippocampal neurons, suggesting pivotal roles for sphingomyelin metabolism and ceramide production in amyloid neurotoxicity. When sphingomyelin levels were reduced by treatment of cells with ISP-1, the production of ceramides in response to Aβ was decreased, which was consistent with sphingomyelin being the source of the ceramides. Ceramides may trigger apoptosis in both physiological and pathological settings. For example, it has been shown that ceramide analogs can induce caspase-3 activation and poly (ADP-ribose) polymerase cleavage (40), and overexpression of the antiapoptotic protein Bcl-2 can prevent ceramide-induced apoptosis (41). In addition, ceramide can act directly on mitochondria to induce membrane permeability transition and release of cytochrome c. Ceramides can also induce the death of cultured hippocampal (42), cortical (43), mesencephalic (15), and motor (44) neurons. Moreover, an increase in ceramide levels is sufficient to cause neurological disease in humans as exemplified by Farber's disease, a disorder resulting from ceramidase deficiency and characterized by mental retardation and motor dysfunction (45). The present findings therefore suggest a role for excessive ceramide production in neuronal death in AD.
Increasing evidence supports the involvement of perturbed cholesterol metabolism in the pathogenesis of AD. Individuals with an apolipoprotein E4 allele are at increased risk of AD, and epidemiological data suggest that individuals prescribed cholesterol-lowering drugs are at reduced risk of AD (7, 8). Oxidative stress may be an important factor that promotes the kinds of derangements of cholesterol and sphingolipid metabolism that occur in AD. Studies of non-neuronal cells have shown that oxidative stress and ceramide production can increase the accumulation of cholesterol in cells (46–49). High levels of free cholesterol can be toxic to cells as demonstrated by the ability of inhibitors of ACAT (an enzyme that converts free cholesterol to cholesterol esters) to induce apoptosis (31, 50) and by the ability of statins to protect neurons against ischemic/oxidative injury (51, 52). In addition to having direct adverse effects in neurons, the increased levels of ceramides in the brain during aging may also promote inflammatory processes (14). The involvement of perturbed sphingomyelin and cholesterol metabolism in the neurotoxic actions of Aβ, and their strong associations with the pathogenesis of AD, suggests a novel approach for therapeutic intervention downstream of Aβ. Inhibitors of serine palmitoyltransferase and sphingomyelinases may prove effective in suppressing the neurodegenerative process.
Acknowledgments
We thank the staff and patients of the Rush Alzheimer's Disease Center. This work was supported by the National Institute on Aging; a grant from the Alzheimer's Association (to M.P.M.); grants from the Rush Alzheimer's Disease Center (to J.K.); National Institutes of Health Grants P30 AG10161 and 1RO3 AG19493-01; grants from the Northwestern Memorial Foundation; the Johns Hopkins Alzheimer's Disease Center; and National Institutes of Health Grant AG05146.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: AD, Alzheimer's disease; Aβ, amyloid β-peptide; MMSE, mini-mental state examination; HNE, 4-hydroxynonenal, ISP-1, myriocin.
References
- 1.DeKosky, S. T. (2001) Clin. Cornerstone 3, 15–26. [DOI] [PubMed] [Google Scholar]
- 2.Mattson, M. P. (1997) Physiol. Rev. 77, 1081–1132. [DOI] [PubMed] [Google Scholar]
- 3.Smith, J. D. (2000) Ann. Med. 32, 118–127. [DOI] [PubMed] [Google Scholar]
- 4.Puglielli, L., Konopka, G., Pack-Chung, E., Ingano, L. A., Berezovska, O., Hyman, B. T., Chang, T. Y., Tanzi, R. E. & Kovacs, D. M. (2001) Nat. Cell Biol. 3, 905–912. [DOI] [PubMed] [Google Scholar]
- 5.Runz, H., Rietdorf, J., Tomic, I., de Bernard, M., Beyreuther, K., Pepperkok, R. & Hartmann, T. (2002) J. Neurosci. 22, 1679–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wahrle, S., Das, P., Nyborg, A. C., McLendon, C., Shoji, M., Kawarabayashi, T., Younkin, L. H., Younkin, S. G. & Golde, T. E. (2002) Neurobiol. Dis. 9, 11–23. [DOI] [PubMed] [Google Scholar]
- 7.Wolozin, B., Kellman, W., Ruosseau, P., Celesia, G. G. & Siegel, G. (2000) Arch. Neurol. 57, 1439–1443. [DOI] [PubMed] [Google Scholar]
- 8.Fassbender, K., Simons, M., Bergmann, C., Stroick, M., Lutjohann, D., Keller, P., Runz, H., Kuhl, S., Bertsch, T., von Bergmann, K., et al. (2001) Proc. Natl. Acad. Sci. USA 98, 5856–5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsui-Pierchala, B. A., Encinas, M., Milbrandt, J. & Johnson, E. M. (2002) Trends Neurosci. 25, 412–447. [DOI] [PubMed] [Google Scholar]
- 10.Toman, R. E., Spiegel, S. & Faden, A. I. (2000) J. Neurotrauma 17, 891–898. [DOI] [PubMed] [Google Scholar]
- 11.Kronke, M. (1999) Chem. Phys. Lipids 102, 157–166. [DOI] [PubMed] [Google Scholar]
- 12.Billis, W., Fuks, Z. & Kolesnick, R. (1998) Recent Prog. Horm. Res. 53, 85–92. [PubMed] [Google Scholar]
- 13.Alessenko, A. V. (2000) Membr. Cell Biol. 13, 303–320. [PubMed] [Google Scholar]
- 14.Yu, Z., Nikolova-Karakashian, M., Zhou, D., Cheng, G., Schuchman, E. H. & Mattson, M. P. (2000) J. Mol. Neurosci. 15, 85–98. [DOI] [PubMed] [Google Scholar]
- 15.Brugg, B., Michel, P. P., Agid, Y. & Ruberg, M. (1996) J. Neurochem. 66, 733–739. [DOI] [PubMed] [Google Scholar]
- 16.Cutler, R. G., Pedersen, W. A., Camandola, S., Rothstein, J. D. & Mattson, M. P. (2002) Ann. Neurol. 52, 448–457. [DOI] [PubMed] [Google Scholar]
- 17.Mark, R. J., Hensley, K., Butterfield, D. A. & Mattson, M. P. (1995) J. Neurosci. 15, 6239–6249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kruman, I., Bruce-Keller, A. J., Bredesen, D. E., Waeg, G. & Mattson, M. P. (1997) J. Neurosci. 17, 5089–5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Floyd, R. A. & Hensley, K. (2002) Neurobiol. Aging 23, 795–807. [DOI] [PubMed] [Google Scholar]
- 20.Ghosh, C., Dick, R. M. & Ali, S. F. (1993) Neurochem. Int. 23, 479–484. [DOI] [PubMed] [Google Scholar]
- 21.Lovell, M. A., Ehmann, W. D., Mattson, M. P. & Markesbery, W. R. (1997) Neurobiol. Aging 18, 457–461. [DOI] [PubMed] [Google Scholar]
- 22.Mattson, M. P. (1998) Trends Neurosci. 21, 53–57. [DOI] [PubMed] [Google Scholar]
- 23.Papaioannou, N., Tooten, P. C., van Ederen, A. M., Bohl, J. R., Rofina, J., Tsangaris, T. & Gruys, E. (2001) Amyloid 8, 11–21. [DOI] [PubMed] [Google Scholar]
- 24.Brown, D. A. & London, E. (1989) Annu. Rev. Cell Dev. Biol. 14, 111–136. [DOI] [PubMed] [Google Scholar]
- 25.Mark, R. J., Pang, Z., Geddes, J. W., Uchida, K. & Mattson, M. P. (1997) J. Neurosci. 17, 1046–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pedersen, W. A., Chan, S. & Mattson, M. P. (2000) J. Neurochem. 74, 1426–1433. [DOI] [PubMed] [Google Scholar]
- 27.Miyake, Y., Kozutsumi, Y., Nakamura, S., Fujita, T. & Kawasaki, T. (1995) Biochem. Biophys. Res. Commun. 211, 396–403. [DOI] [PubMed] [Google Scholar]
- 28.Hanada, K., Nishijima, M., Fujita, T. & Kobayashi, S. (2000) Biochem. Pharmacol. 59, 1211–1216. [DOI] [PubMed] [Google Scholar]
- 29.Mattson, M. P. (2000) Nat. Rev. Mol. Cell Biol. 1, 120–129. [DOI] [PubMed] [Google Scholar]
- 30.Hartfield, P. J., Mayne, G. C. & Murray, A. W. (1997) FEBS Lett. 401, 148–152. [DOI] [PubMed] [Google Scholar]
- 31.Kellner-Weibel, G., Geng, Y. J. & Rothblat, G. H. (1999) Atherosclerosis (Shannon, Irel.) 146, 309–319. [DOI] [PubMed] [Google Scholar]
- 32.Genestier, L., Prigent, A. F., Paillot, R., Ouemeneur, L., Durand, I., Banchereau, J., Rvillard, J. P. & Bonnefoy-Berard, N. (1998) J. Biol. Chem. 273, 5060–5066. [DOI] [PubMed] [Google Scholar]
- 33.Arispe, N. & Doh, M. (2002) FASEB J. 16, 1526–1536. [DOI] [PubMed] [Google Scholar]
- 34.Puglielli, L., Tanzi, R. E. & Kovacs, D. M. (2003) Nat. Neurosci. 6, 345–351. [DOI] [PubMed] [Google Scholar]
- 35.Zhang, D., Ruan, Y. & Beyreuther, K. (1998) Zhonghua Yi Xue Za Zhi. 78, 179–182. [PubMed] [Google Scholar]
- 36.Ehehalt, R., Keller, P., Haass, C., Thiele, C. & Simons, K. (2003) J. Cell Biol. 160, 113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Markesbery, W. R. & Carney, J. M. (1999) Brain Pathol. 9, 133–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dbaibo, G. S., Pushkareva, M. Y., Rachid, R. A., Alter, N., Smyth, M. J., Obeid, L. M. & Hannun, Y. A. (1998) J. Clin. Invest. 102, 329–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoshimura, S., Banno, Y., Nakashima, S., Hayashi, K., Yamakawa, H., Sawada, M., Sakai, N. & Nozawa, Y. (1999) J. Neurochem. 73, 675–683. [DOI] [PubMed] [Google Scholar]
- 40.Smyth, M. J., Perry, D. K., Zhang, J., Poirier, G. G., Hannun, Y. A. & Obeid, L. M. (1996) Biochem. J. 316, 25–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dbaibo, G. S., Perry, D. K., Gamard, C. J., Platt, R., Poirier, G. G., Obeid, L. M. & Hannun, Y. A. (1997) J. Exp. Med. 185, 481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goodman, Y. & Mattson, M. P. (1996) J. Neurochem. 6, 869–872. [DOI] [PubMed] [Google Scholar]
- 43.Willaime, S., Vanhoutte, P., Caboche, J., Lemaigre-Dubreuil, Y., Mariani, J. & Brugg, B. (2001) Eur. J. Neurosci. 13, 2037–2046. [DOI] [PubMed] [Google Scholar]
- 44.Irie, F. & Hirabayashi, Y. (1998) J. Neurosci. Res. 54, 475–485. [DOI] [PubMed] [Google Scholar]
- 45.Bar, J., Linke, T., Ferlinz, K., Neumann, U., Schuchman, E. H. & Sandhoff, K. (2001) Hum. Mutat. 17, 199–209. [DOI] [PubMed] [Google Scholar]
- 46.Stein, O., Oette, K., Dabach, Y., Hollander, G., Ben Naim, M. & Stein, Y. (1992) Biochim. Biophys. Acta 1165, 153–159. [DOI] [PubMed] [Google Scholar]
- 47.Suzukawa, M., Abbey, M., Clifton, P. & Nestel, P. J. (1994) Atherosclerosis (Shannon, Irel.) 110, 77–86. [DOI] [PubMed] [Google Scholar]
- 48.Gesquiere, L., Loreau, N., Minnich, A., Davignon, J. & Blache, D. (1999) Free Radical Biol. Med. 27, 134–145. [DOI] [PubMed] [Google Scholar]
- 49.Zager, R. A. & Kalhorn, T. F. (2000) Am. J. Pathol. 157, 1007–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kellner-Weibel, G., Jerome, W. G., Small, D. M., Warner, G. J., Stoltenborg, J. K., Kearney, M. A., Corjay, M. H., Phillips, M. C. & Rothblat, G. H. Arterioscler. Thromb. Vasc. Biol. 18, 423–431. [DOI] [PubMed]
- 51.Honjo, M., Tanihara, H., Nishijima, K., Kiryu, J., Honda, Y., Yue, B. Y. & Sawamura, T. (2002) Arch. Ophthalmol. 120, 1707–1713. [DOI] [PubMed] [Google Scholar]
- 52.Buxbaum, J. D., Geoghagen, N. S. & Friedhoff, L. T. (2001) J. Alzheimers Dis. 3, 221–229. [DOI] [PubMed] [Google Scholar]