Abstract
Background
A large proportion of patients receiving antiretroviral therapy (ART) in low and middle income countries (LMICs) have unknown treatment outcomes and are classified as lost to follow-up (LTFU). Physical tracing of patients classified as LTFU is common; however, effects of tracing on outcomes remains unclear. The objective of this systematic review is to compare estimates of LTFU, mortality and retention in LMIC in cohorts of patients with and without physical tracing.
Methods and Findings
We systematically identified studies in LMIC programmatic settings using MEDLINE (2003–2011) and HIV conference abstracts (2009–2011). Studies reporting the proportion LTFU 12-months after ART initiation were included. Tracing activities were determined from manuscripts or by contacting study authors. Studies were classified as “tracing studies” if physical tracing was available for the majority of patients. Summary estimates from the 2 groups of studies (tracing and non-tracing) for LTFU, mortality, stop of ART, transfers out, and retention on ART were determined. 261 papers and 616 abstracts were identified of which 39 studies comprising 54 separate cohorts (n = 187,666) met inclusion criteria. Of those, physical tracing was available for 46% of cohorts. Treatment programs with physical tracing activities had lower estimated LTFU (7.6% vs. 15.1%; p<.001), higher estimated mortality (10.5% vs. 6.6%; p = .006), higher retention on ART (80.0 vs. 75.8%; p = .04) and higher retention at the original site (80.0% vs. 72.9%; p = .02).
Conclusions
Knowledge of patient tracing is critical when interpreting program outcomes of LTFU, mortality and retention. The reduction of the proportion LTFU in tracing studies was only partially explained by re-classification of unknown outcomes. These data suggest that tracing may lead to increased re-engagement of patients in care, rather than just improved classification of unknown outcomes.
Introduction
In response to the global HIV epidemic, a public health approach to antiretroviral therapy (ART) has been widely implemented in low- and middle-income countries (LMICs). In 2010, 6.6 million adults and children received ART, representing a 22-fold increase from 2001 [1]. The rapid scale-up of ART is an impressive public health achievement that has led to dramatic declines in HIV related morbidity and mortality [1]–[4].
Frequently reported outcomes for populations receiving ART include the number of: patients alive and on ART, deaths, patients transferring care from one facility to another (‘transfer out’), patients stopping ART (either physician directed or patient initiated) but remaining in care, and patients lost to follow-up (LTFU). [5]–[8] LTFU is a generic term referring to patients who initiate ART but who have unknown treatment outcomes. These unknown treatment outcomes may be divided into 3 general categories: unreported deaths, unknown transfer of care to a different facility without documentation, and disengagement from care [9].
Patient tracing is a commonly used method to improve retention in care and reduce unknown outcomes. Typically in LMICs, tracing involves contacting patients by telephone (telephone tracing), physically visiting their place of residence (physical tracing), or a combination of both. Tracing patients has two potential benefits: 1) linking patients who are disengaged from care back into the health care system, and 2) improved classification of unknown outcomes. By minimizing the number of individuals who disengage from care, programs optimize care by maintaining the greatest possible number of patients on ART, thus decreasing mortality [9] and complications of immunodeficiency. Additionally, patients who have disengaged from care are at increased risk of transmitting HIV due to uncontrolled viremia [10] and for the selection of drug resistance by virtue of ART treatment interruptions [11], [12]. Maximizing the number of patients alive and receiving ART and minimizing the number of patients with unknown outcomes should become an increasingly important public health priority [1], [13], [14].
Program managers are frequently required to report estimates of LTFU, mortality, and retention to ministries of health, funders, and international organizations [5]–[8]. Furthermore, clinicians, program managers and researchers routinely report on LTFU, mortality and retention to quantify the extent of this issue in LMICs [15]–[17]. Patient tracing may result in the improved classification of unknown outcomes allowing for more accurate estimates of LTFU, mortality, and retention. However, the extent to which patient tracing impacts estimates of LTFU, mortality and retention in LMIC remains uncertain. To the authors’ knowledge the only review that stratifies any of these outcomes by tracing status was a mortality estimate from the Antiretroviral Therapy in Lower Income Countries (ART-LINC) Collaboration [18]. All other identified reviews[14], [19]–[21] have synthesized data from multiple studies without incorporating the potential for patient tracing activities to affect estimates of LTFU, mortality or retention.
The proportion of individuals LTFU one year after the initiation of ART has been reported as high as 25–50% in LMICs [22]–[26]. Reasons for LTFU are multi-factorial and include both program and patient factors. Reported predictors of LTFU include evidence of poor nutrition, low CD4 count at diagnosis, the number of doctors available to treat patients, the ability to contact the patient by telephone and decreased levels of community support. [27]–[29] Additional factors such as patient refusal to take ART, adverse events or toxicity related to medication or alternative priorities may also lead to disengagement from ART programs. Furthermore, poor data recording and reporting and information systems that do not permit communication between ART clinics may contribute to high levels of reported LTFU. A lack of communication between record keeping systems may be particularly relevant in settings where different systems are used or unique national ART patient identifiers are not available leading to an inability to identify patients who have transferred out or died. Additionally, in many LMICs deaths go unreported to national death registries, if they exist, and ART programs lack a consistent link between death registries and reporting of population level ART outcomes.
The objective of this systematic review is to compare summary estimates of LTFU, mortality and retention in LMIC, in cohorts of patients with and without physical tracing. In settings with tracing, we hypothesized that summary estimates of LTFU would decrease and estimates of mortality and retention would increase.
Methods
The strategy to identify appropriate studies, abstract data from selected studies and an analytic plan was established in a systematic review protocol.
Search Strategy
All searches were performed using Ovid MEDLINE. Searches were limited to studies published in English from January 2003 through May 2011. Studies assessing outcomes in children (<13 years old) were excluded. The search strategy started by combining all sets of terms under the following Medical Subject Headings (MeSH) to identify HIV infected participants receiving ART: “HIV” or “HIV Infections” or “Antiretroviral Therapy, Highly Active” or “Anti-Retroviral Agents”. Then to identify studies from LMICs we combined all sets of terms under the following MeSH: “Africa” or “Asia” or “Caribbean region” or “Central America” or “Latin America” or “South America”, in addition to the following terms: “resource limited” or “resource constrained” or “developing countries” or “low income countries” or “low and middle income countries” or “Africa” or “Afrika” or "sub Saharan" or “southern Africa” or “Asia” or “Latin America” or “South America”. Terms specific to Eastern Europe were not included. The next step combined different combinations of “lost (or loss) to follow up”, with the terms: “attrition” or “retention”, and all terms under the MeSH “patient dropouts”. Finally, items obtained from the searches for: HIV infected participants, LMICs and Loss to follow-up were combined. The exact search strategy is available within the systematic review protocol provided as a supporting document to this manuscript.
The online conference abstract databases for the 2009 International AIDS Society Conference on HIV Pathogenesis, Treatment and Prevention, the 2010 International AIDS Conference, and the 2009–2011 Conference on Retroviruses and Opportunistic Infections were searched for the terms “lost (or loss) to follow up” and “retention”. These more recent years were chosen to capture additional data reported in abstract form that may not have been published in peer reviewed journals. Reference lists from recent reviews assessing patient retention in ART programs in LMICs were also searched [14], [19].
Study Selection
Original research studies or abstracts reporting on outcomes of HIV infected patients receiving ART in LMICs were included. Studies were included if they were specifically designed to report on LTFU or in cases where it was a secondary finding. Study designs were either cross-sectional or cohort and either prospective or retrospective. All studies included in the analyses reported rates of LTFU for cohorts of individuals who had received care for 12 months after ART initiation and any definition of LTFU was accepted. If cohort studies only reported a median duration of follow up, they were included only if the duration of follow up ranged from 9 to 15 months. When more than one study reported on the same cohort of patients, only the publication containing the most detailed information was included.
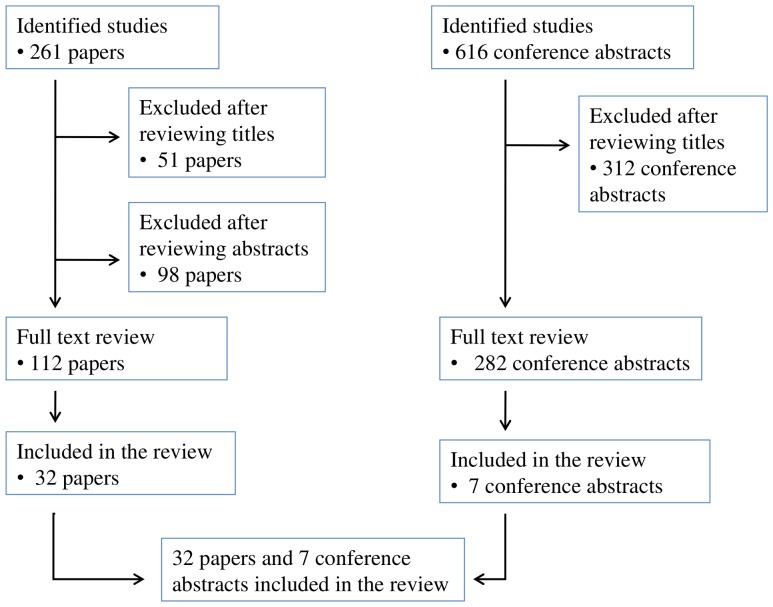
Studies in which the majority of patients were children, patients received mono- or dual-therapy, or that were not performed in LMICs were excluded. Additionally, clinical trials were excluded as the focus of this review was to understand LTFU in service delivery settings. Studies were excluded at one of three steps: after review of the title, the abstract, or the manuscript. The search strategy and study selection is summarized in Figure 1.
Figure 1. Search strategy and study selection.
Data Abstraction and Management
The following data were abstracted from each study: first author, year of publication, country or countries, healthcare setting (public, private, non-governmental organization), need to pay for ART, dates observed, number of clinics, number of patients receiving ART, baseline demographics (age, gender, CD4 count, clinical stage), ART regimen, ART naive prior to ART initiation, study definition of LTFU and the proportion of patients meeting that definition 12 months after initiation of therapy. If reported, the proportion of subjects who died, transferred care to a different facility, or who stopped ART was abstracted. In addition, details of patient tracing were abstracted, and to minimise reporting bias across selected studies, authors not reporting on patient tracing activities were contacted to establish details about tracing. To provide consistency across all studies the denominator of LTFU estimates included all patients who initiated ART.
Data Analysis
Proportions of patients classified as LTFU, died, stopped ART and transferred to a different facility were derived from text, tables and graphs (if exact values were available) within studies. Data presented as incidence density (e.g. person years) were converted to cumulative incidence using standard formulae [30]. Patient ‘retention on ART’ was defined as patients alive and receiving ART at the original site plus the group of patients who have ‘transferred out’. This assumes that patients who are known to have transferred their care to another site providing ART are retained in care. The proportion retained on ART was determined for studies that reported at least the proportion LTFU and proportion died using the following formula: Retained on ART = 1–LTFU - died - stopped ART. Additionally, the term ‘retention at the original site’ defines individuals retained on ART and excludes those who have transferred out. Studies also reporting the proportion transferred out were used to estimate the proportion retained at the original site using the formula: Retained at the original site = 1–LTFU–died–stopped ART–transfer out. For the purpose of this review, if transfer out data were not available for a cohort, the estimates of retained on ART and retained at the original site would be the same, an approach consistent with previous reviews focusing on retention in ART treatment programs [14], [19]. Summary estimates for tracing and non-tracing studies are reported as medians if the group of estimates was non-normally distributed or as weighted means if data were normally distributed. Weighting of each proportion derived from included studies was by the inverse of its variance [1 / (p x (1-p) / n), where p is the proportion and n is the sample size]. Tracing was deemed to have occurred if the activity involved physical tracing of the patient with unknown outcome to her or his residence and if this tracing activity was performed for at least one half of the study population. Non-physical tracing studies may have reported no tracing activities or phone tracing only. When choosing a method to differentiate tracing from non-tracing studies we elected to compare physical versus non-physical tracing studies due to the potential for the face-to-face interaction associated with attending a place of residence to increase the chances of re-engagement into care.
Summary estimates from the 2 groups of studies (tracing and non-tracing) were compared by the Student’s t-test if normally distributed, or the Wilcoxon rank sum test if non-normally distributed for each parameters of interest (LTFU, death, stop of ART, transfer to another facility and retention on ART). A Shapiro-Wilk test p value > 0.05 was used to classify estimates from the tracing and non-tracing groups of studies as normally distributed. No assessment of risk of bias was performed for selected studies. Analyses were conducted using Excel and SAS v9.2 (SAS Institute, Cary, NC).
Results
A total of 261 papers and 616 conference abstracts were identified by the search strategy and of these 39 studies, 32 papers and 7 conference abstracts, met inclusion criteria[4], [16], [17], [22]–[24], [27], [31]–[62] leading to 54 separate cohorts (47 cohorts in 32 papers, and 7 cohorts in 7 abstracts) available for analysis. In 3 papers, data were available for more than one cohort [17], [56], [57] with 2 of these papers reporting on cohorts with and without physical tracing, [17], [57] while the third paper provided data for 2 cohorts that both performed physical tracing [56]. The 39 included studies reported on 17 countries from sub-Saharan Africa, four multi-country studies from sub-Saharan Africa, three countries from Asia and one from Latin America. Published studies contained information to establish tracing status for 18 of the 54 cohorts included in this review. For the remaining 36 cohorts, tracing status was established by contact with study authors. Table 1 presents data on cohorts with physical tracing and Table 2 on cohorts without physical tracing.
Table 1. Cohorts with physical tracing.*.
| Study (Year) | Country (Cohort or data source) | Type of care | Free ART | Dates observed | Sites (n) | Start ART (n) | Baseline features (Median age, % male, median CD4, WHO clinicalstage) | ART regimens& | Time since ART start | Study definition LTFU | LTFU (%) | Died (%) | ART stop (%) | TF out (%) |
| May et al [56] (2010) | Cote d’Ivoire (Abidjan - CEPREF) | NGO | Yes | Initiated Jan 04–Mar 07 | Many | 2117 | 35, 26%, 129, 82% advanced | NR | Mean 11 months | Not attend clinic for > 6 months | 11.5 | 8.7 | NR | NR |
| May et al [56] (2010) | Malawi (Lilongwe) | Public | Yes | Initiated Jan 04–Mar 07 | 1 | 3028 | 36, 41%, 127, 96% advanced | NR | Mean 10 months | Not attend clinic for > 6 months | 12.4 | 8.9 | NR | 14 |
| Thai et al [46] (2009) | Cambodia (Phnom Penh) | Private (non profit) | Yes | Mar 03–Dec 07 | 1 | 1667 | 35, 49.4%, 61, Stage III 39% Stage IV 46% | 100% NNRTI | 12 months | Not attended clinic for 6 consecutive months | 3.9 | 7.6 | NR | NR |
| Bedelu et al [57] (2007) | South Africa (Lusikisiki) | Public, NGO | Yes | Initiated Jan–Jun 05. F/U to Jul 06 | 12 | 595 | NR | NR | Median 12 months | NR | 2.2 | 16.8 | NR | NR |
| Etard et al [40] (2006) | Senegal | Public | Partial | Initiated Aug 98–Apr 02. F/U to Sep 05 | ≥ 3 | 404 | 37, 45%, 128, CDC Stage B 39%, CDC Stage C 55% | 42% PI 95% ART naïve | 12 months | 6 months with no contact, or 6 months with no ART if contacted | 1.7 | 11.6 | NR | NR |
| Ferradini et al [41] (2006) | Malawi (Chiradzulu) | Public, NGO | Yes | 01–April 04 | 1 | 1308 | 35, 36%, 112, Stage III 55% Stage IV 27% | 98% NNRTI 97% ART naïve | 12 months | Not attended clinic > 2 months after last scheduled visit | 5 | 19 | NR | NR |
| Marston et al [43] (2007) | Kenya (Kibera) | Public, NGO | Yes | Feb 03–Feb 05 | 1 | 283 | Mean 36, 30%, 157, Stage III 23% Stage IV 48% | 99% NNRTI 1% PI | 12 months | No clinic visit in > 3 months | 13.0 | 7.0 | NR | NR |
| Coetzee et al [4] (2004) | Sth Africa (Khayelitsha) | Public, NGO | Yes | Initiated May 01–Dec 02, Censored July 03 | 3 | 287 | 31, 30%, 43, Stage III/IV 100% | 99% NNRTI | Median 14 months | Not attended services (clinic or other services) for ≥ 3 months after last scheduled appointment | 0.3 | 13.2 | 3.1 | 1.0 |
| Palombi et al [27] (2009) | Mozambique, Malawi. Guinea-Conakry | Public, NGO | Yes | Initiated Feb 02–Jan 06. F/U to Jun 07 | 5 | 3749 | 34, 38%, 192, Stage III/ IV 37% | 97% NNRTI 3% 3NRTI | Median 15 months | Not attending clinic for > 3 months | 2.8 | 10.5 | NR | NR |
| DeSilva et al [39] (2009) | Nigeria (Jos) | NGO | Yes | Initiated Dec 04–Apr 06. F/U to Dec 06 | 1 | 1552 | 34, 29%, 112, NR | 99% NNRTI 1% PI | Mean 15 months | No clinic records for > 3 months | 8.8 | 6.7 | NR | NR |
| Barth et al [31] (2008) | Sth Africa (Ndlovu) | NGO | Yes | Initiated Sept 03–Apr 06 | 1 | 609 | 35, 29%, 67, Stage III 62% Stage IV 17% | 100% NNRTI | 12 months | NR | 15.0 | 19.0 | NR | NR |
| Moore et al [44] (2010) | Malawi (Blantyre) | Public | Yes | Initiated 05 | 1 | 300 | Mean 36, 39%, Mean 157, Stage IV 29% | 100% NNRTI | 12 months | Failure to attend clinic ≥ 4 weeks after last scheduled appointment | 2.7 | 14.3 | 5.3 | 5.3 |
| Mutevedzi et al [45] (2010) | Sth Africa(Kwa-Zulu Natal) | Public | Yes | Initiated Oct 04–Sept 07 | 16 | 3010 | 34–37, 22%, 91–128,NR | 100% NNRTI | 12 months | No clinic visit for ≥ 90 days | 3.7 | 10.9 | NR | 1.4 |
| Tassie et al [17] (2010) | Kenya (Busia) | NGO | Yes | Initiated Jan–Dec 05 | 1 | 860 | NR | NR | 12 months | No recorded visit for ≥ 90 days from last visit | 8.5 | 6.4 | 3.0 | NR |
| Tassie et al [17] (2010) | Kenya (Hornabay) | NGO | Yes | Initiated Jan–Dec 05 | 1 | 954 | NR | NR | 12 months | No recorded visit for ≥ 90 days from last visit | 10.9 | 10.6 | 1.6 | NR |
| Tassie et al [17] (2010) | Kenya (Kibera) | NGO | Yes | Initiated Jan–Dec 05 | 1 | 435 | NR | NR | 12 months | No recorded visit for ≥ 90 days from last visit | 11.3 | 5.3 | 5.7 | NR |
| Tassie et al [17] (2010) | Kenya (Mathare) | NGO | Yes | Initiated Jan–Dec 05 | 1 | 549 | NR | NR | 12 months | No recorded visit for ≥ 90 days from last visit | 13.5 | 4.2 | 5.8 | NR |
| Tassie et al [17] (2010) | Malawi (Thyolo) | NGO | Yes | Initiated Jan–Dec 05 | 1 | 1359 | NR | NR | 12 months | No recorded visit for ≥ 90 days from last visit | 8.9 | 12.7 | 2.4 | NR |
| Tassie et al [17] (2010) | Nigeria (Lagos) | NGO | Yes | Initiated Jan–Dec 05 | 1 | 713 | NR | NR | 12 months | No recorded visit for ≥ 90 days from last visit | 9.7 | 5.7 | 1.7 | NR |
| Tassie et al [17] (2010) | Zambia (Kapiri Kawama) | NGO | Yes | Initiated Jan–Dec 05 | 1 | 559 | NR | NR | 12 months | No recorded visit for ≥ 90 days from last visit | 3.4 | 12.0 | 0.9 | NR |
| Tassie et al [17] (2010) | Zimbabwe (Bulawayo) | NGO | Yes | Initiated Jan–Dec 05 | 1 | 222 | NR | NR | 12 months | No recorded visit for ≥ 90 days from last visit | 13.5 | 11.5 | 0.5 | NR |
| Tassie et al [17] (2010) | Zimbabwe (Connaught) | Public | Yes | Initiated Jan–Dec 05 | 1 | 378 | NR | NR | 12 months | No recorded visit for ≥ 90 days from last visit | 4.0 | 6.3 | 1.6 | NR |
| Culbert et al [38] (2007) | Congo (Bukavu) | NGO | Yes | Initiated May 02–Jan 06 | 1 | 494 | 37, 34%, 123, Stage III 49% Stage IV 34% | 100% NNRTI | 12 months | NR | 5.4 | 7.9 | NR | NR |
| Johannessen et al [60] (2008) | Tanzania (Manyara) | NGO | Yes | Initiated Oct 03–Nov 06 | 1 | 320 | 35, 30%, NR, Stage III 31% Stage IV 66% | 100% NNRTI | Mean 11 months | Missed appointments for ≥ 3 months | 9.7 | 29.7 | 2.2 | 10.9 |
| Chi et al [35] (2009) | Zambia (Lusaka) | Public | Yes | Initiated Apr 04–Sept 07 | 18 | 37039 | 35, 39%, 110–132, Stage III 59% Stage IV 10% | 100% NNRTI | 12 months | NR | 13.8 | 9.9 | 3.1 | NR |
Defined as physical tracing to the patients place of residence and was available to at least half the study population.
All ART naïve at baseline unless stated Notes: ART, antiretroviral therapy; WHO, World Health Organization; LTFU, lost to follow up; TF, transfer; NR, not reported; F/U, follow up; NGO, non-governmental organization; NNRTI. Non-nucleoside reverse transcriptase inhibitors; PI, protease inhibitors; NRTI, nucleoside reverse transcriptase inhibitors;
Table 2. Cohorts without physical tracing.*.
| Study (Year) | Country (Cohort or data source) | Type of care | Free ART | Dates observed | Sites (n) | Start ART (n) | Baseline features (Median age, % male, median CD4, WHO clinicalstage) | ART regimens& | Time since ART start | Study definition LTFU | LTFU (%) | Died (%) | ART stop (%) | TF out (%) |
| Geng et al [16] (2010) | Uganda (Mbarara) | Public | Yes | Initiated Jan 04–Sept 07 | 1 | 3628 | 35,39%, 95, NR | NR | 12 months | Not attended clinic for > 6 months | 16 | 1.7 | NR | NR |
| Bisson et al [33] (2008) | Botswana (Gaborone - IDCC) | Public | Yes | Initiated Feb 03–August 03 | 1 | 410 | 37, 40%, 81, NR | 97% NNRTI 12% prior ARVs | Median 10 months | Last contact with clinic or pharmacy > 30 days after last scheduled visit | 16.6 | 7.1 | NR | NR |
| Bedelu et al [57] (2007) | South Africa (Lusikisiki) | Public, NGO | Yes | Initiated Jan 05–Jun 05. F/U to Jul 06 | 1 | 430 | NR | NR | Median 12 months | NR | 19.3 | 13.5 | NR | 4.0 |
| Wools-Kaloustian et al [24] (2006) | Kenya (Western) | Public | Some paid | Nov 01–Feb 05 | 8 | 2059 | 37, 40%, 86, Stage III 38% Stage IV 17% | 95% NNRTI | Median 9 months | Not attended clinic > 3 months | 24.5 | 5.4 | NR | NR |
| Wester et al [48] (2005) | Botswana (Gaborone - IDCC) | Public | Yes | Initiated Apr01–Jan 02. F/U to Nov 03 | 1 | 153 | 36, 41%, 96, Stage III 30% Stage IV 47% | 100% NNRTI | 12 months | Miss 2 consecutive visits and then not contactable on 2 phone attempts | 8.4 | 15.3 | NR | 5.2 |
| Charalambous et al [34] (2007) | Sth. Africa | Private (Work) | Yes | Oct 02–Dec 05 | 69 | 2262 | 41, 95%, 158, Stage III 45% Stage IV 27% | “NNRTI” | 12 months | "Stopped treatment" = patient request, LTFU or for ART non-adherence | 8.3 | 4.2 | See LTFU | 5.5 |
| Bisson et al [32] (2006) | Botswana (Gaborone) | Private | Yes | Initiated Dec 99–Jan 04 | 1 | 346 | 37, 42%, 80–113, NR | NR | 12 months | No viralload tests after ART start, then not contactable by phone and not picking up ART | 12.4 | 5.2 | NR | 12.1 |
| Laurent et al [23] (2005) | Cameroon (Douala) | Public/Private | Yes | Oct 00–Dec 03 | 19 | 788 | 39, 48%, 123, CDC stage B 57% CDC stage C 33% | NR 86% ART naïve | Median 13 months | Did not attend in 3 months prior to chart review | 25.1 | 6.6 | NR | NR |
| Karcher et al [42] (2007) | Kenya (Migori) | Public | Yes | Apr 04–Sept 05 | 1 | 124 | 31, 29%, 189, CDC Stage C 46% | “NNRTI” | Median 9 months | Not attended within 4 months after last scheduled appointment | 15.3 | 12.1 | NR | NR |
| Hawkins et al [22] (2007) | Kenya (Nairobi –Saint Mary’s) | NGO | Yes | Initiated Sep 04–Aug 06 | 1 | 1286 | 36, 40.9%, 121 | 99% NNRTI | Median 12 months | Missed clinic visits and failure to collect ART refills for ≥ 3 months | 34.8 | 1.1 | NR | 4.9 |
| Chung et al [36] (2010) | Kenya (Nairobi - Coptic Center) | NGO | Yes | Initiated Mar 06–Dec 07 | 1 | 1231 | NR | NR | 12 months | Not clinic visit > 30 days after last scheduled pick-up or no clinic visit in 120 days if no pharmacy data | 10.0 | NR | NR | NR |
| Toure et al [47] (2008) | Cote d’Ivoire (Abidjan- not CEPREF]) | Public/Private | Partial | Initiated May 04–Feb 07 | 18 | 8094# | 36, 30%, 123, Stage III 69% Stage IV 12% | 94% NNRTI | 12 month | Last contact with care center > 3 months and not known to be dead or TF out | 18 | NR | NR | NR |
| Collini et al [37] (2009) | Ghana (Kumasi) | Public | Partial | Jan 04–Jan 07 | 1 | 237 | Mean 40, 41%, Mean 120, Stage III/IV 78% | “NNRTI” | 12 months | NR | 20.3 | NR | NR | NR |
| Assefa et al [61] (2010) | Ethiopia | Public | Yes | Initiated Sept 03–Oct 07 | 353 | 60476 | NR | 100% NNRTI | 12 months | Not on ART and not known to have died at 12 months | 18.4 | 8.6 | NR | NR |
| Hong et al [58] (2010) | Namibia | Public | Yes | Initiated after Jan 07 | 9 | 1620 | NR | 100% NNRTI | 12 months | Not returned to pharmacy or clinic < 90 days after ART run-out date and have not TF out, stopped, died | 17.5 | NR | NR | NR |
| Sharma et al [62] (2010) | India (Delhi - AIIMS) | Public | Yes | Initiated May 05–Oct 06 | 1 | 631 | Mean 36, 80%, 110, Stage III 51% Stage IV 31% | 100% NNRTI | 12 months | NR | 18.5 | 13.0 | NR | NR |
| Tassie et al [17] (2010) | Cambodia (Kampong Cham) | NGO | Yes | Initiated Jan–Dec 05 | 1 | 606 | NR | NR | 12 months | No recorded visit for ≥ 90 days from last visit | 4.4 | 9.6 | 4.8 | NR |
| Tassie et al [17] (2010) | Cambodia (Phnom Penh) | NGO | Yes | Initiated Jan–Dec 05 | 1 | 610 | NR | NR | 12 months | No recorded visit for ≥ 90 days from last visit | 0.8 | 3.3 | 8.5 | NR |
| Tassie et al [17] (2010) | Uganda (Arua) | NGO | Yes | Initiated Jan–Dec 05 | 1 | 1137 | NR | NR | 12 months | No recorded visit for ≥ 90 days from last visit | 13.4 | 3.9 | 2.9 | NR |
| Tassie et al [17] (2010) | India (YRG) | Public/Private | Yes | Initiated Jan–Dec 05 | 1 | 767 | NR | NR | 12 months | No recorded visit for ≥ 90 days from last visit | 31.9 | 3.6 | 6.0 | NR |
| Tassie et al [17] (2010) | Kenya (AMPATH) | Public | Yes | Initiated Jan–Dec 05 | 1 | 4111 | NR | NR | 12 months | No recorded visit for ≥ 90 days from last visit | 15.0 | 6.7 | 3.8 | NR |
| O’Brien et al [59] (2009) | Congo (Pool) | NGO | Yes | During 07 | 2 | 236 | 37, 31%, 104, Stage III 53%Stage IV 44% | “NNRTI and PI” | Mean 9 months | NR | 8.5 | 12.3 | NR | NR |
| Chinh et al [52] CROI (2010) | Vietnam (Ho Chi Minh City) | Public | Yes | Initiated Sep 05–Dec 07 | 1 | 889 | 30, 77%, 143, Stage III/IV 51% | NR 76% prior ARV | Median 10 months | NR | 4.0 | 5.0 | NR | 1.2 |
| Cortes et al [51] CROI (2010) | Chile (Chilean AIDS cohort) | Public | Yes | Oct 01 –Sept 08 | 29 | 3045 | 37, 85%, NR, NR | 63% NNRTI 15% PI | 12 months | NR | 2.3 | 7.1 | NR | NR |
| Auld et al [55] IAS (2010) | Mozambique (National Sample) | Mixed | NR | 04–07 | 30 | 2596 | 34, 38%, 153, NR | 88% NRTI or NNRTI | 1.3 years | NR | 22.8 | 4.3 | NR | NR |
| Ehmer et al [50] IAS (2009) | Africa+ (Solidarmed) | Mixed | Yes | 05–08 initiation | 8 | 4362 | 38, 35%, 121, Stage III/IV 73% | NR | 12 months | NR | 13.9 | 10.3 | NR | NR |
| Somi et al [49] CROI (2011) | Tanzania (National Sample) | Public | Yes | Initiated Oct 04–Aug 07 | 43 | 2,781 | 37, 32%, 114, Stage III/IV 77% | NR | 12 months | NR | 20.0 | 5.0 | 5 | NR |
| Bertagnolio et al [53] CROI (2011) | Africa (Multiple countries) | Public | NR | 02–10 | 6 | 829 | NR | NR | 12 months | Not returned to pharmacy or clinic < 90 days after ART run-out date and have not TF out, stopped, died | 12.9 | 9.2 | 0.8 | 14.5 |
| Balestre et al [54] CROI 2011 | IeDEA-West Africa^ | NR | NR | 93% of patients after 04 | NR | 19,131 | 40, NR, 159, 85% advanced stage | 87% NNRTI 85% ART naïve | 12 months | NR | 32.8 | 4.3 | NR | NR |
Defined as physical tracing to the patient’s place of residence and was available to at least half the study population. Note that 7 cohorts [17], [32], [36], [48], [52], [62] reported phone only tracing was available to a proportion of the study population, and 4 cohorts [34], [47], [54], [58] reported physical tracing for a minority of the study population & All ART naïve at baseline unless stated.
Sample size determined via contact with study authors.
Benin, Cote d’Ivoire, Gambia, Mali, Nigeria, Senegal.
Lesotho, Mozambique, Tanzania, Zimbabwe Notes: ART, antiretroviral therapy; WHO, World Health Organization; LTFU, lost to follow up; TF, transfer; NR, not reported; F/U, follow up; NGO, non-governmental organization; NNRTI. Non-nucleoside reverse transcriptase inhibitors; PI, protease inhibitors; NRTI, nucleoside reverse transcriptase inhibitors.
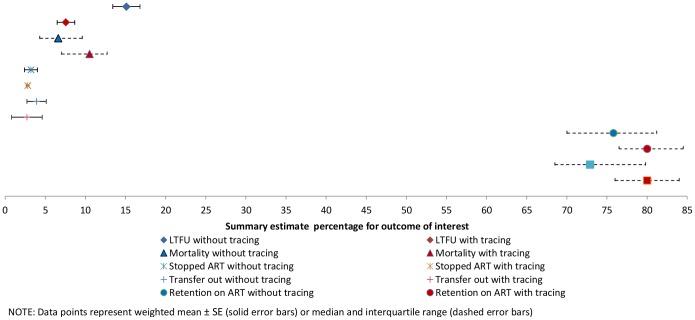
In the 25 cohorts with physical tracing, the weighted mean LTFU was 7.6 % (SE ± 1.1%, range 0.3–15.0%). In the 29 cohorts without physical tracing, the weighted mean LTFU was 15.1% (SE ± 1.7%, range 0.8–34.8%) (Table 3 and Figure 2). The observed difference in summary estimates was statistically significant (p<0.001). Definitions of LTFU were different across different studies but 52% of cohorts (28/54) classified patients as LTFU 3–4 months after their last contact with the ART clinic. Five studies required a 6 month period of being lost and seven used a variety of definitions. Estimates of mortality were significantly higher (p = .006) in cohorts where physical tracing occurred; median estimate of 10.5% (IQR 7.0–12.7%, range 4.2–29.7%,) compared to 6.6% (IQR 4.3–9.6%, range 1.1–15.3%) in cohorts without physical tracing. Weighted mean estimates of ART stop were 2.8% (SE ± 0.2%, range 0.5–5.8%) in the 13 cohorts with physical tracing compared to 3.2% (SE ± 0.8%, range 0.8–8.5%) in the seven cohorts without physical tracing (p = 0.5). The weighted mean estimate of transfer out to another facility was 2.7% (± 1.9%, range 1.0–14.0%) in the five cohorts with tracing and 3.9% (SE ± 1.3%, range 1.2–14.5%) in the seven cohorts without tracing (p = 0.6). A median 80.0% of patients were retained on ART in studies reporting physical tracing (IQR 76.5–84.5%, range 58.4–88.5%) versus 75.8% (IQR 70–81.2%, range 58.5–91.0%) in studies without physical tracing. The median of retention in care at the original ART site for cohorts with physical tracing was 80.0% (IQR 76.0–84.0%, range 47.5–88.5%) versus 72.9% (IQR 68.5–79.8%, range 58.5–90.6%) at clinics without physical tracing. Differences in retention were statistically significant, p = .04 for retention on ART and p = .02 for retention at the original site.
Table 3. Comparison of summary estimates with and without physical tracing.
| Outcome of interest | With tracing | Without tracing | P value# | ||||||
| Cohorts (n) | Starting ART (n) | Range of estimates (%) | Summary estimate* (%) | Cohorts (n) | Starting ART (n) | Range of estimates (%) | Summary estimate* (%) | ||
| LTFU | 25 | 62791 | 0.3–15.0 | 7.6 ± 1.1 | 29 | 124875 | 0.8–34.8 | 15.1 ± 1.7 | < 0.001 |
| Mortality | 25 | 62791 | 4.2–29.7 | 10.5 (7.0–12.7) | 25 | 113693 | 1.1–15.3 | 6.6 (4.3–9.6) | 0.006 |
| Stopped ART | 13 | 43975 | 0.5–5.8 | 2.8 ± 0.2 | 7 | 10841 | 0.8–8.5 | 3.2 ± 0.8 | 0.5 |
| Transfer out | 5 | 6945 | 1.0–14.0 | 2.7 ± 1.9 | 7 | 6195 | 1.2–14.5 | 3.9 ± 1.3 | 0.6 |
| Retention on ART | 25 | 62791 | 58.4–88.5 | 80.0 (76.5–84.5) | 25 | 113693 | 58.5–91.0 | 75.8 (70.0–81.2) | 0.04 |
| Retention at original site | 25 | 62791 | 47.5–88.5 | 80.0 (76.0–84.0) | 25 | 113693 | 58.5–90.6 | 72.9 (68.5–79.8) | 0.02 |
Values represent median (Q1–Q3), or weighted mean ± SE (estimates weighted by the inverse of their variance).
Comparing summary estimates for the 2 groups of studies (tracing and non–tracing) by Wilcoxon rank-sum test for medians or student’s t test for weighted means Notes: LTFU, lost to follow up; ART, antiretroviral therapy.
Figure 2. Plot of summary estimates with and without physical tracing.
Discussion
This review demonstrates lower estimates of LTFU and higher estimates of mortality in LMIC settings where patients receiving ART attend clinics employing physical tracing. The observed differences may be explained by more accurate classification of patients in studies where physical tracing was performed. Specifically, many patients who had previously been classified as LTFU, once traced, were found to have died, thereby contributing to an apparent increased mortality. It remains uncertain by how much the observed decrease in LTFU within physical tracing cohorts was a result of re-engagement of patients back into care versus re-classification of patients with previously unknown outcomes. However, in addition to the significant reduction in LTFU and increase in mortality, we report a significant improvement in retention at the original site. While it is not unexpected that tracing activities would decrease the proportion LTFU and increase mortality estimates due to improved classification of outcomes, the observed improvement in retention at the original site suggests that tracing may have increased the number of patients re-engaged in care. Although the tracing activity would re-classify patients previously thought to be LTFU as transferred out our having died, this reclassification would not alter the estimate of retention at the original site due to its inclusive definition. Therefore, the improvement in retention at the original site is likely not explained by re-classification, but is due to less LTFU, death, or transfers out. Individuals that are re-engaged would have the opportunity to receive the beneficial effects of ART such as improved survival, decreased risk of opportunistic infections, [63] and potentially preventing virological failure and the emergence of HIVDR by limiting treatment interruptions [11], [12]. In addition, the maintenance of an increased proportion of individuals in care and receiving ART is likely to benefit the community by decreasing HIV incidence [64]–[66]. Furthermore, if data on the costs of physical tracing can be obtained, this intervention may potentially be a cost-effective mechanism to re-engage patients into care. Cost-effective analyses of intervention to minimize LTFU and improve survival have been performed but analyses incorporating tracing are not known to the authors at this time. While the qualifications of individuals performing physical tracing is not always reported some included studies did document tracing by peer supporters or people living with HIV without medical qualifications [27], [39], [57] suggesting that physical tracing may prove cost effective in many settings.
The considerable difference in summary estimates between physical and non-physical tracing emphasizes the importance of knowing whether physical tracing is used within an ART program or at a specific ART clinic when interpreting LTFU, mortality or retention data. Estimates of mortality and LTFU are frequently used to assess level of ART program and clinic performance; [5], [6] thus, understanding differences which arise due to physical tracing are important. In addition, the indicator of retention on ART after 12 months of therapy is considered an essential and high impact information when assessing ART program performance [7], [8]. Criticising a program that does not achieve targets for mortality but has functioning tracing programs resulting in few patients with unknown outcomes may not be appropriate. Likewise, reinforcing current practice in settings without tracing and reporting low mortality and higher LTFU rates sends an incorrect message. Furthermore, guidance from the literature in this area is limited as only one previous review was identified that stratified a summary estimate by tracing status. This study by Braitstein et al [18] documented 6.4% mortality with physical tracing and 2.3% without in an LMIC setting whereas we report a mortality of 10.5% with tracing and 6.6% without tracing . Additional reviews report higher 12-month mortality estimates that do not take tracing into account; 14% by Gupta et al [20] and a range of 8–26% mortality by Lawn et al [21]. The reasons for differences in these mortality estimates are unclear, but potentially reflect differences in reporting or improved clinical outcomes. For example, the lower estimates of mortality reported by Braitstein et al [18] are obtained from a smaller number of sites within the ART-LINC collaboration. Additional reviews report estimates of 75–80% retention consistent with findings in this review although tracing status was not documented [14], [19].
This systematic review has some limitations. Settings where physical tracing is available may have increased resources for patients resulting in improved outcomes. Summarizing the effect of tracing from randomised trials containing tracing interventions may provide a more accurate assessment of the impact of tracing on LTFU, mortality and retention by eliminating potential confounding associated with better resourced sites. The authors are unaware of published randomised trials of this nature but data from this review supports the development of randomised trials to quantify the benefits of different tracing strategies including the cost-effectiveness of these strategies. There is also a potential publication bias for settings more likely to publish on LTFU. For example programs associated with academic institutions may be more likely to prepare manuscripts and these programs may have different outcomes from other non-academic settings less inclined to publish their results. Another limitation of this analysis was variability of definitions of LTFU. The majority of studies used a definition of LTFU consistent with international recommendations, [5] yet it is unclear how our findings would have differed if alternative definitions of LTFU had been used. Furthermore, studies classified as physical tracing studies potentially have differing mechanisms to physically trace patients (e.g. number of attempts) which could have influenced outcomes, although the objective of the review was to compare cohorts with and without physical tracing without focussing on specific subgroups within the physical tracing group of studies. Findings from this review may also be limited if additional data are available from other biomedical databases or relevant grey literature. If additional unidentified studies have different findings from the 54 cohorts identified through Ovid Medline and the international HIV conference databases, summary findings could be different. Finally, data on transfer out was only available in a minority of studies despite looking for this data in all selected studies. Estimates of retention at the original site could have potentially changed if complete transfer out data were available. For example a potential bias could exist if cohorts with physical tracing had decreased transfers out which was not documented. This could lead to increased estimates of retention at the original site not necessarily explained by increased re-engagement in care. This limitation emphasises the importance of understanding the proportion transferred out when accurately interpreting and comparing estimates of retention.
In conclusion, physical tracing leads to a reduction in unknown outcomes and likely improved re-engagement in care. Findings from the observational data in this review highlight a critical need for randomised controlled trials to support the effectiveness of patient tracing to improve re-engagement of patients on ART and assess the cost-effectiveness of tracing interventions. Programs providing ART in LMICs should consider physically tracing patients who have become disengaged from care as an important intervention to improve individual outcomes and programmatic evaluation of HIV infected populations receiving ART.
Supporting Information
Physical tracing effects systematic review protocol
(DOC)
Acknowledgments
Some of the authors are staff members of the World Health Organization. The authors alone are responsible for the views expressed in this publication and they do not necessarily represent the decision or stated policy of the World Health Organization.
Funding Statement
JM was supported by a fellowship from Tufts Medical Center Department of Geographic Medicine and Infectious Diseases, and an Australian National Health and Medical Research Council (NHMRC) Postgraduate Scholarship. MRJ was supported by an National Institutes of Health Career Development Award (5K23AI074423-04). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.WHO (2011) Towards universal access : scaling up priority HIV/AIDS interventions in the health sector : progress report 2011. Geneva: World Health Organization.
- 2. Weidle PJ, Malamba S, Mwebaze R, Sozi C, Rukundo G, et al. (2002) Assessment of a pilot antiretroviral drug therapy programme in Uganda: patients' response, survival, and drug resistance. Lancet 360: 34–40. [DOI] [PubMed] [Google Scholar]
- 3. Severe P, Leger P, Charles M, Noel F, Bonhomme G, et al. (2005) Antiretroviral therapy in a thousand patients with AIDS in Haiti. N Engl J Med 353: 2325–2334. [DOI] [PubMed] [Google Scholar]
- 4. Coetzee D, Hildebrand K, Boulle A, Maartens G, Louis F, et al. (2004) Outcomes after two years of providing antiretroviral treatment in Khayelitsha, South Africa. AIDS 18: 887–895. [DOI] [PubMed] [Google Scholar]
- 5.WHO (2010) HIV Drug Resistance Early Warning Indicators.World Health Organization indicators to monitor HIV drug resistance prevention at antiretroviral treatment sites. June 2010 Update. Geneva: World Health Organization.
- 6.WHO (2006) Patient monitoring guidelines for HIV care and antiretroviral therapy. Integrated management of adolescent and adult illness / Integrated management of childhood illness. Geneva: World Health Organization.
- 7.UNAIDS (2009) Monitoring the Declaration of Commitment on HIV/AIDS : guidelines on construction of core indicators : 2010 reporting. Geneva: Joint United Nations Programme on HIV/AIDS (UNAIDS).
- 8.PEPFAR (2009) Next Generation Indicator Reference Guide. Version 1.1. The President’s Emergency Plan for AIDS Relief. Department of Health and Human Services. United States Government.
- 9. Brinkhof MW, Pujades-Rodriguez M, Egger M, Brinkhof MWG, Pujades-Rodriguez M, et al. (2009) Mortality of patients lost to follow-up in antiretroviral treatment programmes in resource-limited settings: systematic review and meta-analysis. PLoS ONE [Electronic Resource] 4: e5790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, et al. (2011) Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 365: 493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Parienti JJ, Massari V, Descamps D, Vabret A, Bouvet E, et al. (2004) Predictors of virologic failure and resistance in HIV-infected patients treated with nevirapine- or efavirenz-based antiretroviral therapy. Clin Infect Dis 38: 1311–1316. [DOI] [PubMed] [Google Scholar]
- 12. Oyugi JH, Byakika-Tusiime J, Ragland K, Laeyendecker O, Mugerwa R, et al. (2007) Treatment interruptions predict resistance in HIV-positive individuals purchasing fixed-dose combination antiretroviral therapy in Kampala, Uganda. AIDS 21: 965–971. [DOI] [PubMed] [Google Scholar]
- 13. Harries AD, Zachariah R, Lawn SD, Rosen S (2010) Strategies to improve patient retention on antiretroviral therapy in sub-Saharan Africa. Trop Med Int Health 15 Suppl 170–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fox MP, Rosen S (2010) Patient retention in antiretroviral therapy programs up to three years on treatment in sub-Saharan Africa, 2007–2009: systematic review. Tropical Medicine & International Health 15 Suppl 11–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cornell M, Grimsrud A, Fairall L, Fox MP, van Cutsem G, et al. (2010) Temporal changes in programme outcomes among adult patients initiating antiretroviral therapy across South Africa, 2002–2007. AIDS 24: 2263–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Geng EH, Bangsberg DR, Musinguzi N, Emenyonu N, Bwana MB, et al. (2010) Understanding reasons for and outcomes of patients lost to follow-up in antiretroviral therapy programs in Africa through a sampling-based approach. Journal of Acquired Immune Deficiency Syndromes: JAIDS 53: 405–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tassie J-M, Malateste K, Pujades-Rodriguez M, Poulet E, Bennett D, et al. (2010) Evaluation of three sampling methods to monitor outcomes of antiretroviral treatment programmes in low- and middle-income countries. PLoS ONE [Electronic Resource] 5: e13899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Braitstein P, Brinkhof MW, Dabis F, Schechter M, Boulle A, et al. (2006) Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet 367: 817–824. [DOI] [PubMed] [Google Scholar]
- 19. Rosen S, Fox MP, Gill CJ (2007) Patient retention in antiretroviral therapy programs in sub-Saharan Africa: a systematic review. PLoS Medicine / Public Library of Science 4: e298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gupta A, Nadkarni G, Yang WT, Chandrasekhar A, Gupte N, et al. (2011) Early mortality in adults initiating antiretroviral therapy (ART) in low- and middle-income countries (LMIC): a systematic review and meta-analysis. PLoS One 6: e28691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lawn SD, Harries AD, Anglaret X, Myer L, Wood R (2008) Early mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa. AIDS 22: 1897–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hawkins C, Achenbach C, Fryda W, Ngare D, Murphy R (2007) Antiretroviral durability and tolerability in HIV-infected adults living in urban Kenya. J Acquir Immune Defic Syndr 45: 304–310. [DOI] [PubMed] [Google Scholar]
- 23. Laurent C, Meilo H, Guiard-Schmid JB, Mapoure Y, Noel JM, et al. (2005) Antiretroviral therapy in public and private routine health care clinics in Cameroon: lessons from the Douala antiretroviral (DARVIR) initiative. Clin Infect Dis 41: 108–111. [DOI] [PubMed] [Google Scholar]
- 24. Wools-Kaloustian K, Kimaiyo S, Diero L, Siika A, Sidle J, et al. (2006) Viability and effectiveness of large-scale HIV treatment initiatives in sub-Saharan Africa: experience from western Kenya. AIDS 20: 41–48. [DOI] [PubMed] [Google Scholar]
- 25. Hingankar N, Thorat S, Deshpande A, Rajasekaran S, Chandrasekar C, et al. (2012) Initial virologic response and HIV drug resistance among HIV-1 infected individuals initiating first-line antiretroviral therapy at two clinics in Chennai and Mumbai India. Clin Infect Dis 54: S348–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ugbena R, Aberle-Grasse J, Diallo K, Bassey O, Jelpe T, et al. (2012) Virological response and HIV-1 drug resistance 12 months after antiretroviral therapy initiation at two clinics in Nigeria Clin Infect Dis. 54: S375–379. [DOI] [PubMed] [Google Scholar]
- 27. Palombi L, Marazzi MC, Guidotti G, Germano P, Buonomo E, et al. (2009) Incidence and predictors of death, retention, and switch to second-line regimens in antiretroviral- treated patients in sub-Saharan African Sites with comprehensive monitoring availability. Clinical Infectious Diseases 48: 115–122. [DOI] [PubMed] [Google Scholar]
- 28. Vella V, Govender T, Dlamini S, Taylor M, Moodley I, et al. (2010) Retrospective study on the critical factors for retaining patients on antiretroviral therapy in KwaZulu-Natal, South Africa. Journal of Acquired Immune Deficiency Syndromes: JAIDS 55: 109–116. [DOI] [PubMed] [Google Scholar]
- 29. Zachariah R, Teck R, Buhendwa L, Fitzerland M, Labana S, et al. (2007) Community support is associated with better antiretroviral treatment outcomes in a resource-limited rural district in Malawi. Trans R Soc Trop Med Hyg 101: 79–84. [DOI] [PubMed] [Google Scholar]
- 30.Oleckno W (2008) Basic Measures of Occurrence in Epidemiology. Epidemiology Concepts and Methods. Long Grove: Waveland Press Inc. 98–99.
- 31. Barth RE, van der Meer JT, Hoepelman AI, Schrooders PA, van de Vijver DA, et al. (2008) Effectiveness of highly active antiretroviral therapy administered by general practitioners in rural South Africa. Eur J Clin Microbiol Infect Dis 27: 977–984. [DOI] [PubMed] [Google Scholar]
- 32. Bisson GP, Frank I, Gross R, Lo Re V, 3rd, Strom JB, et al (2006) Out-of-pocket costs of HAART limit HIV treatment responses in Botswana’s private sector. AIDS 20: 1333–1336. [DOI] [PubMed] [Google Scholar]
- 33. Bisson GP, Gaolathe T, Gross R, Rollins C, Bellamy S, et al. (2008) Overestimates of survival after HAART: implications for global scale-up efforts. PLoS ONE [Electronic Resource] 3: e1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Charalambous S, Innes C, Muirhead D, Kumaranayake L, Fielding K, et al. (2007) Evaluation of a workplace HIV treatment programme in South Africa. AIDS 21 Suppl 3S73–78. [DOI] [PubMed] [Google Scholar]
- 35. Chi BH, Cantrell RA, Zulu I, Mulenga LB, Levy JW, et al. (2009) Adherence to first-line antiretroviral therapy affects non-virologic outcomes among patients on treatment for more than 12 months in Lusaka, Zambia. International Journal of Epidemiology 38: 746–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chung MH, Kohler P, Attwa M, Thiga J, John-Stewart GC, et al. (2010) Comparing clinic retention between residents and nonresidents of Kibera, Kenya. Journal of Acquired Immune Deficiency Syndromes: JAIDS 53: 422–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Collini P, Schwab U, Sarfo S, Obeng-Baah J, Norman B, et al. (2009) Sustained immunological responses to highly active antiretroviral therapy at 36 months in a Ghanaian HIV cohort. Clinical Infectious Diseases 48: 988–991. [DOI] [PubMed] [Google Scholar]
- 38. Culbert H, Tu D, O’Brien DP, Ellman T, Mills C, et al. (2007) HIV treatment in a conflict setting: outcomes and experiences from Bukavu, Democratic Republic of the Congo. PLoS Med 4: e129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. DeSilva MB, Merry SP, Fischer PR, Rohrer JE, Isichei CO, et al. (2009) Youth, unemployment, and male gender predict mortality in AIDS patients started on HAART in Nigeria. AIDS Care 21: 70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Etard JF, Ndiaye I, Thierry-Mieg M, Gueye NF, Gueye PM, et al. (2006) Mortality and causes of death in adults receiving highly active antiretroviral therapy in Senegal: a 7-year cohort study. AIDS 20: 1181–1189. [DOI] [PubMed] [Google Scholar]
- 41. Ferradini L, Jeannin A, Pinoges L, Izopet J, Odhiambo D, et al. (2006) Scaling up of highly active antiretroviral therapy in a rural district of Malawi: an effectiveness assessment. Lancet 367: 1335–1342. [DOI] [PubMed] [Google Scholar]
- 42. Karcher H, Omondi A, Odera J, Kunz A, Harms G (2007) Risk factors for treatment denial and loss to follow-up in an antiretroviral treatment cohort in Kenya. Trop Med Int Health 12: 687–694. [DOI] [PubMed] [Google Scholar]
- 43. Marston BJ, Macharia DK, Nga’nga L, Wangai M, Ilako F, et al. (2007) A program to provide antiretroviral therapy to residents of an urban slum in nairobi, kenya. J Int Assoc Physicians AIDS Care (Chic Ill) 6: 106–112. [DOI] [PubMed] [Google Scholar]
- 44. Moore E, Beadsworth MBJ, Chaponda M, Mhango B, Faragher B, et al. (2010) Favourable one-year ART outcomes in adult Malawians with hepatitis B and C co-infection. Journal of Infection 61: 155–163. [DOI] [PubMed] [Google Scholar]
- 45. Mutevedzi PC, Lessells RJ, Heller T, Barnighausen T, Cooke GS, et al. (2010) Scale-up of a decentralized HIV treatment programme in rural KwaZulu-Natal, South Africa: does rapid expansion affect patient outcomes? Bulletin of the World Health Organization 88: 593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Thai S, Koole O, Un P, Ros S, De Munter P, et al. (2009) Five-year experience with scaling-up access to antiretroviral treatment in an HIV care programme in Cambodia. Tropical Medicine & International Health 14: 1048–1058. [DOI] [PubMed] [Google Scholar]
- 47. Toure S, Kouadio B, Seyler C, Traore M, Dakoury-Dogbo N, et al. (2008) Rapid scaling-up of antiretroviral therapy in 10,000 adults in Cote d’Ivoire: 2-year outcomes and determinants. AIDS 22: 873–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wester CW, Kim S, Bussmann H, Avalos A, Ndwapi N, et al. (2005) Initial response to highly active antiretroviral therapy in HIV-1C-infected adults in a public sector treatment program in Botswana. J Acquir Immune Defic Syndr 40: 336–343. [DOI] [PubMed] [Google Scholar]
- 49.Somi G, Arthur G, Gongo R, Senkoro K, T Kellogg T, et al.. (2011) 24-Month Outcomes for Adults in Tanzania’s National ART Program from 2004 to 2009: Success Maintained during Scale-up, but Should Services Be More Youth Friendly? CROI 2011 Paper #1019.
- 50.Ehmer J, Truniger C, Waldegg G, Mussa I, Mushi A, et al.. (2009) Characteristics and outcomes of HIV-infected patients starting HAART in rural treatment programmes in four sub-Saharan African countries International AIDS Society - Abstract - MOPED045.
- 51.Cortes C, Beltran C, Wolff M (2010) Rate of Mortality, Loss to Follow-up, Viral Suppression, Immune Recovery, and Maintenance of Initial Antiretroviral Regimen at 4 Years: 3045 Chilean Patients. CROI 2010 Paper #523.
- 52.Chinh N, Quang V, Thi Tuyet Nhung V, Colby D (2010) First-line ART Outcomes in HIV-infected Adults in Ho Chi Minh City, Vietnam. CROI 2010 Paper #517.
- 53.Bertagnolio S, Kelley K, Saadani Hassani A, Obeng-Aduasare Y, Jordan M (2011) Surveillance of Transmitted and Acquired HIV Drug Resistance Using WHO Surveys in Resource-limited Settings. CROI 2011 Paper # 52.
- 54.Balestre E, Lokossue A, Eholié S, Sow P, Charurat M, et al.. (2011) Effect of Age on Immunological Response in the First Year of HAART in HIV-1-infected Adults, IeDEA West Africa Collaboration. CROI 2011 Paper # 272.
- 55.Auld A, Mbofana F, Shiraishi R, Sanchez M, Alfredo C, et al.. (2010) Treatment outcomes of Mozambique’s adult antiretroviral therapy program - earlier treatment and co-trimoxazole are important. International AIDS Society - Abstract - TUAB0204.
- 56. May M, Boulle A, Phiri S, Messou E, Myer L, et al. (2010) Prognosis of patients with HIV-1 infection starting antiretroviral therapy in sub-Saharan Africa: a collaborative analysis of scale-up programmes. Lancet 376: 449–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bedelu M, Ford N, Hilderbrand K, Reuter H, Bedelu M, et al. (2007) Implementing antiretroviral therapy in rural communities: the Lusikisiki model of decentralized HIV/AIDS care. Journal of Infectious Diseases 196 Suppl 3S464–468. [DOI] [PubMed] [Google Scholar]
- 58. Hong SY, Jonas A, Dumeni E, Badi A, Pereko D, et al. (2010) Population-based monitoring of HIV drug resistance in Namibia with early warning indicators. Journal of Acquired Immune Deficiency Syndromes: JAIDS 55: 27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. O’Brien DP, Mills C, Hamel C, Ford N, Pottie K (2009) Universal access: the benefits and challenges in bringing integrated HIV care to isolated and conflict affected populations in the Republic of Congo. Confl Health 3: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Johannessen A, Naman E, Ngowi BJ, Sandvik L, Matee MI, et al. (2008) Predictors of mortality in HIV-infected patients starting antiretroviral therapy in a rural hospital in Tanzania. BMC Infect Dis 8: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Assefa Y, Van Damme W, Mariam DH, Kloos H (2010) Toward universal access to HIV counseling and testing and antiretroviral treatment in Ethiopia: looking beyond HIV testing and ART initiation. AIDS Patient Care & Stds 24: 521–525. [DOI] [PubMed] [Google Scholar]
- 62. Sharma SK, Dhooria S, Prasad KT, George N, Ranjan S, et al. (2010) Outcomes of antiretroviral therapy in a northern Indian urban clinic. Bulletin of the World Health Organization 88: 222–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Palella FJ Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, et al. (1998) Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med 338: 853–860. [DOI] [PubMed] [Google Scholar]
- 64. Das M, Chu PL, Santos GM, Scheer S, Vittinghoff E, et al. (2010) Decreases in community viral load are accompanied by reductions in new HIV infections in San Francisco. PLoS One 5: e11068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Montaner JS, Wood E, Kerr T, Lima V, Barrios R, et al. (2010) Expanded highly active antiretroviral therapy coverage among HIV-positive drug users to improve individual and public health outcomes. J Acquir Immune Defic Syndr 55 Suppl 1S5–9. [DOI] [PubMed] [Google Scholar]
- 66.Forgione L, Torzian L (2012) Trends in community viral load, new diagnoses, and estimated incidence of HIV, New York City, 2005–2009. Abstract #1123. CROI. Seattle, USA.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Physical tracing effects systematic review protocol
(DOC)