Abstract
BACKGROUND
Pharmacologic and ablative therapies for atrial fibrillation (AF) have suboptimal efficacy. Newer gene-based approaches that target specific mechanisms underlying AF are likely to be more efficacious in treating AF. Parasympathetic signaling appears to be an important contributor to AF substrate.
OBJECTIVE
The purpose of this study was to develop a nonviral gene-based strategy to selectively inhibit vagal signaling in the left atrium and thereby suppress vagal-induced AF.
METHODS
In eight dogs, plasmid DNA vectors (minigenes) expressing Gαi C-terminal peptide (Gαictp) was injected in the posterior left atrium either alone or in combination with minigene expressing Gαoctp, followed by electroporation. In five control dogs, minigene expressing scrambled peptide (GαRctp) was injected. Vagal- and carbachol-induced left atrial effective refractory periods (ERPs), AF inducibility, and Gαi/octp expression were assessed 3 days following minigene delivery.
RESULTS
Vagal stimulation- and carbachol-induced effective refractory period shortening and AF inducibility were significantly attenuated in atria receiving a Gαi2ctp-expressing minigene and were nearly eliminated in atria receiving both Gαi2ctp- and Gαo1ctp-expressing minigenes.
CONCLUSION
Inhibition of both Gi and Go proteins is necessary to abrogate vagal-induced AF in the left atrium and can be achieved via constitutive expression of Gαi/octps expressed by nonviral plasmid vectors delivered to the posterior left atrium.
Keywords: Atrial fibrillation, Atrial fibrillation inducibility, Autonomic nervous system, Effective refractory period, Muscarinic cholinergic receptor, Pertussis toxin-sensitive G proteins, Vagal signaling
Introduction
Atrial fibrillation (AF) is the most common sustained rhythm disorder of the heart.1,2 In view of the limitations of current treatment options, several investigators have indicated a need for novel therapies that target specific mechanisms underlying AF.2 The autonomic nervous system—specifically the parasympathetic nervous system—is known to be involved in the genesis of AF3,4 and may be a viable therapeutic target in patients with AF.5,6
In the atria, vagal-released acetylcholine (ACh) stimulates primarily type 2 muscarinic cholinergic receptors (M2Rs), which activate heterotrimeric Gαi/oβγ proteins, with resulting dissociation of the Gαi/o subunit from Gβγ. Gβγ activation of IK-ACh leads to significant abbreviation of action potential duration, thereby creating a substrate for reentry.7 In proof-of-concept studies,8 we previously demonstrated that atrial-selective attenuation of vagal signaling can be achieved by a Gαi2 C-terminal peptide (Gαi2ctp) delivered to the posterior left atrium (PLA).9,10 This Gαi2ctp putatively acts by selectively disrupting M2R-Gαi2 coupling, thus impeding Gαi2βγ signal transduction. Although encouraging, the utility of such a peptide-based pharmacotherapy requires sustained and controlled intracellular expression of peptide in the atrial myocardium. Moreover, it was clear in our previous study that inhibition of Gαi2 did not completely abrogate M2R/vagal signaling in the PLA. Indeed, multiple studies have suggested that other pertussis toxin–sensitive Gα subunit isomers, particularly Gαo isomers, contribute to vagal signaling in the atria.11,12 Hence, the present study describes our efforts toward achieving more constitutive administration of not only Gαi2ctp but also Gαo1ctp by incorporating their cDNA into plasmid expression vectors (minigenes), delivering them into canine PLA, and assessing their effects on cholinergic responsiveness.
Methods
All procedures involving animals were approved by the Institutional Animal Care and Use Committee at Northwestern University. The research conforms with the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health (Publication No. 85-23, revised 1996).
Minigene preparation
Cloning of Gαi2ctp, Gαo1ctp, and GαRctp inserts into plasmid backbone
The corresponding cDNA sequences of the last C-terminal 11 amino acids of the Gαi2 subunit, and that for the Gαo1 subunit and that of random-ordered Gαi2ctp (GαRp), were each separately cloned into a pFLAG CMV6a plasmid expression vector (Sigma-Aldrich, St. Louis, MO) to generate Gαi2ctp-Gαo1ctp- and GαRp-expressing plasmid constructs (minigenes) as described elsewhere13 (see brief protocol in Online Data Supplement).
Transformation and plasmid purification
Plasmids were propagated in Escherichia coli and purified using Qiagen Mega-prep kits (Qiagen, Valencia, CA, USA), as described by the manufacturer. Details of transformation and plasmid purification are given in the Online Data Supplement.
Gene injection and in vivo electrophysiologic testing
Gene injection and electroporation
A total of 19 dogs (hounds) were used for this study (12 male, 7 female). Animals were premedicated with acepromazine (0.01–0.02 mg/kg) and induced with propofol (3–7 mg/kg). A median or lateral sternotomy was then performed under general anesthesia (inhaled) with isoflurane (1%–3%). Adequacy of anesthesia was assessed by toe pinch and palpebral reflex.
In a small number of pilot experiments (N = 6), 1 mg of Gαi2ctp or GαRp minigene was injected subepicardially in the PLA (see Results). In the remaining experiments, 15–20 mg of ct-Gαi2ctp (N = 5), 7–10 mg of Gαi2ctp + 7–10mg of Gαo1ctp (N = 3), or GαRp (N = 5) minigenes was injected in the PLA. Minigenes were made up to a volume of 4 mL and injected at multiple sites (6–8 equally spaced sites, 0.5–1 cm apart; a volume of approximately 0.5 mL was injected at each site) in the PLA so as to cover the entire area between the pulmonary veins (PVs). The injected region is anatomically clearly demarcated (the four borders used are base of left atrial appendage, base of left inferior PV, interatrial septum, and atrioventricular groove) and is removed after gene injection. Immediately after gene injection, electroporation was performed at each site of injection as follows. Two gold-plated, needle-style electrodes (10-mm length each) were placed at each gene injection site on the PLA (interelectrode distance 5 mm). Electroporation with performed as previously described in the lung by Dean et al,14 with eight pulses of 1 second at 120–150 V/cm2 (ECM 830, Harvard Bioscience, Holliston, MA, USA).
After minigene delivery, the chest was closed and the animal allowed to recover. Vagal stimulation and electro-physiologic testing were not performed at baseline in order to minimize damage to the vagus nerve.
Terminal electrophysiologic study
Baseline study
Three days after the initial study, the chest was reopened. High-density plaques were applied to the left superior PV (8 × 5 electrodes, 2.5-mm spacing), PLA (7×3 electrodes, 5-mm spacing), and left atrial appendage (LAA; 7×3 electrodes, 5 mm spacing). The PV plaque was placed circumferentially around the vein while the other two plaques were laid flat on the PLA and LAA epicardium. Effective refractory periods (ERPs) were obtained from 5, 6, and 4 sites on the PV, PLA, and LAA plaque, respectively, at baseline.
Vagal stimulation
For vagal stimulation, the left cervical vagus nerve was isolated, a bipolar stainless steel electrode was attached to the nerve, and stimulation was performed at 20 Hz (15–20 V, 2–8 ms) (Grass S44G, Astromed Inc, West War-wick, RI, USA). A vagal response was defined as (1) sinus node slowing by at least 25% or (2) PR prolongation by more than 25% or 2:1 AV block.13 ERP testing was performed in the presence and absence of vagal stimulation (VS).
Carbachol application
We also assessed for ERP shortening by direct application of carbachol (CCh), a nonselective MR agonist, to the PLA. CCh was injected under the subepicardium of the PLA in increasing doses of 3, 10, and 30 μM (also see in vitro CCh dose finding studies). After each dose, ERP testing was performed in the PLA. Long periods of AF were frequently encountered during atrial pacing at higher concentrations of CCh and thus precluded ERP testing at these higher doses.
AF inducibility
AF was defined as an atrial arrhythmia that was irregular in at least one of the recording electrodes. Regular atrial arrhythmias (eg, atrial flutter, atrial tachycardia) were excluded from AF analysis. AF inducibility was measured as the inducibility index and duration of AF episodes after a single extrastimulus.8,15 As previously described, the inducibility index was defined as the number of AF episodes lasting more than 5 seconds induced by a single atrial extrastimulus divided by the total number of single atrial extrastimuli delivered to measure each ERP (at least three for each site).8 The inducibility index was compared for each maneuver. Mean AF duration for each intervention was also assessed.
All data were acquired by a 128-channel mapping system (Prucka CardioLab, GE, Waukesha, WI, USA) at a sampling rate of 977 Hz. All AF episodes induced during extrastimulus testing were stored for offline analysis. After all ERPs had been obtained, minigene injection was performed as described later.
Offline electrogram analysis
Electrograms recorded during the maximum duration AF episodes obtained during extra-stimulus testing were analyzed with dominant frequency (DF) analysis. DF is an estimation of activation rate calculated as the frequency with the most power in the power spectrum. The power spectrum is obtained from the fast Fourier transform of an electrogram after rectification and low-pass filtering (20 Hz). These analyses were performed offline using Matlab (MathWorks, Natick, MA, USA).
Tissue explant assays
Upon finishing the in vivo portion of the study, euthanasia was achieved using a high dose of pentobarbital (>20 cc, fully saturated) to achieve a very deep plane of anesthesia, and the heart removed and perfused with cold cardioplegia solution. The left atrium and PVs were dissected, snap frozen, and subjected to further analysis as detailed in the following.
Transgene expression
mRNA expression
The following primers to detect minigene-expressed mRNA were obtained from IDT (San Diego, CA, USA):
Gαi2ctp: forward AGCTCAAGCTTATCAAGAACAACCT, reverse TACCGGATCCTCAGAAGAGGC
Gαo1ctp: forward AGCTCAAGCTTATTGCCAACAACC, reverse GGTACCGGATCCTCAGTACAAGCC
GαRp: forward CAAGCTTAACGGCATCAAGTGC, reverse GGTACCGGATCCTCACAGCTT
Quantitative real-time polymerase chain reaction was performed to assess for expression of Gαi2ctp, Gαo1ctp, and GαRp expressing minigenes in the PLA following gene injection. GAPDH was used as a reference for sample normalization.
Western blotting
Anti-FLAG antibodies (Sigma) were used to assess for the presence of FLAG-tagged Gαi2ctp in PLA tissue. Calsequestrin-2 was used as a loading control (see Online Data Supplement for details).
Immunohistochemistry
Thin sections (5 μm) of the PLA were obtained for hematoxylin and eosin staining and for immunohistochemistry (the latter to assess for FLAG-tagged Gαi2ctp; see Online Data Supplement for details).
CCh concentration–Ca2+ transient response assay in isolated canine atrial myocytes
Myocyte isolation
Canine right atrial myocytes (from same hearts excised as described earlier) were isolated by colla-genase digestion via a coronary perfusion modified procedure previously described.8,16 Dissociated myocytes were stored in normal Tyrode’s solution at 4°C until use in confocal Ca2+ transient experiments as described.
Ca2+ transients acquisition and CCh administration
As previously described,8 isolated atrial myocytes were incubated with 5–10 μM of the Ca2+ fluorescence dye fluo-4 (Invitrogen, Carlsbad, CA). Action potential (AP)-evoked Ca2+ transients were acquired as confocal X-t line-scan images of uncalibrated fluo-4 fluorescence at a scan rate of 1.92 ms/line-scan. Laser phototoxicity was minimized by scanning at <10% output transmission. Changes in Ca2+ transients in response to serial concentrations of acutely applied CCh (0.01–30 μM) were measured from multiple myocytes per isolation preparation (see online Data Supplement for details).
Statistical analysis
All data are reported as mean ± SE. Comparisons between Gαi2, Gαi2+Gαo1, and GαR dogs (for ERP, AF inducibility) were assessed for significant differences via analysis of variance. DF comparisons between Gαi2 and GαR dogs were made using unpaired t-tests. Before and after comparisons made in the same animals (eg, before and after VS or CCh) were assessed for significant differences via paired t-tests. P ≤.05 was considered significant.
Results
Gαx minigene injection dose vs Gαx minigene gene product expression
In pilot experiments (N = 6), 1–2 mg Gαi2ctp minigene was injected into the PLA, followed immediately by electroporation. However, this pilot minigene injection dose resulted in low–modest Gαi2ctp mRNA expression in the PLA (Online Supplemental Figure 1). An approximately 10× injection dose of minigene (15–20 mg) resulted in significantly greater Gαi2ctp mRNA expression (see Online Supplemental Figure 1) and was used in all subsequent experiments where Gαi2ctp minigene was tested alone. Assessment of mRNA expression in canine PLAs in which Gαi2ctp mini-gene had been delivered (Gαi2ctp minigene alone experiments) suggested a trend toward increased Gαo1 mRNA compared to control PLAs (data not shown). Thus, additional experiments were conducted in which Gαi2ctp mini-gene was delivered together with Gαo1ctp minigene, and an injection dose of 7–10 mg of each minigene was used. As described later, clear electrophysiologic responses were obtained following these latter injection doses of minigenes.
Effects of Gαx minigenes on vagal-induced ERP shortening
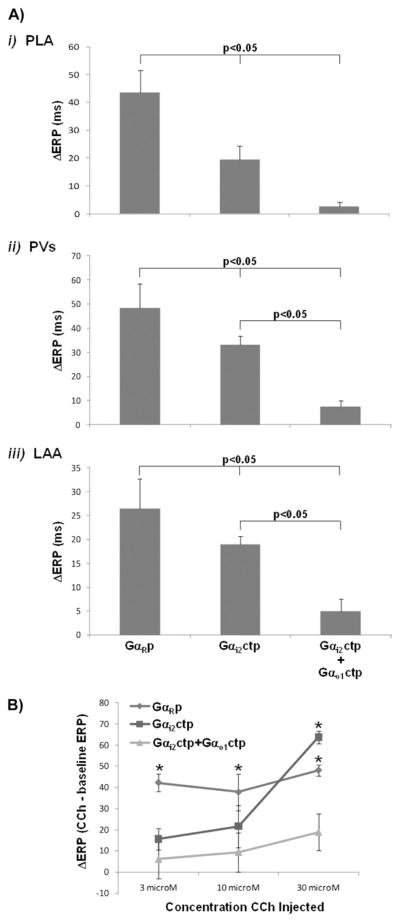
The effects of PLA delivery of Gαi2ctp minigene (N = 5) vs Gαi2ctp + Gαo1ctp minigene (N = 3) vs GαRp minigene (N = 5) on vagal-induced ERP shortening are shown in Figure 1A. Vagal-induce ERP shortening was significantly less in the PLA of Gαi2ctp dogs vs GαRp dogs 19.5 ± 5.0 ms vs 43.6 ± 7.9 ms, P <.05). There was no significant difference in vagal-induced ERP shortening between Gαi2ctp and GαRp dogs in the PV and the LAA. In comparison, in Gαi2ctp+Gαo1ctp dogs, vagal-induced ERP shortening was almost entirely eliminated in the PLA (with shortening significantly less than with Gαi2ctp alone: 2.8 ± 1.5 ms vs 19.5 ± 5.0 ms, P <.01) and was also significantly attenuated in the PV and LAA (Figure 1A).
Figure 1.
Attenuation by minigene-expressing Gαi/octp of vagal stimulation (VS)- and carbachol (CCh)-induced effective refractory period (ERP) shortening. A: i: Compared to GαRcpt, vagal-induced ERP shortening in the posterior left atrium (PLA) is attenuated in Gαi2ctp dogs and is nearly eliminated in Gαi2ctp+ Gαo1ctp dogs. In Gαi2ctp+ Gαo1ctp dogs, vagal-induced ERP shortening was also significantly attenuated in the pulmonary vein (PV) (ii) and left atrial appendage (LAA) (iii). B: ERP shortening in the PLA in response to CCh. In GαRcpt dogs, significant ERP shortening was noted in response to each CCh concentration (3, 10, 30 μM). In contrast, in Gαi2ctp dogs, ERP shortening was noted only at 30 μM CCh. In Gαi2ctp+ Gαo1ctp dogs, there was no significant ERP shortening at any dose of CCh. *P <.05 vs baseline ERP at terminal study.
Effects of Gαx minigenes on CCh-induced ERP shortening
To more specifically assess the action of Gαx minigene delivery on M2R–Gi/o protein coupling and resultant ERP shortening, exogenously applied CCh was used to induce ERP shortening in the PLA in Gαxctp dogs. To arrive at an appropriate CCh dose range for these in vivo experiments, we first determined the concentration–response relationship for CCh to attenuate Ca2+ transient peak amplitude in isolated canine atrial myocytes (see Online Supplemental Figure 2). The IC50 and maximum for this effect was 28 ± 8 nM and 3 μM CCh, respectively. At CCh >3 μM the effect faded, indicating agonist-induced acute M2R desensitization.17 Because our goal was to obtain maximum CCh effect in atrial tissue while minimizing agonist-induced M2R desensitization, we arrived at a small in vivo test range of 3–30 μM CCh to assess ERP shortening following gene delivery in vivo (previous experience in our laboratory has indicated that in vivo dosage is ~3–10× the in vitro dosage). Moreover, as stated earlier, higher doses of CCh administered in vivo resulted in long periods of AF and therefore precluded ERP testing.
Accordingly, CCh (3–30 μM) was directly injected into the PLA. As expected, increasing doses of CCh caused progressively greater ERP shortening in the PLA, but as shown in Figure 1B, CCh-induced ERP shortening was significantly less in Gαi2ctp compared to GαRp dogs at lower CCh concentrations (3 μM, 10 μM). At 30 μM, there was no significant difference in ERP shortening between Gαi2ctp and GαRp dogs. This indicates that 30 μM CCh stimulates M2Rs sufficiently to overcome Gαi2ctp inhibition of M2R–Gi2 signaling (because Gαi2ctp acts as a competitive inhibitor to endogenous M2R–Gαi2 interaction). In Gαi2ctp+Gαo1ctp dogs, there was no significant CCh-induced ERP shortening at any dose of CCh (including the highest dose of 30 μM), with ERP shortening being significantly less than in the other two groups at each dose of CCh (Figure 1B).
Effects of Gαx minigenes on vagal- and CCh-induced AF
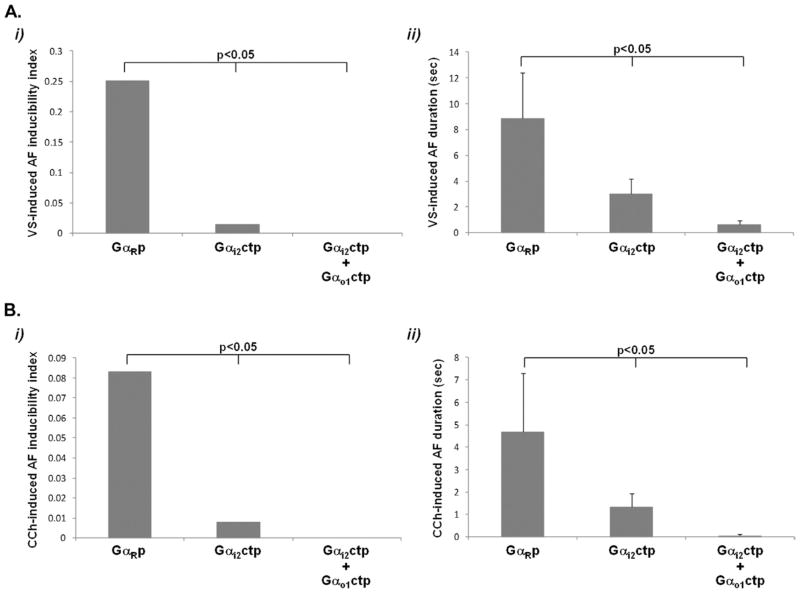
AF inducibility (in response to VS) was significantly less in Gαi2ctp dogs vs GαRp dogs and was lowest (zero) in Gαi2ctp+Gαo1ctp dogs, with not a single episode of AF >5 seconds being induced in this group (Figure 2A). Mean AF duration (in response to VS) was also significantly less in Gαi2ctp dogs vs GαRp dogs and was lowest in Gαi2ctp+Gαo1ctp dogs (Figure 2A). Similarly, AF inducibility and AF duration in response to CCh were significantly less in Gαi2ctp dogs vs GαRp dogs but were lowest in Gαi2ctp+Gαo1ctp dogs (Figure 2B).
Figure 2.
Decrease in vagal stimulation (VS)- and carbachol CCh-induced atrial fibrillation (AF) by minigene-expressing Gαi/o peptide. A: Decrease in VS-induced AF in Gαi2ctp and Gαi2ctp+ Gαo1ctp dogs compared to GαRcpt. Both the AF inducibility index (i) and mean AF duration (ii) showed a progressive decrease in Gαi2ctp and Gαi2ctp+ Gαo1ctp dogs, respectively. B: Decrease in CCh-induced AF in Gαi2ctp dogs and in Gαi2ctp+ Gαo1ctp dogs compared to minigene-expressing GαRcpt. Both the AF inducibility index (i) and mean AF duration (ii) showed a progressive decrease in Gαi2ctp and Gαi2ctp+ Gαo1ctp dogs, respectively.
Effects of Gαi2 expressing minigene on AF characteristics
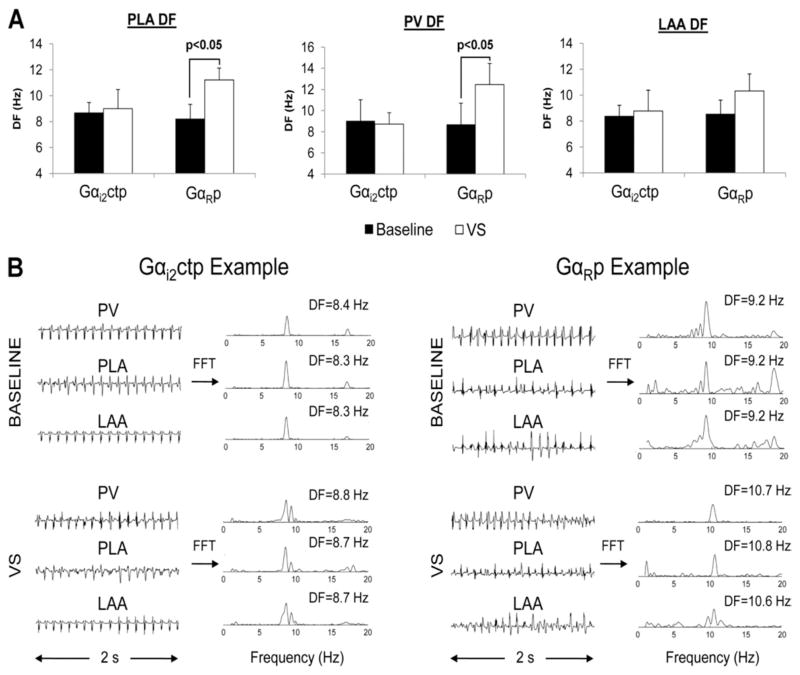
As previously shown by us and others, VS increases DF of AF.8 When AF DF was assessed in Gαi2ctp and GαRp dogs, the VS-induced increase in DF was significantly less in Gαi2ctp dogs vs GαRp dogs (where significant DF was noted in the PLA and PV in response to VS; Figure 3A). Figure 3B shows examples of AF electrograms (with and without VS) from Gαi2ctp vs GαRp dogs. AF DF could not be assessed in dogs receiving Gαi2ctp+Gαo1ctp dogs due to the very small number of AF episodes in these dogs and the very short duration of these episodes.
Figure 3.
Attenuation by minigene-expressing Gαi2ctp of vagal stimulation (VS)-induced changes in atrial fibrillation (AF) dominant frequency (DF). A: VS led to a significant increase in AF DF in the posterior left atrium (PLA) and pulmonary vein (PV) of GαRcpt but not in Gαi2ctp. No significant change in DF was noted in the left atrial appendage (LAA) in either Gαi2ctp or GαRcpt dogs. B: Representative examples of AF electrograms recorded from the PV, PLA, and LAA and their corresponding power spectra. Electrograms recorded from the Gαi2atp group show a modest increase in DF with VS compared to baseline. The GαRp group showed a much larger increase in DF.
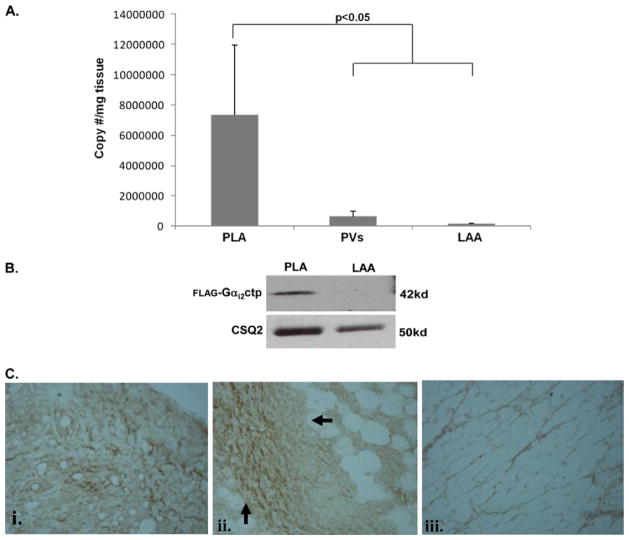
Gαx transgene expression in the left atrium and PVs
Figure 4A shows relative Gαxctp mRNA expression in the PLA, PV, and LAA after minigene delivery into the PLA. Robust expression for Gαi2ctp, Gαo1ctp, and GαRp minigenes was found in the PLA, with minimal expression in the PV (which was adjacent to the area of injection) and no expression in the LAA (remote from site of injection). Anti-FLAG Western blot analysis (Figure 4B) indicated Gαi2ctp (FLAG-tagged) expression in the PLA but not in the LAA. Immunohistochemistry showed evidence of FLAG staining in both myocytes (Figure 4C, subpanel i) and nerve bundles (Figure 4C, subpanel ii) in the PLA. In contrast, no FLAG was detected in the LAA (Figure 4C, subpanel iii). Thus, Gαxctp minigene injection resulted in adequate Gαxctp translation at the site of minigene delivery.
Figure 4.
Verification of Gαx peptide transgene expression in the left atrium. A: Results of polymerase chain reaction on posterior left atrium (PLA), pulmonary vein (PV), and left atrial appendage (LAA) tissue. Transgene expression (for both Gαi2ctp and GαRcpt minigenes) was noted in the PLA. There was minimal expression in the adjoining PV and no expression in the LAA. B: Results of Western blotting for FLAG-tagged Gαi2ctp. FLAG-tagged peptide was detected in the PLA but not in the LAA. Calsequestrin Q is the loading control for each lane. C: Example of immunohistochemistry for FLAG-tagged Gαi2ctp. FLAG-tagged peptide (heavy brown stain) was detected in PLA myocytes (i) and in nerve bundles in the PLA (ii) but not in the LAA (iii).
Discussion
In this study, we demonstrate the feasibility and efficacy of a targeted nonviral gene therapy approach to AF. Using minigene constructs that were delivered to the PLA by direct injection + electroporation, we demonstrate that 3 days after gene injection, (1) vagal responsiveness in the normal PLA was attenuated by a Gαi2ctp expressed in situ by a plasmid expression vector and (2) vagal responsiveness was almost entirely eliminated in the PLA and significantly attenuated elsewhere in the left atrium by a combination of minigenes expressing Gαi2ctp and Gαo1ctp, with a resulting dramatic decrease in vagal-induced AF.
Gene therapy in AF: Prior experience in modification of autonomic signaling via G-protein–related pathways
In an innovative approach, Donahue et al18,19 used an ad-enoviral vector overexpressing Gαi to suppress AV conduction and thereby slow heart rate during AF. Our approach differs from that of Donahue et al in that instead of increasing Gαi activity in the AV node to decrease ventricular rates during AF, we inhibited M2R–Gαi/o interactions in the left atrium with nonviral minigene-expressing Gαi/octps, with the intent of modifying the autonomic substrate in a region of the heart (PLA) that is considered critical to the genesis of AF. Indeed, disruption of M2R–Gαi coupling caused decreased ERP responsiveness and AF inducibility, which became much more apparent upon the additional disruption of M2R–Gαo coupling. Moreover, disruption of M2R–Gαi coupling caused significant attenuation of vagal-induced increase in DF (of the AF that was induced).
Redundancy of G-protein– coupling to M2Rs in the atrium
It is well established that M2R signaling in the atria is transduced by pertussis toxin–sensitive Gi/o proteins. However, because six GTP-binding Gα subunit isoforms are known (Gαi1,2&3, Gαo1,2&3), the specific identity of the Gαi/o isoform(s) that couples to atrial M2RS has not been unequivocally established.11,12,20,21 Indeed, Gαo has been found to be co-localized with Gαi in the porcine atrium in a 1:1 ratio22 and can activate IK-ACh as efficiently as Gαi.23 Such findings corroborate ours in the present study in that inhibition of both Gαi and Gαo seems to be required for complete disruption of M2R responsiveness in the left atrium. To our knowledge, this is the first time disruption of M2R–Gαo coupling has been attempted and shown to significantly contribute to a decrease in vagal-induced AF in the large animal heart.
Gene delivery in the atrium: Viral vs nonviral approaches
Both viral and nonviral delivery methods have relative advantages for use in myocardial gene delivery.24,25 Importantly, a nonviral approach results in a reduced inflammatory and immune response in vivo26 and therefore has a more favorable safety profile. Recent improvements in physical delivery methods (eg, sonoporation, electroporation) have allowed increasing levels of gene transfer and expression with naked DNA, nearing that of viral vectors.26 In this study, we demonstrate that Gαi/octps constitutively expressed via nonviral DNA vectors delivered into the PLA followed by electroporation results in attenuated vagal/M2R-induced ERP shortening and AF.
Current viral approaches may have a potential for long-term gene expression and so may be suited for the treatment of a chronic condition such as AF. For a nonviral gene therapy approach to be a viable option for AF, (1) significantly longer-term gene expression in the atrium than that achieved in the current study would have to be obtained and (2) long-term safety study studies would need to be conducted. Ongoing experiments in our laboratory suggest that longer-acting promoters allow gene expression at least up to a few weeks.27 Longer-term studies need to be performed in order to further investigate the feasibility of a nonviral approach for AF gene therapy.
Other issues to be considered prior to translation of above approach to clinical AF
Of note, IK-ACh is the major effector of M2R stimulation in normal hearts, with a constitutive (agonist-independent) form of IK-ACh (IK-AChc)28 becoming more pronounced in patients with chronic AF.7 Nonetheless, IK-AChc does appear to be G-protein dependent.28 Moreover, as recently shown by us,29 left atrial parasympathetic responsiveness, although diminished in structural heart disease, still appears to play an important role in the maintenance of AF.
Also important is the presence of right atrial-left atrial gradients in IK-ACh activation.30 Local gene therapy may change these gradients, with potentially maladaptive consequences during long-term treatment.
The effect of Gαi/o inhibition on Excitation-Contraction (E-C) coupling also needs to be assessed, especially in light of previous studies suggesting that sympathovagally induced changes in Ca2+ release contribute to arrhythmogenesis.10,31
Study limitations
With the CMV promoter, gene expression was limited in the current study and was assessed only up to 3–4 days. Longer-term studies, essential for such a gene-based approach to be translated to patients with AF, were not performed because cardiac expression in large animal hearts with the CMV promoter may be limited to 1 week.26 However, in preliminary studies, we recently achieved longer-term gene expression with naked DNA using alternative, longer-acting promoters.27
The effect of Gαo inhibition alone on vagal responsiveness was not assessed in this study. However, based on the near complete inhibition of vagal responsiveness seen in response to combined Gαo and Gαi inhibition (as opposed to only partial inhibition noted with Gαi inhibition alone), it appears that Gαo is at least as important as, if not more important than, Gαi in mediating cholinergic responsiveness in the canine left atrium.
Because we performed vagal stimulation only at the terminal study and not at baseline, paired comparisons could not be made (for vagal stimulation) within each animal. However, paired comparisons were made for CCh.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health (NHLBI) Grants 1R01HL093490, 3R01HL093490-01S1, and R21 HL088304; the Everett/O’Connor Trust; and the Dixon Translational Research Award (Northwestern Memorial Hospital).
ABBREVIATIONS
- ACh
acetylcholine
- AF
atrial fibrillation
- CCh
carbachol
- DF
dominant frequency
- ERP
effective refractory period
- Gαi/octp
Gαi/o C-terminal peptide(s)
- GαRp
Gα random-sequence peptide
- IK-ACh
acetylcholine-activated inward rectifying potassium channel
- LAA
left atrial appendage
- M2R
type 2 muscarinic cholinergic receptor
- PLA
posterior left atrium
- PV
pulmonary vein
- VS
vagal stimulation
Appendix
Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.hrthm. 2011.06.018.
References
- 1.Benjamin EJ, Levy D, Vaziri SM, D’Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 1994;271:840–844. [PubMed] [Google Scholar]
- 2.Dobrev D, Nattel S. New antiarrhythmic drugs for treatment of atrial fibrillation. Lancet. 2010;375:1212–1223. doi: 10.1016/S0140-6736(10)60096-7. [DOI] [PubMed] [Google Scholar]
- 3.Sturrock A, Cahill B, Norman K, et al. Transforming growth factor-β1 induces Nox4 NAD(P)H oxidase and reactive oxygen species-dependent proliferation in human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2006;290:L661–L673. doi: 10.1152/ajplung.00269.2005. [DOI] [PubMed] [Google Scholar]
- 4.Ng J, Villuendas R, Cokic I, et al. Autonomic remodeling in the left atrium and pulmonary veins in heart failure—creation of a dynamic substrate for atrial fibrillation. Circ Arrhythm Electrophysiol. 2011;4:388–396. doi: 10.1161/CIRCEP.110.959650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharifov OF, Fedorov VV, Beloshapko GG, Glukhov AV, Yushmanova AV, Rosenshtraukh LV. Roles of adrenergic and cholinergic stimulation in spontaneous atrial fibrillation in dogs. J Am Coll Cardiol. 2004;43:483–490. doi: 10.1016/j.jacc.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 6.Oliveira M, da Silva MN, Geraldes V, et al. Acute vagal modulation of electrophysiology of the atrial and pulmonary veins increases vulnerability to atrial fibrillation. Exp Physiol. 2011;96:125–133. doi: 10.1113/expphysiol.2010.053280. [DOI] [PubMed] [Google Scholar]
- 7.Dobrev D, Graf E, Wettwer E, et al. Molecular basis of downregulation of G-protein-coupled inward rectifying K+ current (IK,ACh) in chronic human atrial fibrillation: decrease in GIRK4 mRNA correlates with reduced IK,ACh and muscarinic receptor-mediated shortening of action potentials. Circulation. 2001;104:2551–2557. doi: 10.1161/hc4601.099466. [DOI] [PubMed] [Google Scholar]
- 8.Aistrup GL, Villuendas R, Ng J, et al. Targeted G-protein inhibition as a novel approach to decrease vagal atrial fibrillation by selective parasympathetic attenuation. Cardiovasc Res. 2009;83:481–492. doi: 10.1093/cvr/cvp148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arora R, Ng J, Ulphani J, et al. Unique autonomic profile of the pulmonary veins and posterior left atrium. J Am Coll Cardiol. 2007;49:1340–1348. doi: 10.1016/j.jacc.2006.10.075. [DOI] [PubMed] [Google Scholar]
- 10.Patterson E, Po SS, Scherlag BJ, Lazzara R. Triggered firing in pulmonary veins initiated by in vitro autonomic nerve stimulation. Heart Rhythm. 2005;2:624–631. doi: 10.1016/j.hrthm.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 11.Ye C, Sowell MO, Vassilev PM, Milstone DS, Mortensen RM. G[alpha]i2, G[alpha]i3and G[alpha] are all required for normal muscarinic inhibition of the cardiac calcium channels in nodal/atrial-like cultured cardiocytes. J Mol Cell Cardiol. 1999;31:1771–1781. doi: 10.1006/jmcc.1999.1015. [DOI] [PubMed] [Google Scholar]
- 12.Boknik P, Grote-Wessels S, Barteska G, et al. Genetic disruption of G proteins, Gi2α or Goα, does not abolish inotropic and chronotropic effects of stimulating muscarinic cholinoceptors in atrium. Br J Pharmacol. 2009;158:1557–1564. doi: 10.1111/j.1476-5381.2009.00441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilchrist A, Li A, Hamm HE. Design and use of C-terminal minigene vectors for studying role of heterotrimeric G proteins. Methods Enzymol. 2002;344:58–69. doi: 10.1016/s0076-6879(02)44705-2. [DOI] [PubMed] [Google Scholar]
- 14.Dean DA, Machado-Aranda D, Blair-Parks K, Yeldandi AV, Young JL. Electroporation as a method for high-level nonviral gene transfer to the lung. Gene Ther. 2003;10:1608–1615. doi: 10.1038/sj.gt.3302053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arora R, Ulphani JS, Villuendas R, et al. Neural substrate for atrial fibrillation: implications for targeted parasympathetic blockade in the posterior left atrium. Am J Physiol Heart Circ Physiol. 2008;294:H134–H144. doi: 10.1152/ajpheart.00732.2007. [DOI] [PubMed] [Google Scholar]
- 16.Yue L, Feng J, Li GR, Nattel S. Transient outward and delayed rectifier currents in canine atrium: properties and role of isolation methods. Am J Physiol Heart Circ Physiol. 1996;270:H2157–H2168. doi: 10.1152/ajpheart.1996.270.6.H2157. [DOI] [PubMed] [Google Scholar]
- 17.Nilius B. Desensitization of the muscarinic receptor in the mammalian atrial myocardium. Biomed Biochim Acta. 1983;42:519–526. [PubMed] [Google Scholar]
- 18.Bauer A, McDonald AD, Nasir K, et al. Inhibitory G protein overexpression provides physiologically relevant heart rate control in persistent atrial fibrillation. Circulation. 2004;110:3115–3120. doi: 10.1161/01.CIR.0000147185.31974.BE. [DOI] [PubMed] [Google Scholar]
- 19.Donahue JK, Heldman AW, Fraser H, et al. Focal modification of electrical conduction in the heart by viral gene transfer. Nat Med. 2000;6:1395–1398. doi: 10.1038/82214. [DOI] [PubMed] [Google Scholar]
- 20.Rudolph U, Spicher K, Birnbaumer L. Adenylyl cyclase inhibition and altered G protein subunit expression and ADP-ribosylation patterns in tissues and cells from Gi2 alpha−/− mice. Proc Natl Acad Sci U S A. 1996;93:3209–3214. doi: 10.1073/pnas.93.8.3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li D, Melnyk P, Feng J, et al. Effects of experimental heart failure on atrial cellular and ionic electrophysiology. Circulation. 2000;101:2631–2638. doi: 10.1161/01.cir.101.22.2631. [DOI] [PubMed] [Google Scholar]
- 22.Ma AW, Pawagi AB, Wells JW. Heterooligomers of the muscarinic receptor and G proteins purified from porcine atria. Biochem Biophys Res Commun. 2008;374:128–133. doi: 10.1016/j.bbrc.2008.06.105. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Q, Pacheco MA, Doupnik CA. Gating properties of GIRK channels activated by Galpha(o)- and Galpha(i)-coupled muscarinic m2 receptors in Xenopus oocytes: the role of receptor precoupling in RGS modulation. J Physiol. 2002;545:355–373. doi: 10.1113/jphysiol.2002.032151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amit G, Kikuchi K, Greener ID, Yang L, Novack V, Donahue JK. Selective molecular potassium channel blockade prevents atrial fibrillation. Circulation. 2010;121:2263–2270. doi: 10.1161/CIRCULATIONAHA.109.911156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lyon AR, Sato M, Hajjar RJ, Samulski RJ, Harding SE. Gene therapy: targeting the myocardium. Heart. 2008;94:89–99. doi: 10.1136/hrt.2007.116483. [DOI] [PubMed] [Google Scholar]
- 26.Dean DA. Nonviral gene transfer to skeletal, smooth, and cardiac muscle in living animals. Am J Physiol Cell Physiol. 2005;289:C233–C245. doi: 10.1152/ajpcell.00613.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cokic IAG, Arapi D, Ng J, et al. A novel minigene-based approach to achieve long term vagal inhibition in the left atrium. Heart Rhythm. 2010;7:S94. [Google Scholar]
- 28.Giordano FJ. Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest. 2005;115:500–508. doi: 10.1172/JCI200524408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dröge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 30.Song Y, Shryock JC, Belardinelli L. An increase of late sodium current induces delayed afterdepolarizations and sustained triggered activity in atrial myocytes. Am J Physiol Heart Circ Physiol. 2008;294:H2031–H2039. doi: 10.1152/ajpheart.01357.2007. [DOI] [PubMed] [Google Scholar]
- 31.Burashnikov A, Antzelevitch C. Reinduction of atrial fibrillation immediately after termination of the arrhythmia is mediated by late phase 3 early afterdepolarization-induced triggered activity. Circulation. 2003;107:2355–2360. doi: 10.1161/01.CIR.0000065578.00869.7C. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.