Abstract
Cannabis is one of the most widely abused substances throughout the world. The primary psychoactive constituent of cannabis, delta 9-tetrahydrocannabinol (▵9_THC), produces a myriad of pharmacological effects in animals and humans. Although it is used as a recreational drug, it can potentially lead to dependence and behavioral disturbances and its heavy use may increase the risk for psychotic disorders.
Many studies that endeavor to understand the mechanism of action of cannabis concentrate on pharmacokinetics and pharmacodynamics of cannabinoids in humans. However, there is limited research on the chronic adverse effects and retention of cannabinoids in human subjects.
Cannabis can be detected in body fluids following exposure through active/passive inhalation and exposure through breastfeeding. Cannabis detection is directly dependent on accurate analytical procedures for detection of metabolites and verification of recent use.
In this review, an attempt has been made to summarize the properties of cannabis and its derivatives, and to discuss the implications of its use with emphasis on bioavailability, limit of detection, carry over period and passive inhalation, important factors for detection and diagnosis.
Keywords: Cannabis, Cannabinoids, Mental disorders, Tetrahydrocannabinol
Cannabis has been used for both recreational and medicinal purposes since several centuries (1–5). Hashish, a cannabis preparation, was found in Egyptian mummies (2). Marijuana, Hashish, Bhang and Ganja are the most widely used illicit drugs in the world. These psychoactive products are obtained from the plant Cannabis sativa (Indian hemp) and some of its subspecies. Cannabis is perceived as a recreational and harmless drug in some countries, even in leading medical journals and in some sections of the lay press (3). However, in most countries, it is categorized as a drug of abuse and its use is strictly prohibited (3, 4).Marijuana comes from leaves, stems, and dried flower buds of the cannabis plant. Hashish is a resin obtained from flowering buds of the hemp plant (5). Most cannabis preparations are either smoked, or taken orally after mixing with other substances (See Table 1).The prevalence of recreational use of cannabis has increased markedly worldwide, particularly among young people. A survey of schoolchildren in United Kingdom showed that more than 40% of 15-16 year old and up to 59% of 18-year-old students admitted to have abused cannabis at least once (6). India, which has a population of just over a billion, has 62.5million alcohol users, 8.75 million cannabis users, two million who use opioids and 0.6 million who use sedatives or hypnotics. A majority of cannabis users in India start abusing cannabis in their adolescence and quit after initial experimentation, while the rest develop dependence (7, 8).Cannabis abuse among younger subjects is associated with poor academic performance and increased school dropout. Many studies have demonstrated that psychosis, violence, aggression, sexual encounters, accidents, and crime are closely associated with cannabis abuse (1–3). Cannabis use have been associated with conduct disorders, attention-deficit hyperactivity disorder (ADHD), and learning disorders. Evidence suggests that cannabis dependence in young people predicts increased risk of using other illicit drugs, under performance in school, and reporting of associated psychotic symptoms(7, 8). Interestingly, the biomedical benefits of cannabis have also been recognized from millennia (5) and it has been shown to have therapeutic potential as an appetite stimulant, antiemetic and antispasmodic (9). Similarly, other clinical conditions where the potential use of cannabis has been suggested include epilepsy, glaucoma and asthma (10).
Table 1.
Different Preparations of Cannabinoids (23)
| No. | Form | Source | Methods of abuse |
|---|---|---|---|
| 1. | Marijuana/Charas/ Ganja | Dried leaves, stalks, flower and seeds | Smoked as joint |
| 2. | Bhang | Fresh leaves and stalk | Mixed with food items and consumed orally |
| 3. | Hashish oil | Leaves, seeds, stem and flowers soaked in oil/solvent | Smoked as joint or consumed orally |
Chemical Components of Cannabis
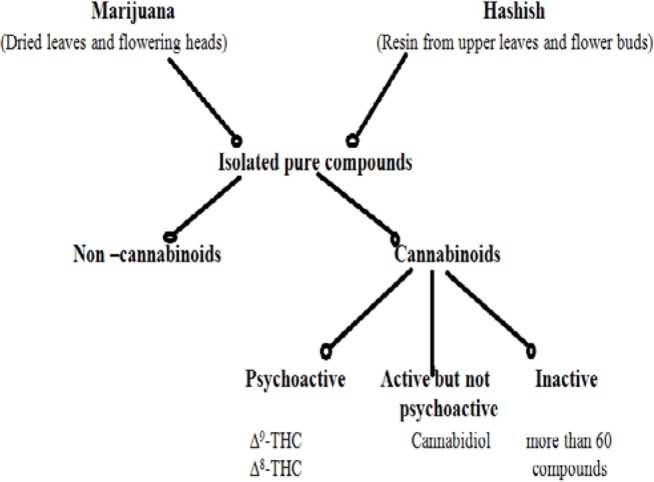
The cannabis plant contains more than 421 chemicals of which 61 are cannabinoids (9, 10) (Figure 1). Interestingly, more than 2000 compounds are produced by pyrolysis during smoking of cannabis (10, 11) and they are represented by different classes of chemicals including nitrogenous compounds, amino acids, hydrocarbons, sugar, terpenes and simple fatty acids. These compounds altogether contribute to the unique pharmacological and toxicological properties of cannabis. A list of the major cannabinoids present in cannabis is listed in Table 2. Among the listed compounds, delta 9-tetrahydrocannabinol (▵9_THC) is considered as the most psychoactive component contributing to the behavioral toxicity of cannabis (11). The aim of the current review is to discuss the properties of cannabinoids, primarily ▵9_THC and its metabolites and the clinical implications thereof.
Figure 1.
Chemical Components of Cannabis
Table 2.
Cannabinoids and their Properties (11)
| Psychoactive components | |
|---|---|
| Name | Effects |
| ▵9-tetrahydrocannabinol | Main psychoactive component; causes psychological and behavioral effects |
| (▵9_ THC) | |
| ▵8_tetrahydrocannabinol( ▵8- THC) | Less psychoactive than ▵9-THC. |
| Cannabinol(CBN) | Less powerful than ▵9_THC |
| 11-hydroxy-▵9_ THC (11-OH-THC) | Liable for psychological effects of cannabis |
| Anandamide (arachidonylethanolamide) | Imitates activity of ▵9_THC and other cannabinoids that interact with cannabinoid receptors. |
| Non-psychoactive components | |
| Cannabidiol (CBD) | Lacks psychoactive properties has anticonvulsant action. |
| Cannabichromene | Not psychoactive |
| (-)▵8- THC-11-oic acid) | Not psychoactive has analgesic activity. |
Pharmacological Actions of Cannabinoids
▵9_THC has a tri-cyclic 21- carbon structure without nitrogen and with two chiral centers in trans-configuration (9). ▵9_THC is volatile viscous oil with high lipid solubility and low aqueous solubility and a pKa of 10.6. The metabolism of ▵9_THC is shown in Figure (2). ▵9_THC is present in cannabis as a mixture of mono-carboxylic acids, which gets readily and efficiently de-carboxylated upon heating (9). It decomposes when exposed to air, heat or light (13) and readily binds to glass and plastic. Therefore, ▵9_THC is usually stored in basic or organic solvents in amber silicate glassware to avoid loss during analytical procedures (14).
Figure 2.
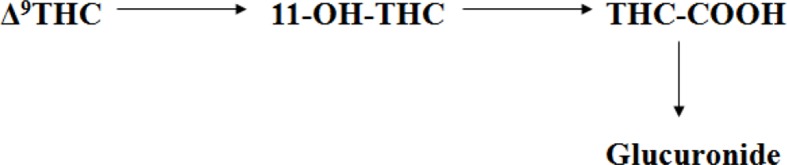
Metabolic route of ▵9-tetrahydrocannabinol (▵9_THC), its primary active metabolite 11-hydroxy-▵9-tetrahydrocannabinol (11-OH-THC) and the primary inactive metabolite, 11-nor-9-carboxy-▵ 9-tetrahydrocannabinol(THC-COOH) 12
Two hypotheses have been proposed to explain the mechanism of in vivo action of ▵9_THC. According to the first, ▵9_THC which is secreted as a glucuronide acts via non-specific interactions with cellular and organelle membranes in the brain supporting a membrane perturbation mechanism (15, 16). The second hypothesis suggests that ▵9_THC interacts with specific cannabinoid receptors (10, 17, 18). Delineating a single mechanism of action is very difficult because molecular analysis has demonstrated ▵9_THC to act on several intracellular targets including opioid and benzodiazepine receptors, prostaglandin synthetic pathway, protein and nucleic acid metabolism (19, 20). Further, cannabinoids inhibit macromolecular metabolism (21, 22) in a dose-dependent manner and have a wide range of effects on enzyme systems, hormone secretion and neurotransmitters (18, 21–23). The evidence of numerous and diffuse in vivo effects support the non-specific interaction hypothesis for THC.
Cannabinoids exert various physiological effects by interacting with specific cannabinoid receptors (CB receptors) present in the brain and periphery (24). CB1 receptors in the brain (25) are particularly concentrated in anatomical regions associated with cognition, memory, reward, anxiety, pain sensory perception, motor co-ordination and endocrine function (26, 27). CB2 receptors are localized to the spleen and other peripheral tissues (28). These receptors may play a role in the immune suppressive actions of cannabinoids. The physiological ligands for these receptors appear to be a family of anandamides (29) which are derivatives of arachidonic acid, related to prostaglandins. There is an endogenous system of cannabinoid receptors and anandamides, which normally modulate neuronal activity by its effect on cyclic-AMP dynamics and transport of Ca+ + and K+ ions (25, 30–32). Although the physiological implications of these ligand-receptor interactions are not completely understood, it is suggested to be connected with opioids, GABAergic, dopaminergic, noradrenergic, serotonergic, cholinergic, glucocorticoid and prostaglandin systems (26, 28, 30, 33). The many effects of exogenous cannabinoids derived from cannabis result from perturbation of this complex system, but the exact mechanism is not clear.
Behavioral and Physiological Effects of Cannabis
Cannabis is known to have behavioral and physiological effects (27–29).Behavioral effects include feeling of euphoria, relaxation, altered time perception, lack of concentration and impaired learning.
Memory and mood changes such as panic and paranoid reactions have also been reported. Physiological effects include rapid changes in heart rate and diastolic blood pressure, conjunctival suffusion, dry mouth and throat, increased appetite, vasodilatation and decreased respiratory rate (31, 32). Cannabis also affects the immune and endocrine system; and its abuse is associated with lung damage and EEG alterations (28, 30, 33, 34, 35).
Cannabis Dependence and Tolerance
Cannabinoids appear to affect the same reward systems as alcohol, cocaine and opioids (34).Evidence for cannabis dependence is now available from epidemiological studies (6, 8) of long-term users (58, 59), clinical populations (75, 77) and controlled experiments on withdrawal and tolerance (35, 36, 37, 38). Tolerance to cannabis can occur in relation to mood, psychomotor performance, sleep, arterial pressure, body temperature, and antiemetic properties. The critical elements of cannabis dependence include preoccupation with its use, compulsion to use and relapse or recurrent use of the substance (39). Over 50% of cannabis users appear to have ‘impaired control’ over their use (40).Symptoms such as irritability, anxiety, craving and disrupted sleep have been reported in 61-96% of cannabis users during abstinence (36, 41, 42, 43).
Psychiatric Conditions Associated with Cannabis Abuse
In addition to producing dependence, cannabis use is associated with a wide range of psychiatric disorders (44).While there is a clear relationship between the use of cannabis and psychosis, different hypotheses for the same have been propounded. One such, which describes psychosis occurring exclusively with cannabis use has limited evidence. There is strong evidence that cannabis use may precipitate schizophrenia or exacerbate its symptoms. There is also reasonable evidence that cannabis use exacerbates the symptoms of psychosis (37).
Heavy cannabis(30-50mg oral and 8-30 mg smoked) use can specifically cause a mania-like psychosis and more generally act as a precipitant for manic relapse in bipolar patients (37, 44, 45). It is possible that cannabis exposure is a contributing factor that interacts with other known and unknown (genetic and environmental) factors culminating in psychiatric illness (46). It is noticed that in many developed countries, persons with severe mental disorders are more likely to use, abuse, and become dependent on psychoactive substances especially cannabis as compared to the general population (47, 48).The same phenomenon has not been established so far in India.
Pharmacokinetics of Cannabis
▵9-THC which is highly lipophilic get distributed in adipose tissue, liver, lung and spleen (12, 49, 50). Hydroxylation of ▵9-THC generates the psychoactive compound 11-hydroxy ▵9_Tetra hydrocannabinol (11-OH-THC) and further oxidation generates the inactive 11-nor-9-carboxy-▵9-tetrahydrocannbinol (THCCOOH). THCCOOH is the compound of interest for diagnostic purposes. It is excreted in urine mainly as a glucuronic acid conjugate (12). ▵9-THC is rapidly absorbed through lungs after inhalation. It quickly reaches high concentration in blood (51). Approximately 90% of THC in blood is circulated in plasma and rest in red blood cells. Following inhalation, ▵9-THC is detectable in plasma within seconds after the first puff and the peak plasma concentration is attained within 3-10 minutes (51–55). However, the bioavailability of ▵9-THC varies according to the depth of inhalation, puff duration and breath-hold. Considering that approximately 30% of THC is assumed to be destroyed by pyrolysis, the systemic bioavailability of THC is ∼23-27% for heavy users (18, 56) and 10-14% for occasional users (48, 49). Maximum ▵9-THC plasma concentration was observed approximately 8 minutes after onset of smoking, while 11-OH-THC peaked at 15 minutes and THC-COOH at 81 minutes. This ▵9-THC concentration rapidly decreases to 1-4 ng/mL within 3-4 hour (57).
In comparison to smoking and inhalation, after oral ingestion, systemic absorption is relatively slow resulting in maximum ▵9-THC plasma concentration within 1-2 hours which could be delayed by few hours in certain cases (56, 58). In some subjects, more than one plasma peak was observed (52, 59). Extensive liver metabolism probably reduces the oral bioavailability of ▵9-THC by 4-12% (53). After oral administration, maximum ▵9-THC plasma concentration was 4.4-11 ng/mL for 20 mg (50) and 2.7-6.3 ng/mL for 15 mg (58, 60). Much higher concentration of 11-OH THC was produced after ingestion than inhalation (56, 58).Following assimilation via the blood, ▵9-THC rapidly penetrates in to fat tissues and highly vascularized tissues including brain and muscle resulting in rapid decrease in plasma concentration (61, 63).This tissue distribution is followed by slow redistribution of it from the deep fat deposits back into the blood stream.
It should be noted that the residual ▵9-THC levels are maintained in the body for a long time following abuse. The half- life of it for an infrequent user is 1.3 days and for frequent users 5-13 days (64). After smoking a cigarette containing 16-34 mg of ▵9-THC, THC-COOH is detectable in plasma for 2-7 days (57, 65). A clinical study carried out among 52 volunteers showed that THC-COOH was detectable in serum from 3.5 to 74.3 hours. Initial concentration was between 14-49 ng/mL(65). This was considerably less than the THC-COOH detection time of 25 days in a single chronic user (66).
Metabolism and Elimination of ▵9-THC
▵9-THC is metabolized in the liver by microsomal hydroxylation and oxidation catalyzed by enzymes of cytochrome P450 (CYP) complex. The average plasma clearance rates have been reported to be 11.8± 3 L/hour for women and 14.9 ±3.7 L/hour for men (59). Others have determined approximately 36 L/hour for naïve cannabis users and 60 L/hour for regular cannabis users12.
More than 65% of cannabis is excreted in the feces and approximately 20% is excreted in urine (58).Most of the cannabis (80-90%) is excreted within 5 days as hydroxylated and carboxylated metabolites (67). There are eighteen acidic metabolites of cannabis identified in urine (68) and most of these metabolites form a conjugate with glucuronic acid, which increases its water solubility. Among the major metabolites (▵9-THC,11-OH-THC, and THCCOOH), THCCOOH is the primary glucuronide conjugate in urine, while 11-OH-THC is the predominant form in feces (51, 69). Since ▵9-THC is extremely soluble in lipids, it results in tubular re-absorption, leading to low renal excretion of unchanged drug. Urinary excretion half-life of THCCOOH was observed to be approximately 30 hours after seven days and 44-60 hours after twelve days of monitoring (69, 70). After smoking approximately 27 mg of ▵9-THC in a cigarette, 11-OH-THC peak concentration was observed in the urine within two hours in the range of 3.2-53.3 ng/mL, peaking at 77.0±329.7 ng/mL after 3 hours and THCCOOH peaking at 179.4ng/mL± 146.9 after 4 hours (71, 72).
Detection and Analysis of Cannabinoids by Different Analytical Techniques
Measurement of cannabinoids is necessary for pharmacokinetic studies, drug treatment, workplace drug testing and drug impaired driving investigations (73).Because of increasing use of cannabis, developing a whole range of efficient testing methods has become essential. Cannabinoids can be detected in saliva, blood, urine, hair and nail using various analytical techniques, including immunoassays (EMIT®, Elisa, fluorescence polarization, radioimmunoassay) (74). Various chromatographic techniques such as Thin Layer Chromatography (TLC) (Foltz and Sunshine, 1990), High Performance Thin layer Chromatography (HPTLC) (72), Gas Chromatography-Mass Spectrometry (GC-MS) (79), high performance liquid chromatography-Mass Spectrometry (HPLC- MS) (79) are reliable in detection and quantitation of various cannabis metabolites.
Urine is the preferred sample because of higher concentration and longer detection time of metabolites in it. Moreover, urine can easily be sampled. Apart from cut off concentration, sensitivity and specificity of assay other factors like route of administration, amount of cannabinoids absorbed, body fat contents rate of metabolism and excretion, degree of dilution and time of specimen collection also influence delectability of ▵9_THC and its metabolites (12, 72).
The cut off value for detection of cannabinoids recommended by the Substance Abuse and Mental Health Services Administration (SAMHSA)” and European threshold of 50 ng/mL was found to be consistent with recent or heavy cannabis abuse (51, 73). Lower concentrations of THCA can be associated with occasional use, carry over period or probable cannabis exposure (70). Immunoassay is adopted as a preliminary method in the drug testing program (78). However, false negative and false positive results occur from structurally related drugs that are recognized by the antibodies or occasionally artifacts such as adulterants affecting pH, detergents and other surfactants (73, 77). For this reason, any positive result using immunoassay must be confirmed by chromatographic techniques (75, 78).Cannabis has a long half-life in humans (67 days) (57). In chronic cannabis users, it is particularly difficult to determine whether a positive result for cannabis represents a new episode of drug use or continued excretion of residual drug (62). Algorithmic models have been devised to determine whether THC levels represent new use or the carry-over from previous use (62, 64). However, these models are not very accurate in discriminating new use and carry-over in chronic users (66).
Interactions of Cannabis with other Drugs of Abuse
Interaction of chronic marijuana with other drugs of abuse has not been studied in detail. It has been demonstrated that there is no cross-tolerance between LSD and ▵9_THC (80, 81).Studies are required to understand and compare the abuse potential of marijuana in isolation and in combination with other drugs and its adverse effects on performance (80, 81). A unique opportunity is available in India for studying various facts of cannabis uses since it is possible to locate people that are abusing cannabis continuously in one form or other over several years, both in isolation, or in combination with other drugs. In such subjects, the pharmacology and the interaction of cannabis with other drugs can be studied (26, 82, 83).
Effects of Indirect Cannabis Exposure
There can be indirect exposure to cannabis through passive smoking. It has to be noted that from approximately 50% of ▵9_THC that survives pyrolysis during the smoking, a major portion (16-53%) is delivered to the smoker, while a lesser amount (6-53%) is released into the air as side stream (57). A passive inhaler in the proximity of the smoker is involuntarily subjected to inhalation of ▵9_THC smoke. In a study, five drugs-free male volunteers with a history of abuse and two marijuana naïve subjects were inactively exposed to the side stream of marihuana smoke81. Analysis of ▵9_THC concentrations in that study confirms that the detection time increased according to the passive dose. The ▵9_THC absorbed by the passive smoking depends on several features related to the condition under which passive inhalation took place (viz. environment, duration, ▵9_THC content, number of smoked joints). There is now a consensus that positive results due to passive inhalation are possible (84).
In an interesting study from Pakistan, major metabolites of cannabis were found in the milk of cows which had grazed upon naturally growing cannabis vegetation (73). Children fed on such milk showed metabolites of cannabis in the urine, suggesting passive consumption through milk (83).
▵9_THC is secreted into human breast milk in moderate amounts (85, 86, 87). A feeding infant would ingest 0.8% of weight adjusted maternal intake of one joint (86–88). Another point to be considered is that cannabis has an effect on the quality and quantity of the breast milk (85, 86). It could inhibit lactation by inhibiting prolactin production via direct action on the mammary gland. However, this data has not been confirmed in human subjects. Clinical and pharmacokinetic data indicate that cannabis use is dangerous during breast-feeding for the child (89). ▵9_ THC can accumulate in human breast milk and infants exposed to marijuana through their mother‘s milk will excrete▵9_ THC in their urine during the first 2-3 weeks (88). Due to the intake of cannabis or other drugs (psychotropic, antiepileptic) by mothers, infant children depending on breast-feeding might exhibit physiological effects such as sedation or reduced muscular tone and other adverse effects (85).
Conclusions
The recreational use of cannabis among youth has increased worldwide over the past few decades. Despite the demonstration of some bio-medical applications, cannabis abuse is associated with different disease conditions including probable risk of developing psychiatric disorders. Hence, there have been significant efforts to identify the toxic factors in cannabis and establish the role of component causes that underlie individual susceptibility to cannabinoid-related psychotic disorders. Secondly, it has necessitated the development of efficient methods to identify and quantify various cannabis metabolites from different body fluids. While immunoassay is adopted as a preliminary test, advanced chromatographic techniques are used for confirmation. Research in the future should focus on the molecular changes induced by acute and long-term exposure to cannabis and the contribution of individual psychoactive components.
Conflict of interest
All the authors declare that they have no conflicts of interest.
Contributors
PS conducted the literature searches and wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
References
- 1.Brecher EM. The Consumers Union Report - Licit and Illicit Drugs. Little, Brown & Co; 1972. [Google Scholar]
- 2.Balabanova S, Parsche F, Pirsig W. First identification of drugs in Egyptian mummies. Naturwissenschaften. 1992;79:358. doi: 10.1007/BF01140178. [DOI] [PubMed] [Google Scholar]
- 3.Grotenhermen F. The toxicology of cannabis and cannabis prohibition. Chem Bio divers. 2007;4:1744–1769. doi: 10.1002/cbdv.200790151. [DOI] [PubMed] [Google Scholar]
- 4.Raharjo TJ, Verpoorte R. Methods for the analysis of cannabinoids in biological materials: a review. Phytochem Anal. 2004;15:79–94. doi: 10.1002/pca.753. [DOI] [PubMed] [Google Scholar]
- 5.Hazekamp A, Grotenhermen F. Review on Clinical Studies with Cannabis and Cannabinoids 2005-2009. Cannabinoids. 2010;5:1–21. [Google Scholar]
- 6.Miller PM, Plant M. Drinking, smoking, and illicit drug use among 15 and 16 year olds in the United Kingdom. BMJ. 1996;313:394–397. doi: 10.1136/bmj.313.7054.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall WD. Cannabis use and the mental health of young people. Aust N Z J Psychiatry. 2006;40:105–113. doi: 10.1080/j.1440-1614.2006.01756.x. [DOI] [PubMed] [Google Scholar]
- 8.Malhotra A, Parthasarathy B. Cannabis Use and Performance in Adolescents. J Indian Assoc Child AdolescMent Health. 2006;2:59–67. [Google Scholar]
- 9.Mechoulam R. Plant cannabinoids: a neglected pharmacological treasure trove. Br J Pharmacol. 2005;146:913–915. doi: 10.1038/sj.bjp.0706415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Appendino G, Chianese G, Taglialatela-Scafati O. Cannabinoids: occurrence and medicinal chemistry. Curr Med Chem. 2011;18:1085–1099. doi: 10.2174/092986711794940888. [DOI] [PubMed] [Google Scholar]
- 11.Perez-Reyes M, White WR, McDonald SA, Hicks RE, Jeffcoat AR, Cook CE. The pharmacologic effects of daily marijuana smoking in humans. Pharmacol Biochem Behav. 1991;40:691–694. doi: 10.1016/0091-3057(91)90384-e. [DOI] [PubMed] [Google Scholar]
- 12.Musshoff F, Madea B. Review of biologic matrices (urine, blood, hair) as indicators of recent or ongoing cannabis use. Ther Drug Monit. 2006;28:155–163. doi: 10.1097/01.ftd.0000197091.07807.22. [DOI] [PubMed] [Google Scholar]
- 13.Skopp G, Potsch L. An investigation of the stability of free and glucuronidated 11-nor-delta9-tetrahydrocannabinol-9-carboxylic acid in authentic urine samples. J Anal Toxicol. 2004;28:35–40. doi: 10.1093/jat/28.1.35. [DOI] [PubMed] [Google Scholar]
- 14.Fenimore DC, Davis CM, Whitford JH, Harrington CA. Vapor phase silylation of laboratory glassware. Analytical Chemistry. 1976;48:2289–90. [Google Scholar]
- 15.Onaivi ES, Ishiguro H, Gong JP, Patel S, Perchuk A, Meozzi PA, et al. Discovery of the presence and functional expression of cannabinoid CB2 receptors in brain. Ann N Y Acad Sci. 2006;1074:514–536. doi: 10.1196/annals.1369.052. [DOI] [PubMed] [Google Scholar]
- 16.Onaivi ES. Neuropsychobiological evidence for the functional presence and expression of cannabinoid CB2 receptors in the brain. Neuropsychobiology. 2006;54:231–246. doi: 10.1159/000100778. [DOI] [PubMed] [Google Scholar]
- 17.Chaperon F, Thiebot MH. Behavioral effects of cannabinoid agents in animals. Crit Rev Neurobiol. 1999;13:243–281. doi: 10.1615/critrevneurobiol.v13.i3.20. [DOI] [PubMed] [Google Scholar]
- 18.Pertwee RG. Pharmacological actions of cannabinoids. Handb Exp Pharmacol. 2005:1–51. doi: 10.1007/3-540-26573-2_1. [DOI] [PubMed] [Google Scholar]
- 19.Burstein S, Hunter SA, Sedor C, Shulman S. Prostaglandins and cannabis--IX. Stimulation of prostaglandin E2 synthesis in human lung fibroblasts by delta 1-tetrahydrocannabinol. BiochemPharmacol. 1982;31:2361–2365. doi: 10.1016/0006-2952(82)90530-5. [DOI] [PubMed] [Google Scholar]
- 20.Grotenhermen F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin Pharmacokinet. 2003;42:327–360. doi: 10.2165/00003088-200342040-00003. [DOI] [PubMed] [Google Scholar]
- 21.Bloom AS. Effect of delta9-tetrahydrocannabinol on the synthesis of dopamine and norepinephrine in mouse brain synaptosomes. J Pharmacol ExpTher. 1982;221:97–103. [PubMed] [Google Scholar]
- 22.Chakravarty I, Sheth AR, Ghosh JJ. Effect of acute delta9-tetrahydrocannabinol treatment on serum luteinizing hormone and prolactin levels in adult female rats. FertilSteril. 1975;26:947–948. doi: 10.1016/s0015-0282(16)41364-6. [DOI] [PubMed] [Google Scholar]
- 23.Mendelson JH, Mello NK. Effects of marijuana on neuroendocrine hormones in human males and females. NIDA Res Monogr. 1984;44:97–114. [PubMed] [Google Scholar]
- 24.Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 25.Grotenhermen F. Cannabinoids. Curr Drug Targets CNS NeurolDisord. 2005;4:507–530. doi: 10.2174/156800705774322111. [DOI] [PubMed] [Google Scholar]
- 26.Adams IB, Martin BR. Cannabis: pharmacology and toxicology in animals and humans. Addiction. 1996;91:1585–1614. [PubMed] [Google Scholar]
- 27.Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, et al. Cannabinoid receptor localization in brain. Proc Natl Acad Sci U S A. 1990;87:1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gardner E, Lowinson JH. Marijuana's interaction with brain reward systems: update 1991. Pharmacol Biochem Behav. 1991;40:571–580. doi: 10.1016/0091-3057(91)90365-9. [DOI] [PubMed] [Google Scholar]
- 29.Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 30.Gardner EL, Vorel SR. Cannabinoid transmission and reward-related events. Neurobiol Dis. 1998;5:502–533. doi: 10.1006/nbdi.1998.0219. [DOI] [PubMed] [Google Scholar]
- 31.Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- 32.Howlett AC, Bidaut-Russell M, Devane WA, Melvin LS, Johnson MR, Herkenham M. The cannabinoid receptor: biochemical, anatomical and behavioral characterization. Trends Neurosci. 1990;13:420–423. doi: 10.1016/0166-2236(90)90124-s. [DOI] [PubMed] [Google Scholar]
- 33.Musty RE, Reggio P, Consroe P. A review of recent advances in cannabinoid research and the 1994 International Symposium on Cannabis and the Cannabinoids. Life Sci. 1995;56:1933–1940. doi: 10.1016/0024-3205(95)00173-4. [DOI] [PubMed] [Google Scholar]
- 34.Wickelgren I. Marijuana: harder than thought? Science. 1997;276:1967–1968. doi: 10.1126/science.276.5321.1967. [DOI] [PubMed] [Google Scholar]
- 35.Ashton CH. Pharmacolgy and effect of cannabis:A brief review. Br J Psychiatry. 2003;45:182–188. [Google Scholar]
- 36.Budney AJ, Hughes JR. The cannabis withdrawal syndrome. CurrOpin Psychiatry. 2006;19:233–238. doi: 10.1097/01.yco.0000218592.00689.e5. [DOI] [PubMed] [Google Scholar]
- 37.Maykut MO. Health consequences of acute and chronic marihuana use. ProgNeuropsychopharmacol Biol Psychiatry. 1985;9:209–238. doi: 10.1016/0278-5846(85)90085-5. [DOI] [PubMed] [Google Scholar]
- 38.Jones RT. Cannabis tolerance and dependence. In: Fehr KO, Kalant H, editors. Cannabis and Health Hazards. Toronto: Toronto Addiction Research Foundation; 1983. [Google Scholar]
- 39.Miller NS, Gold MS. The diagnosis of marijuana (cannabis) dependence. J Subst Abuse Treat. 1989;6:183–192. doi: 10.1016/0740-5472(89)90005-6. [DOI] [PubMed] [Google Scholar]
- 40.Jain R, Balhara YP. Neurobiology of cannabis addiction. Indian J Physiol Pharmacol. 2008;52:217–232. [PubMed] [Google Scholar]
- 41.Haney M. The marijuana withdrawal syndrome: diagnosis and treatment. Curr Psychiatry Rep. 2005;7:360–366. doi: 10.1007/s11920-005-0036-1. [DOI] [PubMed] [Google Scholar]
- 42.Vandrey R, Budney AJ, Kamon JL, Stanger C. Cannabis withdrawal in adolescent treatment seekers. Drug Alcohol Depend. 2005;78:205–210. doi: 10.1016/j.drugalcdep.2004.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hall W, Degenhardt L, Teesson M. Cannabis use and psychotic disorders: an update. Drug Alcohol Rev. 2004;23:433–443. doi: 10.1080/09595230412331324554. [DOI] [PubMed] [Google Scholar]
- 44.Hall W, Degenhardt L, Teesson M. Cannabis use and psychotic disorders: an update. Drug Alcohol Rev. 2004;23:433–443. doi: 10.1080/09595230412331324554. [DOI] [PubMed] [Google Scholar]
- 45.Kulhalli V, Isaac M, Murthy P. Cannabis-related psychosis: Presentation and effect of abstinence. Indian J Psychiatry. 2007;49:256–261. doi: 10.4103/0019-5545.37665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leweke FM, Koethe D. Cannabbis and Psychiatric disoreder: it is not only addiction. Addiction Biol. 2008;13:264–275. doi: 10.1111/j.1369-1600.2008.00106.x. [DOI] [PubMed] [Google Scholar]
- 47.Sewell RA, Skosnik PD, Garcia-Sosa I, Ranganathan M, D'Souza DC. Behavioral, cognitive and psychophysiological effects of cannabinoids: relevance to psychosis and schizophrenia] Rev Bras Psiquiatr. 2010;32(Suppl 1):S15–30. [PubMed] [Google Scholar]
- 48.Castle D, Murray R. Marijuana and madness. First edition. United Kingdom: Cambridge university press; 2004. [Google Scholar]
- 49.McBurney LJ, Bobbie BA, Sepp LA. GC/MS and EMIT analyses for delta 9-tetrahydrocannabinol metabolites in plasma and urine of human subjects. J Anal Toxicol. 1986;10:56–64. doi: 10.1093/jat/10.2.56. [DOI] [PubMed] [Google Scholar]
- 50.Chiarotti M, Costamagna L. Analysis of 11-nor-9-carboxy-delta(9)-tetrahydrocannabinol in biological samples by gas chromatography tandem mass spectrometry (GC/MS-MS) Forensic SciInt. 2000;114:1–6. doi: 10.1016/s0379-0738(00)00248-6. [DOI] [PubMed] [Google Scholar]
- 51.Vandevenne M, Vandenbussche H, Verstraete A. Detection time of drugs of abuse in urine. ActaClinBelg. 2000;55:323–333. doi: 10.1080/17843286.2000.11754319. [DOI] [PubMed] [Google Scholar]
- 52.Law B, Mason PA, Moffat AC, Gleadle RI, King LJ. Forensic aspects of the metabolism and excretion of cannabinoids following oral ingestion of cannabis resin. J Pharm Pharmacol. 1984;36:289–294. doi: 10.1111/j.2042-7158.1984.tb04376.x. [DOI] [PubMed] [Google Scholar]
- 53.Owens SM, McBay AJ, Reisner HM, Perez-Reyes M. 125I radioimmunoassay of delta-9-tetrahydrocannabinol in blood and plasma with a solid-phase second-antibody separation method. Clin Chem. 1981;27:619–624. [PubMed] [Google Scholar]
- 54.Wahlqvist M, Nilsson IM, Sandberg F, Agurell S. Binding of delta-1-tetrahydrocannabinol to human plasma proteins. Bio chem Pharmacol. 1970;19:2579–2584. doi: 10.1016/0006-2952(70)90007-9. [DOI] [PubMed] [Google Scholar]
- 55.Widman M, Agurell S, Ehrnebo M, Jones G. Binding of (+)- and (minus)-delta-1-tetrahydrocannabinols and (minus)-7-hydroxy-delta-1-tetrahydrocannabinol to blood cells and plasma proteins in man. J Pharm Pharmacol. 1974;26:914–916. doi: 10.1111/j.2042-7158.1974.tb09207.x. [DOI] [PubMed] [Google Scholar]
- 56.Hollister LE, Gillespie HK, Ohlsson A, Lindgren JE, Wahlen A, Agurell S. Do plasma concentrations of delta 9-tetrahydrocannabinol reflect the degree of intoxication? J Clin Pharmacol. 1981;21:171S–177S. doi: 10.1002/j.1552-4604.1981.tb02593.x. [DOI] [PubMed] [Google Scholar]
- 57.Huestis MA, Henningfield JE, Cone EJ. Blood cannabinoids. I. Absorption of THC and formation of 11-OH-THC and THCCOOH during and after smoking marijuana. J Anal Toxicol. 1992;16:276–82. doi: 10.1093/jat/16.5.276. [DOI] [PubMed] [Google Scholar]
- 58.Lemberger L, Axelrod J, Kopin IJ. Metabolism and disposition of delta-9-tetrahydrocannabinol in man. Pharmacol Rev. 1971;23:371–380. [PubMed] [Google Scholar]
- 59.Karschner EL, Schwilke EW, Lowe RH, Darwin WD, Herning RI, Cadet JL, et al. Implications of plasma Delta9-tetrahydrocannabinol, 11-hydroxy-THC, and 11-nor-9-carboxy-THC concentrations in chronic cannabis smokers. J Anal Toxicol. 2009;33:469–477. doi: 10.1093/jat/33.8.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kogan NM, Mechoulam R. Cannabinoids in health and disease. Dialogues Clin Neurosci. 2007;9:413–430. doi: 10.31887/DCNS.2007.9.4/nkogan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Haggerty GC, Deskin R, Kurtz PJ, Fentiman AF, Leighty EG. The pharmacological activity of the fatty acid conjugate 11-palmitoyloxy-delta 9-tetrahydrocannabinol. Toxicol Appl Pharmacol. 1986;84:599–606. doi: 10.1016/0041-008x(86)90266-8. [DOI] [PubMed] [Google Scholar]
- 62.Musshoff F, Madea B. Review of biologic matrices (urine, blood, hair) as indicators of recent or ongoing cannabis use. Ther Drug Monit. 2006;28:155–163. doi: 10.1097/01.ftd.0000197091.07807.22. [DOI] [PubMed] [Google Scholar]
- 63.Huestis MA. Pharmacokinetics and metabolism of the plant cannabinoids, delta9-tetrahydrocannabinol, cannabidiol and cannabinol. Handb Exp Pharmacol. 2005:657–690. doi: 10.1007/3-540-26573-2_23. [DOI] [PubMed] [Google Scholar]
- 64.Smith-Kielland A, Skuterud B, Morland J. Urinary excretion of 11-nor-9-carboxy-delta9-tetrahydrocannabinol and cannabinoids in frequent and infrequent drug users. J Anal Toxicol. 1999;23:323–332. doi: 10.1093/jat/23.5.323. [DOI] [PubMed] [Google Scholar]
- 65.Reiter A, Hake J, Meissner C, Rohwer J, Friedrich HJ, Oehmichen M. Time of drug elimination in chronic drug abusers. Case study of 52 patients in a "low-step" detoxification ward. Forensic SciInt. 2001;119:248–253. doi: 10.1016/s0379-0738(00)00437-0. [DOI] [PubMed] [Google Scholar]
- 66.Lowe RH, Abraham TT, Darwin WD, Herning R, Cadet JL, Huestis MA. Extended urinary Delta9-tetrahydrocannabinol excretion in chronic cannabis users precludes use as a biomarker of new drug exposure. Drug Alcohol Depend. 2009;105:24–32. doi: 10.1016/j.drugalcdep.2009.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Goulle JP, Saussereau E, Lacroix C. Delta-9-tetrahydrocannabinol pharmacokinetics] Ann Pharm Fr. 2008;66:232–244. doi: 10.1016/j.pharma.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 68.Halldin MM, Andersson LK, Widman M, Hollister LE. Further urinary metabolites of delta 1-tetrahydrocannabinol in man. Arzneimittelforschung. 1982;32:1135–1138. [PubMed] [Google Scholar]
- 69.Huestis MA, Cone EJ. Urinary excretion half-life of 11-nor-9-carboxy-delta9-tetrahydrocannabinol in humans. Ther Drug Monit. 1998;20:570–576. doi: 10.1097/00007691-199810000-00021. [DOI] [PubMed] [Google Scholar]
- 70.Kelly P, Jones RT. Metabolism of tetrahydrocannabinol in frequent and infrequent marijuana users. J Anal Toxicol. 1992;16:228–235. doi: 10.1093/jat/16.4.228. [DOI] [PubMed] [Google Scholar]
- 71.Johansson EK, Hollister LE, Halldin MM. Urinary elimination half-life of delta-1-tetrahydrocannabinol-7-oic acid in heavy marijuana users after smoking. NIDA Res Monogr. 1989;95:457–458. [PubMed] [Google Scholar]
- 72.Manno JE, Manno BR, Kemp PM, Alford DD, Abukhalaf IK, McWilliams ME, et al. Temporal indication of marijuana use can be estimated from plasma and urine concentrations of delta9-tetrahydrocannabinol, 11-hydroxy-delta9-tetrahydrocannabinol, and 11-nor-delta9-tetrahydrocannabinol-9-carboxylic acid. J Anal Toxicol. 2001;25:538–549. doi: 10.1093/jat/25.7.538. [DOI] [PubMed] [Google Scholar]
- 73.Altunkaya D, Clatworthy AJ, Smith RN, Start IJ. Urinary cannabinoid analysis: comparison of four immunoassays with gas chromatography-mass spectrometry. Forensic Sci Int. 1991;50:15–22. doi: 10.1016/0379-0738(91)90128-6. [DOI] [PubMed] [Google Scholar]
- 74.Fraser AD, Worth D. Monitoring urinary excretion of cannabinoids by fluorescence-polarization immunoassay: a cannabinoid-to-creatinine ratio study. Ther Drug Monit. 2002;24:746–750. doi: 10.1097/00007691-200212000-00011. [DOI] [PubMed] [Google Scholar]
- 75.Kerrigan S, Phillips WH., Jr Comparison of ELISAs for opiates, methamphetamine, cocaine metabolite, benzodiazepines, phencyclidine, and cannabinoids in whole blood and urine. Clin Chem. 2001;47:540–547. [PubMed] [Google Scholar]
- 76.Fraser AD, Worth D. Urinary excretion profiles of 11-nor-9-carboxy-delta9-tetrahydrocannabinol and 11-hydroxy-delta9-THC: cannabinoid metabolites to creatinine ratio study IV. Forensic SciInt. 2004;143:147–152. doi: 10.1016/j.forsciint.2004.02.034. [DOI] [PubMed] [Google Scholar]
- 77.Sharma P, Bharath MM, Murthy P. Qualitative high performance thin layer chromatography (HPTLC) analysis of cannabinoids in urine samples of Cannabis abusers. Indian J Med Res. 2010;132:201–208. [PubMed] [Google Scholar]
- 78.Huestis MA, Mitchell JM, Cone EJ. Detection times of marijuana metabolites in urine by immunoassay and GC-MS. J Anal Toxicol. 1995;19:443–449. doi: 10.1093/jat/19.6.443. [DOI] [PubMed] [Google Scholar]
- 79.Hidvegi E, Somogyi GP. Detection of cannabigerol and its presumptive metabolite in human urine after Cannabis consumption. Pharmazie. 2010;65:408–411. [PubMed] [Google Scholar]
- 80.Hollister LE. Interactions of cannabis with other drugs in man. NIDA Res Monogr. 1986;68:110–116. [PubMed] [Google Scholar]
- 81.Ramsay M, Percy A. Drug misuse declared: results of the 1994 British Crime Survey. London: Home office; 1996. [Google Scholar]
- 82.Huestis MA. Human cannabinoid pharmacokinetics. ChemBiodivers. 2007;4:1770–1804. doi: 10.1002/cbdv.200790152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kalant OJ. Report of the Indian Hemp Drugs Commission, 1893-94: a critical review. Int J Addict. 1972;7:77–96. doi: 10.3109/10826087209026763. [DOI] [PubMed] [Google Scholar]
- 84.Niedbala RS, Kardos KW, Fritch DF, Kunsman KP, Blum KA, Newland GA, et al. Passive cannabis smoke exposure and oral fluid testing. II. Two studies of extreme cannabis smoke exposure in a motor vehicle. J Anal Toxicol. 2005;29:607–615. doi: 10.1093/jat/29.7.607. [DOI] [PubMed] [Google Scholar]
- 85.Garry A, Rigourd V, Amirouche A, Fauroux V, Aubry S, Serreau R. Cannabis and breastfeeding. J Toxicol. 2009;2009:1–5. doi: 10.1155/2009/596149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Perez-Reyes M, Wall ME. Presence of delta9-tetrahydrocannabinol in human milk. N Engl J Med. 1982;307:819–820. doi: 10.1056/NEJM198209233071311. [DOI] [PubMed] [Google Scholar]
- 87.Fernandez-Ruiz J, Gomez M, Hernandez M, de Miguel R, Ramos JA. Cannabinoids and gene expression during brain development. Neurotox Res. 2004;6:389–401. doi: 10.1007/BF03033314. [DOI] [PubMed] [Google Scholar]
- 88.Liston J. Breastfeeding and the use of recreational drugs--alcohol, caffeine, nicotine and marijuana. Breastfeed Rev. 1998;6:27–30. [PubMed] [Google Scholar]