Abstract
Maintaining a finely-balanced network of signaling inputs is critical for the maintenance of pluripotent stem cells. Together, signaling pathways achieve this by maintaining a long-term, proliferative state while suppressing differentiation. Although the major pathways involved in pluripotency have been known for some time, it was not previously clear how they function in concert to maintain stem cell identity. Recent work has identified a signaling network involving cross-talk between PI3K, TGFβ, MAPK and Wnt pathways that culminate in a finely-balanced molecular switch that determines the fate of pluripotent cells.
Introduction
Obtaining a clear and detailed understanding of how cell signaling pathways maintain human pluripotent stem cells (hPSCs) has been difficult due to a number of confounding factors. First, use of disparate culture conditions has led to context-dependent observations. Second, the tools used to evaluate cell signaling in real-time at the single cell level are limiting and consequently, often lead to erroneous conclusions. A third issue relates to the tendency to focus on a specific pathway, rather than attempting to understand the integration and cross-talk between pathways. Fourth, variations in experimental design are often ignored, but if considered carefully can be used to explain discrepancies in the literature. Finally, signaling pathways often have different effects depending on their level of activation- this issue is frequently overlooked and has created considerable confusion. This review will specifically focus on human embryonic stem cells and human induced pluripotent stem cells- often referred to as ‘primed’ pluripotent cells due to their slightly advanced developmental stage relative to naïve or ‘ground state’ pluripotent cells [1–3]. The latter requires a different set of signaling conditions for self-renewal [4,5].
A brief historical perspective
During the late 1990’s the standard method for propagating hPSCs required their culturing on murine embryo fibroblast (MEF) feeder-layers [6]. Our understanding of self-renewal signaling during this period was quite limited and it was only when ‘second generation’, feeder-free, culture systems were introduced that significant advances were made [7]. This latter approach to culturing hPSCs commonly utilized Matrigel or laminin as attachment matrices and incorporated MEF-conditioned media (MEF-CM), fetal calf serum or knockout serum replacement (KSR) supplement, and Fgf2 for maintenance (7). The first MEF-derived bioactivity to be identified was the TGFβ superfamily member, Activin A [8,9]. Other TGFβ family members, including TGFβ and Nodal, can substitute for Activin A and work through the canonical SMAD2,3 pathway that we now know targets genes such as Nanog [10]. Under these feeder-free conditions, fibroblast growth factor 2 (Fgf2) was typically added at ~4ng/ml to supplement the activities in MEF-CM [7,11]. At the time, it was generally assumed that Fgf2 functioned through the MAPK/ERK pathway but as will be discussed later, the scenario is likely to be more complicated.
All of the early feeder-free media formulations included fetal calf serum (FCS) or knockout serum replacement (KSR) supplement [7,11]. These components contain factors such as IGF and insulin that potently stimulate the canonical phosphatidylinositol-3 kinase (PI3K) signaling pathway. As has been shown for murine PSCs [12], PI3K/AKT signaling is critical for maintenance of their human counterparts [13,14]. Other studies have implicated roles for canonical Wnt signaling in self-renewal [15], but details relating to these observations were not fully understood until recently and over time have led to considerable controversy. Together, Fgf2 signaling through MAPK, Activin A signaling through SMAD2,3, insulin/IGF signaling through PI3K and canonical Wnt signaling form the basic framework by which our understanding of self-renewal signaling is generally defined in hPSCs [4]. More recently, studies utilizing chemically-defined media have made the dissection of self-renewal signaling pathways far more feasible and have led to a new level of understanding that has established a robust model for self-renewal signaling in hPSCs [16–18].
Fgf2 and MAPK/ERK signaling in hPSCs: threshold effects?
There have been conflicting reports on the role of MAPK/ERK signaling in hPSCs but potentially, these can be reconciled [19–21]. Under steady-state conditions ERK activity is generally restrained [22,23] but can be transiently activated by factor deprivation, followed by addition of fresh growth factors [18,23]. ERK activity however, resets to lower levels once self-renewal conditions have been re-established. Both high (>50ng/ml) and low (<10ng/ml) levels of Fgf2 maintain a basal level of ERK activity that is compatible with self-renewal [24]. While low Fgf2 achieves this through mild activation of ERK, higher levels also activate the PI3K pathway without further elevating signaling along the MAPK pathway [23]. This scenario is intriguing because PI3K/AKT suppresses MAPK/ERK by a cross-talk mechanism (see Figure 1, 2) and can explain how ERK activity is maintained at a modest level under a wide-range of Fgf2 concentrations. It is still unclear if high doses of Fgf2 signal exclusively through canonical Fgf receptors or through low affinity receptors because detailed receptor activation studies have not been performed. The absolute requirement for exogenous Fgf2 has also recently been questioned because hPSCs can be maintained in its absence [23]. The caveat here however, is that Fgf activities produced by hPSCs themselves could function by an autocrine/paracrine mechanism to activate MAPK/ERK signaling.
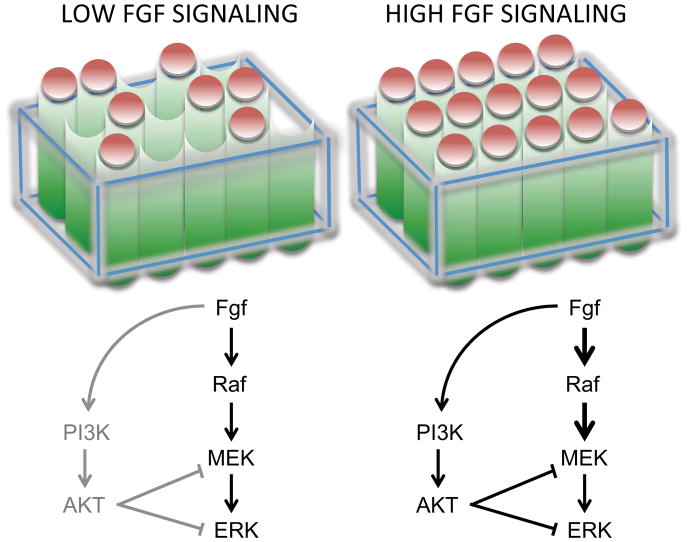
Figure 1. Fgf2 can regulate PI3K and MAPK/ERK in human pluripotent stem cells.
Left: Low concentrations of Fgf2 promote self-renewal by activation of MAPK/ERK signaling. Under these conditions, a threshold of ERK signaling intensity required for differentiation is not exceeded, enabling PSCs to be maintained in a self-renewing state. Right: Elevated Fgf2 signaling drives ERK but now also activates PI3K/AKT- this feeds back to ERK and suppress its activity. This establishes a potential mechanism where ERK activity is maintained within a critical range for self-renewal.
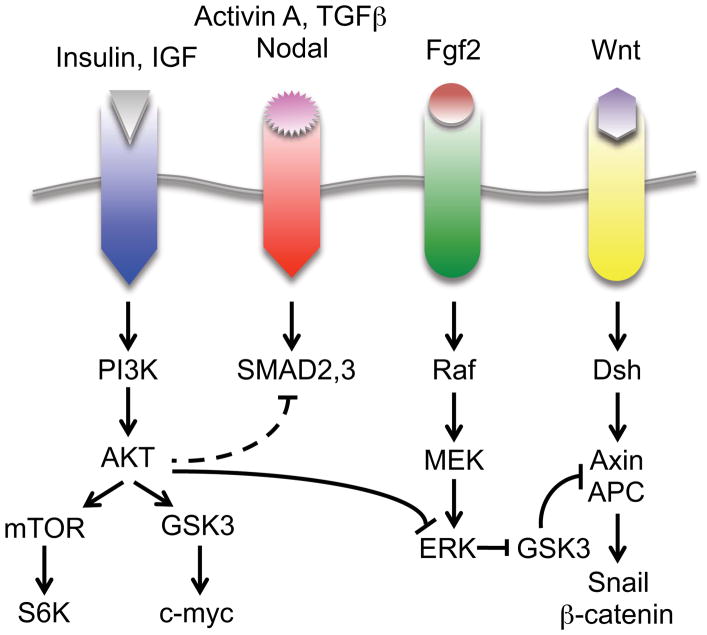
Figure 2. Summary of the signaling network that maintains human pluripotent stem cells.
Cross-talk between PI3K, TGFβ, MAPK and Wnt pathways is shown. The cross-talk network serves to suppress canonical Wnt signaling and to promote pluripotency by maintaining MAPK/ERK activity below a critical threshold. Fgf2 at high concentrations can also activate PI3K (not shown).
In response to a decline in PI3K/AKT signaling ERK activity becomes significantly elevated and plays a key role in the events surrounding early differentiation, consistent with previous reports in murine PSCs [25,26]. The most likely scenario is that low levels of ERK signaling promotes stem cell maintenance but elevated and more sustained signaling promotes differentiation. Downstream targets of ERK signaling required to initiate differentiation pathways were until recently unknown. The identification of GSK3 as an ERK target has shed new light on this question (Figure 2)[23].
The duality of Activin A and SMAD2,3 function
It was unanticipated that Activin A and other TGFβ family members would serve to maintain hPSCs because they have well-defined roles in specifying cell fate in the early embryo. However, studies from several laboratories identified Activin A as a major factor in MEF-CM that contributes to hPSC maintenance [8,9]. Latter generations of media, including most chemically defined medias, also use Activin A or other TGFβ family members for hPSC maintenance [27]. The most well known target of this pathway in hPSCs is NANOG, which is transcriptionally activated by SMAD2,3 [10,28]. Although Activin A is assumed to exert its effects through SMAD2,3 little else is known about this signaling network in hPSCs, including the identities of other target genes. A major dilemma that has only recently been resolved is how SMAD2,3 performs apparently opposing roles in self-renewal and early differentiation [13,29,30]. This apparent duality of function has been resolved by showing that SMAD2,3 activates different sets of target genes in pluripotent and differentiating cells, depending on its level of activation and the availability of cofactors [23,30]. Under self-renewal conditions, PI3K signaling restricts the absolute levels of SMAD2,3 phospho-activation and maintains it within a range compatible with self-renewal. Then, under conditions of reduced PI3K signaling, SMAD2,3 phosphorylation increases, allowing it to activate target genes involved in early differentiation. The exact mechanism for how PI3K modulates SMAD2,3 has not been resolved but this is a good example of how signaling pathways cross-talk in hPSCs (Figures 1,2).
PI3K and AKT signaling: cross-talk with SMAD and ERK
The PI3K pathway uses protein kinase B (PKB/AKT1) to relay intracellular signals generated by factors such as insulin, IGF and in some cases, EGF and FGF-family members. Once activated, AKT signals to other downstream effectors by activating mTOR and by inhibiting GSK3 [4]. As pointed out above, PI3K cross-talks with the TGFβ pathway by an as yet undefined mechanism to maintain SMAD2,3 signaling within a range that is compatible with maintenance of pluripotency. Inhibition of PI3K by addition of small molecules, such as LY294002, or following removal of PI3K activators has numerous effects on signaling pathways in hPSCs, contributing to loss of pluripotency [13,14,23]. Although PI3K/AKT maintains mTOR activity, how this impacts on pluripotency is unclear but its role in regulation of protein synthesis could be important [31]. Addition of rapamycin, an mTOR inhibitor has similar effects to that of LY294002 by inhibiting AKT and subsequently triggers differentiation [13,31]. This suggests that some feedback mechanism exists between mTOR and AKT in hPSCs. In murine PSCs, PI3K supports self-renewal by inhibiting GSK3 through phosphorylation on serine 9. One effect of GSK3 inhibition in this context is to stabilize c-myc, a key regulator of PSC maintenance [32,33]. This mechanism could also apply in hPSCs but has not been evaluated fully.
Our understanding of how PI3K maintains PSCs has dramatically changed following the observation that it is part of an elaborate cross-talk signaling network. Besides impacting on the level of phospho-SMAD2,3 activation, PI3K/AKT suppresses ERK1,2 activity which in turn decreases the activity of GSK3 [23]. Inhibition of GSK3 in this context was particularly surprising because in the canonical PI3K pathway, GSK3 is typically inhibited by AKT and yet in hPSCs, the opposite is the case. It should be remembered that there are multiple pools of GSK3 linked to separate signaling pathways. A major conclusion reached by Singh and colleagues [23] is that PI3K crosstalks with the MAPK/ERK pathway, which in turn reaches over to the canonical Wnt pathway by inhibiting GSK3 (Figure 2). The details of this will be discussed further when the role of Wnt signaling in hPSCs is considered.
Insulin and IGF clearly have the capacity to activate PI3K/AKT but it is interesting that more than one PI3K activator is usually required for maintenance of PSCs. StemPro-based media for example, utilizes IGF and the EGF family member, heregulin – both of these factors signal through PI3K but each is insufficient in the absence of the other. Other defined medias based on mTeSR utilize insulin to drive PI3K and rely on Fgf2 to support this activity. So, why is there a requirement for multiple PI3K activators? While it is possible that insulin, IGF, heregulin and Fgf2 have functions outside of PI3K activation, one possibility is that these factors signal with different temporal kinetics. This could involve the duration of signaling and changes in signaling intensity over time. It will be necessary to perform detailed time-course experiments evaluating the effects of these factors in PI3K signaling to address this question. Although low levels of Fgf2 may have a role in maintaining a low level of ERK activity, at high concentrations it seems to also activate PI3K/AKT signaling. Although not formally shown yet, high doses of Fgf2 are likely to collaborate with insulin/IGF to maintain self-renewal through the PI3K pathway. Since PI3K inhibits MAPK/ERK, Fgf2 at high levels could establish a regulatory loop where enhanced PI3K cross-talks to ERK and suppresses its activity (Figure 1). This would explain how ERK activity is maintained at low levels in pluripotent cells, despite high levels of Fgf2. The details of how Fgf2 controls MAPK and PI3K signaling by establishing different signaling thresholds are unclear and require further investigation.
The Wnt conundrum: why the confusion?
There is considerable evidence in murine PSCs that at least some components of the Wnt pathway are involved in self-renewal. For example, TCF plays a role by repressing genes that antagonize stem cell maintenance [34,35]. Recent studies indicate that β-catenin is not required for this maintenance function [36,37] and moreover, there is only limited evidence implicating Wnt itself in this maintenance pathway. In hPSCs there is little evidence implicating Wnt ligands in self-renewal and recent reports even show that Wnt signaling promote differentiation [23,38,39]. This is consistent with a large amount of evidence in vivo showing that Wnt is required around the time of gastrulation for germ layer formation. So why the confusion? One source of confusion appears to have been generated by overlooking the different pools of GSK3 and their different sensitivities to chemical inhibitors [23,32]. Some inhibitor studies have shown that when GSK3 activity is blocked, pluripotency is stabilized and this has been interpreted to mean that Wnt is important for self-renewal [15]. Another interpretation is that inhibition of the GSK3 pool immediately downstream of AKT is required for stem cell maintenance. This for example, could involve the stabilization of c-myc and other transcription factors in the core pluripotency network. Other studies have now shown that when all pools of GSK3 are inhibited, Wnt signaling through β-catenin is activated and hPSCs differentiate [23,40]. Different pools of GSK3 therefore have very different roles in control of hPSC fate determination. How activation of Wnt signaling collaborates with SMAD2,3 will be discussed below. It now seems that suppression of Wnt signaling stabilizes hPSCs [23,39] and that Wnt effector molecules such as β-catenin promote differentiation [23,40]. Oct4 has even been implicated to play a role in suppression of β-catenin signaling under self-renewing conditions [39], highlighting the opposing roles of pluripotency networks and Wnt-dependent differentiation pathways.
Cross-talk of signaling pathways establishes a stable pluripotent state
Over the last few years we have learned that the analysis of individual signaling pathways has only limited value in predicting their role in cell fate determination. Even if a pathway appears to be activated, this does not tell us whether it is in an activating or repressing mode with regards to stem cell maintenance- ERK signaling is a good example of this. Part of the difficulty in establishing a clear picture of cell signaling networks has been the lack of tools that can measure absolute signaling intensities in real time and at single cell resolution. Another limitation has been the inability to establish how changes in one pathway impact on another in real time and in a quantitative way. Finally, discriminating between different compartments of signaling complexes and how they impact on relaying signals has been difficult. This is illustrated by opposing functions for different pools of GSK3. Despite the technical limitations, we now have a far better conceptual understanding of cell signaling networks in hPSCs. The following will seek to integrate the previous sections and provide a current model for how the major signaling pathways in hPSCs integrate into a stable network.
Figure 2 summarizes our current understanding of how extrinsic signals regulate intracellular signaling pathways in hPSCs. A key aspect of this model is that no individual pathway functions by itself, but is part of an intricate cross-talk network that is subject to threshold and combinatorial effects. This model highlights the role of multiple pools of GSK3. Elevated GSK3 in the Wnt pathway is required to suppress Wnt signaling while low GSK3 activity immediately downstream of AKT is required for stabilized Myc. PI3K has at least three main effects. First, it blocks GSK3 by activating AKT and second by a cross-talk mechanism where it suppresses ERK. It’s third role modulates the extent of SMAD2,3 activation. Although there is evidence for ERK playing a role in pluripotency, this seems to apply only at low Fgf2 signaling intensities. In the presence of elevated signaling through Fgf receptors, Fgf2 activates PI3K and reinforces the negative cross-talk pathway where AKT dampens ERK activation. Finally, ERK cross-talks with the Wnt-associated pool of GSK3 and when activated, inhibits GSK3 allowing for activation of the canonical Wnt pathway. Although signaling networks are likely to change depending on culture conditions, this general model seems to apply for cells grown in the commonly used chemically defined media and for cells cultured in MEF-CM.
Wnt switches SMAD2,3 from being an agonist to an antagonist of self-renewal
One major consequence of Wnt pathway activation is the nuclear accumulation of its transcriptional effectors such as β-catenin. Once stabilized, β-catenin collaborates with SMAD2,3 to target genes required for early differentiation (Figure 3). In effect, β-catenin switches SMAD2,3 from being a key element of the self-renewal machinery to an activity that promotes differentiation [23]. A similar scenario operates in endoderm differentiation where Eomes collaborates with Smad2,3 [30]. This is a reminder of how the same regulators (in this case SMAD2,3) can be used in different contexts to promote contrasting biological outcomes [41].
Figure 3. Wnt switches the activity of SMAD2,3 by redirecting it to new targets.
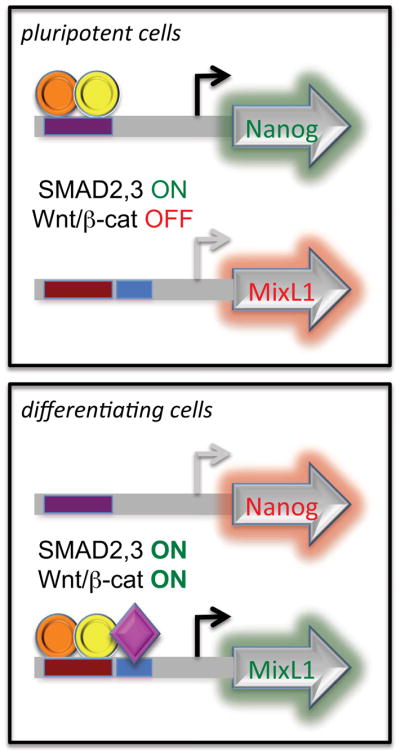
Perturbation of the pluripotency signaling network switches SMAD2,3 from being an activator of pluripotency target genes to an activator of genes required for early differentiation. The switch mechanism arises from the activation of β-catenin which collaborates with SMAD2,3 to activate a different set of target genes. Smad2,3 (orange circle) binds to target sites with its obligatory binding partner Smad4 (yellow circle). Cofactors such as β-catenin and Eomes (purple diamond) can bind to target genes in conjunction with Smad2,3/4 complexes to activate differentiation genes.
Conclusions
Recent studies have provided a robust framework on which we can now base future work relating to signaling networks in pluripotent stem cells. Many questions remain and other pathways not discussed in this review are likely to play important roles. For example, suppression of specific protein kinase C subtypes seems to be a requirement for maintenance of human [42] and murine [43] PSCs. How PKC interacts with other signaling pathways in PSCs is unclear but it will be necessary to establish how it fits into the overall signaling hierarchy. It may also be important to modulate protein kinase A to preserve self-renewal [44]. Another important question is how signaling pathways impact on reprogramming to the pluripotent state. Most reprogramming strategies use culture conditions that are known to support self-renewal and therefore, operate under the general signaling framework described in this review. It is unclear whether these signaling conditions are required for some or all of the reprogramming process or, only once the pluripotent state has been established. The biggest challenges that lie ahead in terms of understanding cell signaling in pluripotency and differentiation relate to the development of new tools that can accurately measure signaling activities in real time at the single cell level. It is only then that we will have a firm grasp on understanding stem cell behavior in response to extracellular cues. In summary, we have learned that signaling in PSCs is a complex, dynamic process where thresholds, temporal changes and combinatorial effects make important contributions to cell fate outcomes.
Highlights.
signaling in pluripotent stem cells is a complex, dynamic process
multiple signaling pathways are required for maintenance of the stem cell state: Fgf/MAPK, TGFβ/SMAD2,3 and insulin/PI3K
these pathways are highly interactive and form a finely-balanced signaling network
thresholds, temporal changes and combinatorial effects make important contributions to cell fate outcomes
Acknowledgments
This work was supported by grants to SD from the National Institute of Child Health and Human Development (RO1 HD049647) and the National Institute for General Medical Sciences (PO1 GM085354).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
- 1.De Los Angeles A, Loh YH, Tesar PJ, Daley GQ. Accessing naïve human pluripotency. Curr Opin Genet Dev. 2012;22:272–282. doi: 10.1016/j.gde.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanna J, Cheng AW, Saha K, Kim J, Lengner CJ, Soldner F, Cassady JP, Muffat J, Carey BW, Jaenisch R. Human embryonic stem cells with biological and epigenetic characteristics similar to those of mouse ESCs. Proc Natl Acad Sci USA. 2010;107:9222–9227. doi: 10.1073/pnas.1004584107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buecker C, Chen HH, Polo JM, Daheron L, Bu L, Barakat TS, Okwieka P, Porter A, Gribnau J, Hochedlinger K, Geijsen N. A murine ESC-like state facilitates transgenesis and homologous recombination in human pluripotent stem cells. Cell Stem Cell. 2010;6:535–546. doi: 10.1016/j.stem.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohtsuka S, Dalton S. Molecular and biological properties of pluripotent embryonic stem cells. Gene Ther. 2008;15:74–81. doi: 10.1038/sj.gt.3303065. [DOI] [PubMed] [Google Scholar]
- 5.Wray J, Kalkan T, Smith AG. The ground state of pluripotency. Biochem Soc Trans. 2010;38:1027–1032. doi: 10.1042/BST0381027. [DOI] [PubMed] [Google Scholar]
- 6*.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. Landmark paper describing the isolation of pluripotent stem cells from human blastocysts. [DOI] [PubMed] [Google Scholar]
- 7*.Xu C, Inokuma MS, Denham J, Golds K, Kundu P, Gold JD, Carpenter MK. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol. 2001;19:971–974. doi: 10.1038/nbt1001-971. An early report demonstrating that human pluripotent stem cells could be cultured under feeder-free conditions. [DOI] [PubMed] [Google Scholar]
- 8.Beattie GM, Lopez AD, Bucay N, Hinton A, Firpo MT, King CC, Hayek A. Activin A maintains pluripotency of human embryonic stem cells in the absence of feeder layers. Stem Cells. 2005;23:489–95. doi: 10.1634/stemcells.2004-0279. [DOI] [PubMed] [Google Scholar]
- 9.Xiao L, Yuan X, Sharkis SJ. Activin A maintains self-renewal and regulates fibroblast growth factor, Wnt, and bone morphogenic protein pathways in human embryonic stem cells. Stem Cells. 2008;24:1476–1486. doi: 10.1634/stemcells.2005-0299. [DOI] [PubMed] [Google Scholar]
- 10**.Xu RH, Sampsell-Barron TL, Gu F, Root S, Peck RM, Pan G, Yu J, Antosiewicz-Bourget J, Tian S, Stewart R, Thomson JA. NANOG is a direct target of TGFbeta/activin-mediated SMAD signaling in human ESCs. Cell Stem Cell. 2008;3:196–206. doi: 10.1016/j.stem.2008.07.001. Reports a mechanistic connection between SMAD2,3 signaling and the core pluripotent transcription machinery. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amit M, Carpenter MK, Inokuma MS, Chiu CP, Harris CP, Waknitz MA, Itskovitz-Eldor J, Thomson JA. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev Biol. 2000;227:271–278. doi: 10.1006/dbio.2000.9912. [DOI] [PubMed] [Google Scholar]
- 12.Watanabe S, Umehara H, Murayama K, Okabe M, Kimura T, Nakano T. Activation of Akt signaling is sufficient to maintain pluripotency in mouse and primate embryonic stem cells. Oncogene. 2006;25:2697–2707. doi: 10.1038/sj.onc.1209307. [DOI] [PubMed] [Google Scholar]
- 13*.McLean AB, D’Amour KA, Jones KL, Krishnamoorthy M, Kulik MJ, Reynolds DM, Sheppard AM, Liu H, Xu Y, Baetge EE, Dalton S. Activin A efficiently specifies definitive endoderm from human embryonic stem cells only when phosphatidylinositol 3-kinase signaling is suppressed. Stem Cells. 2007;25:29–38. doi: 10.1634/stemcells.2006-0219. Identified a role for PI3K in human pluripotent stem cell maintenance and implicated dual roles for Activin A in self-renewal and differentiation. [DOI] [PubMed] [Google Scholar]
- 14.Bendall SC, Stewart MH, Menendez P, George D, Vijayaragavan K, Werbowetski-Ogilvie T, Ramos-Mejia V, Rouleau A, Yang J, Bossé M, Lajoie G, Bhatia M. IGF and FGF cooperatively establish the regulatory stem cell niche of pluripotent human cells in vitro. Nature. 2007;448:1015–1021. doi: 10.1038/nature06027. [DOI] [PubMed] [Google Scholar]
- 15.Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med. 2004;10:55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- 16*.Tsutsui H, Valamehr B, Hindoyan A, Qiao R, Ding X, Guo S, Witte ON, Liu X, Ho CM, Wu H. An optimized small molecule inhibitor cocktail supports long-term maintenance of human embryonic stem cells. Nat Commun. 2011;2:167. doi: 10.1038/ncomms1165. Reports the use of small molecules to maintain human pluripotent cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang L, Schulz TC, Sherrer ES, Dauphin DS, Shin S, Nelson AM, Ware CB, Zhan M, Song CZ, Chen X, Brimble SN, McLean A, Galeano MJ, Uhl EW, D’Amour KA, Chesnut JD, Rao MS, Blau CA, Robins AJ. Self-renewal of human embryonic stem cells requires insulin-like growth factor-1 receptor and ERBB2 receptor signaling. Blood. 2007;110:4111–4119. doi: 10.1182/blood-2007-03-082586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen G, Gulbranson DR, Hou Z, Bolin JM, Ruotti V, Probasco MD, Smuga-Otto K, Howden SE, Diol NR, Propson NE, Wagner R, Lee GO, Antosiewicz-Bourget J, Teng JM, Thomson JA. Chemically defined conditions for human iPSC derivation and culture. Nature Methods. 2012;8:424–429. doi: 10.1038/nmeth.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Armstrong L, Hughes O, Yung S, Hyslop L, Stewart R, Wappler I, Peters H, Walter T, Stojkovic P, Evans J, Stojkovic M, Lako M. The role of PI3K/AKT, MAPK/ERK and NFkappabeta signalling in the maintenance of human embryonic stem cell pluripotency and viability highlighted by transcriptional profiling and functional analysis. Hum Mol Genet. 2006;15:1894–1913. doi: 10.1093/hmg/ddl112. [DOI] [PubMed] [Google Scholar]
- 20.Na J, Furue MK, Andrews PW. Inhibition of ERK1/2 prevents neural and mesendodermal differentiation and promotes human embryonic stem cell self-renewal. Stem Cell Res. 2010;5:157–169. doi: 10.1016/j.scr.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 21.Ding VM, Ling L, Natarajan S, Yap MG, Cool SM, Choo AB. FGF-2 modulates Wnt signaling in undifferentiated hESC and iPS cells through activated PI3-K/GSK3beta signaling. J Cell Physiol. 2010;225:417–428. doi: 10.1002/jcp.22214. [DOI] [PubMed] [Google Scholar]
- 22.Bernardo AS, Faial T, Gardner L, Niakan KK, Ortmann D, Senner CE, Callery EM, Trotter MW, Hemberger M, Smith JC, Bardwell L, Moffett A, Pedersen RA. BRACHYURY and CDX2 mediate BMP-induced differentiation of human and mouse pluripotent stem cells into embryonic and extraembryonic lineages. Cell Stem Cell. 2011;9:144–155. doi: 10.1016/j.stem.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23**.Singh AM, Reynolds D, Cliff T, Ohtsuka S, Mattheyses AL, Sun Y, Menendez L, Kulik M, Dalton S. Signaling network crosstalk in human pluripotent cells: a Smad2/3-regulated switch that controls the balance between self-renewal and differentiation. Cell Stem Cell. 2012;10:312–26. doi: 10.1016/j.stem.2012.01.014. Describes a cross-talk mechanism between the major signaling pathways in human pluripotent cells. Also shows that Smad2,3 is part of a molecular switch that triggers differentiation following activation of the canonical Wnt pathway. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levenstein ME, Ludwig TE, Xu RH, Llanas RA, VanDenHeuvel-Kramer K, Manning D, Thomson JA. Basic fibroblast growth factor support of human embryonic stem cell self-renewal. Stem Cells. 2006;24:568–574. doi: 10.1634/stemcells.2005-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lanner F, Rossant J. The role of FGF/Erk signaling in pluripotent cells. Development. 2010;137:3351–3360. doi: 10.1242/dev.050146. [DOI] [PubMed] [Google Scholar]
- 26.Nichols J, Silva J, Roode M, Smith A. Suppression of Erk signalling promotes ground state pluripotency in the mouse embryo. Development. 2009;136:3215–3222. doi: 10.1242/dev.038893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ludwig TE, Bergendahl V, Levenstein ME, Yu J, Probasco MD, Thomson JA. Feeder-independent culture of human embryonic stem cells. Nat Methods. 2006;3:637–646. doi: 10.1038/nmeth902. [DOI] [PubMed] [Google Scholar]
- 28.Yu P, Pan G, Yu J, Thomson JA. FGF2 sustains NANOG and switches the outcome of BMP4-induced human embryonic stem cell differentiation. Cell Stem Cell. 2011;8:326–34. doi: 10.1016/j.stem.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown S, Teo A, Pauklin S, Hannan N, Cho CH, Lim B, Vardy L, Dunn NR, Trotter M, Pedersen R, Vallier L. Activin/Nodal signaling controls divergent transcriptional networks in human embryonic stem cells and in endoderm progenitors. Stem Cells. 2011;29:1176–1185. doi: 10.1002/stem.666. [DOI] [PubMed] [Google Scholar]
- 30*.Teo AK, Arnold SJ, Trotter MW, Brown S, Ang LT, Chng Z, Robertson EJ, Dunn NR, Vallier L. Pluripotency factors regulate definitive endoderm specification through eomesodermin. Genes Dev. 2011;25:238–250. doi: 10.1101/gad.607311. Reports a role for Eomes in Smad-dependent endoderm differentiation. This highlights a mechanism where Smad2,3 switches from being a regulator of stem cell maintenance to a regulator of differentiation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou J, Su P, Wang L, Chen J, Zimmermann M, Genbacev O, Afonja O, Horne MC, Tanaka T, Duan E, Fisher SJ, Liao J, Chen J, Wang F. mTOR supports long-term self-renewal and suppresses mesoderm and endoderm activities of human embryonic stem cells. Proc Natl Acad Sci USA. 2009;106:7840–7845. doi: 10.1073/pnas.0901854106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh AM, Bechard M, Smith K, Dalton S. Reconciling the different roles of Gsk3β in “naïve” and “primed” pluripotent stem cells. Cell Cycle. 2012;11:2991–2996. doi: 10.4161/cc.21110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cartwright P, McLean C, Sheppard A, Rivett D, Jones K, Dalton S. LIF/STAT3 controls ES cell self-renewal and pluripotency by a Myc-dependent mechanism. Development. 2005;132:885–896. doi: 10.1242/dev.01670. [DOI] [PubMed] [Google Scholar]
- 34.Niwa H. Wnt: what’s needed to maintain pluripotency? Nat Cell Biol. 2011;13:1024–1026. doi: 10.1038/ncb2333. [DOI] [PubMed] [Google Scholar]
- 35.Sokol SY. Maintaining embryonic stem cell pluripotency with Wnt signaling. Development. 2011;138:4341–4350. doi: 10.1242/dev.066209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lyashenko N, Winter M, Migliorini D, Biechele T, Moon RT, Hartmann C. Differential requirement for the dual functions of β-catenin in embryonic stem cell self-renewal and germ layer formation. Nat Cell Biol. 2011;13:753–761. doi: 10.1038/ncb2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wray J, Kalkan T, Gomez-Lopez S, Eckardt D, Cook A, Kemler R, Smith A. Inhibition of glycogen synthase kinase-3 alleviates Tcf3 repression of the pluripotency network and increases embryonic stem cell resistance to differentiation. Nat Cell Biol. 2011;13:838–845. doi: 10.1038/ncb2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bone HK, Nelson AS, Goldring CE, Tosh D, Welham MJ. A novel chemically directed route for the generation of definitive endoderm from human embryonic stem cells based on inhibition of GSK-3. J Cell Sci. 2011;124:1992–2000. doi: 10.1242/jcs.081679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davidson KC, Adams AM, Goodson JM, McDonald CE, Potter JC, Berndt JD, Biechele TL, Taylor RJ, Moon RT. Wnt/β-catenin signaling promotes differentiation, not self-renewal, of human embryonic stem cells and is repressed by Oct4. Proc Natl Acad Sci USA. 2012;109:4485–4490. doi: 10.1073/pnas.1118777109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sumi T, Tsuneyoshi N, Nakatsuji N, Suemori H. Defining early lineage specification of human embryonic stem cells by the orchestrated balance of canonical Wnt/beta-catenin, Activin/Nodal and BMP signaling. Development. 2008;135:2969–2979. doi: 10.1242/dev.021121. [DOI] [PubMed] [Google Scholar]
- 41.Trompouki E, Bowman TV, Lawton LN, Fan ZP, Wu DC, DiBiase A, Martin CS, Cech JN, Sessa AK, Leblanc JL, Li P, Durand EM, Mosimann C, Heffner GC, Daley GQ, Paulson RF, Young RA, Zon LI. Lineage regulators direct BMP and Wnt pathways to cell-specific programs during differentiation and regeneration. Cell. 2011;147:577–589. doi: 10.1016/j.cell.2011.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Feng X, Zhang J, Smuga-Otto K, Tian S, Yu J, Stewart R, Thomson JA. Protein kinase C mediated extraembryonic endoderm differentiation of human embryonic stem cells. Stem Cells. 2012;30:461–470. doi: 10.1002/stem.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43*.Dutta D, Ray S, Home P, Larson M, Wolfe MW, Paul S. Self-renewal versus lineage commitment of embryonic stem cells: protein kinase C signaling shifts the balance. Stem Cells. 2011;29:618–628. doi: 10.1002/stem.605. The first report implicating a role for PKC isoforms in control of pluripotency. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamamizu K, Fujihara M, Tachibana M, Katayama S, Takahashi A, Hara E, Imai H, Shinkai Y, Yamashita JK. Protein Kinase A Determines Timing of Early Differentiation through Epigenetic Regulation with G9a. Cell Stem Cell. 2012;10:759–770. doi: 10.1016/j.stem.2012.02.022. [DOI] [PubMed] [Google Scholar]