Abstract
Chronic immobilization stress (CIS) shortens apical dendritic trees of CA3 pyramidal neurons in the hippocampus of the male rat, and dendritic length may be a determinant of vulnerability to stress. Expression of the polysialylated form of neural cell adhesion molecule (PSA-NCAM) in the hippocampal formation is increased by stress, while PSA removal by Endoneuraminidase-N (endo-N) is known to cause the mossy fibers to defasciculate and synapse ectopically in their CA3 target area. We show here that enzymatic removal of PSA produced a remarkable expansion of dendritic arbors of CA3 pyramidal neurons, with a lesser effect in CA1. This expansion eclipsed the CIS-induced shortening of CA3 dendrites, with the expanded dendrites of both no-stress-endo-N and CIS-endo-N rats being longer than those in no-stress-control rats and much longer than those in CIS-control rats. As predicted by the hypothesis that ENDO-N-induced dendritic expansion might increase vulnerability to excitotoxic challenge, systemic injection with kainic acid, showed markedly increased neuronal degeneration, as assessed by fluorojade B histochemistry, in rats that had been treated with ENDO-N compared to vehicle treated rats throughout the entire hippocampal formation. PSA removal also exacerbated the CIS-induced reduction in body weight and abolished effects of CIS on NPY and NR2B mRNA levels. These findings support the hypothesis that CA3 arbor plasticity plays a protective role during prolonged stress and clarify the role of PSA-NCAM in stress-induced dendritic plasticity.
Keywords: PSA-NCAM, stress, plasticity, dendritic branching, excitotoxicity, cell death
Introduction
Stressors that disrupt or threaten body homeostasis are a universal experience for living organisms (Selye, 1936). One of the major consequences of chronic stress and the activity circulating glucocorticoids as well as endogenous excitatory amino acids is remodeling of dendritic structure in the CA3 region of the hippocampus (McEwen, 1999). Chronic stress in rats causes atrophy of CA3 pyramidal neuron apical dendrites as measured by dendritic length and the number of dendritic branch points (Watanabe, et al., 1992). Dendritic remodeling in CA3 is, at least in part, the result of glutamate release and NMDA receptor activation, as NMDA receptor blockade prevents the response (Magarinos and McEwen, 1995). However, the remodeling response also limits further excitatory input and limits the exposure to excess free radicals produced by extended excitatory amino acid release (de Kloet, et al., 2005, McEwen, 2001).
One molecular player in formation of neuritic outgrowth, branching and terminals is the neural cell adhesion molecule (NCAM) (Washbourne, et al., 2004). The function of NCAM is reflected not only in expression levels of different polypeptide isoforms, but also by its polysialic acid (PSA) moiety, which can exert steric effects on NCAM and its environment (Rutishauser, 2008, Rutishauser and Landmesser, 1996). Importantly, PSA is critical for preventing aberrant NCAM-mediated synaptogenesis, with PSA-deficient transgenic mice exhibiting marked tissue disorganization and perinatal lethality, with lesser effects when NCAM itself is deleted (Weinhold, et al., 2005). In adulthood PSA-NCAM continues to be important for preventing aberrant synaptogenesis, as enzymatic removal of PSA residues from NCAM produces ectopic synaptogenesis and excess mossy fiber innervations in CA3 in developing animals (Seki and Rutishauser, 1998).
PSA-NCAM expression in adult brain is limited largely to areas that undergo neurogenesis and structural plasticity (Bonfanti, 2006). One of the main regions of adult PSA-NCAM expression is the hippocampus (Sandi, 2004). Most expression is in the granule cell layer (GCL) of the DG, but immunoreactivity is also present in the stratum oriens, lucidum, lacunosum-moleculare and radiatum of CA3 and CA1 (Gomez-Climent, et al., 2011, Nacher, et al., 2002). PSA-NCAM plasticity in the hippocampus is directly related to stress and corticosteroid-induced plasticity (Bisaz, et al., 2011). PSA-NCAM expression is inhibited by glucocorticoids (GC), and adrenalectomy increases PSA-NCAM expression (Cremer, et al., 2000, Rodriguez, et al., 1998). Although chronic corticosterone (CORT) decreases hippocampal PSA-NCAM expression, chronic stress biphasically alters PSA-NCAM in the DG, first increasing and then returning to steady state levels (Nacher, et al., 2004, Nacher, et al., 2004, Pham, et al., 2003, Sandi, et al., 2001). This increase in DG PSA-NCAM following chronic stress is accompanied by a decrease in NCAM (Sandi, et al., 2001). Antidepressant treatment by either fluoxetine or imipramine increases PSA-NCAM expression (Sairanen, et al., 2007, Varea, et al., 2007). PSA-NCAM is also altered following injury: e.g., global ischemia produces a glutamate-dependent increase followed by a decrease of hippocampal PSA-NCAM expression (Conrad, et al., 1999, Fox, et al., 2001).
Removal of PSA residues from NCAM appears to reduce physiological plasticity in several different contexts. The majority of these studies have utilized a bacteriophage -derived enzyme ENDO-Neuraminidase (ENDO-N) that cleaves alpha-2, 8-linked polysialic acid from live cells or tissues without detectable toxicity or gross morphological disruption (Rutishauser, et al., 1985, Vimr, et al., 1984). For example, treatment of hippocampal cell cultures with ENDO-N blocks both preferential formation of synapses onto NCAM-expressing cells and the increase in perforated spine synapses associated with NMDA receptor-dependent LTP (Dityatev, et al., 2004), with BDNF supplementation appearing to counteract this deficit (Muller, et al., 2000). Similarly, under conditions of chronic pain, C fibers in lamina II of the spinal cord are often lost and there is an overall decrease in pain sensitivity (El Maarouf, et al., 2005). Removing PSA from NCAM prevents the reduction of these terminals and the reduction in pain sensitivity that is seen in vehicle treated animals (El Maarouf, et al., 2005). Similar prevention of adult synaptic plasticity occurs in the circadian clock in the suprachiasmatic nucleus and in neuro-glial plasticity associated with salt balance and the estrus cycle in the hypothalamus (Hoyk, et al., 2001, Monlezun, et al., 2005, Prosser, et al., 2003). Additionally, upregulation of GAD67 and synaptophysin induced by chronic dopamine 2 receptor activation is prevented by removal of PSA from NCAM residues with endo-N (Castillo-Gomez, et al., 2011). These results suggest that PSA-NCAM may be a major component of multiple forms of structural plasticity in the hippocampal formation.
If PSA-NCAM is sufficient to expand dendritic complexity independent of stress then this would help address whether CA3 pyramidal cell dendritic remodeling is at least in part a protective homeostatic mechanism, or evidence of damage itself. Animals that receive an injection of ibotenic acid (IBO) to CA3 following chronic immobilization stress (CIS) or chronic GC treatment show increased damage compared to non-stressed controls (Conrad, et al., 2004, Conrad, et al., 2007). But it remains an open question whether stress-induced modulation of dendritic morphology exacerbates or protects neurons from excitotoxic cell death. That is, would the stress-induced exacerbation of excitotoxic injury be even worse if the dendrites were even longer.
The goals of this study therefore are threefold. First, to determine whether enzymatic cleavage of PSA residues from NCAM will itself alter dendritic complexity and interact with chronic stress; second, to investigate the neurochemical and behavioral consequences of potentially dissociating dendritic length and volume from the stress response; and, third, to determine whether partially dissociating dendritic remodeling from stress would render neurons differentially vulnerable to excitotoxicity.
Materials and Methods
Animals
Experiments were performed on adult male Sprague-Dawley rats (SD strain; Charles River, Wilmington, MA). Rats were obtained at two months of age, approximately 200–250 g. After arrival, rats were kept for one week 3 per cage to recover from the shipping process. Rats were then single-housed, and had unlimited access to food and water except during experimental manipulations. The cages were maintained on a 12-hour light/dark cycle, with lights on from 7:00 am to 7:00 pm. All experimental manipulations were performed during the light period. All experiments and procedures were conducted according to protocols approved by the Institutional Animal Care and Use Committee (IACUC).
ENDO-N treatment
The enzyme ENDO-Neuraminidase (endoN) was prepared as described previously (El Maarouf and Rutishauser, 2003). Rats were anesthetized with xylazine (0.5 mg/kg; i.p.) and ketamine (0.4 mg/kg; i.m.). A syringe was lowered 0.15mm dorsal to the dura at 0.38mm posterior to bregma and 0.15mm to the right of the midline and 174 U of ENDO-N (in 2μl sterile saline) or sterile saline was slowly injected into the cortical parenchyma. Intracortical injection is sufficient to remove virtually all PSA residues from NCAM throughout the nervous system apparently via diffusion (Ono, et al., 1994, Seki and Rutishauser, 1998). The dose is based on published doses for both intraparenchymal and ICV administration e.g. (Black, et al., 2009).
Chronic Immobilization Stress (CIS)
One week after surgery, animals were subjected to 2 hours of CIS daily for 10 days. Rats were restrained in plastic conical bags similar to commercially available DecapiCones. Control animals were kept in a separate room from stressed animals and handled 2 min/d for 10 d during the immobilization stress period to acclimate them for subsequent behavioral testing. All animals were weighed on the first and the last day of the stress experiment. Twenty-four hours after the final restraint session animals were killed either by transcardial perfusion with paraformaldehyde or rapid decapitation. Trunk blood was collected at tissue collection and the serum was analyzed for corticosterone levels using the Coat-A-Count rat corticosterone kit (Diagnostic Products Corporation).
Golgi Staining and Analysis
Freshly collected brains were processed with the FD Rapid GolgiStain™ Kit (FD Neuro Technologies) according to previously established methods (Glaser and Van der Loos, 1981) that give successful staining of hippocampal pyramidal cells (McLaughlin, et al., 2007). Brains were cut in 200 μm sections (Leica VT 1000S Vibratome) and mounted on Superfrost Plus slides (Fisher Scientific) processed according to the manufacturer’s instructions and coverslipped with DPX.
To select cells for analysis, impregnated neurons had to fit the following characteristics: (1) full impregnation of the cell body and dendrites; (2) relative isolation from surrounding impregnated neurons; (3) located within the segment of CA3b excluding the curvature nearing CA2 and not in CA3c; (4) cell bodies located within the middle third of the tissue section to avoid false endings of dendrites at the edges of the section. Neurons were traced using a Nikon Eclipse E600 microscope using a 40X objective and Neurolucida 8 software (MBF Bioscience).
There are three subtypes of pyramidal neurons in CA3: neurons with a single shaft that is either short (short shaft, SS) or long (long shaft, LS), and neurons with more than one primary shaft (two-shaft, 2S) (Fitch, et al., 1989). Each of the subtypes has a different degree of dendritic complexity, so care was taken to represent the subtypes equally across animals and across experimental groups. For each group, 18–22 neurons were selected and analysis was conducted of total dendritic length and dendritic branch points for the apical dendrites. The Sholl method (Uylings, et al., 1986) was also used to measure apical dendritic complexity in 30 μm intervals.
Immunohistochemistry
Tissue was fixed by transcardial perfusion with 4% paraformaldehyde and free-floating sections were analyzed for PSA-NCAM immunoreactivity. Briefly, sections were incubated overnight at room temperature in mouse monoclonal anti-PSA IgM (1:700; Chemicon). PSA immunoreactivity was visualized with an AlexaFluor 488 conjugated secondary antibody. Sections were analyzed on a Nikon Eclipse E600 microscope using StereoInvestigator software (MBF Bioscience).
In Situ Hybridization and Analysis
Freshly collected and frozen brains were cut at 20 μm on a cryostat and placed on Fisher Superfrost Plus slides. The probes used were the following sequences: NR2A 5′-TCG GGA GTT CCC TTT GGA TTC AGT GCT GAC AGC-3′; NR2B 5′-CAT GTT CTT GGC CGT GCG GAG CAA GCG TAG GAT-3′ NPY 5′-TGC CCG GAC CTG GCC CCT CTG CTC CGC CCC-3′. First a tailing reaction was performed to radioactively label the oligonucleotide probes with 33P –dNTP at the 3′ end using terminal deoxynucleotidyl transferase (Promega) to a specific activity of 5 × 109 cpm/μg. The probe was then purified with the QIA quick spin nucleotide removal kit (Qiagen). Sections were exposed to the probes washed and then air-dried and exposed to Kodak MR autoradiography films for 2 weeks, developed, and analyzed for optical density using MCID (Imaging Research) (Conrad and McEwen, 2000). Hippocampal measurements were taken bilaterally at 2.18 mm caudal to bregma.
Kainate-induced neuronal degeneration
Rats were subjected to either CIS or the control procedure and then half of all animals were treated with endoN as described above. Seizures were induced by injecting rats with 10mg/kg of kainic acid dissolved in saline and 2.5 h later the seizures were halted by i.p. injection of Na phenytoin (50 mg/kg) and then the animals were perfused 48 hours after the induction of the seizures (Kim, et al., 2007).
Fluorojade B Histochemistry
Fluorojade B (FJ) is a fluorescein derivative that labels degenerating neurons. Briefly, slides were dried at room temperature, immersed in a basic ethanol solution (80% containing 1% sodium hydroxide) and then rinsed in 70% ethanol and distilled water (dH20). Slides were then treated with potassium permanganate (.06% in (dH20) for 10 minutes, rinsed with water, and then incubated in Fluorojade B (0.0001% in a 1% acetic acid solution); sections were simultaneously counterstained with DAPI (Sigma-Aldrich, St. Louis, MO, USA), rinsed in dH20, and thoroughly dried on a slide-warmer, cleared for 1 minute in xylene, and coverslipped with DPX (Sigma).
Fluorojade positive cells were counted in multiple hippocampal regions (3 sections at least 150 microns apart approximately 1.85–2.15 mm caudal to bregma; CA1, CA2, CA3, dentate gyrus, dentate hilus, and subiculum) online by an experimenter unaware of the experimental conditions associated with each sample. Both sides of the hippocampus were counted in a single section and averaged.
Data Analysis
Data was analyzed using SPSS utilizing two-way ANOVA and Bonferroni post-tests except for Scholl analysis data on dendritic structure that was analyzed with repeated measures ANOVA. All data are presented as mean ± SEM. P values < 0.05 were considered significant.
Results
ENDO-N injection completely abolishes PSA expression in the hippocampus
In the hippocampus PSA-NCAM expression was mainly concentrated in the innermost part of the granule cell layer, where granule neuronal somata and their apical dendrites appeared labeled. Intense PSA-NCAM expression was detected in the thick processes of the mossy fibers, traversing the hilus and in the stratum lucidum of CA3 (Fig 1A). Thinner processes can also be observed traversing the stratum pyramidale and oriens of this hippocampal region. Some of these thinner processes belong to large PSA-NCAM expressing cells located mainly in the strata radiatum and oriens (Fig. 1B), which resemble those previously described as mature interneurons (Nacher, et al., 2002). Chronic immobilization stress was found to qualitatively reduce PSA immunoreactivity (Supplemental Fig. 1), consistent with previous reports (Pham, et al., 2003). The efficacy of ENDO-N treatment in eliminating PSA content from the hippocampus was then tested. Administration of ENDO-N abolished PSA immunoreactivity in both control and CIS animals (Supplemental Fig. 1), indicating that this treatment is sufficient to markedly suppress PSA expression in the hippocampus. Next we sought to determine whether PSA removal by ENDO-N would alter morphological and neurochemical responses to chronic immobilization stress.
Figure 1.

PSA-NCAM immunohistochemistry in the hippocampal CA3 region. A. Panoramic view showing the presence of intense PSA-NCAM immunostaining in the stratum lucidum. An interneuronal soma (arrow) and several processes, probably belonging to interneurons, can be observed in the stratum radiatum. Some scarce immunoreactive processes can also be found in the stratum oriens. B. Detailed view of the CA3 region. Thick processes belonging to mossy fibers can be observed in the stratum lucidum. Thinner processes also can be seen traversing the stratum pyramidale and oriens. S.L.: stratum lucidum; S.O.: stratum oriens; S.P: stratum pyramidale; S.R.: stratum radiatum. Figure A is a 2D projection of 10 confocal planes and B is a single confocal plane. Scale bar: 100 μm for A and 25 μm for B.
The physiological response to CIS changes significantly with PSA removal
Rats of the age used in this study generally gain body mass over time, and chronic stress significantly reduces the rate of weight gain. Accordingly, chronically immobilized rats gained less body mass during the period of the CIS paradigm (F1, 38=132.44, p<0.000001; Fig. 2). Remarkably, CIS caused even more impairment following PSA removal; body mass growth was completely suppressed (F1, 38=9.36, p<0.005). There was no significant interaction between these variables in preplanned comparisons among stressed groups; the weight gain with ENDO-N and stress was significantly less than with stress alone (F1, 19=7.26, p<0.05). Unstressed ENDO-N-treated rats showed normal weight gain (Fig. 2).
Figure 2.

PSA removal exaggerated CIS effects on body mass growth. Rats at the age used in this study are typically still gaining body mass, and endoN treatment alone did not affect this growth. However, chronic stress slowed this weight gain, and PSA removal by endoN exacerbated this CIS effect. * Indicates significantly different from no stress groups in the same injection type; #Indicates significantly different than stress-endoN group. Body mass growth was completely suppressed by CIS in the absence of PSA. Data are presented as means (± SEM), N=8–9/group.
We next asked whether ENDO-N treatment might alter neuroendocrine responses to CIS. While we found no difference between control and stressed vehicle treated rats in serum CORT levels 24 hours after the last stressor, we found that ENDO-N treatment resulted in an increase in circulating corticosterone concentrations in CIS animals compared to vehicle-treated CIS animals (p < 0.05). Since ENDO-N did not change corticosterone levels in non-stressed rats, these data suggest that ENDO-N selectively increased HPA reactivity following chronic stress (Supplementary Figure 2). This increased HPA axis reactivity was mirrored by reduced expression of anti-stress peptide neuropeptide Y. mRNA levels for the neuropeptide Y (NPY) in CA1 and CA3 subfields of the hippocampus were abolished by ENDO-N treatment, but not those in the lower blade of the dentate gyrus (Supplemental Figure 3).
Expression of NMDA receptors
An additional factor that is altered by chronic stress is the glutamate system in general and the NMDA receptor system in particular (Angata, et al., 2007). We performed mRNA in situ hybridizations to determine if PSA removal and chronic stress alters expression of the gene encoding NMDA receptor subunits. NR2A gene expression in the dentate gyrus was not altered by stress, ENDO-N treatment or the interaction among these variables (P<0.05 in all cases; Fig. 3A). Furthermore, there were no detectable effects of either stress or PSA removal on NR2B gene expression. However, there was a significant interaction between the two variables (F1, 33=5.20, p<0.05; Figure 3b) that was mediated by an opposite effect of stress in vehicle and ENDO-N-treated rats. Specifically, CIS significantly reduced NR2B gene expression in vehicle-treated animals (p<0.05) but tended if anything, to increase it in ENDO-N treated animals.
Figure 3.
Expression of NMDA receptor subunit genes. CIS or endoN did not produce any change in NR2A mRNA levels in the dentate gyrus that could be detectable by in situ hybridization. However, NR2B gene expression was reduced by CIS, an effect that was blocked by endoN treatment. * Indicates significant differences at P<0.05. Data are presented as means (±SEM) N=9–10/group.
Removal of PSA extends dendritic arbors in CA3 pyramidal neurons and occludes their CIS-induced atrophy
The effects of both CIS and PSA removal on dendritic morphology of CA3 pyramidal neurons were examined at the end of the physiological evaluations. The total dendritic length was longer in ENDO-N-treated animals (F1, 42=62.06, p<0.000001; Fig. 4A) compared to vehicle-injected controls and significantly shorter in rats exposed to CIS (F1, 42=9.23, p<0.005). However there was no interaction between the variables (p>0.05). There were also significantly more branching points in ENDO-N-treated rats (F1, 42=50.41, p<0.000001; Fig. 4B) and significantly fewer dendritic intersections in stressed rats compared to control animals (F1, 42=16.20, p<0.00001) with again no interaction between the two variables (p>0.05).
Figure 4.

PSA removal antagonized the specific effect of CIS on dendritic branches.
Top: Representative camera lucida traces from Vehicle and endoN -treated rats that were exposed to ten days of CIS. The dendritic arbors were larger in endoN -treated animals.
Bottom: (A) CIS shortened the total length of CA3 dendritic arbor while PSA removal produced an increase in these measures. With combination of CIS and ENDO-N, the total arbor length was shorter than with endoN alone but still larger that control values. (B) Interestingly, the effects on numbers of branching points followed a similar pattern. (C) Sholl analysis revealed that PSA removal alone produced an elongation of branches located at all distances from the soma (from 0 to 550 μm approximately), while CIS alone specifically shortened branches located near the soma (approximately between 100 and 250 μm). Interestingly, PSA removal completely antagonized this CIS-induced dendrite atrophy. Data are presented as means (±SEM) N=9–10/group.
Sholl analysis of dendritic branch length of CA3 neurons indicated a significant effect of distance from the soma (F21, 756=131.97, p<0.000001; Fig. 4C), with a three-way interaction among ENDO-N treatment, CIS and distance from the soma (F21, 756=1.70, p<0.05). PSA removal alone elongated branches at all distances from the soma (F21, 756=12.7, p<0.000001). However, CIS produced a marked retraction of branches specifically at shorter distances from the soma; and, interestingly, the effect of PSA removal suppressed this CIS effect on branch length. It appears therefore that PSA removal causes significant growth and extension of CA3 dendritic trees, which renders the CIS-induced atrophy incapable of reducing arbor size below its control size. A similar, though far less dramatic expansion of dendrites was found in CA1 pyramidal neurons (Supplemental Fig. 4).
Effects of ENDO-N upon neurotoxicity
In order to determine if ENDO-N altered vulnerability to excitotoxic challenge, rats were injected systemically with the excitotoxin, kainic acid. Neuronal degeneration, as assessed by fluorojade B histochemistry was markedly increased in rats that had been treated with ENDO-N compared to vehicle treated rats both throughout the entire hippocampus (F1, 17=5.10, p<0.05; Figure 5), CA1 field (F1, 17=5.17, p<0.05), and dentate gyrus (F1, 17=7.00, p<0.05). There were no effects of stress or interaction between stress and ENDO-N treatment in any part of the hippocampal formation. Taken together these data indicate that ENDO-N treatment, presumably via PSA-NCAM depletion and possibly by increasing the dendritic arbors of pyramidal neurons, renders the hippocampus more vulnerable to excitotoxic injury.
Figure 5.
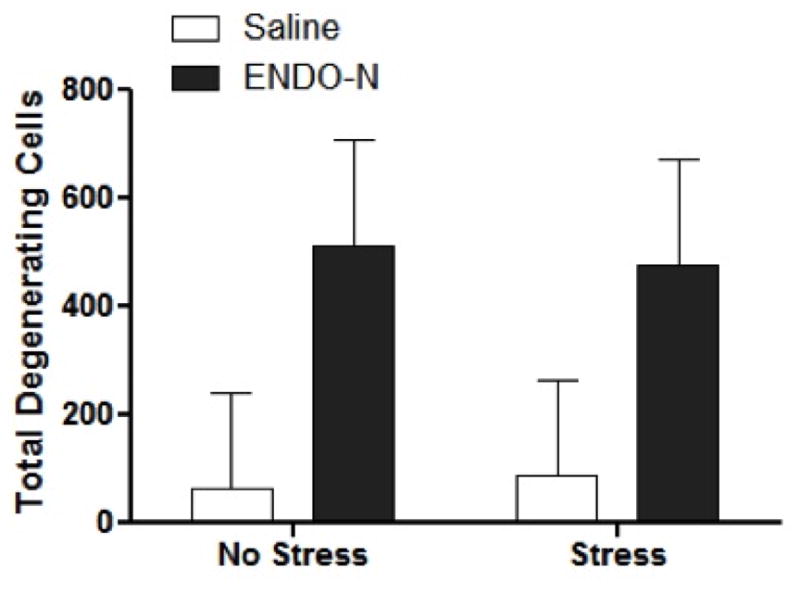
ENDO-N treatment exacerbates kainate-induced hippocampal degeneration independent of stress conditions. EndoN increased cell death independent of stress conditions (p<0.05). Sections were stained with Fluorojade B histochemistry and total degenerating hippocampal cells were counted. Data are presented as means (±SEM) N=4–5/group.
Discussion
Hippocampal dendritic retraction and recovery is a phenomenon that occurs across species and in response to a variety of physiological stimuli (Czeh and Lucassen, 2007, Izquierdo, et al., 2006, Magarinos, et al., 1996, Magarinos, et al., 2006, Popov, et al., 1992, Watanabe, et al., 1992). In the present study in male rats, we confirmed that CIS specifically shortens hippocampal apical branches located at short distances from the soma a portion of the dendrite located in the stratum lucidum, in which most mossy fibers make synaptic contacts, and found that removal of PSA from NCAM in the hippocampus by ENDO-N expands CA3 dendritic branches at all distances from the soma, including an occlusion of the anatomical consequences of CIS. The effect of ENDO-N treatment renders the hippocampus more vulnerable to excitotoxic challenge, apparently independently of the CIS history, and alters some of the physiological and neurochemical responses to CIS.
Possible mechanisms for ENDO-N effects
The adult CA3 is a dynamic region that continuously receives new mossy projections (Seki and Rutishauser, 1998), and this process is likely to involve constant growth and retraction of pyramidal dendrite branches to adjust connectivity. The high levels of PSA that are normally expressed on mossy fibers possibly modulate these plastic events (Burgess, et al., 2008, Seki and Arai, 1999, Seki and Rutishauser, 1998). PSA cleavage by ENDO-N causes mossy fibers to defasciculate into a more spread innervation area, with formation of ectopic synapses, in developing animals (Seki and Rutishauser, 1998). It is also notable that these effects in CA3 appear to cascade down hippocampal circuitry, in that a similar, although far less dramatic, effect with ENDO-N was seen in CA1 apical dendrites, which receive input from the Schaffer collaterals of CA3 pyramidal neurons (Supplemental Fig. 3). Importantly, ENDO-N increased cell death disproportionately in the CA1 field of the hippocampus further reinforcing the importance of the synaptic changes in the dentate and CA3.
Mossy fibers project within the stratum lucidum and typically innervate the proximal apical dendrites of CA3 neurons. CIS specifically shortens branches located in this proximal part of the dendritic tree (Fig. 4), and it is therefore reasonable to suggest that PSA removal occludes the CIS-induced retraction of CA3 dendrites by increasing dendrite-mossy fiber interactions and possibly stabilizing synapses (El Maarouf, et al., 2005, El Maarouf and Rutishauser, 2003).
Although most of the effects of PSA depletion in the hippocampus may be due to structural remodeling of mossy fibers and their connections, a contribution of local interneurons should not be discarded, since a subpopulation of these cells expresses PSA-NCAM in the adult hippocampus (Nacher, et al., 2002). These interneurons have reduced structural features and connectivity when compared with those lacking PSA-NCAM (Gomez-Climent, et al., 2011).
ENDO-N effects on physiological, behavioral and neurochemical responses to CIS
While the ENDO-N-induced changes in hippocampal connectivity did not appear to cause gross physiological alterations in the absence of chronic stress, some aspects of CIS-altered physiology and behavior were amplified by PSA depletion. For example, the CIS-induced inhibition of body mass growth typically seen in rats of the age used in this study was significantly exacerbated by ENDO-N treatment (Ricart-Jane, et al., 2002). Importantly, glucocorticoid concentrations were elevated 24 hours following stress in the endoN treated animals suggesting the possibility that the overall consequences of chronic stress may have been amplified by PSA depletion.
Further evidence for this hypothesis is provided by reduced body mass in endoN treated stressed animals potentially indicating that PSA residues, and possibly by extension dendritic withdrawal buffers the deleterious consequences of chronic stress. Furthermore, ENDO-N treatment abolished the increase in levels of the neuropeptide Y (NPY) in CA1 and CA3b fields of the hippocampus. As NPY has been implicated in resilience to the deleterious effects of stress this phenomenon may provide further indication of enhanced vulnerability to stress following endoN treatment. Taken together, these data suggest that the consequences of chronic stress are buffered by PSA-facilitated dendritic retraction as well as up-regulation of NPY, and, accordingly, that when CIS is combined with the larger dendritic trees caused by PSA removal, some of the consequences of stress, including weight loss, (Ricart-Jane, et al., 2002) may be exacerbated.
One potentially important point is that CORT concentrations were only elevated in CIS-endoN treated animals. The lack of corticosterone elevation in CIS animals that were treated with vehicle is likely due to the return to baseline by 24 hours following the final stress session. Still, this provides further evidence of a potentiated HPA response to CIS in endoN treated animals, as these concentrations were still elevated after the conclusion of CIS.
Positive and negative effects of stress-induced dendritic atrophy
Nevertheless, longer dendrites in ENDO-N treated animals, irrespective of CIS history, led to greater damage of CA3 neurons after kainic acid treatment and this finding indirectly addresses a central question regarding stress-induced dendritic atrophy, namely, whether this reduction in dendritic material represents some type of damage to neurons or an adaptive adjustment that protects neurons from overstimulation by excitatory inputs.
Stressors increase the vulnerability of neurons to a variety of injuries including the direct application of ibotenic acid (Conrad, et al., 2007). Presumably chronic stress increases the vulnerability of neurons to excitotoxic cell death via a number of mechanisms including poorer metabolic function, oxidative stress, predisposition to inflammation and suppression of anti-apoptotic signaling. There is also the possibility that chronic stress increases genomic instability through failure to repress retrotransposon DNA elements (Hunter, et al., 2012).
On the other hand, dendritic retraction may serve to oppose these effects of stress by reducing the metabolic cost of maintaining processes and reducing the exposure to excitatory input. Thus the reduction of CA3 pyramidal arbor size under CIS would serve as a mechanism to lessen transmission as well as reduce consequences of stressful and/or traumatic insults. The fact that the occlusion of CIS-induced retraction in ENDO-N-treated rats correlated with stronger stress effects on body weight and corticosterone supports a protective and buffering role for this retraction.
It is interesting that, in our studies, CIS caused a decrease in DG NR2B expression. This change was suppressed by PSA removal, and is likely to reflect the amount of neurotransmission going through the DG to CA3; nevertheless, CIS did not appear to increase the damage from the excitotoxicity. However, PSA residues inhibit the opening of NMDA receptors containing NR1/NR2B subunits suggesting that ENDO-N treated animals may exhibit significantly greater NMDA mediated excitation in the absence of PSA (Kochlamazashvili, et al., 2012) and this could be an additional factor in increased excitotoxicity after ENDO-N treatment, as PSA-NCAM has been shown to protect cells from NMDA currents and excitotoxicity in vitro (Hammond, et al., 2006).
The possibility that dendritic retraction and changes in vulnerability to excitotoxicity are not directly linked remains. PSA-NCAM depletion interferes with BDNF signaling (Vutskits, et al., 2001) and may alter other aspects of cellular physiology that could render cells vulnerable to excitotoxicity. In any case, a mechanism that would allow the clean dissociation of dendritic plasticity from other aspects of cellular physiology is necessary to fully answer this important question.
The notion of hippocampal plasticity as a mechanism to buffer the effects of stress has been suggested recently with reference to DG neurogenesis (Snyder, et al., 2011). Moreover, an analogous PSA-dependent mechanism appears to exist in the nociceptive system, where non-peptidergic C-terminals retract from their spinal targets under the stress of chronic pain. In this system, PSA removal blocks C-fiber atrophy with a corresponding exaggeration of pain levels (El Maarouf, et al., 2005, El Maarouf and Rutishauser, 2003). These findings imply that the expression of PSA pathways facilitates a protective adjustment in neuronal circuitry to environmental conditions.
Supplementary Material
Highlights.
Chronic stress alters the morphology of hippocampal dendrites
PSA-NCAM is affected by stress and may regulate synaptic plasticity.
PSA depletion increased dendritic length without altering stress-induced changes.
Larger dendrites were associated with greater vulnerability to excitotoxicity.
Acknowledgments
Research support for this project is from NIH Grant MH41256 to BMc. Spanish Ministry of Science and Innovation (MICINN-FEDER) BFU2009-12284/BFI, MICINN-PIM2010ERN-00577/NEUCONNECT in the frame of ERA-NET NEURON” and Generalitat Valenciana ACOMP/2012/229 to JN.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Angata K, Huckaby V, Ranscht B, Terskikh A, Marth JD, Fukuda M. Polysialic acid-directed migration and differentiation of neural precursors are essential for mouse brain development. Mol Cell Biol. 2007;27:6659–6668. doi: 10.1128/MCB.00205-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bisaz R, Schachner M, Sandi C. Causal evidence for the involvement of the neural cell adhesion molecule, NCAM, in chronic stress-induced cognitive impairments. Hippocampus. 2011;21:56–71. doi: 10.1002/hipo.20723. [DOI] [PubMed] [Google Scholar]
- 3.Black MA, Deurveilher S, Seki T, Marsh DR, Rutishauser U, Rafuse VF, Semba K. Role of polysialylated neural cell adhesion molecule in rapid eye movement sleep regulation in rats. Eur J Neurosci. 2009;30:2190–2204. doi: 10.1111/j.1460-9568.2009.07000.x. [DOI] [PubMed] [Google Scholar]
- 4.Bonfanti L. PSA-NCAM in mammalian structural plasticity and neurogenesis. Prog Neurobiol. 2006;80:129–164. doi: 10.1016/j.pneurobio.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Burgess A, Wainwright SR, Shihabuddin LS, Rutishauser U, Seki T, Aubert I. Polysialic acid regulates the clustering, migration, and neuronal differentiation of progenitor cells in the adult hippocampus. Dev Neurobiol. 2008;68:1580–1590. doi: 10.1002/dneu.20681. [DOI] [PubMed] [Google Scholar]
- 6.Castillo-Gomez E, Varea E, Blasco-Ibanez JM, Crespo C, Nacher J. Polysialic Acid is required for dopamine D2 receptor-mediated plasticity involving inhibitory circuits of the rat medial prefrontal cortex. PLoS One. 2011;6:e29516. doi: 10.1371/journal.pone.0029516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conrad CD, Jackson JL, Wise LS. Chronic stress enhances ibotenic acid-induced damage selectively within the hippocampal CA3 region of male, but not female rats. Neuroscience. 2004;125:759–767. doi: 10.1016/j.neuroscience.2004.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conrad CD, LeDoux JE, Magarinos AM, McEwen BS. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav Neurosci. 1999;113:902–913. doi: 10.1037//0735-7044.113.5.902. [DOI] [PubMed] [Google Scholar]
- 9.Conrad CD, McEwen BS. Acute stress increases neuropeptide Y mRNA within the arcuate nucleus and hilus of the dentate gyrus. Brain Res Mol Brain Res. 2000;79:102–109. doi: 10.1016/s0169-328x(00)00105-4. [DOI] [PubMed] [Google Scholar]
- 10.Conrad CD, McLaughlin KJ, Harman JS, Foltz C, Wieczorek L, Lightner E, Wright RL. Chronic glucocorticoids increase hippocampal vulnerability to neurotoxicity under conditions that produce CA3 dendritic retraction but fail to impair spatial recognition memory. J Neurosci. 2007;27:8278–8285. doi: 10.1523/JNEUROSCI.2121-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cremer H, Chazal G, Lledo PM, Rougon G, Montaron MF, Mayo W, Le Moal M, Abrous DN. PSA-NCAM: an important regulator of hippocampal plasticity. Int J Dev Neurosci. 2000;18:213–220. doi: 10.1016/s0736-5748(99)00090-8. [DOI] [PubMed] [Google Scholar]
- 12.Czeh B, Lucassen PJ. What causes the hippocampal volume decrease in depression? Are neurogenesis, glial changes and apoptosis implicated? Eur Arch Psychiatry Clin Neurosci. 2007;257:250–260. doi: 10.1007/s00406-007-0728-0. [DOI] [PubMed] [Google Scholar]
- 13.de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- 14.Dityatev A, Dityateva G, Sytnyk V, Delling M, Toni N, Nikonenko I, Muller D, Schachner M. Polysialylated neural cell adhesion molecule promotes remodeling and formation of hippocampal synapses. J Neurosci. 2004;24:9372–9382. doi: 10.1523/JNEUROSCI.1702-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El Maarouf A, Kolesnikov Y, Pasternak G, Rutishauser U. Polysialic acid-induced plasticity reduces neuropathic insult to the central nervous system. Proc Natl Acad Sci U S A. 2005;102:11516–11520. doi: 10.1073/pnas.0504718102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El Maarouf A, Rutishauser U. Removal of polysialic acid induces aberrant pathways, synaptic vesicle distribution, and terminal arborization of retinotectal axons. J Comp Neurol. 2003;460:203–211. doi: 10.1002/cne.10635. [DOI] [PubMed] [Google Scholar]
- 17.Fitch JM, Juraska JM, Washington LW. The dendritic morphology of pyramidal neurons in the rat hippocampal CA3 area. I. Cell types. Brain Res. 1989;479:105–114. doi: 10.1016/0006-8993(89)91340-1. [DOI] [PubMed] [Google Scholar]
- 18.Fox GB, Kjoller C, Murphy KJ, Regan CM. The modulations of NCAM polysialylation state that follow transient global ischemia are brief on neurons but enduring on glia. J Neuropathol Exp Neurol. 2001;60:132–140. doi: 10.1093/jnen/60.2.132. [DOI] [PubMed] [Google Scholar]
- 19.Glaser EM, Van der Loos H. Analysis of thick brain sections by obverse-reverse computer microscopy: application of a new, high clarity Golgi-Nissl stain. J Neurosci Methods. 1981;4:117–125. doi: 10.1016/0165-0270(81)90045-5. [DOI] [PubMed] [Google Scholar]
- 20.Gomez-Climent MA, Guirado R, Castillo-Gomez E, Varea E, Gutierrez-Mecinas M, Gilabert-Juan J, Garcia-Mompo C, Vidueira S, Sanchez-Mataredona D, Hernandez S, Blasco-Ibanez JM, Crespo C, Rutishauser U, Schachner M, Nacher J. The polysialylated form of the neural cell adhesion molecule (PSA-NCAM) is expressed in a subpopulation of mature cortical interneurons characterized by reduced structural features and connectivity. Cereb Cortex. 2011;21:1028–1041. doi: 10.1093/cercor/bhq177. [DOI] [PubMed] [Google Scholar]
- 21.Hammond MS, Sims C, Parameshwaran K, Suppiramaniam V, Schachner M, Dityatev A. Neural cell adhesion molecule-associated polysialic acid inhibits NR2B-containing N-methyl-D-aspartate receptors and prevents glutamate-induced cell death. J Biol Chem. 2006;281:34859–34869. doi: 10.1074/jbc.M602568200. [DOI] [PubMed] [Google Scholar]
- 22.Hoyk Z, Parducz A, Theodosis DT. The highly sialylated isoform of the neural cell adhesion molecule is required for estradiol-induced morphological synaptic plasticity in the adult arcuate nucleus. Eur J Neurosci. 2001;13:649–656. doi: 10.1046/j.1460-9568.2001.01427.x. [DOI] [PubMed] [Google Scholar]
- 23.Hunter RG, Murakami G, Dewell S, Seligsohn M, Baker ME, Datson NA, McEwen BS, Pfaff DW. Acute stress and hippocampal histone H3 lysine 9 trimethylation, a retrotransposon silencing response. Proc Natl Acad Sci U S A. 2012;109:17657–17662. doi: 10.1073/pnas.1215810109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Izquierdo A, Wellman CL, Holmes A. Brief uncontrollable stress causes dendritic retraction in infralimbic cortex and resistance to fear extinction in mice. J Neurosci. 2006;26:5733–5738. doi: 10.1523/JNEUROSCI.0474-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim TY, Yi JS, Chung SJ, Kim DK, Byun HR, Lee JY, Koh JY. Pyruvate protects against kainate-induced epileptic brain damage in rats. Exp Neurol. 2007;208:159–167. doi: 10.1016/j.expneurol.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 26.Kochlamazashvili G, Bukalo O, Senkov O, Salmen B, Gerardy-Schahn R, Engel AK, Schachner M, Dityatev A. Restoration of synaptic plasticity and learning in young and aged NCAM-deficient mice by enhancing neurotransmission mediated by GluN2A-containing NMDA receptors. J Neurosci. 2012;32:2263–2275. doi: 10.1523/JNEUROSCI.5103-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Magarinos AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience. 1995;69:89–98. doi: 10.1016/0306-4522(95)00259-l. [DOI] [PubMed] [Google Scholar]
- 28.Magarinos AM, McEwen BS, Flugge G, Fuchs E. Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. J Neurosci. 1996;16:3534–3540. doi: 10.1523/JNEUROSCI.16-10-03534.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Magarinos AM, McEwen BS, Saboureau M, Pevet P. Rapid and reversible changes in intrahippocampal connectivity during the course of hibernation in European hamsters. Proc Natl Acad Sci U S A. 2006;103:18775–18780. doi: 10.1073/pnas.0608785103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- 31.McEwen BS. Plasticity of the hippocampus: adaptation to chronic stress and allostatic load. Ann N Y Acad Sci. 2001;933:265–277. doi: 10.1111/j.1749-6632.2001.tb05830.x. [DOI] [PubMed] [Google Scholar]
- 32.McLaughlin KJ, Gomez JL, Baran SE, Conrad CD. The effects of chronic stress on hippocampal morphology and function: an evaluation of chronic restraint paradigms. Brain Res. 2007;1161:56–64. doi: 10.1016/j.brainres.2007.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Monlezun S, Ouali S, Poulain DA, Theodosis DT. Polysialic acid is required for active phases of morphological plasticity of neurosecretory axons and their glia. Mol Cell Neurosci. 2005;29:516–524. doi: 10.1016/j.mcn.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 34.Muller D, Djebbara-Hannas Z, Jourdain P, Vutskits L, Durbec P, Rougon G, Kiss JZ. Brain-derived neurotrophic factor restores long-term potentiation in polysialic acid-neural cell adhesion molecule-deficient hippocampus. Proc Natl Acad Sci U S A. 2000;97:4315–4320. doi: 10.1073/pnas.070022697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nacher J, Blasco-Ibanez JM, McEwen BS. Non-granule PSA-NCAM immunoreactive neurons in the rat hippocampus. Brain Res. 2002;930:1–11. doi: 10.1016/s0006-8993(01)03365-0. [DOI] [PubMed] [Google Scholar]
- 36.Nacher J, Gomez-Climent MA, McEwen B. Chronic non-invasive glucocorticoid administration decreases polysialylated neural cell adhesion molecule expression in the adult rat dentate gyrus. Neurosci Lett. 2004;370:40–44. doi: 10.1016/j.neulet.2004.07.062. [DOI] [PubMed] [Google Scholar]
- 37.Nacher J, Pham K, Gil-Fernandez V, McEwen BS. Chronic restraint stress and chronic corticosterone treatment modulate differentially the expression of molecules related to structural plasticity in the adult rat piriform cortex. Neuroscience. 2004;126:503–509. doi: 10.1016/j.neuroscience.2004.03.038. [DOI] [PubMed] [Google Scholar]
- 38.Ono K, Tomasiewicz H, Magnuson T, Rutishauser U. N-CAM mutation inhibits tangential neuronal migration and is phenocopied by enzymatic removal of polysialic acid. Neuron. 1994;13:595–609. doi: 10.1016/0896-6273(94)90028-0. [DOI] [PubMed] [Google Scholar]
- 39.Pham K, Nacher J, Hof PR, McEwen BS. Repeated restraint stress suppresses neurogenesis and induces biphasic PSA-NCAM expression in the adult rat dentate gyrus. Eur J Neurosci. 2003;17:879–886. doi: 10.1046/j.1460-9568.2003.02513.x. [DOI] [PubMed] [Google Scholar]
- 40.Popov VI, Bocharova LS, Bragin AG. Repeated changes of dendritic morphology in the hippocampus of ground squirrels in the course of hibernation. Neuroscience. 1992;48:45–51. doi: 10.1016/0306-4522(92)90336-z. [DOI] [PubMed] [Google Scholar]
- 41.Prosser RA, Rutishauser U, Ungers G, Fedorkova L, Glass JD. Intrinsic role of polysialylated neural cell adhesion molecule in photic phase resetting of the Mammalian circadian clock. J Neurosci. 2003;23:652–658. doi: 10.1523/JNEUROSCI.23-02-00652.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ricart-Jane D, Rodriguez-Sureda V, Benavides A, Peinado-Onsurbe J, Lopez-Tejero MD, Llobera M. Immobilization stress alters intermediate metabolism and circulating lipoproteins in the rat. Metabolism. 2002;51:925–931. doi: 10.1053/meta.2002.33353. [DOI] [PubMed] [Google Scholar]
- 43.Rodriguez JJ, Montaron MF, Petry KG, Aurousseau C, Marinelli M, Premier S, Rougon G, Le Moal M, Abrous DN. Complex regulation of the expression of the polysialylated form of the neuronal cell adhesion molecule by glucocorticoids in the rat hippocampus. Eur J Neurosci. 1998;10:2994–3006. doi: 10.1046/j.1460-9568.1998.00316.x. [DOI] [PubMed] [Google Scholar]
- 44.Rutishauser U. Polysialic acid in the plasticity of the developing and adult vertebrate nervous system. Nature Rev: Neuroscience. 2008;9:26–35. doi: 10.1038/nrn2285. [DOI] [PubMed] [Google Scholar]
- 45.Rutishauser U, Landmesser L. Polysialic acid in the vertebrate nervous system: a promoter of plasticity in cell-cell interactions. Trends Neurosci. 1996;19:422–427. doi: 10.1016/0166-2236(96)10041-2. [DOI] [PubMed] [Google Scholar]
- 46.Rutishauser U, Watanabe M, Silver J, Troy FA, Vimr ER. Specific alteration of NCAM-mediated cell adhesion by an endoneuraminidase. J Cell Biol. 1985;101:1842–1849. doi: 10.1083/jcb.101.5.1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sairanen M, O’Leary OF, Knuuttila JE, Castren E. Chronic antidepressant treatment selectively increases expression of plasticity-related proteins in the hippocampus and medial prefrontal cortex of the rat. Neuroscience. 2007;144:368–374. doi: 10.1016/j.neuroscience.2006.08.069. [DOI] [PubMed] [Google Scholar]
- 48.Sandi C. Stress, cognitive impairment and cell adhesion molecules. Nat Rev Neurosci. 2004;5:917–930. doi: 10.1038/nrn1555. [DOI] [PubMed] [Google Scholar]
- 49.Sandi C, Merino JJ, Cordero MI, Touyarot K, Venero C. Effects of chronic stress on contextual fear conditioning and the hippocampal expression of the neural cell adhesion molecule, its polysialylation, and L1. Neuroscience. 2001;102:329–339. doi: 10.1016/s0306-4522(00)00484-x. [DOI] [PubMed] [Google Scholar]
- 50.Seki T, Arai Y. Different polysialic acid-neural cell adhesion molecule expression patterns in distinct types of mossy fiber boutons in the adult hippocampus. J Comp Neurol. 1999;410:115–125. doi: 10.1002/(sici)1096-9861(19990719)410:1<115::aid-cne10>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 51.Seki T, Rutishauser U. Removal of polysialic acid-neural cell adhesion molecule induces aberrant mossy fiber innervation and ectopic synaptogenesis in the hippocampus. J Neurosci. 1998;18:3757–3766. doi: 10.1523/JNEUROSCI.18-10-03757.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Selye H. A syndrome produced by diverse nocuous agents. Nature. 1936;138:32–32. doi: 10.1176/jnp.10.2.230a. [DOI] [PubMed] [Google Scholar]
- 53.Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011;476:458–461. doi: 10.1038/nature10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Uylings HB, Ruiz-Marcos A, van Pelt J. The metric analysis of three-dimensional dendritic tree patterns: a methodological review. J Neurosci Methods. 1986;18:127–151. doi: 10.1016/0165-0270(86)90116-0. [DOI] [PubMed] [Google Scholar]
- 55.Varea E, Blasco-Ibanez JM, Gomez-Climent MA, Castillo-Gomez E, Crespo C, Martinez-Guijarro FJ, Nacher J. Chronic fluoxetine treatment increases the expression of PSA-NCAM in the medial prefrontal cortex. Neuropsychopharmacology. 2007;32:803–812. doi: 10.1038/sj.npp.1301183. [DOI] [PubMed] [Google Scholar]
- 56.Vimr ER, McCoy RD, Vollger HF, Wilkison NC, Troy FA. Use of prokaryotic-derived probes to identify poly(sialic acid) in neonatal neuronal membranes. Proc Natl Acad Sci U S A. 1984;81:1971–1975. doi: 10.1073/pnas.81.7.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vutskits L, Djebbara-Hannas Z, Zhang H, Paccaud JP, Durbec P, Rougon G, Muller D, Kiss JZ. PSA-NCAM modulates BDNF-dependent survival and differentiation of cortical neurons. Eur J Neurosci. 2001;13:1391–1402. doi: 10.1046/j.0953-816x.2001.01516.x. [DOI] [PubMed] [Google Scholar]
- 58.Washbourne P, Dityatev A, Scheiffele P, Biederer T, Weiner JA, Christopherson KS, El-Husseini A. Cell adhesion molecules in synapse formation. J Neurosci. 2004;24:9244–9249. doi: 10.1523/JNEUROSCI.3339-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Watanabe Y, Gould E, McEwen BS. Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Res. 1992;588:341–345. doi: 10.1016/0006-8993(92)91597-8. [DOI] [PubMed] [Google Scholar]
- 60.Weinhold B, Seidenfaden R, Rockle I, Muhlenhoff M, Schertzinger F, Conzelmann S, Marth JD, Gerardy-Schahn R, Hildebrandt H. Genetic ablation of polysialic acid causes severe neurodevelopmental defects rescued by deletion of the neural cell adhesion molecule. J Biol Chem. 2005;280:42971–42977. doi: 10.1074/jbc.M511097200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



