Abstract
Relative regional cerebral metabolic rate of glucose in rapid eye movement (REM) sleep and wakefulness was explored in combat veterans with and without PTSD, using positron emission tomography. Hypermetabolism in brain regions involved in arousal regulation, fear responses, and reward processing persist during REM sleep in combat veterans with PTSD.
Keywords: Positron emission tomography, fear response, reward processing
1. Introduction
Posttraumatic stress disorder (PTSD) refers to symptoms of re-experiencing, avoidance, and hyperarousal that persist for more than one month after exposure to a traumatic event, such as combat. Nightmares, a cardinal symptom of PTSD, are prominently phenomenon of rapid-eye movement (REM) sleep. Objective REM sleep disruption has been associated with an increased risk of developing PTSD following trauma exposure (Mellman et al., 2002). Thus, PTSD may be at least partially mediated by REM sleep mechanisms (Germain et al., 2008).
Neuroimaging studies have shown that REM sleep offers a unique entry into the primitive emotional brain on a nightly basis through activation of the limbic system, the seat of emotional memories (Nofzinger et al., 1998). Here, we used [18F]-fluorodeoxyglucose (FDG) PET imaging to explore metabolic changes in the limbic system during wakefulness and REM sleep in combat-exposed military veterans from Operations Enduring and Iraqi Freedom (OEF/OIF), with and without a diagnosis of PTSD.
2. Methods
This study was approved by the University of Pittsburgh Institutional Review Board. Eligible participants were OEF/OIF military veterans between 18 and 50 years-old, medication-free, and free of medical comorbidity. Eighteen veterans provided written, informed consent and completed a detailed diagnostic evaluation, as well as a physical examination with blood work and urine drug screen to determine health status and ascertain the absence of recent substance use. The Clinician Administered PTSD Scale (Blake et al., 1995) was administered to determine current PTSD status and severity. Eight veterans met diagnostic criteria for current PTSD, and 10 did not. The Structured Clinical Interview for DSM Disorders (SCID; First et al., 1996) was used to verify the absence of comorbid Axis I disorders, including mood and alcohol/substance use disorders. All participants were free of psychiatric comorbidity. Current concussive symptoms were assessed with the Military Acute Concussion Evaluation (MACE; French et al., 2008). None endorsed concurrent concussive symptoms at the time of the study. Two veterans with PTSD and one without PTSD endorsed a history head injury with subsequent symptoms suggestive of a concussion, but denied receiving a formal diagnosis. Three other veterans without PTSD and two with PTSD reported being exposed to a blast of an explosion, but denied experiencing any concussive symptom at the time.
Participants slept for three nights in the sleep laboratory. The first night served as a screening night to rule out the presence of obstructive sleep apnea. The second night served as a baseline night, and data collected was used for group comparisons on objective PSG sleep measures. Waking PET scans were acquired on the morning following the second night, two hours after final awakening in the morning. Electroencephalography (EEG) was continuously monitored to ascertain that wakefulness was maintained during the FDG uptake period. On the third and final night, REM sleep PET scans were collected during the second REM sleep period. REM sleep PET scans reflected brain activity over a 20-minute period that began at the onset of the second REM period. Continuous EEG recordings verified REM sleep during the uptake period.
Relative rCMRglc were assessed during wakefulness and REM sleep by using a previously validated FDG PET method (Nofzinger et al., 1997; Nofzinger et al., 1998). Image analysis included statistical parametric mapping (SPM8; Penny et al., 2007) and magnetic resonance-guided whole-brain analyses. Group differences during wakefulness and REM sleep were then evaluated in separate analyses using global metabolism as a covariate. Group (PTSD vs. non-PTSD) by State (REM vs. wake) interactions were conducted to evaluate (1) greater increases and (2) smaller decreases in rCMRglc from wakefulness to REM in veterans PTSD compared to veterans without PTSD. Within-group state differences were evaluated using paired t-test. Results from these analyses are described in the Supplementary Materials.
3. Results
Two of the eight veterans without PTSD and four of the 10 veterans with PTSD did not maintain REM sleep during the FDG uptake period, and were excluded from analyses. Six veterans met diagnostic criteria for current PTSD (M age = 29.1 ± 4.6 years old) and six did not meet diagnostic criteria for PTSD (M age = 29.3 ± 5.4 years old). Mean PTSD duration was 80 months (SD = 63.92 months). During the baseline night, the PTSD group showed a slightly greater percent REM sleep (t(10) = 2.7, p = 0.02) (see Supplementary Materials, Table 1).
The number of 20-second epochs spent in wakefulness, REM sleep, or light sleep (sleep stages 1–2) during the uptake period of the wakefulness or REM scans did not differ between groups; over 90% of uptake periods indeed comprised the targeted physiological state (i.e., wake and REM sleep).
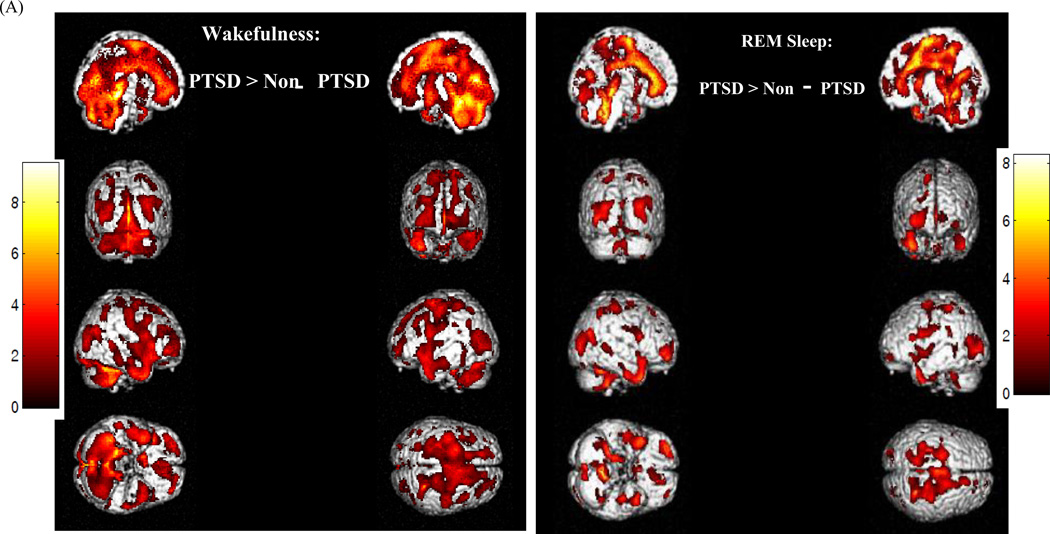
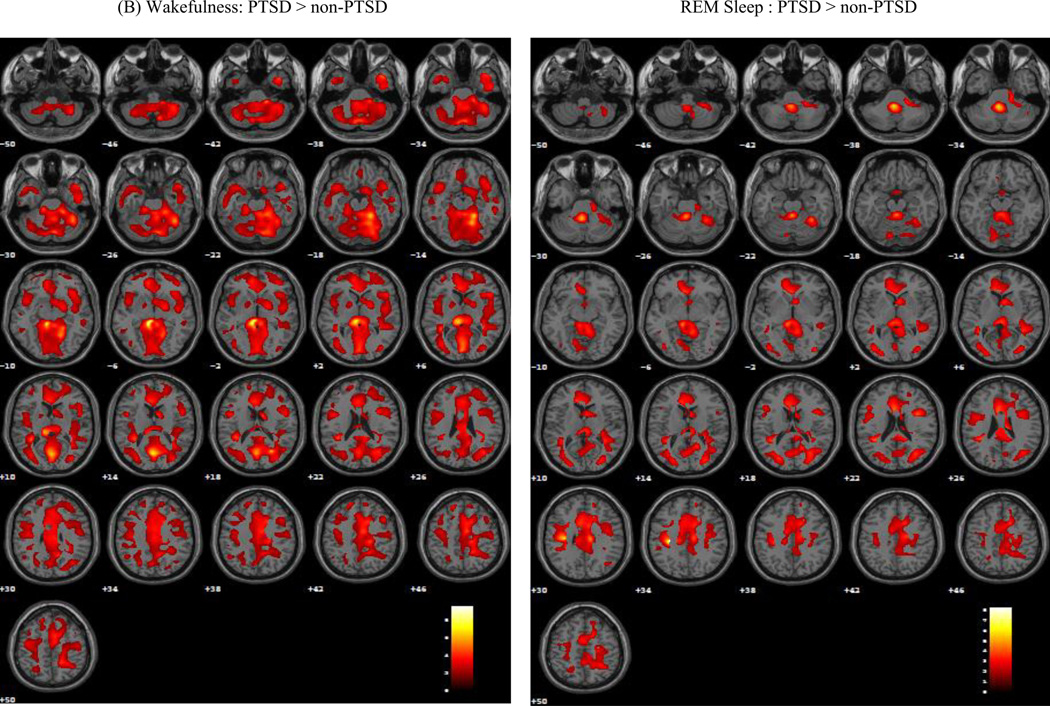
Substantial group differences on relative rCMRglc were observed during both wakefulness and REM sleep (Figure 1). During wakefulness, veterans with PTSD showed significantly greater relative rCMRglc in a large cluster of 70,326 contiguous voxels (MNI coordinates of voxel of maximal significance: −8, −34, −6; Z = 4.70, pFWE < 0.001), and included the premotor and supplementary motor area, medial superior and inferior frontal gyri, orbitofrontal cortex, gyrus rectus, and pars triangularis. This area also extended bilaterally into the anterior, dorsal, and posterior cingulate cortex, and into the rolandic operculum, supramarginal and angular gyri, temporal gyri, and fusiform and parahippocampal gyri. Limbic structures included in this cluster consisted of the hippocampus, amygdala, and insula, as well of the basal ganglia (putamen, caudate, and pallidum), and thalamus. Parietal regions included the postcentral gyrus, and precuneus, extended into the cuneus and occipital gyri. Finally, this region included brainstem regions involved in arousal and REM sleep regulation, including the midbrain reticular formation, left and right locus coeruleus, raphe nuclei and REM-sleep regulating pedunculopontine tegmentum and laterodorsal tegmentum, as well as the cerebellum. Eigenvariate values were extracted from for each veteran and group means were calculated for selected brain regions included in this significant cluster (Figure S2A).
Figure 1.
Render images (A) and axial planes (B) depicting brain regions where veterans with PTSD show greater relative rCMRglc than control veterans without PTSD (CTL) during wakefulness (WAKE) and rapid-eye movement (REM) sleep. Statistical maxima were identified in MNI (x, y, z) coordinates. The alpha level for significance was set at 0.05, with family-wise error (FWE) correction for multiple comparisons.
This pattern persisted during REM sleep (MNI coordinates of voxel of maximal significance: −30, −24, 34; Z = 4.44, pFWE < 0.001), although the extent of this cluster was more restricted (30,865 versus 70,326 contiguous voxels). Frontally, the regions of significance were restricted bilaterally to the medial prefrontal and paralimbic cortices, including premotor and supplementary motor areas, the anterior, medial, and posterior cingulate cortices, and ventrolateral and ventromedial frontal cortex, pars triangularis, and rolandic operculum, and extended into the temporal inferior and superior cortex, and cuneus. Parietal regions included the postcentral gyrus, superior lobule, and the anterior part of the precuneus. The thalamus was also included in the area of significance, as well as the right amygdala, caudate, putamen, pallidum, insula parahippocampal gyrus, and the left hippocampus. Finally, the significant cluster included a large mediodorsal region of the brainstem that comprised the midbrain reticular formation, raphe nuclei, and right locus coeruleus, and cerebellum. Eigenvariate values were also extracted from for each veteran and group means were calculated for selected brain regions included in this significant cluster (Figure S2B).
4. Discussion
The present findings highlight the feasibility of using sleep neuroimaging methods in combat veterans with and without PTSD. A pattern of hypermetabolism in the brainstem, and limbic and cortical regions involved in arousal regulation and in fear response was observed in OEF/OIF veterans with PTSD during wakefulness and REM sleep compared to veterans without PTSD. These findings provide preliminary support for our previously proposed model that identified plausible neural interactions between sleep and the neurocircuitry involved in chronic PTSD (Germain et al., 2008).
Veterans with PTSD also showed hypermetabolism in the basal ganglia during wakefulness and REM sleep. The involvement of the basal ganglia may have particular significance to elucidate the neural underpinnings of the comorbidity between PTSD and mood and addictive disorders (Forbes, 2009; Kalivas and Volkow, 2005).
The cross-sectional nature of this study does not allow for speculation regarding the temporal course of the observed changes in brain metabolism in PTSD: altered brain functions that underlie PTSD symptoms during wakefulness may alter REM sleep, or alterations of REM-sleep mechanisms following trauma may contribute to chronic impairments in fear- and/or reward-related neural circuits, which in turn contribute to PTSD symptoms. If the latter were true, integrity of REM sleep following trauma may be critical to psychological resilience to trauma. Another possibility is that trauma exposure is associated with simultaneous changes in the sleep and arousal regulatory systems and the fear and reward systems during both wakefulness and REM sleep, and that both contribute to trigger and sustain PTSD symptoms.
The present findings highlight the need for animal and human experimental studies to probe the neural mechanisms underlying the possible causal relationships between stress/trauma exposure, sleep and arousal regulation, and PTSD. Normalizing the underlying altered brain systems during both wakefulness and REM sleep in PTSD may be crucial pathways to enhance resilience and hasten the recovery from trauma reactions.
Supplementary Material
Acknowledgements
The authors express special gratitude to military veterans who participated in this study. This work was made possible with the dedication of the research staff: Ryan Stocker, Noelle Rode, and Jean Miewald, as well as the NCTRC staff. The study was supported by the National Institutes of Health (MH083035; PI: Germain; UL1RR024153; PI: Reis) and the Department of Defense (PT073961; PI: Germain). The funding sources were not involved in designing the study, data collection, data analysis, or interpretation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Dr. Germain has served as a consultant for Concurrent Technologies Corporation. Dr. Nofzinger reports equity ownership in Cereve Inc. Drs. Mammen, Price, Herringa, Insana, and Mr. James report no conflict of interest.
References
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a Clinician-Administered PTSD Scale. Journal of Traumatic Stress. 1995;8(1):75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- French L, McCrea M, Baggett M. The Military Acute Concussion Evaluation (MACE) Journal of Special Operations Medicine. 2008;8(1):68–77. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version (SCID-CV) Washington, D.C: American Psychiatric Press, Inc.; 1996. [Google Scholar]
- Forbes EE. Where's the fun in that? Broadening the focus on reward function in depression. Biological Psychiatry 6. 2009;6(3):199–200. doi: 10.1016/j.biopsych.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain A, Buysse DJ, Nofzinger E. Sleep-specific mechanisms underlying posttraumatic stress disorder: integrative review and neurobiological hypotheses. Sleep Medicine Reviews. 2008;12(3):185–195. doi: 10.1016/j.smrv.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. American Journal of Psychiatry. 2005;162(8):1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Mellman TA, Bustamante V, Fins AI, Pigeon WR, Nolan B. REM sleep and the early development of posttraumatic stress disorder. American Journal of Psychiatry. 2002;159(10):1696–1701. doi: 10.1176/appi.ajp.159.10.1696. [DOI] [PubMed] [Google Scholar]
- Nofzinger EA, Mintun MA, Price J, Meltzer CC, Townsend D, Buysse DJ, Reynolds CF, Dachille M, Matzzie J, Kupfer DJ, Moore RY. A method for the assessment of the functional neuroanatomy of human sleep using FDG PET. Brain Research and Brain Research Protocols. 1998;2(3):191–198. doi: 10.1016/s1385-299x(97)00042-1. [DOI] [PubMed] [Google Scholar]
- Nofzinger EA, Mintun MA, Wiseman MB, Kupfer DJ, Moore RY. Forebrain activation in REM sleep: an FDG PET study. Brain Research. 1997;770(1–2):192–201. doi: 10.1016/s0006-8993(97)00807-x. [DOI] [PubMed] [Google Scholar]
- Penny WD, F KJ, Ashburner JT, Kiebel SJ, Nichols TE. Statistical Parametric Mapping: The Analysis of Functional Brain Images. Elsevier; 2007. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.