Abstract
Malignant gliomas are characterized by aggressive tumor growth with a mean survival of 15–18 months and frequently developed resistance to temozolomide. Therefore, strategies that sensitize glioma cells to temozolomide have a high translational impact. We have studied focal adhesion kinase (FAK), a tyrosine kinase and emerging therapeutic target that is known to be highly expressed and activated in glioma. In this report we tested the FAK autophosphorylation inhibitor, Y15 in DBTRG and U87 glioblastoma cells. Y15 significantly decreased viability and clonogenicity in a dose-dependent manner, increased detachment in a dose and time-dependent manner, caused apoptosis and inhibited cell invasion in both cell lines. In addition, Y15 treatment decreased autophosphorylation of FAK in a dose-dependent manner and changed cell morphology by causing cell rounding in DBTRG and U87 cells. Administration of Y15 significantly decreased subcutaneous DBTRG tumor growth with decreased Y397-FAK autophosphorylation, activated caspase-3 and PARP. Y15 was administered in an orthotopic glioma model, leading to an increase in mouse survival. The combination of Y15 with temozolomide was more effective than either agent alone in decreasing viability and activating caspase-8 in DBTRG and U87 cells in vitro. In addition, the combination of Y15 and temozolomide synergistically blocked U87 brain tumor growth in vivo. Thus, pharmacologic blockade of FAK autophosphorylation with the oral administration of a small molecule inhibitor Y15 has a potential to be an effective therapy approach for glioblastoma either alone or in combination with chemotherapy agents such as temozolomide.
Keywords: Focal Adhesion Kinase, Y397 site, autophosphorylation, brain, glioblastoma
Introduction
Malignant glioblastoma is an infiltrative brain tumor where improvements in survival have been largely refractory to advances in surgical and radiological techniques, and focused primarily on the use of temozolomide therapy. The unmet clinical need remains in the development of novel therapies that can cross the blood brain barrier and either directly reduce glioma tumor growth or sensitize gliomas to temozolomide therapy.
Focal Adhesion Kinase (FAK) is a 125 kDa non-receptor tyrosine kinase that was shown to be overexpressed in brain tumors (1). As a coordinator of extracellular inputs (i.e. integrins and growth factor receptors) with intracellular signaling pathways (i.e. sequestration of death receptors and pro-apoptotic proteins such as p53, neurofibromin and others (2–5), FAK is well-established mediator of cell survival and invasion.
The functional role of FAK in glioma cells is based on the observation that glioma cells overexpress FAK with an increased level of FAK autophosphorylation (1). Overexpression of FAK in serum-starved glioblastoma cells results in increased cell motility (6), while expression of Y397-mutant FAK or down-regulation of FAK with FAK siRNA inhibits basal and PDGF-induced cell migration (6). While FAK is a therapeutic target in glioma cells themselves, targeted deletion of FAK in glioma-associated vascular endothelium resulted in a vascular normalization phenotype associated with a reduction in glioblastoma tumor growth (7). The recent development of small molecule inhibitor targeting ATP-binding site of FAK, TAE226, was developed by Novartis and has been shown to increase glioblastoma apoptosis and inhibit tumor growth (8). However, this inhibitor was not specific due to targeting of the conservative ATP-binding domain, which contained the conservative sequences common to other tyrosine kinases, causing inhibition of multiple pathways.
Since FAK has been highly autophosphorylated in glioblastoma, we focused on targeting the FAK autophosphorylation Y397 site with FAK inhibitor Y15 or inhibitor 14, developed in our group and demonstrated specific inhibition of the FAK autophosphorylation site to block tumor growth in non-CNS models (9–11). Y15 specifically inhibited FAK autophosphorylation without affecting other kinases (9). The advantage of this inhibitor is that it targets the main autophosphorylation site of FAK rather than the more conserved ATP-binding domain. Once the Y397 site becomes phosphorylated, SH2-containing proteins such as Src and PI3-Kinase bind to FAK, leading to down-stream signaling, accompanied by functional cellular changes (12).
In this study we tested the effect of the FAK autophosphorylation inhibitor Y15 alone or in combination with temozolomide in DBTRG, a human glioma-derived cell line (7) and U87 glioblastoma cell lines. This is the first report that showed that inhibition of FAK autophosphorylation in glioblastoma has potential to be an effective approach to inhibit glioblastoma tumor growth that is more effective in combination with temozolomide.
Materials and Methods
Cell lines
The early passages of patient-derived human DBTRG glioblastoma cells were from Dr. Brian Eliceiri, described by Dr. Carol Kruse (13), and purchased from American Type Culture Collection. The DBTRG cells were passaged for less than 6 month after resuscitation of frozen aliquots and no other authentication was carried out. The DBTRG cells were maintained in DMEM medium supplemented with 10% fetal bovine serum. 1μg/mL streptomycin, 1μg/mL L-Glutamine, 1μg/mL sodium pyruvate, 1μg/mL nonessential amino acids, and 500 μL of Insulin 10mg/mL. U87 glioblastoma cell line was purchased from ATCC and authenticated by ATCC in 2009 by short tandem repeat analysis. The U87 cells were passaged for less than 6 month after resuscitation of frozen aliquots. The U87 cell line was maintained in MEM medium with 10% fetal bovine serum with 1 μg/ml streptomycin. D37 and U251 glioblatoma cell lines were obtained as a gift from Drs. Michael Ciesielski and Robert Fenstermaker (no authentication was carried by the authors) and maintained in DMEM medium.
Antibodies
Monoclonal anti-FAK antibody, caspase-3, 8 and anti-PARP antibodies were obtained from Millipore, Inc. Polyclonal anti-phospho-Tyrosine 397-FAK were obtained from Biosource Inc. Monoclonal anti-β-actin antibodies were obtained from Sigma.
Small molecule inhibitors
The small molecule inhibitor Y15 (or inhibitor 14), 1,2,4,5-benzenetetraamine tetrahydrocloride was described in (9) and ordered from Sigma Inc. Temozolomide was obtained from Sigma. The control FAK inhibitor, TAE-226 (8) was obtained from Novartis Inc. The Y15-5 derivative 3,3′-Diaminobenzidine tetrahydrochloride was ordered from Sigma, and obtained from Drs. Manivannan Ethiradjan and Ravindra Pandey (Roswell Park Cancer Institute).
Western blotting
1–2×106 cells were plated on 100 mm3 plate, and after 24 hours Y15 and temozolomide compounds were added for 24 hours. Western blotting was performed by the standard procedure (3).
Immunostaining and immunohistochemistry
105 cells were plated on the 24 well plate glass coverslips, and next day Y15 was added to the cells for 24 hours. The immunostaining was performed with phospho-Y397 and FAK and then with secondary Alexa-Red antibodies, as described (14). Actin was stained with Phalloidin-FITC and nuclei were stained with Hoechst. The slides were analyzed under Zeiss Axiovert fluorescent microscope with 100x objective. Immunohistochemistry staining was performed on paraffin-embedded tumor samples in the Pathology Core Facility (Roswell Park Cancer Institute), as described (9).
Cell viability
1×104 cells were plated in a 96-well plate. After 24 hours cells were treated with small molecule compounds for 24 hours. DMSO was added as a negative control. The 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium compound from Promega 96 well Viability kit was used according to the manufacture’s protocol.
Clonogenicity
The 500–1000 cells were plated in 6 well plates. After 24 hours different doses of Y15, Novartis inhibitor TAE226 and Y15-5 derivative at 10 μM doses were added to the cells, which were incubated with inhibitors at 37°C for 1–2 weeks. Then cells were fixed in 25% methanol and stained with Crystal Violet and colonies were counted in two independent experiments.
Detachment
The 2×105 cells were plated in 6 well plates and treated 24 hours after plating with different concentrations of Y15 and incubated for 24–96 hours. The detached and attached cells were collected and counted at different time points under the microscope using a hemacytometer. The percentage of detached cells was calculated by dividing the number of detached cells by the total number of cells in four independent experiments.
Apoptosis
Detached cells were collected and fixed in 3.7% formaldehyde for the apoptosis assay. Detection of apoptosis was done with Hoechst 33342 staining and by Flow cytometry analysis. The 1×106 cells were treated with Y15 and temozolomide compounds for 48 hours and apoptosis was also determined using PE AnnexinV and 7-AAD staining using BD Pharmingen kit according to the manufacturer’s protocol in the Flow Cytometry Facility (Roswell Park Cancer Institute).
Invasion
The cell invasion assay was performed on 24 well plates using Boyden chambers coated with extracellular matrix, ECMatrix using the Chemicon International Inc. kit according to the manufacturer’s protocol. The 1×105 cells in serum free medium were plated into Boyden chamber inserts, and complete medium with 10% serum was added to the bottom of the wells. After 24 hours Y15 was added to the cells for 24 hours, and invaded cells through the chamber pores were stained and processed by colorimetric reading at 560 nm in a 96 well plate on spectrophotometer. The invasion data were normalized to viability MTT data.
Flow cytometry
The 1×106 cells were treated with inhibitors for 48 hours and analyzed by Flow cytometry for cell cycle analysis and apoptosis by the standard procedure in the Flow Cytometry Facility (Roswell Park Cancer Institute).
Tumor growth in xenograft nude mice
Six week old female nude mice were maintained in the animal facility, and all experiments were performed in compliance with NIH animal use guidelines using IACUC protocol approved by the Roswell Park Animal Care Committee. The 2×106 cells were injected into mice subcutaneously. Y15 was introduced by gavage orally at 100 mg/kg dose daily either next day or 6 days after cell injection alone or in combination with temozolomide. Before start of the treatment mice were randomized into four groups (n=5) with an equal average tumor volume equal to 76 mm3. Temozolomide was administered 6.25 mg/kg orally by gavage every day 5 days a week. The control group was treated with 1xPBS. The mice were treated every day 5 days per week for several weeks. Tumor volume in mm3 was determine, as described (9), and at the end of experiment tumor weight was determined in grams. The mice body weight was determined in grams once a week in all tested groups.
Intracranial stereotactic injections and Y15 treatment
Immunodeficient Rag2 knockout mice were used for xenograft studies based on previous studies (15, 16). All animal handling procedures were approved by the University of California San Diego IACUC. After immobilizing the mice in a rodent stereotactic frame, an incision was made in the skin, a burr hole made in the skull, and 0.5×106 of DBTRG tumor cells were injected at a rate of 1–2 microliter/minute using a microsyringe mounted on a stereotactic frame using coordinates of 1 mm lateral and 1 mm anterior to the bregma and 1 mm below the dura. Tumor-bearing mice were injected intraperitoneally with Y15 inhibitor daily starting at seven days post-tumor implantation. Mice (n=10) were given FAK inhibitor Y15 at 30mg/kg (high dose) for four weeks (five days a week) and then switched to 6mg/kg (low dose) for two additional weeks. Equal volume of 1xPBS was injected as control. The brain was harvested from each group, fixed with 10% formalin, and subjected to standard hematoxylin and eosin staining. Survival data were collected in untreated and treated mice.
Statistical analyses
Student’s t test was performed to determine significance. The difference between data with p <0.05 was considered significant. The log-rank test for the Kaplan-Meier survival curve data was performed using Mstat software (17) and the difference between data with p-value <0.05 was considered significant.
Results
Y15 decreased DBTRG and U87 glioblastoma viability and clonogenicity in a dose-dependent manner
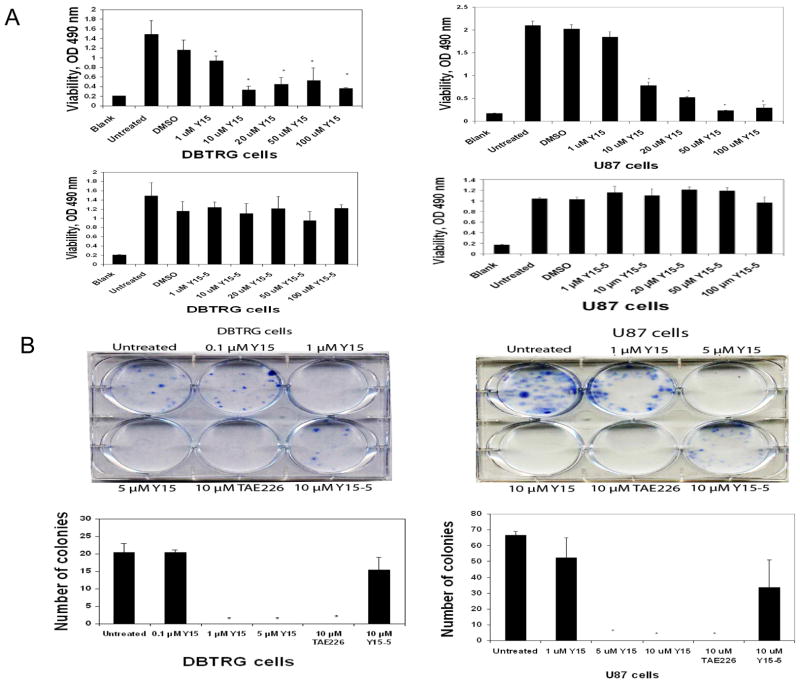
To test the effect of the Y15 FAK inhibitor on glioblastoma cells, DBTRG and U87 cells were treated with different doses of Y15 for 24 hours and MTT assays were performed to determine viability. Y15 significantly decreased viability of DBTRG cells starting at 1 μM dose (Fig. 1A, left upper panel). Y15 also significantly decreased viability of U87 cells in a dose-dependent manner (Fig. 1A, right upper panel). To test efficacy of Y15, we treated DBTRG and U87 cells with Y15 derivative, called Y15-5 and it did not significantly decrease cell viability in contrast to Y15 (Fig. 1 A, lower panels). Thus, Y15 is an effective inhibitor of DBTRG and U87 glioblastoma cell viability.
Figure 1. Y15 decreased DBTRG and U87 glioblastoma cell viability and clonogenicity in a dose-dependent manner.
A. The 1×104 cells were plated in 96 well plates and after 24 hours were treated with Y15 and Y15-5 for 24 hours. Y15 decreased DBTRG and U87 glioblastoma cell viability in a dose-dependent manner, while Y15 derivative, Y15-5 did not. B. Upper panels: Y15 decreased DBTRG and U87 glioblastoma clonogenicity in a dose-dependent manner. Lower panels: Y15 and TAE226 control significantly decrease number of colonies, while Y15-5 did not. *p<0.05, Y15-treated versus untreated cells, Student’s t-test.
To test the effect of Y15 on clonogenicity, DBTRG cells (Fig. 1 B, left) and U87 cells (Fig. 1B, right) were treated with different doses of Y15. For clonogenicity assays we also tested Y15-5 derivative and the Novartis FAK inhibitor, TAE-226. Y15 significantly decreased clonogenicity at 1 μM and 5 μM in DBTRG cells and U87 cells respectively, as well as TAE226 inhibitor at 10 μM, while Y15-5 derivative did not significantly decrease clonogenicity compared with Y15. Thus, Y15 specifically and effectively decreased clonogenicity of DBTRG and U87 cells.
Y15 increased DBTRG and U87 glioblastoma cell detachment in a dose-and time-dependent manner and caused apoptosis
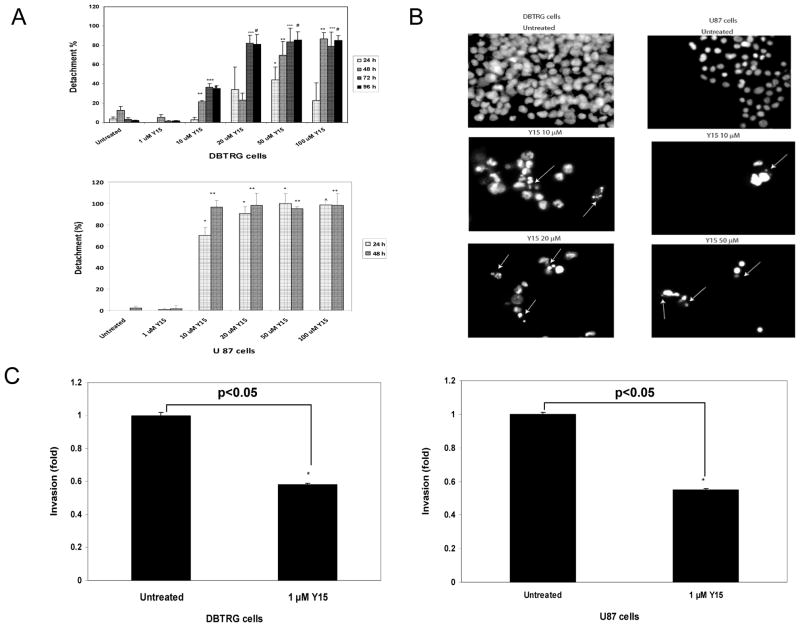
To test the effect of Y15 on cell detachment, we treated DBTRG and U87 cells with different doses of Y15 and performed detachment assays at 24, 48, 72 and 96 hours in DBTRG and at 24 and 48 hours in U87 cells (Fig. 2A). Y15 significantly increased cell detachment at a 10 μM dose in DBTRG cells, reaching an average detachment of 21% at 48 hours and 35–36% at 72–96 hours; and reaching the maximal detachment of 81–82% at 20 μM at 72 and 96 hours (Fig. 2A, upper panel). The same result was obtained at the higher 50 and 100 μM doses, where detachment significantly increased at 48 hours and reached 70% and 86%, respectively; and reached maximum detachment at 50 μM at 72 and 96 hours. In U87 cells, detachment also increased in a dose-dependent and time dependent manner (Fig. 2A, lower panel), reaching maximal level of 96.5% detachment at 10 μM dose at 48 hours. Thus, Y15 effectively caused detachment in DBTRG and U87 cells.
Figure 2.
A. Y15 increased DBTRG and U87 glioblastoma cell detachment in a dose- and time-dependent manner. The 2×105 of DBTRG and U87 cells were plated in 6 well plates, and after 24 hours were treated with different doses of Y15 for 24–96 hours. Y15 increased detachment in a dose- and time-dependent manner in both cell lines. *p<0.05 shows percent of detached cells at 24 hours; ** 48 hours; *** 72 hours and # 96 hours in Y15-treated versus untreated cells, Student’s t-test.
Figure 2B. Y15 caused apoptosis in DBTRG and U87 glioblastoma cells. DBTRG and U87 cells were treated with Y15 at different doses of Y15 for 48 hours and apoptosis was analyzed by Hoechst staining. Arrows show apoptotic fragmented nuclei.
Figure 2C. Y15 decreased DBTRG and U87 glioblastoma invasion. 1×105 of DGTRG and U87 cells were treated and plated in serum-free medium in Boyden chamber inserts using 24 well plates. 24 hours after cell plating 1 μM of Y15 inhibitor was added to the cells for 24 hours. The invasion data were normalized to viability data and expressed relatively to untreated cells. *p<0.05, Y15-treated versus untreated cells. Student’s t-test.
To detect apoptosis DBTRG and U87 cells were analyzed by Hoechst staining and apoptotic nuclei were detected starting at 10 μM dose and increased at 20 μM dose in DBTRG cells (Fig. 2B, left), reaching >90% at higher doses. The apoptotic nuclei were also detected in U87 cells at 10 μM and 50 μM doses (Fig. 2B, right). Thus, Y15 significantly increased glioblastoma cell detachment in a dose- and time-dependent manner and caused cell apoptosis.
Y15 decreased DBTRG and U87 glioblastoma cell invasion
To test if Y15 decreased cell invasion, we treated DBTRG (Fig. 2C, left) and U87 (Fig. 2C, right) cells with 1 μM dose of Y15 and performed an invasion assay using ECMatrix-coated Boyden chambers. To focus only on viable cells, invasion data were normalized to viability data. Y15 significantly decreased cell invasion in both cell lines (Fig. 2C, right and left panels). Thus, Y15 decreased DBTRG and U87 glioblastoma cell invasion in vitro.
Y15 inhibited FAK autophoshorylation, activated caspase-8, changed cell morphology and caused cell rounding in DBTRG and U87 glioblastoma cells
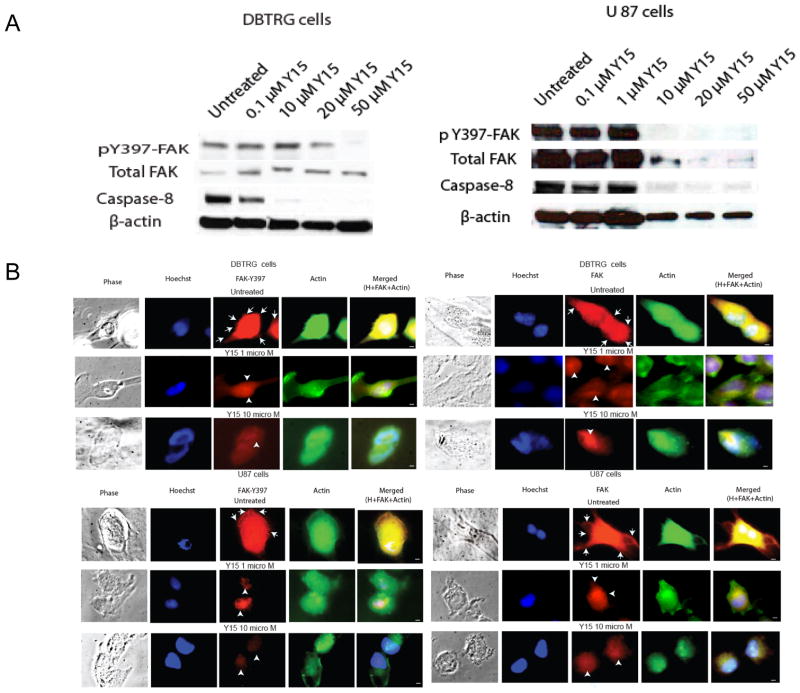
To test the effect of Y15 on FAK autophosphorylation activity in DBTRG and U87 glioma cells, we treated cells with different doses of Y15 and performed Western blotting with phospho-Y397-FAK antibody (Fig. 3A). Y15 effectively decreased phospho-Y397-FAK in a dose-dependent manner in both cell lines, while it did not affect total FAK in DBTRG cells (Fig. 3A, left). Y15 decreased more significantly Y397-FAK then total FAK in U87 cells (Fig. 3A, right). Y15 also decreased uncleaved caspase-8 in DBTRG and in U87 cells starting 10 μM dose (Fig. 3A). Thus, Y15 caused a dose-dependent decrease of FAK autophosphorylation and activated caspase-8 in DBTRG and U87 cells.
Figure 3.
A, B. Y15 decreased Y397-FAK autophosphorylation and activated caspase-8 in DBTRG and U87 glioblastoma cellsA. 1–2×106 cells were treated with Y15 inhibitor in 100 mm3 plates for 24 hours. DBTRG and U87 cells were treated with different doses of Y15 and Western blotting was performed. Y15 significantly decreased Y397-FAK phosphorylation and activated caspase-8 in DBTRG and U87 cells.
B. Decrease and change of Y397-FAK, FAK localization and cell morphology in Y15-treated glioblastoma cells. 1×105 cells were plated on 24 well plates and after 24 hours treated with 1 and 10 μM Y15 for 24 hours. Y397-FAK and FAK was immunostained in DBTRG (upper panels) and U87 (lower panels) with primary anti-Y397-FAK (left) or FAK (right) and then with Alexa-Red secondary antibodies. Actin was stained with Phalloidin-FITC, and nuclei were stained with Hoechst. Phase images are shown on the left and merged images are shown on the right panels. Y15 decreased Y397-FAK and FAK localization in focal adhesions at 1 and 10 μM doses, and changed cell morphology from polarized to rounded in both cell lines. White arrows show focal adhesions; white arrowheads show perinuclear/nuclear staining. 100x magnification; Scale bar: 2 μM.
To test the effect of Y15 on cell morphology, Y397-FAK and FAK localization, we performed immunostaining of FAK, Y397-FAK (red) and actin (green) in DBTRG (Fig. 3B, upper panels) and U87 cells (Fig. 3B, lower panels), treated with 1 and 10 microM of Y15. Y15 caused decrease of phospho-Y397-FAK and decreased localization of Y397-autophosphorylated (Fig. 3B, upper left panels) and total FAK (Fig. 3B, upper right panels) in focal adhesions, filopodia and cytoplasm starting with 1 microM of Y15 in DBTRG cells and increased localization of FAK in the perinuclear/nuclear area. The enlarged merged images of Y397-FAK, FAK and actin staining are shown in Suppl. Fig. S1. In addition, actin staining demonstrated that Y15 changed cell morphology from elongated polarized motile cells to the rounded cells, especially at 10 microM of Y15 (Fig. 3B). The same effects were observed in U87 cells (Fig. 3B). Thus, Y15 decreased Y397-FAK phosphorylation, changed its localization in focal adhesions and cytoplasm, decreased cell polarization and caused cell rounding.
Y15 decreased glioblastoma tumor growth, inhibited FAK autophosphorylation, activated PARP and caspase-3 in tumor xenografts
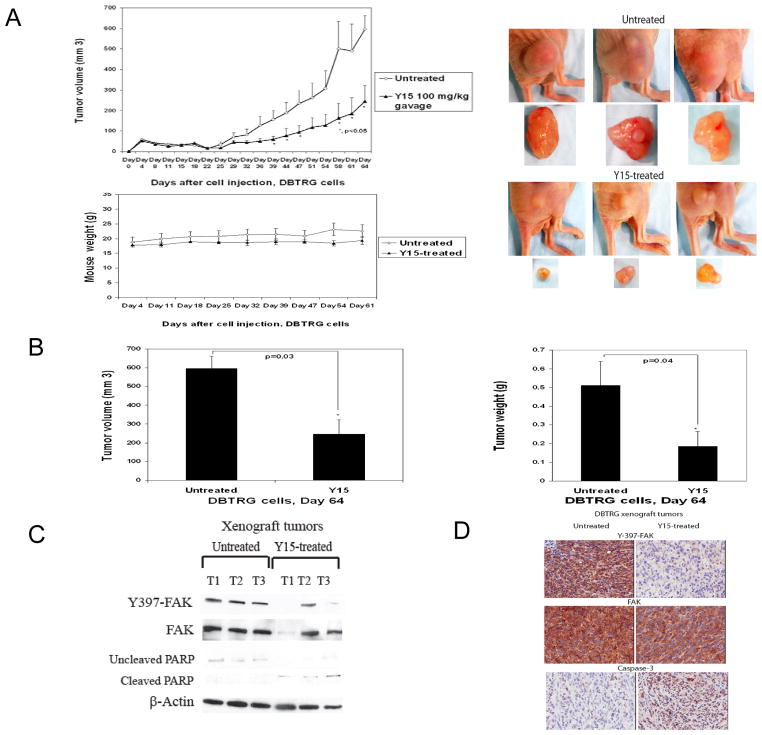
Next, we tested the effect of Y15 on DBTRG glioblastoma tumor growth at 100 mg/kg delivered orally over a 64 day period. Y15 significantly (p<0.05) decreased glioblastoma xenograft tumor growth (Fig. 4A, left upper panel). The tumor sizes were significantly less in Y15-treated mice compared with untreated (PBS-treated) mice (Fig. 4A, right upper panel). Y15 significantly decreased tumor volume (Fig. 4B, left panel) and tumor weight (Fig. 4B, right panel) without effect on mice body weight (Fig. 4A, lower panel).
Figure 4.
A. Y15 decreased glioblastoma tumor growth. A. Y15 decreased tumor growth in DBTRG xenograft model. Upper left panel: 2×106 DBTRG cells were injected into nude mice. Y15 was delivered orally at 100 mg/kg every day 5 days/week for 64 days. *p<0.05 tumor volume in Y15-treated versus untreated. Right upper panel: The Y15 inhibitor significantly decreased the size of Y15-treated tumors. Lower panel: Y15 did not decrease mice body weight. B. Y15 significantly decreased tumor volume and tumor weight. *p<0.05, Y15-treated versus untreated, Student’s t-test. C. Y15 decreases Y397 autophosphorylation and activates PARP cleavage in tumor xenograft tissues by Western blotting. D. Y15 decreases Y397 autophosphorylation and activates caspase-3 in tumor xenograft tissues by immunohistochemical staining.
In addition Y15 significantly decreased Y397-FAK and PARP in Y15-treated compared with untreated mice by Western blotting in most tumor xenografts (Fig. 4C). Immunohistochemical staining analysis demonstrated significantly decreased Y397-FAK and increase of cleaved caspase-3 in Y15-treated tumor samples (Fig. 4D). Thus, Y15 significantly decreased glioblastoma tumor growth at 100 mg/kg by oral delivery in vivo, which was accompanied by decreased autophosphorylation of FAK and activation of caspase-3 and PARP cleavage in Y15-treated xenograft tumors.
Y15 prolonged mice survival in orthotopic xenograft glioblastoma model
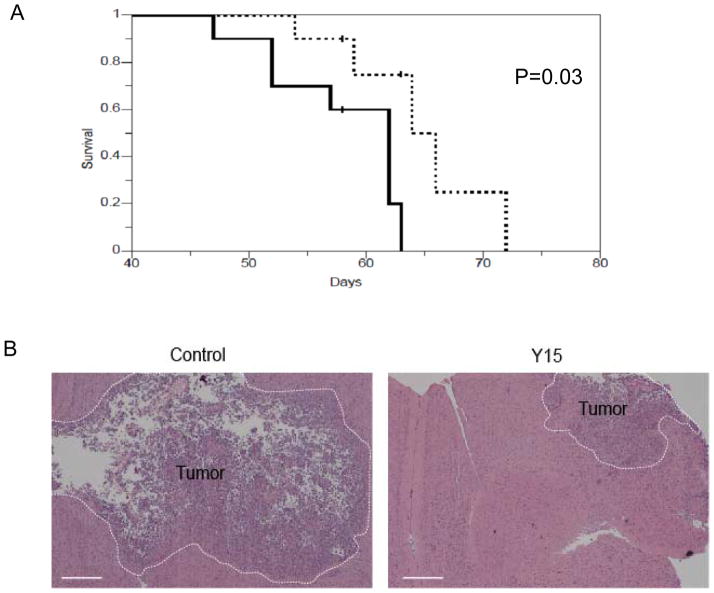
We then determined the effect of Y15 on glioblastoma survival using an orthotopic xenograft model. Mice treated with Y15 demonstrated significantly prolonged survival (p=0.03) in orthotopic xenograft glioblastoma model compared to the control 1xPBS-treated group (Fig. 5A). In addition, harvested brain tumors stained with hematoxylin and eosin demonstrated reduced tumor size (Fig. 5B). Thus, these results on prolonged mice survival in orthotopic mice model confirm the in vivo xenograft studies on reduced tumor growth in Y15-treated mice.
Figure 5. Y15 prolonged survival in orthotopic xenograft model.
The treatment with Y15 orthotopic xenograft model was performed, as described in Materials and Methods. A. Kaplan-Meier survival curve for Y15 inhibitor or PBS treated tumor-bearing mice is shown (solid line – control, dotted line – Y15-treated). Mice treated with Y15 inhibitor demonstrated prolonged survival. (N=10 for each group from three independent experiments, Log-rank test, two-sided P=0.03). B. Hematoxylin and Eosin staining of Y15-treated, tumor-bearing brain demonstrated reduced tumor size compared with the control group. Size bar, 0.5 mm.
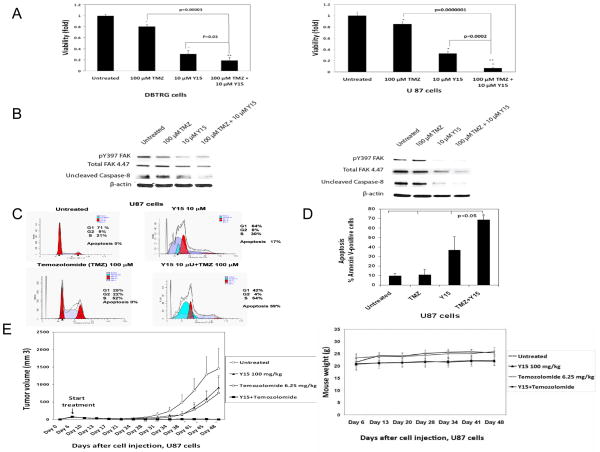
Combination of Y15 and temozolomide decreased cell viability and activated caspase-8 more significantly than each inhibitor alone in DBTRG and U87 cells
To test the combination of the standard chemotherapy drug for treatment of glioblastoma with Y15, we treated DBTRG (Fig. 6A, left) and U87 (Fig. 6, right) cells with 100 μM temozolomide (TMZ) and 10 μM Y15 alone and combination of Y15 and TMZ. The combination of TMZ and Y15 more significantly decreased viability of DBTRG and U87 glioblastoma cells compared with each inhibitor alone and untreated cells (Fig. 6A). The same effect was observed in U251 and D37 glioblastoma cells (Suppl. Fig S2). Thus, combination of Y15 and temozolomide more significantly decreased glioblastoma cell viability compared with each drug alone.
Figure 6.
A. Combination of Y15 plus temozolomide significantly decreased DBTRG and U87 cell viability. Cells were treated with temozolomide (TMZ), Y15 and Y15 plus temozolomide for 24 hours and MTT assay was performed. Bars show mean values ± standard deviations. *p<0.05, Y15, TMZ and Y15+TMZ groups versus untreated cells. **p<0.05, Y15+TMZ versus Y15 and TMZ.
Figure 6B. Combination of Y15 plus temozolomide decreased uncleaved caspase-8 in DBTRG and U87 cells. The cells were treated with Y15, TMZ and Y15+TMZ for 24 hours and Western was performed with Y397-FAK, FAK and caspase-8 antibodies. Y15 decreased Y397-FAK in U87 cells, and combination of Y15 and Temozolomide activated caspase-8 in Y15 plus temozolomide-treated U-87 cells versus untreated, Y15 and TMZ-treated cells.
Figure 6 C, D. Combination of Y15 plus temozolomide increased apoptosis in U87 cells. The 1×106 cells were treated as described in Materials and Methods. Y15 plus temozolomide increased apoptosis by Flow cytometry analysis (C) and by Annexin V/7AAD staining (D) in U87 cells compared with untreated cells. *p<0.05, Y15+TMZ versus Y15, TMZ-treated and untreated cells; p>0.05 in TMZ and Y15-treated versus untreated cells.
Figure 6 E. Combination of Y15 plus temozolomide blocked U87 xenograft tumor growth. Upper panel: The 3×106 U87 cells were injected into mice subcutaneously. At day 6 mice were randomized in four groups (n=5) with an equal average tumor volume in each group equal to 76 mm3. The treatment was started at day 6 (arrow). The representative of two experiments is shown. * Y15+TMZ versus untreated tumors, p<0.05; **p<0.05, Y15+TMZ versus Y15, TMZ and untreated tumors. Lower panel: Mice body weight was not affected by the treatments.
To test the effect of combination of Y15 and temozolomide on apoptosis, we treated DBTRG and U87 cells with Y15, temozolomide, and combination of Y15 and TMZ and performed Western blotting with Y397-FAK, FAK and caspase-8 antibodies (Fig. 6). Y15 decreased Y397-FAK in DBTRG and U87 cells, and caspase-8 was activated more in both cell lines, treated with Y15+TMZ than in cells without TMZ (Fig. 6B). To confirm activation of caspase-8 and find increased apoptosis, we treated U87 cells with a combination of Y15 and temozolomide as well as with each drug alone and analyzed cells by Flow cytometry (Fig. 6C). The combination of Y15 and temozolomide increased apoptosis to 59% in U87 cells compared to untreated, Y15 and temozolomide-treated cells (Fig. 6C). The significant increase of apoptosis in case of Y15 and TMZ combination compared to each agent alone was demonstrated by Annexin V staining (Fig. 6D). Thus, combination of Y15 and temozolomide decreased cell viability and increased apoptosis and activated caspase-8 more significantly than each agent alone in DBTRG and U87 cell lines.
The combination of Y15 and temozolomide blocked tumor growth in U87 xenografts
Since combination of Y15 and temozolomide caused a significant increase of apoptosis in U87 cells, we tested the effect of combination of Y15 and temozolomide in the U87 xenograft model in vivo (Fig. 6E, upper panel). The treatment with Y15, TMZ and combination started at day 6, when the average tumor volume reached 76 mm3 and was equal in each group of mice (Fig. 6E). The combination of Y15 and temozolomide effectively blocked tumor growth in the U87 xenograft model in contrast to Y15 and TMZ. At the end of experiment there were no tumors present in the combination therapy group. The mice body weight was not significantly affected in each treatment group (Fig. 6E, lower panel). We stopped the treatment in this group at day 49 and these tumors did not recur in any of the Y15 and TMZ-treated mice over an additional period of time, for a total 80 days (not shown). Thus, the combination of Y15 with temozolomide synergistically blocks U 87 tumor xenograft growth.
Discussion
This is the first study demonstrating that inhibition of FAK with small molecule inhibitor Y15 is an effective approach in blocking glioblastoma tumor growth, especially in combination with temozolomide.
In this study, the pharmacological blockade of FAK was achieved using an allosteric approach that targeted the major autophosphorylation site of FAK at Y397, and not the active ATP-binding site. This approach has the advantage of higher specificity in targeting FAK because it avoids the sites in the kinase domain that are most highly conserved among tyrosine kinases. The Y15 FAK inhibitor that we used is highly specific to FAK and it did not target other kinases, as we have shown (9). Most of the existing FAK inhibitors target the ATP binding site within the kinase domain and many have shown problems with cross reactivity with other kinases or other off-target effects. For example, TAE226 targeted not only FAK, but IFGR and other kinases and was highly toxic (18). Another inhibitor, PF-271 has been shown to inhibit not only FAK, but also homologous kinase Pyk-2 (19). A recently developed PND-1186 inhibitor, also targeting the ATP-binding site, effectively inhibited not only FAK, but also FLT-3, ACK-1 and Aurora-A (20–21). Thus, in order to effectively target FAK, new small molecule inhibitors and new approaches to this molecule are needed.
The high invasion of brain tumors is one of the leading causes of patient death and resistance to chemotherapy. FAK has been shown to be both highly autophosphorylated in brain tumors (1), and linked to brain tumor invasion (2). We have shown that by targeting the autophosphorylation site of FAK in glioblastoma cell lines we decreased glioma invasion in vitro. In addition, our recent observations in mice with targeted deletions of FAK in the vascular endothelium suggests that FAK blockade may also enhance the perfusion of chemotherapeutics in brain tumors by increasing vascular normalization (7). Thus, our data demonstrating the efficacy of Y15 in decreasing glioma progression may have additional therapeutic benefit when used in combination with temozolomide. The detailed study vascular normalization and invasion in glioblastoma in vivo treated with Y15 will be preformed in the future report.
Thus, these data demonstrated that Y15 is an effective inhibitor in decreasing glioma viability and tumor growth. These results suggest that Y15 may be an effective therapeutic inhibitor for treatment of human glioblastoma in combination with temozolomide.
Supplementary Material
Acknowledgments
The research is supported by Susan Komen for the Cure Foundation grant BCTR0707148 (V.M. Golubovskaya), NCI RO-1 grants CA65910 (W.G. Cance) and NHLBI RO1-73396 (B.P. Eliceiri) and partly by the NCI Cancer Center Support grant to the Roswell Park Cancer Institute (CA 16056).
We would like to thank the Pathology Core Facility at Roswell Park Cancer Institute for immunohistochemical staining. We would like to thank Dr. Paul Wallace and Mr. Craig Jones at Flow Cytometry Core Facility, Roswell Park Cancer Institute for flow cytometry and apoptosis analyses.
References
- 1.Hecker TP, Grammer JR, Gillespie GY, Stewart J, Jr, Gladson CL. Focal adhesion kinase enhances signaling through the Shc/extracellular signal-regulated kinase pathway in anaplastic astrocytoma tumor biopsy samples. Cancer Res. 2002;62:2699–707. [PubMed] [Google Scholar]
- 2.Cance WG, Golubovskaya VM. Focal adhesion kinase versus p53: apoptosis or survival? Sci Signal. 2008;1:pe22. doi: 10.1126/stke.120pe22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Golubovskaya VM, Finch R, Cance WG. Direct Interaction of the N-terminal Domain of Focal Adhesion Kinase with the N-terminal Transactivation Domain of p53. J Biol Chem. 2005;280:25008–21. doi: 10.1074/jbc.M414172200. [DOI] [PubMed] [Google Scholar]
- 4.Golubovskaya VM, Cance WG. Focal adhesion kinase and p53 signaling in cancer cells. Int Rev Cytol. 2007;263:103–53. doi: 10.1016/S0074-7696(07)63003-4. [DOI] [PubMed] [Google Scholar]
- 5.Golubovskaya VM, Cance W. Focal adhesion kinase and p53 signal transduction pathways in cancer. Front Biosci. 2010;15:901–12. doi: 10.2741/3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Natarajan M, Stewart JE, Golemis EA, Pugacheva EN, Alexandropoulos K, Cox BD, et al. HEF1 is a necessary and specific downstream effector of FAK that promotes the migration of glioblastoma cells. Oncogene. 2006;25:1721–32. doi: 10.1038/sj.onc.1209199. [DOI] [PubMed] [Google Scholar]
- 7.Lee J, Borboa AK, Chun HB, Baird A, Eliceiri BP. Conditional deletion of the focal adhesion kinase FAK alters remodeling of the blood-brain barrier in glioma. Cancer Res. 2010;70:10131–40. doi: 10.1158/0008-5472.CAN-10-2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu T-J, LaFortune T, Honda T, Ohmori O, Katakeyama S, Meyer T, et al. Inhibition of both focal adhesion kinase and insulin-like growth factor-I receptor kinase suppresses glioma proliferation in vitro and in vivo. Mol Cancer Ther. 2007;6:1357–67. doi: 10.1158/1535-7163.MCT-06-0476. [DOI] [PubMed] [Google Scholar]
- 9.Golubovskaya VM, Nyberg C, Zheng M, Kweh F, Magis A, Ostrov D, et al. A Small Molecule Inhibitor, 1,2,4,5-Benzenetetraamine Tetrahydrochloride, Targeting the Y397 Site of Focal Adhesion Kinase Decreases Tumor Growth. J Med Chem. 2008;51:7405–16. doi: 10.1021/jm800483v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beierle EA, Ma X, Stewart J, Nyberg C, Trujillo A, Cance WG, et al. Inhibition of focal adhesion kinase decreases tumor growth in human neuroblastoma. Cell Cycle. 2010;9:1005–15. doi: 10.4161/cc.9.5.10936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hochwald SN, Nyberg C, Zheng M, Zheng D, Wood C, Massoll NA, et al. A novel small molecule inhibitor of FAK decreases growth of human pancreatic cancer. Cell Cycle. 2009;8:2435–43. doi: 10.4161/cc.8.15.9145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanks SK, Polte TR. Signaling through focal adhesion kinase. Bioessays. 1997;19:137–45. doi: 10.1002/bies.950190208. [DOI] [PubMed] [Google Scholar]
- 13.Kruse CA, Mitchell DH, Kleinschmidt-DeMasters BK, Franklin WA, Morse HG, Spector EB, et al. Characterization of a continuous human glioma cell line DBTRG-05MG: growth kinetics, karyotype, receptor expression, and tumor suppressor gene analyses. In Vitro Cell Dev Biol. 1992;28A:609–14. doi: 10.1007/BF02631035. [DOI] [PubMed] [Google Scholar]
- 14.Golubovskaya V, Beviglia L, Xu LH, Earp HS, 3rd, Craven R, Cance W. Dual inhibition of focal adhesion kinase and epidermal growth factor receptor pathways cooperatively induces death receptor-mediated apoptosis in human breast cancer cells. J Biol Chem. 2002;277:38978–87. doi: 10.1074/jbc.M205002200. [DOI] [PubMed] [Google Scholar]
- 15.Eliceiri BP, Paul R, Schwartzberg PL, Hood JD, Leng J, Cheresh DA. Selective requirement for Src kinases during VEGF-induced angiogenesis and vascular permeability. Mol Cell. 1999;4:915–24. doi: 10.1016/s1097-2765(00)80221-x. [DOI] [PubMed] [Google Scholar]
- 16.Lund CV, Nguyen MT, Owens GC, Pakchoian AJ, Shaterian A, Kruse CA, et al. Reduced glioma infiltration in Src-deficient mice. J Neurooncol. 2006;78:19–29. doi: 10.1007/s11060-005-9068-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shai A, Pitot HC, Lambert PF. p53 Loss synergizes with estrogen and papillomaviral oncogenes to induce cervical and breast cancers. Cancer Res. 2008;68:2622–31. doi: 10.1158/0008-5472.CAN-07-5266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu W, Bloom DA, Cance WG, Kurenova EV, Golubovskaya VM, Hochwald SN. FAK and IGF-IR interact to provide survival signals in human pancreatic adenocarcinoma cells. Carcinogenesis. 2008;29:1096–107. doi: 10.1093/carcin/bgn026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roberts WG, Ung E, Whalen P, Cooper B, Hulford C, Autry C, et al. Antitumor activity and pharmacology of a selective focal adhesion kinase inhibitor, PF-562,271. Cancer Res. 2008;68:1935–44. doi: 10.1158/0008-5472.CAN-07-5155. [DOI] [PubMed] [Google Scholar]
- 20.Tanjoni I, Walsh C, Uryu S, Tomar A, Nam JO, Mielgo A, et al. PND-1186 FAK inhibitor selectively promotes tumor cell apoptosis in three-dimensional environments. Cancer Biol Ther. 2010;9:764–77. doi: 10.4161/cbt.9.10.11434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walsh C, Tanjoni I, Uryu S, Tomar A, Nam JO, Luo H, et al. Oral delivery of PND-1186 FAK inhibitor decreases tumor growth and spontaneous breast to lung metastasis in pre-clinical models. Cancer Biol Ther. 2010;9:778–90. doi: 10.4161/cbt.9.10.11433. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.