Abstract
Purpose
To determine if the associations among established risk factors and reduced kidney function vary by age.
Methods
We pooled cross-sectional data from 14,788 non-diabetics aged 40–100 years in 4 studies: Cardiovascular Health Study, Health, Aging, and Body Composition Study, Multi-Ethnic Study of Atherosclerosis, and Prevention of Renal and Vascular End-Stage Disease cohort.
Results
Hypertension and low HDL-cholesterol were associated with reduced cystatin C-based estimated glomerular filtration rate (eGFR) across the age spectrum. In adjusted analyses, hypertension was associated with a 2.3 (95% CI 0.1, 4.4), 5.1 (4.1, 6.1), and 6.9 (3.0, 10.4) mL/min/1.73 m2 lower eGFR in participants 40–59, 60–79, and 80+ years, respectively (p-value for interaction <0.001). The association of low HDL-cholesterol with reduced kidney function was also greater in the older age groups: 4.9 (3.5, 6.3), 7.1 (CI 6.0, 8.3), 8.9 (CI 5.4, 11.9) mL/min/1.73 m2 (p-value for interaction <0.001). Smoking and obesity were associated with reduced kidney function in participants under 80 years. All estimates of the potential population impact of the risk factors were modest.
Conclusions
Hypertension, obesity, smoking, and low HDL-cholesterol are modestly associated with reduced kidney function in non-diabetics. The associations of hypertension and HDL-cholesterol with reduced kidney function appear stronger in older adults.
Keywords: Chronic kidney insufficiency, aged, hypertension, cholesterol, obesity, smoking
INTRODUCTION
There is emerging evidence that the risk factor profile for cardiovascular events is different in older compared with younger adults. Some traditional cardiovascular risk factors, such as total cholesterol, hypertension, and obesity, appear to have weaker associations with cardiovascular events in older persons. [1–5] Although prior research has demonstrated associations between high blood pressure, smoking, obesity, and dyslipidemia with reduced kidney function,[6–10] there are fewer data on the associations across the age spectrum. [11]The risk factor profile for reduced kidney function is similar to that of cardiovascular events; therefore one might postulate that these associations may also vary with age. A thorough understanding of the risk factors for reduced kidney function across the age spectrum is essential in order to determine the optimal targets to prevent kidney disease over the life course.
A limitation of some studies of kidney disease in older adults is the use of creatinine-based measures of kidney function. Creatinine is a by-product of muscle mass, and is less accurate for assessing kidney function in older adults, whom often have reduced muscle mass. Cystatin C is an alternative measure of kidney function that is a more accurate estimate of glomerular filtration rate compared with serum creatinine in the elderly, as it is not influenced by muscle mass [12–14].
The present study had two main objectives: 1) to compare the association of major clinical risk factors and reduced kidney function that was evaluated by cystatin C levels and assessed across the age spectrum, and 2) to estimate the population-level impact of the risk factors, based on population intervention models.[15] Since the effect of risk factors across the age spectrum is especially unclear in the development of non-diabetic kidney disease, we limited the present study to non-diabetics. By the combination of data from four studies on nearly 15,000 non-diabetics aged 40–100, we have the opportunity to examine these relationships in a large sample of middle and older aged adults.
SUBJECTS AND METHODS
Study Population
We combined cross-sectional data from 14,788 non-diabetics in the Cardiovascular Health Study (CHS), the Health, Aging, and Body Composition Study (Health ABC), the Multi-Ethnic Study of Atherosclerosis (MESA), and the Prevention of Renal and Vascular End-Stage Disease cohort (PREVEND).
The CHS aimed to evaluate risk factors for the development and progression of cardiovascular disease in the elderly [16]. The study recruited persons from Medicare eligibility lists in Forsyth County, North Carolina; Sacramento County, California; Washington County, Maryland; and Pittsburgh, Pennsylvania in 1989–1990 for the original cohort and 1992–1993 for a supplementary enrollment period designed to increase the number of African-American participants. To be considered eligible for the study, persons had to meet the following criteria: 1) age ≥ 65 years; 2) not institutionalized; 3) expected to remain in the current community for 3 years or longer; 4) not under active treatment for cancer; and 5) able to give informed consent without requiring a proxy respondent.
The Health ABC study was designed to examine the relation between age-related changes of health and body composition and incident functional limitations in initially well-functioning black and white adults aged 70–79 years. Each of the two study sites, Pittsburgh, PA and Memphis, TN, recruited participants from a list of Medicare beneficiaries between April 1997 and June 1998. Inclusion criteria were 1) reported ability to walk one-quarter mile, climb ten steps, and perform basic activities of daily living without difficulty; 2) absence of life-threatening illness; and, 3) plan to remain in the geographic area for at least three years.
MESA is a cohort of men and women, aged 45–84 years, and free of clinical cardiovascular disease at baseline, designed to examine progression from subclinical to clinical cardiovascular disease in adults [17]. The cohort is approximately 38% White, 28% African-American, 23% Hispanic, and 11% Asian (of Chinese descent). Participants were recruited from six field centers (New York, NY, Baltimore, MD, Chicago, IL, Los Angeles, CA, Minneapolis/St. Paul, MN, and Winston Salem, NC) with a variety of population-based approaches from 2000–2002.
The PREVEND study is a prospective cohort that includes inhabitants aged 28–75 of the city of Groningen, The Netherlands in 1997–1998. The study was designed to investigate the natural course of urinary albumin excretion and its impact on renal and cardiovascular disease. The present study includes the sub-sample of the original cohort that is representative of the general population of Groningen [18, 19].
We have previously published results from this Collaborative Study group, and carefully examined study populations and variable definition to ensure comparability across the studies [20]. We restricted the study population to non-diabetic participants aged 40 years and older with measured cystatin C; this included 4,276 participants in the CHS (aged 65–100), 2,471 participants in the Health ABC study (aged 69–80), 5,748 participants in MESA (aged 45–84) and 2,293 participants in PREVEND (aged 40–75).
This study has been approved by all relevant institutional review boards.
Kidney Function
Three of the four studies (CHS, Heath ABC and MESA) measured cystatin C by the same protocol and laboratory at the University of Vermont, and the PREVEND study used the same method at the University of Groningen laboratory. Cystatin-C was measured in all studies by a BNII nephelometer (Dade Behring Inc., Deerfield, IL) that utilizes a particle enhanced immunonepholometric assay (N Latex Cystatin-C) [21]. The assay range is 0.195 to 7.330 mg/L. Intra-assay coefficients of variation (CVs) range from 2.0 – 2.8% and inter-assay CVs range from 2.3 – 3.1%. Samples were measured from frozen plasma (CHS and Health ABC) or serum (MESA and PREVEND) that had been stored at −70°C (CHS, Health ABC, MESA) or −20°C (PREVEND). Cystatin C was measured in 2001 (PREVEND), 2003 (CHS), 2004 (Health ABC), and 2006 (MESA). Studies have shown that cystatin C is stable when frozen, and can withstand several freeze/thaw cycles. To ensure the comparability of the University of Vermont and PREVEND laboratories, we ran a comparison study of 101 samples. The correlation between the sets was 0.97, the CV was 6%, and the mean difference was 0.055 mg/L, which was not significantly associated with the mean concentration.
Creatinine concentration was used as a comparative measure of kidney function and was assayed by means of a colorimetric technique based on the enzymatic method (CHS and PREVEND: Ektachem 700, Eastman Kodak; Health ABC and MESA: Johnson & Johnson VITROS 950 Chemistry Analyzer).
We calculated cystatin C-based estimated glomerular filtration rate (eGFR) based on the cystatin C equation (eGFR = cystatin C−1.19 × 76.7) developed by the CKD-EPI group [22].
Other Variables
Participants were excluded if they had diabetes, defined as self-reported history, fasting glucose ≥126 mg/dL, or oral glucose tolerance test ≥ 200 mg/dL. Plasma glucose values in PREVEND were transformed to whole blood values using an internally validated correction factor [23, 24]. We categorized risk factors as present or absent based on clinically recognized definitions. Risk factors for kidney disease in this analysis included the following: hypertension (self-reported history, medication use, or systolic blood pressure >140 mmHg or diastolic blood pressure >90 mmHg), obesity (body mass index >30 kg/m2), smoking (former or current), high LDL-cholesterol (> 130 mg/dL or medication use) or low HDL-cholesterol (<40 mg/dL in men, <50 mg/dL in women).
Age, sex, and race were determined by self-report. Income was classified into 4 levels: <$12,000 ($10,000 in Health ABC), $12,000–25,000, $25,000–50,000, and $50,000 and over; and a similar 4-level categorical variable in PREVEND participants (in the Netherlands). Alcohol use was classified as none in the past year, <1 drink per week (2 drinks in PREVEND), 1–7 drinks per week, and >1 drink per day. History of cardiovascular disease was defined as a history of coronary artery disease, peripheral vascular disease, or cerebrovascular disease.
Statistical Analysis
We summarized baseline characteristics of participants and risk factors across age groups (40–59, 60–79, and 80+ years). We next calculated the mean difference in cystatin C in participants with and without the risk factors for kidney disease, within each of the age strata. To account for potential confounders we modeled cystatin C as the outcome variable in a linear model that included hypertension, obesity, smoking, high LDL-cholesterol, low HDL-cholesterol, study, age, sex, race, income, alcohol use, and history of cardiovascular disease and heart failure. For the linear models, we used targeted maximum likelihood estimation to estimate the marginal difference in kidney function in participants with and without the risk factors of interest. This estimator is similar to traditional likelihood methods, except it incorporates information from both the outcome model and a model of the risk factor of interest, and will produce consistent estimates if either model is correctly specified [25–27]. These models were conducted within each of the age strata to allow the risk factor and confounder associations to vary across the age. The p-values for the age category and risk factor interactions were calculated from traditional linear regression models, adjusted for all potential confounders. We transformed the marginal differences in cystatin C concentrations into eGFR.
To calculate the population impact of preventing a risk factor, we used population intervention models [15]. This method is similar to a population attributable fraction in that the measure takes into account both the prevalence of the risk factor and the magnitude of the association. For these analyses, we again used linear models with targeted maximum likelihood estimation to estimate the mean difference in level of kidney function between the observed population and a theoretical population in which participants did not have the risk factor, but all other measured characteristics were the same.
To identify the best fit for all models, we used a computer learning algorithm to identify the form of the relationship between the independent and dependent variables, based on a combination of forward and backward model fitting and cross-validation [28].
We calculated standard errors based on bootstrap sampling with 2000 replicates. All analyses were conducted in R (The R Foundation, Vienna, Austria).
RESULTS
Kidney function was lower in the older age groups; median cystatin C-based eGFR was 103 mL/min/1.73m2 in 40–59 year olds, 83 mL/min/1.73m2 in 60–79 year olds, and 66 mL/min/1.73m2 in participants 80 and older (p<0.001). On average, participants 80 years and older, who were predominantly from the CHS, were more likely to be Black, and less likely to be Asian or Latino (Table 1). Older participants also reported lower annual income, and less alcohol use and current smoking. Older participants had slightly lower average body mass index, diastolic blood pressure and HDL-cholesterol, higher systolic blood pressure, LDL-cholesterol and fasting glucose, were more likely to use antihypertensive medications, and were more likely to report a history of cardiovascular disease or heart failure.
Table 1.
Study characteristics by age groups
| 40–59 years (n=4,151) | 60–79 years (n=9,887) | 80+ years (n=750) | |
|---|---|---|---|
| Characteristic | Mean ± SD or n (%) | ||
| Age (years) | 51 ± 5 | 71 ± 5 | 83 ± 3 |
| Female | 2,228 (54%) | 5,642 (57%) | 412 (55%) |
| Race | |||
| Black | 683 (17%) | 2,247 (23%) | 150 (20%) |
| Asian | 337 (8%) | 355(4%) | 29 (4%) |
| Latino | 580 (14%) | 550 (6%) | 47 (6%) |
| White/Other | 2,517 (61%) | 6,732 (68%) | 524 (70%) |
| Income | |||
| <$25,000 | 1,274 (31%) | 4,684 (51%) | 432 (64%) |
| ≥$25,000 | 2,804 (69%) | 4,524 (49%) | 245 (36%) |
| Alcohol use (in past year) | 2,851 (69%) | 5,352 (54%) | 354 (48%) |
| Smoking | |||
| Never | 1,757 (42%) | 4,422 (45%) | 457(61%) |
| Former | 1,395 (34%) | 4,232 (43%) | 259 (34%) |
| Current | 984 (24%) | 1,208 (12%) | 34 (5%) |
| BMI (kg/m2) | 27.5 ± 5.2 | 27.0 ± 4.7 | 25.8 ± 4.3 |
| Systolic Blood Pressure (mmHg) | 120 ± 18 | 134 ± 21 | 142 ± 24 |
| Diastolic Blood Pressure (mmHg) | 73 ± 10 | 72 ± 11 | 69 ± 12 |
| Fasting Glucose (mg/dL) | 89 ± 12 | 96 ± 11 | 99 ± 10 |
| HDL-cholesterol (mg/dL) | 51 ± 15 | 55 ± 16 | 55 ± 16 |
| LDL-cholesterol (mg/dL) | 128 ± 36 | 127 ± 35 | 122 ± 35 |
| Antihypertensive Use | 663 (17%) | 4,329 (44%) | 408 (54%) |
| History of Cardiovascular Disease | 85 (2%) | 2,010 (17%) | 227 (25%) |
| History of Heart Failure | 0 (0%) | 181 (2%) | 34 (5%) |
| Study | |||
| CHS | 0 (0%) | 3,781 (38%) | 495 (66%) |
| Health ABC | 0 (0%) | 2,454 (25%) | 17 (2%) |
| MESA | 2,570 (62%) | 2,940 (30%) | 238 (32%) |
| PREVEND | 1,581 (38%) | 712 (7%) | 0 (0%) |
|
| |||
| Risk Factor | |||
|
| |||
| Hypertension | 1,192 (29%) | 6,172 (62%) | 578 (77%) |
| Smoking (current or former) | 2,379 (58%) | 5,440 (55%) | 293 (39%) |
| Obese | 1,061 (26%) | 2,145 (22%) | 97 (13%) |
| High LDL-Cholesterol | 1,939 (47%) | 4,990 (51%) | 321 (43%) |
| Low HDL-Cholesterol | 1,478 (36%) | 2,748 (29%) | 205 (27%) |
The prevalence of hypertension in participants 80 years and older was over double that of participants 40–59 years (Table 1). In contrast, the prevalence of smoking, obesity, high LDL-cholesterol and low-HDL cholesterol were lower in older age groups compared with younger age groups.
Association of Risk Factors with Reduced Kidney Function, Across Age Groups
The presence of hypertension was associated with reduced cystatin C-based kidney function across all age groups (Table 2), and this association was stronger in the older age groups. In adjusted analyses, hypertension was associated with a 2.3 (95% CI 0.1, 4.4), 5.1 (4.1, 6.1), and 6.9 (3.0, 10.4) mL/min/1.73 m2 lower eGFR in participants 40–59, 60–79, and 80+ years, respectively (p-value for interaction <0.001). The estimates for the association of smoking and reduced kidney function appeared similar across the age spectrum, and reached statistical significance only in adults less than 80 years in both unadjusted and adjusted models. Elevated LDL-cholesterol was only statistically significantly associated with reduced cystatin C-based kidney function in adults aged 60–79 years in unadjusted analyses, and this association did not persist after adjustment for potential confounders. In contrast, low HDL-cholesterol was strongly and significantly associated with reduced cystatin C-based kidney function across the age spectrum, even after adjustment for demographic characteristics and other risk factors. The association of low HDL-cholesterol with reduced kidney function was also greater in the older age groups: 4.9 (3.5, 6.3), 7.1 (CI 6.0, 8.3), 8.9 (CI 5.4, 11.9) mL/min/1.73 m2 (p-value for interaction <0.001). For comparison, we estimated the mean difference in creatinine-based eGFR associated with the risk factors; the estimates were smaller for all of the risk factors except for LDL-cholesterol (Appendix).
Table 2.
Association between risk factors and reduced kidney function in non-diabetics
| Age Group | Hypertension | Smoking | Obesity | High LDL-Cholesterol | Low HDL-Cholesterol | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Difference in eGFR ml/min/1.73m2‡ (95% CI) | ||||||||||
| Unadjusted | ||||||||||
| 40–59 years | −6.1 (−7.7, −4.6) | <0.001 | −1.8 (−3.3, −0.2) | 0.03 | −9.9 (−11.4, −8.4) | <0.001 | −1.3 (−2.9, 0.2) | 0.32 | −7.4 (−8.8, −5.9) | <0.001 |
| 60–79 years | −8.1 (−9.0, −7.2) | <0.001 | −2.9 (−3.9, −1.8) | <0.001 | −5.1 (−6.3, −3.9) | <0.001 | 1.1 (−0.2, 2.2) | 0.05 | −8.6 (−9.6, −7.6) | <0.001 |
| 80+ years | −8.9 (−12.0, −5.5) | <0.001 | −1.4 (−4.8, 2.3) | 0.44 | 1.7 (−3.6, 7.8) | 0.53 | −2.4 (−5.7, 1.2) | 0.18 | −8.9 (−11.9, −5.7) | <0.001 |
| p-value for interaction† | <0.001 | 0.19 | 0.004 | 0.01 | <0.001 | |||||
|
| ||||||||||
| Adjusted* | ||||||||||
| 40–59 years | −2.3 (−4.4, −0.1) | 0.04 | −2.4 (−4.0, −0.8) | 0.003 | −6.6 (−8.4, −4.7) | <0.001 | 0.1 (−1.4, 1.6) | 0.89 | −4.9 (−6.3, −3.5) | <0.001 |
| 60–79 years | −5.1 (−6.1, −4.1) | <0.001 | −2.4 (−3.6, −1.2) | <0.001 | −5.2 (−6.4, −3.9) | <0.001 | 0.8 (−0.3, 1.9) | 0.17 | −7.1 (−8.3, −6.0) | <0.001 |
| 80+ years | −6.9 (−10.4, −3.0) | <0.001 | −1.6 (−5.1, 2.4) | 0.42 | 3.8 (−0.8, 9.1) | 0.11 | −1.9 (−5.1, 1.6) | 0.27 | −8.9 (−11.9, −5.4) | <0.001 |
| p-value for interaction† | <0.001 | 0.84 | 0.01 | 0.16 | <0.001 | |||||
Potential confounders included the risk factors, study, age, sex, race, income, alcohol use, and history of cardiovascular disease and heart failure
Based on targeted maximum likelihood estimation
Based on a traditional linear regression model
Population Intervention Models of Risk Factors
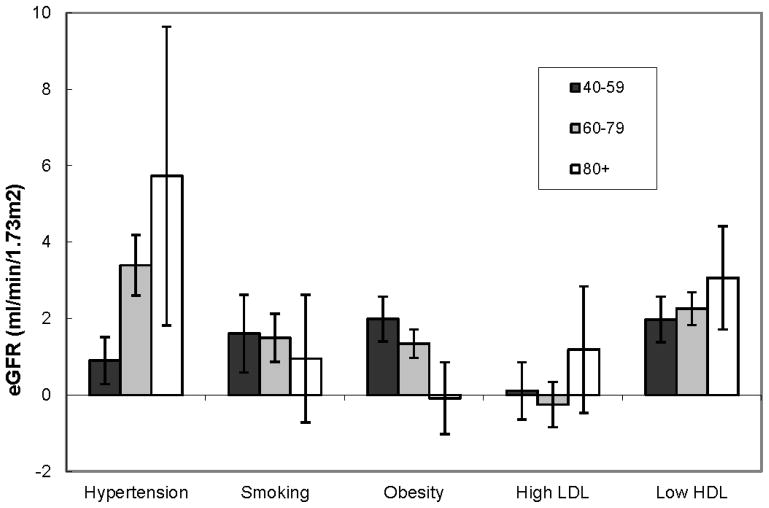
The potential population impact of hypertension was greater in the older age groups (Figure 1). Based on the associations presented above, the theoretical elimination of hypertension would result in a marginal increase of 0.9 (95% CI 0.3, 1.5), 3.4 (95% CI 2.7, 2.1), and 5.7 (95% CI 2.1, 9.0) ml/min/1.73 m2 lower cystatin C-based eGFR in participants 40–59, 60–79, and 80 years, respectively. The estimates of the population level impact of smoking and obesity on kidney function were modest among participants aged 40–59 and 60–79 years, and were not significant in participants 80 and older. The estimated impact of low HDL-cholesterol appeared only slightly greater at older ages: 2.0 (95% CI 1.4, 2.6), 2.3 (95% CI 1.9, 2.7), and 3.1 (95% CI 1.8, 4.3) ml/min/1.73 m2 lower cystatin C-based eGFR in participants 40–59, 60–79, and 80 years, respectively.
Figure 1.
Projected potential population impact of the risk factors on cystatin C-based eGFR in non-diabetics; estimates were adjusted for all risk factors, study, age, sex, race, income, alcohol use, and history of cardiovascular disease and heart failure
DISCUSSION
In a large, multi-study sample of non-diabetics, we found an association of hypertension with reduced kidney function, across the age-spectrum. Due to the increasing prevalence of hypertension with age, the potential population impact of hypertension on reduced kidney function was higher in older compared with younger adults. We also found that low HDL-cholesterol had the strongest association with reduced kidney function; however, the population impact was lower than hypertension because of the relatively smaller prevalence of low HDL-cholesterol. Low HDL-cholesterol is an important cardiovascular risk factor across the age spectrum, and our findings demonstrate that it should be investigated further as a renal risk factor.
In a recent study from NHANES, investigators reported that the association of hypertension with stage 3 or 4 chronic kidney disease diminished with older age (p=0.038 for trend) [11]. The authors used prevalence ratios for the main analysis, which they note are dependent on the baseline risk; but they also reported that a similar trend was observed with absolute difference measures, although the data were not shown. In addition, the NHANES study used creatinine-based measures of kidney function, which are less accurate for estimating eGFR in older adults [12, 14]. In the present study, the estimates for the difference in kidney function associated with hypertension and low HDL-cholesterol were attenuated when we used a creatinine-based eGFR.
We found that low HDL-cholesterol was associated with reduced kidney function, across the age spectrum. Our findings are consistent with prior literature that has reported HDL-cholesterol is an important risk factor for kidney disease [8–10]. Some have hypothesized that a pathologic process similar to atherosclerosis mediates this relationship in the kidney [29]. Additionally, HDL-cholesterol is inversely associated with inflammation, which may also affect kidney function.[30] In contrast, LDL-cholesterol was associated only with reduced creatinine-based kidney function. The evidence for an association of LDL-cholesterol and reduced kidney function has been less robust compared with HDL-cholesterol [10], and randomized clinical trials have found mixed effects of statins in persons with chronic kidney disease [31–33].
We found obesity was associated with reduced kidney function in adults less than 80 years of age. Prior studies have reported that obesity is an important risk factor for kidney disease [7, 8]. It is not surprising that this association was not observed in adults over 80 years, as the effect of obesity on many health outcomes appears to be diminished in older adults. In addition, the prevalence of obesity in the oldest age group was half that of the younger age group, which could indicate those who were susceptible to the adverse effects of obesity might have been lost to competing risks prior to the study initiation. Smoking has been reported to be a risk factor for kidney disease [6, 8], likely due to its adverse effects on the microvasculature. The estimate for the association between smoking and reduced kidney function only reached statistical significance in adults under 80 years, although all estimates were consistently in the harmful direction. The smaller magnitude of the association in the oldest age group could be due to the competing risk of mortality and the high proportion of past smokers compared with current smokers in this age group.
A strength of the present study is the estimation of the potential population impact of hypertension, smoking, obesity, LDL-cholesterol, and HDL-cholesterol, based on population intervention models[15]; this is the first time these models have been applied to examine renal risk factors. This conceptual measure estimates the impact of eliminating the risk factor in a population, under the assumption that the risk factor is causally related to kidney function. Although we cannot determine the direction of causality in the present study, the strength of this method is that it accounts for both the strength of the association of the risk factor with kidney function and the prevalence of the factor in the study population. Traditionally, the population attributable risk has been used as a measure that incorporates both the magnitude of association and prevalence of the risk factor. However, most researchers implement the method incorrectly [34] and do not properly account for confounding. [15] It is noteworthy that the estimates of potential population impact were modest in the present study.
Additional strengths of our study include the combination of data from four large, community-based studies across the age spectrum, the use of cystatin C as a marker of kidney function, the use of difference in mean models, which are not dependent on baseline risk.. The primary limitation of this study is that the use of cross sectional data prohibits us from observing the impact of risk factors over time, and do not allow for conclusions on a causal relationship. It is possible that there is an impact of reduced kidney function on some of the risk factors; for example, kidney disease also likely increases the risk of hypertension. Also, participants with hypertension were frequently using antihypertensive medications, and the cross-sectional design of our study precludes our ability to separate the effects of hypertension and antihypertensive medication use on kidney function. In addition, we simplified the classification of the risk factors into clinically relevant binary variables in order to compare the magnitude of the associations; however, different levels of the risk factors may have different meaning across the age groups. Another limitation is that we did not have information on the history of exposure to the risk factors; it is possible older participants had a longer history of the risk factors, which may have influenced the magnitude of the associations. Given these limitations, our findings provide a strong rationale to prospectively study age dependent effects of risk factor associated decline in kidney function. Finally, our study did not include a direct measure of GFR.
In summary, hypertension, obesity, and low HDL-cholesterol are associated with reduced kidney function in non-diabetics. The associations of hypertension and HDL-cholesterol appear from moderate to strong across the age spectrum, and the population impact of these risk factors is greater at older ages. Our study provides important evidence to consider that risk factors may differ across age groups. Longitudinal studies that evaluate the association of these risk factors and reduced kidney function across the life course will allow investigators to better unravel the causal pathways underlying these associations, and to identify optimal preventive strategies to preserve kidney function at older ages.
Acknowledgments
SUPPORT:
The Cardiovascular Health Study was supported by National Heart, Lung, and Blood Institute (NHLBI) contracts N01-HC-85239, N01-HC-85079 through N01-HC-85086; N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, N01-HC-45133 and NHLBI grant HL080295, with additional contribution from National Institute of Neurological Disorders and Stroke. Additional support was provided through AG-023629, AG-15928, AG-20098, and AG-027058 from the National Institute on Aging (NIA). See also http://www.chs-nhlbi.org/pi.htm. Health ABC was supported through the NIA contracts N01-AG-6-2101, N01-AG-6-2103, and N01-AG-6-2106; NIA grant R01-AG028050, and National Institute of Nursing Research grant R01-NR012459. In addition, this research was supported in part by the Intramural Research Program of the NIH, NIA. MESA was supported by grant R01-HL-63963-01A1 and by contracts N01-HC-95159 through N01-HC-95165 and N01-HC-95169 from the NHLBI. PREVEND is supported by grant E013 of the Dutch Kidney Foundation, Bussum, The Netherlands, and grants T32DK07791 and DK52866 from the National Institute of Diabetes and Digestive, and Kidney Diseases.
This project was also supported by grant R01AG027002 from the NIA. Dr. Odden is supported by a the American Heart Association Western States Affiliate Clinical Research Program and the National Institute on Aging (K01AG039387)
ABBREVIATIONS AND ACRONYMS
- BMI
Body mass index
- CHS
Cardiovascular Health Study
- eGFR
Estimated glomerular filtration rate
- Health ABC
Health, Aging, and Body Composition Study
- HDL
High-density lipoprotein
- LDL
Low-density lipoprotein
- MESA
Multi-Ethnic Study of Atherosclerosis
- PREVEND
Prevention of Renal and Vascular End-Stage Disease cohort
Footnotes
The results presented in this paper are our original work, and have not been published previously in whole or part. The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kronmal RA, Cain KC, Ye Z, Omenn GS. Total serum cholesterol levels and mortality risk as a function of age. A report based on the Framingham data. Arch Intern Med. 1993;153(9):1065–73. [PubMed] [Google Scholar]
- 2.Satish S, Freeman DH, Jr, Ray L, Goodwin JS. The relationship between blood pressure and mortality in the oldest old. J Am Geriatr Soc. 2001;49(4):367–74. doi: 10.1046/j.1532-5415.2001.49078.x. [DOI] [PubMed] [Google Scholar]
- 3.Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338:b1665. doi: 10.1136/bmj.b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rimm EB, Stampfer MJ, Giovannucci E, Ascherio A, Spiegelman D, Colditz GA, et al. Body size and fat distribution as predictors of coronary heart disease among middle-aged and older US men. Am J Epidemiol. 1995;141(12):1117–27. doi: 10.1093/oxfordjournals.aje.a117385. [DOI] [PubMed] [Google Scholar]
- 5.Seeman T, Mendes de Leon C, Berkman L, Ostfeld A. Risk factors for coronary heart disease among older men and women: a prospective study of community-dwelling elderly. Am J Epidemiol. 1993;138(12):1037–49. doi: 10.1093/oxfordjournals.aje.a116822. [DOI] [PubMed] [Google Scholar]
- 6.Briganti EM, Branley P, Chadban SJ, Shaw JE, McNeil JJ, Welborn TA, et al. Smoking is associated with renal impairment and proteinuria in the normal population: the AusDiab kidney study. Australian Diabetes, Obesity and Lifestyle Study. Am J Kidney Dis. 2002;40(4):704–12. doi: 10.1053/ajkd.2002.35677. [DOI] [PubMed] [Google Scholar]
- 7.Ejerblad E, Fored CM, Lindblad P, Fryzek J, McLaughlin JK, Nyren O. Obesity and risk for chronic renal failure. J Am Soc Nephrol. 2006;17(6):1695–702. doi: 10.1681/ASN.2005060638. [DOI] [PubMed] [Google Scholar]
- 8.Fox CS, Larson MG, Leip EP, Culleton B, Wilson PW, Levy D. Predictors of new-onset kidney disease in a community-based population. JAMA. 2004;291(7):844–50. doi: 10.1001/jama.291.7.844. [DOI] [PubMed] [Google Scholar]
- 9.Hunsicker LG, Adler S, Caggiula A, England BK, Greene T, Kusek JW, et al. Predictors of the progression of renal disease in the Modification of Diet in Renal Disease Study. Kidney Int. 1997;51(6):1908–19. doi: 10.1038/ki.1997.260. [DOI] [PubMed] [Google Scholar]
- 10.Muntner P, Coresh J, Smith JC, Eckfeldt J, Klag MJ. Plasma lipids and risk of developing renal dysfunction: the atherosclerosis risk in communities study. Kidney Int. 2000;58(1):293–301. doi: 10.1046/j.1523-1755.2000.00165.x. [DOI] [PubMed] [Google Scholar]
- 11.Islam TM, Fox CS, Mann D, Muntner P. Age-related associations of hypertension and diabetes mellitus with chronic kidney disease. BMC Nephrol. 2009;10:17. doi: 10.1186/1471-2369-10-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dharnidharka VR, Kwon C, Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am J Kidney Dis. 2002;40(2):221–6. doi: 10.1053/ajkd.2002.34487. [DOI] [PubMed] [Google Scholar]
- 13.Shlipak MG, Praught ML, Sarnak MJ. Update on cystatin C: new insights into the importance of mild kidney dysfunction. Curr Opin Nephrol Hypertens. 2006;15(3):270–5. doi: 10.1097/01.mnh.0000222694.07336.92. [DOI] [PubMed] [Google Scholar]
- 14.Fliser D, Ritz E. Serum cystatin C concentration as a marker of renal dysfunction in the elderly. Am J Kidney Dis. 2001;37(1):79–83. doi: 10.1053/ajkd.2001.20628. [DOI] [PubMed] [Google Scholar]
- 15.Hubbard AE, Laan MJ. Population intervention models in causal inference. Biometrika. 2008;95(1):35–47. doi: 10.1093/biomet/asm097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1(3):263–76. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 17.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 18.Gansevoort RT, Verhave JC, Hillege HL, Burgerhof JG, Bakker SJ, de Zeeuw D, et al. The validity of screening based on spot morning urine samples to detect subjects with microalbuminuria in the general population. Kidney Int Suppl. 2005;(94):S28–35. doi: 10.1111/j.1523-1755.2005.09408.x. [DOI] [PubMed] [Google Scholar]
- 19.Hillege HL, Fidler V, Diercks GF, van Gilst WH, de Zeeuw D, van Veldhuisen DJ, et al. Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation. 2002;106(14):1777–82. doi: 10.1161/01.cir.0000031732.78052.81. [DOI] [PubMed] [Google Scholar]
- 20.Odden MC, Tager IB, Gansevoort RT, Bakker SJ, Katz R, Fried LF, et al. Age and cystatin C in healthy adults: a collaborative study. Nephrol Dial Transplant. 2010;25(2):463–9. doi: 10.1093/ndt/gfp474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erlandsen EJ, Randers E, Kristensen JH. Evaluation of the Dade Behring N Latex Cystatin C assay on the Dade Behring Nephelometer II System. Scand J Clin Lab Invest. 1999;59(1):1–8. doi: 10.1080/00365519950185940. [DOI] [PubMed] [Google Scholar]
- 22.Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, et al. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis. 2008;51(3):395–406. doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brantsma AH, Bakker SJ, Hillege HL, de Zeeuw D, de Jong PE, Gansevoort RT. Urinary albumin excretion and its relation with C-reactive protein and the metabolic syndrome in the prediction of type 2 diabetes. Diabetes Care. 2005;28(10):2525–30. doi: 10.2337/diacare.28.10.2525. [DOI] [PubMed] [Google Scholar]
- 24.Burnett RW, D’Orazio P, Fogh-Andersen N, Kuwa K, Kulpmann WR, Larsson L, et al. IFCC recommendation on reporting results for blood glucose. Clin Chim Acta. 2001;307(1–2):205–9. doi: 10.1016/s0009-8981(01)00431-4. [DOI] [PubMed] [Google Scholar]
- 25.van der Laan MJ, Gruber S. Collaborative Double Robust Targeted Maximum Likelihood Estimation. Int J Biostat. 2010;6(1):Article17. doi: 10.2202/1557-4679.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arnold B, Arana B, Mausezahl D, Hubbard A, Colford JM., Jr Evaluation of a pre-existing, 3-year household water treatment and handwashing intervention in rural Guatemala. Int J Epidemiol. 2009;38(6):1651–61. doi: 10.1093/ije/dyp241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gruber S, Van der Laan M. Targeted Maximum Likelihood Estimation: A Gentle Introduction. Int J Biostat. 2009 doi: 10.2202/1557-4679.1181. Paper 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sinisi SE, van der Laan MJ. Deletion/substitution/addition algorithm in learning with applications in genomics. Statistical applications in genetics and molecular biology. 2004;3:Article18. doi: 10.2202/1544-6115.1069. [DOI] [PubMed] [Google Scholar]
- 29.Diamond JR, Karnovsky MJ. Focal and segmental glomerulosclerosis: analogies to atherosclerosis. Kidney Int. 1988;33(5):917–24. doi: 10.1038/ki.1988.87. [DOI] [PubMed] [Google Scholar]
- 30.Barter PJ, Nicholls S, Rye KA, Anantharamaiah GM, Navab M, Fogelman AM. Antiinflammatory properties of HDL. Circ Res. 2004;95(8):764–72. doi: 10.1161/01.RES.0000146094.59640.13. [DOI] [PubMed] [Google Scholar]
- 31.Tonelli M, Isles C, Curhan GC, Tonkin A, Pfeffer MA, Shepherd J, et al. Effect of pravastatin on cardiovascular events in people with chronic kidney disease. Circulation. 2004;110(12):1557–63. doi: 10.1161/01.CIR.0000143892.84582.60. [DOI] [PubMed] [Google Scholar]
- 32.Tonelli M, Moye L, Sacks FM, Cole T, Curhan GC. Effect of pravastatin on loss of renal function in people with moderate chronic renal insufficiency and cardiovascular disease. J Am Soc Nephrol. 2003;14(6):1605–13. doi: 10.1097/01.asn.0000068461.45784.2f. [DOI] [PubMed] [Google Scholar]
- 33.Kendrick J, Shlipak MG, Targher G, Cook T, Lindenfeld J, Chonchol M. Effect of lovastatin on primary prevention of cardiovascular events in mild CKD and kidney function loss: a post hoc analysis of the Air Force/Texas Coronary Atherosclerosis Prevention Study. Am J Kidney Dis. 2010;55(1):42–9. doi: 10.1053/j.ajkd.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flegal KM, Williamson DF, Graubard BI. Using adjusted relative risks to calculate attributable fractions. Am J Public Health. 2006;96(3):398. doi: 10.2105/AJPH.2005.079731. [DOI] [PMC free article] [PubMed] [Google Scholar]