Abstract
The team signaling model for bacterial chemoreceptors proposes that receptor dimers of different detection specificities form mixed trimers of dimers. These receptor “squads” then recruit the cytoplasmic signaling proteins CheA and CheW to form ternary signaling teams, which typically cluster at the poles of the cell. We devised cysteine-directed in vivo crosslinking approaches to ask whether mixed receptor squads could form in the absence of CheA and CheW and, if so, whether the underlying structural interactions conformed to trimer-of-dimers geometry. One approach used cysteine reporters at positions in the serine (Tsr) and aspartate (Tar) receptors that should form disulfide-linked Tsr≈Tar products when juxtaposed at the interface of a mixed trimer. Another approach used a cysteine reporter with trigonal geometry near the trimer contact region and a trifunctional maleimide reagent with a spacer length appropriate for capturing the three axial subunits in a trimer of dimers. Both approaches detected mixed receptor-crosslinking products in cells lacking CheA and CheW. Under these conditions, receptor methylation and ligand-binding state had no discernable effect on crosslinking efficiencies. Crosslinking with the trigonal reporter was rapid and did not increase with longer treatment times or higher reagent concentrations, suggesting that this method produces a short-exposure snapshot of the receptor population. The extent of crosslinking indicated that most of the cell's receptor molecules were organized in higher-order groups. Crosslinking in receptor trimer contact mutants correlated with their signaling behaviors, suggesting that trimers of dimers are both structural and functional precursors of chemoreceptor signaling teams in bacteria.
Keywords: chemotaxis, receptor clustering, signaling teams, trimer of dimers, epistasis
Motile bacteria track gradients of beneficial and harmful chemicals with astounding sensitivity. Escherichia coli, whose chemotactic behavior is best understood, can sense concentration changes of only a few parts per thousand across a five-log concentration range. These small chemical stimuli elicit large changes in motor behavior, corresponding to an ≈50-fold amplification of the sensory signals (1-3). The mechanisms responsible for this prodigious signal gain are not well understood, but networking interactions between chemoreceptors are increasingly thought to play an important role.
Methyl-accepting chemotaxis proteins (MCPs) are the principal chemoreceptors in E. coli and most other bacteria (4). MCP molecules typically have a periplasmic ligand-binding domain for monitoring attractant or repellent levels in the environment and a cytoplasmic signaling domain that communicates with the cell's motor apparatus by a protein phosphorelay. The MCP-signaling domain forms ternary complexes with two cytoplasmic proteins, CheA, a histidine kinase, and CheW, which couples CheA activity to chemoreceptor control. Changes in ligand occupancy modulate CheA activity to trigger motor responses. A sensory adaptation system subsequently restores receptor output to prestimulus levels through the reversible addition or removal of methyl groups on several signaling domain residues.
MCP, CheW, and CheA molecules cluster at the cell poles in E. coli (5). The receptor-signaling complexes may form a 2D lattice held together by receptor-receptor interactions and by bridging connections to CheA and CheW (6, 7). Theoretical (8, 9) and experimental (10, 11) studies show that interactions between chemoreceptors can account for their observed cooperativity and signal gain factors.
A better understanding of network architecture is needed to elucidate the underlying molecular mechanisms of receptor signaling. An x-ray structure of the signaling domain of Tsr, the E. coli serine chemoreceptor, provided an intriguing clue. Kim et al. (12) found Tsr homodimers arranged in trimers of dimers (see Fig. 1). We subsequently showed that single amino acid replacements at any of the highly conserved trimer contact residues could abrogate Tsr signaling in several different ways (13). Some lesions, mainly helix-destabilizing proline replacements, disrupted Tsr clustering, suggesting that trimers of dimers could be building blocks of receptor clusters. Other trimer contact lesions allowed cluster formation but blocked receptor signaling, suggesting that altered trimer-of-dimers geometry could impair receptor function. Finally, some mutant Tsr molecules blocked signaling by heterologous MCPs, implying that different chemoreceptors could join the same functional unit, presumably one based on the trimer of dimers.
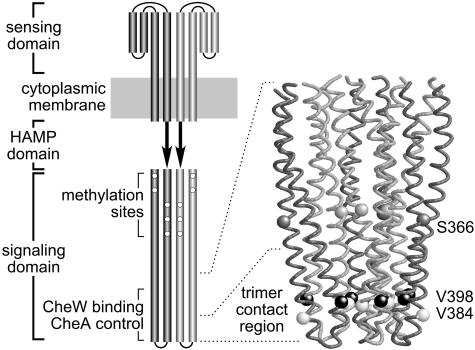
Fig. 1.
Functional architecture of chemoreceptors. (Left) Schematic of an MCP homodimer. Cylindrical segments correspond to α-helices. The periplasmic sensing domain contains two symmetrical ligand-binding sites at the dimer interface (33). Ligand binding to either site shifts one of the transmembrane segments toward the cytoplasm to modulate receptor-signaling activity (34). These transmembrane conformational changes propagate to the cytoplasmic signaling domain through the HAMP domain, whose structure is unknown (35). The signaling domain subunits are arranged in coiled coils that interact to form a four-helix bundle (12). Output signals are generated and regulated by an 80-residue segment centered around the tip of the cytoplasmic domain (36). (Right) The α-carbon backbone of the trimer-of-dimers arrangement at the tip of the signaling domain (12). The light-gray dimer subunits lie on the inside, at the trimer interface; the dark gray subunits lie on the outside. The residues used as cysteine reporter sites are indicated by space-filled α-carbons; residue numbers are for Tsr, the serine chemoreceptor. V398 (black) and V384 (white) constitute a hydrophobic contact pair near the tip; S366 (gray) lies just above the trimer contact region.
The behaviors of Tsr trimer contact mutants suggested that chemoreceptors could function in signaling “teams” (13). The team model proposes that receptor dimers first assemble into higher order groups (”squads”) that can contain receptors of different detection specificities. The receptor squads then recruit CheW and CheA to build signaling teams, which link up, presumably through shared CheW/CheA connections, to form a networked lattice, the receptor cluster. In support of this view, in vivo experiments with a lysine-reactive crosslinking agent revealed physical interactions between Tsr and the aspartate chemoreceptor, Tar (13). Crosslinking was abolished by a proline replacement at one of the Tsr trimer contact sites, implying that Tsr≈Tar crosslinking might occur in mixed trimers of dimers.
To explore the proposition that E. coli receptor squads correspond to trimers of dimers, we devised more incisive cysteine-directed crosslinking approaches, based on unique structural features of the trimer of dimers, for detecting chemoreceptor interactions in vivo. We used the assays to test the following predictions of the receptor team model: (i) that chemoreceptors of different types should crosslink; (ii) that crosslinking efficiency should reflect trimer-of-dimers geometry; (iii) that receptor crosslinking should occur in the absence of CheA and CheW; (iv) that mutant receptors with epistatic properties, i.e., team spoilers, should crosslink to other receptors; and (v) that nonclustering mutant receptors should not crosslink to other receptors (if their clustering defect is at the squad-formation step). All predictions were confirmed, providing strong support for the trimer-of-dimers basis of receptor squads. Our crosslinking methods should be readily applicable to studies of MCP-family chemoreceptors in other prokaryotes.
Materials and Methods
Bacterial Strains. RP3098 [Δ(flhD-flhB)4] (14), RP8606 [Δ(tartap)5201 Δtrg-100], UU1250 [Δtsr-7028 Δ(tar-tap)5201 Δtrg-100 Δaer-1] (13), UU1581 [Δ(flhD-flhB)4 Δtsr-7028 Δtrg-100], and UU1535 [Δtsr-7028 Δ(tar-cheB)2234 Δtrg-100 Δaer-1] are isogenic derivatives of E. coli K12 strain RP437 (15).
Plasmids. Plasmids used were pCJ30, an isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible expression vector derived from pBR322 (16), which confers ampicillin resistance (17); pJC3, a relative of pCJ30 that carries wild-type tsr (13); pCS66, a pACYC184-derived plasmid (18) that confers chloramphenicol resistance and encodes a salicylate-inducible Tar with an Arg-Ser-(His)6 tag at its C terminus (13); pPA705, similar to pCS66, but expressing wild-type Trg under salicylate control (P. Ames, personal communication).
Cysteine-Marked Receptors. Cysteine replacement mutations in receptor genes were constructed with the QuikChange Site-Directed Mutagenesis Kit (Stratagene), with pJC3 (Tsr), pCS66 (Tar), and pPA705 (Trg) as templates. Candidate mutants were verified by sequencing the entire receptor-coding region. Cysteine-bearing derivatives of mutant Tsr (I377P, E385P, and N381W) and Tar (I375P) plasmids were constructed in a similar manner.
Chemotaxis Assays. Tsr and Tar plasmids were assessed for function in strain UU1250 by measuring chemotactic ability on tryptone semisolid agar plates (19). Trg plasmids were assessed for function in strain RP8606 by chemotactic ability on semisolid agar plates containing 0.1% tryptone and 100 μM ribose. All soft agar plates contained appropriate antibiotics (50 μg/ml ampicillin or 12.5 μg/ml chloramphenicol) and variable amounts of the appropriate inducers (IPTG or sodium salicylate). Plates were incubated 7-10 h at 32.5°C.
Disulfide Crosslinking. Cells carrying pCS66 and pJC3 (or their cysteine-bearing derivatives) were grown at 30°C in tryptone broth containing 100 μg/ml ampicillin and 25 μg/ml chloramphenicol. At early-log phase, IPTG (5 μM) and sodium salicylate (0.4 μM) were added to induce Tsr and Tar-(His)6 expression. At late-log phase, cells were harvested by centrifugation and resuspended in KEP buffer (10 mM potassium phosphate, pH 7.0/0.1 mM EDTA) at OD600 = 2. Cell suspensions (0.5 ml) were incubated for 5 min at 30°C, then treated with 0.5 mM diamide (Sigma) for 45 min at 30°C. Reactions were quenched by the addition of 10 mM N-ethylmaleimide (NEM). Cells were pelleted, then lysed by boiling in 60 μl of sample buffer (20) containing 10 mM NEM. Lysate proteins were analyzed by SDS/PAGE as described (21) and visualized by immunoblotting with an antiserum that reacts with the highly conserved MCP-signaling domain (22). Affinity purification and analysis of Tar-(His)6-crosslinking products were carried out as described (13).
Tris-(2-maleimidoethyl-amide) (TMEA) Crosslinking. Cells carrying pJC3 and either pCS66 or pPA705 (or their cysteine-bearing derivatives) were grown and concentrated as described above. Cell samples were treated with 50 μM TMEA (Pierce), usually for 5-15 min, at 30°C. Reactions were quenched with 10 mM NEM, and cells were pelleted, lysed, and analyzed by SDS/PAGE and immunoblotting as described above.
Results
Crosslinking Probes for Receptor Trimers of Dimers. To ask whether MCP molecules form trimers of dimers in vivo, we devised crosslinking assays based on several unique structural features of the trimer (Fig. 1). Our general approach was to introduce single cysteine residues into two different receptors and to analyze their crosslinking products when intact cells were subjected to sulfhydryl-targeted crosslinking conditions. We looked for reporter sites predicted to crosslink in the trimer-of-dimers structure and at which a cysteine replacement could be tolerated with no loss of receptor function. The sites we chose (in Tsr residue numbering) were V384/V398, a pair of hydrophobic trimer contact residues near the tip of the signaling domain, and S366, a residue that lies just above the trimer contact region (Fig. 1). The cysteine-bearing receptors were expressed from compatible plasmids with independently regulatable expression controls. The host cells contained no other receptors nor any of the soluble chemotaxis proteins known to interact with receptor-signaling domains. In particular, the host cells lacked CheA and CheW to explore the proposition that trimers of dimers form without assistance from binding partners, and the MCP-specific modifying enzymes, CheR and CheB, to clamp receptor methylation state and thereby simplify the gel electrophoresis patterns.
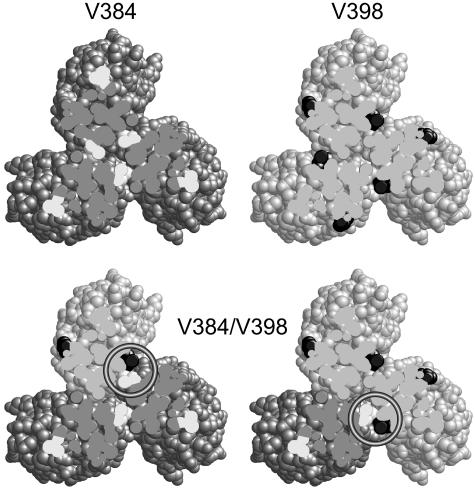
Trimer Contact Residues as Proximity Reporters. Valine residues 384 and 398 of Tsr form a hydrophobic pair at the trimer interface, with V384 from one dimer interacting with V398 in an adjacent dimer (Fig. 2). In this orientation, the 384/398 α-carbons are 6.2 Å apart, well within the range of α-carbon distances for disulfide-bonded cysteines in proteins (23). In contrast, the shortest distance between 384 α-carbons is 11.5 Å, and between 398 α-carbons it is 13.5 Å. Thus, the trimer structure predicts that a receptor containing one of these reporters should form disulfide bonds more readily with a receptor bearing a cysteine at its partner site than it would with an identically marked molecule. To prevent differently marked receptors from exchanging subunits, we introduced cysteine reporters into Tsr and Tar, which are not known to form heterodimers (13). Tsr and Tar molecules also have different gel mobilities, so mixed crosslinking products were expected to have intermediate band positions. Tsr-V398C and its Tar trimer contact partner (V382C, the counterpart of Tsr-V384C) were chosen as the reporters because they retained full function (data not shown).
Fig. 2.
Proximity reporters for the trimer of dimers. Cross sections (viewed from the tip) show the four possible trimer combinations of dark-gray dimers with V384C (white) and light-gray dimers with V398C (black) (Tsr residue numbering). In the crystal structure, the shortest distance between α-carbons at the 384 positions is 11.4 Å; the shortest distance between α-carbons at the 398 positions is 13.8 Å. In mixed trimers that contain both V384C and V398C dimers, a reporter pair (circled) should form at the trimer interface. The distance between their α-carbons is 6.2 Å.
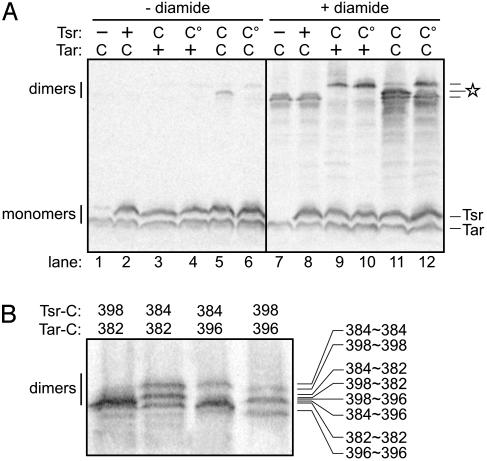
Formation of Interdimer Disulfides in Vivo. We expressed Tsr-V398C and Tar-V382C at physiological levels in strain RP3098 and looked for disulfide-crosslinked molecules after treating the cells with NEM to block unreacted thiol groups. The team model predicts that the cysteine-bearing Tsr and Tar dimers should form four types of trimers (Fig. 2). Trimers with three receptors of the same type should form crosslinks inefficiently, whereas mixed trimers containing receptors of both types should have a pair of cysteines in close proximity at the trimer interface (Fig. 2). In the absence of any treatments to promote formation of disulfide bonds within the cells, a dimer-sized product was seen (faintly) when both receptors carried cysteines (Fig. 3A, lane 5), suggesting that it might correspond to a crosslinked Tsr≈Tar product.
Fig. 3.
Disulfide crosslinking of proximity reporters for the trimer of dimers. Cells expressing various combinations of Tsr and Tar receptors were treated with diamide, and disulfide-crosslinked molecules were analyzed on denaturing gels, as detailed in Materials and Methods. (A) Crosslinking of Tsr to Tar subunits. Tsr symbols: -, no receptor; +, wild-type receptor with no cysteine reporter, induced with 5 μM IPTG; C, V398C, 5 μM IPTG); C°, V398C/I377P, 5 μM IPTG. Tar symbols: +, wild-type receptor with no cysteine reporter, induced with 0.4 μM sodium salicylate; C, V382C, 0.4 μM sodium salicylate. The intermediate dimer band marked with a star represents crosslinked Tsr≈Tar. (B) Dependence of Tsr≈Tar crosslinking on reporter site proximity. Different pairs of Tsr and Tar cysteine reporters were tested for formation of Tsr≈Tar crosslinks. Tsr expression was induced with 5 μM IPTG; Tar expression was induced with 0.4 μM sodium salicylate. Tsr-384 corresponds to Tar-382 in Tar; Tsr-398 in Tsr corresponds to Tar-396. The band position of each crosslinked species is indicated on the right. The complexity of the patterns reflects mobility shifts dependent on the position of the disulfide crosslink.
In the reducing environment of the cytoplasm, disulfide bonds are actively reduced by a number of enzymes (24). To create a less reducing cytoplasmic environment that would promote disulfide bond formation, we incubated the cells with the thiol-specific oxidant diamide (25) before quenching with NEM. On diamide treatment, various dimer-sized products were seen (Fig. 3A, lanes 7-12). Tar-V382C formed rapidly migrating Tar≈Tar dimers (Fig. 3A, lanes 7 and 8) and Tsr-V398C formed slower-migrating Tsr≈Tsr dimers (Fig. 3A, lanes 9 and 10). When both receptors carried cysteines, the predominant dimer band exhibited intermediate mobility, consistent with crosslinked Tsr≈Tar subunits (Fig. 3, lane 11). We confirmed the identity of this species by affinity purifying the Tar(His)6-containing products and showing that on reduction they yielded both Tsr and Tar monomers (data not shown).
The Tsr≈Tar product did not form when Tsr-V398C carried a trimer contact mutation (I377P) that abolishes receptor cluster formation (Fig. 3A, lane 12). However, Tsr-V398C/I377P did produce Tsr≈Tsr crosslinks (Fig. 3A, lane 10). We conclude that crosslinked Tsr≈Tar products do not arise through collisional interactions but rather through specific structural interaction in a higher-order receptor complex. The I377P lesion evidently disrupts interactions with other receptor dimers but does not prevent crosslinking of V398C subunits within a dimer. These findings support our working assumptions that Tsr and Tar cannot form heterodimers and that I377P might block trimer-of-dimers formation.
As structural controls for the proximity reporters, we examined crosslinking between Tsr-V384C/Tar-V382C and between Tsr-V398C/Tar-V396C. When both receptors carried cysteines at the same positions, three crosslinking products formed with comparable efficiencies (Fig. 3B). When the receptors carried cysteines at contact partner positions (i.e., Tsr-V384C/Tar-V396C and Tsr-V398C/Tar-V382C), the Tsr≈Tar species predominated. This result strongly suggests that the trimer of dimers provides the structural basis for Tsr≈Tar crosslinking.
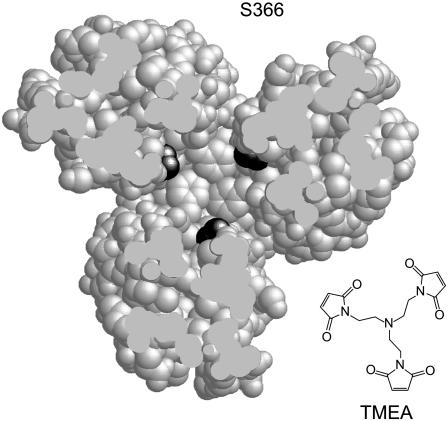
A Trigonal Reporter for the Trimer of Dimers. A unique feature of the trimer of dimers is its trigonal symmetry. For example, just above the trimer contact region, the three dimers flare apart, forming a solvent-accessible space along the central axis of the trimer (Fig. 1). Here, residues in the inner subunits of the dimers can form triangular groups with their side chains projecting into the central space. One of these residues, Tsr-S366, proved suitable as a trigonal crosslinking site (Fig. 4). The α-carbons at the 366 positions on the trimer axis lie 12.8 Å apart, whereas those on the outside of the trimer are very far apart and shielded from one another by the bulk of the trimer (Fig. 4). We reasoned that it might be possible to capture all three axial subunits of a trimer by crosslinking cysteines at position 366 with TMEA, a trifunctional, thiol-specific crosslinking agent (Fig. 4). TMEA has three SH-reactive maleimide groups spaced 10.3 Å apart (Fig. 4). The Tsr-S366C replacement mutant retained full signaling function (data not shown). The corresponding cysteine replacement mutants of Tar (Tar-S364C) and of the low-abundance ribose-galactose receptor Trg (Trg-A374C) retained full function, as well (data not shown).
Fig. 4.
A trigonal reporter site for the trimer of dimers. A cross-section view of the trimer (looking toward the tip) shows the trigonal symmetry of Tsr residue S366 (black) in the inside dimer subunits just above the trimer contact region [which begins at the nested phenylalanines (F373)]. The S366 residues in the outside subunits of the dimers are not visible in this section plane. The distance between S366 α-carbons at the trimer interface is 12.8 Å. The chemical structure of the trifunctional crosslinking reagent TMEA is shown at approximately the same scale. The distance between the thiol-reactive maleimide groups in TMEA is 10.3 Å.
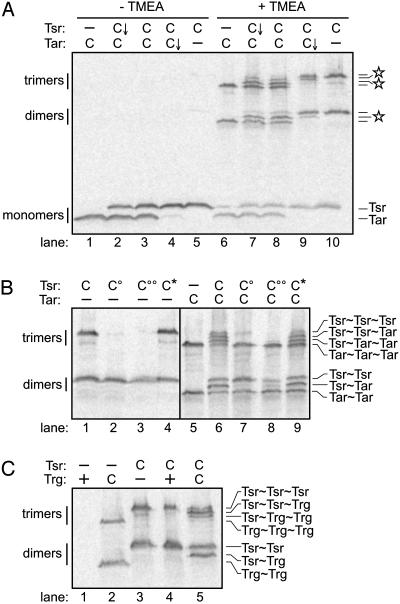
Crosslinking Three Receptor Subunits with TMEA. RP3098 or UU1581 cells carrying Tsr-S366C and/or Tar-S364C expressed at physiological levels were treated with TMEA, then quenched with NEM, and analyzed by gel electrophoresis. Omission of the TMEA treatment produced no crosslinking products from these reporters (Fig. 5A, lanes 1-5). By contrast, TMEA treatment shifted about half of the receptor subunits into crosslinked species (Fig. 5A, lanes 6-10). In cells expressing either Tsr-S366C or Tar-S364C, TMEA generated two- and three-subunit crosslinking products with relative mobilities characteristic of Tsr and Tar (Fig. 5A, lanes 6 and 10). Coexpression of the two reporters produced more complex crosslinking patterns (Fig. 5A, lanes 7-9) with three new bands at intermediate positions (indicated by stars in Fig. 5A). The relative expression levels of Tsr and Tar clearly influenced the patterns, with receptor subunits in the minority appearing predominantly in intermediate bands (Fig. 5A, lanes 7 and 9). We interpret these new species as mixed crosslinking products containing different numbers of Tsr and Tar subunits. On the one hand, they might represent TMEA-crosslinked products from mixed trimers of dimers. On the other hand, they could conceivably have arisen through TMEA-trapped collisional interactions.
Fig. 5.
TMEA crosslinking of a trigonal reporter for the trimer of dimers. Cells expressing various combinations of Tsr and either Tar or Trg receptors were treated with TMEA and analyzed as described in Materials and Methods. (A) Crosslinking of Tsr to Tar subunits. Tsr symbols: -, no receptor; C, S366C, induced with 5 μM IPTG; C↓, S366C, basal (uninduced) expression level. Tar symbols: -, no receptor; C, S364C, induced with 0.4 μM sodium salicylate; C↓, S364C, basal (uninduced) expression level. Stars mark the positions of crosslinked products containing both Tsr and Tar subunits. (B) Effects of trimer contact mutations on TMEA crosslinking. Tsr symbols: -, no receptor; C, S366C, 5 μM IPTG; C°, S366C/I377P, 5 μM IPTG; C°°, S366C/E385P, 5 μM IPTG; C*, S366C/N381W, 5 μM IPTG. Tar symbols: -, no receptor; C, S364C, 0.4 μM sodium salicylate. (C) Crosslinking of Tsr to Trg subunits. Tsr symbols: -, no receptor; C, S366C, 5 μM IPTG. Trg symbols: -, no receptor; +, wild-type receptor; C, A374C. For lanes 1 and 2, Trg expression was induced with 1.2 μM sodium salicylate; for lanes 4 and 5, Trg expression was induced with 0.3 μM sodium salicylate.
To clarify the origin of the intermediate species, we examined the crosslinking behavior of Tsr-S366C derivatives carrying trimer contact mutations (I377P, E385P, and N381W). The crosslinking patterns of Tsr-S366C/N381W, both alone and when coexpressed with Tar-S364C, were identical with those of Tsr-S366C (Fig. 5B, lanes 1 and 6 vs. lanes 4 and 9). N381W is an epistatic lesion that blocks the functions of other receptors, presumably by forming mixed, defective receptor complexes. In contrast, the I377P and E385P lesions abolish receptor clustering and are not epistatic, consistent with defects in trimer-of-dimers formation. Those mutations abolished formation of all three-subunit crosslinking products containing Tsr, either alone (Fig. 5B, lanes 2 and 3) or when coexpressed with Tar-S364C (Fig. 5B, lanes 7 and 8). This result demonstrates that trimer-sized TMEA products form through a higher-order receptor interaction that is abrogated by the I377P and E385P defects. Both mutations also eliminated crosslinked Tsr≈Tar dimers (Fig. 5B, lanes 7 and 8) but still allowed crosslinked Tsr≈Tsr dimers to form (Fig. 5B, lanes 2, 3, 7, and 8). This finding implies that Tsr≈Tar crosslinks form only through dimer-dimer interactions, whereas at least some Tsr≈Tsr crosslinks can evidently form through intradimer subunit interactions. Perhaps the structure-destabilizing effects of the I377P and E385P lesions potentiate collisional crosslinking between receptor subunits. If so, those interactions must still take place within the context of the mutant dimers, because we detected no Tsr≈Tar-crosslinking products when both receptors carried such a lesion (Tsr-S366C/I377P and Tar-S364C/I375P) (data not shown). We cannot say to what extent intradimer crosslinks contribute to the Tsr≈Tsr and Tar≈Tar species formed by wild-type receptors; conceivably, many of those products represent interdimer-crosslinking events.
Trg and other low-abundance chemoreceptors are present in the cell at ≈10% of Tsr and Tar levels. In its simplest form, the team model of receptor signaling predicts that low-abundance receptors should operate almost exclusively in mixed teams with high-abundance receptors. To determine whether Trg can associate in higher-order forms, we tested Trg-A374C (the counterpart of Tsr-S366C) for TMEA crosslinking. [Wild-type Trg contains a cysteine at residue 23 that has no effect on its TMEA-crosslinking pattern (Fig. 5C, lanes 1 and 4).] When expressed alone (at higher than normal expression levels for detection purposes), Trg-A374C formed two- and three-subunit crosslinking products (Fig. 5C, lane 2). When coexpressed at its normal relative expression level with Tsr-S366C, Trg-A374C appeared almost entirely in mixed crosslinking products with Tsr (Fig. 5C, lane 5). This result indicates that most of the Trg molecules in normal cells may, indeed, reside in mixed signaling teams.
Kinetics of Receptor Crosslinking by TMEA. The TMEA experiments presented above were carried out at a concentration of 50 μM, with the cells treated for 5-15 min at 30°C. Time course experiments revealed that the patterns and amounts of crosslinked products did not change significantly with treatments as short as 5 s or as long as 2 h (data not shown). [Addition of NEM immediately before TMEA abolished crosslinking (data not shown), so we know that the quenching effect of NEM was rapid.] Moreover, TMEA concentrations as high as 500 μM did not increase the extent of crosslinking (data not shown). These results demonstrate that TMEA quickly reacts with all receptor subunits available for crosslinking. Thus, TMEA provides a snapshot of the receptor population.
Lack of Methylation, CheA/CheW, and Stimulus Effects on Receptor Crosslinking. In addition to lacking CheA and CheW, the host cells for our crosslinking experiments (RP3098 and UU1581) also lacked CheR (MCP methyltransferase) and CheB (MCP methylesterase) to simplify the receptor gel patterns. In the absence of CheR and CheB function, wild-type receptor molecules have E residues at two methylation sites (Tsr-E304 and Tsr-E493) and Q residues at two other sites (Tsr-Q297 and Tsr-Q311). Ordinarily, CheB deamidates the Q sites, creating E residues, which can then serve as methylation substrates for CheR. Q residues mimic the signaling effects of methylated E, so wild-type (QEQE) receptors behave as if they are half-methylated. To evaluate possible effects of methylation state on formation of higher-order receptor complexes, we examined the TMEA-crosslinking patterns of Tsr-S366C with all E or all Q residues at the methylation sites. The EEEE and QQQQ patterns were indistinguishable from QEQE Tsr-S366C (data not shown), indicating that, under our experimental conditions, methylation state has no detectable influence on receptor trimer-of-dimers formation. We repeated this experiment in UU1535, which lacks CheR/CheB but has CheA/CheW, to see whether those functions had any effect on TMEA crosslinking. All three receptor methylation states produced identical patterns that did not differ from those in the hosts lacking CheA/CheW (data not shown). We also found no change in the disulfide-crosslinking pattern of Tsr-V398C and Tar-V382C when coexpressed in RP3098 in the presence of 1 mM serine and/or 1 mM aspartate (data not shown). We conclude that Tsr≈Tar crosslinking is not appreciably influenced by the methylation state of the receptor molecules, by ligand binding, or by the presence of the CheA and CheW proteins.
Discussion
Receptor Crosslinking in Intact Cells. We have described two receptor crosslinking assays, guided by the x-ray structure of the Tsr-signaling domain (12), that support the view that bacterial chemoreceptors form higher-order groups in vivo. Several considerations indicate that crosslinking reflects structural rather than collisional interactions between receptor molecules. (i) All experiments were performed at or below normal receptor expression levels to minimize nonspecific encounters. (ii) The experiments were performed in cells lacking the CheW and CheA functions needed for receptor clustering, thereby avoiding membrane patches of highly concentrated receptors. (iii) Receptor-crosslinking products were discrete in size and uncontaminated by nonspecific cell proteins. (iv) Crosslinking efficiencies correlated with the relative positions of cysteine reporter sites in the trimer of dimers arrangement. (v) Proline replacements at trimer contact sites eliminated the structural interactions that lead to crosslinks between receptor dimers. (vi) TMEA, a trifunctional crosslinker, captured three receptor subunits with high efficiency. Moreover, the TMEA reaction was complete within a few seconds, consistent with a “snapshot” view of grouped receptor dimers rather than sequential collisional encounters between unaffiliated receptor molecules.
Detection of Receptor Trimers of Dimers in Vivo. We assume that the receptor groups detected by our crosslinking assays are trimers of dimers, although we cannot rigorously exclude another higher-order arrangement. TMEA captured at least half of the cells' receptor subunits in higher-order complexes, consistent with the view that most of the cell's receptor molecules could be organized in trimers of dimers. The extent of crosslinking did not depend on the CheW and CheA components of receptor-signaling complexes or the CheR and CheB enzymes that act on receptor methylation sites. Moreover, crosslinking was not influenced by the methylation states of the receptors or by saturating ligand occupancies. We conclude that at physiological expression levels, and in the absence of cytoplasmic partner proteins, receptors readily form trimers of dimers in vivo.
Homma et al. (26) recently described a crosslinking study of Tar with a cysteine reporter in the periplasmic, ligand-binding domain. They observed Tar oligomers consistent with a trimer-of-dimers interpretation, but their crosslinking signal substantially depended on the presence of CheA and CheW and was significantly reduced on ligand binding. We suggest that their observations do not provide direct evidence for trimers of dimers but rather for a higher-order receptor interaction dependent on receptor clustering, a process known to depend on CheA and CheW. Kim et al. (27) have proposed a model of the receptor network in which the periplasmic domains of neighboring trimer-based receptor-signaling teams contact one another. Those interactions could account for the crosslinking results of Homma et al. (26).
Both the proximity pair and trigonal reporter approaches demonstrated that receptors of different types (Tsr/Tar and Tsr/Trg) can form mixed trimers whose compositions depend mainly on the relative expression levels of the component receptor dimers. Receptors in the minority were usually found in mixed trimers with the majority type, whereas the majority type also formed trimers with all majority members. Receptors did not appear to form trimers preferentially with receptors of the same type. We conclude that trimers of dimers assemble through random recruitment of dimers from the receptor pool. This conclusion implies that low-abundance receptors, such as Trg, will invariably belong to mixed trimers whose other members are high-abundance receptors (Tsr and Tar).
Trimers of Dimers and the Team Model of Receptor Signaling. Our crosslinking results demonstrate that the receptor squads, presumably trimers of dimers, that form in the absence of CheA and CheW are probably precursors of receptor-signaling teams, as specified in the team model (13). The trimer-forming phenotypes of different trimer contact mutants, inferred from their crosslinking patterns, suggest that receptor squads are structural components of receptor-signaling teams. For example, mutant receptors that could not form trimers were also defective in forming signal team clusters. Conversely, an epistatic Tsr mutant formed mixed trimers with Tar, consistent with the team-spoiling model of epistasis (13). Moreover, because most trimer contact lesions abrogate receptor signaling (13), proper trimer-of-dimers geometry may also be important for the operation of receptor-signaling teams.
The architectural relationship between receptor trimers of dimers and signaling teams is not yet clear. On the one hand, the in vitro stoichiometries of receptor-signaling complexes indicate that receptor teams may contain several trimers of dimers (22, 28-31). On the other hand, binding of CheW and CheA to a single trimer of dimers might create a functional signaling unit. A recent modeling study shows that trimer-sized teams can account for the gain factors observed in receptor signaling (32). It should be possible to use in vivo crosslinking approaches like those described here to elucidate the structural features of receptor-signaling teams and their relationship to trimer-of-dimer squads.
Extensions and Limitations of Receptor-Crosslinking Methods. The in vivo crosslinking methods developed in this study should be applicable to MCP-family chemoreceptors in other bacteria. TMEA appears to be a particularly useful reagent, because it readily enters bacterial cells and requires only a single cysteine reporter in the receptor. It is also important to note a potential limitation in these crosslinking approaches: We have not observed trimer-sized crosslinking products when cell membranes were treated with TMEA (data not shown). This difference from the in vivo situation is not caused by occlusion of the reporter sites, because the receptors still formed intradimer crosslinks with normal efficiency. Rather, cell disruption may cause the trimers of dimers to dissociate. Perhaps trimers are stabilized in vivo by molecular crowding effects, which dissipate on loss of the cytoplasmic contents. This scenario may explain why in vitro signaling assays require the use of membranes containing highly overexpressed receptors. Perhaps overexpression packs the receptor molecules densely enough to stabilize trimers of dimers through local crowding effects.
Acknowledgments
We thank Peter Ames (University of Utah) for plasmids, helpful discussions, and comments on the manuscript. This work was supported by National Institutes of Health Research Grant GM19559. The Protein-DNA Core Facility at the University of Utah receives support from National Cancer Institute Grant CA42014 to the Huntsman Cancer Institute.
Abbreviations: MCP, methyl-accepting chemotaxis protein; IPTG, isopropyl-β-d-thiogalactopyranoside; NEM, N-ethylmaleimide; TMEA, Tris-(2-maleimidoethyl)amine.
References
- 1.Block, S. M., Segall, J. E. & Berg, H. C. (1982) Cell 31, 215-226. [DOI] [PubMed] [Google Scholar]
- 2.Jasuja, R., Lin, Y., Trentham, D. R. & Khan, S. (1999) Proc. Natl. Acad. Sci. USA 96, 11346-11351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sourjik, V. & Berg, H. C. (2002) Proc. Natl. Acad. Sci. USA 99, 123-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhulin, I. B. (2001) Adv. Microb. Physiol. 45, 157-198. [DOI] [PubMed] [Google Scholar]
- 5.Maddock, J. R. & Shapiro, L. (1993) Science 259, 1717-1723. [DOI] [PubMed] [Google Scholar]
- 6.Shimizu, T. S., Le Novere, N., Levin, M. D., Beavil, A. J., Sutton, B. J. & Bray, D. (2000) Nat. Cell Biol. 2, 792-796. [DOI] [PubMed] [Google Scholar]
- 7.Levit, M. N., Grebe, T. W. & Stock, J. B. (2002) J. Biol. Chem. 277, 36748-36754. [DOI] [PubMed] [Google Scholar]
- 8.Bray, D., Levin, M. & Morton-Firth, C. (1998) Nature 393, 85-88. [DOI] [PubMed] [Google Scholar]
- 9.Mello, B. A. & Tu, Y. (2003) Proc. Natl. Acad. Sci. USA 100, 8223-8228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gestwicki, J. E. & Kiessling, L. L. (2002) Nature 415, 81-84. [DOI] [PubMed] [Google Scholar]
- 11.Sourjik, V. & Berg, H. C., Nature, in press.
- 12.Kim, K. K., Yokota, H. & Kim, S. H. (1999) Nature 400, 787-792. [DOI] [PubMed] [Google Scholar]
- 13.Ames, P., Studdert, C. A., Reiser, R. H. & Parkinson, J. S. (2002) Proc. Natl. Acad. Sci. USA 99, 7060-7065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith, R. A. & Parkinson, J. S. (1980) Proc. Natl. Acad. Sci. USA 77, 5370-5374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parkinson, J. S. & Houts, S. E. (1982) J. Bacteriol. 151, 106-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bolivar, F., Rodriguez, R., Greene, P. J., Betlach, M. C., Heyneker, H. L. & Boyer, H. W. (1977) Gene 2, 95-113. [PubMed] [Google Scholar]
- 17.Bibikov, S. I., Biran, R., Rudd, K. E. & Parkinson, J. S. (1997) J. Bacteriol. 179, 4075-4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang, A. C. & Cohen, S. N. (1978) J. Bacteriol. 134, 1141-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parkinson, J. S. (1976) J. Bacteriol. 126, 758-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laemmli, U. K. (1970) Nature 227, 680-685. [DOI] [PubMed] [Google Scholar]
- 21.Feng, X., Baumgartner, J. W. & Hazelbauer, G. L. (1997) J. Bacteriol. 179, 6714-6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ames, P. & Parkinson, J. S. (1994) J. Bacteriol. 176, 6340-6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Srinivasan, N., Sowdhamini, R., Ramakrishnan, C. & Balaram, P. (1990) Int. J. Pept. Protein Res. 36, 147-155. [DOI] [PubMed] [Google Scholar]
- 24.Ritz, D. & Beckwith, J. (2001) Annu. Rev. Microbiol. 55, 21-48. [DOI] [PubMed] [Google Scholar]
- 25.Kosower, N. S. & Kosower, E. M. (1995) Methods Enzymol. 251, 123-133. [DOI] [PubMed] [Google Scholar]
- 26.Homma, M., Shiomi, D., Homma, M. & Kawagishi, I. (2004) Proc. Natl. Acad. Sci. USA 101, in press. [DOI] [PMC free article] [PubMed]
- 27.Kim, S. H., Wang, W. & Kim, K. K. (2002) Proc. Natl. Acad. Sci. USA 99, 11611-11615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu, Y., Levit, M., Lurz, R., Surette, M. G. & Stock, J. B. (1997) EMBO J. 16, 7231-7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borkovich, K. A. & Simon, M. I. (1990) Cell 63, 1339-1348. [DOI] [PubMed] [Google Scholar]
- 30.Bornhorst, J. A. & Falke, J. J. (2001) J. Gen. Physiol. 118, 693-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murphy, O. J., III, Yi, X., Weis, R. M. & Thompson, L. K. (2001) J. Biol. Chem. 276, 43262-43269. [DOI] [PubMed] [Google Scholar]
- 32.Albert, R., Chiu, Y. & Othmer, H. G., Biophys. J., in press.
- 33.Milburn, M. V., Prive, G. G., Milligan, D. L., Scott, W. G., Yeh, J., Jancarik, J., Koshland, D. E., Jr., & Kim, S. H. (1991) Science 254, 1342-1347. [DOI] [PubMed] [Google Scholar]
- 34.Falke, J. J. & Hazelbauer, G. L. (2001) Trends Biochem. Sci. 26, 257-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aravind, L. & Ponting, C. P. (1999) FEMS Microbiol. Lett. 176, 111-116. [DOI] [PubMed] [Google Scholar]
- 36.Ames, P., Yu, Y. A. & Parkinson, J. S. (1996) Mol. Microbiol. 19, 737-746. [DOI] [PubMed] [Google Scholar]