Abstract
Major depressive disorder (MDD) is etiologically complex and has a heterogeneous presentation. This heterogeneity hinders the ability of molecular genetic research to reliably detect the small effects conferred by common genetic variation. As a result, significant research efforts have been directed at investigating more homogenous intermediate phenotypes believed to be more proximal to gene function and lie between genes and/or environmental effects and disease processes. In the current review we survey and integrate research on two promising intermediate phenotypes linked to depression: reward processing and stress sensitivity. A synthesis of this burgeoning literature indicates that a molecular genetic approach focused on intermediate phenotypes holds significant promise to fundamentally improve our understanding of the pathophysiology and etiology of depression, which will be required for improved diagnostic definitions and the development of novel and more efficacious treatment and prevention strategies. We conclude by highlighting challenges facing intermediate phenotype research and future development that will be required to propel this pivotal research into new directions.
Introduction
Major depressive disorder (MDD) is a common, recurrent, and etiologically complex mental illness. Family, twin, and adoption studies have shown that this disabling disorder is moderately heritable, with approximately 37% of the variance explained by additive genetic effects (Sullivan et al., 2000). Building upon this evidence, candidate gene and genome-wide association studies (GWAS) have begun to link common DNA sequence variation (polymorphisms; Table 1) to MDD (Caspi et al., 2003; Levinson, 2006; Lohoff, 2010; Sullivan et al., 2009). The potential of such molecular genetic research is undeniable. In addition to furthering our understanding of the genetic basis of depression, molecular genetic research has the potential to inform our etiologic conceptualization of the disorder and identify novel treatment targets. When polymorphisms are of known functionality (i.e., when they have been associated with differences in gene and/or protein expression), they can serve as proxies for individual differences in neurochemistry, which can, in turn, inform our understanding of the molecular processes underlying depression (Hariri, 2009). Additionally, GWAS allow for the identification of novel candidate loci within biological pathways whose role in MDD may have never been suspected (Cichon et al., 2009), while treatment research can uncover genetic variants predicting therapeutic outcomes, bringing psychiatry one step closer to personalized treatment (Fox et al., 2011; Smeraldi et al., 1998). Finally, research on epigenetic factors (Table 1) can inform the mechanisms by which experience, including exposure to stress, shapes biology to influence depression risk (Zhang and Meaney, 2010).
Table 1.
Definitions of common genetics terminology
| Epigenetic effects | Changes in gene expression caused by factors (e.g., methylation affecting gene transcription) other than variation in DNA sequence |
| Epistatic effects | Gene interactions that can affect phenotypes |
| Single Nucleotide Polymorphism (SNP) | A small genetic variation in the DNA sequence. The four nucleotide adenine (A), cytosine (C), thymine (T), and guanine (G) represent the building block of the genetic code. A SNP occurs when a single nucleotide (e.g., C) replaces another nucleotide in a given location (e.g., G). For example, AGTTA is replaced by ACTTA |
| Variable Number of Tandem Repeats (VNTR) | A VNTR is a short nucleotide sequence that is organized into clusters of tandem repeats (e.g., ATGCC, ATGCC, ATGCC). |
With such promise also come substantial challenges (Bogdan et al., in press; Hasler and Northoff, 2011). One of the largest challenges is that individual common polymorphisms, will have, at most, only a small effect on complex polygenetic diseases and related processes, making relationships difficult to uncover and replicate, particularly within small samples characteristic of most non-consortia efforts. This weak penetrance is further compounded by the complexity and heterogeneity of psychiatric diagnoses, including MDD (Hasler et al., 2004; Hasler and Northoff, 2011; Hyman, 2007; Meyer-Lindenberg and Weinberger, 2006).
Specifically, current classification systems for psychopathology (e.g., DSM-IV-TR, American Psychiatric Association, 2000; ICD-10, World Health Organization., 2004) adopt an atheoretical approach with respect to the etiology and pathophysiology of mental illness, and instead rely on symptom clusters and clinical course as diagnostic criteria (Cannon and Keller, 2006; Kendler and Gardner, 1998). While this descriptive nosological system has dramatically improved diagnostic reliability, it also yields heterogeneous disease categories with unknown validity. MDD, as defined by the DSM-IV-TR, requires endorsement of 5 out of 9 symptoms (one of which must be depressed mood or anhedonia), yielding more than 100 unique symptom combinations that presumably reflect distinct pathophysiologies. Moreover, these symptoms are themselves complex and heterogeneous (e.g., anhedonia– classically defined as loss of pleasure or reactivity to pleasurable stimuli – might stem from dysfunction in incentive motivation, consummatory behavior, and/or reward learning; Berridge et al., 2009; Pizzagalli et al., 2008; Treadway and Zald, 2011), disorder-unspecific (e.g., negative affect cuts across anxiety and depressive disorders; Watson et al., 1995), and may even have opposing pathophysiologies (e.g., insomnia vs. hypersomnia). This heterogeneity undoubtedly hinders the search for susceptibility genes because distinct presentations of MDD are likely associated with unique polygenetic clusters and environmental experiences.
To address disorder heterogeneity, many have advocated for the use of intermediate phenotypes (e.g., particular behavioral manifestations or brain function) believed to lie between genes and/or environmental experiences and disease processes (Cannon and Keller, 2006; Hasler et al., 2004; Hasler and Northoff, 2011; Insel and Cuthbert, 2009; Kendler and Neale, 2010; Meyer-Lindenberg and Weinberger, 2006; but see also Flint and Munafo, 2007).1 These intermediate phenotypes are a more accessible target for genetic research because they are presumably not only more specific, quantifiable, and reliable than diagnostic phenotypes, but also more proximal to gene function. Because categorical diagnoses collapse across multiple distinct presentations that may not be characterized by shared pathophysiologies, they will inevitably involve many neural circuits and an exponentially larger number of proteins and genes. As a result, it is unlikely that small effects conferred by single genetic variants affecting the expression/function of a single protein will be detected.
In contrast, by relying upon continuous and quantifiable measures of specific, neurobiologically more homogeneous behavior and brain function, an intermediate phenotypic approach promises to capture biology more directly, thus enhancing penetrance and reducing the number of genes involved in phenotypic expression (Goldman and Ducci, 2007; but see also Flint and Munafo, 2007). Within this context, the National Institute of Mental Health has recently launched the Research Domain Criteria (RDoC) project in an attempt to (1) integrate neuroscience and genetic research into future diagnostic systems, (2) advance our etiologic understanding, and (3) propel treatment development. Specifically, the RDoC project aims to stimulate research conducted on intermediate phenotypes across multiple levels of analyses (from genes to neural circuits to behaviors) and traditionally defined diagnostic borders (Insel et al., 2010; Sanislow et al., 2010). In the spirit of an RDoC conceptualization, this review summarizes and integrates research linking genetic variation to individual differences in reward processing, which is considered one of the most promising intermediate phenotypes of depression (Hasler et al., 2004; Hasler and Northoff, 2011). Moreover, we explore how genetically conferred differences in a second promising intermediate phenotype, stress sensitivity (Hasler et al., 2004; Hasler and Northoff, 2011), may further impact reward processing in the context of stress.
Reward Processing
Anhedoniais a cardinal symptom of depression associated with elevated severity and poor treatment response (American Psychiatric Association, 2000; Hasler et al., 2004; Kasch et al., 2002). Although anhedoniais present in up to 50% of individuals with MDD (Fawcett et al., 1983; Oei et al., 1990; Pelizza and Ferrari, 2009), it transcends diagnostic boundaries and plays prominent roles in other disorders, including schizophrenia and substance abuse (Diekhof et al., 2008; Dowd and Barch, 2010). In the context of a rich theoretical background postulating that a genetic predisposition to hedonic deficits may leave individuals vulnerable to the development of depression (Klein, 1987; Loas, 1996; Meehl, 1975; Myerson, 1922; Willner, 1993) and evidence that reward-related behavior is heritable (Bogdan and Pizzagalli, 2009; Loas, 1996), research has begun to investigate how genetic background and environmental experience contribute to the vast array of individual differences in reward-related behavior and brain function with important implications for our understanding of depression.
Processing and integration of rewards relies on an interconnected dopamine-rich neural network, including the midbrain, amygdala, striatum, anterior cingulate cortex, orbitofrontal cortex, and medial prefrontal cortex (Berridge et al., 2009; Haber and Knutson, 2009; Knutson et al., 2000; O'Doherty et al., 2003). Several studies have linked MDD and elevated depressive symptoms to structural abnormalities (Blood et al., 2010; Pizzagalli et al., 2009) and reduced reward-related activation in this network (Diekhof et al., 2008; Epstein et al., 2006; Forbes et al., 2006; Heller et al., 2009; Keedwell et al., 2005a; Keedwell et al., 2005b), particularly the medial prefrontal cortex (PFC) and striatum (Knutson et al., 2008; Kumar et al., 2008; Pizzagalli et al., 2009; Stoy et al., 2011). Moreover, hypoactivation in these regions, as well as reduced striatal volume, has been linked to anhedonia, suggesting that dysfunction in this network may be associated with the clinical expression of reward processing deficits (Epstein et al., 2006; Keedwell et al., 2005b; Pizzagalli et al., 2009; Stoy et al., 2011). Thus, uncovering factors, including genetic ones, that contribute to variability in reward-related neural activation, is an important step for understanding the etiology of anhedonia, and, potentially, depression (Bijttebier et al., 2011).
Genetics of Reward Processing
Dopamine
Dopamine (DA) plays a critical role in reward processing. A variety of primary and secondary rewards, including food, sex, and drugs can elicit DA release (Egerton et al., 2009; Knutson and Gibbs, 2007; Salimpoor et al., 2011). Initially, DA was thought to code for the hedonic value of reward (Wise, 1978), but more recent research suggests that it is instead involved in coding complex information about appetitive stimuli and facilitating the learning of stimulus-outcome and action-outcome contingencies (Wise, 2008). Schultz and colleagues (2007), in particular, have shown that DA neurons originating in the ventral tegmental area fire in phasic bursts in response to unexpected rewards or predictors indicating that reward delivery is imminent. Thus, DA appears to be primarily involved in reward learning and anticipation of reward, as opposed to hedonic responding.
Following synthesis and release DA may: 1) bind to postsynaptic DA receptors; 2) bind to presynaptic DA autoreceptors; 3) be taken back into presynaptic neurons by the DA transporter (primarily in limbic regions); or 4) be degraded by enzymes (primarily in PFC regions). Functional polymorphisms within genes involved in regulating each of these steps are likely to lead to individual differences in DA function, and more subtle differences in reward-related behavior and neural activation that may contribute to depression. In the current review, we focus on the most studied genes within this system: the DA receptor type 2 (DRD2), DA receptor type 4 (DRD4), dopamine transporter (SLC6A3), and catechol-o-methyltransferase (COMT) genes.
Dopamine Receptor D2 (DA D2, DRD2) and D4 (DA D4, DRD4)
DRD2 is most expressed in the striatum where DA binding inhibits pre- and postsynaptic neurons. Several polymorphisms within DRD2 affect its expression and reward-related reactivity. For instance, the deletion variant of an insertion/deletion polymorphism (rs1799732; −141 Ins/Del) has been associated with enhanced in vivo receptor availability suggesting that the deletion confers increased DA D2 binding sites (Jonsson et al., 1999; but see also Ritchie and Noble, 2003 who reported no differenes in binding across genotype groups and Arinami et al., 1997 who described reduced expression in individuals carrying the deletion variant). The deletion allele has also been associated with enhanced striatal response to reward (Forbes et al., 2009). Similarly, the C allele (i.e., A2) of the DRD2 Taq1A [actually located downstream from DRD2 in the ankyrin repeat and kinase-domain containing 1 (ANKK1) gene]single nucleotide polymorphism (SNP; Table 1), rs1800497, has been associated with relatively increased DA D2 receptor availability (Jonsson et al., 1999; Noble, 2003; Pohjalainen et al., 1998) and increased striatal reactivity to reward (Stice et al., 2008). Collectively, these studies suggest that genetically driven reductions in striatal DA signaling may lead to blunted reward-related neural responsiveness and anhedonic behavior, providing a background against which depression may develop.
Notably, conflicting data exist regarding the molecular function of the DRD2 insertion/deletion polymorphism, with some studies reporting that the deletion is associated with increased DA D2 receptor density in vivo (e.g., Jonsson et al., 1999) but reduced DRD2 expression in vitro (Arinami et al., 1997). The reason that in vivo and in vitro examinations produced opposite patterns of receptor density is unclear and may be related to system-level adaptations that occur in vivo. Consistent with Arinami et al.(1997), neurogenetics research has interpreted the increased striatal response to reward in deletion carriers as reflective of reduced inhibitory DRD2 signaling (Forbes et al., 2009; Nikolova et al., 2011). Moreover, another polymorphic variant, i.e., the A2 (C) allele of the DRD2 Taq1A, associated with relatively increased DA D2 receptor density in vivo (Jonsson et al., 1999; Noble, 2003; Pohjalainen et al., 1998), has been linked to elevated striatal response to reward (Cohen et al., 2005), which is inconsistent with speculations that reduced DRD2 signaling results in greater BOLD response in the ventral striatum due to less inhibitory stimulation. The conflicting functional characterizations of the insertion/deletion DRD2 polymorphism, as well as opposite interpretations of the effect of DRD2 on striatal circuit function across polymorphisms (e.g., ins/del vs. Taq1A), make it difficult to discern the molecular mechanisms by which DRD2 variants may affect reward processing. The DRD2 story only becomes more complicated by evidence of concentration-dependent actions (Williams & Millar, 1990; Trantham-Davidson et al., 2004), synergistic effects between DA D1 and DA D2 receptor binding (Gerfen et al., 1995), and the formation of D1/D2 receptor complexes (Pei et al., 2012) that further moderate the function of the receptors
Unlike DA D2, DA D4 is located primarily in cortical regions. A variable number of tandem repeats (VNTR; Table 1) polymorphism within exon 3 of DRD4 results in alleles ranging in length from 2 to 11. The 7-repeat allele has been linked to reduced DA D4 sensitivity and postsynaptic inhibition (Asghari et al., 1995), elevated ventral striatal reactivity to reward (Forbes et al., 2009), and potentiated approach-related behavior (Garcia et al., 2010; Roussos et al., 2010). These divergent associations between limbic (DRD2) and cortical (DRD4) DA function are consistent with a bidirectional relationship between cortical and limbic DA. Specifically, elevated cortical DA has been associated with reduced limbic DA phasic burst firing critical for reward learning (Bilder et al., 2004; Deutch, 1992; Grace, 1993; Taber et al., 1995). Accordingly, the additive effects of genetically conferred reductions in striatal DA signaling but potentiation in cortical DA signaling may leave individuals particularly vulnerable to reward processing, and in particular reward learning, dysfunction.
Dopamine Transporter (DAT, SLC6A3)
After release into the synapse, excessive DA can be returned to the presynaptic neuron by DAT; as a result, DAT regulates the magnitude and duration of DA available to bind to receptors. DAT is predominantly expressed in the striatum, making it the primary constraint on striatal DA receptor binding. A VNTR within the 3’ untranslated region is the most studied DAT polymorphism. Several in vitro (Brookes et al., 2007) and in vivo (Cheon et al., 2005; Heinz et al., 2000) studies have linked the 9-repeat allele to reduced DAT availability relative to the 10-repeat (but see Martinez et al., 2001; Mill et al., 2005). Thus, the 9-repeat allele likely results in less efficient DA reuptake and increased synaptic DA available to bind to limbic receptors. Consistent with this evidence, 9-repeat allele carriers, who presumably have elevated limbic synaptic DA levels, show increased ventral striatal reactivity to anticipation (Aarts et al., 2010; Dreher et al., 2009; Yacubian et al., 2007) and receipt (Forbes et al., 2009) of rewards. Thus, these studies complement the DRD2 research reviewed above, providing an independent mechanism (enhanced DAT expression) that may also produce reduced reward-related striatal responses.
Catechol-O-methyltransferase (COMT, COMT)
The COMT enzyme degrades DA and other catecholamines. Unlike DAT, which is expressed predominantly in the striatum, COMT is primarily expressed in the PFC. A SNP within COMT results in a Valine (Val) to Methionine (Met) amino acid substitution in the final protein product (Val158Met, rs4680). The 158Met allele is associated with a 3–4 fold reduction in COMT activity (Lotta et al., 1995) and presumably higher DA in the PFC (Chen et al., 2004). Consistent with inhibitory effects of cortical DA on striatal DA transmission, the Val158 allele (associated with reduced cortical DA) has been linked to increased DA synthesis in limbic regions (Akil et al., 2003; Meyer-Lindenberg et al., 2005), flexible reward-related decision making (Krugel et al., 2009), and increased striatal reactivity to unexpectedly high monetary gains (Camara et al., 2010).
In apparent contrast, the 158Met allele (associated with increased cortical DA) has been linked to increased striatal activation during reward anticipation in two studies (Dreher et al., 2009; Yacubian et al., 2007). Recently documented epistatic (Table 1) (Buckholtz et al., 2007) and epigenetic (Ursini et al., 2011) effects, may explain these contradictory results. In addition, these findings raise the possibility that the 158Met allele may be associated with heightened neural reactivity to reward in predictable environments (less dependent on striatal DA bursts inhibited by elevated cortical DA), while the Val158 allele may be associated with more efficient reward learning in the context of changing environmental contingencies (Belsky et al., 2009; Bilder et al., 2004).
Biologically Informed Multilocus DA Profile
The majority of genetic studies of reward processing have examined single polymorphisms independently, with few exceptions examining epistatic effects (Bertolino et al., 2009; Buckholtz et al., 2007; Dreher et al., 2009; Yacubian et al., 2007). However, reward processing, like other complex phenomena, is shaped by a multitude of genetic factors within and across neurotransmitter systems, which may explain the relatively small effects observed in studies of single variants. For example, one could imagine how the effects of a polymorphism conferring increased DRD2 expression could be masked by a variant linked to increased DAT expression because synaptic DA may be cleared more quickly from the synapse by DAT, preventing the possibility of binding at the additional DA D2 receptors.
In an effort to better capture genetically-driven variation across neural systems, additive genetic scores within multiple pathways of a particular system (often referred to as biologically informed multilocus profiles) may be created. Such biologically informed multilocus profiles are similar to genetic risk scores currently used to cumulatively represent genetic associations with disorder (e.g., Purcell et al., 2009). However, there is a key distinction in that biologically-informed multi-locus profiles are determined based upon known associations with biological function and as such, unlike genetic risk profiles, can inform our mechanistic etiologic understanding.
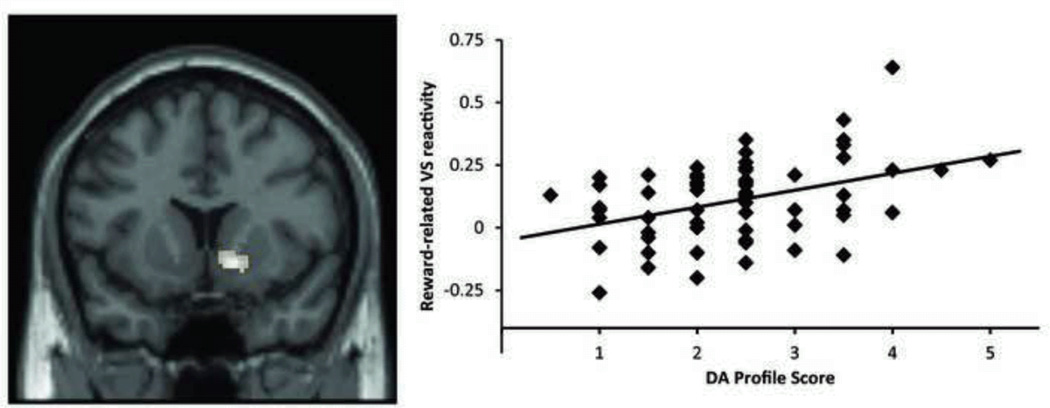
In support of this approach, a recent study showed that a genetic profile for DA signaling based on five of the polymorphic loci reviewed above (COMT Val158Met, DAT1 40-bp VNTR, DRD4 48-bp VNTR, DRD2 −141C Ins/Del, and DRD2 Taq1A) explained nearly 11% of variability in reward-related ventral striatal reactivity, while none of the loci taken individually contributed significantly (Nikolova et al., 2011; Figure 1). Similar multilocus genetic profile approaches could be harnessed to increase power by better capturing genetically driven individual differences within interlinked neural circuits increasing the biological complexity and plausibility of tested models (Bogdan et al., in press).
Figure 1.
A biologically-informed multilocus dopamine profile across five polymorphic loci accounts for 10.9% of the variance in right ventral striatal reactivity (shown on the left) to reward within a card guessing paradigm. Summation used to capture increased DA signaling: DRD2 rs1799732 (deletion = 1, insertion = 0), rs1800497 (C/C = 1, C/T = 0.5, T/T = 0); DRD4 exon 3 VNTR (7-R = 1, all others = 0); SLC6A3 3’ UTR VNTR (9-R = 1, 10/10 = 0); COMT rs4680 (Met/Met = 1, Val/Met = 0.5, Val/Val = 0). Adapted from Nikolova et al. (2011).
With the introduction of biologically informed multilocus profiles, several new challenges arise. First, a limited number of polymorphisms have been functionally characterized, limiting the number of systems that can be presently probed (e.g., 5-HT, DA, HPA axis); those that have been characterized may have limited and conflicting data on their function (e.g., see the discussion of DRD2 receptors above) making their interpretation and assignment difficult. As our functional understanding of individual polymorphisms increases, it may be possible to weight specific polymorphisms differentially according to their effects as opposed to the simple additive approach currently used. Second, such profiles introduce the possibility for a multitude of combinations, making it difficult to determine which polymorphisms to include. When selecting polymorphisms, it will be important to consider linkage disequilibrium across polymorphisms within created profiles to ensure that profiles do not disproportionately weight effects. Along similar lines, it is important to consider the distribution of the profile within samples; for example if rare variants are included, cells with extremely limited numbers of subjects will be created which may produce statistical instability. If these challenges can be overcome, biologically informed multilocus profiles promise to substantially advance our knowledge of individual differences associated with resilience and vulnerability to psychopathology. Moreover, the interaction between profiles could be particularly useful for examining interactions across (e.g., 5-HT × DA) or within (e.g., DA production × DA degradation/reuptake) neural systems to test specified hypotheses.
Beyond DA
In addition to DA, emerging research has emphasized the importance of other neural systems in reward processing. For instance, non-human animal data have linked endocannabinoid function to anticipatory reward processing or “wanting,” as well as consummatory reward processing or “liking” (Berridge et al., 2009). In line with this preclinical evidence, neuroimaging studies have recently highlighted the effects of genetic variation within fatty acid amide hydrolase (FAAH), a membrane bound enzyme that degrades endocannabinoids, on reward-related neural activation. Specifically, the A allele of a common functional SNP in FAAH (rs324420) has been associated with reduced FAAH expression, and presumably increased endocannabinoid signaling (Chiang et al., 2004). Fitting this molecular mechanism, A allele carriers have heightened striatal reactivity to reward (Hariri et al., 2009) and marijuana cues in regular marijuana users (Filbey et al., 2010). Highlighting the impact of multiple genes within a system, Filbey and colleagues (2010) further demonstrated that a SNP (rs2023239) in the cannabinoid type 1 receptor (CNR1) independently and additively (with rs324420) moderated reward-related reactivity to marijuana cues. Other SNPs within CNR1 have been shown to moderate striatal responses and approach behavior (Agrawal et al., 2012; Chakrabarti and Baron-Cohen, 2011; Chakrabarti et al., 2006), emphasizing the potential importance of this system to reward processing.
In addition to these findings, genetic variation in other systems – including the mu-opiod receptor (OPRM1; Lee et al., 2011), TREK1 (KCNK2; Dillon et al., 2010), nitric oxide synthase (NOS1; Hoogman et al., 2011), inhibitory γ-amino butyric acid α2 receptor subunit (GABRA2; Villafuerte et al., 2011), and Bcl-2 (Salvadore et al., 2009)– have been associated with individual differences in reward-related brain structure, neural activation, and/or behavior. As more knowledge about the functional role of common polymorphisms emerges it will be critical to probe biologically-informed multilocus profiles to represent function within distinct neurotransmitter systems (e.g., DA, opiod, endocannabinoid), allowing for examination of synergistic effects.
Links to Depression
As reviewed above, a host of polymorphisms within DA and other systems have been associated with individual differences in reward-related neural activation and behavior, which have, in turn, been linked to clinical depression and depressive symptoms in healthy individuals (Knutson et al., 2008; Pizzagalli et al., 2005; Pizzagalli et al., 2008; Pizzagalli et al., 2009). However, while a few studies report associations between these variants and depression (Liou et al., 2009; López-León et al., 2007), the vast majority of studies do not (Koks et al., 2006; Opmeer et al., 2010) [for review see (Noble, 2003)]. While the lack of cross-association between polymorphisms linked to depression and those linked to reward-related brain function and behavior (in predominantly healthy or clinically unassessed populations) may be disappointing, it is not entirely surprising. In light of evidence suggesting that anhedonia may reach clinical significance in half of patients with MDD (Fawcett et al., 1983; Oei et al., 1990; Pelizza and Ferrari, 2009), a large portion of patients included in studies of depressed individuals will likely not feature reward processing deficits, thereby suppressing the detection of genetic effects increasing vulnerability to MDD via anhedonia-related mechanisms. Such heterogeneity highlights the potential utility of using continuous and specific measures in clinical and healthy populations to better represent distinct disorder components associated with given pathophysiological processes.
Similarly, possible MDD susceptibility genes emerging from GWAS studies not yet been linked to reward processing dysfunction. To the best of our knowledge, the only exception is the piccolo gene (PCLO; Bochdanovits et al., 2009; Hek et al., 2010; Sullivan et al., 2009). Piccolo is a presynaptic scaffolding protein believed to play an important role in neurotransmitter release. Non-human animal research has documented the importance of piccolo to DA function; in addition to being widely expressed in the nucleus accumbens, experimental reductions in piccolo result in heightened reward-related behavior as well as elevated accumbal dopamine (Cen et al., 2008). However, specificity of PCLO is unlikely as it affects general monoamine neurotransmission and has been shown to influence other constructs (e.g., spatial learning, hippocampal long-term potentiation; Ibi et al., 2010). Interestingly, the PCLO genotype associated with depression in GWAS has been linked to individual differences in hypothalamic-pituitary-adrenal (HPA) axis function (Kuehner et al., 2011; Schuhmacher et al., 2011), the body’s central regulator of stress responsiveness. In light of links between stress and anhedonia discussed below, PCLO polymorphisms may be particularly relevant for reward processing deficits in the context of stress.
Conclusions
Consistent with a long-standing theoretical tradition positing that reward processing dysfunction plays a causal role in the development of depression (e.g., Klein, 1987; Loas, 1996; Meehl, 1975), emerging research suggests that intact hedonic capacity and robust neural reward-related circuitry protect against depressive symptoms (Aschbaher et al., 2012; Bijttebier et al., 2011; Nikolova et al., in press). Given these robust links, it is important to understand the factors, including genetic ones that are associated with individual differences in reward-related neural function.
Accumulating research suggests that genetic differences conferring relatively increased subcortical DA or reduced cortical DA signaling (via either receptor availability or synaptic clearance) are associated with enhanced reward-related neural activation and behavior. These findings complement theoretical work suggesting an inverse relationship between cortical and limbic DA (e.g., Bilder et al., 2004). However, much like in MDD, the heterogeneity of reward processing warrants attention. It is now clear that reward processing is not a monolithic phenomenon, but can be parsed into distinct neurochemical, neuroanatomical, and psychological components, including incentive motivation, reward consumption, and reward learning (Berridge et al., 2009). For the most part, molecular genetics research has largely ignored these phenotypic nuances. However, given emerging links between genetic variation within neurochemical systems (e.g., endocannabinoids) linked to specific neurochemical subcomponents of reward processing, it will be important for future neurogenetics research to not only examine polymorphisms within these candidate neural systems, but to also deconstruct their effects on specific reward processing components.
One important concern currently confronting intermediate phenotype research on reward processing is that, for the most part, genetic variants associated with individual differences in reward processing in healthy individuals have not been linked to depression. It will be important to ascertain whether these polymorphisms may be associated with clinically significant anhedonic presentations in patients with depression using refined and construct-specific measurements. Most critically, as our knowledge of genetically-driven variation in reward processing expands, it may be possible to use this knowledge to select and guide more personalized treatments. For example, delineating reward processing dysfunction might suggest a priori selection of psychological (e.g., (Dichter et al., 2009) or pharmacological (e.g., Nestler and Carlezon, 2006; Ossewaarde et al., 2011) interventions directly targeting such abnormalities or used in a preventative context.
Stress Sensitivity
Both retrospective and prospective research has linked stress to depression (Brown and Harris, 1978; Hammen, 2005; Kendler et al., 1999; Muscatell et al., 2009; van Praag et al., 2004). Major stressful life events precede depression in more than 80% of cases (particularly, first depressive episodes) and a general linear relationship between the severity and frequency of negative life events and the probability of a depressive episode has been reported (Mazure, 1998). Interestingly, emerging evidence suggests that stress sensitivity may be related to reward processing dysfunction. A large body of animal literature (Anisman and Matheson, 2005; Willner, 2005) and emerging human research (Berenbaum and Connelly, 1993; Bijttebier et al., 2011; Bogdan and Pizzagalli, 2006; Bogdan et al., 2011; Dillon et al., 2009; Pizzagalli et al., 2007) suggests that stress-induced anhedonia is a promising mechanism underlying the association between stress and depression. Moreover, research suggests that this effect may be particularly relevant for women (Lighthall et al., 2011), consistent with speculation that stress sensitivity phenotypes may be gender specific (Hasler et al., 2004). Complementing this research and providing clues to potential biological mechanisms, studies have linked HPA axis activity to individual differences in DA with non-human animal research suggesting that stress-related HPA axis activation can directly alter DA function (Duval et al., 2006; Pascucci et al., 2007; Piazza et al., 1996). In light of these findings, it is important to understand individual difference variables that may confer vulnerability/resiliency to stress-related deficits in reward processing.
The HPA axis is a key regulator of stress reactivity (de Kloet et al., 2005; Ulrich-Lai and Herman, 2009). Briefly, multiple regulatory pathways converge within the hypothalamus to stimulate corticotropin releasing hormone (CRH) in response to stress perception. CRH binding in the anterior pituitary gland triggers the release of adrenocorticotropic hormone (ACTH), which stimulates cortisol after binding to receptors within the adrenal gland. Cortisol binding then inhibits CRH and ACTH release forming a negative feedback loop wherein the body returns to homeostasis after the stressor. Nearly 50 years of research has demonstrated that depression, among other psychopathologies, is characterized by HPA axis dysfunction (Gibbons and Mc, 1962; Lopez-Duran et al., 2009; Yehuda, 2002). Moreover, in support of the relationship between stress and anhedonia, HPA axis dysfunction is particularly prevalent in individuals with melancholic depression, a severe subtype characterized by anhedonia (Gold and Chrousos, 1999; Gold et al., 2002; Pintor et al., 2007; Stetler and Miller, 2011). Consistent with these associations, emerging research has linked variation in HPA axis activity and stress response with functional and structural differences in striatal and other limbic regions central to reward processing (Jahn et al., 2010; McEwen and Gianaros, 2010; Pruessner et al., 2010; Urry et al., 2006).
Genetics of Stress Sensitivity
Hypothalamic-pituitary-adrenal (HPA) axis
There is substantial individual variability in HPA axis function that is relatively stable over time (Fox et al., 2006; Kudielka et al., 2009; Marquez et al., 2005), suggesting that genetically-conferred variation in HPA axis function may contribute to stable differences in stress responsiveness and hence vulnerability/resilience to the depressogenic effects of stress. Because evidence suggests that HPA axis pathophysiology is largely related to CRH (Binder and Nemeroff, 2009; Hauger et al., 2006), we focus on this critical hormone and neurotransmitter in the next section.
CRH
The CRH system plays a pivotal role in the physiological, behavioral, and cognitive responses to stress. In rodents, CRH administration and overexpression can result in depression-like behavior, while CRH antagonists have antidepressant properties (Hauger et al., 2006; Zieba et al., 2008). Consistent with this evidence, elevated CRH is seen in various forms of stress-related psychopathology, including depression with anhedonic features (Gold and Chrousos, 1999; Pintor et al., 2007). Moreover, emerging research suggests that CRH influences DA and reward-related behavior. Interestingly, this research suggests that CRH increases tonic striatal DA levels and phasic responses to already conditioned stimuli, but may prevent phasic DA bursts from meaningfully associating novel rewards with environmental contingencies (Beckstead et al., 2009; Pecina et al., 2006). This mechanism may explain why stress has been associated with increased habitual responding but blunted novel reward learning (Bogdan and Pizzagalli, 2006; Bogdan et al., 2011; Pecina et al., 2006; Schwabe and Wolf, 2011). In light of evidence most strongly linking CRH pathophysiology to the corticotropin releasing hormone type 1 receptor (CRH R1) (Hauger et al., 2006), we focus on genetic variation within the CRH R1 gene (CRHR1).
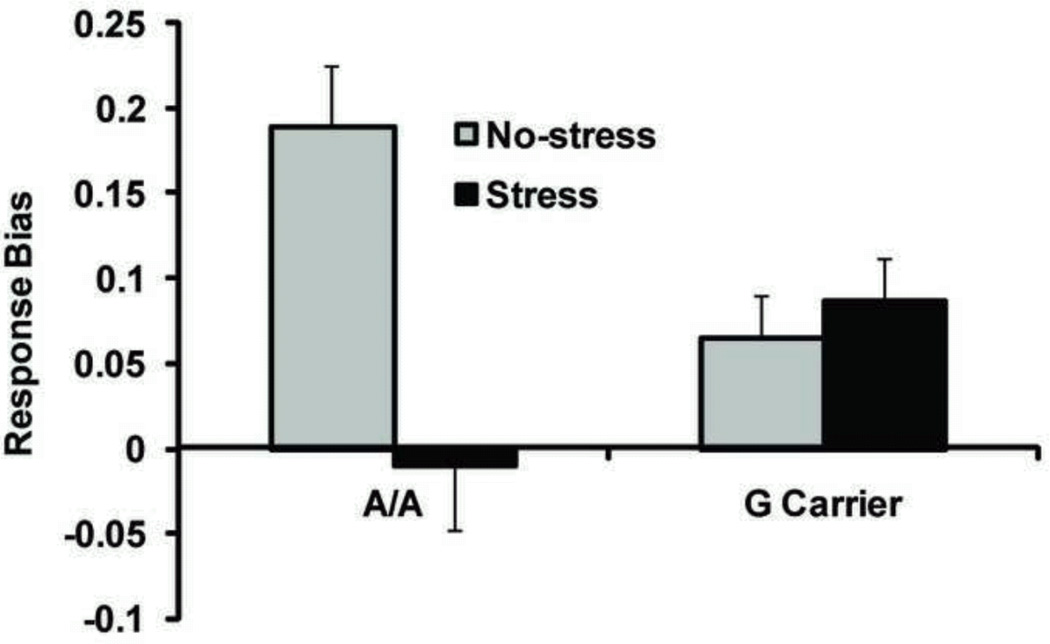
The A allele of a SNP within the promoter of CRHR1 (rs12938031; A/G) has been associated with enhanced CRHR1 mRNA expression as well as diminished HPA axis response to CRH infusion – a pattern also seen in patients with depression (Thode et al., 2009). We have recently extended these findings by reporting that A homozygotes are more susceptible to stress-related behavioral and neural reward learning deficits using an experimentally manipulated within-subject design (Bogdan et al., 2011; Figure 2). Collectively, these studies suggest that genetically driven variation within the HPA axis may confer differences in HPA axis function, as well as vulnerability/resilience to the depressogenic effects of stress, possibly via reward processing disruptions. Further emphasizing the importance of CRHR1 to stress system function and depression, several other reports have linked CRHR1 SNPs to differential HPA axis activation and depression in the context of childhood maltreatment (Blomeyer et al., 2008; Bradley et al., 2008; Polanczyk et al., 2009; Tyrka et al., 2009). Finally, several polymorphisms at different nodes within the HPA axis – including the mineralocorticoid receptor (MR; Bogdan et al., 2010; Kuningas et al., 2007), glucocorticoid receptor (NR3C1; Derijk et al., 2008) and FK506 binding protein (FKBP5; Zimmermann et al., 2011) – have been associated with individual differences in HPA axis function, differences in reward processing and/or depression.
Figure 2.
A homozygosity at rs12938031 within CRHR1 is associated with deficits in reward learning (as represented by response bias) under stress. Stress was manipulated experimentally (threat-of-shock) in a within-subject design. Adapted from Bogdan et al. (2011).
Beyond the HPA axis
A discussion of genetics, stress sensitivity, and depression would be incomplete without inclusion of serotonin transporter research. A repeat polymorphism in the promoter region of the serotonin transporter (5-HTT) gene (SLC6A4) known as the serotonin-transporter-linked polymorphic region (5-HTTLPR) results in common short and long alleles (Heils et al., 1996; Lesch et al., 1996; Murphy et al., 2008). The short allele is associated with reduced 5-HTT mRNA and protein expression, as well as reduced binding (Lesch et al., 1996). A wealth of research suggests that short 5-HTTLPR allele carriers are more vulnerable to environmental circumstances (Caspi et al., 2010). In brief, short allele carriers are characterized by heightened neuroticism (Lesch et al., 1996), elevated threat-related amygdala reactivity (Hariri et al., 2002; Munafo et al., 2008), elevated cortisol upon awakening (Chen et al., 2009), and elevated rates of depression following stressful experiences (Caspi et al., 2003; Caspi et al., 2010; Karg et al., 2011; Kendler et al., 2005; but see Risch et al., 2009). These findings have been replicated in humans and corroborated by preclinical data across species (Caspi et al., 2010; Karg et al., 2011). Collectively, these results have led to the conclusion that the short allele moderates effects of environmental variables (Caspi et al., 2010).
Importantly, the mechanisms underlying the association between 5-HTTLPR and depression in the context of stress remain unknown. Three studies suggest that one potential mechanism, among others, may be stress-related reward processing dysfunctions. In a first study conducted in monkeys, the short allele was associated with risk-aversion under stressful conditions, but risk-seeking in long homozygotes – a pattern interpreted as suggesting enhanced sensitivity to social punishment and reward, respectively (Watson et al., 2009). A second study found that, relative to individuals homozygous for the long allele, human youth with the short allele report lower or higher positive affect in the context of negative or positive parenting, respectively – a finding replicated across three independent cohorts (Hankin et al., 2011). Third, our group described that elevated stress perception regarding an upcoming school exam was associated with reduced reward learning in short allele carriers but enhanced reward learning in long homozygotes (Nikolova et al., 2012).
Links to Depression
HPA axis dysregulation and stress exposure are among the variables most reliably linked to depression, each occurring in up to 80% of MDD patients (Mazure, 1998). As such, several stress-system variants have been linked to depression and other stress-related psychopathologies (Binder, 2009; Caspi et al., 2010; Derijk et al., 2008). Critically, however, these relationships are generally only uncovered when considering the role of the environment. For stress-related disorders such as MDD, genome-wide association studies may be particularly well served to include environmental measures of stress experience. Evidence highlighting the potential of this approach exists in other research fields: for example, the inclusion of environmental factors into GWAS has uncovered novel insights into asthma (Ege et al., 2011). While to our knowledge neuropsychiatriatric GWAS have yet to include environmental effects, this approach promises to be fruitful given: (1) consistent links between stress and psychopathology, (2) candidate gene studies documenting gene × environment interactions (e.g., Caspi et al., 2003), and (3) emerging work documenting that epigenetic effects can be moderated by genotype (Ursini et al., 2011).
Similar to research on reward-related function, the only gene associated with MDD through GWAS known to play a role in the HPA axis is the piccolo gene (PCLO). Specifically, healthy controls with the A allele at rs2522833, which has been linked to depression through GWAS, show blunted cortisol awakening response, elevated diurnal cortisol levels, and reduced changes in HPA axis function following antidepressant treatment (Kuehner et al., 2011; Schuhmacher et al., 2011). Because PCLO affects DA function and the variant linked to MDD has been associated with individual differences in HPA axis function, it is a particularly appealing candidate for investigations probing relations between stress and reward function.
Conclusions
Converging evidence from mouse to man suggests that the depressogenic effects of stress may result, at least partially, from stress-induced anhedonia. Moreover, research has linked HPA axis variability to reward-related brain function (Duval et al., 2006; Pascucci et al., 2007; Piazza et al., 1996). As such, it is important to understand how individual differences may leave individuals more or less vulnerable to stress-related reward system dysfunction. Moreover, because cortisol binding to corticosteroid receptors can access the nucleus and bind to glucocorticoid response elements within genes (thereby affecting the transcription of hundreds of other genes), individual differences in HPA axis function are likely to have widespread and pleiotropic effects.
Polymorphisms within the HPA axis are associated with differential physiological and psychological responses to stress providing putative proxies of HPA axis system activity and elicited activation that may partially explain vulnerability and resiliency to stress. Of note, HPA axis-related polymorphisms that have been associated with increased and prolonged HPA axis activity have been also associated with depression, but generally only in the context of stress exposure. Building upon this work, emergent research raises the possibility that such increased vulnerability to depression may be partially related to a propensity for stress-induced reward processing dysfunction. Importantly, however, research needs to deconstruct which aspects of HPA axis activation are associated with stress-related depression and reward processing dysfunction. Equally important will be to elucidate which aspects of reward processing might be particularly perturbed by stress. Interestingly, research suggests that increased HPA axis activation is associated with blunted novel reward learning (e.g., Bogdan and Pizzagalli, 2006; Bogdan et al., 2011), but a reliance on past impulsive rewarding behaviors (e.g., comfort food consumption, drug use; Brady et al., 2009; George et al., 2010; Ulrich-Lai et al., 2010), which has clear consequences for our understanding of impulse control disorders and substance abuse. As such, the role of stress in the development of anhedonia may be more specific to behaviors that are novel for individuals and may be more specifically associated with novel reward-related learning.
Discussion
Euphoric expectations about the potential of genetic research to rapidly improve our etiological understanding and treatment of mental illness have been largely replaced by sober realizations that individual common polymorphisms will have small, if any, effects on complex polygenetic diseases, making it exceptionally difficult to reliably link them to psychopathology. Critically, the ability to discern these small effects is largely dependent on the phenotypic variables measured and their proximity to the functional consequences of genetic variation. The closer one measures the direct functional consequences of genetic variation, the larger the effect will be (Flint and Munafo, 2007; Goldman and Ducci, 2007); however, the closer one studies the direct functional consequences of polymorphisms (e.g., mRNA expression), the more removed one becomes from clinical relevance. The aim of this review was to survey and integrate molecular genetics research on reward processing and stress sensitivity, two promising depressive intermediate phenotypes, that are not only clinically relevant to MDD and other disorders (Diekhof et al., 2008), but also presumably more closely linked to the functional consequences of genetic variation than DSM-defined MDD.
Importantly, intermediate phenotype and traditional disorder-based genetics research can mutually inform our understanding of psychopathology. The widespread availability and limited cost of psychiatric symptomatology measures facilitate the establishment of large datasets that may be useful for detecting small genetic effects. Indeed, GWAS have begun to link genes never before suspected to MDD, although these associations have not been well replicated (Bosker et al., 2011; Lewis et al., 2010; Lohoff, 2010; Muglia et al., 2010; Rucker et al., 2011; Shi et al., 2011; Shyn et al., 2011; Sullivan et al., 2009). While GWAS of psychiatric diagnoses inform who may be at risk for a particular disorder, they cannot pinpoint the mechanisms by which identified polymorphisms confer risk or resilience. As such, intermediate phenotype neurogenetics research can powerfully complement traditional disorder-based GWAS by evaluating the biological and behavioral mechanisms that may drive associations with psychopathology. As an illustration, we emphasized in the current review how emerging knowledge about the role of PCLO, a gene recently linked to MDD through GWAS, may be used to guide intermediate phenotypic research aimed at uncovering its mechanistic role in MDD.
Challenges and Future Directions
Various challenges confront intermediate phenotype neurogenetics research (Bogdan et al., in press). For example, our ability to research a construct is dependent on how precisely we can measure it (Kendler and Neale, 2010). As such it will be pivotal for future research to establish reliability for intermediate phenotypes. Of the intermediate phenotypes we discussed, HPA axis function has good reliability (Fox et al., 2006; Kudielka et al., 2009; Marquez et al., 2005). We are only aware of two studies investigating the reliability of reward-related reactivity, with one suggesting good reliability (Schacht et al., 2011) and another reporting relatively poor reliability (Fliessbach et al., 2010). In addition to establishing reliability, a major priority will be to establish the validity of intermediate phenotypes. In 1970, Robins and Guze proposed that psychiatric diagnoses are valid when they: 1) have high inter-rater reliability, 2) predict future behavior, 3) predict course, outcome, and treatment response, 4) predict family history, and 5) differentiate between diagnoses.1 Currently, research on intermediate phenotypes has largely ignored these classic validity criteria. Although the field is far from diagnostic classifications of mental illnesses rooted in pathophysiology and etiology, we expect that a close evaluation of promising intermediate phenotypes with respect to these criteria will be pivotal for fulfilling this challenging – yet critical – goal. Ultimately, only an improved understanding of the pathophysiology and etiology of mental illnesses will allow the field to address many unmet needs, including the development of novel and more efficacious intervention and prevention strategies.
Related to establishing validity and reliability of intermediate phenotypes used in neurogenetics research, there is presently widespread variability in paradigms used to measure the same or similar constructs, as well as analysis techniques. For example, studies assessing “reward processing” may use paradigms that can differentiate anticipatory vs. consummatory phases (e.g., Knutson et al., 2000; Dillon et al., 2008), while others may aggregate across these effects (e.g., Hariri et al., 2006). Convergence across studies using different paradigms assessing the same construct will lead to greater confidence in reported findings; however, when a lack of replication is observed it may not necessarily represent an original false positive finding but may reflect experimental or population differences.
Another challenge confronting intermediate phenotype neurogenetics research is obtaining adequate sample sizes to investigate the small effects of common polymorphisms, conduct GWASs, and examine rare variants. Neurogenetics data collection is often time- and resource-intensive (e.g., fMRI); however, collaborations and consortia are beginning to accrue the sample sizes needed to conduct GWAS that include neuroscientific data and consider rare variants.
In this context, although neurogenetics research has primarily focused on common polymorphisms, emerging psychiatric sequencing research has documented large associations between rare variants and depression (Haenisch et al., 2009), as well and other complex polygenic disorders (Rodriguez-Murillo et al., 2012). As such, it is important for neurogenetics research to not only functionally characterize such variants (e.g., associations with gene transcription), but also examine how these rare variants affect intermediate phenotypes. Given how infrequently these variants occur, this represents a major challenge, whereby samples will likely need to be selected based upon the presence of rare variants, potentially through consortia of large imaging genetic studies, or selected recruitment from sequencing studies.
The potential of GWAS on intermediate phenotypes is just beginning to be realized through the combination of datasets and large-scale independent studies. Confronted with the pervasive clinical, etiological, and pathophysiological heterogeneity of psychiatric disorders, neurogenetics research targeting intermediate phenotypes promises to provide a novel window towards a better understanding of psychopathology. Critically, and consistent with the assumption that homogenous intermediate phenotypes are more proximally related to gene function than psychiatric syndromes, unlike the vast majority of GWAS on psychiatric diagnoses, including depression (Lohoff, 2010), many neurogenetics GWAS have identified SNPs reaching genome-wide significance (i.e., 10 × 10−8), with positive replication in some cases (Bakken et al., 2011; Bis et al., 2012; Hodgkinson et al., 2010; Potkin et al., 2009A; Potkin et al., 2009B; Shen et al., 2010; Stein et al., 2012).
Lastly, it will be imperative for neurogenetics research to translate findings to the clinic (Bogdan et al., in press). First, neurogenetics research must document that genetically-associated differences in neural structure, function, and connectivity meaningfully predict differences in psychopathology. An ideal strategy to pursue this would be to conduct longitudinal prospective studies with multiple time points. Additionally, in light of the current largely trial-and-error treatment approach and the significant percentage of patients failing to respond to antidepressant treatments (e.g., Trivedi et al., 2006), it would be particularly valuable for neurogenetics research to inform treatment strategies. In addition to determining for whom a specific treatment may work best, neurogenetics research may contribute to the development of novel treatments alongside non-human animal research. An example of such potential comes from recent research on TREK1 (KCNK2), a background potassium channel. Mice that have TREK1 knockout display resiliency to depression (Heurteaux et al., 2006) and in humans, genetic variation within TREK1 has been linked to differences in reward-related neural function (Dillon et al., 2010) and treatment response to antidepressant medication (Perlis et al., 2008). More recently, researchers (Mazella et al., 2010) have developed a treatment that inhibits TREK1 and has been associated with a positive antidepressant response, increased serotonergic signaling, and hippocampal neurogenesis in rodents. However, the true clinical potential of this novel treatment has yet to be tested in humans. Ultimately, important benchmarks to evaluate the success of neurogenetics research focused on intermediate phenotypes will be directly tied to its ability to inform pathophysiological understandings of psychiatric disorders, identify at-risk individuals, and guide treatment decisions.
Acknowledgements
Acknowledgements and Disclosures
The authors are thankful to Dr. Daniel Dillon for helpful comments on the manuscript and Dr. Ahmad R. Hariri for useful discussions about the material contained herein. Ryan Bogdan was supported by NIDA grant P30 DA023026. Ryan Bogdan and Yuliya Nikolova report no competing interests. Diego Pizzagalli has received consulting fees from ANT North America Inc. (Advanced Neuro Technology), AstraZeneca, Shire, and Ono PharmaUSA, and honoraria from AstraZeneca for studies unrelated to this project.
Footnotes
We chose to use the term “intermediate phenotype” as opposed to “endophenotype” because various criteria including specificity, state-independence, heritability, familial association, cosegregation, biological and clinical plausibility have been proposed as criteria on which to evaluate endophenotypes (Gottesman & Gould, 2003; Hasler et al., 2004). However, in light of evidence of shared pathophysiology across disorders (as well as potential problems in our diagnostic classification), intermediate phenotypes should not be constrained to a particular disorder as currently defined (i.e., exhibit specificity). Moreover, in light of documented GxE and epigenetic regulation, intermediate phenotypes may appear to be state-dependent (e.g., require stress to be unmasked). In an effort to avoid these evaluative criteria we chose to use the term intermediate phenotype instead and emphasize validity criteria from Robins & Guze (1970).
References
- Aarts E, et al. Striatal dopamine mediates the interface between motivational and cognitive control in humans: evidence from genetic imaging. Neuropsychopharmacology. 2010;35:1943–1951. doi: 10.1038/npp.2010.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal A, et al. Cannabinoid receptor genotype moderation of the effects of childhood physical abuse on anhedonia and depression. Arch Gen Psychiatry. doi: 10.1001/archgenpsychiatry.2011.2273. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akil M, et al. Catechol-O-methyltransferase genotype and dopamine regulation in the human brain. J Neurosci. 2003;23:2008–2013. doi: 10.1523/JNEUROSCI.23-06-02008.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders : DSM-IV-TR. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Anisman H, Matheson K. Stress, depression, and anhedonia: Caveats concerning animal models. Neurosci Biobehav Rev. 2005;29:525–546. doi: 10.1016/j.neubiorev.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Asghari V, et al. Modulation of intracellular cyclic AMP levels by different human dopamine D4 receptor variants. J Neurochem. 1995;65:1157–1165. doi: 10.1046/j.1471-4159.1995.65031157.x. [DOI] [PubMed] [Google Scholar]
- Bakken TE, et al. Association of genetic variants on 15q21 with cortical thickness and cognition in schizophrenia. Arch Gen Psychiatry. 2011;68:781–790. doi: 10.1001/archgenpsychiatry.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckstead MJ, et al. CRF Enhancement of GIRK Channel-Mediated Transmission in Dopamine Neurons. Neuropsychopharmacology. 2009;34:1926–1935. doi: 10.1038/npp.2009.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, et al. Vulnerability genes or plasticity genes? Mol Psychiatry. 2009;14:746–754. doi: 10.1038/mp.2009.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenbaum H, Connelly J. The effect of stress on hedonic capacity. J Abnorm Psychol. 1993;102:474–481. doi: 10.1037//0021-843x.102.3.474. [DOI] [PubMed] [Google Scholar]
- Berridge KC, et al. Dissecting components of reward: ‘liking’, ‘wanting’, and learning. Curr Opin in Pharmacol. 2009;9:65–73. doi: 10.1016/j.coph.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolino A, et al. Genetically Determined Interaction between the Dopamine Transporter and the D2 Receptor on Prefronto-Striatal Activity and Volume in Humans. J Neurosci. 2009;29:1224–1234. doi: 10.1523/JNEUROSCI.4858-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijttebier P, et al. Responses to Positive Affect Predict Mood Symptoms in Children Under Conditions of Stress: A Prospective Study. J Abnorm Child Psychol. 2011 doi: 10.1007/s10802-011-9579-2. [DOI] [PubMed] [Google Scholar]
- Bilder RM, et al. The Catechol-O-Methyltransferase Polymorphism: Relations to the Tonic–Phasic Dopamine Hypothesis and Neuropsychiatric Phenotypes. Neuropsychopharmacology. 2004;29:1943–1961. doi: 10.1038/sj.npp.1300542. [DOI] [PubMed] [Google Scholar]
- Binder EB. The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology. 2009;34(Suppl 1):S186–S195. doi: 10.1016/j.psyneuen.2009.05.021. [DOI] [PubMed] [Google Scholar]
- Binder EB, Nemeroff CB. The CRF system, stress, depression and anxiety—insights from human genetic studies. Mol Psychiatry. 2009;15:574–588. doi: 10.1038/mp.2009.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bis JC, et al. Common variants at 12q14 and 12q24 are associated with hippocampal volume. Nat Genet. 2012;44:545–551. doi: 10.1038/ng.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomeyer D, et al. Interaction between CRHR1 gene and stressful life events predicts adolescent heavy alcohol use. Biol Psychiatry. 2008;63:146–151. doi: 10.1016/j.biopsych.2007.04.026. [DOI] [PubMed] [Google Scholar]
- Blood AJ, et al. Microstructural abnormalities in subcortical reward circuitry of subjects with major depressive disorder. PLoS ONE. 2010;5:e13945. doi: 10.1371/journal.pone.0013945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochdanovits Z, et al. Joint reanalysis of 29 correlated SNPs supports the role of PCLO/Piccolo as a causal risk factor for major depressive disorder. Mol Psychiatry. 2009;14:650–652. doi: 10.1038/mp.2009.37. [DOI] [PubMed] [Google Scholar]
- Bogdan R, et al. The impact of mineralocorticoid receptor ISO/VAL genotype (rs5522) and stress on reward learning. Genes Brain Behav. 2010;9:658–667. doi: 10.1111/j.1601-183X.2010.00600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdan R, et al. A neurogenetics approach to understanding individual differences inbrain, behavior, and risk for psychopathology. Mol Psychiatry. doi: 10.1038/mp.2012.35. in press. [DOI] [PubMed] [Google Scholar]
- Bogdan R, Pizzagalli D. Acute Stress Reduces Reward Responsiveness: Implications for Depression. Biol Psychiatry. 2006;60:1147–1154. doi: 10.1016/j.biopsych.2006.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdan R, Pizzagalli DA. The heritability of hedonic capacity and perceived stress: a twin study evaluation of candidate depressive phenotypes. Psychol Med. 2009;39:211–218. doi: 10.1017/S0033291708003619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdan R, et al. Corticotropin-releasing hormone receptor type 1 (CRHR1) genetic variation and stress interact to influence reward learning. J Neurosci. 2011;31:13246–13254. doi: 10.1523/JNEUROSCI.2661-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosker FJ, et al. Poor replication of candidate genes for major depressive disorder using genome-wide association data. Mol Psychiatry. 2011;16:516–532. doi: 10.1038/mp.2010.38. [DOI] [PubMed] [Google Scholar]
- Bradley RG, et al. Influence of child abuse on adult depression: moderation by the corticotropin-releasing hormone receptor gene. Arch Gen Psychiatry. 2008;65:190–200. doi: 10.1001/archgenpsychiatry.2007.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady KT, et al. Response to corticotropin-releasing hormone infusion in cocaine-dependent individuals. Arch Gen Psychiatry. 2009;66:422–430. doi: 10.1001/archgenpsychiatry.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes KJ, et al. Relationship between VNTR polymorphisms of the human dopamine transporter gene and expression in post-mortem midbrain tissue. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:1070–1078. doi: 10.1002/ajmg.b.30572. [DOI] [PubMed] [Google Scholar]
- Brown GW, Harris TO. Social origins of depression : a study of psychiatric disorder in women. New York: Free Press; 1978. [Google Scholar]
- Buckholtz JW, et al. fMRI evidence for functional epistasis between COMT and RGS4. Mol Psychiatry. 2007;12:893–895. 885. doi: 10.1038/sj.mp.4002008. [DOI] [PubMed] [Google Scholar]
- Camara E, et al. The effects of COMT (Val108/158Met) and DRD4 (SNP-521) dopamine genotypes on brain activations related to valence and magnitude of rewards. Cereb Cortex. 2010;20:1985–1996. doi: 10.1093/cercor/bhp263. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Keller MC. Endophenotypes in the Genetic Analyses of Mental Disorders. Annu Rev Clin Psychol. 2006;2:267–290. doi: 10.1146/annurev.clinpsy.2.022305.095232. [DOI] [PubMed] [Google Scholar]
- Caspi A, et al. Genetic sensitivity to the environment: the case of the serotonin transporter gene and its implications for studying complex diseases and traits. Am J Psychiatry. 2010;167:509–527. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, et al. Influence of Life Stress on Depression: Moderation by a Polymorphism in the 5-HTT Gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Cen X, et al. Identification of Piccolo as a regulator of behavioral plasticity and dopamine transporter internalization. Mol Psychiatry. 2008;13:349, 451–463. doi: 10.1038/sj.mp.4002132. [DOI] [PubMed] [Google Scholar]
- Chakrabarti B, Baron-Cohen S. Variation in the human cannabinoid receptor CNR1 gene modulates gaze duration for happy faces. Mol Autism. 2011;2:10. doi: 10.1186/2040-2392-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti B, et al. Variations in the human cannabinoid receptor (CNR1) gene modulate striatal responses to happy faces. Eur J Neurosci. 2006;23:1944–1948. doi: 10.1111/j.1460-9568.2006.04697.x. [DOI] [PubMed] [Google Scholar]
- Chen J, et al. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet. 2004;75:807–821. doi: 10.1086/425589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MC, et al. Serotonin transporter polymorphism predicts waking cortisol in young girls. Psychoneuroendocrinology. 2009;34:681–686. doi: 10.1016/j.psyneuen.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheon KA, et al. The homozygosity for 10-repeat allele at dopamine transporter gene and dopamine transporter density in Korean children with attention deficit hyperactivity disorder: relating to treatment response to methylphenidate. Eur Neuropsychopharmacol. 2005;15:95–101. doi: 10.1016/j.euroneuro.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Chiang KP, et al. Reduced cellular expression and activity of the P129T mutant of human fatty acid amide hydrolase: evidence for a link between defects in the endocannabinoid system and problem drug use. Hum Mol Genet. 2004;13:2113–2119. doi: 10.1093/hmg/ddh216. [DOI] [PubMed] [Google Scholar]
- Cichon S, et al. Genomewide association studies: history, rationale, and prospects for psychiatric disorders. Am J Psychiatry. 2009;166:540–556. doi: 10.1176/appi.ajp.2008.08091354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX, et al. Individual differences in extraversion and dopamine genetics predict neural reward responses. Brain Res Cogn Res. 2005;25:851–861. doi: 10.1016/j.cogbrainres.2005.09.018. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, et al. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- Derijk R, et al. Corticosteroid receptor-gene variants: Modulators of the stress-response and implications for mental health. Eur J Pharmacol. 2008;585:492–501. doi: 10.1016/j.ejphar.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Deutch AY. The regulation of subcortical dopamine systems by the prefrontal cortex: interactions of central dopamine systems and the pathogenesis of schizophrenia. J Neural Transm Suppl. 1992;36:61–89. doi: 10.1007/978-3-7091-9211-5_5. [DOI] [PubMed] [Google Scholar]
- Dichter GS, et al. The Effects of Psychotherapy on Neural Responses to Rewards in Major Depression. Biol Psychiatry. 2009;66:886–897. doi: 10.1016/j.biopsych.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekhof E, et al. Functional neuroimaging of reward processing and decision-making: A review of aberrant motivational and affective processing in addiction and mood disorders. Brain Res Rev. 2008;59:164–184. doi: 10.1016/j.brainresrev.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Dillon DG, et al. Variation in TREK1 gene linked to depression-resistant phenotype is associated with potentiated neural responses to rewards in humans. Hum Brain Mapp. 2010;31:210–221. doi: 10.1002/hbm.20858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon DG, et al. Childhood Adversity Is Associated with Left Basal Ganglia Dysfunction During Reward Anticipation in Adulthood. Biol Psychiatry. 2009;66:206–213. doi: 10.1016/j.biopsych.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd EC, Barch DM. Anhedonia and emotional experience in schizophrenia: neural and behavioral indicators. Biol Psychiatry. 2010;67:902–911. doi: 10.1016/j.biopsych.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher JC, et al. Variation in dopamine genes influences responsivity of the human reward system. Proc Natl Acad Sci USA. 2009;106:617–622. doi: 10.1073/pnas.0805517106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval F, et al. Cortisol hypersecretion in unipolar major depression with melancholic and psychotic features: dopaminergic, noradrenergic and thyroid correlates. Psychoneuroendocrinology. 2006;31:876–888. doi: 10.1016/j.psyneuen.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Ege MJ, et al. Gene-environment interaction for childhood asthma and exposure to farming in Central Europe. J Allergy Clin Immunol. 2011;127:138–144. doi: 10.1016/j.jaci.2010.09.041. 144 e1-4. [DOI] [PubMed] [Google Scholar]
- Egerton A, et al. The dopaminergic basis of human behaviors: A review of molecular imaging studies. Neurosci Biobehav Rev. 2009;33:1109–1132. doi: 10.1016/j.neubiorev.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein J, et al. Lack of ventral striatal response to positive stimuli in depressed versus normal subjects. Am J Psychiatry. 2006;163:1784–1790. doi: 10.1176/ajp.2006.163.10.1784. [DOI] [PubMed] [Google Scholar]
- Fawcett J, et al. Assessing anhedonia in psychiatric patients. Arch Gen Psychiatry. 1983;40:79–84. doi: 10.1001/archpsyc.1983.01790010081010. [DOI] [PubMed] [Google Scholar]
- Filbey FM, et al. Individual and additive effects of the CNR1 and FAAH genes on brain response to marijuana cues. Neuropsychopharmacology. 2010;35:967–975. doi: 10.1038/npp.2009.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliessbach K, et al. Retest reliability of reward-related BOLD signals. Neuroimage. 2010;50:1168–1176. doi: 10.1016/j.neuroimage.2010.01.036. [DOI] [PubMed] [Google Scholar]
- Flint J, Munafo MR. The endophenotype concept in psychiatric genetics. Psychol Med. 2007;37:163–180. doi: 10.1017/S0033291706008750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, et al. Genetic variation in components of dopamine neurotransmission impacts ventral striatal reactivity associated with impulsivity. Mol Psychiatry. 2009;14:60–70. doi: 10.1038/sj.mp.4002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, et al. Reward-related decision-making in pediatric major depressive disorder: an fMRI study. J Child Psychol Psychiatry. 2006;47:1031–1040. doi: 10.1111/j.1469-7610.2006.01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox E, et al. The Serotonin Transporter Gene Alters Sensitivity to Attention Bias Modification: Evidence for a Plasticity Gene. Biol Psychiatry. 2011;70:1049–1054. doi: 10.1016/j.biopsych.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, et al. Reliability of salivary cortisol assessments in cocaine dependent individuals. J Psychopharmacol. 2006;20:650–655. doi: 10.1177/0269881106063474. [DOI] [PubMed] [Google Scholar]
- Garcia JR, et al. Associations between dopamine D4 receptor gene variation with both infidelity and sexual promiscuity. PLoS ONE. 2010;5:e14162. doi: 10.1371/journal.pone.0014162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George SA, et al. CRH-stimulated cortisol release and food intake in healthy, non-obese adults. Psychoneuroendocrinology. 2010;35:607–612. doi: 10.1016/j.psyneuen.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons JL, Mc HP. Plasma cortisol in depressive illness. J Psychiatr Res. 1962;1:162–171. doi: 10.1016/0022-3956(62)90006-7. [DOI] [PubMed] [Google Scholar]
- Gold PW, Chrousos GP. The endocrinology of melancholic and atypical depression: relation to neurocircuitry and somatic consequences. Proc Assoc Am Physicians. 1999;111:22–34. doi: 10.1046/j.1525-1381.1999.09423.x. [DOI] [PubMed] [Google Scholar]
- Gold PW, et al. Divergent endocrine abnormalities in melancholic and atypical depression: clinical and pathophysiologic implications. Endocrinol Metab Clin North Am. 2002;31:37–62. doi: 10.1016/s0889-8529(01)00022-6. vi. [DOI] [PubMed] [Google Scholar]
- Goldman D, Ducci F. Deconstruction of vulnerability to complex diseases: enhanced effect sizes and power of intermediate phenotypes. TheScientificWorldJournal. 2007;7:124–130. doi: 10.1100/tsw.2007.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Grace AA. Cortical regulation of subcortical dopamine systems and its possible relevance to schizophrenia. J Neural Trans. 1993;91:111–134. doi: 10.1007/BF01245228. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The Reward Circuit: Linking Primate Anatomy and Human Imaging. Neuropsychopharmacology. 2009;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haenisch B, et al. Association of major depression with rare functional variants in norepinephrine transporter and serotonin1A receptor genes. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:1013–1016. doi: 10.1002/ajmg.b.30912. [DOI] [PubMed] [Google Scholar]
- Hammen C. Stress and depression. Ann Rev Clin Psychol. 2005;1:293–319. doi: 10.1146/annurev.clinpsy.1.102803.143938. [DOI] [PubMed] [Google Scholar]
- Hankin BL, et al. Differential susceptibility in youth: evidence that 5-HTTLPR × positive parenting is associated with positive affect 'for better and worse'. Translational Psychiatry. 2011;1:e44. doi: 10.1038/tp.2011.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, et al. Preference for immediate over delayed rewards is associated with magnitude of ventral striatal activity. K Neurosci. 2006;26:13213–13217. doi: 10.1523/JNEUROSCI.3446-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR. The Neurobiology of Individual Differences in Complex Behavioral Traits. Ann Rev Neurosci. 2009;32:225–247. doi: 10.1146/annurev.neuro.051508.135335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, et al. Divergent effects of genetic variation in endocannabinoid signaling on human threat- and reward-related brain function. Biol Psychiatry. 2009;66:9–16. doi: 10.1016/j.biopsych.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Hasler G, et al. Discovering Endophenotypes for Major Depression. Neuropsychopharmacology. 2004;29:1765–1781. doi: 10.1038/sj.npp.1300506. [DOI] [PubMed] [Google Scholar]
- Hasler G, Northoff G. Discovering imaging endophenotypes for major depression. Mol Psychiatry. 2011;16:604–619. doi: 10.1038/mp.2011.23. [DOI] [PubMed] [Google Scholar]
- Hauger RL, et al. Corticotropin releasing factor (CRF) receptor signalling in the central nervous system: New molecular targets. CNS Neurol Disord Drug Targets. 2006;5:453–479. doi: 10.2174/187152706777950684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heils A, et al. Allelic variation of human serotonin transporter gene expression. J Neurochem. 1996;66:2621–2624. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- Heinz A, et al. Genotype influences in vivo dopamine transporter availability in human striatum. Neuropsychopharmacology. 2000;22:133–139. doi: 10.1016/S0893-133X(99)00099-8. [DOI] [PubMed] [Google Scholar]
- Hek K, et al. The PCLO gene and depressive disorders: replication in a population-based study. Hum Mol Genet. 2010;19:731–734. doi: 10.1093/hmg/ddp529. [DOI] [PubMed] [Google Scholar]
- Heller AS, et al. Reduced capacity to sustain positive emotion in major depression reflects diminished maintenance of fronto-striatal brain activation. Proc Natl Acad Sci USA. 2009;106:22445–22450. doi: 10.1073/pnas.0910651106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkinson CA, et al. Genome-wide association identifies candidate genes that influence the human electroencephalogram. Proc Natl Acad Sci USA. 2010;107:8695–8700. doi: 10.1073/pnas.0908134107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogman M, et al. Nitric oxide synthase genotype modulation of impulsivity and ventral striatal activity in adult ADHD patients and healthy comparison subjects. Am J Psychiatry. 2011;168:1099–1106. doi: 10.1176/appi.ajp.2011.10101446. [DOI] [PubMed] [Google Scholar]
- Hyman SE. Can neuroscience be integrated into the DSM-V? Nat Rev Neurosci. 2007;8:725–732. doi: 10.1038/nrn2218. [DOI] [PubMed] [Google Scholar]
- Ibi D, et al. Piccolo knockdown-induced impairments of spatial learning and long-term potentiation in the hippocampal CA1 region. Neurochem Intl. 2010;56:77–83. doi: 10.1016/j.neuint.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Insel T, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Insel TR, Cuthbert BN. Endophenotypes: bridging genomic complexity and disorder heterogeneity. Biol Psychiatry. 2009;66:988–989. doi: 10.1016/j.biopsych.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Jahn AL, et al. Subgenual Prefrontal Cortex Activity Predicts Individual Differences in Hypothalamic-Pituitary-Adrenal Activity Across Different Contexts. Biol Psychiatry. 2010;67:175–181. doi: 10.1016/j.biopsych.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson EG, et al. Polymorphisms in the dopamine D2 receptor gene and their relationships to striatal dopamine receptor density of healthy volunteers. Mol Psychiatry. 1999;4:290–296. doi: 10.1038/sj.mp.4000532. [DOI] [PubMed] [Google Scholar]
- Karg K, et al. The Serotonin Transporter Promoter Variant (5-HTTLPR), Stress, and Depression Meta-analysis Revisited: Evidence of Genetic Moderation. Arch Gen Psychiatry. 2011;68:444–454. doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasch KL, et al. Behavioral activation and inhibition systems and the severity and course of depression. J Abnorm Psychol. 2002;111:589–597. doi: 10.1037//0021-843x.111.4.589. [DOI] [PubMed] [Google Scholar]
- Keedwell P, et al. A Double Dissociation of Ventromedial Prefrontal Cortical Responses to Sad and Happy Stimuli in Depressed and Healthy Individuals. Biol Psychiatry. 2005a;58:495–503. doi: 10.1016/j.biopsych.2005.04.035. [DOI] [PubMed] [Google Scholar]
- Keedwell P, et al. The Neural Correlates of Anhedonia in Major Depressive Disorder. Biol Psychiatry. 2005b;58:843–853. doi: 10.1016/j.biopsych.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO., Jr Boundaries of major depression: an evaluation of DSM-IV criteria. Am J Psychiatry. 1998;155:172–177. doi: 10.1176/ajp.155.2.172. [DOI] [PubMed] [Google Scholar]
- Kendler KS, et al. Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry. 1999;156:837–841. doi: 10.1176/ajp.156.6.837. [DOI] [PubMed] [Google Scholar]
- Kendler KS, et al. The interaction of stressful life events and a serotonin transporter polymorphism in the prediction of episodes of major depression: a replication. Arch Gen Psychiatry. 2005;62:529–535. doi: 10.1001/archpsyc.62.5.529. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC. Endophenotype: a conceptual analysis. Mol Psychiatry. 2010;15:789–797. doi: 10.1038/mp.2010.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein DF. Depression and anhedonia. In: Clark DC, Fawcett J, editors. Anhedonia and affect deficit states. NY: PMA Publishing Corporation; 1987. [Google Scholar]
- Knutson B, et al. Neural Responses to Monetary Incentives in Major Depression. Biol Psychiatry. 2008;63:686–692. doi: 10.1016/j.biopsych.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Gibbs SEB. Linking nucleus accumbens dopamine and blood oxygenation. Psychopharmacology. 2007;191:813–822. doi: 10.1007/s00213-006-0686-7. [DOI] [PubMed] [Google Scholar]
- Knutson B, et al. FMRI Visualization of Brain Activity during a Monetary Incentive Delay Task. Neuroimage. 2000;12:20–27. doi: 10.1006/nimg.2000.0593. [DOI] [PubMed] [Google Scholar]
- Koks S, et al. Analysis of SNP profiles in patients with major depressive disorder. Int J Neuropsychopharmacol. 2006;9:167–174. doi: 10.1017/S1461145705005468. [DOI] [PubMed] [Google Scholar]
- Krugel LK, et al. Genetic variation in dopaminergic neuromodulation influences the ability to rapidly and flexibly adapt decisions. Proc Natl Acad Sci USA. 2009;106:17951–17956. doi: 10.1073/pnas.0905191106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudielka B, et al. Why do we respond so differently? Reviewing determinants of human salivary cortisol responses to challenge. Psychoneuroendocrinology. 2009;34:2–18. doi: 10.1016/j.psyneuen.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Kuehner C, et al. PCLO rs 2522833 impacts HPA system activity in healthy young adults. Translational Psychiatry. 2011;1:e10. doi: 10.1038/tp.2011.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, et al. Abnormal temporal difference reward-learning signals in major depression. Brain. 2008;131:2084–2093. doi: 10.1093/brain/awn136. [DOI] [PubMed] [Google Scholar]
- Kuningas M, et al. Mental performance in old age dependent on cortisol and genetic variance in the mineralocorticoid and glucocorticoid receptors. Neuropsychopharmacology. 2007;32:1295–1301. doi: 10.1038/sj.npp.1301260. [DOI] [PubMed] [Google Scholar]
- Lee MR, et al. Functional polymorphism of the mu-opioid receptor gene (OPRM1) influences reinforcement learning in humans. PLoS ONE. 2011;6:e24203. doi: 10.1371/journal.pone.0024203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch KP, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Levinson DF. The genetics of depression: a review. Biol Psychiatry. 2006;60:84–92. doi: 10.1016/j.biopsych.2005.08.024. [DOI] [PubMed] [Google Scholar]
- Lewis CM, et al. Genome-wide association study of major recurrent depression in the U.K. population. Am J Psychiatry. 2010;167:949–957. doi: 10.1176/appi.ajp.2010.09091380. [DOI] [PubMed] [Google Scholar]
- Lighthall NR, et al. Gender differences in reward-related decision processing under stress. Soc Cogn Affect Neurosci. 2011 doi: 10.1093/scan/nsr026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou YJ, et al. Support for the involvement of the KCNK2 gene in major depressive disorder and response to antidepressant treatment. Pharmacogenet Genomics. 2009;19:735–741. doi: 10.1097/FPC.0b013e32832cbe61. [DOI] [PubMed] [Google Scholar]
- Loas G. Vulnerability to depression: a model centered on anhedonia. J Affect Disord. 1996;41:39–53. doi: 10.1016/0165-0327(96)00065-1. [DOI] [PubMed] [Google Scholar]
- Lohoff FW. Overview of the genetics of major depressive disorder. Curr Psychiatry Rep. 2010;12:539–546. doi: 10.1007/s11920-010-0150-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Duran NL, et al. Hypothalamic–pituitary–adrenal axis dysregulation in depressed children and adolescents: A meta-analysis. Psychoneuroendocrinology. 2009;34:1272–1283. doi: 10.1016/j.psyneuen.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-León S, et al. Meta-analyses of genetic studies on major depressive disorder. Mol Psychiatry. 2007;13:772–785. doi: 10.1038/sj.mp.4002088. [DOI] [PubMed] [Google Scholar]
- Lotta T, et al. Kinetics of human soluble and membrane-bound catechol O-methyltransferase: a revised mechanism and description of the thermolabile variant of the enzyme. Biochemistry. 1995;34:4202–4210. doi: 10.1021/bi00013a008. [DOI] [PubMed] [Google Scholar]
- Marquez C, et al. Responsiveness of the hypothalamic-pituitary-adrenal axis to different novel environments is a consistent individual trait in adult male outbred rats. Psychoneuroendocrinology. 2005;30:179–187. doi: 10.1016/j.psyneuen.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Martinez D, et al. The variable number of tandem repeats polymorphism of the dopamine transporter gene is not associated with significant change in dopamine transporter phenotype in humans. Neuropsychopharmacology. 2001;24:553–560. doi: 10.1016/S0893-133X(00)00216-5. [DOI] [PubMed] [Google Scholar]
- Mazure CM. Life stressors as risk factors in depression. Clinical Psychology-Science and Practice. 1998;5:291–313. [Google Scholar]
- McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: Links to socioeconomic status, health, and disease. Ann N Y Acad Sci. 2010;1186:190–222. doi: 10.1111/j.1749-6632.2009.05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehl PE. Hedonic capacity: some conjectures. Bulletin of the Menninger Clinic. 1975;39:295–307. [PubMed] [Google Scholar]
- Meyer-Lindenberg A, et al. Midbrain dopamine and prefrontal function in humans: interaction and modulation by COMT genotype. Nat Neurosci. 2005;8:594–596. doi: 10.1038/nn1438. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Weinberger DR. Intermediate phenotypes and genetic mechanisms of psychiatric disorders. Nat Rev Neurosci. 2006;7:818–827. doi: 10.1038/nrn1993. [DOI] [PubMed] [Google Scholar]
- Mill J, et al. Transient expression analysis of allelic variants of a VNTR in the dopamine transporter gene (DAT1) BMC Genet. 2005;6:3. doi: 10.1186/1471-2156-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muglia P, et al. Genome-wide association study of recurrent major depressive disorder in two European case-control cohorts. Mol Psychiatry. 2010;15:589–601. doi: 10.1038/mp.2008.131. [DOI] [PubMed] [Google Scholar]
- Munafo MR, et al. Serotonin transporter (5-HTTLPR) genotype and amygdala activation: a meta-analysis. Biol Psychiatry. 2008;63:852–857. doi: 10.1016/j.biopsych.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy D, et al. How the serotonin story is being rewritten by new gene-based discoveries principally related to SLC6A4, the serotonin transporter gene, which functions to influence all cellular serotonin systems. Neuropharmacology. 2008;55:932–960. doi: 10.1016/j.neuropharm.2008.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscatell KA, et al. Stressful life events, chronic difficulties, and the symptoms of clinical depression. Journal of Nervous and Mental Disease. 2009;197:154–160. doi: 10.1097/NMD.0b013e318199f77b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myerson A. Anhedonia. Am J Psychiatry. 1922;79:87–103. [Google Scholar]
- Nikolova YS, et al. Multilocus Genetic Profile for Dopamine Signaling Predicts Ventral Striatum Reactivity. Neuropsychopharmacology. 2011;36:1940–1947. doi: 10.1038/npp.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolova YS, et al. Perception of a naturalistic stressor interacts with 5-HTTLPR genotype and gender to impact reward responsiveness. Neuropsychobiol. 2012;65:45–54. doi: 10.1159/000329105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolova YS, et al. Ventral striatal reactivity to reward and recent life stress interact to predict postive affect. Biol Psychiatry. doi: 10.1016/j.biopsych.2012.03.014. in press. [DOI] [PubMed] [Google Scholar]
- Noble EP. D2 dopamine receptor gene in psychiatric and neurologic disorders and its phenotypes. Am J Med Genet B. 2003;116B:103–125. doi: 10.1002/ajmg.b.10005. [DOI] [PubMed] [Google Scholar]
- O'Doherty JP, et al. Temporal difference models and reward-related learning in the human brain. Neuron. 2003;38:329–337. doi: 10.1016/s0896-6273(03)00169-7. [DOI] [PubMed] [Google Scholar]
- Oei TI, et al. Anhedonia, suicide ideation and dexamethasone nonsuppression in depressed patients. J Psychiatr Res. 1990;24:25–35. doi: 10.1016/0022-3956(90)90022-i. [DOI] [PubMed] [Google Scholar]
- Opmeer EM, et al. Depression and the role of genes involved in dopamine metabolism and signalling. Prog Neurobiol. 2010;92:112–133. doi: 10.1016/j.pneurobio.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Ossewaarde L, et al. Two-week administration of the combined serotonin-noradrenaline reuptake inhibitor duloxetine augments functioning of mesolimbic incentive processing circuits. Biol Psychiatry. 2011;70:568–574. doi: 10.1016/j.biopsych.2011.03.041. [DOI] [PubMed] [Google Scholar]
- Pascucci T, et al. The Medial Prefrontal Cortex Determines the Accumbens Dopamine Response to Stress through the Opposing Influences of Norepinephrine and Dopamine. Cereb Cortex. 2007;17:2796–2804. doi: 10.1093/cercor/bhm008. [DOI] [PubMed] [Google Scholar]
- Pecina S, et al. Nucleus accumbens corticotropin-releasing factor increases cue-triggered motivation for sucrose reward: paradoxical positive incentive effects in stress? BMC Biol. 2006;4:8. doi: 10.1186/1741-7007-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelizza L, Ferrari A. Anhedonia in schizophrenia and major depression: state or trait? Ann Gen Psychiatry. 2009;8:22. doi: 10.1186/1744-859X-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlis RH, et al. Pharmacogenetic analysis of genes implicated in rodent models of antidepressant response: Association of TREK1 and treatment resistance in the STAR*)D Study. Neuropsychopharmacology. 2008;33:2810–2819. doi: 10.1038/npp.2008.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, et al. Stress, glucocorticoids, and mesencephalic dopaminergic neurons: a pathophysiological chain determining vulnerability to psychostimulant abuse. NIDA Research Monograph. 1996;163:277–299. [PubMed] [Google Scholar]
- Pintor L, et al. Corticotropin-releasing factor test in melancholic patients in depressed state versus recovery: A comparative study. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1027–1033. doi: 10.1016/j.pnpbp.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, et al. Increased perceived stress is associated with blunted hedonic capacity: potential implications for depression research. Behav Res Ther. 2007;45:2742–2753. doi: 10.1016/j.brat.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, et al. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am J Psychiatry. 2009;166:702–710. doi: 10.1176/appi.ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, et al. Reduced hedonic capacity in major depressive disorder: evidence from a probabilistic reward task. J Psychiatr Res. 2008;43:76–87. doi: 10.1016/j.jpsychires.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, et al. Toward an objective characterization of an anhedonic phenotype: a signal-detection approach. Biol Psychiatry. 2005;57:319–327. doi: 10.1016/j.biopsych.2004.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohjalainen T, et al. The A1 allele of the human D2 dopamine receptor gene predicts low D2 receptor availability in healthy volunteers. Mol Psychiatry. 1998;3:256–260. doi: 10.1038/sj.mp.4000350. [DOI] [PubMed] [Google Scholar]
- Polanczyk G, et al. Protective effect of CRHR1 gene variants on the development of adult depression following childhood maltreatment: replication and extension. Arch Gen Psychiatry. 2009;66:978–985. doi: 10.1001/archgenpsychiatry.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potkin SG, et al. Hippocampal atrophy as a quantitative trait in genome-wide association study identifying novel susceptiility genes for Alzheimer’s disease. PLoS ONE. 2009 A;4:e6501. doi: 10.1371/journal.pone.0006501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potkin SG, et al. A genome-wide association study of schizophrenia using brain activation as a quantitative phenotype. Schizophr Bull. 2009 B;35:96–108. doi: 10.1093/schbul/sbn155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, et al. Stress regulation in the central nervous system: evidence from structural and functional neuroimaging studies in human populations-2008 Curt Richter Award Winner. Psychoneuroendocrinology. 2010;35:179–191. doi: 10.1016/j.psyneuen.2009.02.016. [DOI] [PubMed] [Google Scholar]
- Purcell SM, et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 460:748–752. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Murillo L, et al. The genetic architecture of schizophrenia: Ne wmutations and emerging paradigms. Annu Rev Med. 2012;63:63–80. doi: 10.1146/annurev-med-072010-091100. [DOI] [PubMed] [Google Scholar]
- Risch N, et al. Interaction Between the Serotonin Transporter Gene (5-HTTLPR), Stressful Life Events, and Risk of Depression: A Meta-analysis. JAMA. 2009;301:2462–2471. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie T, Noble EP. Association of seven polymorphisms of the D2 dopamine receptor gene with brain receptor-binding characteristics. Neurochem Res. 2003;28:73–82. doi: 10.1023/a:1021648128758. [DOI] [PubMed] [Google Scholar]
- Robins E, Guze SB. Establoishment of diagnostic validity in psychiatric illness: Its application to schizophrenia. Am J Psychiatry. 1970;126:983–987. doi: 10.1176/ajp.126.7.983. [DOI] [PubMed] [Google Scholar]
- Roussos P, et al. Cognitive and emotional processing associated with the Season of Birth and dopamine D4 receptor gene. Neuropsychologia. 2010;48:3926–3933. doi: 10.1016/j.neuropsychologia.2010.09.021. [DOI] [PubMed] [Google Scholar]
- Rucker JJ, et al. Genome-wide association analysis of copy number variation in recurrent depressive disorder. Mol Psychiatry. 2011 doi: 10.1038/mp.2011.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salimpoor VN, et al. Anatomically distinct dopamine release during anticipation and experience of peak emotion to music. Nat Neurosci. 2011;14:257–262. doi: 10.1038/nn.2726. [DOI] [PubMed] [Google Scholar]
- Salvadore G, et al. Bcl-2 polymorphism influences gray matter volume in the ventral striatum in healthy humans. Biol Psychiatry. 2009;66:804–807. doi: 10.1016/j.biopsych.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanislow CA, et al. Developing constructs for psychopathology research: research domain criteria. J Abnorm Psychology. 2010;119:631–639. doi: 10.1037/a0020909. [DOI] [PubMed] [Google Scholar]
- Schacht JP, et al. Stability of fMRI striatal response to alcohol cues: a hierarchical linear modeling approach. Neuroimage. 2011;56:61–68. doi: 10.1016/j.neuroimage.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuhmacher A, et al. PCLO rs2522833 modulates HPA system response to antidepressant treatment in major depressive disorder. Int J Neuropsychopharmacology. 2011;14:237–245. doi: 10.1017/S1461145710000854. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Wolf OT. Stress-induced modulation of instrumental behavior: from goal-directed to habitual control of action. Behav Brain Res. 2011;219:321–328. doi: 10.1016/j.bbr.2010.12.038. [DOI] [PubMed] [Google Scholar]
- Shen L, et al. Whole genome association study of brain-wide imaging phenotypes for identifying quantitative trait loci in MCI and AD: A study of the ADNI cohort. Neuroimage. 2010;53:1051–1063. doi: 10.1016/j.neuroimage.2010.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, et al. Genome-wide association study of recurrent early-onset major depressive disorder. Mol Psychiatry. 2011;16:193–201. doi: 10.1038/mp.2009.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyn SI, et al. Novel loci for major depression identified by genome-wide association study of Sequenced Treatment Alternatives to Relieve Depression and meta-analysis of three studies. Mol Psychiatry. 2011;16:202–215. doi: 10.1038/mp.2009.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeraldi E, et al. Polymorphism within the promoter of the serotonin transporter gene and antidepressant efficacy of fluvoxamine. Mol Psychiatry. 1998;3:508–511. doi: 10.1038/sj.mp.4000425. [DOI] [PubMed] [Google Scholar]
- Stein JL, et al. Identification of common variants associated with human hippocampal and intracranial volumes. Nat Genet. 2012;44:552–561. doi: 10.1038/ng.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetler C, Miller GE. Depression and hypothalamic-pituitary-adrenal activation: a quantitative summary of four decades of research. Psychosomatic Med. 2011;73:114–126. doi: 10.1097/PSY.0b013e31820ad12b. [DOI] [PubMed] [Google Scholar]
- Stice E, et al. Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science. 2008;322:449–452. doi: 10.1126/science.1161550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoy M, et al. Hyporeactivity of ventral striatum towards incentive stimuli in unmedicated depressed patients normalizes after treatment with escitalopram. J Psychopharmacol. 2011 doi: 10.1177/0269881111416686. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, et al. Genome-wide association for major depressive disorder: a possible role for the presynaptic protein piccolo. Mol Psychiatry. 2009;14:359–375. doi: 10.1038/mp.2008.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PF, et al. Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry. 2000;157:1552–1562. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- Taber MT, et al. Cortical regulation of subcortical dopamine release: mediation via the ventral tegmental area. Journal of Neurochemistry. 1995;65:1407–1410. doi: 10.1046/j.1471-4159.1995.65031407.x. [DOI] [PubMed] [Google Scholar]
- Thode K, et al. Functional effects of polymorphisms in the human corticotropin-releasing hormone receptor 1 (CRHR1) gene; Poster presented at the Annua Meeting of the American Psychiatric Association; 2009. [Google Scholar]
- Treadway MT, Zald DH. Reconsidering anhedonia in depression: Lessons from translational neuroscience. Neurosci Biobehav Rev. 2011;35:537–555. doi: 10.1016/j.neubiorev.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi MH, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: Implications for clinical practice. Am J Psychiatry. 2006;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- Tyrka AR, et al. Interaction of Childhood Maltreatment with the Corticotropin-Releasing Hormone Receptor Gene: Effects on Hypothalamic-Pituitary-Adrenal Axis Reactivity. Biological Psychiatry. 2009;66:681–685. doi: 10.1016/j.biopsych.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM, et al. Pleasurable behaviors reduce stress via brain reward pathways. Proc Natl Acad USA. 2010;107:20529–20534. doi: 10.1073/pnas.1007740107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry HL, et al. Amygdala and Ventromedial Prefrontal Cortex Are Inversely Coupled during Regulation of Negative Affect and Predict the Diurnal Pattern of Cortisol Secretion among Older Adults. J Neurosci. 2006;26:4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursini G, et al. Stress-Related Methylation of the Catechol-O-Methyltransferase Val158 Allele Predicts Human Prefrontal Cognition and Activity. J Neurosci. 2011;31:6692–6698. doi: 10.1523/JNEUROSCI.6631-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag HM, et al. Stress, the brain and depression. New York: Cambridge University Press, Cambridge, UK; 2004. p. 283. pp. xii. [Google Scholar]
- Villafuerte S, et al. Impulsiveness and insula activation during reward anticipation are associated with genetic variants in GABRA2 in a family sample enriched for alcoholism. Mol Psychiatry. 2011 doi: 10.1038/mp.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, et al. Testing a tripartite model: II. Exploring the symptom structure of anxiety and depression in student, adult, and patient samples. J Abnorm Psychol. 1995;104:15–25. doi: 10.1037//0021-843x.104.1.15. [DOI] [PubMed] [Google Scholar]
- Watson KK, et al. Serotonin transporter genotype modulates social reward and punishment in rhesus macaques. PLoS ONE. 2009;4:e4156. doi: 10.1371/journal.pone.0004156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner O, Anhedonia . In: Symptoms of depression. Costello CG, editor. NY: John Wiley & Sons, Inc.; 1993. [Google Scholar]
- Willner P. Chronic Mild Stress (CMS) Revisited: Consistency and Behavioural-Neurobiological Concordance in the Effects of CMS. Neuropsychobiol. 2005;52:90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- Wise RA. Catecholamine theories of reward: a critical review. Brain Res. 1978;152:215–247. doi: 10.1016/0006-8993(78)90253-6. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dopamine and reward: the anhedonia hypothesis 30 years on. Neurotox Res. 2008;14:169–183. doi: 10.1007/BF03033808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. International statistical classification of diseases and related health problems. Geneva: World Health Organization; 2004. [Google Scholar]
- Yacubian J, et al. Gene-gene interaction associated with neural reward sensitivity. Proc Natl Acad Sci USA. 2007;104:8125–8130. doi: 10.1073/pnas.0702029104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R. Post-traumatic stress disorder. N Engl J Med. 2002;346:108–114. doi: 10.1056/NEJMra012941. [DOI] [PubMed] [Google Scholar]
- Zhang TY, Meaney MJ. Epigenetics and the environmental regulation of the genome and its function. Annu Rev Psychol. 2010;61:439–466. doi: 10.1146/annurev.psych.60.110707.163625. C1-3. [DOI] [PubMed] [Google Scholar]
- Zieba B, et al. The behavioural and electrophysiological effects of CRF in rat frontal cortex. Neuropeptides. 2008;42:513–523. doi: 10.1016/j.npep.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Zimmermann P, et al. Interaction of FKBP5 Gene Variants and Adverse Life Events in Predicting Depression Onset: Results From a 10-Year Prospective Community Study. Am J Psychiatry. 2011;168:1107–1116. doi: 10.1176/appi.ajp.2011.10111577. [DOI] [PMC free article] [PubMed] [Google Scholar]