Abstract
Studies suggest that within-person changes in estrogen and progesterone predict changes in binge eating across the menstrual cycle. However, samples have been extremely small (maximum N = 9), and analyses have not examined the interactive effects of hormones that are critical for changes in food intake in animals. The aims of the current study were to examine ovarian hormone interactions in the prediction of within-subject changes in emotional eating in the largest sample of women to date (N = 196). Participants provided daily ratings of emotional eating and saliva samples for hormone measurement for 45 consecutive days. Results confirmed that changes in ovarian hormones predict changes in emotional eating across the menstrual cycle, with a significant estradiol x progesterone interaction. Emotional eating scores were highest during the mid-luteal phase, when progesterone peaks and estradiol demonstrates a secondary peak. Findings extend previous work by highlighting significant interactions between estrogen and progesterone that explain mid-luteal increases in emotional eating. Future work should explore mechanisms (e.g., gene-hormone interactions) that contribute to both within- and between-subject differences in emotional eating.
Recent studies implicate ovarian hormones in risk for disordered eating symptoms (Edler, Lipson, & Keel, 2007; Klump, Culbert, Edler, & Keel, 2008; Lester, Keel, & Lipson, 2003; Racine et al., in press). These investigations have primarily focused on binge eating, given an extensive animal literature demonstrating that experimental manipulations of ovarian hormones cause changes in food intake (Asarian & Geary, 2006). For example, animal studies show significant inverse associations between estrogen and food intake, such that ovariectomy of female animals causes immediate and sustained increases in food intake (Asarian & Geary, 2006). Estradiol treatment reverses these effects (Asarian & Geary, 2006; Kemnitz et al., 1984; Kemnitz, Gibber, Lindsey, & Eisele, 1989). Progesterone appears to antagonize the effects of estrogen, as it has few direct effects on food intake in ovariectomized animals when administered alone (Asarian & Geary, 2006; Wade, 1972), but it attenuates changes in intake caused by exogenous estradiol in ovariectomized animals (Asarian & Geary, 2006; Kemnitz et al., 1989) and it causes increased food intake in intact rats with naturally circulating estradiol (Wade, 1972). Although more research is needed to identify the precise mechanisms of progesterone's effects, findings thus far suggest that progesterone's actions are contingent upon the presence of estrogen, and that progesterone's primary influence is to antagonize the effects of either endogenous or exogenous estrogen on food intake.
Research on hormone effects in animal models of binge eating is still in its infancy, but two rat studies found that ovariectomy specifically increases palatable food intake (Klump et al., 2011b) and administration of a high estradiol/low progesterone treatment decreases intake of high fat food (Yu, Geary, & Corwin, 2008). Early attempts to model these same effects in humans examined differential rates of food intake, binge eating, and emotional eating (i.e., the tendency to eat excessive amounts of food for reasons that are typically endorsed by binge eaters) across menstrual cycle phases characterized by high estrogen (e.g., the follicular/ovulatory phases) versus high progesterone and high estrogen (e.g., the mid-luteal phase) levels. Results consistently supported increased food intake, emotional eating, and binge eating during the mid-luteal phase as compared to the follicular/ovulatory phase (Buffenstein, Poppitt, McDevitt, & Prentice, 1995; Edler et al., 2007; Gladis & Walsh, 1987; Klump et al., 2008; Lester et al., 2003; Price, Torem, & DiMarzio, 1987). Two pilot studies have directly assessed ovarian hormones and found that decreases in estradiol, and increases in progesterone, predict increases in emotional eating (Klump et al., 2008) and binge eating (Edler et al., 2007) across the menstrual cycle. Hormone/binge eating associations were independent of body weight and daily changes in negative affect and were observed for binge episodes in women with bulimia nervosa (BN) (Edler et al., 2007) and emotional eating scores in a non-clinical sample of women (Klump et al., 2008). Thus, they appeared to be present across the full spectrum of dysregulated eating behavior.
Nonetheless, significant limitations of past studies highlight the need for replication. Most importantly, sample sizes in studies utilizing hormone assessments were very small (maximum N = 9). In addition, these studies did not model the interactive effects of hormones on binge/emotional eating risk or confirm that the direction of association is from changes in hormones to changes in binge/emotional eating (rather than the reverse). Animal data strongly suggest that ovarian hormone effects are interactive and cause changes in food intake (Asarian & Geary, 2006). Because menstrual cycle changes in estrogen and progesterone are dictated by the reproductive system, changes in hormones that predict changes in binge and emotional eating should reflect the impact of ovarian hormones on these behaviors rather than the reverse. However, translational studies that model the direction of effects and interactive processes are needed to confirm an etiologic role for ovarian hormones in binge eating and emotional eating risk.
The goal of the current study was to examine within-person associations between changes in hormones and emotional eating across the menstrual cycle in a large, community-based sample of women. We examined the main effects of estrogen and progesterone, as well as their interaction, to determine whether the complex associations observed in animals are present in humans. We analyzed models in which changes in hormones predicted changes in emotional eating, as well as models in which changes in emotional eating predicted changes in hormones. We controlled for body weight and negative affect to ensure that hormone effects were independent of these third variables. We focused on emotional eating rather than binge eating since full binge episodes would be relatively uncommon in our community-based sample, and pilot studies show similar hormone/behavior relationships when analyzing binge frequency in women with BN (Edler et al., 2007) and emotional eating in community women (Klump et al., 2008).
Methods
Participants
Participants were 196 (110 monozygotic (MZ), 86 dizygotic (DZ)) female twins ages 16-25 (M = 17.78, SD = 1.60) drawn from the Twin Study of Hormones and Behavior across the Menstrual Cycle project from the Michigan State University Twin Registry (MSUTR; N ~ 18,000 twins). The MSUTR recruits twins through birth records using methods described previously (Klump & Burt, 2006). Prior (Culbert, Breedlove, Burt, & Klump, 2008) and current analyses indicate that MSUTR twins are demographically representative of the recruitment region (89% Caucasian; 11% African American).
Inclusion/exclusion criteria ensured that we captured natural variations in hormones: 1) menstruation every 22-32 days for the past 6 months; 2) no hormonal, psychotropic or steroid medications within the past 8 weeks; 3) no pregnancy or lactation within the past 6 months; and 4) no history of genetic or medical conditions known to influence hormone functioning or appetite/weight. Despite these criteria, disordered eating scores (e.g., emotional eating, body dissatisfaction) did not differ between our participants and other MSUTR twins (d`s = .02-.18).
Procedures
Participants collected data for 45 consecutive days. Salivary samples were collected every morning within 30 minutes of waking using published methods (Klump et al., 2008). Behavioral ratings were made each evening (after 5:00 pm) using an on-line data collection system or pre-printed scantrons. The timing of data collection ensured that a given day's hormone collection preceded that day's behavioral ratings.
Three in-person visits occurred at the start of the study, mid-way through data collection (~day 23), and at the end of data collection (~day 45). Each visit included a re-assessment of study eligibility, participant height/weight measurements, and collection of samples. Between visits, staff contacted participants 1x/week to answer questions and confirm protocol adherence. These procedures were effective for minimizing drop-outs (3%) and missing data (≤ 6%) and identifying twins who were no longer eligible (3% due to pregnancy or medication use).
Measures
Emotional Eating
Emotional eating was assessed with the Dutch Eating Behavior Questionnaire (DEBQ) (Van Strien, Frijters, Bergers, & Defares, 1986). The DEBQ emotional eating subscale assesses the tendency to eat in response to negative emotions (e.g., “Did you have a desire to eat when you were discouraged?”). This scale was used in the previous community study of ovarian hormones/binge eating (Klump et al., 2008) because it exhibits robust fluctuations across the menstrual cycle (Klump et al., 2008) and is significantly correlated with traditional binge measures (e.g., r's = .55 - .61 with the bulimia scale of the Eating Disorders Inventory) (Van Strien, 1996, 2000) as well as palatable food intake (e.g., ice cream) in the laboratory (van Strien, 2000). Subscale scores also exhibit expected patterns of differential elevations across normal and abnormal groups, where the highest scores are observed in women with BN, followed by overweight women, then normal weight women, and then women with anorexia nervosa (Wardle, 1987). Similar to previous research (Klump et al., 2008), instructions were modified with permission to refer to that day (i.e., “true in relation to you TODAY”). Internal consistency of our modified version was excellent (average α = .90).
Negative Affect
The Negative Affect scale from the Positive and Negative Affect Schedule (Watson, Clark, & Tellegen, 1988) was used to measure negative affect (i.e., depression and anxiety) each day. This scale exhibits good convergent and discriminant validity (Watson et al., 1988) and excellent internal consistency (average α = .85 in our sample).
Hormones
Hormone concentrations were measured in saliva. Saliva collection is a non-invasive method for repeated sampling schedules that is associated with higher compliance and more robust hormone-behavior associations than bloodspots (Edler et al., 2007).
Salivary assays were conducted by Salimetrics, LLC (State College, PA) using enzyme immunoassay kits that show excellent intra- and inter-assay coefficients of variation (estradiol = 7.1 % and 7.5%; progesterone = 6.2% and 7.6%), assay sensitivity (determined by interpolating the mean optical density minus 2 SDs of 10-20 replicates at the 0 pg/mL level; estradiol = 0.10 pg/mL; progesterone = 5 pg/mL) and method accuracy (determined by spike recovery and linearity; estradiol = 104.2% and 99.4%; progesterone = 99.6% and 91.8%). In order to capture key periods of hormonal change (e.g., mid-late follicular through the premenstrual phase) while maximizing the number of subjects assessed, we assayed samples every other day during menstrual bleeding and the early follicular phase when hormone levels were expected to be low and stable.
Body Mass Index (BMI)
BMI (weight (in kilograms)/height (in meters)2) was calculated using height and weight measured during lab assessments with a wall-mounted ruler and digital scale, respectively.
Statistical Analyses
Data Preparation
Analyses of within-person associations between changes in ovarian hormones and emotional eating were modeled after those used by Edler et al. (2007) and Klump et al. (2008). Five-day rolling averages were calculated for hormones, emotional eating, negative affect, and BMI. Rolling averages reduce the influence of hormone pulsatility and increase the signal to noise ratio by minimizing random environmental variations (e.g., decreased opportunities to binge eat) (Gladis & Walsh, 1987; Kassam et al., 1996; Waller et al., 1998). Because BMI was assessed at only three time points, rolling averages were calculated using visit 1 BMI for days in-between visits 1 and 2, visit 2 BMIs for days in-between visits 2 and 3, and visit 3 BMI for the last assessment day (day 45). Changes in weight across the study period were minimal (M = -0.20 lb change, SD = 3.39).
We then converted all of the rolling averages into within-person Z scores based upon each participant's overall mean and standard deviation across data collection. Previous studies (Edler et al., 2007; Gladis & Walsh, 1987; Klump et al., 2008; Lester et al., 2003) have focused on standardized values because they index the extent to which changes in a woman's hormones, relative to her equilibrium, predict changes away from her equilibrium in emotional eating. They also tend to be sensitive to small deviations from equilibria, making them ideal indicators of how within-person changes in hormones predict changes in emotional eating in non-clinical samples of women.
Missing data were handled two ways. We prorated raw scores for behavioral measures when ≤10% of the items were missing, and coded the scores as missing if > 10% of items were missing. We then calculated the behavioral and hormone rolling averages if ≥ 3 days of data were available within the 5-day window. If fewer than 3 days were available, then the average for that day was coded as missing (occurring < 2.6% of the time).
Predictive Associations
We used mixed linear models (MLMs) to examine cycle phase and hormone influences on emotional eating. MLMs are ideal for these analyses as we could examine predictive associations while controlling for covariates (i.e., negative affect and BMI) and the non-independence of the repeated measures and twin data. We controlled for the non-independence of the twin data by estimating random intercepts and allowing them to correlate.1 Because most co-twins were assessed across the same 45-day period, we also estimated a time-specific dyadic correlation that allowed the twins’ residual scores for emotional eating to correlate from day-to-day. For each of these random effects, we specified a compound symmetry covariance structure which estimates a single intercept variance or a single residual variance across twins and time. The models also allowed for random slopes for each of the predictors (i.e., hormones, negative affect, BMI). However, because there was no evidence that these slopes correlated across twins, we fixed the cross-twin correlation to zero for all predictors.
We first used the MLMs to replicate previous findings of higher emotional eating scores during the mid-luteal phase. Cycle phase (i.e., follicular, ovulatory, mid-luteal, premenstrual) was coded based on dates of menstrual bleeding and increases/decreases in hormones (see Edler et al. (2007) for coding methods). Potential phase differences were examined by including phase as a predictor of emotional eating in the MLMs.
We then used MLMs to examine within-person associations between changes in hormones and changes in emotional eating. We first fit a model that tested only the main effects of estradiol, progesterone, and the covariates (i.e., negative affect and BMI). We then fit a second model that included the estradiol x progesterone interaction in addition to all main effects. In order to confirm the direction of association, we fit a third model where changes in emotional eating predicted changes in hormones.
Results
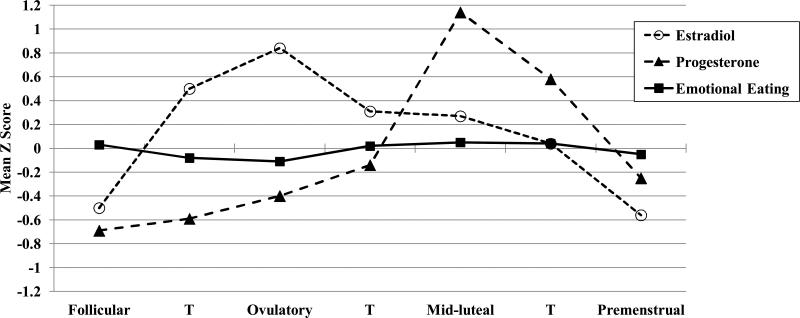
Fluctuations in emotional eating across the menstrual cycle were somewhat more modest than those observed in a clinical sample (Edler et al., 2007), but they nevertheless replicated prior work showing the highest emotional eating scores during the mid-luteal phase and the lowest scores during the ovulatory phase (see Figure 1). MLMs confirmed the presence of a significant phase effect (F(3,5890) = 6.57, p < .001), where emotional eating levels were significantly higher in the mid-luteal as compared to the ovulatory (t(2973) = 4.32, p < .001) and premenstrual (t(2779) = 3.11, p = .002). No significant differences in emotional eating were observed between the mid-luteal phase and follicular phases (t(4168) = 0.52, p = .61), although scores appeared to be higher in the mid-luteal phase.
Figure 1. Emotional Eating, Estradiol, and Progesterone across the Menstrual Cycle.
Mean Z Score = the mean of the 5-day rolling averages calculated within subjects, then averaged across participants; T = transition days that are in-between phases. Mean values within each phase are included for descriptive purposes only, as the daily z scores were included in the mixed linear models for each phase contrast. Mean values for emotional eating z scores (with non-z scored, within-person centered means in parentheses) in each phase are: Follicular: M = 0.03 (.001), SD = 1.11, Ovulatory: M = -0.11 (-.02), SD = 0.98; Mid-luteal: M = 0.05 (.01), SD = 0.93; Premenstrual: M = -0.05 (-.003), SD = 0.82. The number of days included in each phase varied by participant based on their cycle length, but the days roughly corresponded to the following (first day of menstrual bleeding = +1; previous day = -1): Follicular = +3 to +12; Ovulatory = -15 to -12; Mid-luteal = -9 to -5; Premenstrual = -3 to +1.
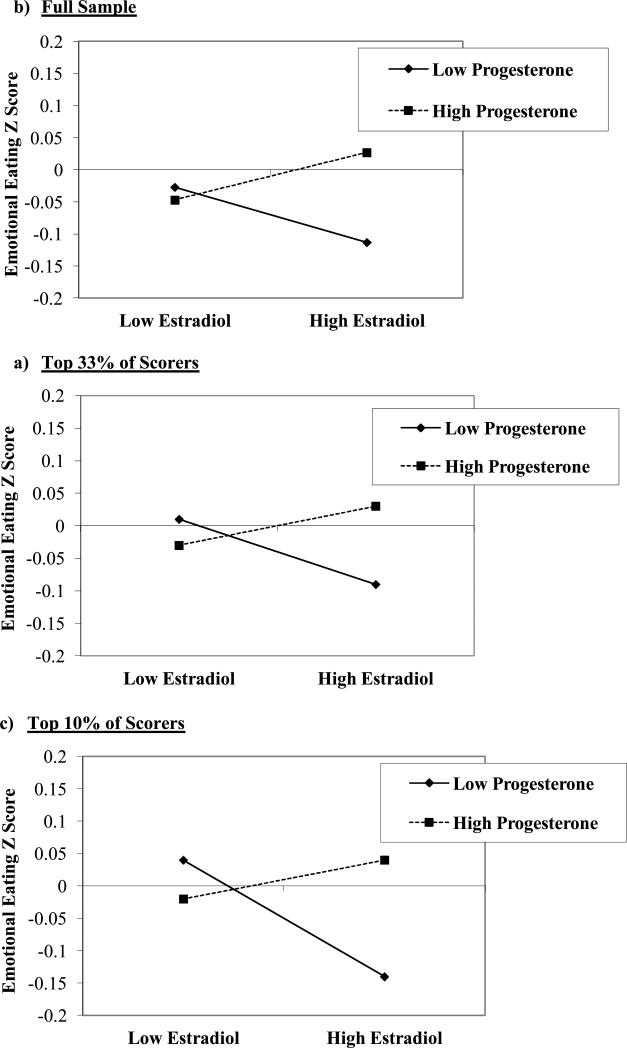
Data in Figure 1 further suggest that changes in ovarian hormones account for these phase differences, as changes in emotional eating appeared to follow changes in hormones. In the main effects model, there was a trend (p = .10) for higher levels of progesterone to predict higher emotional eating scores, even after controlling for the significant effects of both negative affect and BMI (see Table 1). This trend, however, became weaker (p = .20) when the estradiol x progesterone interaction was included. This interaction was significantly different from zero (p = .007) and suggested that within-person changes in estradiol and progesterone interact to influence changes in emotional eating. Using the MLM beta weights (see methods in (Dawson & Richter, 2006), we graphed the estradiol x progesterone interaction and showed that emotional eating scores are elevated when both progesterone and estradiol levels are high (Figure 2). These findings map on to phase analyses showing the highest emotional eating scores during the mid-luteal phase - a phase characterized by the highest progesterone levels of the cycle and a secondary peak in estradiol. Results also corroborated animal work in intact rats suggesting that progesterone causes increased food intake in the presence of estradiol (Wade, 1972); in our data, progesterone had little-to-no effect on emotional eating when estradiol levels were low, but at high estradiol levels, there was a pronounced effect of progesterone on emotional eating. This would explain why emotional eating scores are not higher during the ovulatory phase when estradiol peaks, as progesterone is low during ovulation and is unable to counteract the protective effects of estradiol on emotional eating.
Table 1.
Predictive Associations between Hormones and Emotional Eating.
| Model | b (SE) | t | df | p |
|---|---|---|---|---|
| Main Effects: | ||||
| Intercept | -.03 (.01) | -2.56 | 98 | .01 |
| Estradiol | -.01 (.02) | -0.42 | 204 | .62 |
| Progesterone | .04 (.02) | 1.64 | 206 | .10 |
| Negative Affect | .19 (.03) | 7.48 | 198 | <.001 |
| Body Mass Index (BMI) | .07 (.03) | 2.19 | 190 | .03 |
| Interaction: | ||||
| Intercept | -.04 (.01) | -2.93 | 98 | .004 |
| Estradiol | -.002 (.02) | -0.08 | 207 | .93 |
| Progesterone | .03 (.02) | 1.29 | 209 | .20 |
| Estrogen × Progesterone | .04 (.02) | 2.72 | 198 | .007 |
| Negative Affect | .19 (.03) | 7.42 | 198 | <.001 |
| BMI | .07 (.03) | 2.06 | 189 | .04 |
Figure 2. Interactions between Estradiol and Progesterone in the Prediction of Emotional Eating in the a) Full Sample, b) Top 33% of Scorers, and c) Top 10% of Scorers.
“Emotional Eating Z Score” = 5-day rolling average calculated within subjects, then averaged across participants.
Models examining changes in emotional eating as predictors of hormones supported the hypothesis that the direction of effects is from the hormones to emotional eating rather than the reverse, as emotional eating was not a significant predictor of estradiol (b = -.003 (.02), t(197) = -0.10, p = .92) or progesterone (b = .03 (1.02), t(194) = 1.02, p = .31). To ensure that the ordering of our hormone and emotional eating assessments (i.e., hormones collected each morning; behavioral ratings made each evening) did not influence our results, we re-ran the models using 1-day time lagged scores to examine whether emotional eating on previous night predicted changes in hormones the next morning. The effect of emotional eating was consistently non-significant in these models (p's > .10).
Because effects were more modest than observed previously in clinical samples (Elder et al., 2007), we conducted post hoc analyses examining whether hormone/emotional eating associations are stronger in more disturbed groups (i.e.,, the top 33% (N = 82) and 10% (N = 23) of emotional eating scorers). Despite smaller sample sizes, patterns were nearly identical, with the estradiol x progesterone interaction (top 33%: b = .04 (.02), t(71) = 1.60, p = .11; top 10% group: b = .06 (.04), t(471) = 1.65, p = .09) exerting a stronger influence on emotional eating scores than the hormone main effects (p's ≥ .30). Interactions were strongest in the top 10% group where the beta was 33% higher than in the full sample, and more pronounced differences were observed between high versus low progesterone levels (see Figure 1). The largest differences were still observed at high estradiol levels, although group differences were increased at low estradiol levels as well, where emotional eating scores were highest when progesterone levels were low. These findings again highlight the interactive effects of progesterone and estrogen, as progesterone's influence is limited to times when progesterone is high and estrogen is elevated as well.
Discussion
This is the largest study to date examining within-person changes in ovarian hormones and dysregulated eating across the menstrual cycle. Results replicated previous work suggesting elevated emotional eating scores during the mid-luteal phase and positive associations between changes in progesterone and emotional eating. More importantly, findings extended previous work by showing that interactions between estradiol and progesterone are the strongest predictors of within-subject changes in emotional eating. These interactive effects predicted increased emotional eating during hormonal milieus characterized by high progesterone and high estradiol levels in the full sample as well as more extreme groups.
Observed hormone effects were not due to BMI or changing levels of negative affect, and the direction of association was from changes in hormones to changes in emotional eating. These findings are significant in suggesting potential causal associations between hormonal changes across the reproductive cycle and emotional eating. Moving forward, it will be important to confirm causal influences using a combination of animal and human research. Human research could track changes in binge/emotional eating in response to exogenously administered hormones, and animal studies could provide “purer” tests of hormone effects by administering hormones to ovariectomized animals. In both sets of studies, it will be critical to model the interactive effects of estrogen and progesterone on binge eating risk using physiologic doses of both estradiol and progesterone (Yu et al., 2008) to replicate and extend our work .
Associations between hormones and emotional eating were somewhat smaller than those observed in women with BN (Edler et al., 2007). Our effect sizes were more on par with Klump et al.'s (2008) study of community women that found small-to-moderate associations between emotional eating scores, estradiol, and progesterone levels. Stronger effects in women with BN than community samples might be due to the higher frequency and greater severity of binge eating in clinical samples. Alternatively, differences in binge eating constructs (i.e., binge episodes in women with BN; emotional eating in community samples) could contribute to differential strengths of association. Although our emotional eating scale correlates with traditional binge eating measures and palatable food intake (see Methods), scores on this dimensional measure do not assess binge episodes per se. Importantly, however, we obtained larger effect sizes when we examined our most extreme group, suggesting that differences in the severity of dysregulated eating may account for differences in the size of effects between clinical and community samples.
Interestingly, the differential strength of hormone/eating associations across levels of severity may point to key etiological mechanisms. In particular, they suggest the possible presence of gene-environment interactions in which changes in ovarian hormones predict changes in binge/emotional eating in women who are genetically vulnerable to these extreme phenotypes. This interpretation would help account for the fact that all women who are ovulating experience changes in hormones across the menstrual cycle, but only some develop full-blown binge eating. One primary function of ovarian hormones is to regulate gene transcription of several neurobiological systems disrupted in binge eating (e.g., serotonin) (Ostlund, Keller, & Hurd, 2003; Wilson, Foster, Kronenberg, & Larsen, 1998). Data also suggest significant brain changes (e.g., increased grey matter volume) across the menstrual cycle (Hagemann et al., 2011) that correlate with changes in behavior (e.g., food intake, reward processing) and are thought to be due to the genomic effects of hormones (Protopopescu et al., 2005; Van Vugt, 2010). Given our findings, it may be that associations between menstrual cycle changes in hormones and binge/emotional eating reflect hormone-induced changes in gene transcription in women who are genetically vulnerable to binge eating phenotypes. These genetic changes would become prominent only after hormonal activation at puberty and the onset of menstrual cycles - developmental periods that are associated with both increased phenotypic (Bulik, 2002; Klump et al., 2011a) as well as genetic (Culbert, Burt, McGue, Iacono, & Klump, 2009; Klump, Perkins, Burt, McGue, & Iacono, 2007) risk for binge eating.
Clearly, these hypotheses are speculative, as no studies have examined hormone-induced changes in genetic effects for binge eating or emotional eating. But they represent one set of possible explanations for our phenotypic findings and innovative hypotheses to be examined in future research. This work will need to determine when and for whom ovarian hormones are genetic risk factors for emotional eating by examining whether genetic risk predicts when a vulnerable woman will binge eat across her menstrual cycle as well as who is more likely to binge eat. Differentiation between the when and who will be critical for understanding both maintenance (i.e., mechanisms that explain when a woman is more likely to engage in emotional eating or binge eating) as well as etiologic mechanisms (i.e., factors predicting who is more likely to begin engaging in binge eating) that have important clinical implications. For example, our data and those of others (Edler et al., 2007; Lester et al., 2003) provide important information about when a woman is significantly more likely to binge eat or engage in emotional eating episodes (i.e., during the mid-luteal phase of her cycle). In women who are cycling, this high risk period will occur very regularly (i.e., monthly) and should therefore be an important psychoeducational component of interventions for women with binge eating episodes as well as prevention campaigns aimed at increasing awareness of risk and triggers for dysregulated eating. However, new data on “when” and “who” mechanisms is also needed in order to identify new treatment targets (e.g., hormonal supplements in depression) (Benmansour, Weaver, Barton, Adeniji, & Frazer, 2012; Studd, 2011) and high-risk groups with the potential to significantly increase the effectiveness of intervention and prevention programs for a range of dysregulated eating.
Acknowledgements
This research was supported by grants from the National Institute of Mental Health (NIMH) (R01 MH082054) (KLK, PKK, SAB, MN, CLS, SB) and the Canadian Institute for Health Research (MDR-96630) (SER). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIMH.
Footnotes
To further ensure that our use of twins did not unduly influence results, we conducted analyses in samples of MZ and DZ twins only. The pattern of results was identical to that in the full sample (data not shown).
References
- Asarian L, Geary N. Modulation of appetite by gonadal steroid hormones. Philosophical Transactions of the Royal Society of London. Series B. Biological Sciences. 2006;361(1471):1251–1263. doi: 10.1098/rstb.2006.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benmansour S, Weaver RS, Barton AK, Adeniji OS, Frazer A. Comparison of the effects of estradiol and progesterone on serotonergic function. Biological Psychiatry. 2012;71(7):633–641. doi: 10.1016/j.biopsych.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffenstein R, Poppitt SD, McDevitt RM, Prentice AM. Food intake and the menstrual cycle: A retrospective analysis, with implications for appetite research. Physiology and Behavior. 1995;58(6):1067–1077. doi: 10.1016/0031-9384(95)02003-9. [DOI] [PubMed] [Google Scholar]
- Bulik CM. Eating disorders in adolescents and young adults. Child and Adolescent Psychiatric Clinics. 2002;11:201–218. doi: 10.1016/s1056-4993(01)00004-9. [DOI] [PubMed] [Google Scholar]
- Culbert KM, Breedlove SM, Burt SA, Klump KL. Prenatal hormone exposure and risk for eating disorders: A comparison of opposite- and same-sex twins. Archives of General Psychiatry. 2008;65(3):329–336. doi: 10.1001/archgenpsychiatry.2007.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbert KM, Burt SA, McGue M, Iacono WG, Klump KL. Puberty and the genetic diathesis of disordered eating. Journal of Abnormal Psychology. 2009;118:201–218. doi: 10.1037/a0017207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson JF, Richter AW. Probing three-way interactions in moderated multiple regression: Development and application of a slope difference test. Journal of Applied Psychology. 2006;91:917–926. doi: 10.1037/0021-9010.91.4.917. [DOI] [PubMed] [Google Scholar]
- Edler C, Lipson SF, Keel PK. Ovarian hormones and binge eating in bulimia nervosa. Psychological Medicine. 2007;37(1):131–141. doi: 10.1017/S0033291706008956. [DOI] [PubMed] [Google Scholar]
- Gladis MM, Walsh BT. Premenstrual exacerbation of binge eating in bulimia. American Journal of Psychiatry. 1987;144(12):1592–1595. doi: 10.1176/ajp.144.12.1592. [DOI] [PubMed] [Google Scholar]
- Hagemann G, Ugur T, Schleussner E, Mentzel HJ, Fitzek C, Witte OW, et al. Changes in brain size during the menstrual cycle. PloS one. 2011;6(2):14655. doi: 10.1371/journal.pone.0014655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassam A, Overstreet JW, Snow-Harter C, De Souza MJ, Gold EB, Lasley BL. Identification of anovulation and transient luteal function using a urinary pregnanediol-3-glucuronide ration algorithm. Environmental Health Perspectives. 1996;104(4):408–413. doi: 10.1289/ehp.96104408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemnitz JW, Eisele SG, Lindsay KA, Engle MJ, Perelman RH, Farrell PM. Changes in food intake during menstrual cycles and pregnancy of normal and diabetic rhesus monkeys. Diabetologia. 1984;26:60–64. doi: 10.1007/BF00252265. [DOI] [PubMed] [Google Scholar]
- Kemnitz JW, Gibber JR, Lindsey KA, Eisele SG. Effects of ovarian hormones on eating behavior, body weight, and glucoregulation in rhesus monkeys. Hormones and Behavior. 1989;23:235–250. doi: 10.1016/0018-506x(89)90064-0. [DOI] [PubMed] [Google Scholar]
- Klump KL, Burt SA. The Michigan State University Twin Registry (MSUTR): Genetic, environmental and neurobiological influences on behavior across development. Twin Research and Human Genetics. 2006;9(6):971–977. doi: 10.1375/183242706779462868. [DOI] [PubMed] [Google Scholar]
- Klump KL, Culbert KM, Edler C, Keel PK. Ovarian hormones and binge eating: Exploring associations in community samples. Psychological Medicine. 2008;38(12):1749–1757. doi: 10.1017/S0033291708002997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, Perkins P, Burt SA, McGue M, Iacono WG. Puberty moderates genetic influences on disordered eating. Psychological Medicine. 2007;37:627–634. doi: 10.1017/S0033291707000189. [DOI] [PubMed] [Google Scholar]
- Klump KL, Suisman JL, Culbert KM, Kashy DA, Keel PK, Sisk CL. Binge eating proneness emerges during puberty in female rats: A longitudinal study. Journal of Abnormal Psychology. 2011a;120(4):948–955. doi: 10.1037/a0023600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, Suisman JL, Culbert KM, Kashy DA, Keel PK, Sisk CL. The effects of ovariectomy on binge eating proneness in adult female rats. Hormones and Behavior. 2011b;59(4):585–593. doi: 10.1016/j.yhbeh.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester NA, Keel PK, Lipson SF. Symptom fluctuation in bulimia nervosa: Relation to menstrual-cycle phase and cortisol levels. Psychological Medicine. 2003;33(1):51–60. doi: 10.1017/s0033291702006815. [DOI] [PubMed] [Google Scholar]
- Ostlund H, Keller E, Hurd YL. Estrogen receptor gene expression in relation to neuropsychiatric disorders. Annals of the New York Academy of Sciences. 2003;1007:54–63. doi: 10.1196/annals.1286.006. [DOI] [PubMed] [Google Scholar]
- Price WA, Torem MS, DiMarzio LR. Premenstrual exacerbation of bulimia. Psychosomatics. 1987;28(7):378–379. doi: 10.1016/s0033-3182(87)72511-0. [DOI] [PubMed] [Google Scholar]
- Protopopescu X, Pan H, Altemus M, Tuescher O, Polanecsky M, McEwen B, et al. Orbitofrontal cortext activity related to emotional processing changes across the menstrual cycle. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(44):16060–16065. doi: 10.1073/pnas.0502818102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine SE, Culbert KM, Keel PK, Sisk CL, Burt SA, Klump KL. Differential associations between ovarian hormones and disordered eating symptoms across the menstrual cycle in women. International Journal of Eating Disorders. doi: 10.1002/eat.20941. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studd JW. A guide to the treatment of depression in women by estrogens. The Journal of the International Menopause Society. 2011;14(6):637–642. doi: 10.3109/13697137.2011.609285. [DOI] [PubMed] [Google Scholar]
- Van Strien T. On the relationship between dieting and “obese” and bulimic eating patterns. International Journal of Eating Disorders. 1996;19(1):83–92. doi: 10.1002/(SICI)1098-108X(199601)19:1<83::AID-EAT10>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- van Strien T. Ice cream consumption, tendency toward overeating, and personality. International Journal of Eating Disorders. 2000;28:460–464. doi: 10.1002/1098-108x(200012)28:4<460::aid-eat16>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Van Strien T, Frijters JE, Bergers GP, Defares PB. The Dutch Eating Behavior Questionnaire (DEBQ) for assessment of restrained, emotional, and external eating behavior. The International Journal of Eating Disorders. 1986;5(2):295–315. [Google Scholar]
- Van Vugt DA. Brain imaging study of appetite in the context of obesity and the menstrual cycle. Human Reproduction Update. 2010;16(3):276–292. doi: 10.1093/humupd/dmp051. [DOI] [PubMed] [Google Scholar]
- Wade GN. Gonadal hormones and behavioral regulation of body weight. Physiol Behav. 1972;8(3):523–534. doi: 10.1016/0031-9384(72)90340-x. [DOI] [PubMed] [Google Scholar]
- Waller K, Swan SH, Windham GC, Fenster L, Elkin EP, Lasley BL. Use of urine biomarkers to evaluate menstrual function in healthy premenopausal women. American Journal of Epidemiology. 1998;147(11):1071–1080. doi: 10.1093/oxfordjournals.aje.a009401. [DOI] [PubMed] [Google Scholar]
- Wardle J. Eating style: A validation study of the Dutch Eating Behaviour Questionnaire in normal subjects and women with eating disorders. Journal of Psychosomatic Research. 1987;31(2):161–169. doi: 10.1016/0022-3999(87)90072-9. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54(6):1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wilson JD, Foster DW, Kronenberg HM, Larsen PR. Williams Textbook of Endocrinology. 9th Edition W.B. Saunders Company; Philadelphia, PA: 1998. [Google Scholar]
- Yu Z, Geary N, Corwin RL. Ovarian hormones inhibit fat intake under binge-type conditions in ovariectomized rats. Physiology & Behavior. 2008;95:501–507. doi: 10.1016/j.physbeh.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]