Abstract
Changes in extracellular matrix (ECM) are one of many components that contribute to impaired wound healing in aging. This study examined the effect of age on the glycosaminoglycan hyaluronan (HA) in normal and wounded dermis from young (4–6 month-old) and aged (22–24 month-old) mice. HA content and size was similar in the normal dermis of young and aged mice. Dermal explants labeled with [3H]-glucosamine showed decreased generation of smaller forms of HA in aged explants relative to young explants. Aged mice exhibited delayed wound repair compared with young mice with the greatest differential at 5 days. Expression of hyaluronan synthase (HAS) 2,3 and hyaluronidase (HYAL) 1-3 mRNA in wounds of young and aged mice was similar. There was a trend toward decreased HYAL protein expression in aged wound dermis, which was accompanied by changes in detectable HYAL activity. Total HA content was similar in young and aged wound dermis. There was significantly less HA in the lower MW range (~250 kDa and smaller) in 5-day wound dermis, but not 9-day wound dermis, from aged mice relative to young mice. We propose that decreased cleavage of HA is an additional component of impaired dermal wound healing in aging.
Keywords: Wound healing, Dermis, Age, Mice, Hyaluronan, Hyaluronan Synthase, Hyaluronidase
1. INTRODUCTION
Wound healing is impaired in aging. Age-related changes are multifactorial and include delayed epithelial closure, dysregulation of the immune system, deficient angiogenesis and alterations in the extracellular matrix (ECM) (Reed et al., 1996; Swift et al., 1999 and 2001; Ballas and Davidson 2001; Gosain and Dipietro, 2004). ECM in connective tissue is comprised of numerous proteins that confer strength (such as collagen) in association with nonproteinaceous molecules, the most abundant of which is hyaluronan (HA). HA is a linear polymer of the disaccharide glucuronic acid/N-acetyl glucosamine that is highly hydrophilic and which acts as a scaffold for organization of other ECM macromolecules, thereby mediating ECM assembly and homeostasis (Laurent and Fraser., 1992; Manuskiatti and Maibach., 1996; Toole., 2004; Evanko et al., 2007, 2009; Roughley et al., 2011).
HA is extruded from the surfaces of cells by the action of HA synthases (HAS), of which HAS 2 and 3 are primarily functional in the dermis (Averbeck et al., 2007; Dai et al., 2007; Gebhardt et al., 2010). Native HA can have a molecular weight (MW) as large as 2 × 104 kDa, but it is rapidly cleaved in the extracellular milieu into fragments ranging from 2–25,000 disaccharides by several mechanisms, including the activity of hyaluronidases (HYAL) 1–3 (Ikegami-Kawai and Takahashi., 2002; Stern, 2005; Toole, 2008). HA has distinct biological activities that are determined, in part, by MW. Although this varies by cell type, forms of HA with a MW of 1 × 103 kDa and above (“high MW” HA) are generally thought to inhibit the proliferation and migration of cells. In contrast, lower MW (3–300 kDa) forms of HA usually promote cell proliferation and migration and have been associated with pro-inflammatory processes (Moon et al., 1998; Slevin et al., 2002; Stern, 2005; Bharadwaj et al., 2007; David-Raoudi et al., 2008; Jiang et al., 2011).
Fetal skin contains high levels of high MW HA, which is well described as promoting healing without fibrosis and scar formation (Longaker et al., 1991; Adzick and Lorenz, 1994; Agren et al., 1997). In contrast, the influence of aging on HA content and MW in normal tissues remains incompletely characterized (Ghersetich et al., 1994; Stern and Maibach, 2008) and there are no published reports of the properties of HA in aged dermal wounds. There is evidence that in normal tissues cleavage of HA into lower MW forms is altered by aging (Miyamato and Nagase, 1984; Ghersetich et al., 1994; Meyer and Stern, 1994; Stern and Maibach, 2008; Simpson et al., 2009; Robert et al., 2010), which leads to the question of whether similar, age-associated differences in HA may exist during dermal wound repair. In the present study, we used an established mouse model to define the influence of age, and associated mechanisms, on HA content and MW distribution in normal and wounded dermis.
2. RESULTS
2.1. HA content and size is similar in the dermis of mice of different ages
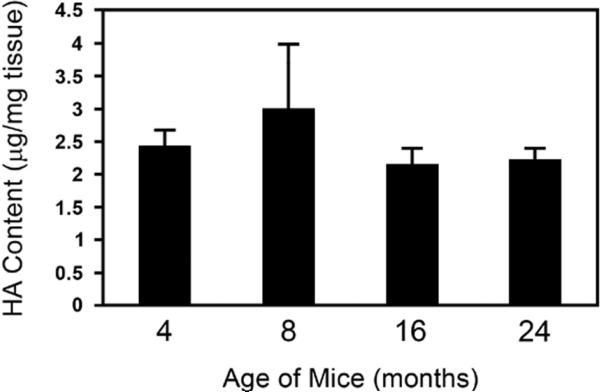
We began our studies of HA by measuring HA content and MW range in the skin from mice 4, 8, 16, and 24 months of age, an accepted model for the study of cutaneous wound repair and aging (Brubaker et al., 2011). The epidermis was removed in order to selectively measure dermal HA. Within this age range, we found that the content of HA in normal dermis was similar among the age groups (2.43+/-0.24, 3.01+/-0.97, 2.14+/-0.25, 2.24+/-0.15 µg/mg tissue, respectively; error bars are mean +/- SD) (Figure 1). The size distribution of HA in normal dermis also did not differ significantly among the age groups (data not shown).
Figure 1.
HA content in normal dermis was not significantly different with age. HA content in normal dermis from mice of 4, 8, 16 and 24 months as analyzed by quantitative ELISA was similar among all age groups (2.43+/-0.24, 3.01+/-0.97, 2.14+/-0.25, 2.24+/-0.15 μg total HA/mg tissue, respectively; error bars are mean +/- SD, n=5-6 in each age group). HA size distribution was also similar in young and aged mice in normal dermis.
2.2 Dermal explants from aged mice are less able to generate lower MW forms of HA
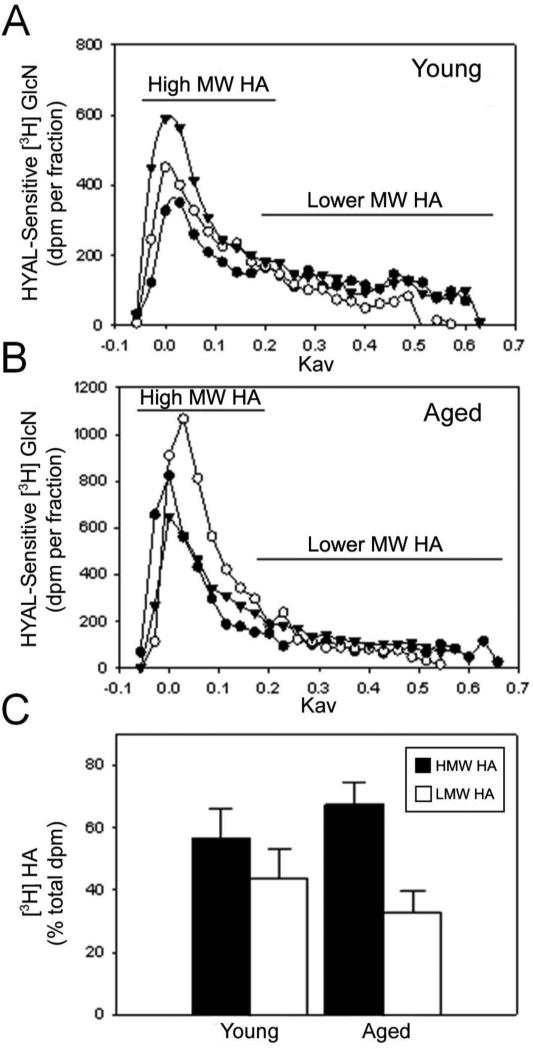
To begin to study the effect of injury on HA size, we obtained dermal explants from the skin of young and aged mice, wounded them in culture and then exposed them for 48 hours in the presence of [3H]-glucosamine to radiolabel newly-synthesized HA. The HA was purified from the culture supernates and resolved by chromatography to generate profiles of MW (Figure 2A, B). Subsequently, the chromatographic fraction data was distributed into two pools representing ranges of “high” and “lower” MW HA (Figure 2C). Quantification of these distributions showed that the HA synthesized by the dermis of young mice was more enriched in lower MW HA (~250kDa and smaller) (43.4% of total, synthesized HA was in the lower MW pool) compared to the dermis of aged mice (32.6% of total, synthesized HA was in the lower MW pool) (Figure 2C).
Figure 2.
Dermal explants from aged mice were less able to generate lower MW forms of HA than those from young mice. Dermal explants from young (A) and aged mice (B) were wounded and cultured for 48 hours in [3H]-glucosamine ([3H]GlcN) and the radiolabeled HA (purified from the culture supernates) resolved by Sephacryl S-1000 column chromatography. Results are expressed as the distribution HYAL-sensitive [3H]GlcN per fraction. Three replicates (black circles, white circles, black triangles) per group were analyzed. For each replicate shown in panels A and B, the fractions were distributed into two pools representing high MW HA with a Kav of -0.05–0.15 (HMW HA–black bars) and lower MW HA with a Kav 0.16–0.6 (LMW HA–white bars) and the total dpm for each pool measured and expressed as the percentage of the total dpm (C). Error bars represent the mean +/- standard deviation of the three replicates for each sample. The samples from young mice contained a higher percentage of lower MW HA than the samples from aged mice (C).
2.3 Dermal wound repair is delayed in aging
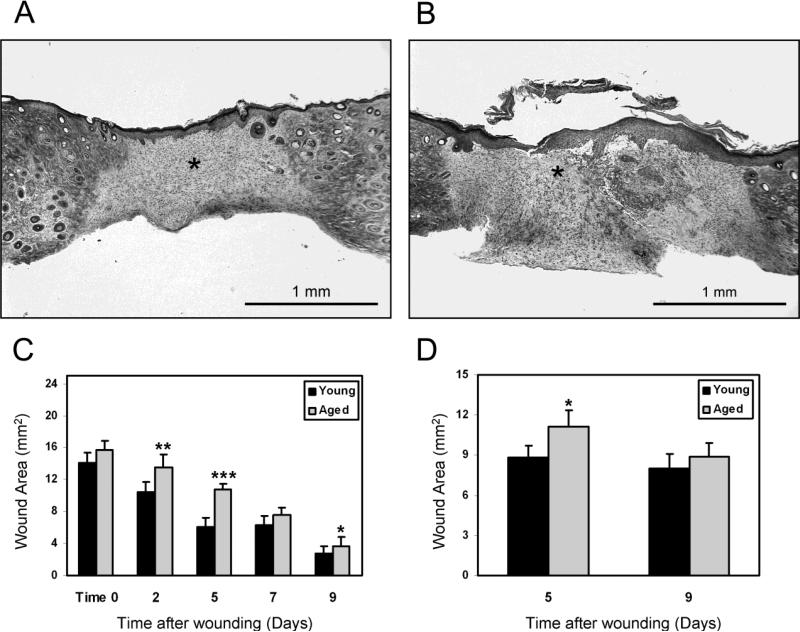
For studies of wound repair, we used a well-characterized model of full-thickness cutaneous wounds in young and aged mice to establish that our protocol matched publicized historical data (Figure 3A and 3B) (Reed et al., 1996; Swift et al., 1999 and 2001). As expected, aged mice showed decreased rates of epidermal closure compared with young mice throughout the 9-day period of wound healing (Figure 3C), with the greatest differential noted at 5 days. Repair of dermal wounds was also significantly delayed in aged mice relative to young mice at 5 days, but not after 9 days (Figure 3D). The rate of cutaneous wound repair by middle-aged mice was intermediate to that of the young and aged mice (data not shown). Consequently, middle-aged mice were not analyzed further.
Figure 3.
Repair of dermal wounds was delayed in aged mice versus young mice. Representative examples of cutaneous wounds from young (A) and aged (B) mice are shown as cross-sections stained with Masson's Trichrome. The wound dermis is indicated by asterisk (Bar = 1mm). Epidermal closure 0–9 days after wounding was significantly delayed in aged relative to young mice (*p < 0.05, error bars are mean+/-SEM; **p = 0.004, error bars are mean+/-SEM; ***p = 0.00006, error bars are mean+/-SEM, n=10 mice per age group up to 5 days, then n=5 per age group at 7 and 9 days due to euthanasia timepoints) (C). Dermal repair was significantly delayed in aged mice versus young mice, as measured by area of wounded dermis at 5 days, but was no longer significant by 9 days after wounding, (*p < 0.05, error bars are mean+/-SEM, n=5 mice per age group and time point) (D).
2.4 Aged mice have a lower proportion of low MW forms of HA than do young mice during dermal wound repair
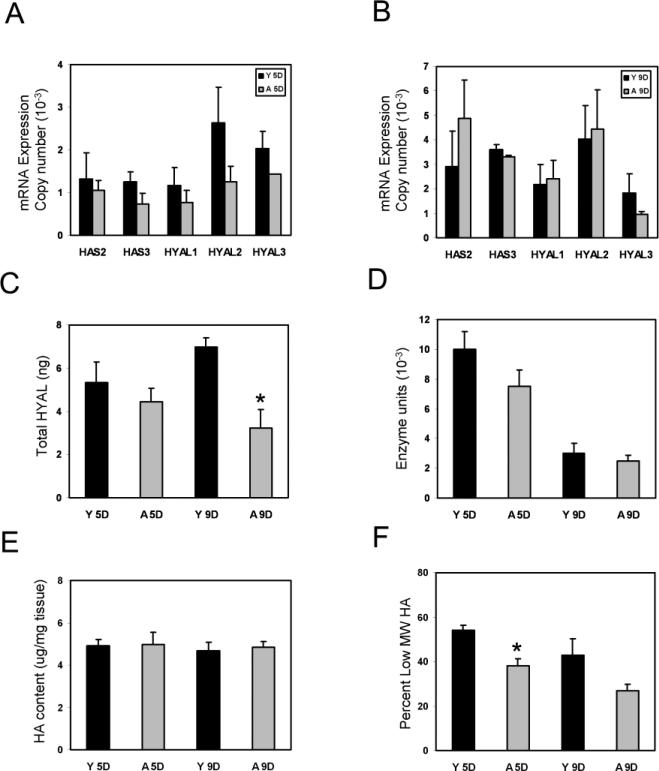
Many variables determine HA content and size. The expression of mRNAs for the primary HAS that synthesize HA in the dermis, HAS 2 and 3, (Gebhardt et al., 2010) did not differ significantly with age in 5-day or 9-day wound dermis (Figure 4A, 4B). HA size primarily reflects the activity of HYAL 1-3 (West et al., 1997; Averbeck et al., 2007; de la Motte et al., 2009; Gebhardt et al., 2010). Expression of HYAL mRNAs was also similar in the young and aged 5-day and 9-day wound dermis (Figure 4A, 4B). There was a trend toward lower expression of HYAL total protein (as determined by quantitative ELISA per 90µg total protein), in the aged wounds at 5 days, which was significant in 9-day wound dermis (5.33+/-0.9ng versus 4.4ng+/-0.6ng at 5 days and 6.97+/-0.43ng versus 3.22+/-0.85ng at 9 days, error bars are mean+/- SD, n=5 each, *p<0.05) (Figure 4C). Measures of HYAL activity were able to detect subtle differences in young and aged wound dermis at days 5 and 9, but did not find statistically significant differences in the 5-day or 9-day wounds despite a linear detection range of 2×10-4 units to 2 units per 25μg total protein (Figure 4D).
Figure 4.
Aged mice have a lower proportion of low MW forms of HA than do young mice during dermal wound repair. Wound dermis showed a trend of less HAS 2, 3 and HYAL 1–3 mRNAs in the aged wounds relative to the young wounds that was not statistically significant (A, B). Expression of total HYAL protein in the dermis of young and aged mice 5 days after wounding was measured by quantitative ELISA (90μg total protein) and no significant difference was noted in the 5-day samples, but there was a significant decrease by 9 days (5.33+/-0.9ng versus 4.4ng+/-0.6ng at 5 days and 6.97+/-0.43ng versus 3.22+/-0.85ng at 9 days, error bars are mean+/- SD, n=5 each, *p<0.05) (C). There was a trend toward decreased HYAL activity in aged dermal wounds relative to young dermal wounds based on an assay with a detection range of 2×10-4 units to 2 units per 25μg total protein (D). Wound dermis of young and aged mice contained similar quantities of total HA (4.7-4.9 μg per mg tissue versus 4.0-5.5 μg per mg tissue in young versus aged wound dermis, respectively, error bars are mean +/- SEM (E). HA size was then determined by gel electrophoresis of equivalent amounts of HA extracted from the wound dermis of each young and aged mouse (n=5 in each group). Densitometric analysis of individual samples from each mouse demonstrated that 5-day dermal wounds of young mice had a significantly greater percentage of total HA in the wound that was ~250 kDa and smaller than that of 5-day wounds from aged mice (54+/-2% versus 38+/-3% at 5 days, error bars are mean+/- SEM, n=5 each, *p<0.05) (F). Samples from 9-day wounds did not show statistically significant differences in HA size between wound dermis of young and aged mice (43+/-8% versus 27+/-3% at 9 days, error bars are mean+/- SEM, n=5 each) (F).
Quantitation of total HA content during dermal wound repair demonstrated that, as in normal dermis, the HA content within wound dermis of young and aged mice was similar for both groups of mice at 5 and 9 days after wounding (Figure 4E). Wound dermis of young and aged mice had roughly twice the HA content per unit weight of tissue than normal dermis. In young and aged mice, there was 4.1-5.0 μg of HA per mg dry weight of 5- and 9-day wound dermis (Figure 4E) versus 2.14-3.01 μg of HA per mg dry weight of normal dermis (Figure 1).
The biological effects of HA are strongly influenced by its MW. For example, high MW forms of HA generally inhibit tissue growth, whereas lower MW HA promotes angiogenesis, inflammatory responses, and usually stimulates cell proliferation and migration. We evaluated the size of HA in the dermis of 5-day and 9-day wounds of young and aged mice using agarose gel electrophoresis in combination with densitometric scans of the stained gels. Five mice were evaluated individually for each of the 4 groups (i.e., young versus aged at 5 days and 9 days after wounding), for a total of 20 mice. Results were highly consistent among individual mice within each group. The size profile of HA extracted from 5-day wound dermis of young mice showed greater HA content in the lower MW HA range (~250 kDa and smaller) than that seen in corresponding extracts of 5-day wound dermis from aged mice (54+/-2% versus 38+/-3% at 5 days, error bars are mean+/- SEM, n=5 each, *p<0.05) (Figure 4F). In contrast, the size profiles of HA in extracts of 9-day wound dermis from young and aged mice were similar (43+/-8% versus 27+/-3% at 9 days, error bars are mean+/- SEM, n=5 each) (Figure 4F).
3. DISCUSSION
It has been proposed that aging of the skin is associated with changes in HA content that result in a less hydrated and structurally sound ECM (Miyamato and Nagase, 1984; Weigel et al., 1986; Ghersetich et al., 1994; Meyer and Stern, 1994; Manuskiatti and Maibach, 1996; Stern and Maibach, 2008; Simpson et al., 2009). Following synthesis, extracellular HA is rapidly cleaved into smaller fragments by several mechanisms, including the activity of HYAL (West et al., 1997; Averbeck et al., 2007; Harada and Takahashi, 2007; de La Motte et al., 2009; Girish et al., 2009; Gebhardt et al., 2010). Age-related changes in HA size are also of relevance, as the effects of HA on cell behaviors are determined by the degree to which high MW forms of HA are cleaved into smaller fragments (West et al., 1985; Sattar et al., 1994; Montesano et al., 1996; Deed et al., 1997; Gao et al., 2008).
In this study we found that HA content and size are similar in normal dermis, but that HA from dermal explants of young mice tended toward lower MWs than that of HA from corresponding dermal explants of aged mice. The age-related difference in the MW of HA in explanted dermis suggests that age could affect the size profile of HA under other conditions of stress, such as wound repair. To specifically examine the effect of age on HA during dermal wound healing, we utilized a well-characterized murine model of full-thickness cutaneous wounds (Reed et al., 1996 and 2006; Swift et al., 1999 and 2001). Such wounds in aged mice generally exhibit deficiencies in repair processes relative to wounds in young mice, particularly during the early and middle stages of healing. These deficiencies become less acute toward the later stages of healing, as was noted in this study. Interestingly, we observed that wound dermis of young and aged mice contained similar quantities of HA at both the middle (5-day) and later (9-day) stages of healing. However, we did find a significant age-related difference in the MW of HA in wound dermis, such that wounds from young mice at the 5-day stage had a significantly greater amount of HA in the size range of ~250 kDa and smaller than did 5-day wounds from aged mice. This difference was not seen in 9-day wounds – a point where differences in the extent of dermal wound repair between young and aged mice was no longer significant, as was shown in Figure 3D.
Several potential mechanisms contribute to differences in HA size. One consideration is the relative expression and activity of the HAS enzymes responsible for HA synthesis in the dermis (Gebhardt et al., 2010), as HAS 2 is thought to generate larger forms of HA than HAS 3 and has variable expression with aging (Stern, 2005; Oh et al., 2011). We did not detect significant differences in HAS 2 and 3 mRNA between young and aged mouse dermal wounds. Another possibility is that decreased fibroblast density in aged dermal wounds (Reed et al., 1996; Swift et al., 1999; Gosain and DiPietro, 2004) contributes to the presence of larger forms of HA (Chen et al., 1989). The fact that HA size did not vary significantly in normal dermis from aged mice, which is also known to have decreased fibroblast density (Reed et al., 1996; Gosain and DiPietro, 2004), suggests density did not mediate age-related differences in HA size in wound dermis. Additional mechanisms include age-related alterations in receptor mediator clearance and turnover of HA. Changes in HA size in this context reflect expression and activity of CD44, RHAMM and other HA receptors – a process that is best studied using exogenously administered labeled HA (Harada and Takahashi 2007; Jadin et al., 2012; Afratis et al., 2012). Lastly, a major determinant of HA size in the dermis is HYAL activity, an enzymatic process that is mediated primarily by the expression and regulation of HYAL 1, 2, and 3 (Averbeck et al., 2007; Gebhardt et al., 2010). Levels of the specific mRNAs corresponding to HYAL 1, 2, and 3 – the three isoforms that mediate HA cleavage in dermis– were not significantly different in young compared to aged wound dermis, but there was a trend toward increased expression of the three HYAL isoforms in young wound dermis, a result that was mimicked in quantitation of total HYAL protein in 5-day and 9-day wounds of young and aged mice. HYAL enzymatic activity was also measured in young and aged dermal explants and wound dermis. Although there were trends toward decreased activity in aged dermal explants, the differences did not achieve statistical significance. It is unlikely this reflects lack of sensitivity given the large detection range of our standard curve, but even small changes in enzyme international units could reflect important biologic changes that are not reflected in statistical measures extrapolated from measures of optical density. Nonetheless these data suggest additional age-related changes that determine HA size in the wound dermis, such as circulating endogenous inhibitors of HYAL activity and/or HA binding proteins that protect HA against cleavage by HYAL (Jha et al., 2004; Evanko et al., 2007; Girish et al., 2009). The view that age-related deficits are multifactorial, with small decrements in many stages, is a recurring theme in studies of aging and tissue repair (Sadoun and Reed 2003; Gosain and DiPietro, 2004).
Although it is generally accepted that many mechanisms interact to influence wound repair in aging, we suggest that decreased lower MW HA (~250 kDa and smaller) in 5-day wounds could contribute to impaired dermal healing in aged hosts. In support of this hypothesis, “small” and “medium” forms of HA have been reported to promote proliferation of connective tissue cells, such as fibroblasts (Moon et al., 1998; David Raoudi et al., 2008). Correspondingly, we have observed that addition of 200-300 kDa HA significantly increased the proliferation of aged human fibroblasts in vitro (Reed et al., unpublished observations). In fetal wounds there is persistence of high MW HA, relative to wounds of post natal, young animals, that contributes to a scarless form of healing (Longaker et al., 1991; Adzick and Lorenz, 1994; Agren et al., 1997; West et al., 1997). This effect is obviated by exogenous HYAL, which degrades high MW HA and promotes collagen deposition (Mast et al., 1992). In addition to direct effects on cell behaviors, the difference in HA size with age is likely relevant to the production of smaller forms of HA, such as oligomers. Smaller forms of HA, whether added directly or generated by addition of HYAL, are known to promote angiogenesis and granulation tissue formation (Sattar et al., 1994; West et al., 1985; Montesano et al., 1996; Deed et al., 1997; Iocono et al., 1998; Slevin et al., 2002; Gao et al., 2008), a process that we and many others have noted is significantly impaired in aging (Rivard et al., 1999, Swift et al., 1999; Edelberg et al., 2002; Sadoun and Reed 2003).
In summary, we found no age-associated differences in HA content or MW in normal dermis from young and aged mice. Using dermal explants and a well established model of dermal wound repair in aging, we noted that although the HA content was similar, there was less lower MW HA in the dermis of aged mice in comparison to corresponding dermis in young mice. We propose that lower levels of dermal HA processing contribute to delayed wound healing in aging.
4. EXPERIMENTAL PROCEDURES
4.1. Animals
Male C57/BL6 mice ranging from 4 to 24 months of age were originally obtained from the NIA Aged Rodent Colony (http://www.nia.nih.gov/search/site/Rodentcolony). The Office of Animal Welfare at the University of Washington approved the care of mice and all procedures.
4.2. Normal Dermis and Dermal Wound model
Normal dermis and dermal explants were derived from mice of young (4-8 months), middle aged (12-16 months), and aged (>20 months) age groups (n=3-6 mice in each group).
For wound repair studies, young (4–6 month), middle-aged (12–14 month), and aged (22– 24 month) mice (n=10 in each group) received two dorsal, 6 mm full-thickness dermal wounds as previously described (Reed et al., 1996; 2006). At time 0 and at 2, 5, 7 and 9 days after wounding, the wounds of the living animals were digitally photographed from above and the wound areas measured from the images using ImageJ (NIH). Results were recorded in square pixels and then converted to area (mm2). Groups of mice were euthanized at 5 and 9 days after wounding (Reed et al., 2006) and the wound tissue harvested using a 6 mm biopsy punch. Each wound plug was divided in half: one half was processed for histology and the other half was stripped of epidermis and analyzed for HA content and MW, and for expression of mRNAs for specific HYAL, as described below.
4.3. Histology
Wound isolates were embedded in paraffin and sectioned at 5 μm. Sections from the middle of the wound were mounted on slides, de-paraffinized, and stained with Masson's Trichrome. Wound regions were identified in the sections by the presence of co-localized collagen (blue) and fibrin (red), which clearly demarcated the wounded dermis. Wound areas were measured in square pixels by analysis of digital images with ImageJ and then converted to area (mm2). Significance of the data was calculated using Student's t-test.
HA was identified in de-paraffinized, mounted sections by blocking the sections in 2% goat serum and exposing them to biotinylated hyaluronic acid binding protein (bHABP). The bound bHABP was visualized with a Vectastain® avidin-biotin complex (ABC) kit (Vector Laboratories, Burlingame, CA), in conjunction with 3,3'-diaminobenzidine (DAB).
4.4. Measurement of total HA content
To assess total HA content, normal dermis or portions of wounds from each mouse (stripped of epidermis) were homogenized with cell lysis buffer (Qiagen, Valencia, CA). Individual extracts were standardized to a total protein content of 100 μg (BCA assay, Pierce/Thermo Fisher Scientific, Inc., Rockford, IL) and then were lyophilized, rehydrated with 100 mM ammonium acetate (pH 7.0), digested with proteinase K (250 μg/ml) to degrade endogenous proteins and proteoglycans, heated to 100°C for 20 minutes to inactivate the proteinase K, and then assayed for HA by a competitive enzyme-linked sorbent assay (ELSA) using bHABP (Wilkinson et al., 2004; Sakr et al., 2008; Jarvelainen et al., 2009). HA reactivity in the ELSA was quantitated according to a standard curve derived from purified HA (Underhill et al., 1993; Wilkinson, 2004; Jarvelainen et al., 2009).
4.5. Determination of HA size
To evaluate the size profile of newly-synthesized HA in injured dermis, freshly obtained and finely minced dermal explants were radiolabeled for 48 hours with 40mCi/ml of [3H]-glucosamine in Dulbecco's Modified Eagle Medium (DMEM – Gibco®-Invitrogen, Grand Island, NY) with 5% fetal bovine serum. Supernates were isolated by centrifugation and digested with proteinase K (250 μg/ml) for 24 hours at 60°C. Following digestion, proteinase K was inactivated by heating to 100°C for 20 minutes. HA and other glycosaminoglycans (GAGs) were separated from unincorporated [3H]-glucosamine by chromatography on Sephadex G-50 columns. Macromolecular fractions containing identical [3H] counts were incubated with or without 0.5 U/ml of Streptomyces HYAL (Sigma-Aldrich, St. Louis, MO) for 18 hours at 37°C and analyzed by size exclusion chromatography on a 1.2 × 58 cm Sephacryl S-1000 column. Fractions were eluted in 0.5 M sodium acetate/0.025% CHAPS, pH 7.0, and the radioactivity was measured by liquid scintillation counting. For each fraction, radioactivity associated with HA (defined as HYAL-sensitive GAG) was calculated by subtracting HYAL-resistant radioactivity from that of the undigested total.
To measure the MW of HA in normal and wound dermis, HA extracts of dermis (each containing 10 μg of HA) were electrophoresed into a 1.2% agarose gel (ISC BioExpress, Kaysville, UT) at 60V for 6 hours. For this study, 4 groups of mice were wounded (young and aged, euthanized after 5 days and 9 days) for a total of 20 individual mouse wounds that were analyzed separately. The gels were stained overnight at room temperature with 0.005% Stains-All (Sigma-Aldrich) in 50% ethanol, and destained in water. The MW profiles of the HA bands, relative to the position of purified HA MW standards, were determined by scanning densitometry.
4.6. Measurement of HAS and HYAL mRNAs
Measurement of mRNAs corresponding to mouse HAS 2 and 3 and HYAL 1–3 was performed by RT-PCR. Total cellular RNA from mouse dermis was extracted using Trizol (Invitrogen). RNA purity and integrity was assessed by spectrophotometric analysis and denaturating agarose/MOPS gels. A total of 1 μg of RNA was reverse transcribed using an iScript kit (Bio-Rad Laboratories, Hercules, CA). RT-PCR was performed using an ABI 7900 RTPCR instrument with SYBR Green Master Mix (Bio-Rad) for mouse HAS 2, HAS 3, HYAL 1, HYAL 2 and HYAL 3. All experiments were performed in triplicate and normalized to GAPDH mRNA. Fluorescent signals were analyzed during each of 40 cycles consisting of denaturation (95°C, 15 seconds), annealing (54°C, 15 seconds). Relative quantitation was calculated using the comparative threshold cycle method.
4.7. Measurement of total HYAL
Samples of 5-day wound dermis were harvested from each mouse and homogenized in NP-40 cell lysis buffer (Cat. # FNN0021, Invitrogen). Equivalent amounts (90 μg) of total protein (determined by BCA assay) were loaded per well in a 96-well ELISA plate and a mouse-specific HYAL ELISA performed per the manufacturer's instructions (Cat. # BG-MUS11250, Novatein Biosciences, Inc., Cambridge, MA). Total HYAL expression was quantified against a standard curve generated by mouse HYAL with a linear range extending from 3.12– 25 ng/ml per manufacturer's instructions.
4.8. Measurement of HYAL activity
A modification of a previously published assay was utilized to measure HYAL activity (Frost and Stern, 1997). Plates (96-well) were coated with HA-BSA (0.2mg/ml) and then blocked with 10% FBS in PBS for 1 hour. After washing with PBS, serially diluted HYAL standards (Sigma) and equal amounts of protein from the dermal explant media (25μg) and wound dermis (25μg) were added (as determined by Coomassie Plus and BCA [both Pierce], respectively) for 1 hour at 37°C. Samples were then incubated with bHABP (Millipore) at 3μg/ml in 10% FBS in PBS for 1 hour at room temperature. Subsequently, the plates were washed and incubated for 20 minutes with peroxidase-labelled streptavidin (1:500 dilution in 10%FBS in PBS), then washed and developed using 0.03% H2O2, 0.05mg/ml 2,2 Azino-bis (Sigma) in 0.1M sodium citrate, pH 4.2, and the optical density read at 405nm after 20 minutes. Units of HYAL activity were calculated from a standard curve and had a detection range of 2×10-4 units to 2 units per 25μg total protein.
Highlights.
HA content and size was similar in the normal dermis of young and aged mice.
Labeled dermal explants showed decreased generation of smaller forms of HA in aged explants relative to young explants.
Total HA content was similar in young and aged wound dermis.
There was significantly less lower MW HA in 5 day wound dermis from aged mice relative to young mice.
Acknowledgements
R21 AG033391 NIA/NIH (MJR, RBV)
UW Royalty Research Fund (MJR)
P01 HL018645 NHLBI/NIH (TNW)
The authors wish to thank Drs. Ingrid A. Harten, Susan Potter-Perigo, and Stephen P. Evanko for helpful discussions and Dr. Virginia M. Green for editorial assistance.
Abbreviations
- HA
hyaluronan
- HAS
hyaluronan synthase
- HYAL
hyaluronidase
- MW
molecular weight
- ECM
extracellular matrix
- GAG
glycosaminoglycan
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adzick NS, Lorenz HP. Cells, matrix, growth factors, and the surgeon. The biology of scarless fetal wound repair. Ann. Surg. 1994;220:10–8. doi: 10.1097/00000658-199407000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afratis N, Gialeli C, Nikitovic D, Tsegenidis T, Karousou E, Theocharis AD, Pavão MS, Tzanakakis GN, Karamanos NK. Glycosaminoglycans: key players in cancer cell biology and treatment. FEBS J. 2012;279:1177–1197. doi: 10.1111/j.1742-4658.2012.08529.x. [DOI] [PubMed] [Google Scholar]
- Agren UM, Tammi M, Ryynänen M, Tammi R. Developmentally programmed expression of hyaluronan in human skin and its appendages. J Invest Dermatol. 1997;109:219–224. doi: 10.1111/1523-1747.ep12319412. [DOI] [PubMed] [Google Scholar]
- Averbeck M, Gebhardt CA, Voigt S, Beilharz S, Anderegg U, Termeer CC, Sleeman JP, Simon JC. Differential regulation of hyaluronan metabolism in the epidermal and dermal compartments of human skin by UVB irradiation. J. Invest. Dermatol. 2007;127:687–697. doi: 10.1038/sj.jid.5700614. [DOI] [PubMed] [Google Scholar]
- Ballas CB, Davidson JM. Delayed wound healing in aged rats is associated with increased collagen gel remodeling and contraction by skin fibroblasts, not with differences in apoptotic or myofibroblast cell populations. Wound Repair. Regen. 2001;9:223–237. doi: 10.1046/j.1524-475x.2001.00223.x. [DOI] [PubMed] [Google Scholar]
- Bharadwaj AG, Rector K, Simpson MA. Inducible hyaluronan production reveals differential effects on prostate tumor cell growth and tumor angiogenesis. J. Biol. Chem. 2007;282:20561–20572. doi: 10.1074/jbc.M702964200. [DOI] [PubMed] [Google Scholar]
- Brubaker AL, Palmer JL, Kovacs EJ. Age-related Dysregulation of Inflammation and Innate Immunity: Lessons Learned from Rodent Models. Aging Dis. 2011;2:346–360. [PMC free article] [PubMed] [Google Scholar]
- Chen WY, Grant ME, Schor AM, Schor SL. Differences between adult and fetal fibroblasts in the regulation of hyaluronate synthesis: correlation with migratory activity. J. Cell Sci. 1989;94:577–584. doi: 10.1242/jcs.94.3.577. [DOI] [PubMed] [Google Scholar]
- Dai G, Freudenberger T, Zipper P, Melchior A, Grether-Beck S, Rabausch B, de Groot J, Twarock S, Hanenberg H, Homey B, Krutmann J, Reifenberger J, Fischer JW. Chronic ultraviolet B irradiation causes loss of hyaluronic acid from mouse dermis because of down-regulation of hyaluronic acid synthases. Am. J. Pathol. 2007;171:1451–1461. doi: 10.2353/ajpath.2007.070136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David-Raoudi M, Tranchepain F, Deschrevel B, Vincent JC, Bogdanowicz P, Boumediene K, Pujol JP. Differential effects of hyaluronan and its fragments on fibroblasts: relation to wound healing. Wound Repair. Regen. 2008;16:274–287. doi: 10.1111/j.1524-475X.2007.00342.x. [DOI] [PubMed] [Google Scholar]
- de la Motte C, Nigro J, Vasanji A, Rho H, Kessler S, Bandyopadhyay S, Danese S, Fiocchi C, Stern R. Platelet-derived hyaluronidase 2 cleaves hyaluronan into fragments that trigger monocyte-mediated production of proinflammatory cytokines. Am. J. Pathol. 2009;174:2254–2264. doi: 10.2353/ajpath.2009.080831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deed R, Rooney P, Kumar P, Norton JD, Smith J, Freemont AJ, Kumar S. Early-response gene signalling is induced by angiogenic oligosaccharides of hyaluronan in endothelial cells. Inhibition by non-angiogenic, high-molecular-weight hyaluronan. Int. J. Cancer. 1997;71:251–256. doi: 10.1002/(sici)1097-0215(19970410)71:2<251::aid-ijc21>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Edelberg J,M, Lee SH, Kaur M, Tang L, Feirt NM, McCabe S, Bramwell O, Wong SC, Hong MK. Platelet-derived growth factor-AB limits the extent of myocardial infarction in a rat model: feasibility of restoring impaired angiogenic capacity in the aging heart. Circulation. 2002;105:608–613. doi: 10.1161/hc0502.103672. [DOI] [PubMed] [Google Scholar]
- Evanko SP, Tammi MI, Tammi RH, Wight TN. Hyaluronan-dependent pericellular matrix. Adv. Drug. Deliv. Rev. 2007;59:1351–1365. doi: 10.1016/j.addr.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evanko SP, Potter-Perigo S, Johnson PY, Wight TN. Organization of hyaluronan and versican in the extracellular matrix of human fibroblasts treated with the viral mimetic poly I:C. J. Histochem. Cytochem. 2009;57:1041–1060. doi: 10.1369/jhc.2009.953802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost GI, Stern R. A microtiter-based assay for hyaluronidase activity not requiring specialized reagents. Anal. Biochem. 1997;251:263–269. doi: 10.1006/abio.1997.2262. [DOI] [PubMed] [Google Scholar]
- Gao F, Yang CX, Mo W, Liu YW, He YQ. Hyaluronan oligosaccharides are potential stimulators to angiogenesis via RHAMM mediated signal pathway in wound healing. Clin. Invest. Med. 2008;31:E106–116. doi: 10.25011/cim.v31i3.3467. [DOI] [PubMed] [Google Scholar]
- Gebhardt C, Averbeck M, Diedenhofen N, Willenberg A, Anderegg U, Sleeman JP, Simon JC. Dermal hyaluronan is rapidly reduced by topical treatment with glucocorticoids. J. Invest. Dermatol. 2010;130:141–149. doi: 10.1038/jid.2009.210. [DOI] [PubMed] [Google Scholar]
- Ghersetich I, Lotti T, Campanile G, Grappone C, Dini G. Hyaluronic acid in cutaneous intrinsic aging. Int. J. Dermatol. 1994;33:119–122. doi: 10.1111/j.1365-4362.1994.tb01540.x. [DOI] [PubMed] [Google Scholar]
- Girish KS, Kemparaju K, Nagaraju S, Vishwanath BS. Hyaluronidase inhibitors: a biological and therapeutic perspective. Curr. Med. Chem. 2009;16:2261–2288. doi: 10.2174/092986709788453078. [DOI] [PubMed] [Google Scholar]
- Gosain A, DiPietro LA. Aging and wound healing. World J. Surg. 2004;28:321–326. doi: 10.1007/s00268-003-7397-6. [DOI] [PubMed] [Google Scholar]
- Harada H, Takahashi M. CD44-dependent intracellular and extracellular catabolism of hyaluronic acid by hyaluronidase-1 and -2. J. Biol. Chem. 2007;282:5597–5607. doi: 10.1074/jbc.M608358200. [DOI] [PubMed] [Google Scholar]
- Ikegami-Kawai M, Takahashi T. Microanalysis of hyaluronan oligosaccharides by polyacrylamide gel electrophoresis and its application to assay of hyaluronidase activity. Anal. Biochem. 2002;311:157–165. doi: 10.1016/s0003-2697(02)00425-6. [DOI] [PubMed] [Google Scholar]
- Iocono JA, Krummel TM, Keefer KA, Allison GM, Paul H. Repeated additions of hyaluronan alters granulation tissue deposition in sponge implants in mice. Wound Repair Regen. 1998;6:442–448. doi: 10.1046/j.1524-475x.1998.60506.x. [DOI] [PubMed] [Google Scholar]
- Jadin L, Bookbinder LH, Frost G,I. A comprehensive model of hyaluronan turnover in the mouse. Matrix Biol. 2012;31:81–89. doi: 10.1016/j.matbio.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Jarvelainen H, Sainio A, Koulu M, Wight TN, Penttinen R. Extracellular matrix molecules: potential targets in pharmacotherapy. Pharmacol. Rev. 2009;61:198–223. doi: 10.1124/pr.109.001289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha BK, Mitra N, Rana R, Surolia A, Salunke DM, Datta K. pH and cation-induced thermodynamic stability of human hyaluronan binding protein 1 regulates its hyaluronan affinity. J. Biol. Chem. 2004;279:23061–23072. doi: 10.1074/jbc.M310676200. [DOI] [PubMed] [Google Scholar]
- Jiang D, Liang J, Noble PW. Hyaluronan as an immune regulator in human diseases. Physiol. Rev. 2011;1:221–264. doi: 10.1152/physrev.00052.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent TC, Fraser JR. Hyaluronan. Faseb J. 1992;6:2397–2404. [PubMed] [Google Scholar]
- Longaker MT, Chiu ES, Adzick NS, Stern M, Harrison MR, Stern R. Studies in fetal wound healing. V. A prolonged presence of hyaluronic acid characterizes fetal wound fluid. Ann. Surg. 1991;213:292–6. doi: 10.1097/00000658-199104000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuskiatti W, Maibach HI. Hyaluronic acid and skin: wound healing and aging. Int. J. Dermatol. 1996;35:539–544. doi: 10.1111/j.1365-4362.1996.tb03650.x. [DOI] [PubMed] [Google Scholar]
- Mast BA, Haynes JH, Krummel TM, Diegelmann RF, Cohen IK. In vivo degradation of fetal wound hyaluronic acid results in increased fibroplasia, collagen deposition, and neovascularization. Plast. Reconstr. Surg. 1992;89:503–509. doi: 10.1097/00006534-199203000-00019. [DOI] [PubMed] [Google Scholar]
- Meyer LJ, Stern R. Age-dependent changes of hyaluronan in human skin. J. Invest. Dermatol. 1994;102:385–389. doi: 10.1111/1523-1747.ep12371800. [DOI] [PubMed] [Google Scholar]
- Miyamoto I, Nagase S. Age-related changes in the molecular weight of hyaluronic acid from rat skin. Jikken. Dobutsu. 1984;33:481–485. doi: 10.1538/expanim1978.33.4_481. [DOI] [PubMed] [Google Scholar]
- Moon SO, Lee JH, Kim TJ. Changes in the expression of c-myc, RB and tyrosine-phosphorylated proteins during proliferation of NIH 3T3 cells induced by hyaluronic acid. Exp. Mol. Med. 1998;30:29–33. doi: 10.1038/emm.1998.4. [DOI] [PubMed] [Google Scholar]
- Montesano R, Kumar S, Orci L, Pepper MS. Synergistic effect of hyaluronan oligosaccharides and vascular endothelial growth factor on angiogenesis in vitro. Lab. Invest. 1996;75:249–262. [PubMed] [Google Scholar]
- Oh JH, Kim YK, Jung JY, Shin JE, Chung JH. Changes in glycosaminoglycans and related proteoglycans in intrinsically aged human skin in vivo. Exp. Dermatol. 2011;20:454–456. doi: 10.1111/j.1600-0625.2011.01258.x. [DOI] [PubMed] [Google Scholar]
- Reed MJ, Penn PE, Li Y, Birnbaum R, Vernon RB, Johnson TS, Pendergrass WR, Sage EH, Abrass IB, Wolf NS. Enhanced cell proliferation and biosynthesis mediate improved wound repair in refed, caloric-restricted mice. Mech. Ageing Dev. 1996;89:21–43. doi: 10.1016/0047-6374(96)01737-x. [DOI] [PubMed] [Google Scholar]
- Reed MJ, Karres N, Eyman D, Vernon RB, Edelberg JM. Age-related differences in repair of dermal wounds and myocardial infarcts attenuate during the later stages of healing. In Vivo. 2006;20:801–806. [PubMed] [Google Scholar]
- Rivard A, Fabre JE, Silver M, Chen D, Murohara T, Kearney M, Magner M, Asahara T, Isner JM. Age-dependent impairment of angiogenesis. Circulation. 1999;99:111–120. doi: 10.1161/01.cir.99.1.111. [DOI] [PubMed] [Google Scholar]
- Robert L, Robert AM, Renard G. Biological effects of hyaluronan in connective tissues, eye, skin, venous wall. Role in aging. Pathol. Biol. (Paris) 2010;58:187–198. doi: 10.1016/j.patbio.2009.09.010. [DOI] [PubMed] [Google Scholar]
- Roughley PJ, Lamplugh L, Lee ER, Matsumoto K, Yamaguchi Y. The role of hyaluronan produced by Has2 gene expression in development of the spine. Spine (Phila Pa 1976) 2011;36:E914–920. doi: 10.1097/BRS.0b013e3181f1e84f. [DOI] [PubMed] [Google Scholar]
- Sadoun E, Reed MJ. Impaired angiogenesis in aging is associated with alterations in vessel density, matrix composition, inflammatory response, and growth factor expression. J. Histochem. Cytochem. 2003;51:1119–1130. doi: 10.1177/002215540305100902. [DOI] [PubMed] [Google Scholar]
- Sakr SW, Potter-Perigo S, Kinsella MG, Johnson PY, Braun KR, Goueffic Y, Rosenfeld ME, Wight TN. Hyaluronan accumulation is elevated in cultures of low density lipoprotein receptor-deficient cells and is altered by manipulation of cell cholesterol content. J. Biol. Chem. 2008;283:36195–36204. doi: 10.1074/jbc.M807772200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattar A, Rooney P, Kumar S, Pye D, West DC, Scott I, Ledger P. Application of angiogenic oligosaccharides of hyaluronan increases blood vessel numbers in rat skin. J. Invest. Dermatol. 1994;103:576–579. doi: 10.1111/1523-1747.ep12396880. [DOI] [PubMed] [Google Scholar]
- Simpson RM, Meran S, Thomas D, Stephens P, Bowen T, Steadman R, Phillips A. Age-related changes in pericellular hyaluronan organization leads to impaired dermal fibroblast to myofibroblast differentiation. Am. J. Pathol. 2009;175:915–928. doi: 10.2353/ajpath.2009.090045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slevin M, Kumar S, Gaffney J. Angiogenic oligosaccharides of hyaluronan induce multiple signaling pathways affecting vascular endothelial cell mitogenic and wound healing responses. J. Biol. Chem. 2002;277:41046–41059. doi: 10.1074/jbc.M109443200. [DOI] [PubMed] [Google Scholar]
- Stern R. Hyaluronan metabolism: a major paradox in cancer biology. Pathol. Biol. 2005;53:372–382. doi: 10.1016/j.patbio.2004.12.021. [DOI] [PubMed] [Google Scholar]
- Stern R, Maibach HI. Hyaluronan in skin: aspects of aging and its pharmacologic modulation. Clin. Dermatol. 2008;26:106–122. doi: 10.1016/j.clindermatol.2007.09.013. [DOI] [PubMed] [Google Scholar]
- Swift ME, Kleinman HK, DiPietro LA. Impaired wound repair and delayed angiogenesis in aged mice. Lab. Invest. 1999;79:1479–1487. [PubMed] [Google Scholar]
- Swift ME, Burns AL, Gray KL, DiPietro LA. Age-related alterations in the inflammatory response to dermal injury. J. Invest. Dermatol. 2001;117:1027–1035. doi: 10.1046/j.0022-202x.2001.01539.x. [DOI] [PubMed] [Google Scholar]
- Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nat. Rev. Cancer. 2004;4:528–539. doi: 10.1038/nrc1391. [DOI] [PubMed] [Google Scholar]
- Toole BP, Ghatak S, Misra S. Hyaluronan oligosaccharides as a potential anticancer therapeutic. Curr. Pharm. Biotechnol. 2008;9:249–252. doi: 10.2174/138920108785161569. [DOI] [PubMed] [Google Scholar]
- Underhill CB, Nguyen HA, Shizari M, Culty M. CD44 positive macrophages take up hyaluronan during lung development. Dev. Biol. 1993;155:324–336. doi: 10.1006/dbio.1993.1032. [DOI] [PubMed] [Google Scholar]
- Weigel PH, Fuller GM, LeBoeuf RD. A model for the role of hyaluronic acid and fibrin in the early events during the inflammatory response and wound healing. J. Theor. Biol. 1986;119:219–234. doi: 10.1016/s0022-5193(86)80076-5. [DOI] [PubMed] [Google Scholar]
- West DC, Hampson IN, Arnold F, Kumar S. Angiogenesis induced by degradation products of hyaluronic acid. Science. 1985;228:1324–1326. doi: 10.1126/science.2408340. [DOI] [PubMed] [Google Scholar]
- West DC, Shaw DM, Lorenz P, Adzick NS, Longaker MT. Fibrotic healing of adult and late gestation fetal wounds correlates with increased hyaluronidase activity and removal of hyaluronan. Int. J. Biochem. Cell. Biol. 1997;29:201–210. doi: 10.1016/s1357-2725(96)00133-1. [DOI] [PubMed] [Google Scholar]
- Wilkinson TS, Potter-Perigo S, Tsoi C, Altman LC, Wight T,N. Pro- and anti-inflammatory factors cooperate to control hyaluronan synthesis in lung fibroblasts. Am. J. Respir. Cell. Mol. Biol. 2004;31:92–99. doi: 10.1165/rcmb.2003-0380OC. [DOI] [PubMed] [Google Scholar]