Abstract
Pre-clinical development of therapy for acute ischemic stroke requires robust animal models; the rodent middle cerebral artery occlusion (MCAo) model using a nylon filament inserted into the internal carotid artery is the most popular. Drug screening requires targeted delivery of test substance in a controlled manner. To address these needs, we developed a novel method for delivering substances directly into the ischemic brain during MCAo in the awake rat. An indwelling catheter is placed in the common carotid artery ipsilateral to the occlusion at the time of the surgical placement of the occluding filament. The internal and common carotid arteries are left patent to allow superfusion anterograde. The surgeries can be completed quickly to allow rapid recovery from anesthesia; tests substances can be infused at any given time for any given duration. To simulate clinical scenarios, the occluding filament can be removed minutes or hours later (reperfusion) followed by therapeutic infusions. By delivering drug intra-arterially to the target tissue, “first pass” loss in the liver is reduced and drug effects are concentrated in the ischemic zone. To validate our method, rats were infused with Evans blue dye either intra-arterially or intravenously during a 4 hour MCAo. After a 30 minute reperfusion period, the dye was extracted from each hemisphere and quantitated with a spectrophotometer. Significantly more dye was measured in the ischemic hemispheres that received the dye intra-arterially.
Keywords: Cerebral ischemia, stroke, middle cerebral artery occlusion, intraluminal filament, drug delivery
1. Introduction
As more treatments for human stroke fail in clinical trials (Tymianski, 2010), indictments of pre-clinical stroke models have emerged. For example, putative stroke treatments are often given to test animals immediately after ischemia onset, an impossible clinical scenario (Fisher and Tatlisumak, 2005). Patients are not anesthetized during stroke, and thus pre-clinical models must be developed to allow drug infusions in awake animals because anesthetic have neuroprotective effects (Kawaguchi et al., 2005). Study sample sizes may be constrained because test substances might be in short supply; therefore, to allow larger group sizes, intra-arterial drug delivery could enable testing with much lower quantities in each subject.
Conventional methods of drug screening in an animal model of stroke are often conducted by occluding the middle cerebral artery (Macrae, 2011)and delivering the test substance by intravenous or intraperitoneal injection. These methods allow high-throughput modeling, as they are easy to perform, but the obvious problem is that the distribution of the test substances are unpredictable and often dependent on peripheral drug metabolism(Alavijeh et al., 2005). A large quantity of test substance will be metabolized or excreted or both by the liver or kidney before reaching the brain. As a result, a larger dose of test substance is needed to achieve an effective level in the brain. Such larger doses might be accompanied by side effects and complications in other tissues. To solve these issues, there is a need to develop a simple model that allows target delivery of test substances to the brain in a controllable manner. Here we establish a new method for implanting a catheter in the carotid artery that will deliver test substances intra-arterially to ischemic brain.
2. Materials and Methods
2.1 Animals and Evans blue preparation
Adult male Sprague Dawley rats weighing 290–310 grams (obtained from Harlan Laboratories, San Diego, CA) were used in this experiment. Animals were housed under standard conditions (21–23 °C, 12 h light–dark cycle) with unlimited access to standard food and water. All procedures were carried out in accordance with the Institutional Animal Care and Use Committee guidelines. A solution of 4% Evans blue (40mg/mL) was prepared in sterile 0.9% sodium chloride and was protected from light.
2.2 Pre-surgical preparation
2.2.1 Suture and catheter preparation
4-0 Ethilon monofilament suture was heat blunted by waving each filament briefly through a flame; the tip diameter was measured under a microscope (Fig. 1.A) and recorded for later use. To prepare the infusion catheter, 60cm of 2 French silicone tubing (0.3mm ID × 0.6mm OD; Access Technologies; Cat.No. BC-2S) was attached to a 27guage female luer stub adaptor (Access Technologies Cat.No. LSA-27). The adaptor was then attached to a 1mL syringe filled with saline. Two 3 French retention beads (Access Technologies; Cat.No. RB-3S) were sleeved over the distal end of the catheter. The plunger was depressed to expel all of the air and load the catheter line with saline. A bevel was cut on the distal end of the catheter to aid in insertion into the vessel (Fig. 1.B).
Figure 1.
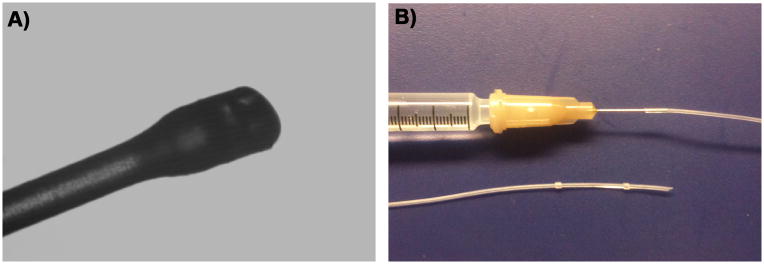
A. 4-0 Ethilon monofilament with tip heat blunted. The widest part of this particular occlusion monofilament measures 308um. B. Catheter set-up. Top: Saline filled 1 cc syringe with a 27 gauge luer stub adaptor and catheter attached. Bottom: 2 French silicone catheter (0.3mmID × 0.6mm OD) with 3 French retention beads sleeved over the distal beveled end.
2.2.2 Animal preparation
Twenty-one animals were weighed and randomly assigned to one of four groups: intra-arterial (IA) infusion during MCAo (n=8), intravenous (IV) infusion during MCAo (n=8), IA infusion without MCAo (IA sham group n=2), and IV infusion without MCAo (IV sham group, n=3). To increase the likelihood of successful MCA occlusion (a known issue with this method), for each animal a heat blunted filament was selected based on the previously measured tip diameter, ranging between 290–310μm. Through extensive trial-and-error, we found a 1:1 ratio to be the most reliable method to select suture size i.e., a 300μm suture tip would be used with a 300g rat. The suture was marked 1.7cm from tip using a wax pencil. This mark served as a visual aid for placement depth when advancing the suture up the internal carotid artery (ICA) later. Anesthesia was induced with 4% isoflurane, in 70% N2O and 30% O2, in a closed plastic induction box in a chemical fume hood. Once induced, the animal was then transferred to the surgery table and fitted into a nose cone with a bite bar for stabilization throughout surgery. The vacuum line for gas scavenging was set to 5L/min. Isoflurane was then reduced to 2–2.5% for maintenance. An empty10cc syringe was placed under the neck to keep the airway open and to aid in the exposure of the carotids. The anesthetized animal then received intraperitoneal (IP) injection of 0.05mg/kg atropine and 0.4mg/kg carprofen subcutaneously (SQ) and the eyes were lubricated with a petrolatum ophthalmic ointment. A lubricated thermometer was then inserted into the rectum for the servo-controlled warming blanket to regulate core body temperature to 37° C. The fur on the ventral cervical area from the mandibles to the sternum was shaved. Another area dorsally between the shoulder blades also was shaved (catheter exit site). From both areas, loose hair was removed with a lint roller, and the skin swabbed with betadine, followed by 70% alcohol. Sterile instruments were organized so that they were within easy reach. All materials and the surgical procedures were performed under sterile conditions.
2.3 Reversible Middle Cerebral Artery Occlusion Surgery
2.3.1 Anesthetic Depth Assessment
Before starting surgery, the depth of anesthesia was assessed by pinching the interdigital skin. If no response was elicited, the surgery was started. If the animal responded, the isoflurane was slightly increased (0.5%), a minute would elapse and the interdigital skin was pinched again.
2.3.2 Approach
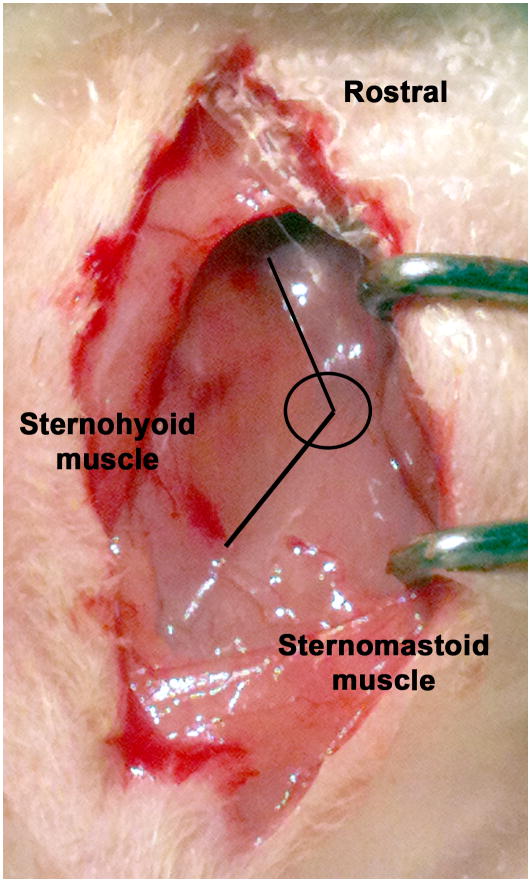
Under the operating microscope with 7X magnification, a midline skin incision was made cranial to the sternum. Blunt dissection was used to expose the intersection of the digastric, sternohyoid, and sternomastoid muscles (Fig. 2). This triangular intersection reliably marks the underlying carotid bifurcation. Cotton tip applicators were used to blot any blood from small vessels. A retractor was positioned so that the sternomastoid and mandibular glands were gently retracted laterally. Blunt dissection was used in the center of the intersection to expose the pulsating common carotid artery (CCA) directly underneath. 4-0 silk was then looped around the belly of the omohyoid muscle that lies directly over and diagonal to the CCA to retract the muscle medially (Supplementary Fig. S1). The weight of hemostats clamped on the ends of the suture kept the muscle retracted medially to improve the exposure of the CCA. The external carotid (ECA), the ICA, and the CCA were bluntly dissected and the surrounding fascia was carefully separated.
Figure 2.
Blunt dissection near the intersection of the sternomastoid and the sternohyoid muscles exposed the underlying carotid bifurcation. Using these landmarks sped dissection and eliminated unnecessary exploration.
2.3.3 Middle Cerebral Artery Occlusion (both IV and IA groups)
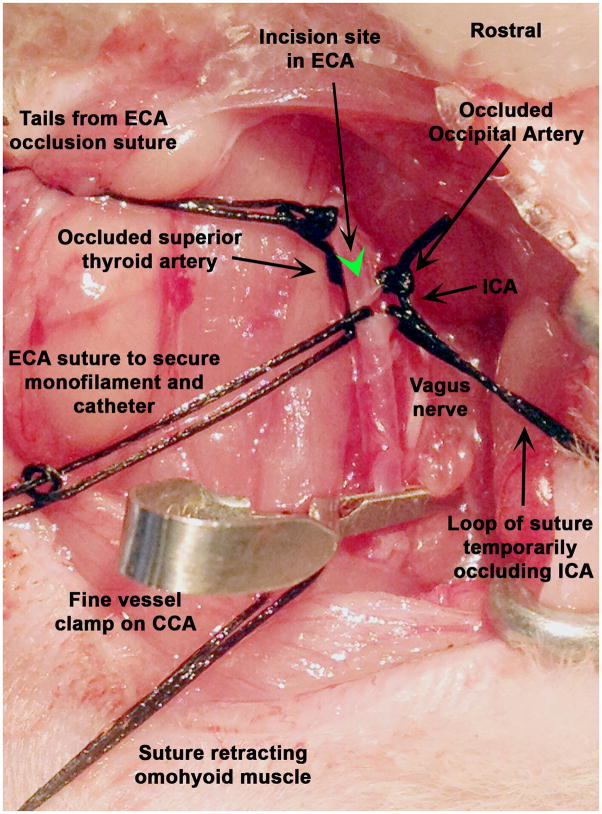
The superior thyroid artery was ligated using 4-0 silk. The tails from this suture were used to gently retract the ECA ventrally to free it from surrounding tissue. The ECA was then ligated with 4-0 silk distal to the superior thyroid artery origin; the tails from the suture on the superior thyroid were then cut. The tails from the suture on the ECA were kept long for securing the catheter later, after placement. The occipital artery was then ligated near its origin using 4-0 silk. A loop of 4-0 silk was placed around the ICA but the knot was not tightened. A loop of silk was placed around the ECA immediately above the bifurcation of the CCA however the knot was not pulled tight. The weight of hemostats at the ends of the sutures occluded the vessels without having to tighten knots or use vascular clamps. Gentle dissection rostrally along the ICA exposed the pterygopalatine artery (PPA), which courses more laterally, thus allowing better visualization of the more medially placed ICA. This rostral dissection along the ICA aided in the insertion of the occlusion filament later. All distal vessels should be identified and retracted before clamping the CCA while the vessels are still distended with blood. A cotton tipped applicator was used to gently free the vagus nerve from the fascia surrounding the CCA. To temporarily occlude the CCA, a Micro Serrefine vessel clamp (Fine Science Tools; Cat. No. 18055-04) was placed as proximally as possible to ensure the deepest seating of the intra-arterial catheter. The vagus nerve was visualized thoroughly to ensure that it had not been included in the clamps’ teeth. Vagal stimulation will cause the animals to gasp for air or cardiac arrest (Heiser, 2007). If gasping, bradycardia, or arrest occurred, the clamp was removed, the vagus nerve was more carefully freed from the surrounding fascia and the clamp was replaced. At this point there were two sutures with tails cut (the superior thyroid and the occipital artery) and three with tails weighted with hemostats (occluded ECA, ICA, and the loop above the bifurcation on the ECA) on the carotids arteries (Fig. 3).
Figure 3.
Set up for the arteriotomy into the external carotid artery (ECA). Note that the vagus nerve has not been included in the clamp on the common carotid.
Using Vannus Spring scissors (3mm cutting edge; Fine Science Tools; Cat. No. 215000-00), an arteriotomy was made in the ECA distal to the bifurcation of the CCA between the two suture loops (one was tightened and one was loose at that point). A small amount of blood oozed from the incision. The arteriotomy was not made extremely close to the bifurcation because fitting a secure suture proximal to the incision but distal to the bifurcation would prove difficult. If excessive or pulsing blood was observed, the ICA was checked to ensure that it was properly occluded. The occlusion monofilament was inserted into the ECA pointing towards the CCA and then curved up towards the ICA. Loosening the loop around the ECA aided in the passage through the bifurcation. Using a finely pointed cotton tip applicator, the filament was gently guided up the ICA (Fig. 4) and the loop on the ECA was re-tightened (not tied). The loop on the ICA was loosened slightly to let the filament pass and this allowed some blood to flow out of the incision in the ECA. Once the filament passed the loop on the ICA, cotton tipped applicators were used to absorb any blood to maintain a clear view of the course of the filament in the ICA. A small narrow cotton tipped applicator was used to gently occlude the origin of the PPA and guide the filament medially up the ICA (Fig. 4). The filament was advanced until the mark (1.7cm) reached the bifurcation of the ICA and ECA. Resistance was felt as the tip entered the proximal segment of the anterior cerebral artery and thus blocked the ostium of the middle cerebral artery (MCA). If the filament could only be advanced 0.5–0.7 cm, it had entered the PPA, and was withdrawn until the tip could be visualized at the loop on the ICA. A cotton tipped applicator was placed a bit more ventrally to block the PPA and the filament was again advanced up the ICA. Once the filament had been advanced the correct distance, the occlusion time was noted. The filament was trimmed so that 5–7mm was extending out of the ECA.
Figure 4.
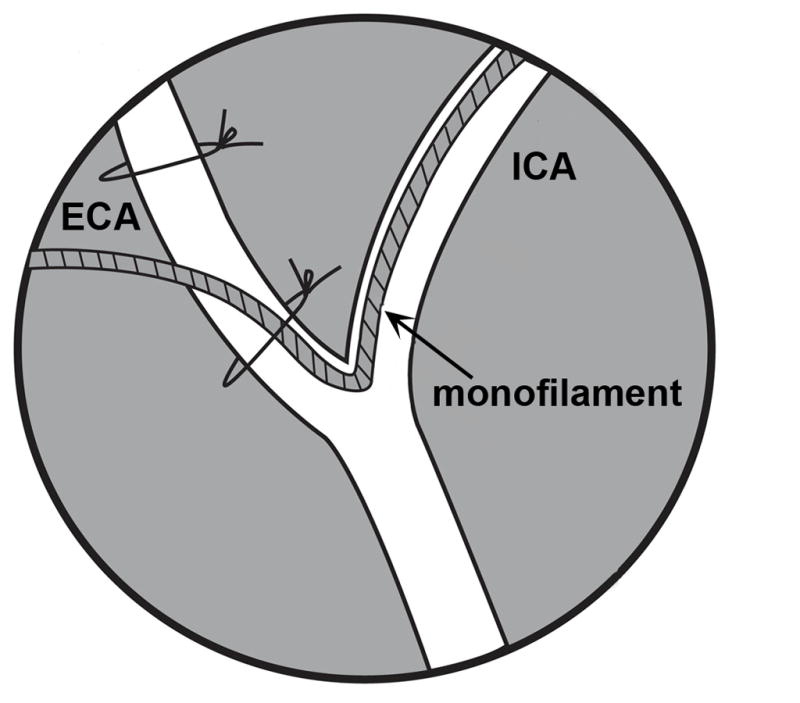
Schematic showing the monofilament placed in the external (ECA) and coursing up the internal (ICA) carotid arteries.
2.4 Placement of Catheters
2.4.1 Placement of jugular catheter
In the group of animals that were to receive jugular infusions, the suture surrounding the monofilament and ECA was then tightened to secure the monofilament. Catheters were then placed in the jugular vein as described previously (Heiser, 2007). Briefly, on the same side of the occlusion, the jugular vein was identified, dissected and ligated. Through a small venotomy, the silicone catheter was advanced approximately 12mm and secured with two sutures; one distal to the venotomy and one proximal. The catheter was then tunneled around the side of the neck and secured as described for the arterial catheter. The sham IV group did not receive MCAo, but did receive the jugular catheter. If the animal was in the intra-arterial group (both MCAo and sham), a catheter was placed in the common carotid as described below.
2.4.2 Placement of intra-arterial catheter
A catheter was placed in the CCA for both the group that received the MCAo and the sham group that received the IA infusion.
First, the suture loop around the ECA just above the bifurcation was loosened. The catheter was then introduced into the incision in the ECA. Inserting the catheter behind the filament eased the insertion. Care was taken not to pull or push on the filament as it was not secured. The catheter was advanced and a cotton tipped applicator was used to guide the tip down the CCA oriented retrograde (Fig. 5). The catheter had a tendency to follow the course of the filament up the ICA anterograde. The catheter was advanced as far down the CCA as possible. The placement of the proximal vessel clamp limited the extent of the catheter advancement down the CCA. Next the loop of suture around the ECA (that also encircled the filament and the catheter) was tightened, but not excessively as this would occlude the catheter. This suture must be proximal to the incision in the ECA or bleeding would occur.
Figure 5.
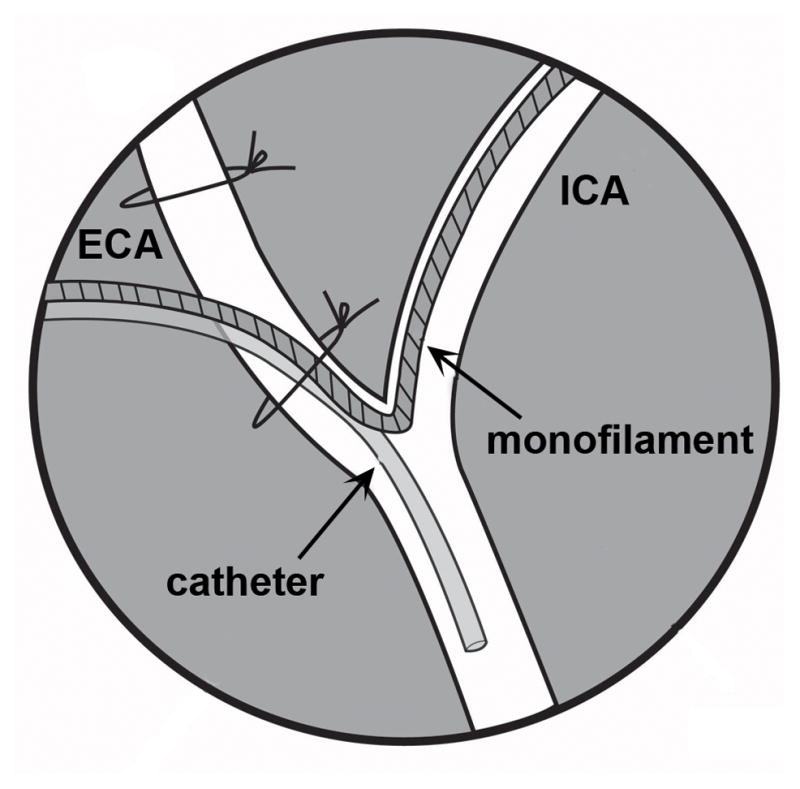
Schematic of catheter placement in the common carotid artery (CCA).
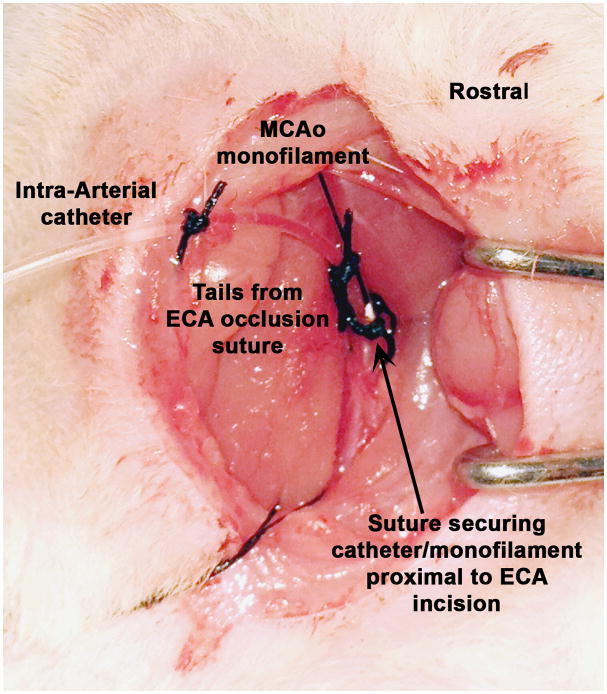
Rubber-shod hemostats (hemostat with plastic tubing covering the teeth; Supplementary Fig. S2) were clamped on the catheter close to the syringe. The loop of suture from the ICA was then removed. To ensure the catheter had not been occluded by the suture on the ECA, the rubber-shod hemostats were briefly unclamped from the catheter and the syringe pulled to check if blood could be withdrawn into the line. The blood was then slowly flushed back through the line. The rubber-shod hemostats were re-clamped on the catheter. Once the first suture had been tightened, the first retention bead was moved down the catheter so that it sat immediately above the incision in the ECA and above the first suture. The tails from the suture that occluded the ECA were then looped back around the ECA to now include the catheter and another knot was made (Fig. 6). This suture was positioned right above the retention bead. Carefully and slowly, the clamp from the CCA was removed. The ECA incision was observed for bleeding. Any bleeding indicated that the first suture around the ECA was insufficient. If this was the case, another loop of 4-0 silk suture was passed around the ECA/catheter/filament, ensuring that the suture is proximal to the incision, and tightened (but not overly tight as to occlude the catheter).
Figure 6.
Catheter and monofilament in place and secured.
2.4.2. Securing catheter and exit from body
A small segment of 4-0 silk suture was passed through the superficial fascia in the neck lateral to catheter exit site and was used to secure the catheter to the body wall. This suture was positioned right above the second retention bead. Due to the direction of the catheter exiting from the ECA, it was usually best to tunnel the catheter around the opposite side of the body. Care was taken to ensure that there was enough slack so that when the animal flexed its neck there would not be excessive force on the catheter or pressure on the trachea. The animal was then turned over to lateral recumbency (for a left MCAo the animal would be turned to left recumbency i.e. the catheter would be tunneled out the right side of the neck). A small cut was then made on the nape of the neck. Sharp hemostats, starting closed and staying close to the skin, were then pushed through to the ventral incision. The end of the catheter was grabbed with the hemostats and rubber-shod hemostats were unclamped. The catheter was removed from the stub adaptor and pulled through the tunnel to back of neck and out through the small skin incision. Care was taken not to pull the catheter too tight as this would either occlude the trachea or pull the catheter from the CCA; considerable slack was left in the catheter to allow free movement. The rubber-shod hemostats were replaced on the catheter and the terminal segment, where the hemostats were clamped (the silicone was possibly punctured), was trimmed. The catheter was then reattached to the adaptor on the syringe. The animal was turned to dorsal recumbency and the skin incision was closed with 4-0 Prolene being careful to avoid the catheter with the suture needle while closing. A single suture was placed in the skin (and encircled the catheter as well) to close the incision on the nape of the neck. Care was taken to not tighten the knot excessively. The entire procedure took approximately (MCAo and catheter placement) 20–25 minutes.
2.5 Recovery
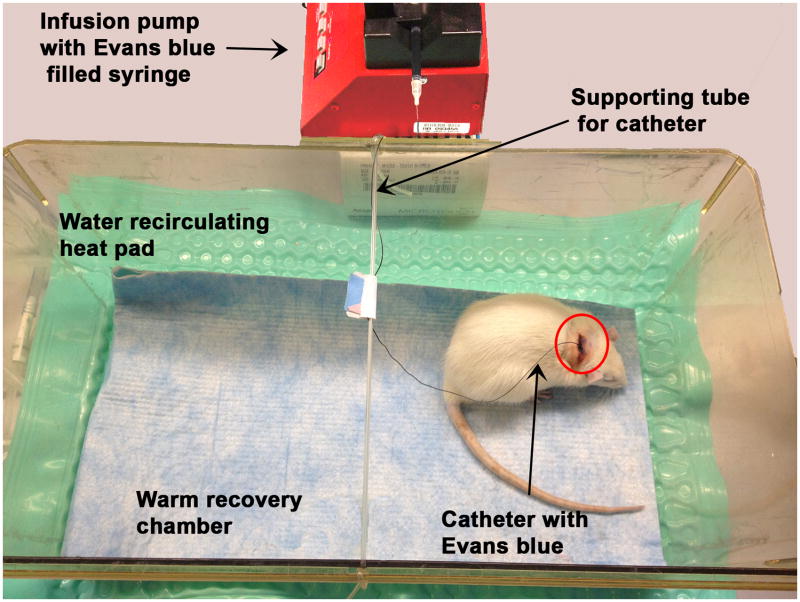
The animal was removed from anesthesia and transferred to a pre-warmed recovery chamber. 5cc Lactated ringers saline was administered subcutaneously. A syringe containing 4% Evans blue was attached to the catheter and the syringe plunger was slowly depressed until the Evans blue filled the entire catheter line. The syringe was placed into a syringe pump (Braintree Scientific, Inc.; Cat. No. BS-300) and the desired rate was set (here 0.2mL/hr or 26.67mg/kg/hr). The animal remained tethered in the recovery cage receiving a continuous infusion of Evans blue during the entire 4 hour MCAo. A piece of tubing stretched across the top of the recovery cage provided a support to wrap the catheter up and over on its way to the syringe pump (Fig. 7). MCAo animals will often circle and the torque from the twisting on the catheter may dislodge the catheter, therefore the animals were monitored closely, especially immediately upon waking up.
Figure 7.
Rat in warmed recovery chamber receiving infusion of Evans blue for the period of the middle cerebral artery occlusion. Note the tubing stretched across the top of the cage to serve as a support for the catheter line.
2.6 Reperfusion and catheter removal
2.6.1 Reperfusion
At the end of the 4 hours occlusion, the pump was stopped and the catheter was clamped with the rubber-shod hemostats. The adaptor was removed from the syringe and a cap (Becton Dickinson Vascular Access; Cat. No. 388161) was placed on the adaptor. The rubber-shod hemostats were removed. The necessary neurologic exams were then preformed (forelimb withdrawal, twisting to one side when suspended by tail and circling). Anesthesia was induced as previously described and the anesthetized animal was then moved to the surgery table. The skin was prepped as before. The ventral skin incision was reopened and cotton tipped applicators were used to absorb any serum that had accumulated. Retractors were placed to gently pull the sternomastoid and mandibular glands laterally. A loop of 4-0 silk was looped around the ICA and the ends of the suture were weighted with hemostats to occlude the ICA temporarily. The CCA was re-clamped with a fine vessel clamp. Suture was passed around the ECA/catheter/filament complex (ECA/filament in the animals receiving IV infusions) but not tightened at that time. The filament was removed from the ICA slowly.
2.6.2 Removal of catheter
In the animals that received IA infusion, the sutures that were holding the catheter in place were cut and the catheter was slowly removed from the CCA. The catheter may have been included in the vessel clamp but with a little effort, would come loose. Care was taken to not pull too hard as tearing of the CCA might occur. The suture around the ECA was then tightened. Next, the loop of suture around the ICA was removed and the ECA was observed for bleeding. The clamp from the CCA was then removed and again the ECA was checked for bleeding. The jugular catheter was also removed from the animals that received an IV infusion. After removal of the retention beads from the end, the catheter was pulled out the back of the animal through the skin incision in the neck (it was unnecessary to remove the suture from the nape of the neck if the retention beads had been removed from the end first). Finally, the ventral skin incision was closed with 4-0 Prolene. The anesthesia was discontinued and the animal was moved to the pre-warmed recovery chamber. The entire reperfusion process took about 10 minutes.
2.7 Neurologic Exam
We used a standardized examination of rat neurologic function modified from a published method (Bederson et al., 1986). First we looked for the animal to withdraw a forelimb when suspended by its tail. While suspended, we also looked for the animal to twist towards the contra-lateral side of the occlusion. Finally, we observed the animal for circling behavior. For each abnormal finding, we gave the score of 1 point for a total possible score of 3.
2.8 Sacrifice and Evans blue quantification
After a 30 minutes reperfusion period, the animals were sacrificed with an overdose of anesthetic administered intra-peritoneally, followed by trans-cardiac perfusion with warm 0.9% saline. Each hemisphere was weighed and then homogenized in 10X phosphate buffered saline (PBS). An equal amount of cold 60% trichloroacetic acid (TCA; dissolved in water) was added; the sample was vortexed vigorously for 2 minutes and then incubated at 4°C for 30 minutes. Following 10 minutes of centrifugation at 18,000rpm at 4°C, a 1.5mL aliquot of the supernatant was measured in the spectrophotometer at 620nm (Kaya and Ahishali, 2011). A set of standard samples were prepared in the same way by adding 0, 0.1, 0.2, 0.4, 0.8, 1.6, 3.2, 6.4, and 12.8μg/mL Evans blue, respectively to a 50:50 mixture of 60%TCA and 10X PBS, and aliquots measured at 620nm in the spectrophotmeter. The OD620 for each brain sample was compared to the standard curve and the concentration of contained Evans blue was estimated in μg/ml. For calculation of Evans blue dye IV sham was considered as control and the average OD of IV sham was subtracted from the rest of the groups for normalizing the background OD. The quantity of Evans blue per gram of tissue was then estimated by multiplying the sample’s concentration by the volume of suspension liquid and then divided by the wet weight of the hemisphere.
3. Results and Discussion
3.1 Neurologic results
All animals, except the sham group, and except one animal in the IV group which received a score of 2, exhibited sign of stroke and received a score of 3.
3.2 Amount of dye leaked into each hemisphere
Both sham animals (IV and IA) had no to minimal amounts of Evans blue dye leakage in both the left and right hemispheres. Increased Evans blue dye leakage was observed in the brains of the group receiving dye intra-arterially compared to the group receiving via intravenous (Figure 8A). The group that received the dye intravenously during the MCAo had an average±SD of 2.04±1.424 in the ipsilateral and 0.355±0.667μg/g of dye in the contralateral hemispheres. The group that received the dye intra-arterially during the MCAo had an average± SD of 4.963±3.334 in the ipsilateral and 1.329±1.026μg/g of dye in the contralateral hemispheres. A two-tailed student t-test revealed a significant difference between the IA and IV groups (P=0.047) and IA and Sham group (P=0.004) (Fig. 8b).
Figure 8.
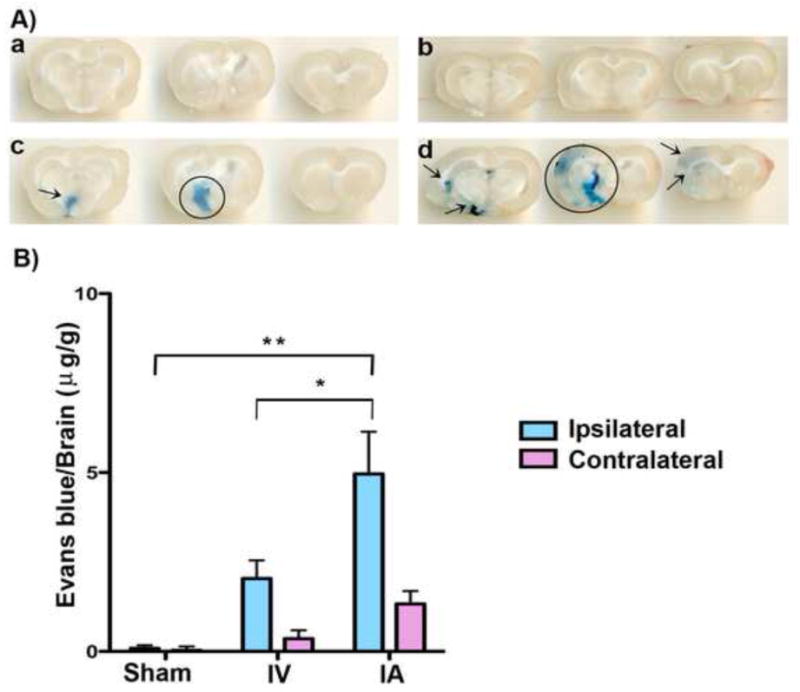
A) Representative photomicrographs illustrating increased Evans blue extravasation into the Intra-arterial (IA) infusion (d) delivered brain, compared to IA sham (b), intravenous (IV) sham (a) and IV infusion (c). Evans blue extravasation is indicated by circle and arrows. B) Measurements of leaked Evans blue dye for the four test groups. IA infusion delivered significantly more substances compared to intravenous IV delivery to the ischemic brain (**two-tailed student t-test, P<0.01).
3.3 Discussion
This combined occlusion/infusion model takes advantage of the fact that a nylon occluding monofilament leaves a residual flow of about 20% in the hemisphere so there is sufficient residual blood flow that will take the substance from the carotid artery to the branches of middle cerebral artery (Yang and Betz, 1994). The animals that received the Evans blue dye intra-arterially had significantly more dye in the ipsilateral, ischemic hemisphere than the animals that received the infusion intravenously. A molecule of the size of Evans blue (molecule weight 961Da in its free form or 66KDa when bound to serum albumin) can enter the brain through the BBB opened during acute phase of ischemia (Belayev et al., 1996; Chen et al., 2010). In experimental set-ups that delay drug infusion until after de-occlusion, all of the restored blood flow carries the test substance anterograde into the ischemic territory.
There are several benefits of the intra-arterial delivery approach over intravenous delivery: firstly, arterial infusion targets the ischemic region through an endogenous mechanism of BBB opening; secondlyit increases the efficiency of the delivery and as a result decreases the total amount of substances needed (Joshi et al., 2008). This efficiency is especially important when test substances may be expensive or in short supply. Further, smaller test infusions may reduce potential systemic side effects. Finally, intra-arterial delivery allows the investigator to infuse the substances in a controlled manner at a given time for a specified duration without confounds from variable absorption (e.g. after intra-peritoneal administration) or variable metabolism (e.g. after intravenous administration). We have used this model successfully in a number of studies (Chen et al., 2010; Chen et al., 2012).
Supplementary Material
Highlights.
We describe a novel method to deliver test substances directly into the ischemic area of the brain during stroke in rats.
This method provides a powerful tool in the pre-clinical testing of ischemic stroke therapies.
We validate our method by comparing the amount of dye present in the ischemic brain after intra-arterial verses intravenous administration routes.
We have successfully used this method in over 100 rats.
Acknowledgments
This study was supported by a grant from NINDS R01NSO754930-01
Footnotes
Conflict of Interest
The authors have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alavijeh MS, Chishty M, Qaiser MZ, Palmer AM. Drug metabolism and pharmacokinetics, the blood-brain barrier, and central nervous system drug discovery. NeuroRx. 2005;2:554–71. doi: 10.1602/neurorx.2.4.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–6. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- Belayev L, Alonso OF, Busto R, Zhao W, Ginsberg MD. Middle cerebral artery occlusion in the rat by intraluminal suture. Neurological and pathological evaluation of an improved model. Stroke. 1996;27:1616–22. doi: 10.1161/01.str.27.9.1616. discussion 23. [DOI] [PubMed] [Google Scholar]
- Chen B, Cheng Q, Yang K, Lyden PD. Thrombin mediates severe neurovascular injury during ischemia. Stroke. 2010;41:2348–52. doi: 10.1161/STROKEAHA.110.584920. [DOI] [PubMed] [Google Scholar]
- Chen B, Friedman B, Whitney MA, Winkle JA, Lei IF, Olson ES, Cheng Q, Pereira B, Zhao L, Tsien RY, Lyden PD. Thrombin activity associated with neuronal damage during acute focal ischemia. J Neurosci. 2012;32:7622–31. doi: 10.1523/JNEUROSCI.0369-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M, Tatlisumak T. Use of animal models has not contributed to development of acute stroke therapies: con. Stroke. 2005;36:2324–5. doi: 10.1161/01.STR.0000179039.76922.e8. [DOI] [PubMed] [Google Scholar]
- Heiser A. Rat Jugular Vein and Carotid Artery Catheterization for Acute Survival Studies. Vol. 33. Spring Science+Business Media; New York: 2007. pp. 7–48. [Google Scholar]
- Joshi S, Meyers PM, Ornstein E. Intracarotid delivery of drugs: the potential and the pitfalls. Anesthesiology. 2008;109:543–64. doi: 10.1097/ALN.0b013e318182c81b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi M, Furuya H, Patel PM. Neuroprotective effects of anesthetic agents. J Anesth. 2005;19:150–6. doi: 10.1007/s00540-005-0305-5. [DOI] [PubMed] [Google Scholar]
- Kaya M, Ahishali B. Assessment of permeability in barrier type of endothelium in brain using tracers: Evans blue, sodium fluorescein, and horseradish peroxidase. Methods Mol Biol. 2011;763:369–82. doi: 10.1007/978-1-61779-191-8_25. [DOI] [PubMed] [Google Scholar]
- Macrae IM. Preclinical stroke research--advantages and disadvantages of the most common rodent models of focal ischaemia. Br J Pharmacol. 2011;164:1062–78. doi: 10.1111/j.1476-5381.2011.01398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tymianski M. Can molecular and cellular neuroprotection be translated into therapies for patients?: yes, but not the way we tried it before. Stroke. 2010;41:S87–90. doi: 10.1161/STROKEAHA.110.595496. [DOI] [PubMed] [Google Scholar]
- Yang GY, Betz AL. Reperfusion-induced injury to the blood-brain barrier after middle cerebral artery occlusion in rats. Stroke. 1994;25:1658–64. doi: 10.1161/01.str.25.8.1658. discussion 64–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.