Abstract
We have previously shown that hepatitis B virus (HBV) replication is controlled by noncytolytic mechanisms that depend primarily on the effector functions of the CD8+ T cell response, especially the production of IFN-γ in the liver. The mechanisms that control the nuclear pool of viral covalently closed circular DNA (cccDNA) transcriptional template of HBV, which must be eliminated to eradicate infection, have been difficult to resolve. To examine those mechanisms, we quantitated intrahepatic HBV cccDNA levels in acutely infected chimpanzees whose virological, immunological, and pathological features were previously described. Our results demonstrate that the elimination kinetics of the cccDNA are more rapid than the elimination of HBV antigen-positive hepatocytes during the early phase of viral clearance, and they coincide with the influx of small numbers of IFN-γ producing CD8+ T cells into the liver. In contrast, terminal clearance of the cccDNA is associated with the peak of liver disease and hepatocellular turnover and with a surge of IFN-γ producing CD8+ T cells in the liver. Collectively, these results suggest that cccDNA clearance is a two-step process mediated by the cellular immune response. The first step reduces the pool of cccDNA molecules noncytolytically, probably by eliminating their relaxed circular DNA precursors and perhaps by destabilizing them. The second step enhances this process by destroying infected hepatocytes and triggering their turnover. Surprisingly, despite this multipronged response, traces of cccDNA persist indefinitely in the liver, likely providing a continuous antigenic stimulus that confers lifelong immunity.
Hepadnaviruses are small enveloped hepatotropic DNA viruses containing a relaxed circular DNA genome, which is converted into a covalently closed circular DNA (cccDNA) episome in the nucleus of infected cells (1). The cccDNA molecule serves as the transcriptional template for production of viral RNAs that are exported to the cytoplasm and encode the viral structural and nonstructural proteins, including the reverse transcriptase/DNA polymerase. Reverse transcription of the viral pregenomic (pg)RNA and second-strand DNA synthesis occurs in the cytoplasm within viral capsids formed by the HBV core protein (HBcAg). Capsids containing the mature relaxed circular DNA genome are either enveloped and exported as virions, or they deliver their DNA content to the nucleus, thereby amplifying the pool of cccDNA molecules to 10-50 copies per cell (2-5). The factors that regulate the cccDNA content of infected cells are not very well understood. In the duck hepatitis B virus (DHBV) model, the cccDNA level is regulated by the viral envelope proteins (6). Evidence from the woodchuck hepatitis virus (WHV) system suggests that cccDNA clearance is tightly associated with the destruction and turnover of infected hepatocytes (7, 8). The possibility that cccDNA may also be susceptible to control by immune-mediated noncytolytic mechanisms is an open question at this time, at least partially due to uncertainty regarding its half-life.
Noncytolytic immune-mediated mechanisms that inhibit HBV replication have been well described in HBV transgenic mice (9) and in HBV-infected chimpanzees (10, 11). In mouse experiments, it was shown that HBV-specific CD8+ T cells and other stimuli that trigger the production of IFN-γ or IFN-α/β in the liver rapidly inhibit viral replication by eliminating the pgRNA-containing capsids from the cytoplasm of the hepatocyte. In addition, the viral RNA is eliminated from the cell by a delayed posttranscriptional mechanism mediated by IFN-γ and tumor necrosis factor α (12, 13). Unfortunately, HBV transgenic mice do not produce viral cccDNA, so it has not been possible to examine the mechanism(s) that eliminate it from the cell in that model.
To further elucidate the pathogenetic mechanisms responsible for viral clearance, we have monitored the virological and immunological features of HBV infection on a weekly basis in the liver of acutely infected chimpanzees. In early studies, we demonstrated that viral clearance is tightly associated with the entry of HBV-specific T cells into the liver, and that viral replication is rapidly inhibited as much as 50-fold in the absence of significant liver disease or hepatocellular turnover, both of which peak much later in the infection and coincide with its termination (11). Furthermore, we showed that the kinetics of inhibition of viral replication as well as the kinetics of disease pathogenesis and final viral clearance could be greatly delayed by the infusion of monoclonal anti-CD8 antibodies that rapidly but transiently deplete CD8+ T cells from the animal (10). Although the viral cccDNA appeared to be cleared with similar kinetics as the viral replicative DNA intermediates in those studies, the results were not quantitative, so we could not determine the precise clearance kinetics of the cccDNA from the liver (10, 11).
In the current study, therefore, we used quantitative PCR methodology to monitor the amplification and elimination of HBV cccDNA purified from the liver of two chimpanzees on a weekly basis throughout the course of infection. The results were compared to the kinetics of intrahepatic viral gene expression and replication, the number of HBcAg-positive hepatocytes, and histological and biochemical evidence of liver disease and hepatocellular turnover in the same animals. The results suggest that viral replication and the cccDNA content of the liver are suppressed by a CD8-dependent noncytopathic process during the early phase of viral clearance. The data also indicate that these events are later supplemented and amplified by a CD8-dependent cytolytic process and hepatocellular turnover that coincide with the peak of liver disease. We conclude that the HBV cccDNA episome is susceptible to noncytopathic and cytopathic control mechanisms, and that both processes appear to be required to terminate the infection.
Materials and Methods
Chimpanzees. Chimpanzees (Ch)1627 and 1620, two healthy young adult HBV-seronegative chimpanzees, were used in this study. The animals were handled according to humane use and care guidelines specified by law and approved by the Animal Care and Use Committees of the National Institute of Allergy and Infectious Diseases, The Scripps Research Institute, and Bioqual, Incorporated (Rockville, MD), where they were housed. Bioqual, Incorporated is an American Association for Accreditation of Laboratory Animal Care International-accredited institution under contract to the National Institute of Allergy and Infectious Diseases. Both animals were inoculated with 108 genome equivalents of a monoclonal HBV (genotype ayw) contained in pooled serum from HBV transgenic mice (14). Before inoculation and each week thereafter, blood was obtained by venipuncture and analyzed for serum alanine aminotransferase (sALT) activity, as described (15).
CD8+ T Cell Depletion. CD8 depletion was achieved in Ch1620 by i.v. administration of a humanized chimeric monoclonal anti-human CD8 antibody cM-T807 (16) just before the peak of infection, as described (10).
Liver Biopsy. Before infection and each week thereafter, liver tissue was obtained by hepatic needle biopsy. In most instances, several tissue fragments 5 mm in length were obtained. One fragment was fixed in 10% zinc formalin solution for subsequent histological examination; and the other fragments were snap-frozen and shipped to The Scripps Research Institute by overnight express for subsequent RNA, DNA, and cccDNA isolation.
Immunhistochemical Analysis. Formalin-fixed liver biopsy fragments were paraffin-embedded, sectioned (3 μm), stained for hematoxylin/eosin, and intracellular distribution of HBcAg and proliferating cell nuclear antigen (PCNA) was assessed exactly as described (11, 15).
HBV DNA Detection in the Liver. Total liver DNA was extracted from frozen liver biopsy samples as described (11), and the levels of HBV DNA replicative intermediates were determined by quantitative real-time PCR by using a Bio-Rad iCycler system exactly as described (10).
Isolation and Quantitation of Intrahepatic cccDNA. Low-molecular-weight nucleic acids comprising HBV cccDNA and host mitochondrial DNA were isolated from liver biopsies, as described (17, 18). Briefly, liver biopsies were homogenized in a Potter-Elvehjem tissue grinder with 200 μl of homogenization buffer [50 mM Tris·HCl (pH 8)/1 mM EDTA/0.2% Nonidet P-40/0.15 M NaCl]. Nuclei in the homogenate were lysed by the addition of 200 μl of lysis buffer [6% (vol/vol) SDS/0.1 M NaOH]. The reaction was mixed thoroughly and incubated for 30 min at 37°C with occasional mixing. The alkaline lysate was neutralized by the addition of 100 μl of neutralization buffer [3 M KAc (pH 4.8)] and centrifuged for 15 min at 4°C at 12,000 × g. The supernatant was extracted with 600 μl of water-saturated phenol (pH 4.5) followed by chloroform extraction, and nucleic acids were precipitated with isopropanol in the presence of 10 μg of Escherichia coli tRNA. The precipitate was dissolved in 50 μl of TE [10 mM Tris·HCl (pH 7.5)/1 mM EDTA]. Fifteen microliters of the isolated low molecular-weight DNA samples was used for HBV-specific Southern blot analysis, as described (14), and the cccDNA content in 5 μl of appropriately diluted sample was determined by HBV-specific quantitative real-time PCR, as described (10). Another 5 μl of appropriately diluted low-molecular-weight DNA sample as well as an aliquot of total liver DNA were used for mitochondrial DNA quantitation by SYBR-green PCR by using SYBR Green PCR Master Mix (Applied Biosystems), 200 nM forward primer PT mito 8686 (5′ CCCTCTCGGCCCTCCTAATAACCT-3′), and 200 nM reverse primer PT mito 8796 (5′-GCCTTCTCGTATAACATCGCGTCA-3′). Ten-fold serial dilutions (108-103 copies) of PCR-amplified mitochondrial DNA fragment were used as standards in parallel mitochondrial DNA-specific PCR reactions. Quadruplicate PCR reactions were performed for all time points. The mitochondrial DNA content was expressed as copies of mitochondrial DNA per genomic DNA content of a cell (≈6 pg). This number was used to calculate cccDNA copies/hepatocyte, assuming a proportionality of the liver size with that of humans, adjusted for weight (4 × 1010 hepatocytes). The linearity of the HBV-specific PCR allowed for quantitation of ≥0.01 cccDNA copies per hepatocyte.
RNA Isolation and RNase Protection Assay. Frozen liver tissues were mechanically pulverized, and RNA was extracted by the acid-guanidinium phenol-chloroform method (19). Total RNA (20 μg) was analyzed by the RNase protection assay for IFN-γ, CD3 and L32 mRNA expression (11), and quantitation of HBV gene expression (20), as described.
Results
HBV cccDNA Content in Serial Liver Biopsies. To monitor intrahepatic cccDNA content during the course of acute HBV infection, low-molecular-weight cccDNA was isolated from serial liver biopsies from Ch1627 and Ch1620 (10) and subjected to Southern blot analysis, as described in Materials and Methods. A representative sample from Ch1620 obtained at the peak of infection (Fig. 1, lane 1) shows one major DNA species migrating with the expected molecular weight of cccDNA when compared to HBV DNA replicative intermediates present in total DNA derived from the same chimpanzee (lane 3). Importantly, the DNA species in the cccDNA sample were converted into linear DNA by a single restriction enzyme digest (Fig. 1, lane 2) when compared with a linear HBV fragment (lane 4), confirming they indeed represented cccDNA. For subsequent quantitation of cccDNA, we subjected isolated low-molecular-weight DNA from all samples to HBV- and mitochondrial-DNA-specific quantitative real-time PCR, and we calculated the liver cccDNA content as the number of cccDNA copies per hepatocyte, as described in Materials and Methods.
Fig. 1.
Southern blot analysis of cccDNA isolated from chimpanzee liver biopsies. Low-molecular-weight DNA was isolated from a liver biopsy obtained from Ch1620 at the peak of infection (week 12), as described in Materials and Methods. Ten microliters of extracted nucleic acids was subjected to HBV-specific Southern blot analysis (lane 1). Another 10 μl of the week 8 sample was linearized by XhoI digestion in a 100-μl reaction before Southern blot analysis (lane 2). Lane 3 shows HBV replicative intermediates in 10 μg of total HBV DNA (T) in the liver of Ch1620 in week 12. A linear EcoRI HBV fragment (F) was included as a size marker (lane 4). rcDNA, relaxed circular HBV DNA; linear, one genome-copy-length HBV fragment; ssDNA, single-stranded HBV DNA.
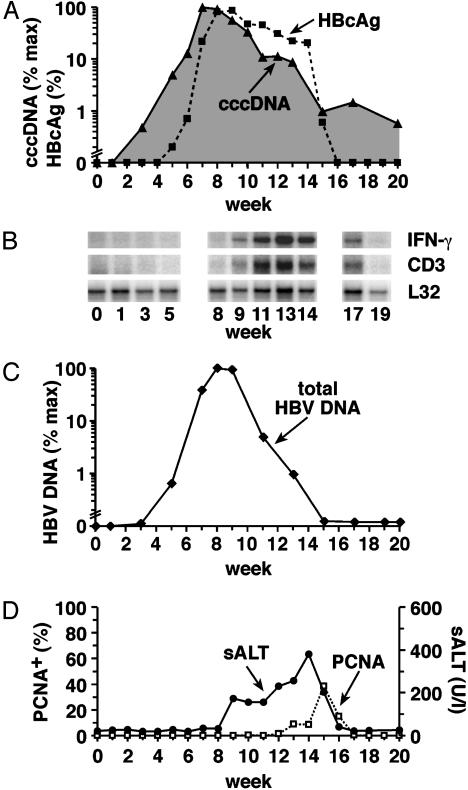
Kinetics of Viral Expansion. As shown in Figs. 2A and 3A, the viral cccDNA was first detected in the liver of both chimpanzees ≈3-4 weeks after inoculation, and it increased rapidly with a doubling time of 3.8-4.2 days until reaching peak levels 7-9 weeks later. The kinetics of expansion of viral pgRNA (Fig. 3C) and replicative DNA intermediates in the liver (Figs. 2C and 3C) and HBcAg-positive hepatocytes (Figs. 2 A and 3A) were either nearly identical to the cccDNA or slightly delayed, as expected. At the peak of infection, 86% and 99% of the hepatocytes were HBcAg-positive in Ch1627 and Ch1620, respectively, and each contained on average ≈10 copies of cccDNA per cell. The virus remained at peak levels until CD3 and IFN-γ mRNA appeared in the liver (Figs. 2B and 3B), whereupon all of the viral components began to decline, albeit with differing kinetics.
Fig. 2.
Kinetics of HBV cccDNA during acute HBV infection in the control Ch1627. (A) Intrahepatic HBV cccDNA (shaded area with black triangles) was quantitated by real-time PCR and is expressed as a percentage (%max) of the corresponding peak of cccDNA in the liver of this animal. HBcAg-positive hepatocytes (black squares) are expressed as a percentage (%) of the total number of hepatocytes. (B) Total RNA isolated from liver biopsy samples was analyzed for the expression of IFN-γ, CD3, and L32 by a RNase protection assay, as described in Materials and Methods. The L32 signals reflect the amount of RNA used in the assay. (C) Total HBV DNA replicative intermediates (black diamonds) were quantitated by real-time PCR and are expressed as a percentage (%max) of the corresponding peak value. (D) PCNA-positive (open squares) hepatocytes are displayed as a percentage (%) of the total number of hepatocytes. sALT activity (black circles) is expressed in units per liter (U/l).
Fig. 3.
Kinetics of HBV cccDNA during acute HBV infection and CD8 depletion in Ch1620. (A) Intrahepatic HBV cccDNA and HBcAg-positive hepatocytes are displayed as described in the legend to Fig. 2 A. The number of CD3+ CD8+ T cells is expressed as a percentage of the total number of CD3+ T cells in the peripheral blood. (B) Analysis of IFN-γ, CD3, and L32 expression as described in Fig. 2B.(C) Intrahepatic levels of pgRNA were determined by RNase protection assay. Total HBV DNA (black diamonds) and pgRNA (open circles) are expressed as a percentage (%max) of their corresponding peak value. (D) PCNA-positive hepatocytes are expressed as a percentage of the total number of hepatocytes and sALT activity (black circles) are displayed as units per liter (U/l). See the legend to Fig. 2 for all other details. The arrow indicates time of CD8 depletion.
Kinetics of Viral Contraction. Control Ch1627. As shown in Fig. 2B, CD3 and IFN-γ mRNA were first detectable in the liver between weeks 7 and 9 after infection, at which time the number of HBcAg-positive hepatocytes was still rising (Fig. 2 A), suggesting that viral spread was still occurring at that time. Nonetheless, the cccDNA content of the liver began to fall during that interval (Fig. 2 A), suggesting that the copy number was falling in each infected cell. Importantly, there was little or no evidence of liver disease or hepatocellular turnover at that time (Fig. 2D), suggesting that the cccDNA content of the liver was regulated by noncytopathic mechanisms during and immediately after the peak of infection in this animal. Consistent with this notion, the cccDNA was observed to fall much more rapidly than the number of HBcAg-positive hepatocytes during weeks 9-11 after infection (Fig. 2 A), coinciding with an increase in the CD3 and IFN-γ mRNA content of the liver (Fig. 2B) and a slight increase in sALT activity but in the absence of any evidence of liver cell turnover (Fig. 2D). Expressed quantitatively, as shown in Table 1, the cccDNA content of the liver decreased 8.6-fold between weeks 8-12, whereas the number of HBcAg-positive hepatocytes fell only 3.2-fold, suggesting that noncytolytic mechanisms were probably responsible for cccDNA control at this time. It is important to note that the HBV DNA replicative intermediates (identified as total HBV DNA in Fig. 2C) decreased 33-fold during this period (Table 1), indicating they were more susceptible to noncytolytic control than the cccDNA. After week 12, we observed a peak in the hepatic content of CD3 and IFN-γ mRNA (Fig. 2B), a surge in the severity of liver disease, and the onset of a significant degree of hepatocellular turnover (Fig. 2D). This coincided with a progressive decrease in both the hepatic content of cccDNA and HBcAg-positive hepatocytes (Fig. 2 A). Actually, the number of HBcAg-positive hepatocytes decreased >100-fold between weeks 12 and 17, whereas the cccDNA decreased only 11-fold during the same period, suggesting that cytopathic mechanisms were playing a dominant role in the clearance of the cccDNA as well as the replicative intermediates at that point. It is noteworthy that, despite the cooperative effects of these noncytopathic and cytopathic events, the cccDNA was not completely eliminated, remaining detectable at ≈0.1 copies per hepatocyte in week 20 (Fig. 2 A) and persisting in the liver at 0.01-0.1 copies per hepatocyte for >2 years after infection (data not shown).
Table 1. Changes in virological markers during elimination of HBV from the liver of acutely infected chimpanzees.
| Chimpanzee | Weeks | cccDNA* | pgRNA* | Total HBV DNA*† | HBcAg*‡ |
|---|---|---|---|---|---|
| 1627 | 8-12 | 8.6 | ND§ | 33.0 | 3.2 |
| 12-17 | 11.0 | ND§ | ≥50 | ≥100 | |
| 1620 | 12-18 | 7.3 | 7.3 | 40.0 | 1.5 |
| 18-28 | 2.5 | 2.7 | 1.6 | 3.3 | |
| 28-32 | 9.0 | ≥10 | ≥50 | ≥30 |
Fold decrease.
All forms of DNA replicative intermediates.
Percentage of HBcAg-positive hepatocytes.
Not determined.
CD8-depleted Ch1620. The sequential nature of the noncytopathic and cytopathic events illustrated in Ch1627 was more easily observed in Ch1620, in which the entire course of infection, including the clearance process, was delayed due to the transient depletion of CD8+ T cells in this animal. As shown in Fig. 3A, cccDNA did not start to decline until week 12, coinciding with the reappearance of CD8+ cells in the blood (Fig. 3A) and the initial appearance of CD3 and IFN-γ mRNA in the liver (Fig. 3B). Importantly, as shown in Table 1, there was a 7.3-fold decrease in cccDNA and a 40-fold decrease in HBV DNA replicative intermediates between weeks 12 and 18, whereas the number of HBcAg-positive hepatocytes decreased only 1.5-fold during that time. It is noteworthy that the 1.5-fold decline in HBcAg-positive hepatocytes was delayed until weeks 14-18 in this animal (Fig. 3A), and it coincided with an increase in the sALT levels (Fig. 3D), although there was little or no evidence of liver cell turnover at this time, suggesting that the extent of liver cell destruction was minimal. Collectively, those results suggest that the cccDNA and replicative DNA intermediates in the liver decrease due to CD8 T cell-mediated noncytolytic mechanisms during the early phase of viral clearance. Over the next 10 weeks, the hepatic content of the viral cccDNA (Fig. 3A), replicative DNA intermediates (Fig. 3C), and HBcAg-positive hepatocytes (Fig. 3A) stabilized at a plateau that coincided with an unexpected stalling of the CD8+ cells at subnormal levels (Fig. 3A). This, in turn, corresponded with a low-grade liver disease and a surprisingly small degree of hepatocellular turnover, suggesting that the destructive process was modest, and that viral clearance was balanced by viral spread during this period. Suddenly, however, in weeks 28-29, the CD8+ cells surged back to baseline levels, accompanied by a surge of liver disease and a >30-fold decrease in HBcAg-positive hepatocytes, whereas the cccDNA content decreased only 9-fold during the same period (Table 1). This suggests that CD8-mediated cytopathic events played a major role in the clearance of both species at this point in the infection. Despite this, the cccDNA was not completely eliminated, because it was detectable at 0.01-0.1 copies per cell for >2 years after the infection (data not shown).
Discussion
The intrinsic half-life of the hepadnaviral cccDNA transcriptional template is a matter of some conjecture. Experiments in DHBV-infected primary duck hepatocytes and in a conditional DHBV replication system suggest that the half-life could be as short as 2-5 days (21, 22). However, cccDNA has been reported to be very stable with a half-life of at least 33-50 days in WHV-infected woodchucks and hepatocyte cultures (3, 23). If cccDNA half-life is short, it could be susceptible to elimination by mechanisms that reduce its rate of production. If its half-life is long, mechanisms that either destroy and dilute the cccDNA or that otherwise decrease its stability would be necessary for elimination.
In chronically infected ducks treated with hepatocytotoxic reverse transcriptase inhibitors (24), in WHV-infected woodchucks treated with inhibitors of viral DNA replication (3, 25), and in acutely WHV-infected woodchucks undergoing viral clearance (4, 7), there is strong evidence that cccDNA elimination depends on the death and turnover of infected hepatocytes. In other studies performed in HBV transgenic mice (9, 15, 26) and HBV-infected chimpanzees (10, 11), it has been shown that viral replication is strongly and rapidly inhibited noncytopathically by the antiviral effects of IFN-α/β and IFN-γ, which eliminate the replicative DNA precursors of cccDNA from the cell (13). Because the transgenic mice do not produce the viral cccDNA, its susceptibility to noncytopathic control could not be determined in those experiments.
Noncytolytic control of cccDNA, at least theoretically, is possible if its half-life is relatively short, because the noncytolytic elimination of the replicative DNA intermediates that serve as its precursors could reduce its steady-state content in the cell. Furthermore, irrespective of the length of cccDNA half-life under noninflammatory conditions, it is also theoretically possible that IFN-induced intracellular events that are triggered during acute viral hepatitis might destabilize preformed cccDNA without killing the cell. The need to examine this important unresolved question provided the impetus for the current study.
Collectively, the current results suggest that the content of HBV cccDNA and HBV replicative DNA intermediates in the liver is controlled by both cytolytic and noncytolytic mechanisms during immune-mediated clearance of HBV infection. Importantly, it appears that both viral DNA species are primarily controlled by noncytolytic mechanism(s) during the early phase of viral clearance, whereas cytolytic clearance mechanisms are dominant later in the infection. The results also suggest that the replicative DNA intermediates are more sensitive to noncytolytic immune-mediated inhibition than the cccDNA.
The argument in favor of a noncytolytic cccDNA clearance process early in the infection must take into account the temporally associated increase in sALT activity, a marker of hepatocellular injury. Indeed, the results clearly suggest that cytolytic cccDNA clearance mechanisms are operative during this phase of the infection. However, the data also suggest that cccDNA clearance can be explained only partly by that process, and that noncytolytic mechanisms are responsible for most of the reduction in cccDNA content during that period. The reasoning behind that argument is based primarily on the observation that the cccDNA content of the liver decreases much more than the HBcAg-positive hepatocytes during early viral clearance.
The HBcAg results in this study reflect the number of hepatocytes that contain nuclear core antigen. We have previously shown, in an HBcAg transgenic mouse model (27), that nuclear HBcAg is released into the cytoplasm of dividing hepatocytes when the nuclear membrane dissolves, and it is not detectable in the nucleus when the nuclear envelope reforms in the regenerating daughter cells. Accordingly, in that model, the absence of nuclear HBcAg is the hallmark of prior liver cell death and regeneration. Based on those observations, one would expect the number of nuclear HBcAg-positive hepatocytes to decline to the same extent as the cccDNA content in the infected chimpanzees if cell death and regeneration were the main reasons behind the early reduction in cccDNA. We would also expect to see an increase in the number of PCNA-positive hepatocytes, which did not happen.
Instead, the cccDNA decreased much more than the HBcAg-positive hepatocytes during the early stages of clearance. This is illustrated by the appearance of a widening gap between the cccDNA and HBcAg profiles beginning at week 9 in Ch1627 (Fig. 2 A) and week 12 in Ch1620 (Fig. 3A). As mentioned earlier, the early decrease of HBcAg-positive hepatocytes suggests that cytolytic events also contributed to early cccDNA clearance in these animals. However, the number of HBcAg-positive hepatocytes decreased only 1.5- and 3.2-fold in Ch1620 and Ch1627, whereas the cccDNA content decreased 7.3- and 8.6-fold, respectively, during that period (Table 1). That observation is supported by the absence of evidence of significant cell turnover (i.e., PCNA-positive hepatocytes) at that time (Figs. 2D and 3D).
In our opinion, the results suggest that the early phase of cccDNA clearance can best be explained by a process in which CD8-dependent immunologically mediated noncytolytic antiviral mechanisms are dominant. If that interpretation is correct, it suggests a model in which IFN-γ either destabilizes the cccDNA or prevents its replenishment by inhibiting viral replication and reducing the pool of relaxed circular DNA molecules that serve as precursors of cccDNA. If the latter is correct, our data would suggest that HBV cccDNA has an intrinsic half-life of 9-14 days in the chimpanzee liver. This is within the range of half-lives reported for the cccDNA of DHBV (2-5 days) and WHV (33-50 days) in their respective hosts (3, 21, 22).
In addition to the foregoing, it is noteworthy that the ratio of intrahepatic pgRNA to cccDNA did not change throughout the infection in Ch1620 (Fig. 3 A and C and Table 1), suggesting that the transcription of the cccDNA template and the half-life of the viral RNA were not deregulated during viral clearance. It is also notable that the strong decrease of HBV replicative DNA intermediates relative to cccDNA and pgRNA during the early clearance phase suggests that HBV replication is regulated posttranscriptionally, probably by a mechanism that prevents the formation or accelerates the degradation of pgRNA-containing capsids, as we have described in the HBV transgenic mouse model (13, 15).
In contrast to the early phase of viral clearance, HBcAg-positive hepatocytes are removed more rapidly than the cccDNA, and there is clear evidence of liver disease and cell turnover late in the infection, indicating that cytolytic clearance mechanisms are probably dominant at that time. Because IFN-γ expression is still high at this point, it is likely that noncytolytic clearance mechanisms are also active during that period, but their impact on viral replication is probably masked by the cytolytic process late in the infection. Interestingly, however, despite the destruction and regeneration of hepatocytes during the final stages of acute hepatitis, the cccDNA persists indefinitely at very low levels after resolution of the infection, as has been reported in WHV-infected woodchucks (4). The mechanism responsible for persistence of this cccDNA reservoir remains to be determined. If it is transcriptionally active, however, it is probably responsible for the life-long immunity to HBV that follows resolution of acute hepatitis (28, 29).
Acknowledgments
We thank M. Shapiro and Dr. M. St. Claire (Bioqual, Incorporated) for animal care, Ms. C. Steiger for coordination and handling of biopsies, R. Koch and R. Engle for technical assistance, and Dr. A. McLachlan (The Scripps Research Institute) for providing the necessary material to perform HBV-specific RNase protection assays. We thank Dr. Jesse Summers for critical reading of the manuscript and helpful advice. This study was supported by Grants AI20001 and CA76403 and contracts N01-AI-52705, N01-AI-45180, and N01-CO-56000 from the National Institutes of Health, and by the Sam and Rose Stein Charitable Trust. R.T. was also supported by Grants TH 719/1-1 and TH 719/2-1 (Emmy Noether Program) from the Deutsche Forschungsgemeinschaft, Bonn, Germany, and by a postdoctoral training fellowship from the Cancer Research Institute, New York. H.C.S. was supported by a grant from the Fritz Thyssen Foundation, Köln, Germany. This is manuscript number 16262-MEM from The Scripps Research Institute.
Abbreviations: cccDNA, covalently closed circular DNA; pgRNA, pregenomic RNA; HBV, hepatitis B virus; DHBV, duck HBV; WHV, woodchuck hepatitis virus; PCNA, proliferating cell nuclear antigen; Chn, chimpanzee n; sALT, serum alanine aminotransferase.
References
- 1.Seeger, C. & Mason, W. S. (2000) Microbiol. Mol. Biol. Rev. 64, 51-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jilbert, A. R., Wu, T. T., England, J. M., Hall, P. M., Carp, N. Z., O'Connell, A. P. & Mason, W. S. (1992) J. Virol. 66, 1377-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu, Y., Yamamoto, T., Cullen, J., Saputelli, J., Aldrich, C. E., Miller, D. S., Litwin, S., Furman, P. A., Jilbert, A. R. & Mason, W. S. (2001) J. Virol. 75, 311-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kajino, K., Jilbert, A. R., Saputelli, J., Aldrich, C. E., Cullen, J. & Mason, W. S. (1994) J. Virol. 68, 5792-5803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu, Q., Guo, J. T. & Seeger, C. (2003) J. Virol. 77, 9204-9210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lenhoff, R. J. & Summers, J. (1994) J. Virol. 68, 4565-4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo, J. T., Zhou, H., Liu, C., Aldrich, C., Saputelli, J., Whitaker, T., Barrasa, M. I., Mason, W. S. & Seeger, C. (2000) J. Virol. 74, 1495-1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Summers, J., Jilbert, A. R., Yang, W., Aldrich, C. E., Saputelli, J., Litwin, S., Toll, E. & Mason, W. S. (2003) Proc. Natl. Acad. Sci. USA 100, 11652-11659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guidotti, L. G. & Chisari, F. V. (2001) Annu. Rev. Immunol. 19, 65-91. [DOI] [PubMed] [Google Scholar]
- 10.Thimme, R., Wieland, S., Steiger, C., Ghrayeb, J., Reimann, K. A., Purcell, R. H. & Chisari, F. V. (2003) J. Virol. 77, 68-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guidotti, L. G., Rochford, R., Chung, J., Shapiro, M., Purcell, R. & Chisari, F. V. (1999) Science 284, 825-829. [DOI] [PubMed] [Google Scholar]
- 12.Guidotti, L. G., Ando, K., Hobbs, M. V., Ishikawa, T., Runkel, L., Schreiber, R. D. & Chisari, F. V. (1994) Proc. Natl. Acad. Sci. USA 91, 3764-3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wieland, S. F., Guidotti, L. G. & Chisari, F. V. (2000) J. Virol. 74, 4165-4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guidotti, L. G., Matzke, B., Schaller, H. & Chisari, F. V. (1995) J. Virol. 69, 6158-6169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guidotti, L. G., Ishikawa, T., Hobbs, M. V., Matzke, B., Schreiber, R. & Chisari, F. V. (1996) Immunity 4, 25-36. [DOI] [PubMed] [Google Scholar]
- 16.Schmitz, J. E., Kuroda, M. J., Santra, S., Sasseville, V. G., Simon, M. A., Lifton, M. A., Racz, P., Tenner-Racz, K., Dalesandro, M., Scallon, B. J., et al. (1999) Science 283, 857-860. [DOI] [PubMed] [Google Scholar]
- 17.Raney, A. K., Eggers, C. M., Kline, E. F., Guidotti, L. G., Pontoglio, M., Yaniv, M. & McLachlan, A. (2001) J. Virol. 75, 2900-2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang, Y. Y. & Summers, J. (2000) J. Virol. 74, 5257-5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chomczynski, P. & Sacchi, N. (1987) Anal. Biochem. 162, 156-159. [DOI] [PubMed] [Google Scholar]
- 20.Tang, H. & McLachlan, A. (2001) Proc. Natl. Acad. Sci. USA 98, 1841-1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Civitico, G. M. & Locarnini, S. A. (1994) Virology 203, 81-89. [DOI] [PubMed] [Google Scholar]
- 22.Guo, J. T., Pryce, M., Wang, X., Barrasa, M. I., Hu, J. & Seeger, C. (2003) J. Virol. 77, 1885-1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moraleda, G., Saputelli, J., Aldrich, C. E., Averett, D., Condreay, L. & Mason, W. S. (1997) J. Virol. 71, 9392-9399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fourel, I., Cullen, J. M., Saputelli, J., Aldrich, C. E., Schaffer, P., Averett, D. R., Pugh, J. & Mason, W. S. (1994) J. Virol. 68, 8321-8330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peek, S. F., Cote, P. J., Jacob, J. R., Toshkov, I. A., Hornbuckle, W. E., Baldwin, B. H., Wells, F. V., Chu, C. K., Gerin, J. L., Tennant, B. C., et al. (2001) Hepatology 33, 254-266. [DOI] [PubMed] [Google Scholar]
- 26.McClary, H., Koch, R., Chisari, F. V. & Guidotti, L. G. (2000) J. Virol. 74, 2255-2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guidotti, L. G., Martinez, V., Loh, Y. T., Rogler, C. E. & Chisari, F. V. (1994) J. Virol. 68, 5469-5475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rehermann, B., Fowler, P., Sidney, J., Person, J., Redeker, A., Brown, M., Moss, B., Sette, A. & Chisari, F. V. (1995) J. Exp. Med. 181, 1047-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rehermann, B., Ferrari, C., Pasquinelli, C. & Chisari, F. V. (1996) Nat. Med. 2, 1104-1108. [DOI] [PubMed] [Google Scholar]