Abstract
Merkel cell carcinoma (MCC) is an aggressive cutaneous malignancy linked to a contributory virus (Merkel cell polyomavirus/MCPyV). Multiple epidemiologic studies have established an increased incidence of MCC among persons with systemic immune suppression. Several forms of immune suppression are associated with increased MCC incidence, including hematologic malignancies, HIV/AIDS, and immunosuppressive medications for autoimmune disease or transplant. Indeed, immune suppressed persons represent approximately 10% of the MCC patients, a significant over-representation relative to the general population. We hypothesized that immune suppressed patients may have a poorer MCC-specific prognosis and examined a cohort of 471 patients with a combined follow-up of 1427 years (median 2.1 years). Immune suppressed persons (n=41) demonstrated reduced MCC-specific survival (40% at 3 years) compared to persons with no known systemic immune suppression (n=430; 74% MCC-specific survival at 3 years). By competing risk regression analysis, immune suppression was a stage-independent predictor of worsened MCC-specific survival (hazard ratio 3.8, p < 0.01). Immune-suppressed persons thus have both an increased chance of developing MCC and poorer MCC-specific survival. It may be appropriate to follow these higher-risk individuals more closely, and, when clinically feasible, there may be benefit of diminishing iatrogenic systemic immune suppression.
INTRODUCTION
Merkel cell carcinoma (MCC) is a neuroendocrine skin cancer with a prognosis poorer than that of melanoma. In 2008, a polyomavirus (Merkel cell polyomavirus, MCPyV) was reported to be a likely causative agent for the majority of MCCs(Feng et al., 2008); this has subsequently been well established by multiple international groups(Becker et al.; Foulongne et al.; Garneski et al.). Most MCC tumors depend on persistent expression of viral T-antigen oncoproteins,(Houben et al.; Shuda et al.;Shuda et al.) that are targets for the cellular(Iyer et al.) and humoral immune system(Paulson et al.).
It has been well established that immune suppression is associated with increased risk of developing MCC. Indeed, immune suppressed persons make up approximately 10% of the MCC patient population (Heath et al.), and it is this link that led to the search for, and eventual discovery of, Merkel cell polyomavirus(Arora et al.). Multiple forms of systemic immune suppression have been linked with an increased incidence of MCC, including chronic lymphocytic leukemia (CLL) and other hematologic malignancies(Brewer et al.; Heath et al.), HIV/AIDS (particularly prior to the widespread adoption of effective antiretrovirals)(Engels et al.), solid organ transplant(Penn and First), and autoimmune disease (with associated treatment regimens)(Hemminki et al.).
Conversely, and consistent with a role for anti-viral immune responses in protecting against MCC progression, CD8+ and CD3+ intratumoral lymphocyte responses have been found to be associated with improved MCC outcomes(Paulson et al.; Sihto et al.). In both of these studies, patients with robust lymphocyte infiltration into the tumor make up a minority of patients but exhibit outstanding MCC-specific survival.
One form of systemic immune suppression, CLL, has recently been associated with reduced MCC survival in a national cancer registry(Brewer et al.). However, to our knowledge, the effect of chronic immune suppression more broadly on MCC outcomes has not been examined. We hypothesized that systemic immune suppression would be associated with worsened MCC-specific survival in a stage-independent manner.
RESULTS
Frequency and distribution of systemic immune suppression amongst MCC patients
Amongst 471 persons with Merkel cell carcinoma from the United States, a total of 41 (8.7%) had clinically recognized systemic immune suppression. Immune suppressed patients were similar to those without immune suppression in terms of age at diagnosis and stage of disease at presentation (Table 1), but differed in terms of gender distribution with immune suppressed persons more likely to be male (80% vs. 59%; p < 0.01). Multiple forms of systemic immune suppression were represented including CLL (n=16; 3% of MCC patient cohort), other hematologic malignancies (n=5; 1%), HIV/AIDS (n=5; 1%), and long-term immunosuppressive medication regimens used for autoimmune disease (n=3; 1%) or solid organ transplant (n=12; 3%).
Table 1. Demographics.
Immune suppressed and non-immune suppressed patient groups were similar in terms of stage at diagnosis and patient age but differed in their gender distributions, with immune suppressed patients being more likely to be male. Age quartiles for the 25th, 50th, 75th percentile were 63 years, 72 years, 79 years for the not immune suppressed group and 58 years, 67 years, 77 years for the immune suppressed group. Comparisons made using Fisher’s Exact Test. N=471.
| Not immune suppressed (n= 430) |
Immune suppressed (n=41) |
P-value | |||
|---|---|---|---|---|---|
| Characteristic | N | Percent | N | Percent | |
| STAGE AT DIAGNOSIS | |||||
| Local | 242 | 56% | 23 | 56% | 0.94 (N.S.) |
| Regional | 147 | 34% | 15 | 37% | |
| Distant | 41 | 10% | 3 | 7% | |
| SEX | |||||
| Female | 177 | 41% | 8 | 20% | <0.01 |
| Male | 253 | 59% | 33 | 80% | |
| AGE AT DIAGNOSIS | |||||
| < 65 years | 127 | 30% | 17 | 41% | 0.16 (N.S.) |
| >= 65 years | 303 | 70% | 24 | 59% | |
Persons with systemic immune suppression and MCC have diminished overall survival
A combined 1427 years of follow-up was available for the 471 patients with MCC (median 2.1 years). Persons with MCC and systemic immune suppression had worsened overall survival as compared to persons with MCC and no systemic immune suppression (hazard ratio 2.1; p < 0.01). 3 year overall survival was 33% in the immune suppressed group and 59% in the comparison group.
Persons with systemic immune suppression have worsened Merkel cell carcinoma specific survival
We hypothesized that persons with systemic immune suppression would have worsened Merkel cell carcinoma–specific survival as compared to persons without systemic immune suppression due to failed immune surveillance of the cancer. We also reasoned that the groups would very likely have different rates of non-MCC death. Therefore, in order to account for possible differences in the death rate from other causes between the two groups, we performed competing risk regression analysis where only deaths from MCC were considered to be events, and deaths from other causes (including non-MCC deaths related to the immune suppression process) were considered to be competing events.
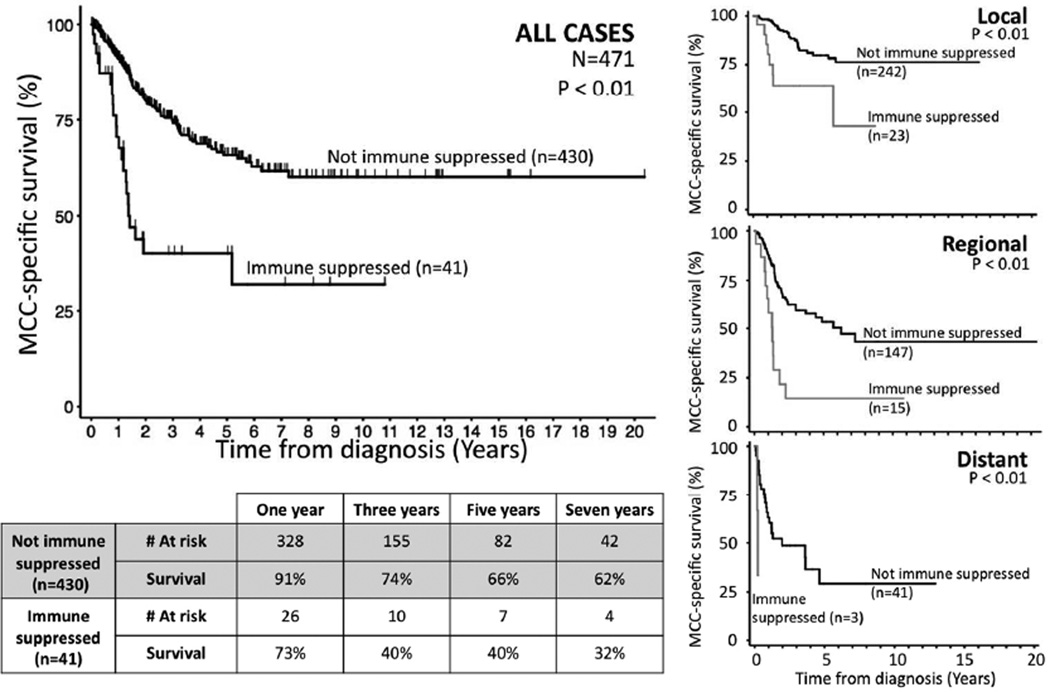
Immune suppressed persons (n=41) had statistically significantly worsened MCC-specific survival as compared to persons without immune suppression (n=430) (hazard ratio 3.0; 95% confidence interval 1.8–4.8; p < 0.01; Table 2). Furthermore, this difference was clinically appreciable, with immune suppressed patients having a three-year survival proportion that was approximately half that of the non-immune suppressed patients (40% vs. 74%; p < 0.05 for point comparison; Figure 1).
Table 2. Multivariate Competing Risk Regression Analyses Demonstrate Immune Suppression Is an Independent Predictor of Poor Merkel Cell Carcinoma Specific Survival.
Left column: univariate analyses considering each listed variable. Right column: multivariate analysis including all listed variables.
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Characteristic | HR | 95% CI | HR | 95% CI |
| EXTENT OF DISEASE AT PRESENTATION | ||||
| Regional (vs. local) | 3.4* | 2.3–5.0 | 3.5* | 2.3–5.2 |
| Distant (vs. local) | 6.3* | 3.6–10.8 | 7.4* | 4.2–13.1 |
| SEX | ||||
| Female (vs. Male) | 0.8 | 0.6–1.2 | 0.9 | 0.6–1.4 |
| AGE AT DIAGNOSIS | ||||
| Age (per year older) | 1.00 | 0.98–1.01 | 1.00 | 0.99–1.02 |
| SYSTEMIC IMMUNE SUPPRESSION | ||||
| Immunosuppressed (vs. not) | 3.0* | 1.8–4.8 | 3.8* | 2.2–6.4 |
HR = hazard ratio; * indicates p < 0.05. CI = confidence interval. N = 471.
Figure 1. Merkel cell carcinoma survival and immune suppression.
Large graph: Persons with immune suppression (n=41) had significantly worsened Merkel cell carcinoma (MCC) specific survival as compared to those without systemic immune suppression (n=430) on univariate (hazard ratio 3.0; p < 0.01) and multivariate (hazard ratio 3.8; p < 0.01) competing risk regression analyses (Table 2). Numbers at risk at one, three, five, and seven years indicated below. Small graphs: Effects of immune suppression persisted across stage at presentation.
Our patient population represents a non-overlapping mixture of patients enrolled either as individuals (n=228) or as part of records review in institutional sets(n=243). To ensure that our results were similar between the two categories of patients, we looked at each subgroup independently, and found that in each case immune suppression was a significant predictor of worsened outcome (records based enrollment: hazard ratio 2.5; p = 0.01)(individual enrollment: hazard ratio 4.0; p < 0.01).
Immune suppression is a stage-independent predictor of diminished MCC-specific survival
We observed significantly reduced Merkel cell carcinoma-specific survival among immune suppressed patients at all stages of presentation (Figure 1). In order to formally test whether immune suppression represents an independent predictor of MCC outcome, we performed multivariate competing risk regression analysis accounting for stage at presentation (local-regional-distant stage), age at diagnosis, and sex in addition to immune suppression (Table 2). Immune suppression represented a significant independent predictor of worsened MCC-specific survival (hazard ratio 3.8; 95% CI 2.2–6.4; p < 0.01).
We initially performed our analysis using local-regional-distant stage in order to minimize the number of variables in the model. However, we repeated this analysis using the current American Joint Committee on Cancer (AJCC) 7th Edition staging (Lemos et al.) instead in order to determine whether immune suppression adds information to current consensus staging. 395 of the 471 patients had sufficient information to determine AJCC substage at presentation. Again, immune suppression was a significant stage-independent predictor of poorer MCC-specific outcome (hazard ratio 4.2; 95% CI 2.4–7.4; p < 0.01; Supplemental Table 1). We performed a third analysis also including lymphovascular invasion status in the model (data available for 149 patients), results were similar and remained significant (hazard ratio 8.9; 95% CI 3.8–21.2; P < 0.01).
DISCUSSION
Merkel cell carcinoma is an aggressive skin cancer. At least 75% of MCC cases have a viral etiology. It has been well established that multiple forms of immune suppression (including HIV, hematologic malignancies, and immunosuppressive medications) are linked with an increased risk of developing MCC(Engels et al.; Heath et al.; Hemminki et al.; Lanoy et al.; Lanoy and Engels; Penn and First; Quaglino et al.; Sihto et al.). However, the effect of systemic immune suppression on MCC prognosis has not been well studied.
We find that systemic immune suppression is a stage-independent predictor of worsened survival among patients with Merkel cell carcinoma. To account for possible differences in overall health between immune suppressed and non-immune suppressed individuals, we performed competing risk regression analysis specifically considering the cause of death. Notably, three year MCC-specific survival was nearly twice as good in the non-immune suppressed group, suggesting systemic immune suppression is of significant clinical importance.
Persons with systemic immune suppression have been found to be at increased risk of developing other skin cancers, including squamous cell carcinoma (SCC), basal cell carcinoma (BCC), and melanoma. SCC incidence is at least 50-fold increased among solid organ transplant recipients(Moloney et al.) and transplant patients with metastatic disease have a poorer SCC prognosis as compared to immune competent patients(Martinez et al.). Furthermore, melanoma has been associated with poorer outcomes among immunocompromised populations as compared to healthy populations(Matin et al.), and this melanoma-associated mortality significantly increases the total mortality of the immune suppressed patients(Alam et al.). Combined with our findings regarding MCC, these data highlight the importance of carefully following the skin of immune suppressed persons and having a low threshold for biopsy of suspicious lesions.
Limitations
Our study had several limitations. Many of the patients were enrolled through referral to tertiary centers thus suggesting a source of ascertainment bias. To mitigate this as much as possible, we limited inclusion to patients who presented within 180 days of diagnosis. Given the variable nature of human disease, we were unable to control for the relative degree of immune suppression between various immune suppressed patients. Finally, although MCC is increasing in incidence it remains an uncommon disease. Although our study size of 471 is large for MCC, we still did not have a sufficient number of immune suppressed patients to determine the relative impact of each of the various forms of immune suppression on survival.
Immune suppression is associated with both increased MCC incidence and worsened MCC outcome. Conversely, strong intratumoral immune responses are associated with improved MCC survival(Paulson et al.; Sihto et al.). Given these associations and the known viral immune targets in MCC, immune therapy holds significant promise for the future treatment of MCC. At this time, in the treatment of patients with immune suppression and MCC, it would appear prudent to follow these higher-risk patients very closely also consider reducing or modifying iatrogenic immune suppression whenever feasible given the clinical context.
METHODS
Patient enrollment
All studies were institutional review board approved (FHCRC IRB# 6585), performed in accordance with Helsinki principles, and written informed patient consent was obtained. All MCC patients in the FHCRC repository of data and specimens were considered for inclusion and all were enrolled in the United States. Enrollment criteria included (all must be present): a diagnosis of Merkel cell carcinoma confirmed by two pathologists, presence of follow-up information, known stage at diagnosis (local-regional-distant), known age at diagnosis, known sex, and known immune suppression status. Patients in the repository enroll in one of two approaches: either as individuals (through our tertiary referral clinic and the internet) or through records review as part of a patient set from one of several institutions (including a 186 patient cohort from Kaiser Permanente, a large integrated health care delivery system in Northern California). Patients were represented only once. Patients enrolling as individuals after 180 days of diagnosis were eliminated in order to reduce ascertainment bias. A total of 471 unique patients with Merkel cell carcinoma met all criteria (243 from institution cohorts and 228 as individuals); demographics are described in Table 1.
Statistical analyses
Demographic and stage information was compared between immune suppressed and non-immune suppressed persons using Fisher’s exact test. Overall survival analyses (considering deaths from any cause to be events) were performed with Cox regression. Disease-specific survival analyses were performed with competing risk regression. For competing risk regression, deaths from Merkel cell carcinoma were considered to be events (n=126), deaths from other causes were considered to be competing events (n=75), deaths from unknown causes were censored on the day of death (n=31), and living patients were censored on the date of last follow-up (n=239). Kaplan-Meier curves were generated in order to visually compare survival between groups. Statistical analyses were performed with Stata version 11.0 software for Macintosh. A p-value of 0.05 was considered to be the cutpoint for statistical significance.
Supplementary Material
Acknowledgments
Funding: NIH-RC2CA147820 (PN), American Cancer Society RSG-08-115-01-CCE (PN), NIH-K24-CA139052 (PN), Michael Piepkorn Endowment, Cora May Poncin foundation (KP), MCC Patient Gift Fund, David & Rosalind Bloom Fund for MCC
Abbreviations
- MCC
Merkel cell carcinoma
- CLL
chronic lymphocytic leukemia
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
References
- Alam M, Brown RN, Silber DH, Mullen GM, Feldman DS, Oren RM, et al. Increased incidence and mortality associated with skin cancers after cardiac transplant. Am J Transplant. 11:1488–1497. doi: 10.1111/j.1600-6143.2011.03598.x. [DOI] [PubMed] [Google Scholar]
- Arora R, Chang Y, Moore PS. MCV and Merkel cell carcinoma: a molecular success story. Curr Opin Virol. 2012 doi: 10.1016/j.coviro.2012.05.007. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JC, Houben R, Ugurel S, Trefzer U, Pfohler C, Schrama D. MC polyomavirus is frequently present in Merkel cell carcinoma of European patients. J Invest Dermatol. 2009;129:248–250. doi: 10.1038/jid.2008.198. [DOI] [PubMed] [Google Scholar]
- Brewer JD, Shanafelt TD, Otley CC, Roenigk RK, Cerhan JR, Kay NE, et al. Chronic lymphocytic leukemia is associated with decreased survival of patients with malignant melanoma and Merkel cell carcinoma in a SEER population-based study. J Clin Oncol. 2012;30:843–849. doi: 10.1200/JCO.2011.34.9605. [DOI] [PubMed] [Google Scholar]
- Engels EA, Frisch M, Goedert JJ, Biggar RJ, Miller RW. Merkel cell carcinoma and HIV infection. Lancet. 2002;359:497–498. doi: 10.1016/S0140-6736(02)07668-7. [DOI] [PubMed] [Google Scholar]
- Feng H, Shuda M, Chang Y, Moore PS. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319:1096–1100. doi: 10.1126/science.1152586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulongne V, Kluger N, Dereure O, Brieu N, Guillot B, Segondy M. Merkel cell polyomavirus and Merkel cell carcinoma, France. Emerg Infect Dis. 2008;14:1491–1493. doi: 10.3201/eid1409.080651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garneski KM, Warcola AH, Feng Q, Kiviat NB, Leonard JH, Nghiem P. Merkel cell polyomavirus is more frequently present in North American than Australian Merkel cell carcinoma tumors. J Invest Dermatol. 2009;129:246–248. doi: 10.1038/jid.2008.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath M, Jaimes N, Lemos B, Mostaghimi A, Wang LC, Penas PF, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol. 2008;58:375–381. doi: 10.1016/j.jaad.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemminki K, Liu X, Ji J, Sundquist J, Sundquist K. Kaposi sarcoma and Merkel cell carcinoma after autoimmune disease. Int J Cancer. 2012 doi: 10.1002/ijc.27376. [DOI] [PubMed] [Google Scholar]
- Houben R, Shuda M, Weinkam R, Schrama D, Feng H, Chang Y, et al. Merkel cell polyomavirusinfected Merkel cell carcinoma cells require expression of viral T antigens. J Virol. 2010;84:7064–7072. doi: 10.1128/JVI.02400-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer JG, Afanasiev OK, McClurkan C, Paulson K, Nagase K, Jing L, et al. Merkel cell polyomavirusspecific CD8 and CD4 T-cell responses identified in Merkel cell carcinomas and blood. Clin Cancer Res. 2011;17:6671–6680. doi: 10.1158/1078-0432.CCR-11-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanoy E, Dores GM, Madeleine MM, Toro JR, Fraumeni JF, Jr, Engels EA. Epidemiology of nonkeratinocytic skin cancers among persons with AIDS in the United States. AIDS. 2009;23:385–393. doi: 10.1097/QAD.0b013e3283213046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanoy E, Engels EA. Skin cancers associated with autoimmune conditions among elderly adults. Br J Cancer. 103:112–114. doi: 10.1038/sj.bjc.6605733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos BD, Storer BE, Iyer JG, Phillips JL, Bichakjian CK, Fang LC, et al. Pathologic nodal evaluation improves prognostic accuracy in Merkel cell carcinoma: analysis of 5823 cases as the basis of the first consensus staging system. J Am Acad Dermatol. 2010;63:751–761. doi: 10.1016/j.jaad.2010.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez JC, Otley CC, Stasko T, Euvrard S, Brown C, Schanbacher CF, et al. Defining the clinical course of metastatic skin cancer in organ transplant recipients: a multicenter collaborative study. Arch Dermatol. 2003;139:301–306. doi: 10.1001/archderm.139.3.301. [DOI] [PubMed] [Google Scholar]
- Matin RN, Mesher D, Proby CM, McGregor JM, Bouwes Bavinck JN, del Marmol V, et al. Melanoma in organ transplant recipients: clinicopathological features and outcome in 100 cases. Am J Transplant. 2008;8:1891–1900. doi: 10.1111/j.1600-6143.2008.02326.x. [DOI] [PubMed] [Google Scholar]
- Moloney FJ, Comber H, O'Lorcain P, O'Kelly P, Conlon PJ, Murphy GM. A population-based study of skin cancer incidence and prevalence in renal transplant recipients. Br J Dermatol. 2006;154:498–504. doi: 10.1111/j.1365-2133.2005.07021.x. [DOI] [PubMed] [Google Scholar]
- Paulson KG, Carter JJ, Johnson LG, Cahill KW, Iyer JG, Schrama D, et al. Antibodies to merkel cell polyomavirus T antigen oncoproteins reflect tumor burden in merkel cell carcinoma patients. Cancer Res. 2010;70:8388–8397. doi: 10.1158/0008-5472.CAN-10-2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson KG, Iyer JG, Tegeder AR, Thibodeau R, Schelter J, Koba S, et al. Transcriptome-wide studies of merkel cell carcinoma and validation of intratumoral CD8+ lymphocyte invasion as an independent predictor of survival. J Clin Oncol. 2011;29:1539–1546. doi: 10.1200/JCO.2010.30.6308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn I, First MR. Merkel's cell carcinoma in organ recipients: report of 41 cases. Transplantation. 1999;68:1717–1721. doi: 10.1097/00007890-199912150-00015. [DOI] [PubMed] [Google Scholar]
- Quaglino D, Di Leonardo G, Lalli G, Pasqualoni E, Di Simone S, Vecchio L, et al. Association between chronic lymphocytic leukaemia and secondary tumours: unusual occurrence of a neuroendocrine (Merkell cell) carcinoma. Eur Rev Med Pharmacol Sci. 1997;1:11–16. [PubMed] [Google Scholar]
- Shuda M, Arora R, Kwun HJ, Feng H, Sarid R, Fernandez-Figueras MT, et al. Human Merkel cell polyomavirus infection I.MCV T antigen expression in Merkel cell carcinoma, lymphoid tissues and lymphoid tumors. Int J Cancer. 2009;125:1243–1249. doi: 10.1002/ijc.24510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuda M, Kwun HJ, Feng H, Chang Y, Moore PS. Human Merkel cell polyomavirus small T antigen is an oncoprotein targeting the 4E-BP1 translation regulator. J Clin Invest. 2011;121:3623–3634. doi: 10.1172/JCI46323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sihto H, Bohling T, Kavola H, Koljonen V, Salmi M, Jalkanen S, et al. Tumor Infiltrating Immune Cells And Outcome of Merkel Cell Carcinoma: A Population-based Study. Clin Cancer Res. 2012 doi: 10.1158/1078-0432.CCR-11-3020. Online ahead of print. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.