Abstract
Rationale: The function of the P2X7 nucleotide receptor protects against exacerbation in people with mild-intermittent asthma during viral illnesses, but the impact of disease severity and maintenance therapy has not been studied.
Objectives: To evaluate the association between P2X7, asthma exacerbations, and incomplete symptom control in a more diverse population.
Methods: A matched P2RX7 genetic case-control was performed with samples from Asthma Clinical Research Network trial participants enrolled before July 2006, and P2X7 pore activity was determined in whole blood samples as an ancillary study to two trials completed subsequently.
Measurements and Main Results: A total of 187 exacerbations were studied in 742 subjects, and the change in asthma symptom burden was studied in an additional 110 subjects during a trial of inhaled corticosteroids (ICS) dose optimization. African American carriers of the minor G allele of the rs2230911 loss-of-function single nucleotide polymorphism were more likely to have a history of prednisone use in the previous 12 months, with adjustment for ICS and long-acting β2-agonists use (odds ratio, 2.7; 95% confidence interval, 1.2–6.2; P = 0.018). Despite medium-dose ICS, attenuated pore function predicted earlier exacerbations in incompletely controlled patients with moderate asthma (hazard ratio, 3.2; confidence interval, 1.1–9.3; P = 0.033). After establishing control with low-dose ICS in patients with mild asthma, those with attenuated pore function had more asthma symptoms, rescue albuterol use, and FEV1 reversal (P < 0.001, 0.03, and 0.03, respectively) during the ICS adjustment phase.
Conclusions: P2X7 pore function protects against exacerbations of asthma and loss of control, independent of baseline severity and the maintenance therapy.
Keywords: asthma, P2X7, exacerbation, Asthma Clinical Research Network, corticosteroids
At a Glance Commentary
Scientific Knowledge on the Subject
Previous literature shows that the nucleotide receptor P2X7 regulates the development of murine airway allergic hypersensitivity and yet protects against virus-induced exacerbations in patients with mild-intermittent asthma. The influences of inhaled corticosteroids or other asthma therapies have not been evaluated.
What This Study Adds to the Field
Attenuated P2X7 pore function occurs in approximately 35% of patients with asthma and increases the risk of exacerbation or loss of control despite maintenance therapy. These data suggest that this receptor may be involved in the resolution of airway inflammation and that the immune mechanisms contributing to exacerbations are distinct from those related to asthma inception.
The release of ATP during cell injury is a danger signal to the airway inflammatory response (1). Levels of ATP in the airway are elevated after allergen challenge in humans and mice, contributing to production of dendritic cell–derived cytokines important to efficient recruitment of eosinophils and lymphocytes and the subsequent development of airway hyperresponsiveness to methacholine (2). In addition, airway ATP levels are likely elevated during virus-induced asthma exacerbations in humans (3) and serve as an airway biomarker of neutrophilic inflammation during infection (4). The nucleotide receptor P2X7 regulates this process. This receptor is a trimeric, nonselective cation channel expressed by leukocytes and epithelial cells that opens a larger pore on full activation (size restriction 900 Da) (5, 6). Data by Müller and coworkers (7) in the ovalbumin-sensitized mouse model suggest that inhibition of P2X7 partially attenuates dendritic cell–dependent influx of eosinophils and lymphocytes and the development of airway hyperresponsiveness and remodeling. Consistent with a role for this receptor in compartmental recruitment of granulocytes, we have previously shown that P2X7 pore function correlates with early recruitment of neutrophils in nasal lavage samples from patients with asthma experiencing a viral-induced cold (8).
The gene for this receptor (P2RX7) localizes to chromosomal position 12q24 (9), a minor asthma locus containing multiple related genes (10–16). P2RX7 is highly polymorphic with more than 800 single nucleotide polymorphisms (SNPs) (17). At least 10 loss-of-function alleles have been validated in recombinant expression systems (18–22); however, the frequency of the minor alleles for most of these variants is rare (minor allele frequencies <3%) and contributes to relatively small blocks of linkage disequilibrium for this gene (23, 24). To circumvent these problems, we have developed a functional screening assay for P2X7 pore activity that detects the presence of five of the most common loss-of-function genotypes, and now permits analyses of multicenter studies (25, 26).
In patients with mild-intermittent asthma at baseline, attenuated P2X7 pore function is associated with a 15-fold increased risk of exacerbations in the setting of a rhinovirus cold (8). In accord with this, bronchial epithelial expression of P2X7 confers a small protective effect in terms of limiting human rhinovirus replication (27). The direction of these effects in our human studies are in some ways opposite to that predicted from the role of P2X7 in regulating ovalbumin-induced airway hypersensitivity and inflammation in the mouse. Additionally, the ability of maintenance asthma therapy with inhaled corticosteroids (ICS) to reduce the risk of exacerbation has not been evaluated with stratification by P2X7 function. Given this background, and because early in vitro experiments suggested that corticosteroids have minimal impact on P2X7 pore activity, we predicted that normal P2X7 function protects against asthma exacerbations in adults with established disease, independent of the asthma maintenance therapy.
Methods
Human Subject Participation
We studied three cohorts within the Asthma Clinical Research Network (ACRN): (1) a cross section of participants enrolled in ACRN trials before July 2006, with mild-to-moderate severity at baseline and a diverse mix of maintenance therapy; (2) incompletely controlled patients despite medium-dose ICS during the run-in; and (3) well-controlled patients using low-dose ICS followed during an ICS-adjustment strategy trial. Details of these trials have been previously published (28–30). Table E1 in the online supplement also has basic descriptors of these cohorts. Briefly, ACRN enrolled adults with physician-diagnosed asthma; harmonized phenotypic data and follow-up were available across the studies. Asthma exacerbations were defined as new administration of oral prednisone or other systemic corticosteroids. These analyses were approved by the ACRN Steering Committee and Data and Safety Monitoring Board. Sample collection was described in the trial consent form, approved by the local Internal Review Board of each clinical center and signed by each participant.
Measurement P2X7 Pore Activity in Whole-Blood Samples in Multicenter Trials
Collection of a citrate-anticoagulated whole-blood sample from the subjects in the two prospective cohorts was independent of the randomization process for the parent trials. The methods of our pore assay in whole-blood monocytes have been described, including refinements to accommodate loss of viability caused by overnight shipping; nonviable cells were excluded after pore closure by the addition of propidium iodide (23, 25, 26). The median fluorescence intensity of YO-PRO-1 associated with viable CD14+ cells treated with the P2X7 ligand BzATP was used as the measure of pore activity, with a receiver operator curve defined threshold between low and normal pore activity of 382 median fluorescence intensity (31). Pore function was independent of participant age, sex, ethnicity, and ACRN Center (see Figure E1). Description of the genotyping methods is provided in the online supplement.
Statistical Analysis
Phenotype data were managed at Data Coordinating Centers of the ACRN with a database that does not include P2RX7 genetic data. One author from the Data Coordinating Centers (E.L.) remained masked to the genetic data and performed matching of the cases with control subjects lacking the history of prednisone. Matching was performed on the basis of participant-reported ethnicity, sex, and percent predicted FEV1 at the screening visits. Given our focus on validated loss-of-function alleles for the ACRN analysis and the trimeric nature of the receptor that allows cooperative ligand binding (32, 33), the dominant model was chosen for the primary analysis. The Cochran-Mantel-Haenszel test was used for a matched case-control analysis of allele frequencies in the ACRN cross-sectional population. Multivariable logistic regression to model case-control status was adjusted for the match identifier. For the pore assay functional analysis, Kaplan-Meier models of the time to first exacerbation was performed using the log-rank test, and the time to multiple events was evaluated by a repeated measures proportional hazards regression model. The changes in secondary endpoints over the duration of these trials were evaluated by a repeated measures analysis of covariance model with comparisons made between the low and normal pore groups. For all considerations, a P value less than 0.05 was considered significant without adjustment for multiple comparisons.
Results
P2RX7 Genetic Association with Exacerbations in a Cross Section of ACRN Participants at Enrollment Is Independent of Maintenance ICS
From 1,435 genotyped samples, we identified 170 case subjects who were randomized in ACRN trials, and also had a history of prednisone use in the 12 months before enrollment. Additionally, 481 control subjects were matched from this cohort on the basis of race, sex, and FEV1. The distribution of cases to control subjects was as follows: 151 cases with three matched control subjects each, nine cases with two matched control subjects each, and 10 cases with one matched control subject. Table 1 shows the distribution of ethnicity and the baseline phenotype variables. There was a numeric trend for the cases to have slightly lower percent-predicted FEV1 values and slightly higher exhaled nitric oxide measurements. Methacholine responsiveness, sputum eosinophils, and serum IgE values were not different, but the cases were more likely to be taking ICS or long-acting β2 agonists (LABA) before trial enrollment (Table 1).
TABLE 1.
ACRN PARTICIPANT SCREENING VARIABLES FOR THE MATCHED CASE-CONTROL ANALYSIS
| Prednisone Use in the Prior 12 Months (Cases) | No Prednisone Use in the Prior 12 Months (Control Subjects) | P Value | |
| Total, n | 170 | 481 | |
| White, n | 103 | 305 | |
| African American, n | 40 | 110 | |
| Hispanic, n | 17 | 45 | |
| Asian/Pacific Islander, n | 7 | 17 | |
| Other, n | 3 | 4 | |
| % Female | 67.6 | 67.2 | |
| Age, yr | 33 ± 10 | 32 ± 11 | 0.581 |
| % Predicted FEV1 | 80.4 ± 14.6 | 82.7 ± 13.5 | 0.067 |
| Methacholine PC20, mg/ml | 0.8 (0.3–2.1) | 0.9 (0.4–2.3) | 0.359 |
| FeNO, ppb (n = 151, 421) | 15.8 (10.8–25.8) | 14.7 (9.3–22) | 0.062 |
| % Sputum EOS (n = 67, 207) | 0.3 (0–1.7) | 0.4 (0–1.6) | 0.987 |
| IgE, IU/ml (n = 52, 220) | 156 (44–512) | 141 (48–333) | 0.288 |
| ICS use, % | 88.7 | 65.4 | <0.001 |
| LABA use, % | 50.3 | 26.7 | <0.001 |
| LTRA use, % | 13.9 | 13.2 | 0.819 |
Definition of abbreviations: ACRN = Asthma Clinical Research Network; EOS = eosinophils; FeNO = fraction of exhaled nitric oxide; ICS = inhaled corticosteroids; LABA = long-acting β2 agonists; LTRA = leukotriene receptor antagonists; PC20 = provocative concentration of methacholine.
Cases were defined as the acute use of prednisone in the 12 months before ACRN trial entry. Control subjects were matched by ethnicity, sex, and percent predicted FEV1. Spirometry data are reported as the means and standard deviations, whereas the median values and interquartile ranges are shown for the variables requiring log transformation before analysis of variance. The percent of sputum eosinophils is also reported as the median and interquartile range with comparison by the Kruskal-Wallis test. Categorical use of any or no ICS, LABA, or LTRA was compared in the two groups by a chi-square test.
The association of exacerbation status with the minor allele frequencies of the 10 genotyped SNPs is shown in Table 2, with adjustment for the matching variable. The minor allele for rs2230911 was enriched in the cases compared with control subjects, an effect that is driven largely by African American participants. The presence of a minor allele for rs2230911 remained significantly associated with a history of prednisone use in African Americans using a multivariate logistic regression model with adjustment for ICS and LABA use (odds ratio, 2.70; 95% confidence interval, 1.18–6.17; P = 0.018).
TABLE 2.
LOSS-OF-FUNCTION P2RX7 MINOR ALLELE FREQUENCIES IN MATCHED ACRN CASES AND CONTROL SUBJECTS
| P2RX7 SNP (Minor Allele, Genotyping Call Rate) | MAF Cases | MAF Control Subjects | Cochran-Mantel-Haenszel Odds Ratio (95% Confidence Interval) | P Value |
| rs656612 (C, 0.969) | 0.348 | 0.390 | 0.76 (0.53–1.09) | 0.121 |
| rs1653583 (T, 0.975) | 0.087 | 0.085 | 0.92 (0.56–1.50) | 0.709 |
| rs28360447 (A, 0.988)* | 0.003 | 0.013 | 0.23 (0.03–1.79) | 0.105 |
| rs507085 (G, 0.985) | 0.276 | 0.290 | 0.85 (0.60–1.21) | 0.329 |
| rs28360457 (A, 0.982)* | 0.015 | 0.013 | 1.17 (0.41–3.38) | 0.816 |
| rs1718119 (T, 0.959) | 0.352 | 0.393 | 0.74 (0.51–1.06) | 0.139 |
| rs2230911 (G, 0.978)* | 0.154 | 0.109 | 1.74 (1.16–2.60) | 0.012 |
| 911 White only | 0.078 | 0.089 | 1.02 (0.55–1.89) | 1.000 |
| 911 African American only | 0.262 | 0.126 | 3.44 (1.61–7.38) | <0.001 |
| 911 Hispanic only | 0.294 | 0.170 | 2.68 (0.88–8.49) | 0.081 |
| rs2230912 (G, 0.986) | 0.132 | 0.131 | 0.96 (0.63–1.44) | 0.791 |
| rs3751143 (G, 0.972)* | 0.151 | 0.156 | 0.96 (0.65–1.42) | 0.814 |
| rs1653624 (A, 0.982)* | 0.018 | 0.013 | 1.42 (0.52–3.84) | 0.416 |
Definition of abbreviations: ACRN = Asthma Clinical Research Network; MAF = minor allele frequencies; SNP = single-nucleotide polymorphisms.
The MAF are listed for the total population because ethnicity was a strict matching variable for the case-control pair definitions. Blocking on the match variable, the Cochran-Mantel-Haenszel odds ratio, and confidence intervals for the dominant model are presented with corresponding P values.
Attenuated Pore Function and the Time to Exacerbation in Symptomatic Patients with Moderately Severe Asthma on Medium-Dose ICS
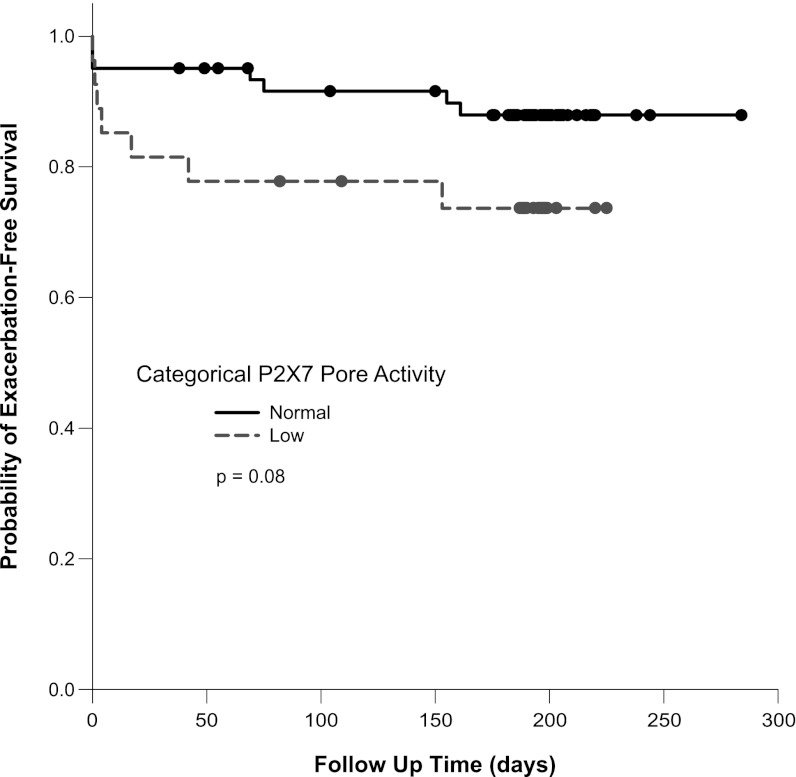
Given that rs2330911 has been previously validated as a loss-of-function allele (21, 22), we implemented our pore assay to increase the sample size efficiency in a prospective trial. To assess whether ICS treatment mitigates against this P2X7-dependent risk of exacerbation, we performed an ancillary study to an ACRN trial that targeted symptomatic patients with moderately severe disease who were believed to be at risk for exacerbation. Participants in this trial could not have had a cold within 6 weeks of enrollment, but were required to have incompletely controlled asthma symptoms despite medium-dose ICS at the end of the run-in period (29). Sixty-one subjects had normal pore activity and 27 had attenuated function; 28 and 33 of the normal pore subjects were assigned to placebo and clarithromycin treatments in the parent trial, respectively, whereas this distribution was 15 and 12 for the low pore group (P = 0.403). During the course of the trial, 17 exacerbations occurred in 14 participants who also had available pore assay data. Seven (25.9%) of 27 participants with low pore function had an exacerbation despite maintenance ICS, whereas this occurred in only 7 (11.5%) of 61 with normal pore activity. Figure 1 shows a Kaplan-Meier analysis of the time to the first exacerbation, with a trend toward an earlier exacerbation time in the low pore group. Of note, four of these first-exacerbation events occurred on the day of the bronchoscopy; excluding these events results in a log-rank P value of 0.027. Additionally, by allowing for multiple events including these procedure-associated prednisone bursts, a proportional hazards model demonstrates a hazard ratio of 3.2 (95% confidence interval, 1.1–9.3; P = 0.033) associating low pore activity with exacerbations. We also assessed the impact of pore function on the change in asthma control during the course of the trial in participants who were only receiving ICS as their maintenance therapy. Specifically, a logistic regression model showed an inverse relationship between continuous P2X7 pore function and the likelihood of not having symptomatic improvement (change in Juniper Asthma Control Questionnaire score, >0.5) while on a fixed dose, medium-strength ICS (P = 0.049). The direction of this effect suggests that patients with low pore activity are less likely than those with normal P2X7 function to receive symptomatic improvement on ICS during the context of a clinical trial.
Figure 1.
Kaplan-Meier analysis of time to exacerbation in symptomatic patients with moderate asthma at baseline. The survival curves are shown separated by categories of P2X7 pore function defined by the receiver-operator curve generated threshold. The solid and the dashed lines represent the normal and low pore groups, respectively. Dots along these lines are the times of patient censure caused by completing participation without an exacerbation, including both the 16 weeks of the study medication and the run-out period; the variable time of completion is caused by variability in the length of the run-out. Six of 61 and 2 of 27 subjects in the normal and low pore groups dropped out of the study before completion of the treatment period (P = 0.7). The comparison of event rates was performed with correction for multiple events in a repeated measures proportional hazards regression model.
Attenuated Pore Function and the Return of Asthma Symptoms in a Mild-to-Moderate Population during Maintenance Therapy Adjustment
These genetic and functional data suggest that individuals with low pore activity might have a greater propensity to have asthma symptoms at baseline or be less responsive to ICS. To determine whether patients with asthma and low P2X7 pore function are more likely to have asthma symptoms return after a period of control, we performed an ancillary study to an ACRN trial that enrolled subjects with a mild-to-moderate asthma who could achieve good symptom control during the run-in period while taking a low-dose ICS as the only maintenance therapy (30). This trial assessed provider-based, fraction of exhaled nitric oxide–based, and symptom-based strategies for ICS dose adjustment designed to guide decisions regarding step-up and step-down therapy in this population, with the main result being a similar number of treatment failures among the three arms (30). Pore activity was assessed from 110 of the subjects in this trial at the time of randomization to three different ICS-adjustment strategies, and the proportion of subjects with low pore activity did not differ among the three arms of the trial (0.40, 0.35, and 0.34; P > 0.1). Table 3 shows the change in several variables related to asthma control during the 36 weeks of follow-up in these participants, stratified by pore function. Neither group had a significant change in their Juniper Asthma Control Questionnaire score over the course of the trial. By contrast, the individuals with low pore activity experienced a small increase in their daily symptom scores over the course of the trial, which corresponded with an increase in rescue albuterol use and an increase in the bronchodilator-induced percent reversal of FEV1; these changes were smaller in the normal pore group such that the comparison between low and normal status remained significant (Table 3). Both groups had an increase in exhaled nitric oxide and had trends toward being more responsive to methacholine over the course of the trial, but these parameters did not differ according to P2X7 pore status. Of note, the 30-day average exposure to ICS was not different between the two groups (mean ± standard deviation of the natural log of 30-d ICS dose; low pore, 7.42 ± 1.38, normal pore 7.25 ± 1.21; P = 0.488).
TABLE 3.
CHANGE IN ASTHMA CONTROL VARIABLES DURING ICS ADJUSTMENT IN PATIENTS WITH MILD ASTHMA STRATIFIED BY PORE FUNCTION
| Change in Endpoints during ICS Adjustment | Low Pore (n = 40) | Normal Pore (n = 70) | Low to Normal |
| Juniper Asthma Control Questionnaire Average | 0.10 (−0.14 to 0.35); 0.41 | −0.07 (−0.26, 0.11); 0.44 | 0.18 (−0.13 to 0.48); 0.26 |
| Daily Symptom Diary Scores | 0.07 (0.05 to 0.09); <0.001 | 0.00 (−0.01 to 0.02); 0.97 | 0.07 (0.05 to 0.10); <0.001 |
| Rescue albuterol use (puffs/day) | 0.26 (0.13 to 0.39); <0.001 | 0.09 (0.01 to 0.18); 0.03 | 0.17 (0.01 to 0.32); 0.03 |
| FEV1-albuterol reversal (4 puffs, %) | 2.62 (0.75 to 4.50); 0.007 | 0.10 (−1.28 to 1.48); 0.89 | 2.52 (0.19 to 4.85); 0.03 |
| Natural log(FeNO, ppb) | 0.40 (0.10 to 0.70); 0.008 | 0.51 (0.29 to 0.73); <0.001 | −0.11 (−0.48 to 0.26); 0.57 |
| Log2(PC20, mg/ml) | −0.61 (−1.24 to 0.01); 0.05 | −0.39 (−0.85 to 0.07); 0.10 | −0.23 (−1.00 to 0.55); 0.56 |
Definition of abbreviations: FeNO = fraction of exhaled nitric oxide; ICS = inhaled corticosteroids; PC20 = provocative concentration of methacholine.
All data are reported as estimate (95% confidence interval); P value. Data reflect the change in each endpoint from randomization to the end of the treatment period, with the last column representing the differences between the low and normal pore groups. The P values and 95% confidence intervals are reported from repeated measures analysis of covariance models.
Discussion
Our initial observations with mild-intermittent people with asthma are extended in several ways by the main findings of this analysis. First, adult African American carriers of a loss-of-function rs2230911 G allele (attenuates P2X7 function in recombinant expression systems [21, 22]) have an increased frequency of prednisone use in the past year, even after adjustment for ICS and LABA use. Second, attenuated whole-blood monocyte pore function was associated with a more rapid rate of exacerbation in a moderately severe population despite medium-dose ICS. In contrast to our previous work in people with mild asthma enrolled at the time of a cold experiencing a loss of control, the present analysis focused on prednisone use, to be consistent with guideline definition of severe exacerbation. These events are likely representative of exacerbations from a diverse set of triggers, particularly in that cold symptoms were not required for study enrollment. Third, individuals with low pore activity have a higher asthma symptom burden than those with normal P2X7 activity despite ICS in our prospective studies involving mild and moderate populations. These data also confirm that attenuated whole-blood monocyte pore function is common in patients with asthma, representing 35% of the participants in the two trials combined. Whether P2X7 function is a biomarker for ICS responsiveness is an intriguing possibility that requires further study.
Our study has limitations. First, the genetic case-control design was retrospective and the prospective functional data were collected as an ancillary study. We did not detect differences in baseline characteristics among subjects in the parent trial with or without participation in the ancillary study (P > 0.1 in all cases). Nonetheless, this design restricted our ability to collect additional samples for mechanistic studies. Second, we cannot rule out a confounding influence of genetic admixture in our African American cohort. The ACRN registry included participants from 11 centers across the country, and there were an average of 2.75 control subjects for each case, such that we think this potential confounding is likely to be small. Finally, there are likely to be other P2RX7 alleles associated with this effect. Our pore assay robustly detects the five most common loss-of-function genotypes, but has a relatively low positive predictive value because of the presence of samples with additional loss-of-function variants (23). As such, it is likely that replication efforts to test the P2RX7 genetic association to exacerbation risk will yield other SNPs, an aspect of reproducibility that is more common for genes with rare functional variants (34). Despite these limitations, our genetic data in combination with the use of the whole-blood monocyte P2X7 pore assay as an epidemiologic tool significantly strengthens our findings. Deep sequencing of this gene in a larger study with uniform participant entry criteria and prednisone-dependent exacerbation data will likely define which P2RX7 SNPs confer the most risk.
Several potential mechanisms are worth consideration regarding P2X7-dependent protection from exacerbations and asthma symptoms. First, activation of inflammasome-dependent caspases leading to proteolytic cleavage of IL-1 family cytokines is a cardinal feature of P2X7. How the production of more IL-1β or IL-18 by persons with normal P2X7 function would be protective from exacerbations is unclear. Additionally, this effect on IL-1β production is only notable at early time points in vitro, whereas differences in IL-10 production between normal and low pore groups are sustained for at least 24 hours (25, 35). Second, we have shown that the early recruitment of neutrophils to the upper airway directly correlates with P2X7 function and is protective of exacerbation, potentially by limiting the spread of infection to the lung (8). Interestingly, P2X7 is involved in the early steps of granulocyte extravasation before the activation of the Mac-1/ICAM-1 integrin receptors involved in neutrophil adherence to endothelial cells, such that the knock-out mouse has diminished PMN adherence (36). Other possible P2X7-dependent mechanisms that may be relevant during infectious triggers of acute asthmatic inflammation include differences in the rates of epithelial cell apoptosis or macrophage phagocytosis of spent inflammatory cells (37–39). In this case, delayed epithelial cell apoptosis in individuals with attenuated pore function could provide an advantage to viral replication (40, 41). Similarly, inefficient P2X7-dependent macrophage phagocytosis of apoptotic epithelial cells or neutrophils would likely prolong the inflammation in the lower airway, consistent with observations in patients with severe asthma (42, 43). In conclusion, P2X7 function helps protect a diverse group of patients with asthma from exacerbation and may be important for baseline symptom control while on maintenance ICS; the precise mechanism warrants further study.
Supplementary Material
Acknowledgments
The authors thank the participants of these studies for their commitment to asthma research and the study coordinators for their dedication to make this work possible. They also thank Dr. Scott Weiss for DNA banking services performed for the Asthma Clinical Research Network, and for comments pertaining to the manuscript. Dr. Esteban Burchard at University of California, San Francisco, and Dr. Kenny Beckman the staff at the Children’s Hospital Oakland Research Institute Center for Genetics facilitated the genotyping. Drs. Robert Zeiger, Robert Strunk, Christina Rebordosa, and Fernando Martinez have also provided helpful insight for the manuscript.
Footnotes
Supported by NIH NHLBI U10 HL074212, NIH NHLBI K23 HL081492, and NIH NCRR 1 UL1RR025011.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201204-0750OC on November 9, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Willart MA, Lambrecht BN. The danger within: endogenous danger signals, atopy and asthma. Clin Exp Allergy 2009;39:12–19 [DOI] [PubMed] [Google Scholar]
- 2.Idzko M, Hammad H, van Nimwegen M, Kool M, Willart MA, Muskens F, Hoogsteden HC, Luttmann W, Ferrari D, Di Virgilio F, et al. Extracellular ATP triggers and maintains asthmatic airway inflammation by activating dendritic cells. Nat Med 2007;13:913–919 [DOI] [PubMed] [Google Scholar]
- 3.Wark PA, Johnston SL, Moric I, Simpson JL, Hensley MJ, Gibson PG. Neutrophil degranulation and cell lysis is associated with clinical severity in virus-induced asthma. Eur Respir J 2002;19:68–75 [DOI] [PubMed] [Google Scholar]
- 4.Esther CR, Jr, Alexis NE, Clas ML, Lazarowski ER, Donaldson SH, Ribeiro CM, Moore CG, Davis SD, Boucher RC. Extracellular purines are biomarkers of neutrophilic airway inflammation. Eur Respir J 2008;31:949–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Surprenant A, Rassendren F, Kawashima E, North RA, Buell G. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7). Science 1996;272:735–738 [DOI] [PubMed] [Google Scholar]
- 6.Di Virgilio F. Liaisons dangereuses: P2X(7) and the inflammasome. Trends Pharmacol Sci 2007;28:465–472 [DOI] [PubMed] [Google Scholar]
- 7.Müller T, Paula Vieira R, Grimm M, Durk T, Cicko S, Zeiser R, Jakob T, Martin SF, Blumenthal B, Sorichter S, et al. A potential role for P2X7R in allergic airway inflammation in mice and humans. Am J Respir Cell Mol Biol 2011;44:456–464 [DOI] [PubMed] [Google Scholar]
- 8.Denlinger LC, Shi L, Guadarrama A, Schell K, Green D, Morrin A, Hogan K, Sorkness RL, Busse WW, Gern JE. Attenuated P2X7 pore function as a risk factor for virus-induced loss of asthma control. Am J Respir Crit Care Med 2009;179:265–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buell GN, Talabot F, Gos A, Lorenz J, Lai E, Morris MA, Antonarakis SE. Gene structure and chromosomal localization of the human P2X7 receptor. Receptors Channels 1998;5:347–354 [PubMed] [Google Scholar]
- 10.Barnes KC, Neely JD, Duffy DL, Freidhoff LR, Breazeale DR, Schou C, Naidu RP, Levett PN, Renault B, Kucherlapati R, et al. Linkage of asthma and total serum IgE concentration to markers on chromosome 12q: evidence from Afro-Caribbean and caucasian populations. Genomics 1996;37:41–50 [DOI] [PubMed] [Google Scholar]
- 11.Dizier MH, Besse-Schmittler C, Guilloud-Bataille M, Annesi-Maesano I, Boussaha M, Bousquet J, Charpin D, Degioanni A, Gormand F, Grimfeld A, et al. Genome screen for asthma and related phenotypes in the French EGEA study. Am J Respir Crit Care Med 2000;162:1812–1818 [DOI] [PubMed] [Google Scholar]
- 12.Xu J, Postma DS, Howard TD, Koppelman GH, Zheng SL, Stine OC, Bleecker ER, Meyers DA. Major genes regulating total serum immunoglobulin E levels in families with asthma. Am J Hum Genet 2000;67:1163–1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raby BA, Silverman EK, Lazarus R, Lange C, Kwiatkowski DJ, Weiss ST. Chromosome 12q harbors multiple genetic loci related to asthma and asthma-related phenotypes. Hum Mol Genet 2003;12:1973–1979 [DOI] [PubMed] [Google Scholar]
- 14.Shao C, Suzuki Y, Kamada F, Kanno K, Tamari M, Hasegawa K, Aoki Y, Kure S, Yang X, Endo H, et al. Linkage and association of childhood asthma with the chromosome 12 genes. J Hum Genet 2004;49:115–122 [DOI] [PubMed] [Google Scholar]
- 15.Brasch-Andersen C, Tan Q, Borglum AD, Haagerup A, Larsen TR, Vestbo J, Kruse TA. Significant linkage to chromosome 12q24.32-q24.33 and identification of sfrs8 as a possible asthma susceptibility gene. Thorax 2006;61:874–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Celedon JC, Soto-Quiros ME, Avila L, Lake SL, Liang C, Fournier E, Spesny M, Hersh CP, Sylvia JS, Hudson TJ, et al. Significant linkage to airway responsiveness on chromosome 12q24 in families of children with asthma in Costa Rica. Hum Genet 2007;120:691–699 [DOI] [PubMed] [Google Scholar]
- 17.Fuller SJ, Stokes L, Skarratt KK, Gu BJ, Wiley JS. Genetics of the P2X7 receptor and human disease. Purinergic Signal 2009;5:257–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gu BJ, Zhang W, Worthington RA, Sluyter R, Dao-Ung P, Petrou S, Barden JA, Wiley JS. A glu-496 to ALA polymorphism leads to loss of function of the human P2X7 receptor. J Biol Chem 2001;276:11135–11142 [DOI] [PubMed] [Google Scholar]
- 19.Wiley JS, Dao-Ung LP, Li C, Shemon AN, Gu BJ, Smart ML, Fuller SJ, Barden JA, Petrou S, Sluyter R. An ile-568 to as polymorphism prevents normal trafficking and function of the human P2X7 receptor. J Biol Chem 2003;278:17108–17113 [DOI] [PubMed] [Google Scholar]
- 20.Gu BJ, Sluyter R, Skarratt KK, Shemon AN, Dao-Ung LP, Fuller SJ, Barden JA, Clarke AL, Petrou S, Wiley JS. An arg307 to glen polymorphism within the ATP-binding site causes loss of function of the human P2X7 receptor. J Biol Chem 2004;279:31287–31295 [DOI] [PubMed] [Google Scholar]
- 21.Shemon AN, Sluyter R, Fernando SL, Clarke AL, Dao-Ung LP, Skarratt KK, Saunders BM, Tan KS, Gu BJ, Fuller SJ, et al. A thr357 to ser polymorphism in homozygous and compound heterozygous subjects causes absent or reduced P2X7 function and impairs ATP-induced mycobacterial killing by macrophages. J Biol Chem 2006;281:2079–2086 [DOI] [PubMed] [Google Scholar]
- 22.Roger S, Mei ZZ, Baldwin JM, Dong L, Bradley H, Baldwin SA, Surprenant A, Jiang LH. Single nucleotide polymorphisms that were identified in affective mood disorders affect ATP-activated P2X7 receptor functions. J Psychiatr Res 2010;44:347–355 [DOI] [PubMed] [Google Scholar]
- 23.Denlinger LC, Coursin DB, Schell K, Angelini G, Green DN, Guadarrama AG, Halsey J, Prabhu U, Hogan KJ, Bertics PJ. Human P2X7 pore function predicts allele linkage disequilibrium. Clin Chem 2006;52:995–1004 [DOI] [PubMed] [Google Scholar]
- 24.Stokes L, Fuller SJ, Sluyter R, Skarratt KK, Gu BJ, Wiley JS. Two haplotypes of the P2X(7) receptor containing the ala-348 to Thr polymorphism exhibit a gain-of-function effect and enhanced interleukin-1beta secretion. FASEB J 2010;24:2916–2927 [DOI] [PubMed] [Google Scholar]
- 25.Denlinger LC, Angelini G, Schell K, Green DN, Guadarrama AG, Prabhu U, Coursin DB, Bertics PJ, Hogan K. Detection of human P2X7 nucleotide receptor polymorphisms by a novel monocyte pore assay predictive of alterations in lipopolysaccharide-induced cytokine production. J Immunol 2005;174:4424–4431 [DOI] [PubMed] [Google Scholar]
- 26.Korpi-Steiner NL, Sheerar D, Puffer EB, Urben C, Boyd J, Guadarrama A, Schell K, Denlinger LC. Standardized method to minimize variability in a functional P2X(7) flow cytometric assay for a multi-center clinical trial. Cytometry B Clin Cytom 2008;74:319–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi L, Manthei DM, Guadarrama AG, Lenertz LY, Denlinger LC. Rhinovirus-induced IL-1beta release from bronchial epithelial cells is independent of functional P2X7. Am J Respir Cell Mol Biol 2012;47:363–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Denlinger LC, Sorkness CA, Chinchilli VM, Lemanske RF., Jr Guideline-defining asthma clinical trials of the National Heart, Lung, and Blood Institute's Asthma Clinical Research Network and Childhood Asthma Research and Education Network. J Allergy Clin Immunol 2007;119:3–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sutherland ER, King TS, Icitovic N, Ameredes BT, Bleecker E, Boushey HA, Calhoun WJ, Castro M, Cherniack RM, Chinchilli VM, et al. A trial of clarithromycin for the treatment of suboptimally controlled asthma. J Allergy Clin Immunol 2010;126:747–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Calhoun WJ, Ameredes BT, King TS, Icitovic N, Bleecker E, Castro M, Cherniack RM, Chinchilli VM, Craig TJ, Denlinger L, et al. Comparison of inhaled corticosteroid adjustment strategies for long term asthma management: the ACRN BASALT trial. JAMA 2012;308:987–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manthei DM, Jackson DJ, Evans MD, Gangnon RE, Tisler CJ, Gern JE, Lemanske RF, Jr, Denlinger LC. Protection from asthma in a high-risk birth cohort by attenuated P2X7 function. J Allergy Clin Immunol 2012;130:496–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Michel AD, Chambers LJ, Clay WC, Condreay JP, Walter DS, Chessell IP. Direct labelling of the human P2X7 receptor and identification of positive and negative cooperativity of binding. Br J Pharmacol 2007;151:103–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yan Z, Khadra A, Li S, Tomic M, Sherman A, Stojilkovic SS. Experimental characterization and mathematical modeling of P2X7 receptor channel gating. J Neurosci 2010;30:14213–14224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Q, Zhang H, Yu K. Approaches for evaluating rare polymorphisms in genetic association studies. Hum Hered 2010;69:219–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perregaux DG, Gabel CA. Post-translational processing of murine IL-1: evidence that ATP-induced release of IL-1 alpha and IL-1 beta occurs via a similar mechanism. J Immunol 1998;160:2469–2477 [PubMed] [Google Scholar]
- 36.McDonald B, Pittman K, Menezes GB, Hirota SA, Slaba I, Waterhouse CC, Beck PL, Muruve DA, Kubes P. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science 2010;330:362–366 [DOI] [PubMed] [Google Scholar]
- 37.Welter-Stahl L, da Silva CM, Schachter J, Persechini PM, Souza HS, Ojcius DM, Coutinho-Silva R. Expression of purinergic receptors and modulation of P2X7 function by the inflammatory cytokine IFNgamma in human epithelial cells. Biochim Biophys Acta 2009;1788:1176–1187 [DOI] [PubMed] [Google Scholar]
- 38.Gorodeski GI. P2X7-mediated chemoprevention of epithelial cancers. Expert Opin Ther Targets 2009;13:1313–1332 [DOI] [PubMed] [Google Scholar]
- 39.Gu BJ, Saunders BM, Jursik C, Wiley JS. The P2X7-nonmuscle myosin membrane complex regulates phagocytosis of nonopsonized particles and bacteria by a pathway attenuated by extracellular ATP. Blood 2010;115:1621–1631 [DOI] [PubMed] [Google Scholar]
- 40.Wark PA, Johnston SL, Bucchieri F, Powell R, Puddicombe S, Laza-Stanca V, Holgate ST, Davies DE. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med 2005;201:937–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tesfaigzi Y. Roles of apoptosis in airway epithelia. Am J Respir Cell Mol Biol 2006;34:537–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huynh ML, Malcolm KC, Kotaru C, Tilstra JA, Westcott JY, Fadok VA, Wenzel SE. Defective apoptotic cell phagocytosis attenuates prostaglandin E2 and 15-hydroxyeicosatetraenoic acid in severe asthma alveolar macrophages. Am J Respir Crit Care Med 2005;172:972–979 [DOI] [PubMed] [Google Scholar]
- 43.Fitzpatrick AM, Holguin F, Teague WG, Brown LA. Alveolar macrophage phagocytosis is impaired in children with poorly controlled asthma. J Allergy Clin Immunol 2008;121:1372–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.