Abstract
The neurotransmitter acetylcholine is considered essential for proper functioning of the hippocampus-dependent declarative memory system, and it represents a major neuropharmacological target for the treatment of memory deficits, such as those in Alzheimer's disease. During slow-wave sleep (SWS), however, declarative memory consolidation is particularly strong, while acetylcholine levels in the hippocampus drop to a minimum. Observations in rats led to the hypothesis that the low cholinergic tone during SWS is necessary for the replay of new memories in the hippocampus and their long-term storage in neocortical networks. However, this low tone should not affect nondeclarative memory systems. In this study, increasing central nervous cholinergic activation during SWS-rich sleep by posttrial infusion of 0.75 mg of the cholinesterase inhibitor physostigmine completely blocked SWS-related consolidation of declarative memories for word pairs in human subjects. The treatment did not interfere with consolidation of a nondeclarative mirror tracing task. Also, physostigmine did not alter memory consolidation during waking, when the endogenous central nervous cholinergic tone is maximal. These findings are in line with predictions that a low cholinergic tone during SWS is essential for declarative memory consolidation.
Memory relies on a consolidation process that is thought to benefit from sleep (1-5). This long-held view recently has received substantial support from animal and human studies, suggesting that reprocessing of newly acquired material within hippocampal and neocortical networks takes place during sleep and could be a basis for long-term memory consolidation (6-10). In this regard, several studies point to a particular relevance of slow-wave sleep (SWS). In rats, spatiotemporal patterns of neuronal activity observed in hippocampal CA1 neurons during encoding of a spatial maze are replayed during subsequent periods of SWS (11, 12). In humans, declarative memory for word pairs and spatial locations, which depends on the hippocampus (13), improved more across periods of SWS-rich sleep compared with retention periods containing large amounts of rapid eye movement (REM) sleep or wakefulness (4, 14). Nondeclarative tasks benefited mainly from periods rich in REM sleep (4, 15).
A striking paradox derives from the fact that SWS is characterized by suppression of cholinergic activity (deriving from basal forebrain and tegmental neurons and spreading to the entire neocortex and hippocampus) to an absolute minimum, compared with the high cholinergic tone during wakefulness and REM sleep (16, 17). Undiminished cholinergic activity is thought to be a prerequisite for memory function: A global reduction in cholinergic neurotransmission, either of pathologic origin as in Alzheimer's disease or experimentally induced, e.g., by an antagonist at the cholinergic synapse like scopolamine, distinctly impairs memory function (18-20). On the other hand, improved memory can be found after enhancing cholinergic tone with the cholinesterase inhibitor physostigmine, which reduces acetylcholine breakdown (21, 22). Blocking cholinergic activity with scopolamine during REM sleep in rats induces deficits in the consolidation of an avoidance task learned before sleep (23). Memory retrieval, in contrast, might not be impaired by scopolamine (24). To our knowledge, no studies have investigated the relationship between the low cholinergic tone during SWS and memory consolidation.
A model presented by Buzsaki (7, 25) and Hasselmo (26) proposes that during the sharp-wave activity of SWS newly acquired declarative memories stored in the hippocampus are reactivated and transferred to neocortical networks. This process is thought to require a silencing of cholinergic activity (27), which releases cholinergic suppression on excitatory feedback synapses in the hippocampal CA3 region and on efferent projections spreading activation from CA3 to CA1, the entorhinal cortex, and neocortex (28). This model predicts that elevated levels of cholinergic activity during SWS will disrupt memory processing. This study's aim is to verify the hypothesis that increasing the central nervous cholinergic tone during a period of SWS-rich sleep disturbs SWS-related consolidation of declarative memories. To control the specificity of this effect, a declarative (hippocampus-dependent) and a nondeclarative (hippocampus-independent) memory task were selected. Additionally, subjects in a control experiment did not sleep between learning and recall.
Methods
Subjects, Design, and Procedure. Healthy men between the ages of 18 and 35 (n = 29) participated in one adaptation and two experimental nights. On each experimental night, after insertion of venous catheters for substance administration and blood collection and application of electrodes for polysomnography, the subjects learned two memory tasks from 10:00 to 10:30 p.m. Then, subjects in the sleep experiment (n = 18) went to bed, lights were turned off at 11:00 p.m., and subjects slept for the first half of the night. Sleep onset occurred on average at 11:15 p.m., and subjects were awakened 3 h after sleep onset. Recall testing took place 30 min after awakening at ≈2:45 a.m.; i.e., the retention interval was ≈4.45 h. The subjects in the wake control experiment (n = 11) also learned from 10:00 to 10:30 p.m. but then stayed awake during the interval between learning and recall testing. At learning and recall testing, all subjects were asked to rate their subjective drowsiness, fatigue, tenseness, and motivation on five-point scales. After sleep, they were asked to rate their sleep quality.
Memory Tasks. Two different memory tasks were chosen, a declarative paired-associate wordlist task and a nondeclarative mirror tracing task, which are known to be differentially influenced by periods with high amounts of SWS and REM sleep (for a detailed description, see ref. 4). The wordlist task consisted of 40 pairs of semantically related German words, which the subject had to learn to a criterion of at least 60% (see Table 1, which is published as supporting information on the PNAS web site). The word pairs were presented sequentially on a computer screen for 5 s each, with an intertrial interval of 100 ms. After presentation of the complete list, recall was tested immediately. The first word of each pair was presented, and the subject had to name the second one. Afterward, the correct answer was displayed for 2 s, allowing the subject to correct his memory when necessary. When the 60% criterion was not reached, recall testing was repeated. Note that because of the presentation of the correct word directly after the answer, performance increased from learning to recall. The mirror tracing task required the subject to trace several figures that he could see only in a mirror. The time needed for completion of the figures (speed) and the number of deviations from the prescribed 0.8-cm-wide path (accuracy) were recorded. Subjects were instructed to trace the lines of the figures as fast and as accurately as possible. Before tracing the actual figures, subjects were trained by tracing a simple star-shaped figure until they could draw it in <30 s with <12 errors. Both the average of all six figures and the results of the last figure drawn before sleep and the first one after sleep are given.
Substance Administration. Beginning with sleep onset (sleep experiment) or at 11:15 p.m. (wake control experiment), subjects received an infusion of physostigmine (0.75 mg) over 2 h dissolved in 50 ml of saline solution on one night and 50 ml of saline solution (placebo) on another. Thus, in the sleep group, cholinergic activity was increased during the first part of sleep, which is a period with a high percentage of SWS.
The dose was chosen with the goal of minimizing peripheral side effects (e.g., salivation and bradycardia) and influence on the phenotypic sleep pattern, especially on awakening. It was comparable with doses used in other studies finding memory effects after physostigmine administration (e.g., see ref. 21). In prestudy trials, doses of 1 mg and higher over 2 h led to increased waking and REM sleep. On the other hand, doses of <0.75 mg over 2 h were considered too low to be effective. Additionally, directly before going to sleep subjects received a single oral dose (10 mg) of butylscopolamine or placebo, which does not pass the blood-brain barrier, to counteract peripheral side effects of physostigmine. In a standardized interview, subjects did not report any substantial side effects. Substance administration was in a balanced, randomized, double-blind fashion. The duration of drug administration was chosen on the basis of the 20-30 min elimination half-life of physostigmine in blood (29). Thus, it was ascertained that the substance was effective only during sleep, not during retrieval testing, which took place 1.5 h after the end of substance administration. In an additional experiment, 12 subjects received, in randomized and balanced order, only a single oral dose (10 mg) of butylscopolamine or placebo after learning the wordlist, directly before going to bed. Procedures were otherwise as in the main experiments.
Sleep Recordings, Hormone Assays, and Statistical Analysis. Sleep was recorded polysomnographically. Recordings were scored offline by two independent raters according to standard criteria (30). Discrepant scorings were decided with the aid of a third rater. Additionally, the number of sleep spindles in sleep stage 2 (S2) was counted manually by two independent raters. Throughout the whole experimental period, blood samples were taken every 30 min via long plastic tubes from an adjacent room without disturbing the subject's sleep. Blood samples were immediately centrifuged, and plasma and serum were stored at -20°C until assay. Cortisol was measured from serum by using an ELISA (Enzymun-Test Cortisol, Roche Diagnostics). Peripheral norepinephrine levels were determined in 14 subjects from EDTA plasma by high-performance liquid chromatography (Waters) with electrochemical detection. Statistical testing for all tasks relied on three-factorial ANOVA with two within-subject factors (substance, pretest/posttest) and one between-group factor (sleep/wake). Where appropriate, conditions were compared with t tests for within group comparisons. The main analyses for the sleep experiment were restricted to a subsample of subjects that showed no change in sleep parameters (n = 11). However, results for all subjects are also given.
Results
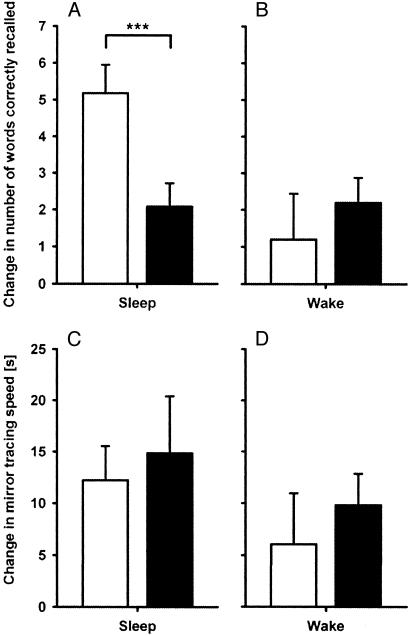
Effects of Physostigmine on Sleep-Associated Memory. The central finding of this study is that, during sleep, memory for the declarative wordlist task distinctly decreased after administration of physostigmine as compared with placebo. In the sleep experiments, subjects at learning needed on average (mean ± SEM) 1.6 ± 0.3 trials and 1.7 ± 0.3 trials to reach the criterion of 60% correct in the placebo and physostigmine conditions, respectively (P > 0.5). Also, the number of initially learned words was comparable for both conditions (26.6 ± 1.3 words after placebo vs. 28.2 ± 1.7 words after physostigmine, P > 0.25). After sleep, recall improved on average by 5.2 ± 0.8 words when placebo was given, but only by 2.1 ± 0.6 words after physostigmine (P < 0.001) (Fig. 1A). The decrease in retention rate caused by physostigmine corresponds to a total loss of the benefit sleep has compared with wakefulness under placebo conditions (see below). The administration of butylscopolamine alone did not affect wordlist recall after sleep as compared with that of placebo (initial learning, 27.3 ± 0.6 words after butylscopolamine vs. 29.6 ± 0.7 words after placebo; increase after sleep, 5.0 ± 1.1 words after butylscopolamine vs. 4.2 ± 0.7 words after placebo; P > 0.35).
Fig. 1.
Memory performance after physostigmine and placebo were administered during sleep and wake periods. Memory is indicated by the difference in performance between learning and recall sessions (physostigmine, filled bars; placebo, open bars). (A and B) As expected from previous studies, placebo-treated subjects retained significantly more words under the sleep than the wake condition (P < 0.001). Physostigmine completely eliminated the consolidating effect of sleep on hippocampus-dependent declarative memory (P < 0.001), whereas it had no effect during wakefulness (P > 0.40). (C and D) Hippocampus-independent memory for mirror tracing performance showed no detrimental effect of physostigmine during either sleep or wakefulness (P > 0.40).
On the other hand, neither speed nor accuracy in the nondeclarative mirror tracing task decreased after physostigmine administration (Fig. 1C). Before sleep, subjects in the placebo and physostigmine conditions, respectively, needed on average 68.2 ± 5.5 s and 73.5 ± 9.1 s to trace the figures (P > 0.60). The speed increase at recall testing was likewise comparable for both conditions (12.2 ± 3.3 s vs. 14.2 ± 5.6 s, P > 0.75). The average number of errors before sleep in the placebo and physostigmine conditions, respectively, was 4.2 ± 1.0 and 7.2 ± 2.6 (P > 0.10); at recall testing the number decreased by 1.2 ± 0.5 and 3.8 ± 1.5 (P > 0.12). When comparing only the last pre- and first postsession trials to remove within-session learning effects, speed increased at recall testing by 5.6 ± 2.6 s and 4.9 ± 3.1 s in the placebo and physostigmine conditions, respectively (P > 0.80). The number of errors at recall testing decreased by 2.0 ± 0.7 and by 2.7 ± 0.8 in both conditions (P > 0.40).
The results for the entire sample (n = 18), including the subjects displaying physostigmine-related alterations in sleep (see below), are very similar to those for only the good sleepers. At learning before sleep, trials to reach the 60% criterion (1.5 ± 0.4 trials vs. 1.7 ± 0.3 trials, P > 0.25) and number of learned words (26.8 ± 0.9 words vs. 28.2 ± 1.1 words, P > 0.15) were comparable for placebo and physostigmine, respectively. At recall testing, however, the number of words correctly recalled improved by 5.4 ± 0.7 words after placebo and by only 2.6 ± 0.4 words after physostigmine (P < 0.001). In the mirror tracing task, the average speed for the six figures at learning (71.1 ± 4.7 s vs. 67.8 ± 6.0 s, P > 0.70) and the gain over sleep (15.5 ± 3.3 s vs. 11.8 ± 3.6 s, P > 0.50) did not differ between the placebo and the physostigmine conditions, respectively. The number of errors at learning (4.8 ± 0.7 errors vs. 6.8 ± 1.6 errors, P > 0.20) was at recall testing after sleep slightly less reduced after placebo (1.4 ± 0.5 errors) than after physostigmine (3.2 ± 1.0 errors, P = 0.10).
Effects of Physostigmine on Sleep and Hormones. In the 11 subjects selected for analysis, sleep parameters did not differ between placebo and physostigmine conditions: awake, 2.3 ± 1.4% vs. 1.0 ± 0.4%, P > 0.30; S1, 11.4 ± 3.4% vs. 11.7 ± 2.9%, P > 0.90; S2, 47.5 ± 4.4% vs. 50.3 ± 3.0%, P > 0.60; S3, 14.3 ± 2.2% vs. 13.5 ± 1.5%, P > 0.60; S4, 11.6 ± 2.4% vs. 10.2 ± 1.6%, P > 0.35; and REM sleep, 11.4 ± 2.5% vs. 12.4 ± 1.9%, P > 0.70. In the entire sample of 18 subjects, however, there was a decrease in sleep depth after physostigmine administration as indicated by reduced time spent in SWS (S3, 14.4 ± 1.7% vs. 11.1 ± 1.2%, P < 0.05; S4, 15.7 ± 2.0% vs. 10.0 ± 1.4%, P < 0.01). There were no differences between the conditions for the other sleep stages: awake, 1.8 ± 0.9% vs. 2.4 ± 0.9%, P > 0.50; S1, 10.4 ± 2.5% vs. 14.7 ± 2.9%, P > 0.15; S2, 43.5 ± 2.9% vs. 47.3 ± 2.9%, P > 0.30; and REM sleep, 13.0 ± 1.7% vs. 13.3 ± 1.6%, P > 0.80. The number of sleep spindles per 30-s epoch of S2 sleep was increased by physostigmine administration (1.53 ± 0.13 vs. 1.89 ± 0.20, P < 0.05). This change in spindle activity was not related to changes in declarative memory performance (r = -0.24, P > 0.35).
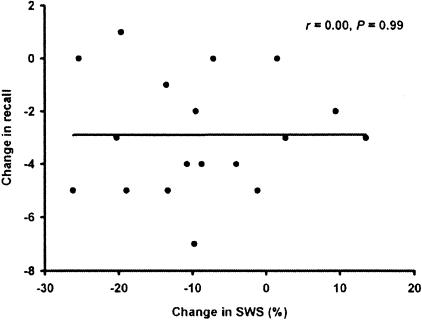
To further examine possible connections between the change in SWS and in learning performance after physostigmine administration compared with placebo, the correlation was calculated for the entire sample (n = 18). This analysis did not indicate any relationship (r = 0.00, P = 0.99) (Fig. 2). Subjective ratings of sleep and also of subjective state, including fatigue and drowsiness before and after sleep and well-restedness after sleep, did not differ between physostigmine and placebo conditions (P > 0.50). Subjects could not tell which treatment they received during the night. Their judgments in this respect did not differ significantly from chance (P > 0.65).
Fig. 2.
Correlation between the change in SWS and word recall after physostigmine as compared with placebo administration in the entire subject sample (n = 18). Although most subjects experienced a reduction in SWS, this reduction was not correlated with impaired recall performance. This finding shows that the effect of physostigmine on SWS is independent of its effect on memory performance.
Because previous studies indicate that SWS-related consolidation of declarative memory is suppressed by cortisol (31), blood concentrations of cortisol were determined. However, concentrations of cortisol did not differ between the placebo and physostigmine conditions. Average plasma cortisol concentrations in the placebo and physostigmine conditions, respectively, were at learning 3.07 ± 0.5 mg/dl vs. 3.35 ± 0.5 mg/dl, during sleep 1.96 ± 0.31 mg/dl vs. 1.94 ± 0.30 mg/dl, and at retrieval 10.96 ± 1.42 mg/dl vs. 9.60 ± 0.72 mg/dl (P > 0.25). As an indicator of catecholaminergic activity, known to influence memory consolidation (32), peripheral norepinephrine levels were assessed. Average levels were comparable in both the placebo and physostigmine conditions, respectively, and were at learning 144 ± 15 pg/ml vs. 148 ± 10 pg/ml, during sleep 102 ± 11 pg/ml vs. 100 ± 8 pg/ml, and at retrieval 191 ± 17 pg/ml vs. 201 ± 17 pg/ml (P > 0.75).
Memory Effects of Physostigmine During Wakefulness. In the wake control experiment, subjects received the same treatment and tasks as in the sleep experiment, but they stayed awake between learning and retrieval. Here, the elevation of central cholinergic tone did not result in decreased memory performance but, on average, in a nonsignificant increase in wordlist recall as compared with that in placebo conditions (Fig. 1B). Before the wake interval, in the placebo and physostigmine conditions, respectively, subjects learned 28.8 ± 1.1 words vs. 28.6 ± 1.1 words. Increases in recall after this interval were small and comparable for both conditions (1.2 ± 1.3 words vs. 2.2 ± 0.7 words, P > 0.40).
Procedural memory for the mirror tracing task was not influenced by physostigmine administration. Neither speed nor accuracy differed between conditions (Fig. 1D). Average speed before the wake interval in the placebo and physostigmine conditions, respectively, was 67.72 ± 7.60 s vs. 73.45 ± 4.34 s and thereafter was increased by 6.06 ± 4.90 s vs. 9.82 ± 3.04 s (P > 0.50). The average number of errors (which, at learning, was 5.20 ± 0.88 errors vs. 5.32 ± 1.21 errors) at recall testing after the wake interval was reduced by 1.56 ± 0.41 errors vs. 1.78 ± 0.76 errors (P > 0.81). When comparing only the last pre- and first postsession trial, speed decreased nonsignificantly (P > 0.10) by 4.2 ± 2.3 s vs. 1.2 ± 3.4 s (P > 0.45 in the placebo and physostigmine conditions, respectively). The number of errors at recall testing after the wake interval was reduced by 1.9 ± 0.8 errors vs. 1.9 ± 1.0 errors (P > 0.90). Thus, both analyses yield the result that performance was slightly inferior after wakefulness, compared with after sleep, and did not differ between treatments.
The selective impairment of physostigmine on sleep-related declarative memory function was confirmed by an overall ANOVA including the sleep and wake control experiments. It showed for the wordlist task a significant pretest/posttest effect (P < 0.001) and a pretest/posttest × sleep interaction (P < 0.05), replicating previous studies that demonstrated better memory performance after sleep than after wakefulness. Additionally, there was a significant three-way interaction of substance × pretest/posttest × sleep (P < 0.01), reflecting that only during sleep does physostigmine have an impact on declarative memory formation. For the mirror tracing task, ANOVA revealed only a significant pretest/posttest effect (P < 0.001 for speed and accuracy). All other main effects and interactions were nonsignificant (P > 0.40 for speed and P > 0.15 for accuracy).
Discussion
The main outcome of the present study is that the SWS-associated enhancement of hippocampus-dependent declarative memory, found in previous studies and confirmed here, does not appear when the central cholinergic tone is increased by administration of physostigmine during a period of SWS-rich sleep. During wakefulness, the same treatment has no negative effect. These effects were distinct from effects on hippocampus-independent procedural memory in a mirror tracing task, which was not impaired by physostigmine during sleep and wakefulness. This pattern of results fits well with models of a hippocampal-neocortical dialogue (7, 26), which consider acetylcholine an important modulator of the direction of information flow between hippocampus and neocortex during sleep and wakefulness. During wakefulness, strong cholinergic activity suppresses hippocampal-neocortical feedback, but not neocortical-hippocampal flow (28). During SWS, which in the animal literature refers to all non-REM sleep, and also during quiet wakefulness in rodents, cholinergic suppression is released (16). The loss of cholinergic tone disinhibits hippocampal feedback synapses, and activity coming from the hippocampus, especially brief, large-amplitude sharp waves, can spread toward the entorhinal cortex and neocortex. This process has been proposed as underlying the integration of new memories into existing neocortical networks (7, 33). This framework assumes that, whereas acquisition of declarative information during the wake state needs the higher cholinergic tone, the long-term storage and integration of the materials into neocortical networks require a period of release from cholinergic suppression of feedback transmission in the hippocampus. Although our results provide confirmatory evidence for this integrative model of sleep-related memory function, it is conceptual in nature and needs further testing in various aspects.
Whereas postlearning administration of cholinergic agonists has not been studied in humans, some studies in animals report enhancing effects on long-term memory when cholinergic receptor agonists are administered immediately after learning (34). These studies, however, used tasks like passive avoidance that seem less dependent on hippocampal functioning and did not specifically control for sleep and wakefulness; for that reason these studies are difficult to compare. Here, posttrial physostigmine proved ineffective when subjects remained awake, indicating an effect selectively on a sleep-dependent type of consolidation. This finding would not exclude the possibility of strengthening effects of such substances on, for example, emotionally aversive memories, especially when given shortly after acquisition.
The main definition for SWS comes from its phenotypical appearance of slow, large electroencephalogram (EEG) waves, but several other factors vary in concurrence with these EEG signs, including cholinergic activity, the focus of our study. Previous animal studies led to the prediction that acetylcholine impairs memory consolidation at a hippocampal level during SWS. Here we show in humans that central nervous cholinergic activation during a period of SWS-rich sleep does in fact impair hippocampus-dependent declarative memory consolidation, although we cannot show directly the site of action in humans. Nevertheless, we excluded several possible mediating factors like peripheral norepinephrine levels, sleep depth (by using subjects with comparable sleep architecture on both nights), or subjective sleepiness and fatigue. Because the cholinergic transmitter system is involved in the regulation of the sleep/wake state (35), increasing the general central cholinergic tone usually leads to a concomitant change in sleep stage from non-REM to REM sleep and to wakefulness (36). Therefore, a dissociation of direct cholinergic effects on memory consolidation from effects mediated by a change in sleep stage can be achieved only with difficulty. However, several facts speak in favor of a direct cholinergic effect on memory consolidation in this study. First, we chose a dosage that would only minimally affect sleep. In fact, in prestudy trials, even small increases in the rate of the infusion produced long-lasting increases in early REM sleep. Notably, after physostigmine, we found an increase in spindle activity. Because the thalamocortical mechanisms underlying spindle activity are thought to enhance cortical memory integration (37, 38), this finding adds support to the view that physostigmine blocked declarative memory consolidation at a hippocampal, rather than a thalamoneocortical, stage. More importantly, the results are identical in the subsample without sleep disturbances and in the entire sample. There is no correlation between the decrease in SWS and in memory performance. In addition, performance was impaired only in the declarative memory task, whereas procedural memory remained unaffected, evidence against a nonspecific mediation of this effect. Therefore, it seems justified to conclude that the loss of sleep-related declarative memory consolidation did not result from the loss of a small percentage of SWS.
The lack of correlation between the amount of SWS and declarative memory performance may be somewhat surprising, but it is consistent with previous studies (reviewed in refs. 39 and 40) and can be explained when SWS is seen as a permissive factor for memory consolidation: SWS always occurs without regard to the actual amount of learning that has taken place. Accordingly, SWS does not increase after extensive declarative learning, and there is no increase in memory performance with increasing amounts of SWS in normal subjects (38, 41). Only when large amounts of SWS are missing can impaired memory consolidation be seen (4, 14). On the other hand, if physostigmine has already blocked memory replay on a hippocampal level, any reduction in SWS (on the neocortical level) should be without further effect, thus preventing a correlation between SWS and memory consolidation.
Together, our findings show that the changes in central nervous cholinergic activity during wakefulness and sleep have a functional significance. As predicted, suppression of acetylcholine during a SWS-rich period is a necessary condition for sleep-related declarative memory consolidation to occur. This finding also implies that the administration of cholinesterase inhibitors before sleep in patients with Alzheimer's disease should be reconsidered.
Supplementary Material
Acknowledgments
We thank Drs. G. Buzsaki and M. Hasselmo for valuable comments on an earlier version of the manuscript. This work was supported by the Deutsche Forschungsgemeinschaft.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: SWS, slow-wave sleep; REM, rapid eye movement; Sn, sleep stage n.
See Commentary on page 1795.
References
- 1.McGaugh, J. L. (2000) Science 287, 248-251. [DOI] [PubMed] [Google Scholar]
- 2.Van Ormer, E. B. (1933) Psychol. Bull. 30, 415-439. [Google Scholar]
- 3.Graves, E. A. (1936) J. Exp. Psychol. 19, 316-322. [Google Scholar]
- 4.Plihal, W. & Born, J. (1997) J. Cognit. Neurosci. 9, 534-547. [DOI] [PubMed] [Google Scholar]
- 5.Stickgold, R., Hobson, J. A., Fosse, R. & Fosse, M. (2001) Science 294, 1052-1057. [DOI] [PubMed] [Google Scholar]
- 6.Pavlides, C. & Winson, J. (1989) J. Neurosci. 9, 2907-2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buzsaki, G. (1996) Cereb. Cortex 6, 81-92. [DOI] [PubMed] [Google Scholar]
- 8.Lee, A. K. & Wilson, M. A. (2002) Neuron 36, 1183-1194. [DOI] [PubMed] [Google Scholar]
- 9.Maquet, P. (2001) Science 294, 1048-1052. [DOI] [PubMed] [Google Scholar]
- 10.Benington, J. H. & Frank, M. G. (2003) Prog. Neurobiol. 69, 71-101. [DOI] [PubMed] [Google Scholar]
- 11.Skaggs, W. E. & McNaughton, B. L. (1996) Science 271, 1870-1873. [DOI] [PubMed] [Google Scholar]
- 12.Nadasdy, Z., Hirase, H., Czurko, A., Csicsvari, J. & Buzsaki, G. (1999) J. Neurosci. 19, 9497-9507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Squire, L. R. (1998) C. R. Acad. Sci. Ser. III 321, 153-156. [DOI] [PubMed] [Google Scholar]
- 14.Fowler, M. J., Sullivan, M. J. & Ekstrand, B. R. (1973) Science 179, 302-304. [DOI] [PubMed] [Google Scholar]
- 15.Fischer, S., Hallschmid, M., Elsner, A. L. & Born, J. (2002) Proc. Natl. Acad. Sci. USA 99, 11987-11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marrosu, F., Portas, C., Mascia, M. S., Casu, M. A., Fa, M., Giagheddu, M., Imperato, A. & Gessa, G. L. (1995) Brain Res. 671, 329-332. [DOI] [PubMed] [Google Scholar]
- 17.Pace-Schott, E. F. & Hobson, J. A. (2002) Nat. Rev. Neurosci. 3, 591-605. [DOI] [PubMed] [Google Scholar]
- 18.Coyle, J. T., Price, D. L. & DeLong, M. R. (1983) Science 219, 1184-1190. [DOI] [PubMed] [Google Scholar]
- 19.Sitaram, N., Weingartner, H. & Gillin, J. C. (1978) Science 201, 274-276. [DOI] [PubMed] [Google Scholar]
- 20.Iversen, S. D. (1998) C. R. Acad. Sci. Ser. III 321, 209-215. [DOI] [PubMed] [Google Scholar]
- 21.Davis, K. L., Mohs, R. C., Tinklenberg, J. R., Pfefferbaum, A., Hollister, L. E. & Kopell, B. S. (1978) Science 201, 272-274. [DOI] [PubMed] [Google Scholar]
- 22.Santucci, A. C., Kanof, P. D. & Haroutunian, V. (1989) Psychopharmacology 99, 70-74. [DOI] [PubMed] [Google Scholar]
- 23.Smith, C., Tenn, C. & Annett, R. (1991) Can. J. Psychol. 45, 115-124. [DOI] [PubMed] [Google Scholar]
- 24.Hasselmo, M. E. & Wyble, B. P. (1997) Behav. Brain Res. 89, 1-34. [DOI] [PubMed] [Google Scholar]
- 25.Buzsaki, G. (1989) Neuroscience 31, 551-570. [DOI] [PubMed] [Google Scholar]
- 26.Hasselmo, M. E. (1999) Trends Cognit. Sci. 3, 351-359. [DOI] [PubMed] [Google Scholar]
- 27.Buzsaki, G. (1986) Brain Res. 398, 242-252. [DOI] [PubMed] [Google Scholar]
- 28.Hasselmo, M. E., Schnell, E. & Barkai, E. (1995) J. Neurosci. 15, 5249-5262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aquilonius, S. M. & Hartvig, P. (1986) Clin. Pharmacokinet. 11, 236-249. [DOI] [PubMed] [Google Scholar]
- 30.Rechtschaffen, A. & Kales, A. (1968) A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects (Brain Inf. Service, Univ. of California, Los Angeles).
- 31.Plihal, W. & Born, J. (1999) Neuroreport 10, 2741-2747. [DOI] [PubMed] [Google Scholar]
- 32.McGaugh, J. L. & Roozendaal, B. (2002) Curr. Opin. Neurobiol. 12, 205-210. [DOI] [PubMed] [Google Scholar]
- 33.McClelland, J. L., McNaughton, B. L. & O'Reilly, R. C. (1995) Psychol. Rev. 102, 419-457. [DOI] [PubMed] [Google Scholar]
- 34.Power, A. E., Vazdarjanova, A. & McGaugh, J. L. (2003) Neurobiol. Learn. Mem. 80, 178-193. [DOI] [PubMed] [Google Scholar]
- 35.Hobson, J. A., Stickgold, R. & Pace-Schott, E. F. (1998) Neuroreport 9, R1-R14. [DOI] [PubMed] [Google Scholar]
- 36.Sitaram, N., Wyatt, R. J., Dawson, S. & Gillin, J. C. (1976) Science 191, 1281-1283. [DOI] [PubMed] [Google Scholar]
- 37.Sejnowski, T. J. & Destexhe, A. (2000) Brain Res. 886, 208-223. [DOI] [PubMed] [Google Scholar]
- 38.Gais, S., Molle, M., Helms, K. & Born, J. (2002) J. Neurosci. 22, 6830-6834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith, C. (2001) Sleep Med. Rev. 5, 491-506. [DOI] [PubMed] [Google Scholar]
- 40.Peigneux, P., Laureys, S., Delbeuck, X. & Maquet, P. (2001) Neuroreport 12, A111-A124. [DOI] [PubMed] [Google Scholar]
- 41.Meier-Koll, A., Bussmann, B., Schmidt, C. & Neuschwander, D. (1999) Percept. Mot. Skills 88, 1141-1159. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.