Abstract
Rationale
Cues associated with rewards bias attention towards them, and can motivate drug-seeking and drug-taking behavior. There is, however, considerable individual variation in the extent to which cues associated with rewards acquire motivational properties. For example, only in some rats does a localizable food cue become attractive, eliciting approach towards it, and “wanted”, in the sense that it serves as an effective conditioned reinforcer.
Objectives
We asked whether the propensity of animals to attribute incentive salience to a food cue predicts the extent to which a classically conditioned cocaine cue acquires incentive motivational properties.
Methods
First, a Pavlovian conditioned approach procedure was used to identify rats prone to attribute incentive salience to a food cue. We then measured the extent to which a classically conditioned cocaine cue acquired two properties of an incentive stimulus: (1) the ability to elicit approach towards it, and (2) the ability to reinstate drug-seeking behavior, using an extinction-reinstatement procedure (i.e., to act as a conditioned reinforcer).
Results
We found that a classically conditioned cocaine cue became more attractive, in that it elicited greater approach toward it, and more desired, in that it supported more drug-seeking behavior under extinction conditions, in individuals prone to attribute incentive salience to a food cue.
Conclusions
We conclude that rats vary in their propensity to attribute incentive salience to both food and cocaine cues, and it is possible to predict, prior to any drug experience, in which rats a cocaine cue will acquire the strongest motivational control over behavior.
Keywords: addiction, cocaine, sign-tracking, goal-tracking, incentive salience, Pavlovian conditioning
INTRODUCTION
There is considerable individual variation in the extent to which cues associated with rewards acquire motivational control over behavior (Boakes 1977; Johnson 1974; Mahler and de Wit 2010; Robinson and Flagel 2009; Schachter 1968; Tomie et al. 2000). In a series of studies using food as the unconditioned stimulus (US), we have shown that only in some rats (“sign-trackers”, STs; Hearst and Jenkins 1974) does a food cue itself (conditioned stimulus, CS) become attractive, eliciting approach and engagement with it, and “wanted”, in the sense that animals will work to get it (Robinson and Flagel 2009). In other rats (“goal-trackers”, GTs; Boakes 1977) the cue evokes a conditioned response (CR), but the CR consists of approach behavior directed towards the location where food will be delivered, rather than towards the food cue itself, and in GTs a food cue is a less effective conditioned reinforcer (Robinson and Flagel 2009). We have suggested that this phenotypic variation is due, at least in part, to intrinsic individual variation in the propensity to attribute incentive motivational properties (incentive salience) to reward cues (Flagel et al. 2009; Flagel et al. 2011b; Meyer et al. 2012a; Robinson and Flagel 2009; Saunders and Robinson 2010; Yager and Robinson 2010).
Although there is now considerable evidence for individual variation in the extent to which a classically conditioned food cue is attributed with incentive salience, there is much less information concerning individual variation in the extent to which drug cues acquire incentive motivational properties. We previously reported that a cocaine cue produces greater reinstatement of drug-seeking behavior (after extinction) in STs than GTs (Saunders and Robinson 2010), but in that study, as in most studies on drug reinstatement, the cocaine cue acquired its motivational properties in an instrumental (self-administration) setting (See 2005 for review). Although drug cues may reinstate drug-seeking behavior whether the cue was associated with drug administration in either an instrumental setting (i.e., contingent upon an action) or a Pavlovian setting (independent of an action) (Kruzich et al. 2001; See 2005) these may involve different psychological and even neurobiological processes (Cardinal et al. 2002; Everitt et al. 2001; Parkinson et al. 1999). One purpose of the experiments reported here, therefore, was to determine if STs and GTs differ in the extent to which a cocaine cue acquires incentive motivational properties when the cue is associated with cocaine administration using classic Pavlovian conditioning procedures; i.e., independent of any action. We assessed two different properties of an incentive stimulus (Cardinal et al. 2002): (1) the extent to which it comes to elicit approach towards it (Uslaner et al. 2006), and (2) the degree to which the cue reinforces actions to get it, using an extinction-reinstatement procedure (Kruzich et al. 2001). In addition to measuring conditioned approach behavior we also quantified a second CR, conditioned orienting behavior, which may not require that the cue be attributed with incentive salience (Saunders and Robinson 2012). This allowed us to assess if rats learned the CS-US association, even if the CS failed to elicit approach into close proximity with it.
METHODS
Subjects
A total of 207 (Exp. 1 initial N = 111, Exp. 2 initial N = 96) male Sprague-Dawley rats (Harlan, Haslett, Michigan) weighing 250-275g upon arrival were individually housed in a climate-controlled colony room on a 12-hr light/12-hr dark cycle (lights on at 0800 hr). Food and water were available ad libitum. After arrival, rats were given 1 week to acclimate to the colony room before testing commenced. All experiments followed the principles of laboratory animals care specified by Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research National Research Council (2003).
Pavlovian Training Using Food as the US
A summary of the experimental design is shown in Figure 1.
Fig. 1.
Schematic illustration of the experimental design. Independent groups of rats were used for each experiment. Each numbered box represents an individual session/day. (a) Following Pavlovian training with a food unconditioned stimulus (US), rats underwent subsequent Pavlovain training with a cocaine US during which rats received non-contingent cue-light (CS)-US presentations. (b) Following Pavlovian training with a food US, rats were trained to self-administer cocaine (US) in the absence of any explicit cue. During subsequent Pavlovian conditioning sessions rats received non-contingent cue-light(CS)-cocaine (US) presentations. Depending on the experimental phase, an active nose poke produced the US (acquisition), no US (extinction), or the CS but no US (reinstatement).
Pavlovian training procedure
Rats were initially trained using a Pavlovian conditioned approach (PCA) procedure and equipment described previously (Flagel et al. 2007; Saunders and Robinson 2012). Briefly, rats were trained over 5 consecutive daily sessions consisting of 25 trials/session. Each trial consisted of insertion of an illuminated lever (lever-CS) into the chamber for 8s. Retraction of the lever was immediately followed by the delivery of a single 45-mg banana-flavored pellet (the US) into the food magazine. CS-US pairings occurred on a variable time (VT) 90 (30-150s) schedule. No instrumental response was required by the rat to initiate delivery of the food pellet. Lever deflections, magazine entries, latency to the first lever deflection, and latency to the first magazine entry during CS presentation were quantified.
Pavlovian conditioned approach (PCA) index
Following completion of Pavlovian training, animals were assigned to one of three groups based on whether they preferentially interacted with the lever-CS (‘sign-trackers’, STs), preferentially interacted with the food magazine during the lever-CS presentation (‘goal-trackers’, GTs), or had no strong preference for the lever-CS or food magazine (‘intermediate group’, IG). This was quantified using a composite Pavlovian conditioned approach (PCA) index, based on performance on days 4 and 5 of training, as described previously (Lomanowska et al. 2011; Meyer et al. 2012a). Briefly, the PCA Index score consisted of the average of three measures of conditioned approach behavior: (1) the probability of contacting either the lever-CS or food magazine during a trial [P(lever)-P(food magazine)]; (2) the response bias for contacting the lever-CS or food magazine during a trial [(#lever deflections - #food magazine entries)/(#lever deflections + #food magazine entries)]; and (3) the mean latency to contact the lever or enter the food magazine during a trial [(magazine contract latency - lever deflection latency)/8]. This produces values ranging from −1.0 to +1.0, where a score of +1 indicates an animal made a ST CR on every trial, a score of −1 that an animal made a GT CR on every trial and a score of 0 that an animal distributed ST and GT responses 50:50. For purposes of classification, rats with scores of −1.0 to −0.3 were operationally classed as GTs and rats with scores of +0.3 to +1.0 were classed as STs. Rats that were within the range of −0.29 to +0.29, whose behavior vacillated between the lever-CS and food magazine, were classified as intermediates (IGs) and were not used further because we were interested in comparing rats that differed strongly in their propensity to attribute incentive salience to food cues (Meyer et al. 2012a). Of the 207 number of rats screened for this experiment 109 were classed STs, 45 IGs, and 53 GTs, and the distribution of PCA Index scores were similar to that reported previously (Meyer et al. 2012a; Saunders and Robinson 2011; Saunders and Robinson 2012).
Video Analysis
For a subset of rats the 1st, 3rd, and 5th session were video recorded using a digital recording system and the video was subsequently scored offline and analyzed for orientation to the CS in 8 STs and 8 GTs. An orienting response was scored if a rat made a head and/or body movement in the direction of the lever-CS during the CS period, even if it did not approach into close proximity the lever-CS. Of course, if a rat approached and engaged the CS, as indicated by a lever deflection, an orienting response would also be scored, as this always preceded approach. Thus, we were able to quantify the acquisition of two different CRs: 1) an orienting CR, and 2) an approach CR.
Experiment 1: Pavlovian Approach Using Cocaine as the US
Surgery
Following Pavlovian training using food as the US, chronic indwelling catheters were implanted into the jugular vein of STs and GTs as described previously (Crombag et al. 2000).
Apparatus
Behavioral testing was conducted in chambers identical to those used to screen animals for ST and GT, except the food magazine and lever were removed from the chamber and two stimulus lights were placed on the left and right sides of the wall opposite the white houselight, 13.5 cm above the stainless steel grid floor. The side of the stimulus light designated to serve as a CS (i.e. to be paired with cocaine infusion) was counterbalanced between rats. A syringe pump, located outside the sound attenuating chamber and connected to rats’ catheter back ports, delivered cocaine infusions. The infusion tubing was suspended into the chamber via a swivel mechanism, which allowed rats free movement in the chamber.
Pavlovian training procedures
Prior to training rats were assigned to either Paired (CS and US presented together) or Unpaired groups (US explicitly not paired with presentation of the CS). Before Pavlovian training began, rats were first habituated to the presentation of the stimulus light (light-CS) and infusion procedure to decrease otherwise high levels of responding to what were novel stimuli (Uslaner et al. 2006). The habituation session consisted of 25 individual trials (VT 90s schedule) during which both stimulus lights were simultaneously illuminated for either 10 or 20s (see below for further description) and coincided with activation of the infusion pump and an intravenous (IV) infusion of saline (50 μL delivered in 2.8s). Fifteen days of Pavlovian conditioning, using cocaine as the US, commenced the following day (see Fig. 1a for the overall design). Each session consisted of eight trials (CS-US presentations) occurring on a VT schedule with a mean of 900s (840-960s). For rats in the Paired group, each light-CS presentation was paired with an intravenous infusion of either 0.2 or 0.4 mg/kg of cocaine HCl (weight of the salt, dissolved in 0.9% saline in 50μl over 2.8s). Of course, no action was required to initiate either illumination of the light or the cocaine injection. For rats tested with 0.4 mg/kg, each trial consisted of illumination of the CS for 10s and cocaine delivery coincided with the onset of the CS. This experiment was conducted first, and therefore, we had scored the video prior to conducting a second experiment with a lower dose. Based on the video we decided that it would be advantageous if rats had a little more time available to make an approach response. Thus, for the second experiment with a dose of 0.2 mg/kg we increased the length of time the stimulus light was illuminated to 20s, and cocaine delivery began 10s after CS onset. Rats in the Unpaired groups received non-contingent infusions of 0.4 mg/kg cocaine that were explicitly not paired with illumination of the CS (cocaine was administered on a VT schedule with a mean of 180s after the CS was extinguished).
Video Analysis
Video was scored offline by an observer blind to the experimental condition for two different conditioned responses (CRs). (1) Conditioned Orientation: an orienting response was scored if the rat made a head and/or body movement in the direction of the CS during the CS period, regardless of whether the rat approached the CS. (2) Conditioned Approach: an approach response was scored if during the CS period a rat moved towards the CS, bringing its nose to within 1cm of the light. To do this a rat had to rear, lifting both paws off the floor, towards the light. Thus, if an approach response were scored on a given trial an orienting response would also be scored, as orienting always preceded approach. However, an orienting response could occur in the absence of an approach response. Rats were removed from analysis if their catheter lost patency (ST n = 4, GT n = 1).
Experiment 2: Individual Variation in Pavlovian Cue-Induced Reinstatement of Drug-seeking Behavior
The basic experimental design for Experiment 2 is shown in Fig. 1b. For this experiment an independent cohort of rats were trained on the Pavlovian task using food as the US to identify STs and GTs, and subsequently prepared with I.V. catheters, exactly as described for experiment 1.
Apparatus
For self-administration, extinction, and reinstatement testing the food magazine and lever were removed from the chamber and replaced with two nose-poke ports located 3 cm above the floor on the left and right sides of the wall opposite the houselight. A stimulus light was mounted above each nose-poke port, 13.5 cm above the floor. Removable waste trays were filled with corn cob bedding, a pine scented air-freshener was placed in the chamber, a red houselight was used, and the floor was made of stainless steel bars. For Pavlovian training sessions, the nose-poke ports were removed from the chambers, the removable waste trays were emptied, a vanilla scented air-freshener was placed in the chamber, a white houselight was used, and the floor was made of wire mesh to create a context different from that used for self-administration sessions. This was done to reduce any effect of context conditioning acquired during the Pavlovian conditioning sessions from influencing responding during the reinstatement test.
Self-Administration Training
Rats were trained to make an instrumental response (a nose poke) to receive an intravenous injection of cocaine (0.4 mg/kg/infusion over 2.8s) on a fixed ratio (FR) 1 20s time-out schedule of reinforcement. Responses into the active port during the time-out, or into the inactive port, had no programmed consequence. Importantly, no explicit discrete cue was associated with the drug infusion during self-administration sessions. Rather than restricting the length of the session, rats were required to earn a fixed number of infusions each day (infusion criterion, IC), which increased across days, as described previously (Saunders and Robinson 2010; 2011). This was done to ensure that all rats received exactly the same number of drug infusions.
Pavlovian conditioning procedures
Following acquisition of stable self-administration behavior over 12 days of training, the nose poke ports were removed and rats underwent two sessions of Pavlovian training with cocaine as the US. Each Pavlovian session was separated by three days of self-administration at IC 40 (see Fig. 1b). Prior to Pavlovian conditioning, rats were assigned to either Paired or Unpaired groups, matched based on the length of time to complete self-administration sessions averaged over the final two days of training at IC 40. Each Pavlovian training session consisted of 20 CS-US presentations. For rats in the Paired group, the light-CS was illuminated for 20s and cocaine delivery (0.2 mg/kg over 2.8s) coincided with the onset of the CS. Rats in the Unpaired group received non-contingent infusions of cocaine that were explicitly not paired with CS presentation (cocaine was administered on VT schedule with a mean of 120 s after the light-CS was extinguished). Following the second Pavlovian conditioning session, rats were again allowed to self-administer cocaine at IC 40 for three additional days to re-stabilize behavior. Thus, in this experiment the CS that predicted cocaine delivery was not present during instrumental (self-administration) sessions, but was associated with cocaine in two separate Pavlovian training sessions, and the Pavlovian context was distinct from the self-administration context.
Extinction and Reinstatement
After the last self-administration session at IC 40, rats underwent ten daily 60-min sessions of extinction training. During extinction, responses into the nose ports had no consequences. The day after the final extinction session, rats were tested for Pavlovian cue-induced reinstatement of drug-seeking behavior (Fig. 1b). During this session, responses into the active nose poke resulted in illumination of the cocaine cue (CS) for 5s and activation of the infusion pump, but no cocaine delivery.
Statistical Analysis
Linear mixed-models (LMM) analysis was used for all repeated measures data (Verbeke and Molenberghs 2000). The covariance structure was explored and modeled for each dependent variable. Analysis of variance was used to analyze dose-response data for conditioned orientation, conditioned approach, and to compare reinstatement responding. When main effects were found post hoc comparisons were made using Fisher’s LSD test. Statistical significance was set at p < 0.05.
RESULTS
Individual Variation in Pavlovian Conditioned Approach Behavior to a Food Cue
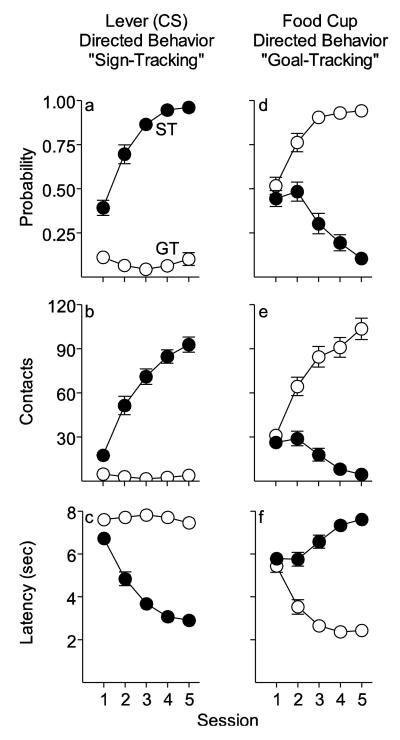
Two distinct phenotypes emerged as a result of Pavlovian training using food as the US, as reported previously (Robinson and Flagel 2009). For a subset of rats presentation of the lever-CS came to evoke a sign-tracking (ST) CR, consisting of reliable and rapid approach to the lever-CS (Figs. 2a and 2c) and vigorous engagement with it (Fig. 2b). In contrast, for another subset of rats, presentation of the lever-CS rarely elicited approach to it. Rather, presentation of the lever-CS elicited a goal-tracking (GT) CR that consisted of reliable and rapid approach to the food magazine (Figs. 2d and 2f) and vigorous engagement with it (Fig. 2e). Individual variation in the topography of the conditioned approach responses that developed with Pavlovian training is clearly evident by examining the change in the PCA Index scores in STs and GTs over days of training (Fig. 3a).
Fig. 2.
Behavior directed towards the lever-CS vs. the location of food delivery (the food magazine) during Pavlovian training in rats designated as sign-trackers (STs) or goal-trackers (GTs). The mean ± SEM for: (a) probability of approaching the lever-CS during the 8s CS period, (b) number of lever contacts, (c) latency to first lever contact after CS presentation, (d) probability of approaching the food magazine during the 8s CS period, (e) number of food magazine entries during the 8s CS period, and (f) latency to the first food magazine entry after CS presentation. For all measures there was a significant effect of group (ST or GT), session, and a group × session interaction (p’s < 0.001)
Fig. 3.
Comparison of development of conditioned approach and conditioned orientation towards the lever-CS during Pavlovian training in sign-trackers (STs, n =8) and goal-trackers (GTs, n =8). Data represent the means ± SEM. (a) PCA index scores for STs and GTs across 5 sessions of training. A score of 1.0 reflects a complete bias for interaction with the lever-CS (STs), a score of −1.0 reflects complete bias for interaction with the food magazine during lever-CS presentation (GTs), and a score of zero reflects that responses were directed equally to both locations. (b) Probability of orientation to the food cue (lever-CS), during the CS period, across training sessions.
Both STs and GTs Learn a Conditioned Orienting Response
In contrast to variation in the topography of conditioned approach behavior, both STs and GTs developed a conditioned orienting response to the lever-CS across sessions [F(2, 25.52) = 31.95, p < 0.001], before then approaching either the lever or the food magazine, respectively, and the two groups did not differ (Fig. 3b). Indeed, on trials when GTs had their head in the food magazine prior to presentation of the CS they would typically remove their head from the food magazine when the lever-CS was presented, glance at the lever, and then turn back to the food magazine. Thus, both STs and GTs learned a conditioned orienting response directed towards the lever-CS, but only in STs did the lever-CS become sufficiently attractive to draw a rat into close proximity with it, and only STs vigorously engaged the lever-CS (Figs. 2 and 3).
Individual Variation in Conditioned Approach to a Cocaine Cue, but not Conditioned Orientation
When cocaine is used as a US, rather than food, rats typically do not physically engage a lever-CS, therefore, they do not reliably deflect it. Instead, a sign-tracking CR consists of approach to the CS, and sniffing and investigation of it (Flagel et al. 2010; Uslaner et al. 2006). Thus, when using cocaine as the US we scored a CS-directed approach response (a ST CR) if a rat brought its nose to within 1 cm of the light-CS during the CS period, which required it to rear. In contrast, conditioned orientation was defined as a head and/or body movement in the direction of the light-CS upon CS presentation, regardless of whether the rat reared, bringing it into close proximity to the light.
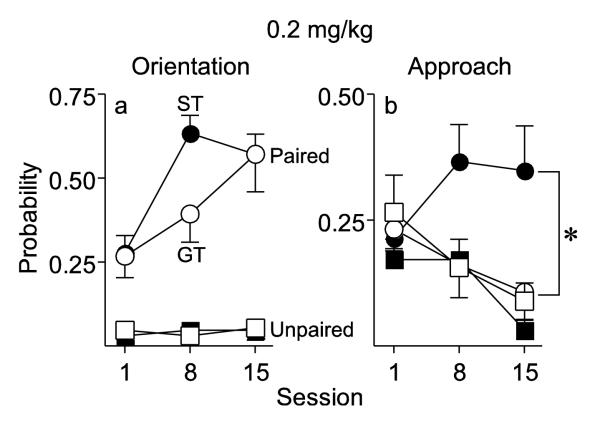
Conditioned Orientation (0.2 mg/kg)
Figure 4a illustrates the probability of conditioned orientation across training sessions when using 0.2 mg/kg cocaine as the US. As can be seen in Fig. 4a, at this dose both Paired STs and GTs learned a conditioned orienting response, as indicated by a significant increase in the probability of orienting behavior across sessions [F(2, 34.42) = 11.07, p <0.001], and there were no group differences. Additionally, both STs and GTs showed a significant increase in the probability of orienting to the cocaine cue across sessions, relative to their respective Unpaired control groups [pairing × session interactions; STs: F(2, 60) = 5.67, p = 0.006; GTs: F(2, 33.74) = 3.91; p = 0.03].
Fig. 4.
CS-directed orientation and approach to a cue associated with a non-contingent intravenous injection of 0.2 mg/kg cocaine in rats that received paired (ST n = 14, GT n = 7) or unpaired (ST n = 8, GT n = 8) cue-drug presentations. Data represent the means ± SEM. (a) The probability to orient to the cocaine cue during light-CS presentation. (b) The probability to approach the cocaine cue during the light-CS presentation. *, indicates a significant group difference between Paired STs and GTs, p < 0.05
Conditioned Approach (0.2 mg/kg)
Figure 4b illustrates the probability of conditioned approach across training sessions when using 0.2 mg/kg cocaine as the US. Fig. 4b shows that STs and GTs differed in the extent to which the cocaine cue elicited an approach CR [effect of group, F(1, 57) = 4.93; p = 0.03]. At this dose STs continued to approach the CS across sessions, whereas GTs showed a significant decrease in the probability of approaching the cocaine cue across sessions [effect of session, F(2, 10.51) = 4.38, p = 0.041]. STs also had a higher probability of approaching the cocaine cue across sessions, relative to their Unpaired control group [pairing × session interaction, F(2, 40) = 3.88, p = 0.029], whereas the Paired and Unpaired GT groups did not differ, decreasing approach similarly across sessions.
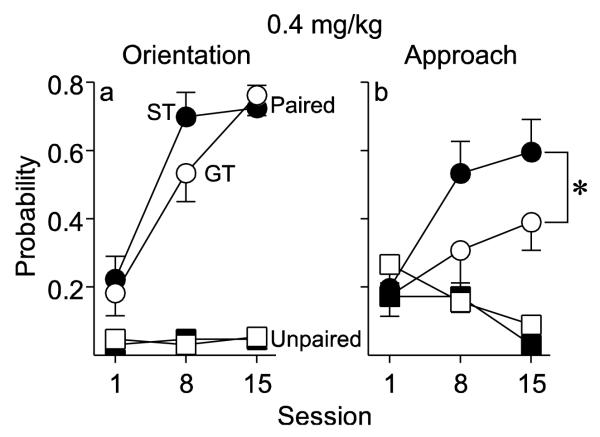
Conditioned Orientation (0.4 mg/kg)
Fig. 5a shows that when using 0.4 mg/kg cocaine as the US both Paired STs and GTs acquired a conditioned orienting response, as indicated by a significant increase in the probability of orienting behavior across sessions [F(2, 19) =, p < 0.001], and the two groups did not differ. In addition, both STs and GTs showed a significant increase in probability of orienting to the cocaine cue across sessions, relative to their respective Unpaired control groups [pairing × session interactions; STs: F(2, 16) = 14.1, p < 0.001; GTs: F(2, 50) = 10.84, p < 0.001].
Fig. 5.
CS-directed orientation and approach to a cue associated with a non-contingent intravenous injection of 0.4 mg/kg cocaine in rats that received paired (ST n = 10, GT n = 11) or unpaired (ST n = 8, GT n = 8) cue-drug presentations. Data represent the means ± SEM. (a) The probability to orient to the cocaine cue during light-CS presentation. (b) The probability to approach the cocaine cue during the light-CS presentation. *, indicates a significant group difference between Paired STs and GTs, p < 0.05
Conditioned Approach (0.4 mg/kg)
In contrast to conditioned orientation, Fig. 5b shows that STs did differ from GTs in the probability of approaching the CS [effect of group, F(1, 52.17) = 4.44, p = 0.04]. Indeed, the effect of session was statistically significant for STs [F(2, 9) = 19.34, p = 0.001] but not GTs. Finally, STs also showed a significant increase in the probability of approaching the cocaine cue across sessions, relative to their Unpaired control group [pairing × session interaction, F(2, 22.77) = 8.52, p = 0.002], whereas Paired and Unpaired GTs did not statistically differ. Importantly, neither STs nor GTs in the Unpaired group developed an orienting CR or an approach CR.
Figure 6 summarizes the dose-response functions for the probability of conditioned orientation (Panel a) and conditioned approach (Panel b) on the final day of training. For conditioned orienting a two-way analysis of variance (ANOVA) revealed that there were no differences between STs and GTs, and the probability of this CR increased as a function of dose in both groups [F(1, 38) = 5.67, p = 0.022]. However, Fig. 6b shows that the cocaine cue elicited greater approach behavior in STs than in GTs [F(1, 38) = 6.03, p = 0.019], although the probability of approach increased equally in STs and GTs as a function of dose. We separately analyzed dose-response data for STs and GTs and included unpaired control animals in this analysis. A one-way ANOVA showed a significant effect of treatment group for both STs and GTs on performance of a conditioned approach CR on the final day of training [STs, F(2, 29) = 9.03, p = 0.001; GTs, F(2, 22) = 6.4, p = 0.006]. However, post-hoc analysis (Fisher’s LSD) revealed that, on the final day of testing, Paired STs differed from Unpaired STs at both doses tested (p’s < 0.05) while Paired GTs only differed from Unpaired GTs only at the highest dose tested [0.2 mg/kg, p = 0.985; 0.4 mg/kg, p = 0.015].
Fig. 6.
Dose-response functions for the probability of conditioned orientation (a) and conditioned approach (b) on the final day of training. Data represent the means ± SEM. Each data point represents an independent group of rats
Acquisition and Extinction of Cocaine Self-Administration in STs and GTs
An independent cohort of rats underwent Pavlovian training with food as the US to identify STs and GTs as previously described (data not shown) and rats were subsequently prepared with IV catheters. Rats were then trained to nose poke for an IV cocaine infusion, but during self-administration sessions no cue was explicitly paired with drug delivery. Rats in each group received the same number of response-reinforcer pairings by requiring them to take a fixed number of drug injections each session. Thus, any differences in the acquisition of self-administration would be evident in the average number of cocaine infusions taken per minute (rate). There were no group differences in rate of responding at any infusion criterion (Fig. 7b). There were also no group differences in active or inactive responses/session and both groups learned to discriminate between the active and inactive ports (Fig. 7a). Following stable responding at IC 40 for two days, rats underwent two days of Pavlovian conditioning with cocaine as the US as described in the Methods. After each Pavlovian conditioning session, rats were returned to an FR1 schedule at IC 40 to re-stabilize behavior. During these sessions there were no group differences in the rate of self-administration or the number of responses (data not shown).
Fig. 7.
Acquisition of cocaine self-administration behavior in sign-trackers (n = 25) and goal-trackers (n = 16). (a) Mean ± SEM number of active and inactive responses for infusion criteria 5 and 10 (0.4 mg/kg/inf) and infusion criteria 20 and 40 (0.2 mg/kg/inf). (b) Mean ± SEM number of cocaine infusions/min at each infusion criterion
Following the final day of self-administration testing at IC 40, rats underwent ten sessions of extinction training during which responses into the active port no longer produced cocaine. Figure 8 shows that there were no group differences in the rate of extinction and all groups extinguished to the same low level of responding [effect of session, F(9, 37) = 13.24, p < 0.001].
Fig. 8.
Mean ± SEM number of active responses during extinction of responding for cocaine in Paired sign-trackers (n = 14) and goal-trackers (n = 8) and Unpaired sign-trackers (n = 11) and goal-trackers (n = 8)
A Pavlovian Cocaine Cue Produced more Robust Reinstatement of Drug-Seeking Behavior in STs than GTs
Following extinction training all rats were tested for the ability of response-dependent presentation of the Pavlovian cocaine cue (light-CS) to reinforce drug-seeking behavior. During this test, during which no cocaine was delivered, responses into the active port produced presentation of the light-CS previously either paired or unpaired with cocaine injections. All groups reinstated responding, in that the number of responses into the active port were greater than those into the inactive port (Fig. 9). However, Paired STs showed greater reinstatement of responding than Paired GTs, as indicated by a significant group × pairing interaction [F(1, 37) = 7.22, p = 0.011].
Fig. 9.
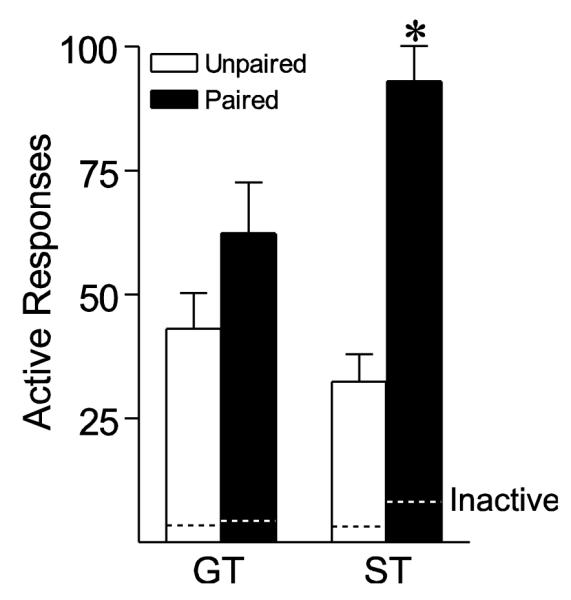
Cocaine cue reinstatement test. Following extinction, all animals were given a single 60 min cue reinstatement test session, in which active responses resulted in presentation of the cue previously paired or unpaired with non-contingent cocaine delivery. Mean ± SEM number of active responses in Unpaired (white bars) GTs (n = 8) and STs (n = 11) and Paired (black bars) GTs (n = 8) and STs (n = 14). The dashed lines indicate the mean number of responses in the inactive port. *, indicates significant difference, p < 0.05
DISCUSSION
We previously reported that there is considerable individual variation in the extent to which a Pavlovian food cue acquires the properties of an incentive stimulus (Meyer et al. 2012a; Robinson and Flagel 2009; Yager and Robinson 2010). Here we asked whether variation in the propensity to attribute incentive salience to a food cue predicts the extent to which a classically conditioned cocaine cue acquires motivational properties. We found that a classically conditioned cocaine cue was more attractive, in that it elicited approach towards it, and more desirable, in that it reinforced actions to get it, in STs than GTs. Importantly, even though GTs did not reliably approach the cocaine cue they did learn the CS-US association, as indicated by acquisition of a conditioned orienting response, similar to that seen when a food cue is used as the US (Zener 1937, and the present study). These findings, together with our previous reports, indicate that there is considerable individual variation in the extent to which a cocaine cue acquires motivational control over behavior (Flagel et al. 2010; Meyer et al. 2012b; Saunders and Robinson 2010).
One limitation when using intravenous drug as the US and approach as the CR is that there is no explicit “goal” to approach (this is also the case when using electrical brain stimulation as the US; Peterson et al. 1972). Thus, it can be difficult to determine whether GTs do not readily approach a cocaine cue because they do not learn the CS-US association as well as STs, or because the cocaine cue is not attributed with sufficient incentive salience to draw them into close proximity to it. To begin to address this issue we quantified, for the first time, acquisition of a conditioned orienting response as an alternative measure of learning the CS-US association (Grastyan and Vereczkei 1974; Sokolov 1963). Importantly, STs and GTs did not differ in the acquisition of an orienting CR, when either a food cue or a cocaine cue was used as the US.
Conditioned orientation was not simply a reflexive reaction to a change in the environment (Sokolov 1963), as rats that received unpaired CS-US presentations did not orient to the cue. It has also been argued that sign-tracking behavior is simply an elaboration of a conditioned orienting response (Buzsaki 1982; Grastyan and Buzsaki 1979; Grastyan and Vereczkei 1974; Holland 1980). However, our data do not support this view because these two CRs were dissociable. When using food as the US, GTs oriented to the food cue, but then approached the food magazine, not the cue. Interestingly, Zener (1937) described a similar effect seventy-five years ago, observing that some dogs would respond to the CS with “an initial glance at the bell” before fixating on the food pan. Thus, absence of an approach CR does not mean that the CS-US association was not acquired. For example, when using an auditory CS, rats will orient to the sound source and display enhanced general activity but will not approach the auditory stimulus (Cleland and Davey 1983; Holland 1977; Rescorla 1988). We suggest, therefore, that the reason GTs do not readily approach the cocaine cue is not because they fail to learn the CS-US association, but because they do not attribute sufficient incentive salience to the cue for it to become powerfully attractive.
Although STs and GTs did not differ in the acquisition of a conditioned orienting response they did differ in the extent to which the cocaine cue acquired incentive motivational properties, based on two independent measures. First, STs and GTs differed in the extent to which the cocaine cue evoked a conditioned approach response, defined as coming into close proximity with the cue. With a low dose of cocaine GTs did not show any evidence of conditioned approach, although with a higher dose they showed some approach, although less than that seen in STs. Second, the Pavlovian cocaine cue was more effective in reinforcing drug-seeking behavior following extinction of self-administration behavior. It is unlikely that these differences between STs and GTs were due to differences in the inherent reinforcing properties of cocaine during conditioning because they did not differ in the acquisition of self-administration behavior, as we have reported previously (Saunders and Robinson 2010; 2011). In addition, STs and GTs did not differ in exposure to cocaine or the number of cue-drug pairings, because we utilized procedures that held these variables constant.
It is interesting to note that during the Pavlovian cocaine cue reinstatement test, both Paired and Unpaired rats reinstated responding to some extent, in that responses in the active port were greater than in the inactive port in all groups, and indeed, in GTs there was no difference between Paired and Unpaired groups. However, in STs the cocaine cue did reinstate stronger drug-seeking behavior in Paired relative to Unpaired animals. Nevertheless, there are a number of reasons why Unpaired animals may have showed more active than inactive responses. First, a light stimulus is itself inherently reinforcing and will sustain instrumental responding in the absence of any other reinforcer (Olsen and Winder 2009; Stewart 1960) and this may have been sufficient to maintain low levels of responding in all groups. Second, drugs have relatively long durations of action and so it is difficult to “unpair” a discrete CS and a drug US, unless the inter-trial interval is very long – well beyond the half-life of the drug. Although we used a relatively long inter-trial interval, it may have been short enough so that during the Pavlovian conditioning sessions brain levels of cocaine may have sometimes still been elevated during CS presentation, even in the Unpaired groups, resulting is some association between the CS and US. A third possibility is that some responding was maintained by context conditioning, that is, an association between cocaine and the context in which it was experienced. However, this seems less likely because the Pavlovian conditioning sessions were conducted in a context different from the self-administration and reinstatement context.
Our finding that a Pavlovian cocaine cue acquired greater incentive motivational value in STs than GTs is consistent with previous studies in which a cocaine cue acquired incentive motivational properties in an instrumental setting. Saunders and Robinson (2010) used a self-administration procedure, where responding resulted in both delivery of a light cue and cocaine, and reported that following extinction the light cue produced greater cue-induced reinstatement in STs than GTs. Furthermore, Meyer et al. (2012b) used a conditioned cue preference procedure, where a non-contingent cocaine injection was paired with a tactile floor cue (Cunningham et al. 1993), and found that only STs developed a preference for the cocaine-associated floor. With this procedure, approach to the cocaine-associated floor was likely due to its conditioned reinforcing properties. Our results are also consistent with studies using rats selectively bred for reactivity to a novel environment (Flagel et al. 2010). Selectively bred high responder rats (bHRs) are almost exclusively STs and selectively bred low responder rats (bLRs) are almost exclusively GTs. Flagel et. al (2010) reported that bHR/STs developed a sign-tracking CR to a cue associated with an i.v. cocaine infusion whereas bLR/GTs did not.
The neurobiological basis of individual variation in the propensity to attribute incentive salience to food and drug cues is not known. There is, however, considerable evidence that dopamine (DA) systems are involved in the assignment of incentive value to rewards and their associated stimuli (Berridge 2012; Berridge and Robinson 1998; Cardinal et al. 2002), and STs and GTs do differ on some measures of DA function. For example, STs show greater expression of dopamine D1 receptor mRNA in the nucleus accumbens initially and lower levels of DA transporter and tyrosine hydroxylase in the ventral tegmental area and D2 mRNA in the nucleus accumbens relative to GTs following Pavlovian conditioned approach training (Flagel et al. 2007). Additionally, learning a sign-tracking CR is DA-dependent but learning a goal-tracking CR is not (Flagel et al. 2011b). Furthermore, Flagel, Clark and colleagues (2011b) showed that over the course of Pavlovain training with food as the US, there is a transfer of a phasic DA signal from the food-US to the lever-CS in STs but not GTs, in the accumbens core. STs and GTs also differ in what brain regions are engaged by food cues. For example, presentation of a Pavlovian food cue elicits greater c-fos mRNA expression in STs in both the dorsal and ventral striatum as well as the orbitofrontal cortex and thalamus (Flagel et al. 2011a). Interestingly, in human studies, differences in dopamine transporter genotype is associated with differential activation of the mesocorticolimbic reward circuitry and behavioral responses elicited by smoking cues (Franklin et al. 2008). These studies are consistent with the hypothesis that individual variation in dopaminergic signaling may contribute to individual variation in the propensity to attribute incentive salience to reward cues, but this issue clearly requires further study.
The influence of drug cues on behavior have traditionally been studied using self-administration procedures, in which an animal works for both presentation of the cue and the delivery of the drug reward (de Wit and Stewart 1981; Shaham et al. 2003). However, there are many complex psychological processes that interact to contribute to behavior in an instrumental setting (e.g.; see Fig. 2 in Cardinal et al. 2002). For example, it is difficult to parse whether a reward-associated cue is eliciting the next response or if it is reinforcing the prior response. Importantly, in the “real world” of substance abusers, drug cues often precede actions that result in acquiring and taking drugs. Indeed, the psychological and neurobiological processes controlling behavior may be quite different in Pavlovian vs. instrumental settings. For example, there is evidence that neural responses, as measured by immediate early gene expression, differ in response to the presentation of a Pavlovian CS vs. presentation of a CS acquired in an instrumental setting (Thomas et al. 2003; Thomas and Everitt 2001). Stimuli associated with drug self-administration do acquire incentive motivational properties, serving as conditioned reinforcers (Di Ciano and Everitt 2004) and spurring behavior (Shaham et al. 2003), but it is also important to determine whether purely Pavlovian drug cues can similarly motivate behavior, as these stimuli may be potent instigators of relapse in addicts. It has been reported that a Pavlovian cocaine cue can become both attractive, eliciting approach towards it (Uslaner et al. 2006), and desired, in that animals will work for presentation of the cue (Kruzich et al. 2001). However, in these studies there was considerable individual variation in the extent to which the cocaine cue acquired motivational control over behavior. The data reported here suggest that this variation is due, at least in part, to individual differences in the propensity to attribute incentive salience to a Pavlovian CS, transforming it from a mere CS into a potent incentive stimulus (Meyer et al. 2012a).
In conclusion, the extent to which a classically conditioned cocaine cue acquires motivational control over behavior is predicted by the propensity of an individual to attribute incentive salience to a food cue. Indeed, similar variation in the response to smoking cues was recently reported in humans - smokers who reported the highest craving to food cues when food deprived also reported the highest craving to smoking cues during abstinence (Mahler and de Wit 2010). Thus, some individuals are prone to attribute incentive salience to reward cues and this is true whether reward cues are presented in an instrumental or Pavlovian setting (Meyer et al. 2012b; Saunders and Robinson 2010; Yager and Robinson 2010). Individuals prone to attribute incentive salience to drug cues may be especially vulnerable to addiction, as in these individuals drug cues would most powerfully motivate drug-seeking and drug-taking behavior.
ACKNOWLEDGEMENTS
This research was supported by National Institute on Drug Abuse grants to L.M.Y (F31 DA030799) and T.E.R. (R37 DA004294 and P01 DA031656). The authors report no financial conflict of interest. The content is solely the responsibility of the authors, and does not necessarily represent the official views of the National Institute on Drug Abuse or the National Institutes of Health. All research was conducted in concordance with current local and national US laws. We thank Anusari Dewasurendra for assistance with behavioral testing, and Drs. Shelly Flagel, Vedran Lovic, and Paul Meyer for helpful comments on an earlier draft of this manuscript.
Funding Acknowledgments
This research was supported by National Institute on Drug Abuse grants to L.M.Y (F31 DA030799) and T.E.R. (R37 DA004294 and P01 DA031656).
REFERENCES
- Berridge KC. From prediction error to incentive salience: Mesolimbic computation of reward motivation. Eur J Neurosci. 2012;35:1124–43. doi: 10.1111/j.1460-9568.2012.07990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Rev. 1998;28:309–69. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Boakes R. Performance on learning to associate a stimulus with positive reinforcement. In: Davis H, Hurwits H, editors. Operant-Pavlovian Interactions. Lawrence Erlbaum Associates; Hillsdale: 1977. pp. 67–97. [Google Scholar]
- Buzsaki G. The “Where is it?” reflex: Autoshaping the orienting response. J Exp Anal Behav. 1982;37:461–484. doi: 10.1901/jeab.1982.37-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: The role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Cleland GG, Davey GCL. Autoshaping in the rat: The effects of localizable visual and auditory signals for food. J Exp Anal Behav. 1983;40:47–56. doi: 10.1901/jeab.1983.40-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, Badiani A, Maren S, Robinson TE. The role of contextual versus discrete drug-associated cues in promoting the induction of psychomotor sensitization to intravenous amphetamine. Behav Brain Res. 2000;116:1–22. doi: 10.1016/s0166-4328(00)00243-6. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Niehus JS, Noble D. Species difference in sensitivity to ethanol’s hedonic effects. Alcohol. 1993;10:97–102. doi: 10.1016/0741-8329(93)90087-5. [DOI] [PubMed] [Google Scholar]
- de Wit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology (Berl) 1981;75:134–143. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Conditioned reinforcing properties of stimuli paired with self-administered cocaine, heroin or sucrose: Implications for the persistence of addictive behaviour. Neuropharmacology. 2004;47:202–213. doi: 10.1016/j.neuropharm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Dickinson A, Robbins TW. The neuropsychological basis of addictive behaviour. Brain Res Rev. 2001;36:129–138. doi: 10.1016/s0165-0173(01)00088-1. [DOI] [PubMed] [Google Scholar]
- Flagel SB, Akil H, Robinson TE. Individual differences in the attribution of incentive salience to reward-related cues: Implications for addiction. Neuropharmacology. 2009;56:139–148. doi: 10.1016/j.neuropharm.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Cameron CM, Pickup KN, Watson SJ, Akil H, Robinson TE. A food predictive cue must be attributed with incentive salience for it to induce c-fos mRNA expression in cortico-striatal-thalamic brain regions. Neuroscience. 2011a;196:80–96. doi: 10.1016/j.neuroscience.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, Akers CA, Clinton SM, Phillips PEM, Akil H. A selective role for dopamine in stimulus-reward learning. Nature. 2011b;469:53–57. doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Robinson TE, Clark JJ, Clinton SM, Watson SJ, Seeman P, Phillips PEM, Akil H. An animal model of genetic vulnerability to behavioral disinhibition and responsiveness to reward-related cues: Implications for addiction. Neuropsychopharmacology. 2010;35:388–400. doi: 10.1038/npp.2009.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Watson SJ, Robinson TE, Akil H. Individual differences in the propensity to approach signals vs goals promote different adaptations in the dopamine system of rats. Psychopharmacology (Berl) 2007;191:599–607. doi: 10.1007/s00213-006-0535-8. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Lohoff FW, Wang Z, Sciortino N, Harper D, Li Y, Jens W, Cruz J, Kampman K, Ehrman R, Berrettini W, Detre JA, O’Brien CP, Childress AR. DAT genotype modulates brain and behavioral responses elicited by cigarette cues. Neuropsychopharmacology. 2008;34:717–728. doi: 10.1038/npp.2008.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grastyan E, Buzsaki G. The orienting-exploratory response hypothesis of discriminative conditioning. Acta Neurobiol Exp (Wars) 1979;39:491–501. [PubMed] [Google Scholar]
- Grastyan E, Vereczkei L. Effects of spatial separation of the conditioned signal from the reinforcement: A demonstration of the conditioned character of the orienting response or the orientational character of conditioning. Behav Biol. 1974;10:121–146. doi: 10.1016/s0091-6773(74)91725-8. [DOI] [PubMed] [Google Scholar]
- Hearst E, Jenkins HM. Sign Tracking: the stimulus-reinforcer relation and directed action. Monograph of the Psychonomic Society; Austin: 1974. [Google Scholar]
- Holland PC. Conditioned stimulus as a determinant of form of Pavlovian conditioned response. J Exp Psychol Anim Behav Process. 1977;3:77–104. doi: 10.1037//0097-7403.3.1.77. [DOI] [PubMed] [Google Scholar]
- Holland PC. Infuence of visual conditioned stimulus characteristics on the form of Pavlovian appetitive conditioned responding in rats. J Exp Psychol Anim Behav Process. 1980;6:81–97. [PubMed] [Google Scholar]
- Johnson WG. Effect of cue prominence and subject weight on human food-directed performance. J Pers Soc Psychol. 1974;29:843–848. doi: 10.1037/h0036390. [DOI] [PubMed] [Google Scholar]
- Kruzich PJ, Congleton KM, See RE. Conditioned reinstatement of drug-seeking behavior with a discrete compound stimulus classically conditioned with intravenous cocaine. Behav Neurosci. 2001;115:1086–1092. doi: 10.1037//0735-7044.115.5.1086. [DOI] [PubMed] [Google Scholar]
- Lomanowska AM, Lovic V, Rankine MJ, Mooney SJ, Robinson TE, Kraemer GW. Inadequate early social experience increases the incentive salience of reward-related cues in adulthood. Behav Brain Res. 2011;220:91–99. doi: 10.1016/j.bbr.2011.01.033. [DOI] [PubMed] [Google Scholar]
- Mahler SV, de Wit H. Cue-reactors: Individual differences in cue-induced craving after food or smoking abstinence. PLoS ONE. 2010;5:e15475. doi: 10.1371/journal.pone.0015475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer PJ, Lovic V, Saunders BT, Yager LM, Flagel SB, Morrow JD, Robinson TE. Quantifying individual variation in the propensity to attribute incentive salience to reward cues. PLoS ONE. 2012a;7:e38987. doi: 10.1371/journal.pone.0038987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer PJ, Ma ST, Robinson TE. A cocaine cue is more preferred and evokes more frequency-modulated 50-kHz ultrasonic vocalizations in rats prone to attribute incentive salience to a food cue. Psychopharmacology (Berl) 2012b;219:999–1009. doi: 10.1007/s00213-011-2429-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen CM, Winder DG. Operant sensation seeking engages similar neural substrates to operant drug seeking in C57 mice. Neuropsychopharmacology. 2009;34:1685–94. doi: 10.1038/npp.2008.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson JA, Olmstead MC, Burns LH, Robbins TW, Everitt BJ. Dissociation in effects of lesions of the nucleus accumbens core and shell on appetitive Pavlovian approach behavior and the potentiation of conditioned reinforcement and locomotor activity by D-Amphetamine. J Neurosci. 1999;19:2401–2411. doi: 10.1523/JNEUROSCI.19-06-02401.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson GB, Ackil JE, Frommer GP, Hearst ES. Conditioned approach and contact behavior toward signals for food or brain-stimulation reinforcement. Science. 1972;177:1009–1011. doi: 10.1126/science.177.4053.1009. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Pavlovian conditioining-It’s not what you think it is. Am Psychol. 1988;43:151–160. doi: 10.1037//0003-066x.43.3.151. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Flagel SB. Dissociating the predictive and incentive motivational properties of reward-related cues through the study of individual differences. Biol Psychiatry. 2009;65:869–873. doi: 10.1016/j.biopsych.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. A cocaine cue acts as an incentive stimulus in some but not others: Implications for addiction. Biol Psychiatry. 2010;67:730–736. doi: 10.1016/j.biopsych.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. Individual variation in the motivational properties of cocaine. Neuropsychopharmacology. 2011;36:1668–1676. doi: 10.1038/npp.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. The role of dopamine in the accumbens core in the expression of Pavlovian-conditioned responses. Eur J Neurosci. 2012 doi: 10.1111/j.1460-9568.2012.08217.x. 10.1111/j.1460-9568.2012.08217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter S. Obesity and eating-Internal and external cues differentially affect eating behavior of obese and normal subjects. Science. 1968;161:751–756. doi: 10.1126/science.161.3843.751. [DOI] [PubMed] [Google Scholar]
- See RE. Neural substrates of cocaine-cue associations that trigger relapse. Eur J Pharmacol. 2005;526:140–146. doi: 10.1016/j.ejphar.2005.09.034. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, de Wit H, Stewart J. The reinstatement model of drug relapse: History, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Sokolov EN. Perception and the conditioned reflex. Pergamon Press, Pergamon Press; 1963. [Google Scholar]
- Stewart J. Reinforcing effects of light as a function of intensity and reinforcement schedule. J Comp Physiol Psychol. 1960;53:187–193. doi: 10.1037/h0047315. [DOI] [PubMed] [Google Scholar]
- Thomas KL, Arroyo M, Everitt BJ. Induction of the learning and plasticity-associated gene Zif268 following exposure to a discrete cocaine-associated stimulus. Eur J Neurosci. 2003;17:1964–1972. doi: 10.1046/j.1460-9568.2003.02617.x. [DOI] [PubMed] [Google Scholar]
- Thomas KL, Everitt BJ. Limbic-cortical-ventral striatal activation during retrieval of a discrete cocaine-associated stimulus: A cellular imaging study with gamma protein kinase C expression. J Neurosci. 2001;21:2526–35. doi: 10.1523/JNEUROSCI.21-07-02526.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomie A, Aguado AS, Pohorecky LA, Benjamin D. Individual differences in Pavlovian autoshaping of lever pressing in rats predict stress-induced corticosterone release and mesolimbic levels of monoamines. Pharmacol Biochem Behav. 2000;65:509–517. doi: 10.1016/s0091-3057(99)00241-5. [DOI] [PubMed] [Google Scholar]
- Uslaner JM, Acerbo MJ, Jones SA, Robinson TE. The attribution of incentive salience to a stimulus that signals an intravenous injection of cocaine. Behav Brain Res. 2006;169:320–324. doi: 10.1016/j.bbr.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Verbeke G, Molenberghs G. Linear Mixed Models for Longitudinal Data. Springer, Springer; 2000. [Google Scholar]
- Yager LM, Robinson TE. Cue-induced reinstatement of food seeking in rats that differ in their propensity to attribute incentive salience to food cues. Behav Brain Res. 2010;214:30–34. doi: 10.1016/j.bbr.2010.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zener K. The significance of behavior accompanying conditioned salivary secretion for theories of the conditioned response. Am J Psychol. 1937;50:384–403. [Google Scholar]