Abstract
Interactions between separate synaptic inputs converging on the same target appear to contribute to the fine-tuning of information processing in the central nervous system. Intersynaptic crosstalk is made possible by transmitter spillover from the synaptic cleft and its diffusion over a distance to neighboring synapses. This is the case for glutamate, which inhibits γ-aminobutyric acid (GABA)ergic transmission in several brain regions through the activation of presynaptic receptors. Such heterosynaptic modulation depends on factors that influence diffusion in the extracellular space (ECS). Because glial cells represent a physical barrier to diffusion and, in addition, are essential for glutamate uptake, we investigated the physiological contribution of the astrocytic environment of neurons to glutamate-mediated intersynaptic communication in the brain. Here we show that the reduced astrocytic coverage of magnocellular neurons occurring in the supraoptic nucleus of lactating rats facilitates diffusion in the ECS, as revealed by tortuosity and volume fraction measurements. Under these conditions, glutamate spillover, monitored through metabotropic glutamate receptor-mediated depression of GABAergic transmission, is greatly enhanced. Conversely, impeding diffusion with dextran largely prevents crosstalk between glutamatergic and GABAergic afferent inputs. Astrocytes, therefore, by hindering diffusion in the ECS, regulate intersynaptic communication between neighboring synapses and, probably, overall volume transmission in the brain.
There is growing evidence that transmitters do not always remain sequestered within the synaptic cleft but can diffuse and exert their action at distant receptors located extrasynaptically and even on adjacent synapses (1-3). Such interactions between independent synaptic inputs, known as intersynaptic crosstalk, have been shown in several brain regions where synaptically released glutamate induces the inhibition of γ-aminobutyric acid (GABA)ergic and glutamatergic transmission through the activation of presynaptic metabotropic, kainate or α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors (4-8). These forms of heterosynaptic modulation depend on the extent of neurotransmitter diffusion onto neighboring synapses. Such extrasynaptic regulatory processes are likely therefore to be influenced by surrounding astrocytes that not only ensure most of transmitter uptake through high-affinity transporters (9) but also represent a physical barrier to diffusion in the extracellular space (ECS), hindering the movement of substances within the tissue (10-12).
To examine this issue in a physiological context, we took advantage of a model in which glial coverage of neurons and of their afferent synapses is modified in response to biological stimulations. It is now established that during conditions of increased oxytocin secretion, like lactation, astrocytic coverage of supraoptic nucleus (SON) neurons in the hypothalamus significantly diminishes (13, 14). We previously reported that this relative absence of glial processes around glutamatergic synapses in the SON enhanced the level of ambient glutamate, which modified excitatory neurotransmission through the tonic activation of presynaptic metabotropic glutamate receptors (mGluRs) (15). The question arises whether this astroglial remodeling favors neurotransmitter diffusion and, as a result, facilitates crosstalk between neighboring synapses.
This question was answered by using two different approaches. One consisted of assessing ECS diffusion through the measurement of tortuosity, volume fraction, and nonspecific transport. The other relied on the ability of glutamate released from glutamatergic inputs to activate mGluRs located on adjacent GABAergic terminals. Here we report that reduction of the astrocytic coverage of SON neurons indeed dramatically favors diffusion in the tissue and, as a consequence, glutamate-induced heterosynaptic depression of GABAergic transmission. On the other hand, this form of intersynaptic crosstalk was largely prevented when diffusion in the ECS was limited by introduction of a large neutral molecule like dextran into the ECS. These data, therefore, indicate that the astrocytic environment of neurons is a critical regulator of communication between neighboring synapses and, probably, of overall extrasynaptic transmission in the central nervous system.
Methods
Diffusion Parameter Measurements. Hypothalamic coronal slices (400 μm) that included the supraoptic nuclei were obtained as described (16) from virgin (3- to 5-month-old) and lactating (8th to 9th day of lactation) Wistar rats. Briefly, rats were anaesthetized with isoflurane and decapitated. The brain was quickly removed and placed in ice-cold artificial cerebrospinal fluid (aCSF) saturated with 95% O2 and 5% CO2. After dissection, slices were hemisected along the midline and maintained at 30-34°C for at least 1 h in a submerged chamber containing aCSF before being transferred to a recording chamber. aCSF (pH 7.4) contained 117 mM NaCl, 3 mM KCl, 35 mM NaHCO3, 1.25 mM Na2HPO4, 1.3 mM MgCl2, 1.5 mM CaCl2, 10 mM glucose, and 0.1 mM tetramethylammonium (TMA+). Diffusion parameters in the ECS were measured as described (17). In brief, an extracellular marker such as TMA+, to which cell membranes are relatively impermeable, was administered into the tissue by iontophoresis. Its concentration was measured by a TMA+-selective microelectrode filled with an ion exchanger (Corning 477317) at the tip and backfilled with 100 mM TMA+ chloride. The iontophoretic pipette, filled with 100 mM TMA+, was glued parallel to the TMA+-selective electrode with a tip separation of ≈100 μm. Iontophoresis was performed by applying a +200-nA current for 60 s. Diffusion curves were fitted by using a nonlinear curve-fitting simple algorithm with the program voltoro (kindly provided by C. Nicholson, New York University, New York) to yield the volume fraction (α), tortuosity (α), and nonspecific uptake (k′). Before recordings in tissue, diffusion curves were recorded in 0.3% agar gel dissolved in 150 mM NaCl/3mMKCl/1 mM TMA-Cl to calibrate the measurements.
In our experiments, there was no change in nonspecific uptake of TMA+.
Electrophysiological Recordings. Acute slices (300 μm) were perfused with aCSF containing 123 mM NaCl, 2.5 mM KCl, 1 mM Na2HPO4, 26.2 mM NaHCO3, 1.3 mM MgCl2, 2.5 mM CaCl2, and 10 mM glucose (pH 7.4; 295 mosM·kg-1) equilibrated with 95% O2/5% CO2. Magnocellular neurons were visually identified by using differential interference contrast microscopy [Olympus (Rungis, France) BX50]. Patch-clamp recording pipettes (3-5 MΩ) were filled with an intracellular solution containing 150 mM Cs-methanesulfonate, 10 mM Hepes, 1 mM EGTA, 1.3 mM MgCl2, and 0.1 mM CaCl2 (pH 7.1-7.2 adjusted with CsOH; 295 mosM·kg-1). Membrane currents were recorded by using a Multiclamp 700A amplifier (Axon Instruments, Foster City, CA). Signals were filtered at 2 kHz and digitized at 5 kHz via a DigiData 1322 interface (Axon Instruments). Series resistance (6-20 MΩ) was monitored online, and cells were excluded from data analysis if a >15% change occurred during the course of the experiment. Data were collected and analyzed online by using pclamp 9 software (Axon Instruments). Recordings were obtained in the presence of d-2-amino-5-phosphonopentanoic acid (D-AP5) (50 μM) and of GABA-B receptor antagonists (200 μM saclofen, 200 μM 2-hydroxy-saclofen, or 10 μM CGP 54626). The first stimulating electrode (S1) was placed close to the ventral glial limitans at the base of the nucleus, whereas the second one (S2) was placed in an area outside the SON dorsal to the recording site (see Fig. 2 A). To make sure that the two pathways involved independent sets of synapses, we verified that the stimulation of one of the pathways was not affected by prior (60-ms) stimulation of the other pathway. When the two paths shared some synapses, facilitation of transmitter release occurring during this test resulted in a change in amplitude of the second response, just as is the case during a paired-pulse paradigm. The protocol for examining the heterosynaptic action of glutamate on GABAergic transmission consisted of stimulating the first pathway (S1) when holding the neuron at -70 mV to record α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor-mediated currents and to make sure that glutamate release was elicited. A second pathway (S2) was stimulated 200 ms later with the neuron clamped at 0 mV to record an inhibitory postsynaptic current (IPSC) (see Fig. 2 A). Delays shorter than 200 ms were not used, because changing the membrane potential from -70 to 0 mV induced a shift in holding current that interfered with the measurement of the evoked IPSC. These experiments were performed in the presence of a GABA-B receptor antagonist to avoid presynaptic changes that could result from the activation of these receptors by GABA spillover associated with the conditioning train (18).
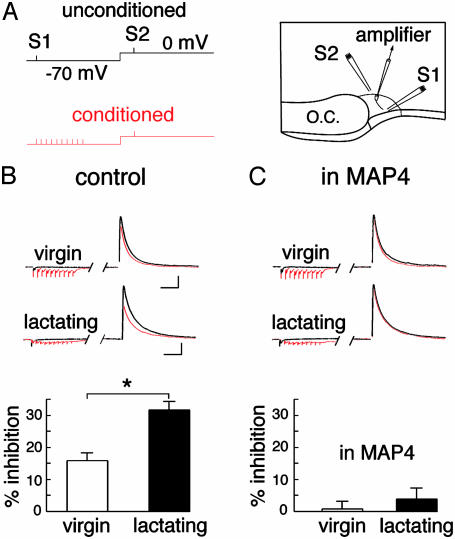
Fig. 2.
Heterosynaptic depression of evoked IPSCs. (A) Experimental set-up illustrating the paradigm of stimulation (Left) and the position of the stimulating electrodes (Right; see Methods). (B) Sample traces (Upper) and summary histograms (Lower) illustrating the heterosynaptic depression obtained in virgin (n = 18) and lactating (n = 18) rats. (C) Sample traces (Upper) and summary histograms (Lower) illustrating the heterosynaptic depression obtained in virgin (n = 8) and lactating (n = 6) animals in the presence of MAP4. (Bars = 200 pA, 40 ms.)
The paired-pulse facilitation ratio was calculated as the amplitude ratio of the second IPSC over the first IPSC elicited 60 ms later. The coefficient of variation (CV) of IPSCs was calculated as CV = SD/mean. Data were compared statistically by using either the nonparametric Mann-Whitney test or the parametric paired Student t test accordingly. Significance was assessed at P < 0.05. All data are reported as means ± SEM.
Drugs. All drugs were bath-applied. Appropriate stock solutions were made and diluted with aCSF just before application. Drugs used were CGP 54626, d-AP5, 2-hydroxy-saclofen, (S)-2-amino-2-methyl-4-phosphonobutanoic acid (MAP4), saclofen (Tocris, Cookson, Bristol, U.K.), and dextran (Sigma).
Results
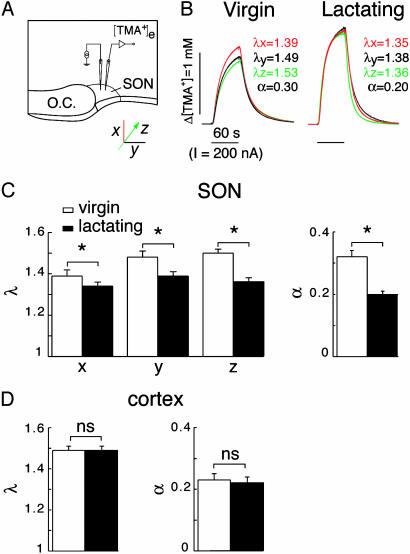
We first investigated the influence of the astrocytic environment on intersynaptic communication by examining whether the glial remodeling characterizing the SON of lactating rats (13, 14) affected ECS diffusion parameters. Using the real-time TMA+ iontophoretic method (10, 11), tortuosity (α), volume fraction (α), and nonspecific uptake (k′) were compared in the ventrodorsal (x axis), mediolateral (y axis), and rostrocaudal (z axis) planes of the SON in acute slices obtained from virgin and lactating animals (Fig. 1A). Tortuosity, a measure of restriction on diffusion in the ECS, was different along the three axes in virgin rats, indicating that the tissue was anisotropic (αx = 1.39 ± 0.03, n = 11; αy = 1.48 ± 0.03, n = 11; αz = 1.50 ± 0.02, n = 6; Fig. 1 B and C). The volume of tissue available for diffusion, α, had a mean value of 0.32 ± 0.02 (n = 11), and nonspecific uptake was 7.09 ± 0.38 × 10-3·s-1. That diffusion was not so much hindered along the x axis probably reflects the particular ventrodorsal orientation of most astrocytic processes in this nucleus (19). In contrast, in the SON of lactating rats, whereas nonspecific uptake was unchanged (k′ = 6.04 ± 0.82 × 10-3·s-1), both tortuosity and volume fraction were significantly reduced (αx = 1.34 ± 0.02, n = 9; αy = 1.39 ± 0.02, n = 9; αz = 1.36 ± 0.02, n = 5; α = 0.20 ± 0.01, n = 9; P < 0.05; Fig. 1 B and C), and diffusion anisotropy disappeared. The variations in diffusion parameters were related to morphological remodeling in the SON, because tortuosity and volume fraction remained unaffected by lactation in the cortex (Fig. 1D). In the latter, α, which was equivalent along the three axes as expected from an isotropic structure (20), α, and k′ were not significantly different in the cortex of virgin and lactating rats (α = 1.49 ± 0.02 and 1.49 ± 0.02, α = 0.23 ± 0.02 and 0.22 ± 0.02, k′= 5.04 ± 0.54 and 3.71 ± 0.81 × 10-3·s-1 for virgin and lactating animals, respectively; P > 0.05; n = 13 and 12). Our data thus reveal that glial remodeling not only modifies the geometry but also facilitates diffusion in the ECS of the SON.
Fig. 1.
Lactation induces changes in diffusion parameters of the ECS in the SON. (A) Experimental set-up illustrating the real-time TMA+ iontophoretic method applied to the SON in acute slices. Measurements were made along three perpendicular axes (x, y, and z), as illustrated. O.C., optic chiasm. (B) Example of diffusion curves obtained in virgin and lactating rats. Values for tortuosity and volume fraction extracted from three curves recorded along the x, y, and z axes are indicated. (C) Histograms summarizing tortuosity (α) (Left) and volume fraction (α)(Right) measured in the SON of virgin (n = 6-11) and lactating (five to nine) rats. (D) Histograms of tortuosity (Left) and volume fraction (Right) measured in the cortex of virgin (n = 13) and lactating (n = 12) rats.
If diffusion in the ECS is enhanced in the SON of lactating rats, then spillover of neurotransmitters onto neighboring synapses should be facilitated. To test this hypothesis, we first examined whether we could detect intersynaptic crosstalk in the SON. This was done by taking advantage of the presence of group III mGluRs on GABAergic terminals in the SON (21), whose activation with a specific agonist, or with endogenous glutamate after transporter blockade (22), inhibits GABA release. Interestingly, resting glutamate levels are not sufficient to activate these receptors in the SON of either virgin or lactating animals. This is not surprising, because most glutamate-mediated heterosynaptic modulations described in the literature have been obtained in response to the concerted release of glutamate vesicles (1-8).
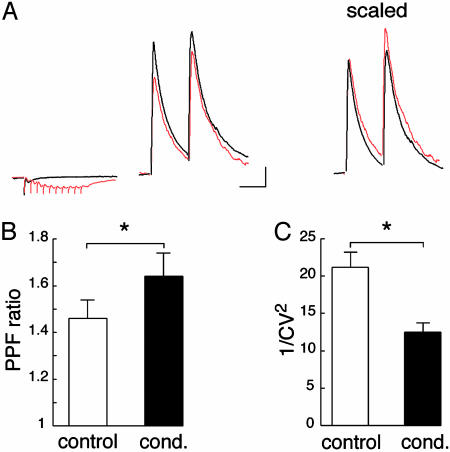
To determine whether these mGluRs sense glutamate released from excitatory synapses, whole-cell voltage-clamp recordings were performed on SON neurons while stimulating two independent synaptic pathways. We examined whether the sustained release of glutamate, elicited through a brief high-frequency (100 Hz) train of 10 stimuli applied to one pathway, affected GABA-A receptor-mediated IPSCs evoked 200 ms later by stimulation of the other pathway (Fig. 2A). In the SON of virgin rats in which astrocytic coverage of SON neurons is extensive, the conditioning train induced a significant reduction in IPSC amplitude (-16.5 ± 1.9%; n = 18; P < 0.05; Fig. 2B). The origin of this depression was investigated by analyzing the paired-pulse facilitation ratio (PPR), and trial-to-trial fluctuation of the evoked-GABAergic responses. After the conditioning train, PPR was increased by 11.1 ± 2.8% (n = 16; P < 0.05; Fig. 3 A and B), whereas the inverse of the squared coefficient of variation (1/CV2) (CV, coefficient of variation) of IPSC amplitude, which is an index of changes in presynaptic function (23), was reduced by 38.7 ± 4.7% (n = 18; P < 0.05; Fig. 3C). Taken together, these results indicate that the conditioning train causes a presynaptic inhibition of evoked IPSCs.
Fig. 3.
Heterosynaptic depression of GABAergic transmission has a presynaptic origin. (A) Sample traces illustrating paired-pulse facilitation obtained under control conditions and after a conditioning train (Left). Superimposition of the two traces scaled to the first IPSC obtained under control conditions reveals that paired-pulse facilitation is increased following the conditioning train (Right). (Bars = 200 pA, 40 ms.) (B) Histogram summarizing paired-pulse facilitation ratio under control conditions and following the conditioning train (cond.) (n = 16). (C) Summary histogram of trial-to-trial fluctuation of IPSC amplitude (expressed as 1/CV2) (CV, coefficient of variation) measured under control conditions and following the conditioning train (n = 18).
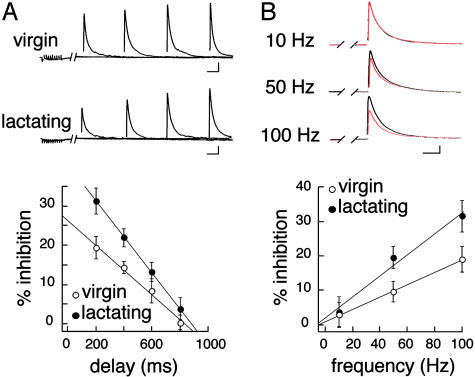
The involvement of mGluRs in this depression was verified by applying MAP4 (250 μM), a specific group III mGluR antagonist (15, 21, 22). In its presence, heterosynaptic depression was completely blocked (-0.7 ± 2.4%; n = 8; P > 0.05; Fig. 2C), providing evidence that IPSC inhibition was due to mGluR activation by synaptically released glutamate. To estimate how long glutamate exerted its inhibitory effect on GABA transmission, we varied the delay (from 200 to 800 ms) between the beginning of the conditioning train and the IPSC (Fig. 4A). With increasing delay, the depression of evoked IPSCs was reduced, to be completely abolished for delays >600 ms. This shows that, for hundreds of milliseconds after the conditioning train, glutamate levels remained elevated in the vicinity of GABA synapses, or that the metabotropic signaling pathway was still active. If such heterosynaptic depression depends on the amount of glutamate being released, it should be sensitive to the stimulation frequency within the conditioning train. Decreasing the frequency from 100 to 50 Hz reduced the mGluR-mediated inhibition of IPSC amplitude from 19.0 ± 3.6% to 9.7 ± 3.0% (n = 10), whereas 10-Hz conditioning trains did not affect IPSC amplitude (Fig. 4B). The heterosynaptic action of glutamate on GABA release in the SON, therefore, is a frequency-dependent mechanism similar to that reported in the cerebellum (4, 7) and the hippocampus (5).
Fig. 4.
Modalities of induction of heterosynaptic depression in SON neurons. (A) Example of two recordings obtained from a virgin and a lactating animal in which the delay between the conditioning train and the IPSC was varied (Upper). The summary graph illustrates the relationship between delay and IPSC inhibition in virgin (n = 7-13) and lactating (n = 10-14) animals (Lower). (Bars = 200 pA, 50 ms.) (B) Example of recordings from one experiment in which the 10 stimuli within the conditioning train were successively applied at 10, 50, and 100 Hz (Upper). The summary graph (Lower) shows the frequency dependence of heterosynaptic depression in virgin (n = 8-10) and lactating (n = 10-11) rats. (Bars = 200 pA, 30 ms.)
Our results indicate that group III mGluRs located on GABAergic terminals can sense glutamate spillover from excitatory afferent inputs. If the astrocytic environment influences diffusion in the ECS, as suggested by our diffusion measurements, intersynaptic crosstalk due to transmitter spillover should be favored by a physiological reduction of glial coverage of SON neurons such as that occurring during lactation (13-15). In the case of glutamate, this process should be further exacerbated by the reduced uptake of the excitatory amino acid associated with glial withdrawal (15). We therefore used the mGluR-mediated heterosynaptic depression of IPSCs as an assay for glutamate spillover and found that in lactating rats, the reduction in amplitude of the IPSC evoked 200 ms after a conditioning train (10 stimuli at 100 Hz) was significantly larger (-32.8 ± 2.7%; n = 18; P < 0.05; Fig. 2B) than in virgin animals. This phenomenon was largely prevented by MAP4 (-4.7 ± 3.2%; n = 6; Fig. 2C), indicating that the same presynaptic mechanism was responsible for IPSC inhibition in the SON of virgin and lactating rats. As to the induction of such a depression, we found that the relationship between IPSC inhibition and delay was shifted toward larger inhibitory values in lactating animals (Fig. 4A). Similarly, decreasing stimulation frequency within the train from 100 to 50 Hz reduced the depression from 31.5 ± 4.5 to 19.6 ± 3.2% (n = 11), a value still larger than that measured in virgin rats (Fig. 4B). The action of glutamate at a distance from its release sites, therefore, is remarkably facilitated in the SON of lactating animals, in agreement with the enhanced diffusion that would occur in the ECS after removal of a barrier such as astrocytic processes.
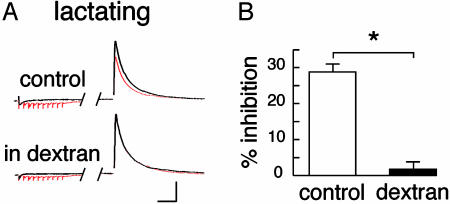
To test whether a reduction of diffusion in the ECS interferes with this form of communication between neighboring synapses, we added 5% dextran (40 kDa) in the perfusing solution. The presence of this neutral macromolecule increases viscosity and should reduce the coefficient of diffusion of glutamate in the ECS (24, 25). As illustrated in Fig. 5, the heterosynaptic depression of evoked IPSCs measured in the SON of lactating rats was abolished in the presence of dextran (from 28.8 ± 2.1% to 1.7 ± 2.0%; n = 6). Thus, ECS diffusion parameters directly influence mGluR-mediated depression of GABA release by regulating the action of glutamate at a distance from its release sites.
Fig. 5.
Diffusion in the ECS affects glutamate spillover. (A) Sample traces obtained from a lactating rat in the absence and presence of dextran in the extracellular solution. (Bars = 200 pA, 40 ms.) (B) Histogram summarizing the heterosynaptic depression in the absence and presence of dextran.
Discussion
The main finding of this study is that communication between independent synapses is sensitive to the astrocytic environment of neurons. This conclusion emerges from the observation that physiological withdrawal of glial processes from the vicinity of synapses facilitates diffusion in the ECS and the action of glutamate on neighboring GABAergic synapses.
The real-time TMA+ iontophoretic method (10) allowed us to determine that reduction in the astrocytic coverage of SON neurons during lactation (13, 14) modified ECS geometry and diffusion parameters. More specifically, tortuosity was decreased compared to that measured in virgin animals, indicating that diffusion was hindered less in the SON of lactating rats. Moreover, it appeared that diffusion in the SON switched from anisotropic in virgin to isotropic in lactating rats. In other words, diffusion not only was enhanced but also became equivalent in all directions as a result of the remodeling. This is likely to reflect the reduction of astrocytic coverage of neurons as well as the reorganization of astrocyte processes, whose orientation switches from vertical to horizontal (26). Diffusion measurements also revealed that the volume fraction, which reflects the space available for diffusion in the SON, was significantly decreased in lactating rats. Such a change in α may be due to the increased proportion of direct neuronal surface membrane juxtapositions without glia interpositions that occurs in lactating rats (13, 14). It must be noted that at these juxtapositions, the interstice between plasma membranes remains normal (≈10 nm) (13), but the withdrawal of astrocytic processes reduces the overall interneuronal space from two interstices, in the presence of an interposed glial process, to a single one.
Do these physiological changes in the diffusion properties of the tissue affect extrasynaptic communication and, more specifically, intersynaptic crosstalk mediated by glutamate? To answer this question, we took advantage of the mGluRs present on inhibitory terminals (21, 22) to monitor the heterosynaptic modulation of GABAergic transmission by glutamate spillover in the SON. In virgin animals, where there is extensive glial coverage of SON neurons, sustained release of glutamate caused a consistent and reversible inhibition of evoked IPSCs. This phenomenon was observed in the presence of GABA-B receptor antagonists and was completely prevented by MAP4, indicating that it depended exclusively on the activation of presynaptic group III mGluRs on inhibitory terminals. Furthermore, the depression was sensitive to the frequency of stimulation within the conditioning train as well as to the delay between this train and the evoked IPSC. These observations imply that this form of intersynaptic crosstalk between glutamatergic and GABAergic afferent inputs depends on the concentration of glutamate reaching GABAergic terminals. Our data are consistent with the idea that glutamate concentration at a distance from release sites is dropping rapidly to negligible levels, and that a concerted release of several glutamate vesicles is required to activate those remote mGluRs. This is in agreement with our previous finding showing that resting glutamate concentrations were not sufficient to activate tonically these receptors (22).
The heterosynaptic depression of GABA release mediated by glutamate spillover appeared to be regulated by the astrocytic environment of neurons, because this depression was found to be of a much greater magnitude in lactating rats, under conditions where overall diffusion in the SON is enhanced. This is consistent with the reduced tortuosity and volume fraction of the tissue, as well as with the reduced glutamate uptake (15), that are expected to result in a higher concentration of the excitatory amino acid at a distance from its release site. Assuming this to be true, reducing the coefficient of diffusion of glutamate should limit the concentration of the excitatory amino reaching neighboring synapses and, as a consequence, alter heterosynaptic modulation. This is exactly what we observed when we slowed diffusion in the ECS with dextran (24, 25), a treatment that completely prevented IPSC depression by glutamate spillover in lactating animals.
Taken together, our data indicate therefore that glutamate-mediated heterosynaptic modulation of GABAergic transmission is a frequency-dependent mechanism whose gain is inversely correlated to the degree of astrocytic coverage of SON neurons. The glial environment of neurons, therefore, not only contributes to local modulation of synaptic efficacy at excitatory inputs by controlling glutamate clearance (15) but also represents an important regulator of glutamatergic communication between independent synapses by setting the parameters of diffusion in the ECS.
The physiological relevance of heterosynaptic modulation of GABA release by glutamate remains speculative. As has been proposed in the cerebellum (4) and in the hippocampus (5), such a phenomenon could boost transmission of excitatory information during intense fiber firing by inhibiting adjacent GABA synapses, thereby producing a local disinhibition of the postsynaptic cell. In addition, the spillover of glutamate is also likely to inhibit transmitter release at neighboring glutamatergic terminals expressing mGluRs (15, 21). This will prevent the postsynaptic neuron from being contaminated by nonrelevant signals and will serve to increase the signal-to-noise ratio for pertinent information (8). In the SON, glutamate spillover is likely to occur during the brief (2- to 4-s) high-frequency (40- to 80-Hz) bursts of afferent glutamatergic potentials believed to mediate transmission of the suckling information (27) to oxytocin neurons as well as during the increase in glutamatergic synaptic activity induced by osmotic stimulation (28). If this were the case, the reduced astrocytic coverage of neurons characterizing the SON of lactating and dehydrated animals (13, 14) would favor heterosynaptic inhibition of GABA release. Changes in diffusion properties and in glutamate spillover associated with anatomical remodelings are thus likely to improve neurohypophysial hormone release in response to suckling and dehydration. These changes, therefore, may be an essential mechanism for physiological functions such as milk ejection and body fluid homeostasis. Astrocytic regulation of diffusion in the ECS could be relevant in other brain regions in which similar physiological neuroglial remodeling has been documented (11, 13, 29, 30). It is highly likely, then, that astrocytes not only influence communication between independent adjacent synapses but, more generally, also constitute a potent regulator of extrasynaptic or volume transmission (12, 31) for a variety of active substances including neurotransmitters, peptides, endocannabinoids, or neurosteroids.
Acknowledgments
We thank P. Chavis, O. Manzoni, F. Nagy, and D. Theodosis for helpful comments on an earlier version of the manuscript and P. Ciofi for discussion. This work was supported by grants from the Institut National de la Santé et de la Recherche Médicale and the Conseil Régional d'Aquitaine (to S.H.R.O.), from the Ministry of Education, Youth, and Sport of the Czech Republic (Grants LN 00A065, J13/98:11100004), and from the Academy of Sciences of the Czech Republic (Grant AV0Z5039906, to E.S.). S.H.R.O. is the recipient of an Action Concertée Incitative Jeunes Chercheurs from the Ministère de la Recherche. R.P. is supported by a studentship from the Ministère de l′Education Nationale, de la Recherche, et de la Technologie.
Abbreviations: ECS, extracellular space; IPSC, inhibitory postsynaptic current; GABA, γ-aminobutyric acid; MAP4, (S)-2-amino-2-methyl-4-phosphonobutanoic acid; mGluR, metabotropic glutamate receptor; SON, supraoptic nucleus; TMA+, tetramethylammonium; aCSF, artificial cerebrospinal fluid.
References
- 1.Barbour, B. & Häusser, M. (1997) Trends Neurosci. 20, 377-384. [DOI] [PubMed] [Google Scholar]
- 2.Rusakov, D. A., Kullmann, D. M. & Stewart, M. G. (1999) Trends Neurosci. 22, 382-388. [DOI] [PubMed] [Google Scholar]
- 3.Isaacson, J. S. (2000) Curr. Biol. 10, R475-R477. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell, S. J. & Silver, R. A. (2000) Nature 404, 498-502. [DOI] [PubMed] [Google Scholar]
- 5.Semyanov, A. & Kullmann, D. M. (2000) Neuron 25, 663-672. [DOI] [PubMed] [Google Scholar]
- 6.Min, M.-Y., Meylan, Z. & Kullmann, D. M. (1999) Proc. Natl. Acad. Sci. USA 96, 9932-9937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Satake, S., Saitow, F., Yamada, J. & Konishi, S. (2000) Nat. Neurosci. 3, 551-558. [DOI] [PubMed] [Google Scholar]
- 8.Vogt, K. E. & Nicoll, R. A. (1999) Proc. Natl. Acad. Sci. USA 96, 1118-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schousboe, A. (2003) Neurochem. Res. 28, 347-352. [DOI] [PubMed] [Google Scholar]
- 10.Nicholson, C. & Syková, E. (1998) Trends Neurosci. 21, 207-215. [DOI] [PubMed] [Google Scholar]
- 11.Roitbak, T. & Syková, E. (1999) Glia 28, 40-48. [DOI] [PubMed] [Google Scholar]
- 12.Syková, E. (2001) Prog. Brain Res. 132, 339-363. [DOI] [PubMed] [Google Scholar]
- 13.Theodosis, D. T. & Poulain, D. A. (1993) Neuroscience 57, 501-535. [DOI] [PubMed] [Google Scholar]
- 14.Hatton, G. I. (1997) Annu. Rev. Neurosci. 20, 375-397. [DOI] [PubMed] [Google Scholar]
- 15.Oliet, S. H. R., Piet, R. & Poulain, D. A. (2001) Science 292, 923-926. [DOI] [PubMed] [Google Scholar]
- 16.Oliet, S. H. R. & Poulain, D. A. (1999) J. Physiol. 520, 815-825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nicholson, C. & Phillips, J. M. (1981) J. Physiol. 321, 225-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mouginot, D., Kombian, S. B. & Pittman, Q. J. (1998) J. Neurophysiol. 79, 1508-1517. [DOI] [PubMed] [Google Scholar]
- 19.Bonfanti, L., Poulain, D. A. & Theodosis, D. T. (1993) J. Neuroendocrinol. 5, 1-5. [DOI] [PubMed] [Google Scholar]
- 20.Voriásek, I. & Syková, E. (1997) J. Neurophysiol. 78, 912-919. [DOI] [PubMed] [Google Scholar]
- 21.Schrader, L. A. & Tasker, J. G. (1997) J. Neurophysiol. 77, 527-536. [DOI] [PubMed] [Google Scholar]
- 22.Piet, R., Bonhomme, R., Theodosis, D. T., Poulain, D. A. & Oliet, S. H. R. (2003) Eur. J. Neurosci. 17, 1777-1785. [DOI] [PubMed] [Google Scholar]
- 23.Malinow, R. & Tsien, R. W. (1990) Nature 346, 177-180. [DOI] [PubMed] [Google Scholar]
- 24.Min, M.-Y., Rusakov, D. A. & Kullmann, D. M. (1998) Neuron 21, 561-570. [DOI] [PubMed] [Google Scholar]
- 25.Perrais, D. & Ropert, N. (2000) Eur. J. Neurosci. 12, 400-404. [DOI] [PubMed] [Google Scholar]
- 26.Bobak, J. B. & Salm, A. K. (1996) J. Comp. Neurol. 376, 188-197. [DOI] [PubMed] [Google Scholar]
- 27.Jourdain, P., Israel, J.-M., Dupouy, B., Oliet, S. H., Allard, M., Vitiello, S., Theodosis, D. T. & Poulain, D. A. (1998) J. Neurosci. 18, 6641-6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richard, D. & Bourque, C. W. (1995) J. Physiol. 489, 567-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laming, P. R., Kimelberg, H., Robinson, S., Salm, A., Hawrylak, N., Muller, C., Roots, B. & Ng, K. (2000) Neurosci. Biobehav. Rev. 24, 295-340. [DOI] [PubMed] [Google Scholar]
- 30.Syková, E., Mazel, T., Hasenöhrl, R. U., Harvey, A. R., Simonová, Z., Mulders, W. H. A. M. & Huston, J. P. (2002) Hippocampus 12, 469-479. [DOI] [PubMed] [Google Scholar]
- 31.Nicholson, C. (2000) Prog. Brain Res. 125, 437-446. [DOI] [PubMed] [Google Scholar]