Abstract
We have previously demonstrated that immunization with inactivated Francisella tularensis, a Category A intracellular mucosal pathogen, combined with IgG2a anti-F. tularensis monoclonal antibody, enhances protection against subsequent F. tularensis challenge. To understand the mechanism(s) involved, we examined the binding, internalization, presentation, and in vivo trafficking of inactivated F. tularensis in the presence and absence of opsonizing monoclonal antibody. We found that when inactivated F. tularensis is combined with anti-F. tularensis monoclonal antibody, presentation to F. tularensis-specific T cells is enhanced, this enhancement is Fc receptor-dependent, and requires a physical linkage between the monoclonal antibody and the inactivated F. tularensis immunogen. This enhanced presentation is due, in part, to enhanced binding and internalization of inactivated F. tularensis by antigen presenting cells, and involves interactions with multiple Fc receptor types. Furthermore, targeting inactivated F. tularensis to Fc receptors enhances dendritic cell maturation and extends the time period over which antigen presenting cells stimulate T cells. In vivo trafficking studies reveal enhanced transport of inactivated F. tularensis immunogen to the Nasal Associated Lymphoid Tissue in the presence of monoclonal antibody, which is FcRn-dependent. In summary, these are the first comprehensive studies using a single vaccine protection model/immunogen to establish the array of mechanisms involved in enhanced immunity/protection mediated by an Fc receptor-targeted mucosal immunogen. These results demonstrate that multiple cellular/immune mechanisms contribute to Fc receptor-enhanced immunity.
Keywords: Fc receptors, Vaccine, Mucosal, F. tularensis, Antigen presentation, Trafficking
Introduction
Francisella tularensis (Ft) is a Category A mucosal pathogen that represents a significant bioterror threat 1. We have previously demonstrated that intranasal (i.n.) administration of immune complexes (ICs) composed of inactivated Ft (iFt) and IgG2a monoclonal antibody (mAb) specific for iFt LPS, in which free/non-iFt bound mAb has been removed (mAb-iFt ICs), enhances the immune response to, and protection against Ft challenge 2. We further demonstrated that the enhanced protection observed was Fc receptor (FcR)-dependent. In addition, this was the first study to demonstrate that targeting an immunogen to FcR i.n. enhances protection against a mucosal infectious disease challenge. Subsequent studies have verified the potential for using FcR-targeted immunogens as effective mucosal vaccines 3–6.
While numerous mechanisms, including enhanced Ag presentation, have been predicted to be involved in FcR-enhanced immune responses, comprehensive studies using a proven FcR-targeted mucosal vaccine strategy to actually define these specific mechanisms have not been conducted 7–10. Some of the proposed mechanisms involved include the following: It has been proposed that complement-mediated binding of antibody (Ab)-Ag ICs to APCs, not FcR binding, may be responsible for Ab-Ag IC-mediated immune enhancement. However, this has been contradicted in studies using FcR knockout (KO) mice, in which FcR were required for immune enhancement 9. Alternatively, up regulation of MHC class II and second signal molecules, as a result of FcR cross-linking on APCs, could also lead to enhanced Ag presentation. However, in previous in vitro studies using human IgG-Ag ICs and monocytes as APCs, we did not observe increased expression of these molecules as a consequence of IC-FcR interaction 11. Studies using dendritic cells (DCs) however, have indicated that Ab-Ag ICs can induce DC maturation 12, although this was later contradicted by a study suggesting IC interaction with the inhibitory FcR (FcγRIIB) blocks DC maturation induced by ICs 13. In addition, as previously indicated above, more efficient Ag-binding and internalization is believed to play an important role in IC-enhanced immune responses. However, this is dependent on not only the concentration of ligand, but the valency of the ligand, the number of FcR expressed on the APC surface, and whether or not the particular FcγR is occupied with serum IgG 9, 14. In the latter case, the amount of FcR cross-linking required to induce internalization appears to actually be reduced when FcγRI is occupied 9, 14. Engaging FcR on APCs can also induce cytokine production 15–17. The cytokine milieu can then determine the type and degree of the immune response. For example, studies, in which the activating FcγRIIA on monocyte-derived DCs was ligated, resulted in secretion of IL-10 and IL-6 (stimulates B cells and plasma cells), and TNF alpha and IL-8 (chemoattractants). However, the cytokines produced can vary with the source of DCs and the ratio of activating and inhibitory (FcγRIIB) receptors engaged 12, 18. Furthermore, the impact of Ag targeting to FcR on Ag persistence, which is observed when Ag is targeted to the B cell receptor 19, or Ag trafficking to lymphoid tissues in vivo 8, 9, remains unclear. More recently, and contrary to prior belief, studies have clearly demonstrated the neonatal Fc receptor (FcRn), which binds IgG Fc, is present in the epithelium of the nasal mucosa in adult mice 4, and that targeting Ags to FcRn via this route also enhances the immune responses to, and protection against, mucosal pathogens 4, 5. This is also consistent with our previous studies indicating the presence of FcRn is critical for the enhanced protection that we observe when utilizing mAb-iFt ICs as protective mucosal immunogen 2.
Thus, we sought to clarify the mechanism(s) involved in FcR-enhanced immunity, including the specific role of FcRn, by conducting a comprehensive analysis of the numerous potential mechanisms involved in FcR-targeted vaccination using a single FcR-targeted model immunogen, which has been proven to be effective in not only enhancing the immune response in vivo, but also producing protection against subsequent infectious disease challenge.
Results
Enhanced Protection Against Ft Challenge Following i.n. Administration of mAb + iFt ICs
We have previously demonstrated that mAb-iFt ICs from which free/unbound mAb has been removed via centrifugation, when administered i.n., enhance protection against subsequent i.n. challenge with Ft 2. Furthermore, enhanced protection was not observed when administering either mAb alone or F(ab′)2 mAb-iFt ICs 2. To facilitate the conduct of these specific studies, as well as future use of this approach as a vaccine strategy, we first determined whether anti-iFt mAb ± iFt ICs, in which free/unbound anti-iFt mAb is not removed (mAb + iFt ICs), could be used in place of mAb-iFt ICs (free/unbound mAb removed) 2. In figure 1A Western Blot was utilized to verify the formation of ICs following incubation of anti-iFt mAb with iFt. To accomplish this using Western Blot, it was necessary to remove free/unbound mAb, as was done in our previously published studies. Importantly, the incubation steps prior to centrifugation were the same in both our published studies in which free mAb was removed (mAb-iFt ICs) and the present study in which free mAb is not removed (mAb + iFt ICs). Thus, the ICs detected in Fig. 1A reflect ICs formed regardless of whether free/unbound mAb is or is not removed. Most importantly, the sole purpose of this experiment was to verify IC formation. In fact, ICs were formed and there was a significant increase in IC formation in the presence of 5 μg versus 1μg of mAb (Fig. 1A). We then immunized and boosted mice i.n. with these mixtures and challenged 14 days later i.n. with either Ft LVS (Fig. 1B) or Ft SchuS4 (Fig. 1C). Similar to studies using mAb-iFt ICs (free/unbound mAb removed) 2, a significant enhancement of protection against both strains of Ft was observed with mAb + iFt ICs (free/unbound mAb present). Also, consistent with previous studies using mAb-iFt ICs, while mAb + iFt ICs made with 1 μg mAb were sufficient to protect against Ft LVS challenge, mAb + iFt ICs made with 5 μgs mAb were required to protect against Ft SchuS4 challenge. Thus, use of mAb + iFt ICs represents a relatively simple and straightforward approach for targeting immunogens to FcR. This is particularly the case in those instances where a protective Ag cannot be identified and the use of inactivated organisms is required, such as is currently the case with Ft.
Figure 1. Enhanced protection is observed using anti-iFt mAb + iFt ICs.
Prior to immunization, 1×109 iFt organisms were incubated at 4°C overnight with 0, 1 or 5 μg/ml of anti-iFt mAb in PBS. Following the incubation, iFt and mAb + iFt ICs were washed (to remove free mAb) and analyzed by SDS PAGE and Western Blot to verify mAb-iFt IC formation (A). 2×107 iFt organisms alone or complexed with anti-iFt mAb were administered i.n. to C57BL/6 mice. The mice were boosted on day 21 and challenged on day 35 with either 20,000 CFU of Ft LVS (B) or boosted on days 14 and 28 and challenged on day 42 with 21 CFU of Ft SchuS4 (C). Survival was monitored for 21 days. The p value for iFt versus mAb + iFt ICs in B (Ft LVS challenge) was < 0.05. The p value for iFt versus mAb + iFt ICs in C (Ft SchuS4 challenge, 5 μg) was < 0.02.
Enhanced T cell Responses in the Presence of Ft-Specific mAb
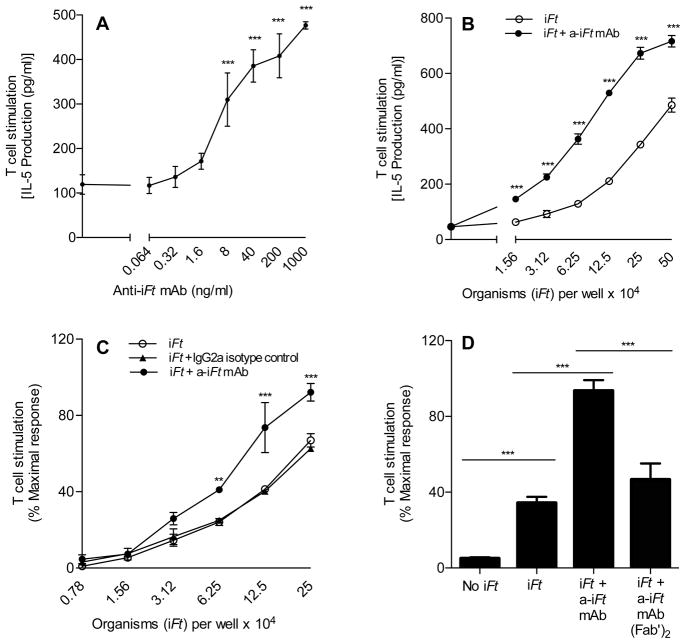
One of the mechanisms by which mAb + iFt ICs may enhance immune responses in vivo is through the induction of enhanced T cell activation. Thus, we examined the response of an Ft-specific T cell hybridoma (FT256D10) to APCs incubated with either iFt alone or a mAb + iFt ICs. The T cells were incubated with Peritoneal Exudate Cells (PECs)/Macrophages (MØ) obtained from Balb/c mice, which are histocompatible with the T cell hybridoma, and either iFt or anti-iFt mAb + iFt ICs. After 24 hours, the supernatant was collected and IL-5 levels (a marker of the T cell response) were measured (Fig. 2). We observed that the level of IL-5 in supernatants from T cells and APCs incubated with mAb + iFt ICs was significantly higher than in the supernatants of T cells and APCs incubated with iFt alone (Fig. 2) and that the enhancement was both mAb (Fig. 2A) and iFt (Fig. 2B) concentration dependent. Alternatively put, mAb in the absence of sufficient amounts of Ag did not enhance T cell activation, indicating mAb alone does not stimulate the enhanced T cell activation observed when both mAb + iFt ICs are present. Furthermore, enhancement was eliminated if the iFt-specific mAb was replaced with a non-specific isotype (Ig2a) control mAb (Fig. 2C), or if the Fc portion of the iFt-specific mAb was removed (Fig. 2D). Thus, enhanced presentation of iFt is one mechanism by which mAb + iFt ICs can exert their effect on the immune response, and this effect is Fc-dependent.
Figure 2. Presentation of iFt to Ft-specific T cells is enhanced in the presence of anti-iFt mAb.
Ft-specific T cell hybridoma cells (1×105 cells/well) were cultured with Balb/c MØs (2×105 cells/well) in the presence of: increasing concentrations of anti-iFt mAb and a fixed concentration of iFt organisms (4×105 per well) (A), increasing amounts of iFt organisms plus and minus 1 μg/ml of anti-iFt mAb (B), increasing amounts of iFt organisms either alone, plus 1 μg/ml of anti-iFt mAb, or plus a non-specific IgG2a isotype control mAb (C), media, 4×105 iFt/well, or 4×105 iFt/well plus 0.04 μg/ml of anti-iFt mAb or 0.04 μg/ml of F(ab′)2 anti-iFt mAb (D). Production of IL-5 by Ft-specific T cells was measured in all figures (A–D) as an indicator of T cell response to iFt by CBA. In the case of (C) and (D) data was normalized between experiments by expressing results as percent maximal response. Results are representative of three independent experiments. (***) p value < 0.001.
Anti-iFt mAb Mediates Enhanced iFt Binding to APC
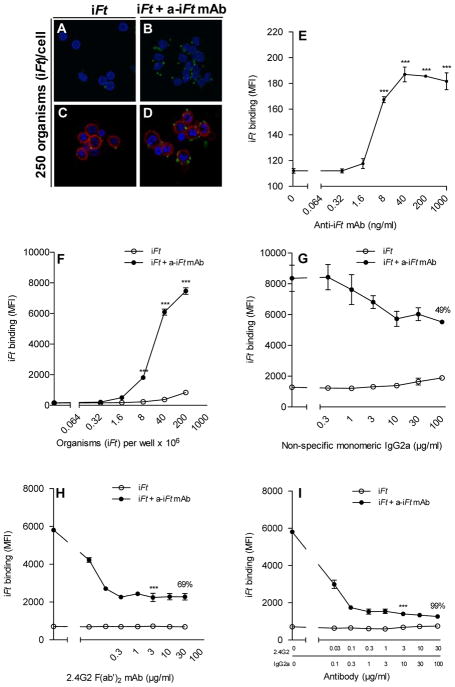
One factor that can contribute to enhanced processing and presentation of iFt by APCs, is increased binding of iFt to APCs in the presence of mAb. We thus tested whether in the presence of mAb-iFt there is increased iFt binding to APCs via FcR, as compared to iFt alone. To demonstrate visually, enhanced binding of mAb + iFt ICs versus iFt alone to FcR, FcR-bearing MØs were incubated with media, iFt, or mAb + iFt ICs at 4°C and unbound iFt removed. In fact, we observed a significant increase in the number of bound bacteria in the presence of anti-iFt mAb + iFt ICs versus iFt alone (Figs. 3A–D). This increase was dependent on both mAb (Fig. 3E) and iFt (Fig. 3F) concentration. Furthermore, approximately 50% of the mAb-mediated iFt binding could be blocked with monomeric IgG2a (Fig. 3G). Monomeric IgG2a primarily blocks the high affinity FcγRI. The mAb 2.4G2, which blocks FcγRII and FcγRIII, blocked approximately 70% of the mAb-iFt binding (Fig. 3H). When combining monomeric IgG2a with mAb 2.4G2, nearly 100% of mAb-mediated iFt binding was eliminated (Fig. 3I). Thus, enhanced binding of iFt to APCs in the form of mAb + iFt ICs represents another contributing mechanism by which mAb + iFt ICs impact the immune response via FcR-enhanced delivery of Ag to APCs.
Figure 3. Anti-iFt mAb enhances FcR-dependent binding of iFt to APCs.
Z projections of Balb/c MØs incubated for 2 hours at 4°C with either iFt alone or anti-iFt mAb + iFt ICs. The iFt (green) (GFP expressing inactivated Ft organisms) can be seen on the cells’ surface (red) labeled with CTB Alexa 647 and the nuclei (blue) have been stained with DAPI. The images were acquired on an Olympus IX 81 confocal microscope (A–D). Balb/c MØs were incubated at 4°C for 2 hours in the presence of either increasing concentrations of anti-iFt mAb and 5×105 iFt organisms per well (E), or increasing concentrations of iFt organisms plus or minus 1 μg/ml of anti-iFt mAb (F). Prior to the addition of the iFt organisms in the presence or absence of anti-iFt mAb, FcγR were blocked for 1 hour at 4°C with monomeric IgG2a (FcγRI) (G), F(ab′)2 2.4G2 mAb (FcγRII and FcγRIII) (H), or both IgG2a and F(ab′)2 2.4G2 mAb (I). Binding of GFP-expressing iFt organisms was detected by flow cytometry using a BD LSRII flow cytometer (E–I). Results are representative of three independent experiments. (***) p value < 0.001.
A Physical Linkage Between mAb and Ag is Required for FcR-Mediated Enhancement of Ag Presentation by APCs
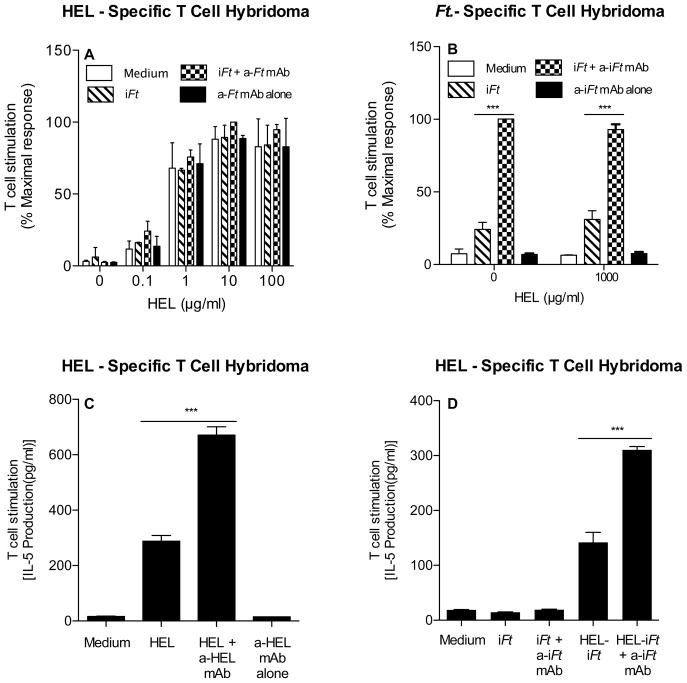
We also considered the possibility that the impact of mAb + iFt ICs on the presentation of iFt was not dependent on iFt being directly targeted to FcγR, but rather due to signaling events initiated by cross-linking FcγR. Thus, we determined whether mAb-iFt added to APCs could enhance the presentation of an Ag other than iFt, specifically Hen Egg Lysozyme (HEL). In this case, an HEL-specific T cell hybridoma (LY50.5) was cultured in the presence of APCs and increasing concentrations of HEL containing either media, iFt, mAb-iFt, or mAb alone. After 24 hours, supernatant was collected and IL-5 levels were measured. As shown in Fig. 4, the presence of mAb-iFt did not impact the level of IL-5 produced by the T cell hybridoma in response to HEL, regardless of whether media, iFt, mAb-iFt, or mAb alone was present (Fig, 4A). The latter was not due to a failure of the mAb-iFt to mediate enhanced presentation of iFt (Fig. 4B). Furthermore, when HEL-specific mAb was bound to HEL, HEL presentation was enhanced (Fig. 4C), as was the presentation of HEL when iFt was chemically labeled with HEL and anti-iFt mAb bound (Fig. 4D). Finally, consistent with the inability of mAb-iFt to stimulate enhanced presentation of HEL, neither MHC class II expression or expression of second signal molecules on these APCs, were enhanced in the presence of mAb-iFt (data not shown). Thus, cross-linking FcR with mAb + iFt ICs alone is insufficient to enhance Ag presentation by APCs. Rather, a direct linkage between the Ag being presented and the mAb is required.
Figure 4. A physical linkage between mAb and Ag is required for FcR-mediated enhancement of Ag presentation by APCs.
HEL-specific T cell hybridoma cells (1×105 cells/well) were cultured with C3H/HeN MØs (2×105 cells/well) in the presence of media, 5×105 iFt organisms/well alone or plus 1μg/ml of anti-iFt mAb and increasing amounts of HEL (A). Ft-specific T cell hybridoma cells (1×105 cells/well) were cultured with Balb/c MØs (2×105 cells/well) in the presence or absence of 1 mg/ml of HEL in media, 5×105 iFt organisms/well alone or plus 1μg/ml of anti-iFt mAb (B). HEL specific T cell hybridoma cells (1×105 cells/well) were cultured with C3H/HeN MØs (2×105 cells/well) in the presence of media, 0.1 mg/ml of HEL, 0.1 mg/ml of HEL plus anti-HEL mAb (2D1), or anti-HEL mAb alone (2D1) (C). HEL-specific T cell hybridoma cells (1×105 cells/well) were cultured with C3H/HeN MØs (2×105 cells/well) in the presence of media, 2×107 iFt organisms plus or minus anti-iFt mAb, or 2×107 HEL conjugated iFt organisms plus or minus anti-iFt mAb (D). Production of IL-5 by Ft-specific T cells was measured in all figures (A–D) as an indicator of T cell response to iFt by CBA. Data was normalized between experiments by expressing results as percent maximal response. Results in 2A and 2B represent the combined data from two independent experiments. All other results are representative of three independent experiments. (***) p value < 0.001.
The Rate of iFt Internalization is Increased in the Presence of Anti-iFt mAb
Another mechanism by which iFt processing and presentation could be enhanced, is via an increased rate of iFt internalization by APCs. To address this possibility, the amount of iFt internalized by APCs was analyzed utilizing flow cytometry. Cells were pulsed for one hour with 200 iFt organisms/APC at 4°C on a rocker. Cells were then washed and incubated at 37°C for varying periods of time. Once the incubation was complete, the cells were cooled immediately on ice, washed, and incubated with pronase in order to remove the non-internalized iFt. Once the non-internalized iFt was removed from the surface of the cells, they were washed again and fixed. The mean fluorescence intensity (MFI) was then determined for each time point. As shown in Fig. 5, it took approximately 3–4 hours for internalization to be detected in the form of increased fluorescence of pronase-stripped cells, when pulsing with iFt alone (Fig. 5A). However, the increase was much stronger and more rapid when anti-iFt mAb was present, occurring in five minutes or less (Fig. 5B). Importantly, the rate of internalization for iFt alone remained similar even when the number of organisms/APC was increased by 10 fold. (Fig. 4C). Thus, not only is iFt binding to APCs enhanced in the presence of iFt-specific mAb, but also the rate of iFt internalization by APCs.
Figure 5. Internalization of iFt is enhanced in the presence of anti-iFt mAb.
Balb/c MØs (2×105 cells) were pulsed with 200 iFt organisms/MØ in the absence (A) or presence (B) of anti-iFt mAb for 1 hour at 4°C. After the pulse, the unbound iFt was removed and the MØs were incubated at 37°C to allow internalization of the iFt. At the end of each 37°C incubation time point, the non-internalized iFt was stripped from the cell surface using pronase, and the amount of internalized iFt was measured by flow cytometry (A–B). In addition, MØs were also pulsed with 2000 iFt organisms/MØ in the absence of anti-iFt mAb. The experiment was then carried out as in A and B. Internalization is reflected as increased MFI following incubation and pronase treatment, as compared to pronase-treated cells at time zero. Results are representative of three independent experiments. (**) p value < 0.01, (***) p value < 0.001.
Enhanced Presentation of iFt Persists in the Presence of anti-iFt mAb
Since we observed an increased T cell response, increased binding, and more rapid internalization of the iFt in the presence of anti-iFt mAb, we wanted to know how these changes impact the kinetics of Ag processing and presentation. To examine this, APCs were allowed to take up and process iFt in the presence and absence of mAb for 0, 1, 2, 4, 8, 22 and 26 hours. Following each incubation time point, APCs were fixed and incubated with T cells as a read out of iFt processing. After 24 hours of APC plus T cell culture, the supernatant was collected and IL 5 was measured. As early as 4 hrs after iFt pulse, the T cell response in the presence of mAb was increased above that of iFt alone (Figs. 6A). Furthermore, the length of time processed iFt was available on the surface of the APCs for presentation was also increased (Figs. 6A). To further investigate the latter observation, APCs were allowed to internalize and process the Ag for 8 hours, then the non-bound/non-internalized Ag was washed away and the cells were either fixed immediately or fixed after 15, 18, 21 or 36 hours of additional incubation. As demonstrated in Figure 6B, when iFt is internalized in the presence of anti-iFt mAb, up to 36 hours after free Ag is removed, an enhanced response by T cells to processed iFt is observed, as compared to APCs pulsed with iFt alone. Thus, the enhanced iFt presentation induced by mAb-iFt begins as soon as 4 hours post iFt addition and also persists up to 36 hours after the iFt pulse.
Figure 6. Enhanced presentation of iFt persists in the presence of anti-iFt mAb.
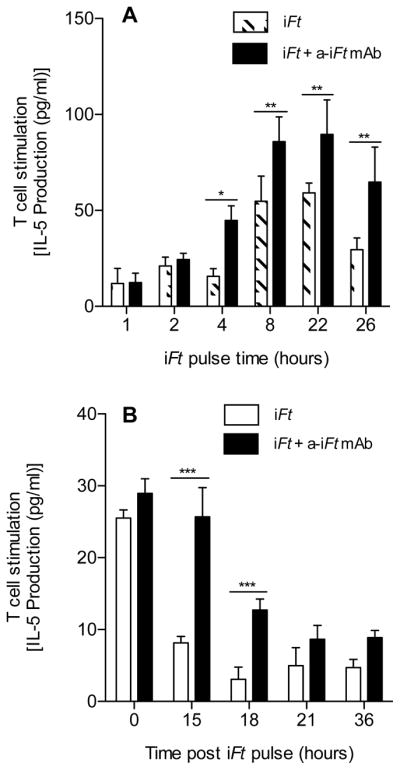
Balb/c MØs (2×105 cells) were cultured in media alone or media containing 1×106 iFt organisms in the presence or absence of 1 μg/ml of anti-iFt mAb at 37°C in 5% CO2 for 0, 1, 2, 4, 8, 22 or 26 hours. At each time point the cells were then fixed for 15 minutes at room temperature in 1% paraformaldehyde, washed, and stored at 4°C until all time points were completed. Cells were then counted and adjusted to 2×106 cells/ml, 100 μl of the latter were then added to the wells of a 96 well plate containing the Ft-specific T cell hybridoma at 1×105 cells/well. The plate was then incubated at 37°C in 5% CO2 in a humidity chamber for 24 hours, the supernatant was collected, and assayed for IL-5 using CBA. The average of three independent experiments is presented (A). Balb/c MØs (2×105 cells) were cultured in media alone or media containing 1×106 iFt organisms in the presence or absence of 1 μg/ml of anti-iFt mAb for 8 hours at 37°C in 5% CO2. After the incubation, the cells were washed 3 times with PBS and further incubated at 37°C in 5% CO2 for 0, 15, 18, 21 or 36 hours, at which point they were fixed with 1% paraformaldehyde, washed with PBS and then stored at 4°C until all time points were completed. Subsequently, cells were counted and adjusted to 2×106 cells/ml and 100 μl of the latter were then added to the wells of a 96 well plate containing the Ft-specific T cell hybridoma at 1×105 cells/well. The plate was incubated at 37°C in 5% CO2 in a humidity chamber for 24 hours and the supernatant was collected. The supernatants were assayed for IL-5 using CBA (B). Results are representative of three independent experiments. (*) p value < 0.05, (**) p value < 0.01, (***) p value < 0.001.
Enhanced iFt Binding, Presentation, and iFt Persistence is Also Observed With DCs in the Presence of mAb + iFt ICs
Since all the studies up to this point had focused on PECs/MØs as APCs, we wanted to know whether mAb + iFt ICs would have a similar impact on DCs. Interestingly, the level of enhanced binding (Fig. 7A) and enhanced presentation (Fig. 7B) was even more dramatic when using DCs as APCs. In addition, in contrast to MØs, the expression of MHC class II and second signal molecules (DC maturation markers) was increased in the presence of mAb-iFt versus iFt (Fig. 7C). However, iFt alone also induced a significant increase in the expression of these markers (Fig. 7C). Furthermore, the higher levels of iFt presentation by DCs, induced in the presence of mAb-iFt, also demonstrated similar persistence to that of MØs (Figs. 6 and 7D). Thus, with the exception of MHC class II, CD80, and CD86 expression, which is enhanced by mAb + iFt ICs on DCs, but not on MØs (data not shown), the impact of mAb + iFt ICs on iFt binding, presentation, and Ag persistence, is similar to that of MØs.
Figure 7. Enhanced iFt binding, presentation, and persistence are also observed when using DCs as APCs.
Balb/c bone marrow-derived DCs were incubated for 2 hours at 4°C in the presence of increasing concentrations of iFt organisms plus or minus 1μg/ml of anti-iFt mAb. Binding of GFP-expressing iFt organisms to DCs was detected by flow cytometry using a BD LSRII flow cytometer (A). Ft-specific T cell hybridoma cells were cultured with Balb/c bone marrow-derived DCs in the presence of increasing concentrations of iFt organisms in the presence or absence of 1 μg/ml of anti-iFt mAb (B). Balb/c bone marrow-derived DCs (5×105) were cultured for 24 hours at 37°C in 5% CO2 in the presence of media alone or 1×107 iFt organisms plus or minus 1 μg/ml of anti-iFt mAb. Following the incubation, cells were washed PBS/BSA and stained for CD83, CD80, CD86, DEC205, and MHC class II. Fluorescence was detected by flow cytometry on a LSRII flow cytometer (C). Balb/c bone marrow-derived DCs were pulsed for 12 hours at 37°C in 5% CO2 with media alone, iFt organisms (MOI 2.5), or iFt organisms plus 1 μg/ml of anti-iFt mAb. After the pulse, the DCs were washed three times with media and incubated with the Ft-specific T cell hybridoma cells (2:1 ratio) at 37°C in 5% CO2. Every 24 hours the media from each well was collected and stored at −20°C. Fresh media was added to the wells each day after collecting the sample. This was done for 4 days. Production of IL-5 by Ft-specific T cells was measured as an indicator of T cell response to iFt by CBA and normalized between experiments by expressing results as percent maximal response (D). Results are representative of three independent experiments. (**) p value < 0.01, (***) p value < 0.001.
Enhanced Localization of iFt to NALT in vivo
It had been previously hypothesized that one potential impact of targeting Ag to Fc receptors in vivo may be to enhance Ag trafficking to lymphoid organs. Given that our immunizations are i.n., we wanted to test this hypothesis with regards to Nasal Associated Lymphoid Tissue (NALT). Utilizing qPCR to detect iFt localization after i.n. administration of iFt and mAb-iFt, we observed nearly a 10 fold increase in the amount of iFt trafficking to the NALT by 30 minutes post-administration of mAb-iFt, as compared to iFt alone (Fig. 8A). Furthermore, prior indications that FcRn can play a significant role in Ag transport across the epithelium in adults 4, 5, 8, 20–23, was confirmed in the case of mAb + iFt ICs (Fig. 8B). Specifically, when using FcRn KO mice in similar trafficking studies, the enhanced trafficking of mAb-iFt observed with WT mice (Fig. 8A) was eliminated (Fig. 8B). Thus, another mechanism by which mAb + iFt ICs exert their effect on the immune response is via enhanced transport of iFt to the NALT via FcRn.
Figure 8. Trafficking of iFt to the NALT is enhanced and is FcRn-dependent in the presence of anti-iFt mAb.
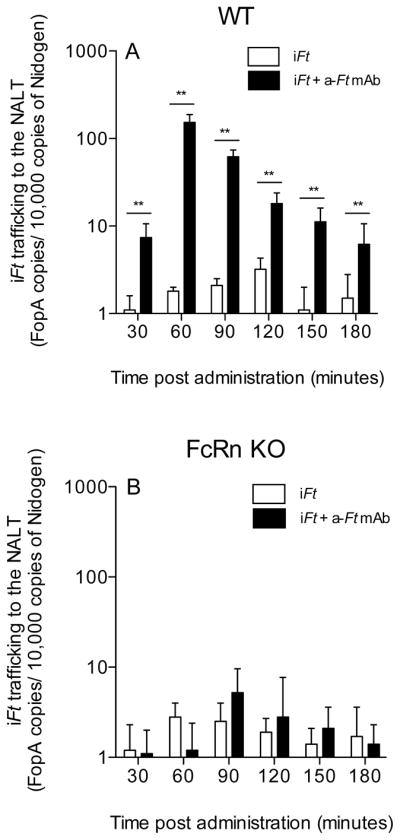
C57BL/6 (A) or FcRn deficient (B) mice were immunized i.n. with either iFt (2 × 107 CFUs/mouse) plus or minus anti-iFt mAb (1ug/ml). At 30 minute intervals the NALT from a single mouse from each group was harvested. Genomic DNA was isolated and the number of FopA gene copies present was determined by multiplex real-time PCR. Equal amounts of total chromosomal DNA were added to each reaction (50 ng/reaction). Each sample was run in triplicate and the results were then normalized against 10,000 copies of the mouse gene, Nidogen. Data in A is representative of three independent experiments. (**) p value < 0.01.
Discussion
Numerous studies have demonstrated that targeting Ag to FcR can enhance the immune response to Ag 12, 24–28. In 2008, we demonstrated for the first time, that targeting Ag to FcR i.n. could also enhance protection against a subsequent challenge with the highly virulent mucosal pathogen Ft 2. Utilizing this model, we now provide the first comprehensive study examining the potential mechanism(s) that contribute to this immune enhancement.
Numerous studies have suggested one mechanism of immune enhancement involves enhanced presentation of the FcR-targeted immunogen 17, 21, which is generally believed to be the primary contributor. However, a comprehensive study using an established FcR-targeted protective immunogen, such as mAb-iFt, has not been conducted to determine if this is in fact the case. In this study, we verify that FcR-dependent enhanced presentation is a significant component of the enhanced response to FcR targeted iFt (Fig. 2), and that targeting of iFt to FcR on APCs enhances both iFt binding and internalization (Figs. 3 and 5). We also demonstrate that the observed FcR-mediated enhancement in iFt presentation requires a physical linkage between iFt and the mAb that engages the FcR (Figs. 4A–D). In other words, simply cross-linking FcR is not sufficient to enhance Ag presentation. This is consistent with the idea that enhanced Ag binding and Ag internalization is critical to the enhanced presentation observed. The fact that cross-linking MØ FcR with mAb-iFt did not enhance expression of MHC class II or second signal molecules (data not shown) is also consistent with this observation and previous observations by this laboratory in which similar results were obtained using human IgG-Ag ICs and human monocytes 11. However, the impact of ICs on DC maturation is more controversial. While some studies have indicated that DC maturation can be enhanced in the presence of ICs 12, more recent studies have suggested ICs inhibit DC maturation via engagement of FcγRIIB, the inhibitory FcR 13. In fact, despite the failure of mAb + iFt ICs to enhance MHC class II and second signal molecule expression on MØs, DC maturation (indicated by upregulation of both MHC class II and second signal molecules), was enhanced in the presence of mAb + iFt ICs (Fig. 7C). The latter is likely explained by differences between these and other published studies, and the numerous variables involved. Specifically, the study, which demonstrates inhibition, utilized ICs composed of OVA and rabbit anti-OVA IgG, combined with human monocyte-derived DCs, as apposed to our studies, which utilized ICs composed of mouse IgG2a mAb + iFt ICs and mouse DCs. In this regard, the size of the ICs, the valency, and differences in inter- and cross-species binding of IgG isotypes to FcR, all significantly impact, which FcR (activating versus inhibitory) are engaged 8, 9, 12, 17. It is likely in our case, engagement and activation of the stimulatory FcRs dominates that of the inhibitory FcR, resulting in the enhanced DC maturation we observe.
This paper also demonstrates for the first time that targeting Ag to FcR results in Ag presentation persisting at higher levels for an extended period of time (Figs. 6 and 7). As with enhanced Ag binding and presentation, this was observed for both MØs and DCs (Figs. 6 and 7). In fact, a similar observation has been made when targeting Ag to the B cell Ag receptor 19. Thus, this may represent a common mechanism used by many APCs to enhance and prolong the immune response, and thus immune protection. Importantly, despite the fact mAb + iFt ICs enhance T cell activation, we have not observed any physical side effects following immunization with ICs, whether or not free mAb is present. Histological analysis of lung tissues and BAL 1, 3, and 6 days post immunization also show no significant differences in cellular infiltration (nearly absent) between PBS, iFt, or mAb + iFt IC-immunized mice (Data not shown). Never the less, it is likely that the inflammatory response is enhanced within the NALT following administration of ICs, as reflected in the resultant enhanced humoral and cellular immune responses.
It has also been proposed that an additional mechanism by which FcR-targeted immunogens enhance the immune response is by facilitating the trafficking of Ag to lymphoid tissues 9. In the case of mAb-iFt this does hold true, in that iFt transport to the NALT was enhanced in the presence of anti-iFt mAb and was FcRn-dependent. (Fig. 8B). In fact, since our original publication in 2008 2, other laboratories have published papers demonstrating that targeting Ag to FcR i.n. can enhance the protective response to other pathogens, and that this can be accomplished by targeting Ag specifically to FcRn 3–5. FcRn is not only expressed in the nasal epithelium, but can also mediate the transport of Ag/immunogen across epithelial barriers to the underlying lymphoid tissues in adults 20, 22, 23. Furthermore, in our 2008 study, enhanced protection following i.n. immunization with mAb-iFt was lost in FcRn KO mice, suggesting FcRn was playing a key role 2. Thus, our data support the hypothesis that mAb + iFt ICs first engage FcRn for transepithelial transport to the NALT, followed by engagement of FcγR on APCs within the NALT. However, further trafficking studies will be needed to specifically verify this hypothesis. It should also be noted Ags can also be transported by M cells 29, which may also play a role. In fact, in regard to the latter, we have recently published studies in which engagement of FcRn cannot occur, but never the less enhanced mucosal immunity is still observed when targeting the immunogen to FcγRI on mucosal APCs 6.
In summary, utilizing a proven FcR-targeted protective mucosal immunogen 2, we demonstrate that a number of mechanisms contribute to the enhanced immunity and protection observed when targeting an immunogen to FcR. Specifically, FcR targeting mediates enhanced Ag binding, as well as more rapid Ag internalization, leading to enhanced activation of Ag-specific T cells. Consistent with this, Ag must be physically attached to the FcR-targeting component (in this case mAb) to observe enhanced presentation. In addition, not only is the T cell response enhanced, but also that enhancement is maintained over an extended period of time. Furthermore, DC maturation is also enhanced, as well as the FcRn-mediated trafficking of iFt to NALT. Thus, the impact of targeting Ag to FcR is multi-pronged with each mechanism involved having the potential to significantly contribute to enhanced immunity and protection. By understanding and further optimizing each of these events, one has the potential to further improve on FcR targeting as an effective mucosal vaccine platform. Furthermore, the fact that mAb + iFt ICs are as effective as mAb-iFt ICs in which free mAb has been removed [2 and Fig. 1] will further facilitate the application of this specific strategy in a clinical setting.
Materials and Methods
Reagents
The Ag HEL and Pronase were purchased from Sigma (St. Louis, MO). Endotoxins were removed from HEL using Detoxi-Gel from Pierce (Rockford, IL). Cell-Tak was purchased from BD Biosciences (San Diego, CA) and used as directed by the vendor. Cholera Toxin Subunit B (recombinant)-Alexa Fluor 647 conjugate was purchased from Invitrogen (Carlsbad, CA). The mouse IgG2a anti-Ft LPS mAb used to make mAb + iFt ICs, was purchased from Fitzgerald (cat # 10-F02, clone# M0232621, Acton, MA). F(ab′)2 mAb against Ft LPS was prepared using an F(ab′)2 preparation kit from Pierce according to vendor instructions. Anti-mouse F4/80 Ab was purchased from Invitrogen. Anti-mouse CD16/32 (2.4G2) F(ab′)2 Ab was purchased from BD Biosciences. Anti-mouse MHC class II (I-A/I-E), CD83 and DEC205 Ab were purchased from eBioscience (San Diego, CA). Anti-mouse CD80 and CD86 Ab were purchased from BioLegend (San Diego, CA). IgG2a isotype control Ab was purchased from MP Biomedicals, LLC (Solon, OH). All other isotype control Abs were purchased from BioLegend.
Cells
Either PEC/MØs or DCs were used as APCs in these studies. PEC/MØs were obtained from Balb/c or C3H/HeN mice. DCs were derived from Balb/c bone marrow cells and cultured for one week in the media described below supplemented with 50 ng/ml of FLT3 (R&D Systems, Minneapolis, MN). The Ft-specific T cell hybridoma (FT256D10) is specific for an Ft ribosomal protein-derived peptide, and was provided by Dr. Jeffrey Frelinger (University of North Carolina at Chapel Hill). The hybridoma was cultured in RPMI 1640 (CellGro, Manassas, VA) containing 10% FBS (HyClone, Logan, UT), 2mM L-Glutamine (CellGro), MEM non essential aminoacids (CellGro), 1 mM Sodium Pyruvate, 50 μM 2-ME, 100 U/ml Penicillin, 100 μg/ml Streptomicin (Gibco), and 500 μg/ml of Hygromycin B (CellGro). The HEL-specific T cell hybridoma (Ly50.5) and Balb/c bone marrow-derived DCs were cultured in the same media without the addition of Hygromycin.
Mice
Balb/c, C57BL/6, and C3H/HeN mice were purchased from Taconic Laboratories (Hudson, NY). All mice were housed at the Animal Resources Facility at Albany Medical College. The mice were used at 6–10 weeks of age. All protocols were reviewed by the Albany Medical College Ethics Committee utilizing NIH standards.
Inactivation and Labeling of Ft
Inactivated Ft (iFt) was generated by growing GFP-expressing Ft LVS in MHB medium (BD Biosciences) up to a density of 1×109 CFU/ml. The culture was then spun down at 22,000g for 20 minutes at 4°C, and washed 3 times with PBS, resuspended in 2% paraformaldehyde (Sigma) and incubated 2 hours at room temperature on a rocker. Bacteria were then washed 3 more times with PBS and 1×109 organisms were plated on a chocolate agar plate (BD Biosciences) to confirm inactivation. The plate was then incubated for 7 days at 37°C. HEL labeling of iFt was done by using a Rapid Conjugation Kit (AbD Serotec, Raleigh, NC). Briefly, 1×1010 iFt organisms were resuspended in 200 μl of PBS and 20 μl of modifier reagent (provided with the kit) along with 500 to 1000 μg of HEL dissolved in PBS. This mixture was then incubated at room temperature overnight. The following day 20 μl of quencher (provided with the kit) were added to the iFt organisms and this was incubated for 30 minutes at room temperature. Then the labeled iFt was washed with PBS 2 times. The final concentration of iFt organisms was determined by OD at 610 nm, HEL labeling was verified by ELISA and anti-iFt mAb binding to the HEL-labeled iFt was verified by flow cytometry and ELISA.
Generation of mAb + iFt ICs
To generate mAb + iFt ICs, 1×109 iFt LVS organisms were incubated at 4°C overnight on a rocker with 0, 1, or 5 μg/ml of IgG2a anti-iFt LPS mAb in PBS. Following the incubation, iFt or mAb + iFt ICs were administered to mice i.n.
SDS-PAGE and Western Blot Analysis
The IgG2a anti-Ft LPS mAb and iFt were incubated as previously indicated 2 and as briefly described above. To avoid detection of free mAb by the goat anti-mouse mAb used to detect ICs, following the incubation of mAb + iFt ICs, free/unbound mAb was removed via centrifugation. Samples of iFt [10μg (~1×108 organisms of iFt or mAb-iFt ICs)] were mixed with Laemelli sample buffer and boiled for 10 minutes prior to resolution through 4–12 % gradient SDS-PAGE pre-cast gels (Invitrogen). The running buffer was NuPAGE MES SDS buffer from Invitrogen; gels were run at 120 Volts. Resolved samples were transferred to nitrocellulose membranes. Membranes were blocked for 1 hour with PBS, 0.05% Tween 20, 2.5% horse serum and 1% casein. Biotinylated goat anti-mouse Ig heavy (γ) chain from Southern Biotech (Birmingham, AL) was used as the primary Ab for overnight incubations at a dilution of 1:1,000 in blocking buffer; streptavidin-conjugated HRP at a dilution of 1:5000 for 1 hr was used for detection. Development of the chemiluminescent substrate (SuperSignal West Pico, Pierce, Rockford, IL) was visualized using an Alpha Innotech imaging system in movie mode. Densitometric analysis of developed blots was performed on the same system. Following development of the Ig heavy chain (HC) signals, we re-probed the membranes for total FopA (a constitutively-expressed Francisella protein) and quantified the data as surface mAb/total FopA. These ratios were normalized to the corresponding ratios from mAb-iFt -1 μg of mAb.
Immunization and Challenge Studies
C57BL/6 mice were immunized on days 0 and 21 (Ft LVS challenge) or days 0, 14 and 28 (Ft SchuS4 challenge) with 2×107 iFt organisms alone or in the form of mAb + iFt ICs utilizing IgG2a anti-iFt LPS mAb. On day 35 (Ft LVS) or day 42 (Ft SchuS4) the mice were challenged with 20,000 CFU of live Ft LVS or 21 CFU of live Ft SchuS4. Following challenge survival was monitored for 21 days.
Ag Presentation Assays
APCs (2×105) from Balb/c mice and the Ft-specific T cell hybridoma cells (FT25 6D10) (1×105) were added in T cell medium to the wells of a 96-well plate containing iFt alone or iFt pre incubated 2 hours at 37°C with whole anti-iFt mAb (1 μg/ml), an F(ab′)2 anti-iFt mAb, or an IgG2a isotype control Ab. The plate was then incubated at 37°C in 5% CO2 in a humidity chamber for 24 hours and supernatants were collected. The supernatants were then assayed for IL-5 using BD Biosciences Cytometric Bead Array (CBA) following vendor instructions. In the case of Ag presentation assays specific for HEL, APCs (2×105) from C3H/HeN mice and the HEL-specific T cell hybridoma cells (LY50.5) (1×105) were added in T cell medium to the wells of a 96-well plate containing endotoxin free HEL and iFt alone or iFt pre-incubated with 1 μg/ml of anti-iFt mAb. Alternatively, HEL-conjugated iFt alone or pre-incubated with 1 μg/ml of anti-iFt mAb was added to the wells. The plates were then incubated and supernatants harvested and analyzed as indicated above.
Monitoring Ag Presentation Kinetics/Persistence
Three approaches were used to measure persistence of Ag presentation. In the first instance, APCs (2×105) were cultured in the presence of (1×106) iFt organisms alone or with 1 μg/ml of anti-iFt mAb for either 0, 1, 2, 4, 8, 22, or 26 hours at 37C in 5% CO2. At each specified time point, the APCs were fixed with 1% paraformaldehyde (Sigma), washed 3 times with media and maintained at 4°C until the end of the time course. Once the final time point was reached and all APCs were fixed, they were recounted and 2×105 APCs were added to the wells of a 96 well plate containing 1×105 Ft-specific T cell hybridoma cells. Supernatants were collected 24 hours later and IL-5 was measured using CBA. In the second instance, APCs (2×105) were cultured in the presence of (1×106) iFt organisms alone or with 1 μg/ml of anti-iFt mAb for 8 hours at 37°C in 5% CO2. Following the incubation, the APCs were washed 3 times with media and incubated at 37°C for another 0, 15, 18, 21, or 36 hours, fixed with 1% paraformaldehyde (Sigma), washed 3 times with media and kept at 4°C until the end of the time course. Once the last time point was reached and all APCs were fixed, they were re-counted and 2×105 APCs were added to the wells of a 96 well plate containing 1×105 Ft-specific T cell hybridoma cells. Supernatant were then collected 24 hours later and IL-5 was measured as described above. In the third instance, APCs (2×106) were cultured in the presence of (5×106) iFt organisms alone or with 1 μg/ml of anti-iFt mAb for 12 hours at 37°C in 5% CO2. Then, the APCs were washed 3 times with media and seeded on the wells of a 96 well plate containing the Ft-specific T cell hybridoma cells (1:1 ratio). Each day for the following 4 days supernatants were collected and the wells were replenished with fresh media. IL-5 was measured as described above. In some instances data was normalized using percent maximal response to facilitate comparisons between figures or to combine data from multiple experiments into a single figure. This was done by taking cytokine values for individual samples and dividing by the maximal cytokine value obtained for the experiment. These values were then multiplied by 100 in order to calculate percent maximal response.
iFt Binding Studies
Binding of iFt to APCs in the presence and absence of anti-iFt-specific mAb, was visualized by flow cytometry. Experiments were carried out at 4°C to prevent iFt internalization. Briefly, 2×105 APCs were added to the wells of a 96-well plate. Cells were washed two times with PBS containing 2mg/ml BSA and 0.1% azide, and then 100 μl of PBS-BSA-azide containing iFt alone or iFt plus 1 μg/ml of anti-iFt mAb was added to the wells. The plate was then incubated for 2 hours at 4°C on a rocker, the cells were then washed with PBS-BSA-azide 3 times, fixed with 2% paraformaldehyde and subsequently analyzed on either a FACSCanto or LSRII flow cytometer (BD Biosciences). Alternatively, to demonstrate FcR specificity of the observed binding, the APCs were pre-incubated with non-specific monomeric mouse IgG2a (blocks FcγRI), F(ab′)2 anti-mouse CD16/32 (2.4G2) (blocks FcγRII and FcγRIII), or both IgG2a and F(ab′)2 2.4G2 mAb, for 90 minutes on ice prior to adding iFt plus or minus anti-iFt mAb. To visualize iFt binding using fluorescence microscopy, after the 2 hour incubation and subsequent washes, the cells were resuspended in PBS-azide containing Cholera Toxin subunit B-Alexa Fluor 647 conjugate (5ug/ml), which labels cell membranes, incubated for 15 minutes at 4°C and then fixed. After fixation cells were attached to a coverslip previously coated with CellTak (BD Biosciences) and mounted on a slide. Observation and image acquisition was done using an Olympus IX 81 confocal microscope (Olympus America Corporation, Center Valley, CA).
Internalization Studies
Internalization of iFt in the presence and absence of anti-iFt mAb was visualized by flow cytometry. Briefly, APCs (2×105) were pulsed with 200 to 2000 iFt organisms/APC plus and minus 1 μg/ml anti-iFt mAb for 1 hour at 4°C in HEPES-RPMI-10% FBS. After the pulse, the cells were washed 3 times with 250μl of cold HEPES-RPMI, resuspended in culture media, and incubated at 37°C for the desired time. Once the incubation was complete, the cell suspension was cooled immediately on ice and washed 2 times with PBS BSA 0.1% azide. The cells were then resuspended in 100 μl of 400 μg/ml pronase [in PBS plus 0.1% azide (Prevents further iFt internalization)] and subsequently incubated at 37°C for 15 minutes to strip from the cell surface the non-internalized iFt. After Pronase treatment, the cells were washed and fixed with 2% paraformaldehyde. The samples were analyzed on a FACSCanto flow cytometer (BD Biosciences).
Surface Marker Expression by APCs
APCs (2×105) were added to the wells of a non-binding surface 96 well plate and incubated at 37°C in 5% CO2 in the presence of iFt alone or anti-iFt mAb + iFt ICs. After 24 hours the cells were washed 3 times with PBS-BSA-azide, resuspended in blocking buffer [PBS-BSA-azide plus 30 μg/ml of normal mouse IgG (Sigma)] and incubated on ice for 30 minutes. Cells were then washed 3 times with PBS-BSA-azide and fluorescently labeled Ab to MHC class II, F4/80, CD80, CD83, DEC205 or CD86 or their corresponding isotype controls were added. The cells were then incubated on ice for 30 minutes, washed, and then fixed with 2% paraformaldehyde. Cells were then analyzed by flow cytometry on an LSRII flow cytometer (BD Biosciences).
In Vivo Trafficking of iFt and iFt in mAb + iFt ICs
Wildtype C57BL/6 mice or FcRn deficient mice on the same genetic background, were immunized i.n. with either iFt (2 × 107 organisms/mouse) or anti-iFt mAb + iFt ICs at a mAb concentration of 1 μg/ml. At 30 minute intervals for a period of 3 hours, a single mouse from each group was euthanized, and the NALT was harvested. To detect the trafficking of iFt, total Ft genomic DNA was isolated from the NALT samples using the DNeasy Blood and Tissue kit (Qiagen, Valencia, CA). A BioRad iQ5 Multicolor Real-Time PCR Detection System (Hercules, CA) was used to amplify and detect the Francisella gene, fopA, as an indication of iFt trafficking to the NALT. The amount of target Francisella DNA was determined based upon calculations using standard curves established with plasmids containing the cloned fragment of the Ft fopA gene. More specifically, total Ft genomic DNA was obtained by using the DNeasy Blood and Tissue kit, and the 600 bp fopA gene was amplified by PCR. Both the forward (ACTTATAGCGCTTTGACTAACAAGGAC) and reverse (CTGCTGGTATTAAGCAATGTGAAGGC) primers were designed and purchased by Integrated DNA Technologies (Coralville, IA). The desired PCR product (tac ttatagcgct ttgactaaca aggacaatac ttggggtcct caagatagaa ctggccagtg gtacttaggt gtagatgcta acggtctagc tggaactcct aactctccat caggtgctgg tgctaacttc acaatcggtt ataacatcaa taaatacttc gctgtacagt acaaccaatt agttggtaga gtatttgctg gtttaggtga aggtgttgta aactttagta ataatactat gtttactcca tatgctgcag gtggtgctgg ttgggcaaat ctagcaggtc aagcaacagg tgcttgggat gtgggtggtg gtcttaagtt tgaactatct agaaatgttc aagcaagtgt tgactacaga tatatccaaa caatggcacc tagtaatatt tctggtgcta atggcagagc gggtactaac atgattggtg ctggtttaac atggttcttt ggtggcaaag atactactaa taatgacact ggtaatattc aggataatgg tgcgactaca gctgctcaaa ctgttgctat gccaactatt gatgagtcta agtatgtttt acctgctggt attaagcaat gtgaaggc) was gel purified and cloned in pCR4-TOPO plasmid vector (Invitrogen, Carlsbad, CA), and was subsequently used to transform chemically competent DH5a E. coli cells. Kanamycin resistant E. coli colonies were selected and the plasmid DNA was isolated, sequenced, and used as a real-time PCR standard. The numbers of fopA copies from each sample were normalized against 10,000 copies of the mouse gene Nidogen.
Statistical Analysis
The Log-Rank (Mantel-Cox) test was used for survival curves. One-way analysis of variances (ANOVA) or the unpaired, one-tailed student t-test was used for the remaining figures. GraphPad Prism 4 provided the software for the statistical analysis (San Diego, CA).
Acknowledgments
These studies were funded by NIH grants (P01 AI056320 and R01 AI076408), as well as a grant from the Army (BAA W11NF-11-1-0274).
References
- 1.McLendon MK, Apicella MA, Allen LA. Francisella tularensis: taxonomy, genetics, and Immunopathogenesis of a potential agent of biowarfare. Annu Rev Microbiol. 2006;60:167–85. doi: 10.1146/annurev.micro.60.080805.142126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rawool DB, Bitsaktsis C, Li Y, Gosselin DR, Lin Y, Kurkure NV, et al. Utilization of Fc receptors as a mucosal vaccine strategy against an intracellular bacterium, Francisella tularensis. J Immunol. 2008;180:5548–57. doi: 10.4049/jimmunol.180.8.5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGhee JR. A mucosal gateway for vaccines. Nat Biotechnol. 2011;29:136–8. doi: 10.1038/nbt.1766. [DOI] [PubMed] [Google Scholar]
- 4.Lu L, Palaniyandi S, Zeng R, Bai Y, Liu X, Wang Y, et al. A neonatal Fc receptor-targeted mucosal vaccine strategy effectively induces HIV-1 antigen-specific immunity to genital infection. J Virol. 2011;85:10542–53. doi: 10.1128/JVI.05441-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ye L, Zeng R, Bai Y, Roopenian DC, Zhu X. Efficient mucosal vaccination mediated by the neonatal Fc receptor. Nat Biotechnol. 2011;29:158–63. doi: 10.1038/nbt.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bitsaktsis C, Iglesias BV, Li Y, Colino J, Snapper CM, Hollingshead SK, et al. Mucosal immunization with an unadjuvanted vaccine that targets Streptococcus pneumoniae PspA to human Fcgamma receptor type I protects against pneumococcal infection through complement-and lactoferrin-mediated bactericidal activity. Infect Immun. 2012;80:1166–80. doi: 10.1128/IAI.05511-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amigorena S, Bonnerot C. Fc receptors for IgG and antigen presentation on MHC class I and class II molecules. Semin Immunol. 1999;11:385–90. doi: 10.1006/smim.1999.0196. [DOI] [PubMed] [Google Scholar]
- 8.Gosselin EJ, Bitsaktsis C, Li Y, Iglesias BV. Fc receptor-targeted mucosal vaccination as a novel strategy for the generation of enhanced immunity against mucosal and non-mucosal pathogens. Arch Immunol Ther Exp (Warsz) 2009;57:311–23. doi: 10.1007/s00005-009-0040-y. [DOI] [PubMed] [Google Scholar]
- 9.Heyman B. Regulation of antibody responses via antibodies, complement, and Fc receptors. Annu Rev Immunol. 2000;18:709–37. doi: 10.1146/annurev.immunol.18.1.709. [DOI] [PubMed] [Google Scholar]
- 10.van der Poel CE, Spaapen RM, van de Winkel JG, Leusen JH. Functional characteristics of the high affinity IgG receptor, FcgammaRI. J Immunol. 2011;186:2699–704. doi: 10.4049/jimmunol.1003526. [DOI] [PubMed] [Google Scholar]
- 11.Jelley-Gibbs DM, Plitnick LM, Gosselin EJ. Differences in IgG subclass do not effect immune complex-enhanced T cell activation despite differential binding to antigen presenting cells. Hum Immunol. 1999;60:469–78. doi: 10.1016/s0198-8859(99)00023-3. [DOI] [PubMed] [Google Scholar]
- 12.Boruchov AM, Heller G, Veri MC, Bonvini E, Ravetch JV, Young JW. Activating and inhibitory IgG Fc receptors on human DCs mediate opposing functions. J Clin Invest. 2005;115:2914–23. doi: 10.1172/JCI24772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laborde EA, Vanzulli S, Beigier-Bompadre M, Isturiz MA, Ruggiero RA, Fourcade MG, et al. Immune complexes inhibit differentiation, maturation, and function of human monocyte-derived dendritic cells. J Immunol. 2007;179:673–81. doi: 10.4049/jimmunol.179.1.673. [DOI] [PubMed] [Google Scholar]
- 14.Guyre CA, Keler T, Swink SL, Vitale LA, Graziano RF, Fanger MW. Receptor modulation by Fc gamma RI-specific fusion proteins is dependent on receptor number and modified by IgG. J Immunol. 2001;167:6303–11. doi: 10.4049/jimmunol.167.11.6303. [DOI] [PubMed] [Google Scholar]
- 15.Fridman WH. Regulation of B-cell activation and antigen presentation by Fc receptors. Curr Opin Immunol. 1993;5:355–60. doi: 10.1016/0952-7915(93)90053-u. [DOI] [PubMed] [Google Scholar]
- 16.Guriec N, Daniel C, Le Ster K, Hardy E, Berthou C. Cytokine-regulated expression and inhibitory function of FcgammaRIIB1 and -B2 receptors in human dendritic cells. J Leukoc Biol. 2006;79:59–70. doi: 10.1189/jlb.0305155. [DOI] [PubMed] [Google Scholar]
- 17.Nimmerjahn F, Ravetch JV. Fc-receptors as regulators of immunity. Adv Immunol. 2007;96:179–204. doi: 10.1016/S0065-2776(07)96005-8. [DOI] [PubMed] [Google Scholar]
- 18.Bayry J, Lacroix-Desmazes S, Kazatchkine MD, Hermine O, Tough DF, Kaveri SV. Modulation of dendritic cell maturation and function by B lymphocytes. J Immunol. 2005;175:15–20. doi: 10.4049/jimmunol.175.1.15. [DOI] [PubMed] [Google Scholar]
- 19.Gondre-Lewis TA, Moquin AE, Drake JR. Prolonged antigen persistence within nonterminal late endocytic compartments of antigen-specific B lymphocytes. J Immunol. 2001;166:6657–64. doi: 10.4049/jimmunol.166.11.6657. [DOI] [PubMed] [Google Scholar]
- 20.Bitonti AJ, Dumont JA, Low SC, Peters RT, Kropp KE, Palombella VJ, et al. Pulmonary delivery of an erythropoietin Fc fusion protein in non-human primates through an immunoglobulin transport pathway. Proc Natl Acad Sci U S A. 2004;101:9763–8. doi: 10.1073/pnas.0403235101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu X, Lu L, Yang Z, Palaniyandi S, Zeng R, Gao LY, et al. The neonatal FcR-mediated presentation of immune-complexed antigen is associated with endosomal and phagosomal pH and antigen stability in macrophages and dendritic cells. J Immunol. 2011;186:4674–86. doi: 10.4049/jimmunol.1003584. [DOI] [PubMed] [Google Scholar]
- 22.Spiekermann GM, Finn PW, Ward ES, Dumont J, Dickinson BL, Blumberg RS, et al. Receptor-mediated immunoglobulin G transport across mucosal barriers in adult life: functional expression of FcRn in the mammalian lung. J Exp Med. 2002;196:303–10. doi: 10.1084/jem.20020400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshida M, Claypool SM, Wagner JS, Mizoguchi E, Mizoguchi A, Roopenian DC, et al. Human neonatal Fc receptor mediates transport of IgG into luminal secretions for delivery of antigens to mucosal dendritic cells. Immunity. 2004;20:769–83. doi: 10.1016/j.immuni.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 24.White DM, Pellett S, Jensen MA, Tepp WH, Johnson EA, Arnason BG. Rapid immune responses to a botulinum neurotoxin Hc subunit vaccine through in vivo targeting to antigen-presenting cells. Infect Immun. 2011;79:3388–96. doi: 10.1128/IAI.00166-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adamova E, Walsh MC, Gosselin DR, Hale K, Preissler MT, Graziano RF, et al. Enhanced antigen-specific antibody and cytokine responses when targeting antigen to human FcGAMMA receptor type I using an anti-human FcGAMMA receptor type I-streptavidin fusion protein in an adjuvant-free system. Immunol Invest. 2005;34:417–29. doi: 10.1080/08820130500265372. [DOI] [PubMed] [Google Scholar]
- 26.Gosselin EJ, Wardwell K, Gosselin DR, Alter N, Fisher JL, Guyre PM. Enhanced antigen presentation using human Fc gamma receptor (monocyte/macrophage)-specific immunogens. J Immunol. 1992;149:3477–81. [PubMed] [Google Scholar]
- 27.Guyre PM, Graziano RF, Goldstein J, Wallace PK, Morganelli PM, Wardwell K, et al. Increased potency of Fc-receptor-targeted antigens. Cancer Immunol Immunother. 1997;45:146–8. doi: 10.1007/s002620050418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heijnen IA, van Vugt MJ, Fanger NA, Graziano RF, de Wit TP, Hofhuis FM, et al. Antigen targeting to myeloid-specific human Fc gamma RI/CD64 triggers enhanced antibody responses in transgenic mice. J Clin Invest. 1996;97:331–8. doi: 10.1172/JCI118420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Man AL, Prieto-Garcia ME, Nicoletti C. Improving M cell mediated transport across mucosal barriers: do certain bacteria hold the keys? Immunology. 2004;113:15–22. doi: 10.1111/j.1365-2567.2004.01964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]