Abstract
Six different adjuvants, each in combination with inactivated polio vaccine (IPV) produced with attenuated Sabin strains (sIPV), were evaluated for their ability to enhance virus neutralizing antibody titers (VNTs) in the rat potency model. The increase of VNTs was on average 3-, 15-, 24-fold with adjuvants after one immunization (serotype 1, 2, and 3, respectively). Also after a boost immunization the VNTs of adjuvanted sIPV were on average another 7- 20- 27 times higher than after two inoculations of sIPV without adjuvant. The results indicate that it is feasible to increase the potency of inactivated polio vaccines by using adjuvants.
Keywords: sabin IPV, oil-in water, PagL, AlOH3, replicon particles, MPL
1. Introduction
After wild polioviruses are eradicated, the live-attenuated oral poliovirus vaccine (OPV) viruses will be the only source of live poliovirus in the world. These attenuated strains can revert to neurovirulence and cause paralysis. In addition, this neurovirulent virus may then spread among a population as circulating vaccine-derived polioviruses (cVDPV) [1]. To minimize the risks of cVDPV, OPV use should eventually stop. Instead, safe and affordable IPV or, from a biosafety in manufacturing viewpoint, preferably sIPV, will be necessary for countries that wish to continue polio immunization. The National Institute of Public Health and the Environment (RIVM) has developed a candidate sIPV vaccine [2, 3] The production costs of sIPV are currently estimated equivalent to that for IPV, which is about 20-fold more expensive than OPV [4]. To replace OPV, costs should decrease further, for instance by antigen reduction using adjuvants.
Preclinical studies have already demonstrated the enhancing effect of some adjuvants in combination with IPV. Both oil-in-water emulsion and aluminium hydroxide enhance potency in rats [4]. Ivanow et al described the enhanced mucosal and systemic immune responses by 1,25-Dihydroxyvitamin D3 [5]. Also, CpG oligodeoxynucleotides as well as aluminium hydroxide can significantly enhance both the humoral and cellular immune responses to sIPV in mice [6]. In the current study, we optimised sIPV potency through formulation with six adjuvants: aluminium hydroxide, two squalene-in-water emulsions, two lipopolysaccharide (LPS) derivatives, and Venezuelan equine encephalitis (VEE) replicon particles (GVI3000).
Aluminium salts are the most frequently used vaccine adjuvants. They may serve as an antigen depot, resulting in slow release of the antigen if it is adsorbed. Therefore, high adsorption levels are usually favourable and the degree of adsorption should be determined [7].
Emulsions are widely known as potent adjuvant systems [8–10]. Oil-in-water emulsions contain metabolisable oil like squalene, e.g. MF59® (Novartis Vaccines and Diagnostics), which has been used extensively in influenza vaccines.
Two LPS-derivatives were also included in this study. LPS is a TLR4 ligand and stimulates a variety of cells to produce cytokines and chemokines that control dendritic cell trafficking and maturation. Because of its toxicity, LPS cannot be used as an adjuvant. However, chemically detoxified LPS, monophosphoryl lipid A (MPLA), is at least 100 times less toxic than LPS but retains strong adjuvant activity [11]. MPLA in combination with aluminium salts is present in the HPV vaccine Cervarix® and HBV vaccine Fendrix® (GlaxoSmithKline Biologicals). Genetically modified PagL LPS is obtained through the expression of the Bordetella bronchiseptica PagL gene in Neisseria meningitidis. PagL expression leads to Lipid A deacylation at the 3 position and a reduced toxicity compared to wild type LPS [12].
GVI3000 lacks genes for VEE structural proteins, which are provided in trans during production, but retains the information for replication [13]. GVI3000 is infectious, undergoes only a single round of replication and cannot further propagate. The viral RNA and/or partially dsRNA replication intermediates, may act as an adjuvant by stimulating the innate immune response in dendritic cells [14, 15] . In mouse models, GVI3000 enhances humoral and cellular responses in both systemic and mucosal compartments [8, 14].
To achieve dose sparing we tested the different adjuvant formulations with sIPV in the standard rat IPV potency test, which consists of one immunization, measuring VNTs in serum [16]. We also added a second immunization to determine the effect of boosting.
2. Materials and methods
2.1 Vaccine preparation
sIPV was produced under cGMP according to a slightly modified wild type (wt) IPV production process [2]. Sabin poliovirus (type 1: LSc 2ab KP2; type 2: P712 Ch2ab-KP2; type 3: Pfizer 457-III) virus was produced on Vero cells, grown on microcarriers in a bioreactor. Virus was purified by filtration, gel permeation chromatography and DEAE ion exchange chromatography (GE Healthcare). Formaldehyde inactivation was performed according to WHO requirements [17]. The vaccine was administered in 0.4–0.6–1.2 DU/rat for type 1, 2, and 3 respectively (unless stated differently) which corresponds with 10 ng for type 1, 50 ng for type 2, and 50 ng virus protein for type 3 [3].
2.2 Adjuvant formulations
Aluminium salts The adsorption behaviour of monovalent sIPV to aluminium phosphate (Adjuphos 2%, Brenntag, Denmark) and aluminium hydroxide (Alhydrogel 2%, Brenntag, Denmark) was determined as a function of temperature (4°C and 22°C), incubation time (0.5–16 h), NaCl concentration (25-144-500 mM) and phosphate concentration (1-10-100 mM). Prior to adsorption the D-antigen concentrations of the individual monovalent Sabin IPV batches were diluted to 10-16-32 DU/ human dose for type 1, 2, and 3, respectively, and adjusted to pH 7. Adsorption was determined by measuring the D-antigen in supernatant of pelleted vaccine. D-antigen concentrations were measured with biosensor analysis [3]. For immunization studies, aluminium salt was diluted with vaccine medium to 0.5 mg aluminium salt per dose.
Oil in water (o/w) emulsion #1 (EM030) was manufactured at IDRI, Seattle, USA [18] to contain 10% (v/v) squalene, 1.8% (v/v) glycerol, 1.9% (w/v) egg phosphatidylcholine and 0.09% (w/v) Pluronic® F68 in 25mM ammonium phosphate buffer, pH ~5.5. Particle size (dynamic light scattering (DLS), was ~95 nm (polydispersity index (PI) 0.04). The emulsion was diluted with vaccine medium to 2% (v/v) squalene and mixed gently for 30 seconds prior to immunization. The pH of the final vaccine was ~6.9.
O/w emulsion #2 (SWE02) was manufactured by the Vaccine Formulation Laboratory [19, 20] at the University of Lausanne (Epalinges, Switzerland) and contained 3.9% (w/v) squalene, 0.5% (w/v) Tween® 80 and 0.5% (w/v) Span® 85 in 10mM citrate buffer. The particle size was as determined by DLS 140±3 nm (PI 0.07). The emulsion was diluted 1:1 with antigen and mixed gently for 30 seconds prior to immunization. The pH of the final vaccine was ~6.5.
PagL LPS was generated at RIVM as decribed previously [12]. PagL LPS concentration in the vaccine was 1 μg/vaccine dose.
Monophosphoryl Lipid A (MPLA) (InvivoGen, San Diego, USA) was added to the vaccine at a final concentration of 1 μg/vaccine dose prior to immunization.
GVI3000 replicon particles were manufactured at Global Vaccines Inc. (Research Triangle Park, NC 27709, United States) [13, 21, 22]. GVI3000 was produced by electroporation of BHK-21 cells with the replicon genome along with two helper RNAs. The replicon particles used in this study were packaged in the wild-type (V3000) envelope [23]. After purification, the absence of detectable propagation-competent virus was confirmed by cytopathic effect assay. GVI3000 was titered by immunofluorescence of VEE non-structural proteins in infected BHK-21 cells. Prior to immunization GVI3000 was mixed with antigen-medium solution in a final concentration of 105 infectious units (IU)/vaccine. GVI3000 was previously referred to as VRP (Tonkin in Vaccine 2010 and 2012).
2.3 Immunizations
Outbred Rivm:TOX rats, 175–250g, were used. The experiments were performed using males and females (1:1) which are evenly distributed between all groups. Groups of 14–16 rats were immunized twice 4 weeks apart, intramuscularly (i.m.) with 100μl in the hind limb.
The animals were bled 21 days after the first immunization by orbital puncture and 21 days after the boost immunization by heart puncture. Sera were stored at −20°C.
2.4 Virus neutralization assay
Neutralizing antibodies against all three poliovirus types were measured separately inoculating Vero cells with 100 CCID50 of the wild-type strains (Mahoney, MEF-1 and Saukett). The sera were inactivated at 56°C for 30 minutes prior to testing. Two-fold serial dilutions were made from 21 (1:2) to 224 (1:16777216). Serum/virus (100 CCID50/50 μl) mixtures were incubated 3 hours at 36°C and 5% CO2 and subsequently overnight at 5°C. 50 μl 2×105 Vero cells/ml was added. After 7 days of incubation (36°C and 5% CO2) the plates were stained and fixed with crystal violet and the results were read macroscopically. Virus neutralizing titers were expressed as the last serum dilution that has an intact monolayer (no signs of cytopathogenic effect)[24].
2.5 Serological testing
Serology was performed for sIPV type 2 only. The poliovirus-specific IgG subclasses were determined with a sandwich ELISA. Biosensor analysis was used to determine the dissociation rate of poliovirus-specific rat sera. More detailed descriptions of the serological assays are provided as supplementary material and methods.
2.6 Statistical analysis
The Mann-Whitney two-tailed test was used for statistical analysis (GraphPad Prism 5.00 for Windows (GraphPad Software, San Diego, USA). p values < 0.05 were considered significant.
3. Results
3.1. Aluminium phosphate or aluminium hydroxide
Adsorption to aluminium hydroxide was 100% for all serotypes at low phosphate concentration, whereas temperature, time and NaCl-concentration had no effect in the range of parameters tested here. Adsorption to aluminium phosphate was serotype-dependent, pH-dependent and often incomplete (results not shown). Importantly, the potency of sIPV was affected by the degree of adsorption (Table I). At pH 7 the relative potency of sIPV as measured in the regular rat potency test [16], formulated with either aluminium phosphate or aluminium hydroxide was approximately 3-fold increased for type 1 and type 2. Also the potency of type 3, formulated with aluminium hydroxide increased by a factor of two. However, aluminium phosphate had no effect on type 3 potency. This is in line with the degree of adsorption of polio type 1, 2 and 3: all three types adsorbed 100% to aluminium hydroxide. Type 3 did not adsorb to aluminium phosphate at all and type 2 adsorbed 43±9% (n=3) to aluminium phosphate. Based on these results further work was done with aluminium hydroxide.
Table I.
Relative potency of plain or adsorbed sIPVa against WHO reference Pu91-01 (40-8-32 DU/dose) with a potency of 1 for types 1, 2, and 3, respectively
| DU/dose | Relative potency (95% confidence interval)b |
|||||
|---|---|---|---|---|---|---|
| Type | Nominal | Plain | + Aluminium Phosphate | Adsorption to Aluminium Phosphate (%) | + Aluminium Hydroxide | Adsorption to Aluminium Hydroxide (%) |
| Type 1 | 20 | 1.63 | 5.16 | 100 | 4.92 | 100 |
| (0.65 – 3.62) | (3.02 – 10.41) | (2.48 – 9.11) | ||||
| Type 2 | 40 | 0.12 | 0.38 | 43 ± 9c | 0.40 | 100 |
| (0.06 – 0.20) | (0.26 – 0.59) | (0.25 – 0.64) | ||||
| Type 3 | 32 | 0.97 | 0.80 | 0 | 1.60 | 100 |
| (0.57 – 1.67) | (0.51 – 1.23) | (1.19 – 2.17) | ||||
Experimental vaccine
The relative potency was calculated with the parallel line method. A relative potency of 1 means equal potency (per DU) as compared to the reference preparation
(n=3)
For a good comparison of the enhancing effect of the different adjuvants we have chosen a vaccine dose that is low without adjuvant in the linear region of the dose response curve (0.4, 0.6, and 1.2 DU for sIPV type 1, 2 and 3, respectively) except for the aluminum hydroxide experiments. Because these batches were part of a clinical trial, the potency of these batches was tested in the regular rat potency test with several doses of D-antigen (fig. 1). A dose response could be seen with sIPV although the VNTs were not significantly different between the highest doses of type 1 (10 DU and 3.3 DU), type 2 (16 DU and 5.3 DU) and type 3 (32 DU and 10.7 DU) (fig. 1). The adjuvanted sIPV induced a significantly higher VNT than plain sIPV for type 2 at all doses and for serotype 3 at a dose of 3.6 DU. Aluminium hydroxide had no significant effect on sIPV type 1 although the adjuvanted type 1 VNTs tended to be higher than the VNTs of plain sIPV type 1.
Fig. 1.
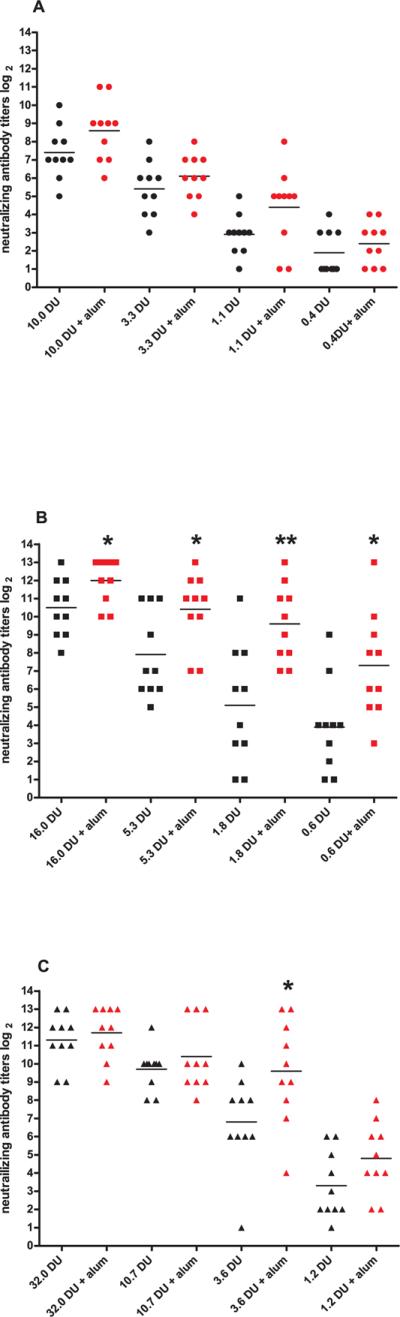
(wt polio)Virus neutralizing antibody titers from rats (n=10/group) 21 days after one immunization with various doses of inactivated Sabin polio vaccine (with or without aluminium hydroxide) type 1 (A), type 2 (B), and type 3 (C), respectively. Each symbol represents the titer of one individual rat and means are indicated by horizontal bars. Sera that were positive at the highest dilution (1:4096), i.e. titer is equal or larger than 13 are represented as 13(log2); sera that were negative at the 1:2 dilution are designated as 1. Statistical significance to sIPV was determined by the Mann-Whitney two-tailed test using GraphPad Prism version 5. *P <0.05, **P <0.01.
3.2 Oil in water (o/w) emulsion #1 (EM030)
Rats (15 per group) were immunized twice at an interval of 28 days with 0.4, 0.6, and 1.2 DU for sIPV type 1, 2 and 3, respectively. Sera obtained at day 21 showed that emulsion #1 addition to sIPV significantly enhanced the VNT response for antibodies against serotype 2 and 3 but not antibodies against serotype 1 (Fig. 2A). A clear booster effect was seen at day 49 for both plain and adjuvanted sIPV. Although VNT levels at day 49 were already enhanced for plain sIPV (group means of about 29 or higher, Fig. 2B), for adjuvanted sIPV type 2 and 3 the increase of VNTs was still significant.
Fig. 2.
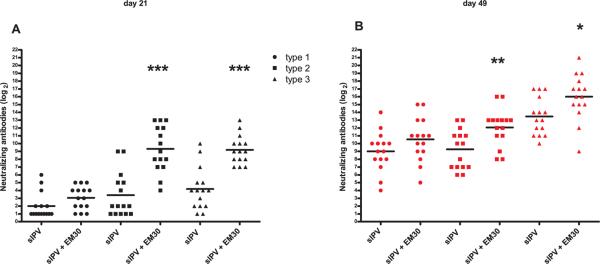
(wt polio)Virus neutralization antibody titers from rats (n=15/group) 21 days following a prime (A) and boost (B) immunization (day 28) with 0.4–0.6–1.2 DU/rat for sIPV type 1, 2 and 3 (with or without EM030), respectively. Each symbol represents the titer of one individual rat and means are indicated by horizontal bars. Sera that were negative at the 1:2 dilution are designated as 1. Statistical significance to sIPV was determined by the Mann-Whitney two-tailed test using GraphPad Prism version 5. *P <0.05, **P <0.01, ***P<0.001.
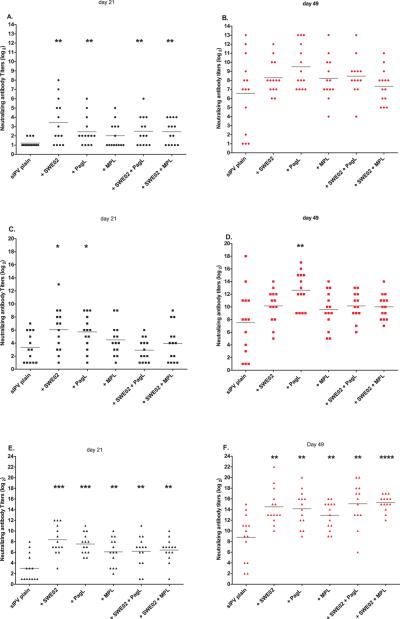
3.3 O/w emulsion #2 (SWE02), PagL LPS, MPLA
sIPV was mixed with PagL LPS or MPLA or SWE02 or with combinations of these adjuvants. Figure 3 shows a significant increase in VNT on day 21 against all three serotypes when rats were immunized with sIPV and SWE02 or PagL LPS. The increase in VNT was not significantly different between these two types of adjuvant. The combination o/w emulsion and PagL LPS or MPLA did not enhance the virus neutralizing antibody titers further.
Fig. 3.
(wt polio)Virus neutralization antibody titers against poliovirus type 1 (A, B), type 2(C, D) or type 3 (E, F) from rats (n=14/group) 21 days following a prime (A, C, E) and boost (B, D, F) immunization (day 28) with 0.4–0.6–1.2 DU/rat sIPV type 1, 2 and 3 respectively (with or without adjuvant). Each symbol represents the titer of one individual rat and means are indicated by horizontal bars. Sera that were negative at the 1:2 dilution are designated as 1. Statistical significance to sIPV was determined by the Mann-Whitney two-tailed test using GraphPad Prism version 5. *P <0.05, **P <0.01, ***P<0.001, ****P<0.0001.
Post-boost VNTs on day 49 were clearly increased for all adjuvants with sIPV and sIPV alone compared to day 21 (Fig.3B, 3D and 3F). After boost, VNTs of all adjuvanted sIPV groups were significantly higher than plain sIPV for type 3, PagL LPS for type 2, but none for type 1. Differences between the kind of adjuvant or between single adjuvant and combinations of adjuvants were not significant (one-way analysis of variance, α=0.05).
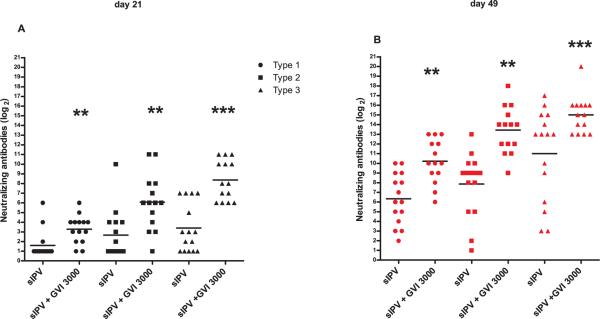
3.4 GVI 3000
sIPV was also mixed with 105 I.U. GVI3000 adjuvant in a prime and boost rat potency experiment identical in design to Figures 2, 3, and 4. GVI3000 was the only adjuvant that significantly enhanced the VNTs to all three poliovirus serotypes at both day 21 and day 49 compared to vaccine alone (Fig. 4). At day 21 VNTs increased 2-8-32 fold for type 1, 2 and 3 respectively. The increase in VNT was even higher at day 49, from 16-fold (type 1 and 3) to 32-fold (type 2). In addition, a clear booster effect was seen for both plain and adjuvanted sIPV at day 49 compared to day 21.
Fig. 4.
(wt polio)Virus neutralization antibody titers against poliovirus type 1, type 2 or type 3 from rats (n=14/group) 21 days following a prime (A) and boost (B) immunization (day 28) with 0.4-0.6-1.2 DU/rat sIPV type 1, 2 and 3 respectively (with or without GVI3000). Each symbol represents an individual titer and the line indicates the group mean. Statistical significance relative to plain sIPV was determined by the Mann-Whitney two-tailed test using GraphPad Prism version 5. *P <0.05, **P <0.01, ***P<0.001.
3.5 Serology
Rat sera of the experiment in which sIPV type 2 was mixed with PagL LPS or MPLA and/or SWE02 were serologically tested. IgG2b titers were significantly enhanced after administration of o/w emulsion in rat sera at both day 21 and day 49 (Supplemental Fig. S1A, B). PagL LPS induced significantly more IgG1 and IgG2a after prime and boost immunization (Fig. S1E–H). PagL LPS and MPLA and mixtures of these adjuvants with SWE02 boosted the IgG2c response after prime immunisation. After boost immunisation IgG2c titers were also significantly enhanced by PagL but significantly decreased by SWE02+MPL (Supplemental Fig.S1C, D).
The real-time binding of serum antibodies to antigen allows monitoring the association and dissociation of antibodies. Antibody avidity was estimated by measuring percentage loss of antibodies bound to immobilized type 2 wt polio vaccine using a biosensor analysis. Surprisingly, at day 49 a higher loss of antibody binding was found as compared to day 21 which was significant (P<0.05) for the SWE02 group and the SWE02+PagL LPS group (p<0.0001), indicating that antibodies induced by a boost immunization tend to have a lower avidity than antibodies after prime immunization (Supplemental Fig. S2). However, the poliovirus specific-antibodies induced by adjuvanted IPV tend to be more avid than antibodies induced by sIPV alone.
4. Discussion
Six adjuvants have been compared in this study. Table 2 shows a summary of the adjuvant effect of the investigated adjuvants. The increase in VNT of the aluminum hydroxide adjuvanted sIPV (1-, 8-, and 4-fold for type 1, 2, and 3 sIPV respectively), is more or less in agreement with previous findings [4, 6]. The adjuvant effect of MPLA was similar to that of aluminium hydroxide. In the experiments with o/w emulsions (EM030 and SWE02), we saw significantly higher responses compared to plain sIPV to all three serotypes after a prime immunization at low doses of adjuvanted sIPV. By further serum dilution, we saw that EM030 induced a 60-fold increase in anti-type 2 antibody titers after prime immunization compared to 2- to 11-fold increase with other adjuvants. SWE02 however, induced a mere 6-fold increase of anti-type 2 antibody titer (Table 2). Baldwin et al [4] used the same o/w emulsions in their study with wt IPV. In that study, stable emulsion (SE), which is the same as EM030, also induced greater antibody titers against type 2 than EM1, which is the same as SWE02. O/w emulsions like EM030 and SWE02 have all different components and their adjuvanticity usually varies a lot with the type of antigen, buffer, pH, route of administration, animal model etc.[18, 25–27]. The adjuvant effect at day 21 of SWE02, PagL LPS, and GVI3000 are close together with fold increases of 2–5, 5–11 and 24–42 for Sabin type 1, 2 and 3, respectively.
Table II.
Increase in VNTa adjuvanted sIPV compared with plain sIPV
| day 21 x--fold | day 49 x--fold | ||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Rat Experiment | Adjuvant/plain | Type 1 | Type 2 | Type 3 | Type 1 | Type 2 | Type 3 |
| 1 | Al(OH)3b | 1 | 8*c | 4 | - | - | - |
|
| |||||||
| 2 | Plain sIPV | 1 | 1 | 1 | 128d | 60 | 630 |
| EM030 | 2 | 60* | 32* | 384 | 420* | 3780* | |
|
| |||||||
| 3 | Plain sIPV | 1 | 1 | 1 | 42 | 17 | 56 |
| SWE02 | 5* | 6* | 42* | 126 | 102 | 2912* | |
| PagL LPS | 2* | 5* | 24* | 294 | 578* | 2352* | |
| MPLA | 2 | 2 | 9* | 210 | 68 | 952* | |
|
| |||||||
| 4 | Plain sIPV | 1 | 1 | 1 | 26 | 37 | 194 |
| GVI3000 | 3* | 11* | 32* | 390* | 1813* | 3104* | |
The increase in VNTs of the adjuvanted groups is based on the difference between the mean 2log score of the adjuvanted group at day 21 and 49, respectively, and the mean 2log score of the plain sIPV group at day 21
For the aluminium hydroxide experiment we used the data obtained with 0.4 −0.6 −1.2 DU for serotype 1, 2, and 3, respectively
The asterisks depict the significant differences of the adjuvanted groups compared to plain sIPV at day 21 and day 49, respectively
The increase in VNT of plain sIPV at day 49 is based on the difference between the mean 2log score of the plain sIPV group at day 49 and the mean 2log score of the plain sIPV group at day 21
Although PagL LPS caused significant increases in VNTs which was comparable to the immunogenicity responses caused by o/w emulsions, combinations of PagL LPS or MPLA in o/w emulsion did not induce higher antibody responses than the single adjuvants. Interestingly, Garçon et al [28] showed that the formulation of malaria-antigen with o/w emulsion in combination with 3D-MPL was also not significantly different from the formulation of malaria vaccine with o/w emulsion alone. Only, when QS21 was added to the formulation the adjuvant system (AS02: o/w, 3D-MPL and QS21) was capable of eliciting high antibody titers.
OPV, better than IPV/sIPV, can induce mucosal immunity to poliovirus infection in the gut, which also helps prevent transmission [29]. The GVI3000 adjuvant uniquely induces mucosal immunity in mice even when delivered intramuscularly with IPV [Jorquera, manuscript in preparation];[13]. Thus, a GVI3000-sIPV vaccine may be ideal to replace OPV without losing the ability to induce mucosal immunity and additionally allowing for antigen sparing of sIPV.
Contrary to the finding of Baldwin et al [4] a second administration of plain sIPV boosted the VNT response enormously. In addition, a clear enhancing effect of the adjuvants of experiment 2–4 was seen after boost (Table 2).The variation in VNT response after a boost of sIPV alone between three experiments was substantial. This may be attributed to a large animal-to-animal variability (the use of outbred rats).
Aluminium salts and oil-in-water emulsions are usually considered as Th2-adjuvants ([8, 30] whereas MPLA and PagL LPS are usually more Th1-inducing adjuvants [8, 31]. In the rat IgG1 antibodies can be assigned to a Th2- and IgG2b and IgG2c to a Th1-dependent immune response, where IgG2a is also indicative of a Th1 pathway [32, 33]. Remarkably, in our study it is just the opposite: oil-in water emulsion SWE02 boosted a polio-specific IgG2b response (Supplemental Fig. S1A–B) where PagL LPS boosted a clear IgG1 and IgG2a response (Supplemental Fig. S1 E–H). As little is known about poliovirus specific T cell responses in rats, further research will be necessary to determine the relative roles of antibodies and the influence of adjuvants on cellular immunity in rats.
PagL LPS and GVI3000 are both adjuvants that have not yet been clinically tested but both may contribute to a wider choice of adjuvants in the future.
Summarizing, the results of this study indicate that it is feasible to increase the potency of sIPV by using adjuvants. Nevertheless, although we demonstrated that most of the adjuvants are dose sparing, since cost-reduction is a main issue in replacing OPV with sIPV a thorough investigation into production costs of each adjuvant is recommended.
Supplementary Material
Highlights
Six adjuvants with Sabin inactivated poliovirus vaccine were evaluated in rats
The increase of neutralizing antibodies was 3-,15-, 24-fold for type 1, 2 and 3
After boost the increase was another 7-, 20-, 27-fold
It is feasible to increase the potency of polio vaccine by adjuvants
Acknowledgements
The authors are grateful to Lonneke Levels for statistical support and Debbie Brugmans for technical assistance. This work was supported by the World Health Organization using funds provided by a grant from the Bill and Melinda Gates Foundation, by a U.S. National Institute of Health grant U01-AI070976 to GVI, and by the Bill and Melinda Gates Foundation grant #42387 to IDRI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Sutter RW, Caceres VM, Mas Lago P. The role of routine polio immunization in the post-certification era. Bull World Health Organ. 2004 Jan;82(1):31–9. [PMC free article] [PubMed] [Google Scholar]
- [2].Bakker WA, Thomassen YE, van't Oever AG, Westdijk J, van Oijen MG, Sundermann LC, et al. Inactivated polio vaccine development for technology transfer using attenuated Sabin poliovirus strains to shift from Salk-IPV to Sabin-IPV. Vaccine. 2011 Sep 22;29(41):7188–96. doi: 10.1016/j.vaccine.2011.05.079. [DOI] [PubMed] [Google Scholar]
- [3].Westdijk J, Brugmans D, Martin J, van't Oever A, Bakker WA, Levels L, et al. Characterization and standardization of Sabin based inactivated polio vaccine: proposal for a new antigen unit for inactivated polio vaccines. Vaccine. 2011 Apr 18;29(18):3390–7. doi: 10.1016/j.vaccine.2011.02.085. [DOI] [PubMed] [Google Scholar]
- [4].Baldwin SL, Fox CB, Pallansch MA, Coler RN, Reed SG, Friede M. Increased potency of an inactivated trivalent polio vaccine with oil-in-water emulsions. Vaccine. 2011 Jan 17;29(4):644–9. doi: 10.1016/j.vaccine.2010.11.043. [DOI] [PubMed] [Google Scholar]
- [5].Ivanov AP, Dragunsky EM, Chumakov KM. 1,25-dihydroxyvitamin d3 enhances systemic and mucosal immune responses to inactivated poliovirus vaccine in mice. J Infect Dis. 2006 Feb 15;193(4):598–600. doi: 10.1086/499970. [DOI] [PubMed] [Google Scholar]
- [6].Yang C, Shi H, Zhou J, Liang Y, Xu H. CpG oligodeoxynucleotides are a potent adjuvant for an inactivated polio vaccine produced from Sabin strains of poliovirus. Vaccine. 2009 Aug 31; doi: 10.1016/j.vaccine.2009.08.047. [DOI] [PubMed] [Google Scholar]
- [7].(EMEA) EMA. Committee for medicinal products for human use (CHMP) Guideline on adjuvants in vaccines for human use. 2004 EMEA/CHMP/VEG/134716/2004. [Google Scholar]
- [8].Wilson-Welder JH, Torres MP, Kipper MJ, Mallapragada SK, Wannemuehler MJ, Narasimhan B. Vaccine adjuvants: current challenges and future approaches. J Pharm Sci. 2009 Apr;98(4):1278–316. doi: 10.1002/jps.21523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Mutwiri G, Gerdts V, van Drunen Littel-van den Hurk S, Auray G, Eng N, Garlapati S, et al. Combination adjuvants: the next generation of adjuvants? Expert Rev Vaccines. 2011 Jan;10(1):95–107. doi: 10.1586/erv.10.154. [DOI] [PubMed] [Google Scholar]
- [10].Fox CB. Squalene emulsions for parenteral vaccine and drug delivery. Molecules. 2009;14(9):3286–312. doi: 10.3390/molecules14093286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ribi E, Amano K, Cantrell J. Preparation and antitumor activity of nontoxic lipid A. Cancer Immunol Immunother. 1982;12(2):91–6. [Google Scholar]
- [12].Arenas J, van Dijken H, Kuipers B, Hamstra HJ, Tommassen J, van der Ley P. Coincorporation of LpxL1 and PagL mutant lipopolysaccharides into liposomes with Neisseria meningitidis opacity protein: influence on endotoxic and adjuvant activity. Clin Vaccine Immunol. 2010 Apr;17(4):487–95. doi: 10.1128/CVI.00423-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Tonkin DR, Jorquera P, Todd T, Beard CW, Johnston RE, Barro M. Alphavirus replicon-based enhancement of mucosal and systemic immunity is linked to the innate response generated by primary immunization. Vaccine. 2010 Apr 19;28(18):3238–46. doi: 10.1016/j.vaccine.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Thompson JM, Whitmore AC, Konopka JL, Collier ML, Richmond EM, Davis NL, et al. Mucosal and systemic adjuvant activity of alphavirus replicon particles. Proc Natl Acad Sci U S A. 2006 Mar 7;103(10):3722–7. doi: 10.1073/pnas.0600287103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Tonkin DR, Whitmore A, Johnston RE, Barro M. Infected dendritic cells are sufficient to mediate the adjuvant activity generated by Venezuelan equine encephalitis virus replicon particles. Vaccine. 2012 Jun 22;30(30):4532–42. doi: 10.1016/j.vaccine.2012.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].van Steenis G, van Wezel AL, Sekhuis VM. Potency testing of killed polio vaccine in rats. Dev Biol Stand. 1981;47:119–28. [PubMed] [Google Scholar]
- [17].WHO. World Health Organisation Requirements for poliomyelitis vaccine (inactivated) World Health Organ Tech Rep Series. 2002;910:32e65. Annex 2. [Google Scholar]
- [18].Fox CB, Anderson RC, Dutill TS, Goto Y, Reed SG, Vedvick TS. Monitoring the effects of component structure and source on formulation stability and adjuvant activity of oil-in-water emulsions. Colloids Surf B Biointerfaces. 2008 Aug 1;65(1):98–105. doi: 10.1016/j.colsurfb.2008.03.003. [DOI] [PubMed] [Google Scholar]
- [19].Collin N, Dubois PM. The Vaccine Formulation Laboratory: a platform for access to adjuvants. Vaccine. 2011 Jul 1;29(Suppl 1):A37–9. doi: 10.1016/j.vaccine.2011.04.125. [DOI] [PubMed] [Google Scholar]
- [20].Ventura R, Brunner L, Heriyanto B, de Boer O, O'Hara M, Huynh C, et al. Technology transfer of an oil-in-water vaccine-adjuvant for strengthening pandemic influenza preparedness in Indonesia. Vaccine. 2012 Aug 7; doi: 10.1016/j.vaccine.2012.07.074. [DOI] [PubMed] [Google Scholar]
- [21].Pushko P, Parker M, Ludwig GV, Davis NL, Johnston RE, Smith JF. Replicon-helper systems from attenuated Venezuelan equine encephalitis virus: expression of heterologous genes in vitro and immunization against heterologous pathogens in vivo. Virology. 1997 Dec 22;239(2):389–401. doi: 10.1006/viro.1997.8878. [DOI] [PubMed] [Google Scholar]
- [22].Davis NL, Caley IJ, Brown KW, Betts MR, Irlbeck DM, McGrath KM, et al. Vaccination of macaques against pathogenic simian immunodeficiency virus with Venezuelan equine encephalitis virus replicon particles. J Virol. 2000 Jan;74(1):371–8. doi: 10.1128/jvi.74.1.371-378.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Davis NL, Powell N, Greenwald GF, Willis LV, Johnson BJ, Smith JF, et al. Attenuating mutations in the E2 glycoprotein gene of Venezuelan equine encephalitis virus: construction of single and multiple mutants in a full-length cDNA clone. Virology. 1991 Jul;183(1):20–31. doi: 10.1016/0042-6822(91)90114-q. [DOI] [PubMed] [Google Scholar]
- [24].Albrecht P, van Steenis G, van Wezel AL, Salk J. Standardization of poliovirus neutralizing antibody tests. Rev Infect Dis. 1984 May-Jun;6(Suppl 2):S540–4. doi: 10.1093/clinids/6.supplement_2.s540. [DOI] [PubMed] [Google Scholar]
- [25].Fox CB, Lin S, Sivananthan SJ, Dutill TS, Forseth KT, Reed SG, et al. Effects of emulsifier concentration, composition, and order of addition in squalene-phosphatidylcholine oil-in-water emulsions. Pharm Dev Technol. 2011 Oct;16(5):511–9. doi: 10.3109/10837450.2010.495397. [DOI] [PubMed] [Google Scholar]
- [26].Fox CB, Baldwin SL, Duthie MS, Reed SG, Vedvick TS. Immunomodulatory and physical effects of oil composition in vaccine adjuvant emulsions. Vaccine. 2011 Nov 28;29(51):9563–72. doi: 10.1016/j.vaccine.2011.08.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Fox CB, Baldwin SL, Duthie MS, Reed SG, Vedvick TS. Immunomodulatory and Physical Effects of Phospholipid Composition in Vaccine Adjuvant Emulsions. AAPS PharmSciTech. 2012 Mar 14; doi: 10.1208/s12249-012-9771-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Garcon N, Heppner DG, Cohen J. Development of RTS,S/AS02: a purified subunit-based malaria vaccine candidate formulated with a novel adjuvant. Expert Rev Vaccines. 2003 Apr;2(2):231–8. doi: 10.1586/14760584.2.2.231. [DOI] [PubMed] [Google Scholar]
- [29].Onorato IM, Modlin JF, McBean AM, Thoms ML, Losonsky GA, Bernier RH. Mucosal immunity induced by enhance-potency inactivated and oral polio vaccines. J Infect Dis. 1991 Jan;163(1):1–6. doi: 10.1093/infdis/163.1.1. [DOI] [PubMed] [Google Scholar]
- [30].Lambrecht BN, Kool M, Willart MA, Hammad H. Mechanism of action of clinically approved adjuvants. Curr Opin Immunol. 2009 Feb;21(1):23–9. doi: 10.1016/j.coi.2009.01.004. [DOI] [PubMed] [Google Scholar]
- [31].Steeghs L, Keestra AM, van Mourik A, Uronen-Hansson H, van der Ley P, Callard R, et al. Differential activation of human and mouse Toll-like receptor 4 by the adjuvant candidate LpxL1 of Neisseria meningitidis. Infect Immun. 2008 Aug;76(8):3801–7. doi: 10.1128/IAI.00005-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Gracie JA, Bradley JA. Interleukin-12 induces interferon-γ-dependent switching of IgG alloantibody subclass. Eur J Immunol. 1996;26(6):1217–21. doi: 10.1002/eji.1830260605. [DOI] [PubMed] [Google Scholar]
- [33].Jakel T, Khoprasert Y, Kliemt D, Mackenstedt U. Immunoglobulin subclass responses of wild brown rats to Sarcocystis singaporensis. Int J Parasitol. 2001 Mar;31(3):273–83. doi: 10.1016/s0020-7519(00)00172-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.