Abstract
Alcohol dependence (AD) is a common neuropsychiatric disorder with high heritability. A number of studies have analyzed the association between the Taq1A polymorphism (located in the gene cluster ANKK1/DRD2) and AD. In the present study, we conducted a large-scale meta-analysis to confirm the association between the Taq1A polymorphism and the risk for AD in over 18,000 subjects included in 61 case-control studies that were published up to August 2012. Our meta-analysis demonstrated both allelic and genotypic association between the Taq1A polymorphism and AD susceptibility [allelic: P(Z)=1.1×10−5, OR=1.19; genotypic: P(Z)=3.2×10−5, OR=1.24]. The association remained significant after adjustment for publication bias using the trim and fill method. Sensitivity analysis showed that the effect size of the Taq1A polymorphism on AD risk was moderate and not influenced by any individual study. The pooled odds ratio from published studies decreased with the year of publication but stabilized after the year 2001. Subgroup analysis indicated that publication bias could be influenced by racial ancestry. In summary, this large-scale meta-analysis confirmed the association between the Taq1A polymorphism and AD. Future studies are required to investigate the functional significance of the ANKK1/DRD2 Taq1A polymorphism in AD.
Keywords: Alcohol dependence, meta-analysis, ANKK1 and DRD2 gene cluster, the Taq1A polymorphism
Introduction
Dopamine plays a key role in alcohol dependence (AD) pathophysiology due to its involvement in reward behavior (Wise and Rompre 1989). Dysfunction of dopaminergic neurotransmission in the brain likely contributes to the pathophysiology of other neuropsychiatric disorders as well (Hummel and Unterwald 2002; Kienast and Heinz 2006). Family, twin, and adoption studies suggest that more than 50% of the population variance in AD is due to genetic factors (Prescott and Kendler 1999). Variation in genes encoding proteins involved in dopaminergic neurotransmission has been found to contribute to the risk for developing alcohol dependence, most likely through altered reward processing and sensitivity to alcohol exposure (Bontempi et al. 2007; Fiorentini et al. 2002). The dopamine receptor D2, encoded by the gene DRD2, is a presynaptic as well as postsynaptic G protein-coupled receptor located on dopaminergic neurons. Both in vivo and in vitro experiments have demonstrated that DRD2 is a susceptibility gene for AD (Kienast and Heinz 2006; Wise and Rompre 1989).
The Taq1A polymorphism [also known as rs1800497 (C/T)] is located in the gene cluster ANKK1/DRD2 on chromosome 11q23.2. The minor A1 allele of the Taq1A polymorphism (or the T allele of rs1800497) was found to be associated with a reduced number of dopamine binding sites in the brain (Pohjalainen et al. 1998). Altered D2 receptor expression due to the Taq1A polymorphism may confer vulnerability to substance (alcohol or drug) dependence and certain neuropsychiatric disorders. A number of studies have analyzed the association between this polymorphism and AD. Blum et al. (1990) investigated the association between the Taq1A polymorphism and AD in a sample of 35 alcoholics and 35 non-alcoholics and found an over eight-fold increased risk of AD in subjects carrying the A1 allele (or the T allele) of the Taq1A polymorphism. This finding was supported by several follow-up studies (Amadeo et al. 1993; Berggren et al. 2006; Comings et al. 1991; Hietala et al. 1997; Ovchinnikov et al. 1999; Parsian et al. 1991). Nevertheless, conflicting results have also been reported (Anghelescu et al. 2001; Bolos et al. 1990; Cook et al. 1992; Gelernter et al. 1991; Goldman et al. 1992; Sander et al. 1999). The above studies were mainly conducted in European (and European American) populations. Additionally, the association of the Taq1A polymorphism and AD was examined in Asian and other non-European populations; however, the results were negative (Arinami et al. 1993; Chen et al. 1996; Lee et al. 1997; Lu et al. 1996; Matsushita et al. 2001; Shaikh et al. 2001). As of 2006, over 40 studies had examined the potential role of the Taq1A polymorphism in AD, yielding inconsistent results.
Subsequently, three relatively large meta-analyses examined the association between the Taq1A polymorphism and AD by combing data from studies published between 1990 and 2006. Munafo et al. (2007) analyzed the data from 40 published studies including 4,962 alcoholic and 5,253 comparison controls, and found that the A1 allele of the Taq1A polymorphism conferred a moderate risk for AD in both European (OR=1.19) and East Asian (OR=1.17) populations. Smith et al. (2008) included over 9,000 participants from 44 published studies, and found that subjects with the presence of the A1 allele of the Taq1A polymorphism (i.e., carrying genotype A1A1 or A1A2) had a significantly higher risk of AD than those with absence of the A1 allele (i.e., carrying genotype A2A2). Le Foll et al. (2009) re-analyzed the data from 5,395 patients and 4,304 controls recruited for 40 published studies and observed similar results. The three meta-analyses provided further evidence of a moderate effect of the Taq1A polymorphism on the risk for AD. They also demonstrated a significant between-study heterogeneity and publication bias, which could possibly be explained by different ethnic backgrounds or lacking of ethnic-matched controls.
Since 2006, 16 new studies evaluating the association of the Taq1A polymorphism with AD have been published. These studies included 7,756 new subjects (3,807 cases and 3,949 controls). However, no further meta-analyses have been conducted to investigate whether the association between the Taq1A polymorphism and AD remains significant. In the present study, we performed a large-scale meta-analysis to validate the association between the Taq1A polymorphism and AD by including data from studies that were published from 1990 until now (August 2012).
Methods
Literature search and inclusion of eligible studies
Studies that investigated the association of the TaqA1 polymorphism with AD were selected from the electronic database PubMed/MEDLINE (the US National Library of Medicine) and included in the present meta-analysis. They were published from 1990 to August, 2012. The search strategy was based on the following terms: “dopamine receptor D2”, “DRD2”, “alcohol”, “alcoholics”, “alcohol dependence”, “association”, and “associated”. Abstracts of studies retrieved from the primary search were then browsed to see whether these studies analyzed the association between the Taq1A polymorphism and AD.
Once the published studies were selected, all reference papers (including reviewed papers) that were cited in these studies were further examined to identify additional articles that were not indexed by the PubMed/MEDLINE database. Duplications were discarded. Studies were considered to be eligible for the present meta-analysis if the following information was available: (1) a valid diagnostic tool (e.g., DSM-III-R, DSM-IV, and ICD-10) for AD; (2) genotyping methods [e.g., the restriction fragment length polymorphism (RFLP) technique or the TaqMan Method] were described; (3) genotyping data of the Taq1A polymorphism were provided to calculate odds ratios (ORs) and 95% confidential intervals (95% CIs); and (4) consent forms from study subjects and ethics approvals from local committees were obtained. If two studies contained overlapping samples, the study with a larger sample size was kept for this meta-analysis.
Data extraction
For each eligible study, the following data were extracted using standard forms: (1) authors and publication year; (2) ancestry or race information; (3) diagnostic criteria for alcoholics and comparison controls; (4) number of patients and comparison controls; (5) genotyping data of the Taq1A polymorphism in case and control groups; (6) mean age and sex ratio in case and control groups; and (7) statement of hardy-Weinberg equilibrium (HWE) test. In our meta-analysis, ancestry or race was coded as European, Asian or Others (American Indians, mixed population, or not stated).
Statistical analysis
Allelic and genotypic data from eligible studies were summarized in two by two tables for meta-analyses. Odds ratios (ORs) and 95% confidential intervals (95% CIs) were calculated for individual studies using Pearson’s Chi-squared test. Since previous studies found a significant effect of the A1 allele of the Taq1A polymorphism on risk for AD, we carried out both allelic [the A1 allele (or the T allele) vs. the A2 allele (or the C allele)] and genotypic (dominant model: A1A1+A1A2 vs.A2A2) meta-analysis using data from all eligible studies.
We also performed subgroup analyses stratified by ancestry (i.e., European or Asian) or diagnostic criteria (i.e., DSM-III-R, DSM-IV, or ICD-10). Heterogeneity between studies was estimated using two methods (Kienast and Heinz 2006): the Cochran’s Q test and the I-square (I2) test. Cochran’s Q test was calculated as the weighted sum of squared differences between individual study effects and the pooled effect across studies, weighting the contribution of each study by its inverse variance; the Q statistics followed a chi-square distribution with n-1 degrees of freedom, where n was the number of studies. The I2 statistic [I2 = 100% × (Q−df)/Q] described the percentage of variation across studies due to heterogeneity rather than chance. It indicated an evidence of heterogeneity between studies if I2 ≥40%.
When between-study heterogeneity existed, the random effect model (or the DerSimonian and Laird method) was applied (DerSimonian and Laird 1986); otherwise, the fixed model (the Mantel-Haenszel method) was applied (DerSimonian and Laird 1986). The significance of the pooled ORs was examined by the Z-test. In order to check whether the result was excessively influenced by any individual study, sensitivity analyses were conducted by repeating the meta-analysis with one study being omitted at a time. To assess publication bias, the graphic method (or the funnel plot) (Egger et al. 1997; Galbraith 1988) was used. If there was evidence of publication bias, the trim and fill method (Duval and Tweedie 2000) was applied to adjust the meta-analysis results by imputing data from presumed missing studies.
Results
Description of eligible studies
A total of 504 articles were retrieved. Sixty-seven studies met the inclusion criteria for this meta-analysis. Three studies (Cruz et al. 1995; Karaoguz et al. 2004; Yang et al. 2007) were excluded because genotyping data were not available. Three other studies (Comings 1998; Konishi et al. 2004a; Noble et al. 1991) were also excluded because of sample overlapping with previous studies. After filtration, 61 studies (refer to Supplementary Table S1) were considered eligible for the present meta-analysis. Among these 61 studies, 36 analyzed the association between the TaqA1 polymorphism and AD in the European population (Amadeo et al. 1993; Amadeo et al. 2000; Anghelescu et al. 2001; Bau et al. 2000; Berggren et al. 2006; Bolos et al. 1990; Comings et al. 1991; Comings et al. 1994; Cook et al. 1992; Finckh et al. 1996; Foley et al. 2004; Freire et al. 2006; Geijer et al. 1994; Gelernter and Kranzler 1999; Gelernter et al. 1991; Goldman et al. 1992; Gorwood et al. 2000a; Gorwood et al. 2000b; Heinz et al. 1996; Hietala et al. 1997; Kasiakogia-Worlley et al. 2011; Konishi et al. 2004b; Kovanen et al. 2010; Kraschewski et al. 2009; Landgren et al. 2011; Lawford et al. 1997; Limosin et al. 2002; Ovchinnikov et al. 1999; Parsian et al. 1991; Pastorelli et al. 2001; Ponce et al. 2008; Samochowiec et al. 2008; Samochowiec et al. 2000; Sander et al. 1995; Sander et al. 1999; Schellekens et al. 2012); 18 analyzed the association between the TaqA1 polymorphism and AD in the Asian population (Arinami et al. 1993; Bhaskar et al. 2010; Chen et al. 1996; Chen et al. 1997; Huang et al. 2007; Ishiguro et al. 1998; Joe et al. 2008; Kono et al. 1997; Lee et al. 1997; Lu et al. 1996; Lu et al. 2010; Lu et al. 2001; Matsushita et al. 2001; Namkoong et al. 2008; Prasad et al. 2010; Shaikh et al. 2001; Wang et al. 2007; Wu et al. 2008); and seven analyzed the association between the TaqA1 polymorphism and AD in American Indians (Goldman et al. 1993; Goldman et al. 1997), a mix population (Blum et al. 1991; Blum et al. 1990; Sakai et al. 2007), or other populations that were not stated (Neiswanger et al. 1995; Noble et al. 1994).
A total of 9,590 alcoholic cases and 9,140 comparison controls recruited from the above 61 case-control studies were included in the present meta-analysis. The frequency of the minor A1 allele of the Taq1A polymorphism varied substantially in different populations. In European control subjects, the frequency of the A1 allele was about 19% (6% – 44%), which was significantly lower than that in the Asian control subjects (about 38%, 22% – 47%; P <0.001, data not show). Moreover, both European and Asian alcoholic subjects showed a higher frequency of the A1 allele (European, 22%, 12–45%; Asian, 42%, 27–51%) when compared with their respective ethnic control subjects. Additionally, in two American Indian studies, the A1 allele was the major allele of the Taq1A polymorphism (cases, 58–66%; controls, 61–67%).
Meta-analysis results
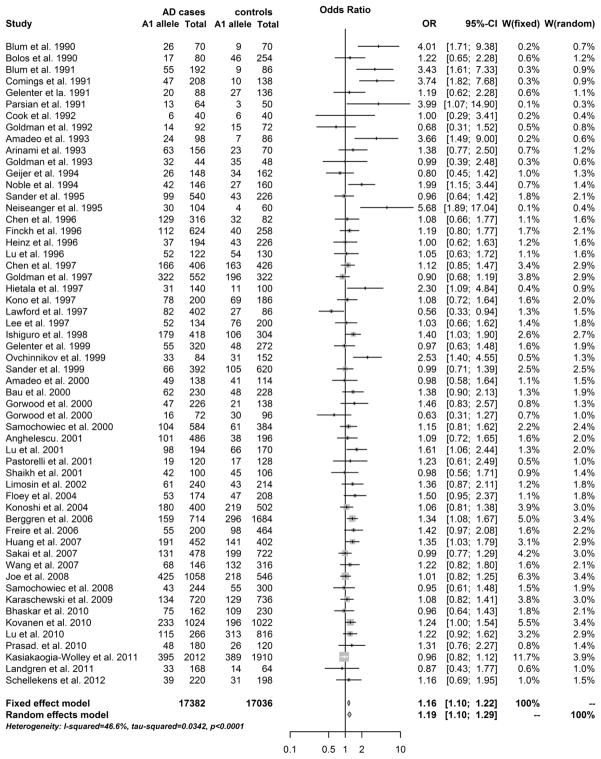
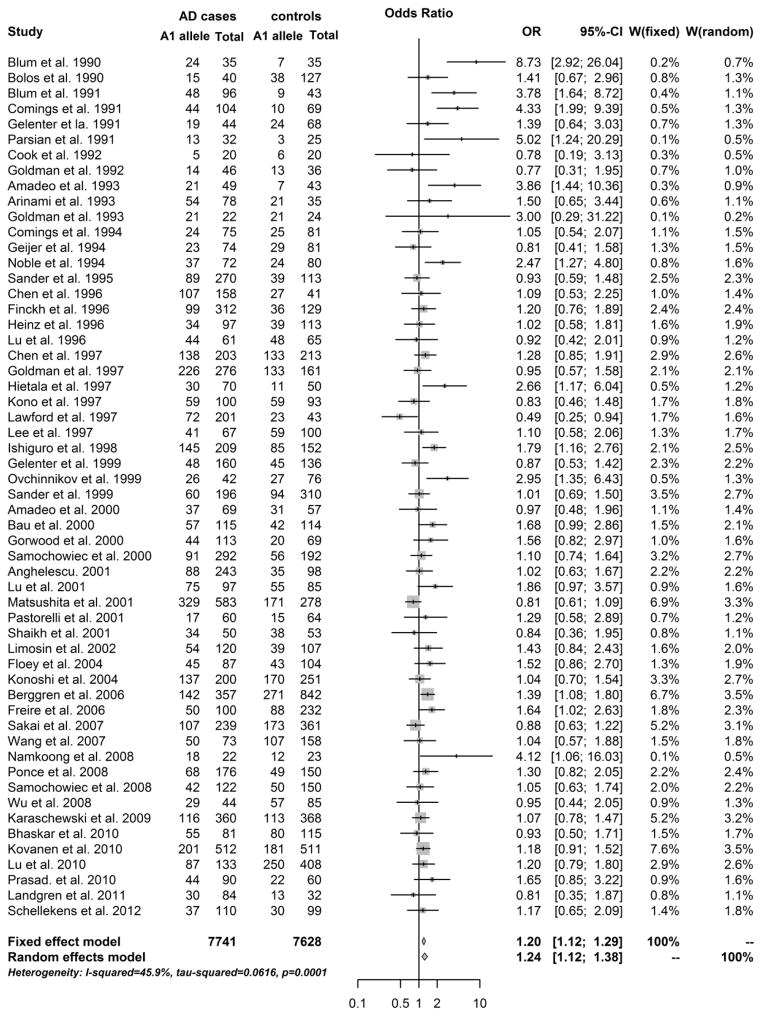
The allelic and genotypic meta-analysis results are summarized in Table 1. Among the 61 studies included in this meta-analysis, five studies only had allelic data (A1 vs. A2) and five studies only had genotypic data (A11+A12 vs. A12). Other 51 studies had both allelic and genotypic data. Thus, 56 studies were included in allelic meta-analysis. The OR of individual studies and the pooled OR are shown in a forest plot (Figure 1). There was strong evidence of heterogeneity between studies [P(Q) < 0.0001, I2 = 46.6%]. The random effects model was therefore applied in the data analysis. The A1 allele showed a significant association with AD [Z = 4.41, P(Z) = 1.1 × 10−5, OR = 1.19] (Table 1). Similarly, 56 studies were included in genotypic meta-analysis. The OR of individual studies and the pooled OR are shown in a forest plot (Figure 2). There was also strong evidence of heterogeneity between studies [P(Q) < 0.0001, I2 = 45.9%]. Under the random effects model, subjects carrying the A1 allele (or with genotype A1A1 or A1A2) showed a higher risk for AD compared to subjects without the allele A1 (i.e., with genotype A2A2) [Z = 4.16, P(Z) = 3.2 × 10−5, OR = 1.24] (Table 1).
Table 1.
Results of meta-analyses including all and subgroup studies stratified by ancestry and diagnosis criteria
| na | Zb | P(Z)c | OR (95%CI)d | P(Q)e | I2 (95%CI)f | P(B)g | |
|---|---|---|---|---|---|---|---|
| Allelic association analysis (A1 vs. A2): | |||||||
| All studies h | 56 | 4.41 | 1.1 × 10−5 | 1.19 (1.10–1.29) | <0.0001 | 46.6 (26.8–61.0) | 0.010 |
| Allelic studies with diagnosis 1i | 51 | 4.34 | 1.4 × 10−5 | 1.21 (1.11–1.31) | <0.0001 | 47.2 (26.7–62.0) | 0.054 |
| Allelic studies with diagnosis 2j | 47 | 5.45 | < 6.0 × 10−7 | 1.18 (1.11–1.25) | <0.0001 | 49.2 (28.8–63.8) | 0.069 |
| European studies | 34 | 2.93 | 0.003 | 1.16 (1.06–1.29) | 0.002 | 45.7 (18.7–63.8) | 0.183 |
| Allelic studies with diagnosis 1i | 30 | 2.71 | 0.007 | 1.17 (1.05–1.32) | 0.003 | 46.4 (17.7–65.1) | 0.681 |
| Allelic studies with diagnosis 2j | 27 | 2.19 | 0.029 | 1.15 (1.02–1.31) | 0.003 | 47.8 (18.2–66.7) | 0.856 |
| Asian studies | 15 | 3.32 | 9.0× 10−4 | 1.17 (1.07–1.28) | 0.813 | 0.0 (0.0–30.0) | 0.794 |
| Allelic studies with diagnosis 1i | 14 | 3.46 | 5.0× 10−4 | 1.18 (1.07–1.30) | 0.824 | 0.0 (0.0–29.5) | 0.633 |
| Allelic studies with diagnosis 2j | 13 | 3.52 | 4.0× 10−4 | 1.19 (1.08–1.31) | 0.797 | 0.0 (0.0–33.6) | 0.427 |
| Genotypic association analysis (A1A1+A1A2 vs. A2A2): | |||||||
| All studies h | 56 | 4.16 | 3.2 × 10−5 | 1.24 (1.13–1.38) | 0.0001 | 45.9 (25.9–60.6) | 0.014 |
| Genotypic studies with diagnosis 1i | 52 | 4.02 | 5.8 × 10−5 | 1.25 (1.13–1.46) | <0.0001 | 49.1 (29.7–63.1) | 0.016 |
| Genotypic studies with diagnosis 2j | 48 | 3.66 | 3.0 × 10−4 | 1.24 (1.10–1.39) | <0.0001 | 50.6 (31.0–64.6) | 0.034 |
| European studies | 34 | 3.42 | 6.0 × 10−4 | 1.23 (1.09–1.38) | 0.0187 | 36.6 (3.80–58.2) | 0.316 |
| Genotypic studies with diagnosis 1i | 31 | 3.13 | 0.002 | 1.23 (1.08–1.40) | 0.009 | 41.4 (10.0–61.9) | 0.375 |
| Genotypic studies with diagnosis 2j | 28 | 2.69 | 0.007 | 1.21 (1.05–1.39) | 0.011 | 42.3 (9.5–63.2) | 0.677 |
| Asian studies | 16 | 1.73 | 0.083 | 1.13 (0.98–1.29) | 0.186 | 23.6 (0.0–58.0) | 0.191 |
| Genotypic studies with diagnosis 1i | 15 | 1.83 | 0.067 | 1.14 (0.99–1.31) | 0.156 | 27.2 (0.0–60.8) | 0.175 |
| Genotypic studies with diagnosis 2j | 14 | 1.93 | 0.538 | 1.15 (0.99–1.32) | 0.132 | 30.5 (0.0–63.3) | 0.129 |
Number of studies included.
Statistics of Z test.
Significance of pooled effect size.
Odds ratio with 95 % confidential interval.
Significance of heterogeneity test between studies using Cochran’s Q test.
I2 statistic with 95 % confidential interval (test of heterogeneity between studies).
Significance of publication bias test.
Studies including all eligible European, Asian and other studies
Subjects were screened using stated diagnostic criteria.
Subjects were screened using the standard DSM-III-R, DSM-IV, or ICD10 diagnostic tool.
Fig. 1. Forest plot of allelic meta-analysis results across all studies.
The counts of the A1 allele and total alleles of the Taq1A polymorphism in alcohol dependence (AD) and control groups from eligible studies were presented by rows. The bars with squares in the middle represent 95% confidence intervals (CIs) and odds ratios (ORs). The central vertical solid line indicates the ORs (equal to 1) for the null hypothesis. The estimated pooled effect size (represented by the diamond symbol) underneath the plot was calculated under the fixed effects model and the random effects model.
Fig. 2. Forest plot of genotypic meta-analysis results across all studies.
The counts of genotypes A1A1+A1A2 and all genotypes of the Taq1A polymorphism in alcohol dependence (AD) and control groups from eligible studies were presented by rows. The bars with squares in the middle represent 95% confidence intervals (CIs) and odds ratios (ORs). The central vertical solid line represents ORs (equal to 1) for the null hypothesis. The estimated pooled effect size (represented by the diamond symbol) underneath the plot was calculated under the fixed effects model and the random effects model.
Subgroup analyses stratified by race and diagnostic criteria
When the meta-analysis was limited to subgroups according to ethnic (ancestral) background (i.e., European or Asian), only the Asian population studies did not show between-study heterogeneity [Allelic: P(Q) = 0.813, I2 = 0.0%; Genotypic: P(Q) = 0.186, I2 = 23.6%]. Allelic meta-analysis indicated that the association between the Taq1A polymorphism and AD was positive in both European and Asian subgroups [European: Z = 2.93, P(Z) = 0.003, OR = 1.16; Asian: Z = 3.32, P(Z) = 9.0×10−4, OR = 1.17). Similar results were obtained from genotypic meta-analysis in the European subgroup but not in the Asian subgroup [European: Z = 3.42, P(Z) = 6×10−4; Asian: Z = 1.73, P(Z) = 0.083] (Table 1). When the meta-analysis was categorized in terms of clearly stated diagnostic criteria (diagnosis 1) or the standard DSM-III-R, DSM-IV, or ICD-10 criteria (diagnosis 2), between-study heterogeneity still existed in European studies [Allelic (diagnosis 1): P(Q) = 0.003 and I2 = 46.4%; Allelic (diagnosis 2): P(Q) = 0.003 and I2 = 47.8%; Genotypic (diagnosis 1): P(Q) = 0.009 and I2 = 41.4%; Genotypic (diagnosis 2): P(Q) = 0.011 and I2 = 42.3%] but not in Asian studies [P(Q) > 0.05 for both allelic and genotypic studies with either diagnosis 1 or diagnosis 2] (Table 1). Nevertheless, both allelic and genotypic meta-analysis results remained significant when studies were stratified by either diagnosis 1 or diagnosis 2 [P(Z) < 0.01], except genotypic analysis in Asian studies [diagnosis 1: Z = 1.83, P(Z) = 0.067; diagnosis 2: Z = 1.93, P(Z) = 0.538] (Table 1).
Sensitivity and accumulative analysis
To examine whether the association between the TaqA1 polymorphism and AD was influenced by individual studies, both allelic and genotypic meta-analyses were repeated under the random effects model after omitting one individual study at a time. Sensitivity analysis showed that our meta-analysis was stable and the results remained unchanged (Supplementary Figure S1).
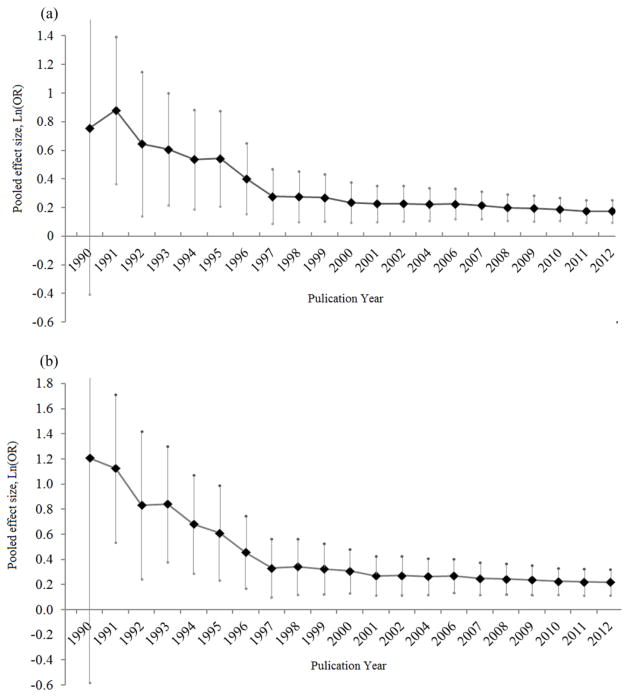
To test whether the pooled effect of the Taq1A polymorphism on the risk for AD varied by the publication year, pooled ORs and 95% CIs from all studies were calculated along with the year of publication. As shown in Figure 3, the pooled OR was high in early studies published in 1990 and was then decreased substantially in later years. The OR became stable after the year 1997. A similar cumulative curve was observed in genotypic meta-analyses.
Fig. 3. Plots of pooled effect size with the publication year.
The pooled effect size [Ln(OR)] of theTaq1A polymorphism for risk of alcohol dependence was plotted against the publication year in allelic (Fig. 3a) and genotypic (Fig. 3b) meta analyses. The X-axis represents the year when all studies were included to calculate the pooled effect size. Each vertical line with a diamond symbol represents the 95% confidential interval and the pooled effect size.
Identification of publication bias
Both graphic and statistical methods were applied to analyze the publication bias that potentially existed in the global as well as the subgroup analyses (Supplementary Figure S2). In the funnel plot, the effect size (OR) of each study was plotted against standard errors of the effect size (OR). If publication bias exists, we would expect the funnel plot to be asymmetrical. The Egger test (Egger et al. 1997) was also used to quantitatively estimate the symmetry of the funnel plot by linear regression analysis. As shown in Table 1, publication bias existed in both allelic and genotypic meta-analyses when all studies were included [allelic: P(B) = 0.010; genotypic: P(B) = 0.014]. However, when the analysis was conducted in studies grouped by racial ancestry, the publication bias disappeared in both European [allelic: P(B) = 0.183; genotypic: P(B) = 0.316] and Asian [allelic: P(B) = 0.794; genotypic: P(B) = 0.191] studies. Furthermore, diagnostic criteria were not found to cause significant publication bias in either European or Asian studies [P(B) > 0.05, for both allelic and genotypic analyses].
Since publication bias was shown in studies selected for this meta-analysis, the Trim and Fill method was applied to adjust for the meta-analysis results by adding missing negative results. In allelic meta-analysis, the association between the Taq1A polymorphism and AD was still positive in all studies and studies with the two diagnostic criteria [All studies: Z = 2.78, P(Z) = 0.005, OR = 1.13; diagnostic 1: Z = 4.06, P(Z) = 5.0 × 10−5, OR = 1.20; diagnostic 2: Z=3.69, P(Z) = 2.0 ×10−4, OR=1.19]. In genotypic meta-analysis, the association between the Taq1A polymorphism and AD also remained significant [All studies: Z = 2.83, P(Z) = 0.005, OR = 1.18; diagnostic 1: Z = 2.66, P(Z) =0.008, OR=1.18; diagnostic 2: Z=2.67, P(Z) =0.007, OR=1.19].
Discussion
Since Blum et al. (1990) initially reported a large effect of the A1 allele of the Taq1A polymorphism on risk for AD, a number of follow-up studies successfully validated this finding, although negative results were also obtained in some studies. Moreover, three meta-analyses of studies confirmed the association and demonstrated a moderate effect of the Taq1A polymorphism on AD (Le Foll et al. 2009; Munafo et al. 2007; Smith et al. 2008). However, the conclusion was far from certain because of inconsistent findings and the limited sample size of previous studies. We performed the largest meta-analysis so far concerning the association between the Taq1A polymorphism and AD. Sixty-one eligible studies published up to 2012 were considered, gathering a total of 18,730 subjects including 9,590 cases and 9,140 comparison controls. Our results provide strong evidence of the association between the Taq1A polymorphism and AD, especially in the European population. The association between the Taq1A polymorphism and AD remains significant after adjustment for publication bias using the trim and fill method. Sensitivity analysis shows that the results from both allelic and genotypic meta-analyses are stable and not influenced by any individual study. As the year of publication progressed, and more eligible studies were included, pooled ORs decreased remarkably compared to the first positive report in 1990 and become constant after the year 2001 (Figure 3). The trend curve showed that the Taq1A polymorphism has a moderate impact on AD in the population, across all studies.
Both our allelic and genotypic meta-analyses confirmed previous findings concerning the significant association between the Taq1A polymorphism and AD, except the genotypic meta-analysis in Asian studies (refer to Table 1). The reason for the negative finding obtained in the genotypic subgroup meta-analysis in subjects with Asian ancestry is unknown, but here we present some possible explanations. First, allelic and genotypic analyses are two different but related methods used in genetic association studies. Allelic association studies are based on comparing allele frequencies while genotypic association studies are based on comparing genotype distributions between cases and controls. Moreover, genotypic analysis can examine the effect of polymorphisms on diseases under different genetic models (e.g., dominant, recessive, or co-dominant). Second, the frequency of the minor A1 allele of the Taq1A polymorphism varies substantially in different populations and a higher frequency (over 40%) of the A1 allele was shown in the Asian population, suggesting that the association between the Taq1A polymorphism and AD could be race-specific. Third, the observed association between the Taq1A polymorphism and AD may result from functional variants that are in tight linkage disequilibrium (LD) with the TaqA1 polymorphism, which was originally considered to be the cause for the DRD2 restriction fragment length polymorphism (RFLP) (Blum et al. 1990; Bolos et al. 1990).
The exact location of the TaqA1 polymorphism was not specified until the ankyrin repeat and kinase domain containing-1 gene (ANKK1) was identified (Neville et al. 2004). Now it is known that the Taq1A polymorphism causes a missense mutation (Glu713Lys) in the conserved 11th ankyrin repeat site. According to the ANKK1 and DRD2 gene cluster LD information from the HapMap database, the Taq1A polymorphism is actually located in a LD block that extends to the DRD2 gene region especially in the European population. However, it was not found to be in close LD with other AD-associated variants in ANKK1 (Dick et al. 2007). Since the Taq1A polymorphism does not affect the DRD2 protein sequence, Le Foll et al. (2009) proposed that the Taq1A polymorphism might simply be a marker linked to a functional polymorphism for AD. Nevertheless, positron emission tomography (PET) and high performance liquid chromatography (HPLC) studies showed that the Taq1A polymorphism could affect DRD2 availability (Hirvonen et al. 2004) and the dopamine metabolite homovanillic acid level (Ponce et al. 2004), suggesting a functional role the Taq1A polymorphism in regulating DRD2 expression either directly or indirectly. Moreover, functional variants in ANKK1 were found to influence expression levels of nuclear factor-kappaB (NF-kB) (Huang et al. 2009), which can regulate the transcription of DRD2 (Bontempi et al. 2007; Fiorentini et al. 2002). Additionally, the Taq1A polymorphism was predicted to influence the aggregation of the ANKK1 protein (Ghosh et al. 2012). Thus, the positive association between the Taq1A polymorphism and AD is likely due to three possible reasons: (1) the Taq1A polymorphism is in tight LD with causative variants for AD, as suggested by Le Foll et al. (2009); (2) the Taq1A polymorphism, which is located in the downstream region of DRD2 (Yang et al. 2007), is potentially being harbored in the regulatory region of DRD2 ; and (3) the Taq1A polymorphism, which is a nonsynonymous variant in exon 8 of ANKK1 (Neville et al. 2004), may influence the activity of ANKK1 which subsequently affects DRD2 expression. Additionally, besides ANKK1 and DRD2, other genes grouped in the same chromosomal region harboring the Taq1A polymorphism (such as TTC12 and NCAM1) might be involved in AD (Dick et al. 2007; Gelernter et al. 2006).
Publication bias in meta-analyses may be partially influenced by population admixture. In the present meta-analysis, seven of sixty-one studies analyzed populations that were classified as “Others” (American Indians, mixed population, or not stated). When the meta-analysis was performed using all studies including different ethnic groups, publication bias was shown to exist; however, publication bias disappeared when studies were limited to European or Asian subgroup studies. Additionally, we did not observe a significant effect of diagnostic criteria on publication bias.
To estimate the type II error, a retrospective statistical power analysis was calculated. For allelic analysis, given the effect size of 1.19 and the type I error of 0.05, the minimal sample size would be 3,201 subjects for the European population and 2,162 subjects for the Asian population to achieve an expected statistical power of 80%. For genotypic analysis, given the effect size of 1.24 and the type I error of 0.05, the minimal sample size would be about 1,500 subjects for both European and Asian populations to achieve an expected statistical power of 80%. The above results suggest that most studies included in this meta-analysis did not have sufficient statistical power to detect the association between the TaqA1 polymorphism and AD because of a moderate effect size of the TaqA1 polymorphism. This likely explains the inconsistent findings in previous studies.
Two limitations should be addressed in this meta-analysis. First, a large heterogeneity was observed in European studies. This could be due to other potential differences between studies, for example, sex, age, family history, severity of AD, or unscreened controls. Only a few studies included in this meta-analysis provided information regarding these variables. It is therefore difficult to investigate the influence of the above covariates in causing heterogeneity between studies. Moreover, the potential interaction of ANKK1/DRD2 with other genes may also lead to heterogeneity between studies. Recent studies demonstrated that dopamine receptor D2 (coded by DRD2) and dopamine receptor D4 (coded by DRD4) were able to form functional heteromers and the heteromerization process was modulated by polymorphisms in DRD2 and DRD4 (Gonzalez et al. 2012; Mota et al. 2012). Interestingly, the DRD2/DRD4 heteromers were found to influence genetic susceptibility to AD (Mota et al. 2012). Second, publication bias is not the only explanation for the asymmetric funnel plot. The aforementioned covariates could contribute to publication bias as well, resulting in false positive findings. Additionally, selection bias could also have occurred because only studies published in English were taken into consideration. Thus, to have a better understanding of the association between the Taq1A polymorphism and AD, an improved meta-analysis could be conducted using more sophisticated analysis methods for controlling of between-study heterogeneity and publication bias.
Conclusions
In summary, our meta-analysis provides further evidence supporting a moderate effect size of the Taq1A polymorphism on AD across populations. Some of the heterogeneity between studies and publication bias might be due to differences in the racial ancestry of the different populations studied. Retrospective statistical power analysis suggested that the sample size for future studies of the association between the Taq1A polymorphism and AD should be at least 1,500 subjects in order to have sufficient statistical power. Moreover, findings from previous studies and this large meta-analysis suggest that, in future studies, we need to (1) explore the molecular mechanism by which the TaqA1 polymorphism influences risk for AD, (2) examine the biological function of those variants which are located in the ANKK1/DRD2 gene cluster region and in tight LD with the TaqA1 polymorphism, and (3) deep-sequence the ANKK1/DRD2 gene region to identify novel functional variants which are potentially linked to the TaqA1 polymorphism.
Supplementary Material
Acknowledgments
This study was supported by the National Institute of Health (NIH) Grants K99/R00 DA022891 and the grant from the Alcoholic Beverage Medical Research Foundation (ABMRF).
Footnotes
Conflict of Interest
The authors have no conflict of interest to report.
References
- Amadeo S, Abbar M, Fourcade ML, Waksman G, Leroux MG, Madec A, Selin M, Champiat JC, Brethome A, Leclaire Y, et al. D2 dopamine receptor gene and alcoholism. J Psychiatr Res. 1993;27:173–9. doi: 10.1016/0022-3956(93)90005-m. [DOI] [PubMed] [Google Scholar]
- Amadeo S, Noble EP, Fourcade-Amadeo ML, Tetaria C, Brugiroux MF, Nicolas L, Deparis X, Elbaz A, Zhang X, Ritchie T, Martin PV, Mallet J. Association of D2 dopamine receptor and alcohol dehydrogenase 2 genes with Polynesian alcoholics. Eur Psychiatry. 2000;15:97–102. doi: 10.1016/s0924-9338(00)00206-6. [DOI] [PubMed] [Google Scholar]
- Anghelescu I, Germeyer S, Muller MJ, Klawe C, Singer P, Dahmen N, Wetzel H, Himmerich H, Szegedi A. No association between the dopamine d2 receptor taqi a1 allele and earlier age of onset of alcohol dependence according to different specified criteria. Alcohol Clin Exp Res. 2001;25:805–9. [PubMed] [Google Scholar]
- Arinami T, Itokawa M, Komiyama T, Mitsushio H, Mori H, Mifune H, Hamaguchi H, Toru M. Association between severity of alcoholism and the A1 allele of the dopamine D2 receptor gene TaqI A RFLP in Japanese. Biol Psychiatry. 1993;33:108–14. doi: 10.1016/0006-3223(93)90309-2. [DOI] [PubMed] [Google Scholar]
- Bau CH, Almeida S, Hutz MH. The TaqI A1 allele of the dopamine D2 receptor gene and alcoholism in Brazil: association and interaction with stress and harm avoidance on severity prediction. Am J Med Genet. 2000;96:302–6. doi: 10.1002/1096-8628(20000612)96:3<302::aid-ajmg13>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Berggren U, Fahlke C, Aronsson E, Karanti A, Eriksson M, Blennow K, Thelle D, Zetterberg H, Balldin J. The taqI DRD2 A1 allele is associated with alcohol-dependence although its effect size is small. Alcohol Alcohol. 2006;41:479–85. doi: 10.1093/alcalc/agl043. [DOI] [PubMed] [Google Scholar]
- Bhaskar LV, Thangaraj K, Non AL, Singh L, Rao VR. Population-based case-control study of DRD2 gene polymorphisms and alcoholism. J Addict Dis. 2010;29:475–80. doi: 10.1080/10550887.2010.509274. [DOI] [PubMed] [Google Scholar]
- Blum K, Noble EP, Sheridan PJ, Finley O, Montgomery A, Ritchie T, Ozkaragoz T, Fitch RJ, Sadlack F, Sheffield D, et al. Association of the A1 allele of the D2 dopamine receptor gene with severe alcoholism. Alcohol. 1991;8:409–16. doi: 10.1016/0741-8329(91)90693-q. [DOI] [PubMed] [Google Scholar]
- Blum K, Noble EP, Sheridan PJ, Montgomery A, Ritchie T, Jagadeeswaran P, Nogami H, Briggs AH, Cohn JB. Allelic association of human dopamine D2 receptor gene in alcoholism. JAMA. 1990;263:2055–60. [PubMed] [Google Scholar]
- Bolos AM, Dean M, Lucas-Derse S, Ramsburg M, Brown GL, Goldman D. Population and pedigree studies reveal a lack of association between the dopamine D2 receptor gene and alcoholism. JAMA. 1990;264:3156–60. [PubMed] [Google Scholar]
- Bontempi S, Fiorentini C, Busi C, Guerra N, Spano P, Missale C. Identification and characterization of two nuclear factor-kappaB sites in the regulatory region of the dopamine D2 receptor. Endocrinology. 2007;148:2563–70. doi: 10.1210/en.2006-1618. [DOI] [PubMed] [Google Scholar]
- Chen CH, Chien SH, Hwu HG. Lack of association between TaqI A1 allele of dopamine D2 receptor gene and alcohol-use disorders in atayal natives of Taiwan. Am J Med Genet. 1996;67:488–90. doi: 10.1002/(SICI)1096-8628(19960920)67:5<488::AID-AJMG10>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Chen WJ, Lu ML, Hsu YP, Chen CC, Yu JM, Cheng AT. Dopamine D2 receptor gene and alcoholism among four aboriginal groups and Han in Taiwan. Am J Med Genet. 1997;74:129–36. [PubMed] [Google Scholar]
- Comings DE. Why different rules are required for polygenic inheritance: lessons from studies of the DRD2 gene. Alcohol. 1998;16:61–70. doi: 10.1016/s0741-8329(97)00178-x. [DOI] [PubMed] [Google Scholar]
- Comings DE, Comings BG, Muhleman D, Dietz G, Shahbahrami B, Tast D, Knell E, Kocsis P, Baumgarten R, Kovacs BW, et al. The dopamine D2 receptor locus as a modifying gene in neuropsychiatric disorders. JAMA. 1991;266:1793–800. [PubMed] [Google Scholar]
- Comings DE, Muhleman D, Ahn C, Gysin R, Flanagan SD. The dopamine D2 receptor gene: a genetic risk factor in substance abuse. Drug Alcohol Depend. 1994;34:175–80. doi: 10.1016/0376-8716(94)90154-6. [DOI] [PubMed] [Google Scholar]
- Cook BL, Wang ZW, Crowe RR, Hauser R, Freimer M. Alcoholism and the D2 receptor gene. Alcohol Clin Exp Res. 1992;16:806–9. doi: 10.1111/j.1530-0277.1992.tb00683.x. [DOI] [PubMed] [Google Scholar]
- Cruz C, Camarena B, Mejia JM, Paez F, Eroza V, Ramon De La Fuente J, Kershenobich D, Nicolini H. The dopamine D2 receptor gene TaqI A1 polymorphism and alcoholism in a Mexican population. Arch Med Res. 1995;26:421–6. [PubMed] [Google Scholar]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Dick DM, Wang JC, Plunkett J, Aliev F, Hinrichs A, Bertelsen S, Budde JP, Goldstein EL, Kaplan D, Edenberg HJ, Nurnberger J, Jr, Hesselbrock V, Schuckit M, Kuperman S, Tischfield J, Porjesz B, Begleiter H, Bierut LJ, Goate A. Family-based association analyses of alcohol dependence phenotypes across DRD2 and neighboring gene ANKK1. Alcohol Clin Exp Res. 2007;31:1645–53. doi: 10.1111/j.1530-0277.2007.00470.x. [DOI] [PubMed] [Google Scholar]
- Duval S, Tweedie R. A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. Journal of the American Statistical Association. 2000:89–98. [Google Scholar]
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finckh U, von Widdern O, Giraldo-Velasquez M, Podschus J, Dufeu P, Sander T, Harms H, Schmidt LG, Rommelspacher H, Rolfs A. No association of the structural dopamine D2 receptor (DRD2) variant 311Cys with alcoholism. Alcohol Clin Exp Res. 1996;20:528–32. doi: 10.1111/j.1530-0277.1996.tb01087.x. [DOI] [PubMed] [Google Scholar]
- Fiorentini C, Guerra N, Facchetti M, Finardi A, Tiberio L, Schiaffonati L, Spano P, Missale C. Nerve growth factor regulates dopamine D(2) receptor expression in prolactinoma cell lines via p75(NGFR)-mediated activation of nuclear factor-kappaB. Mol Endocrinol. 2002;16:353–66. doi: 10.1210/mend.16.2.0773. [DOI] [PubMed] [Google Scholar]
- Foley PF, Loh EW, Innes DJ, Williams SM, Tannenberg AE, Harper CG, Dodd PR. Association studies of neurotransmitter gene polymorphisms in alcoholic Caucasians. Ann N Y Acad Sci. 2004;1025:39–46. doi: 10.1196/annals.1316.005. [DOI] [PubMed] [Google Scholar]
- Freire MT, Marques FZ, Hutz MH, Bau CH. Polymorphisms in the DBH and DRD2 gene regions and smoking behavior. Eur Arch Psychiatry Clin Neurosci. 2006;256:93–7. doi: 10.1007/s00406-005-0610-x. [DOI] [PubMed] [Google Scholar]
- Galbraith R. Graphical display of estimates having differing standard errors. Technometrics. 1988:271–281. [Google Scholar]
- Geijer T, Neiman J, Rydberg U, Gyllander A, Jonsson E, Sedvall G, Valverius P, Terenius L. Dopamine D2-receptor gene polymorphisms in Scandinavian chronic alcoholics. Eur Arch Psychiatry Clin Neurosci. 1994;244:26–32. doi: 10.1007/BF02279808. [DOI] [PubMed] [Google Scholar]
- Gelernter J, Kranzler H. D2 dopamine receptor gene (DRD2) allele and haplotype frequencies in alcohol dependent and control subjects: no association with phenotype or severity of phenotype. Neuropsychopharmacology. 1999;20:640–9. doi: 10.1016/S0893-133X(98)00110-9. [DOI] [PubMed] [Google Scholar]
- Gelernter J, O’Malley S, Risch N, Kranzler HR, Krystal J, Merikangas K, Kennedy JL, Kidd KK. No association between an allele at the D2 dopamine receptor gene (DRD2) and alcoholism. JAMA. 1991;266:1801–7. [PubMed] [Google Scholar]
- Gelernter J, Yu Y, Weiss R, Brady K, Panhuysen C, Yang BZ, Kranzler HR, Farrer L. Haplotype spanning TTC12 and ANKK1, flanked by the DRD2 and NCAM1 loci, is strongly associated to nicotine dependence in two distinct American populations. Hum Mol Genet. 2006;15:3498–507. doi: 10.1093/hmg/ddl426. [DOI] [PubMed] [Google Scholar]
- Ghosh J, Pradhan S, Mittal B. Identification of a Novel ANKK1 and Other Dopaminergic (DRD2 and DBH) Gene Variants in Migraine Susceptibility. Neuromolecular Med. 2012 doi: 10.1007/s12017-012-8195-9. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Goldman D, Brown GL, Albaugh B, Robin R, Goodson S, Trunzo M, Akhtar L, Lucas-Derse S, Long J, Linnoila M, et al. DRD2 dopamine receptor genotype, linkage disequilibrium, and alcoholism in American Indians and other populations. Alcohol Clin Exp Res. 1993;17:199–204. doi: 10.1111/j.1530-0277.1993.tb00749.x. [DOI] [PubMed] [Google Scholar]
- Goldman D, Dean M, Brown GL, Bolos AM, Tokola R, Virkkunen M, Linnoila M. D2 dopamine receptor genotype and cerebrospinal fluid homovanillic acid, 5-hydroxyindoleacetic acid and 3-methoxy-4-hydroxyphenylglycol in alcoholics in Finland and the United States. Acta Psychiatr Scand. 1992;86:351–7. doi: 10.1111/j.1600-0447.1992.tb03279.x. [DOI] [PubMed] [Google Scholar]
- Goldman D, Urbanek M, Guenther D, Robin R, Long JC. Linkage and association of a functional DRD2 variant [Ser311Cys] and DRD2 markers to alcoholism, substance abuse and schizophrenia in Southwestern American Indians. Am J Med Genet. 1997;74:386–94. doi: 10.1002/(sici)1096-8628(19970725)74:4<386::aid-ajmg9>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Gonzalez S, Rangel-Barajas C, Peper M, Lorenzo R, Moreno E, Ciruela F, Borycz J, Ortiz J, Lluis C, Franco R, McCormick PJ, Volkow ND, Rubinstein M, Floran B, Ferre S. Dopamine D4 receptor, but not the ADHD-associated D4.7 variant, forms functional heteromers with the dopamine D2S receptor in the brain. Mol Psychiatry. 2012;17:650–62. doi: 10.1038/mp.2011.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorwood P, Batel P, Gouya L, Courtois F, Feingold J, Ades J. Reappraisal of the association between the DRD2 gene, alcoholism and addiction. Eur Psychiatry. 2000a;15:90–6. doi: 10.1016/s0924-9338(00)00207-8. [DOI] [PubMed] [Google Scholar]
- Gorwood P, Bellivier F, Ades J, Leboyer M. The DRD2 gene and the risk for alcohol dependence in bipolar patients. Eur Psychiatry. 2000b;15:103–8. doi: 10.1016/s0924-9338(00)00205-4. [DOI] [PubMed] [Google Scholar]
- Heinz A, Sander T, Harms H, Finckh U, Kuhn S, Dufeu P, Dettling M, Graf K, Rolfs A, Rommelspacher H, Schmidt LG. Lack of allelic association of dopamine D1 and D2 (TaqIA) receptor gene polymorphisms with reduced dopaminergic sensitivity to alcoholism. Alcohol Clin Exp Res. 1996;20:1109–13. doi: 10.1111/j.1530-0277.1996.tb01954.x. [DOI] [PubMed] [Google Scholar]
- Hietala J, Pohjalainen T, Heikkila-Kallio U, West C, Salaspuro M, Syvalahti E. Allelic association between D2 but not D1 dopamine receptor gene and alcoholism in Finland. Psychiatr Genet. 1997;7:19–25. doi: 10.1097/00041444-199700710-00003. [DOI] [PubMed] [Google Scholar]
- Hirvonen M, Laakso A, Nagren K, Rinne JO, Pohjalainen T, Hietala J. C957T polymorphism of the dopamine D2 receptor (DRD2) gene affects striatal DRD2 availability in vivo. Mol Psychiatry. 2004;9:1060–1. doi: 10.1038/sj.mp.4001561. [DOI] [PubMed] [Google Scholar]
- Huang SY, Lin WW, Wan FJ, Chang AJ, Ko HC, Wang TJ, Wu PL, Lu RB. Monoamine oxidase-A polymorphisms might modify the association between the dopamine D2 receptor gene and alcohol dependence. J Psychiatry Neurosci. 2007;32:185–92. [PMC free article] [PubMed] [Google Scholar]
- Huang W, Payne TJ, Ma JZ, Beuten J, Dupont RT, Inohara N, Li MD. Significant association of ANKK1 and detection of a functional polymorphism with nicotine dependence in an African-American sample. Neuropsychopharmacology. 2009;34:319–30. doi: 10.1038/npp.2008.37. [DOI] [PubMed] [Google Scholar]
- Hummel M, Unterwald EM. D1 dopamine receptor: a putative neurochemical and behavioral link to cocaine action. J Cell Physiol. 2002;191:17–27. doi: 10.1002/jcp.10078. [DOI] [PubMed] [Google Scholar]
- Ishiguro H, Arinami T, Saito T, Akazawa S, Enomoto M, Mitushio H, Fujishiro H, Tada K, Akimoto Y, Mifune H, Shioduka S, Hamaguchi H, Toru M, Shibuya H. Association study between the -141C Ins/Del and TaqI A polymorphisms of the dopamine D2 receptor gene and alcoholism. Alcohol Clin Exp Res. 1998;22:845–8. [PubMed] [Google Scholar]
- Joe KH, Kim DJ, Park BL, Yoon S, Lee HK, Kim TS, Cheon YH, Gwon DH, Cho SN, Lee HW, Namgung S, Shin HD. Genetic association of DRD2 polymorphisms with anxiety scores among alcohol-dependent patients. Biochem Biophys Res Commun. 2008;371:591–5. doi: 10.1016/j.bbrc.2008.02.076. [DOI] [PubMed] [Google Scholar]
- Karaoguz MY, Cosar B, Sahin FI. Lack of Association Between TaqIA RFLP of the Dopamine D2 Receptor Gene and Alcoholism in Type 2 Turkish Alcoholics. Gene & Genomics. 2004;26:149–154. [Google Scholar]
- Kasiakogia-Worlley K, McQuillin A, Lydall GJ, Patel S, Kottalgi G, Gunwardena P, Cherian R, Rao H, Hillman A, Gobikrishnan N, Douglas E, Qureshi SY, Jauhar S, Ball D, Okane A, Owens L, Dedman A, Sharp SI, Kandaswamy R, Guerrini I, Thomson AD, Smith I, Dar K, Morgan MY, Gurling HM. Lack of allelic association between markers at the DRD2 and ANKK1 gene loci with the alcohol-dependence syndrome and criminal activity. Psychiatr Genet. 2011;21:323–4. doi: 10.1097/YPG.0b013e3283458a68. [DOI] [PubMed] [Google Scholar]
- Kienast T, Heinz A. Dopamine and the diseased brain. CNS Neurol Disord Drug Targets. 2006;5:109–31. doi: 10.2174/187152706784111560. [DOI] [PubMed] [Google Scholar]
- Konishi T, Calvillo M, Leng AS, Lin KM, Wan YJ. Polymorphisms of the dopamine D2 receptor, serotonin transporter, and GABA(A) receptor beta(3) subunit genes and alcoholism in Mexican-Americans. Alcohol. 2004a;32:45–52. doi: 10.1016/j.alcohol.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Konishi T, Luo HR, Calvillo M, Mayo MS, Lin KM, Wan YJ. ADH1B*1, ADH1C*2, DRD2 (-141C Ins), and 5-HTTLPR are associated with alcoholism in Mexican American men living in Los Angeles. Alcohol Clin Exp Res. 2004b;28:1145–52. doi: 10.1097/01.alc.0000134231.48395.42. [DOI] [PubMed] [Google Scholar]
- Kono Y, Yoneda H, Sakai T, Nonomura Y, Inayama Y, Koh J, Sakai J, Inada Y, Imamichi H, Asaba H. Association between early-onset alcoholism and the dopamine D2 receptor gene. Am J Med Genet. 1997;74:179–82. doi: 10.1002/(sici)1096-8628(19970418)74:2<179::aid-ajmg13>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Kovanen L, Saarikoski ST, Haukka J, Pirkola S, Aromaa A, Lonnqvist J, Partonen T. Circadian clock gene polymorphisms in alcohol use disorders and alcohol consumption. Alcohol Alcohol. 2010;45:303–11. doi: 10.1093/alcalc/agq035. [DOI] [PubMed] [Google Scholar]
- Kraschewski A, Reese J, Anghelescu I, Winterer G, Schmidt LG, Gallinat J, Finckh U, Rommelspacher H, Wernicke C. Association of the dopamine D2 receptor gene with alcohol dependence: haplotypes and subgroups of alcoholics as key factors for understanding receptor function. Pharmacogenet Genomics. 2009;19:513–27. doi: 10.1097/fpc.0b013e32832d7fd3. [DOI] [PubMed] [Google Scholar]
- Landgren S, Berglund K, Jerlhag E, Fahlke C, Balldin J, Berggren U, Zetterberg H, Blennow K, Engel JA. Reward-related genes and personality traits in alcohol-dependent individuals: a pilot case control study. Neuropsychobiology. 2011;64:38–46. doi: 10.1159/000324992. [DOI] [PubMed] [Google Scholar]
- Lawford BR, Young RM, Rowell JA, Gibson JN, Feeney GF, Ritchie TL, Syndulko K, Noble EP. Association of the D2 dopamine receptor A1 allele with alcoholism: medical severity of alcoholism and type of controls. Biol Psychiatry. 1997;41:386–93. doi: 10.1016/S0006-3223(96)00478-7. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Gallo A, Le Strat Y, Lu L, Gorwood P. Genetics of dopamine receptors and drug addiction: a comprehensive review. Behav Pharmacol. 2009;20:1–17. doi: 10.1097/FBP.0b013e3283242f05. [DOI] [PubMed] [Google Scholar]
- Lee MS, Lee KJ, Kwak DI. No association between the dopamine D2 receptor gene and Korean alcoholism. Psychiatr Genet. 1997;7:93–5. doi: 10.1097/00041444-199722000-00007. [DOI] [PubMed] [Google Scholar]
- Limosin F, Gorwood P, Loze JY, Dubertret C, Gouya L, Deybach JC, Ades J. Male limited association of the dopamine receptor D2 gene TaqI a polymorphism and alcohol dependence. Am J Med Genet. 2002;112:343–6. doi: 10.1002/ajmg.10712. [DOI] [PubMed] [Google Scholar]
- Lu RB, Ko HC, Chang FM, Castiglione CM, Schoolfield G, Pakstis AJ, Kidd JR, Kidd KK. No association between alcoholism and multiple polymorphisms at the dopamine D2 receptor gene (DRD2) in three distinct Taiwanese populations. Biol Psychiatry. 1996;39:419–29. doi: 10.1016/0006-3223(95)00182-4. [DOI] [PubMed] [Google Scholar]
- Lu RB, Lee JF, Huang SY, Lee SY, Chang YH, Kuo PH, Chen SL, Chen SH, Chu CH, Lin WW, Wu PL, Ko HC. Interaction between ALDH2*1*1 and DRD2/ANKK1 TaqI A1A1 genes may be associated with antisocial personality disorder not co-morbid with alcoholism. Addict Biol. 2012;17:865–874. doi: 10.1111/j.1369-1600.2010.00268.x. [DOI] [PubMed] [Google Scholar]
- Lu RB, Lee JF, Ko HC, Lin WW. Dopamine D2 receptor gene (DRD2) is associated with alcoholism with conduct disorder. Alcohol Clin Exp Res. 2001;25:177–84. [PubMed] [Google Scholar]
- Matsushita S, Muramatsu T, Murayama M, Nakane J, Higuchi S. Alcoholism, ALDH2*2 allele and the A1 allele of the dopamine D2 receptor gene: an association study. Psychiatry Res. 2001;104:19–26. doi: 10.1016/s0165-1781(01)00290-6. [DOI] [PubMed] [Google Scholar]
- Mota NR, Rovaris DL, Bertuzzi GP, Contini V, Vitola ES, Grevet EH, Roman T, Callegari-Jacques SM, Hutz MH, Bau CH. DRD2/DRD4 heteromerization may influence genetic susceptibility to alcohol dependence. Mol Psychiatry. 2012 doi: 10.1038/mp.2012.50. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Munafo MR, Matheson IJ, Flint J. Association of the DRD2 gene Taq1A polymorphism and alcoholism: a meta-analysis of case-control studies and evidence of publication bias. Mol Psychiatry. 2007;12:454–61. doi: 10.1038/sj.mp.4001938. [DOI] [PubMed] [Google Scholar]
- Namkoong K, Cheon KA, Kim JW, Jun JY, Lee JY. Association study of dopamine D2, D4 receptor gene, GABAA receptor beta subunit gene, serotonin transporter gene polymorphism with children of alcoholics in Korea: a preliminary study. Alcohol. 2008;42:77–81. doi: 10.1016/j.alcohol.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Neiswanger K, Hill SY, Kaplan BB. Association and linkage studies of the TAQI A1 allele at the dopamine D2 receptor gene in samples of female and male alcoholics. Am J Med Genet. 1995;60:267–71. doi: 10.1002/ajmg.1320600402. [DOI] [PubMed] [Google Scholar]
- Neville MJ, Johnstone EC, Walton RT. Identification and characterization of ANKK1: a novel kinase gene closely linked to DRD2 on chromosome band 11q23.1. Hum Mutat. 2004;23:540–5. doi: 10.1002/humu.20039. [DOI] [PubMed] [Google Scholar]
- Noble EP, Blum K, Ritchie T, Montgomery A, Sheridan PJ. Allelic association of the D2 dopamine receptor gene with receptor-binding characteristics in alcoholism. Arch Gen Psychiatry. 1991;48:648–54. doi: 10.1001/archpsyc.1991.01810310066012. [DOI] [PubMed] [Google Scholar]
- Noble EP, Syndulko K, Fitch RJ, Ritchie T, Bohlman MC, Guth P, Sheridan PJ, Montgomery A, Heinzmann C, Sparkes RS, et al. D2 dopamine receptor TaqI A alleles in medically ill alcoholic and nonalcoholic patients. Alcohol Alcohol. 1994;29:729–44. [PubMed] [Google Scholar]
- Ovchinnikov IV, Druzina E, Ovtchinnikova O, Zagorovskaya T, Nebarakova T, Anokhina IP. Polymorphism of dopamine D2 and D4 receptor genes and Slavic-surnamed alcoholic patients. Addict Biol. 1999;4:399–404. doi: 10.1080/13556219971380. [DOI] [PubMed] [Google Scholar]
- Parsian A, Todd RD, Devor EJ, O’Malley KL, Suarez BK, Reich T, Cloninger CR. Alcoholism and alleles of the human D2 dopamine receptor locus. Studies of association and linkage. Arch Gen Psychiatry. 1991;48:655–63. doi: 10.1001/archpsyc.1991.01810310073013. [DOI] [PubMed] [Google Scholar]
- Pastorelli R, Bardazzi G, Saieva C, Cerri A, Gestri D, Allamani A, Airoldi L, Palli D. Genetic determinants of alcohol addiction and metabolism: a survey in Italy. Alcohol Clin Exp Res. 2001;25:221–7. [PubMed] [Google Scholar]
- Pohjalainen T, Rinne JO, Nagren K, Lehikoinen P, Anttila K, Syvalahti EK, Hietala J. The A1 allele of the human D2 dopamine receptor gene predicts low D2 receptor availability in healthy volunteers. Mol Psychiatry. 1998;3:256–60. doi: 10.1038/sj.mp.4000350. [DOI] [PubMed] [Google Scholar]
- Ponce G, Hoenicka J, Jimenez-Arriero MA, Rodriguez-Jimenez R, Aragues M, Martin-Sune N, Huertas E, Palomo T. DRD2 and ANKK1 genotype in alcohol-dependent patients with psychopathic traits: association and interaction study. Br J Psychiatry. 2008;193:121–5. doi: 10.1192/bjp.bp.107.041582. [DOI] [PubMed] [Google Scholar]
- Ponce G, Hoenicka J, Rodriguez-Jimenez R, Gozalo A, Jimenez M, Monasor R, Aragues M, Rubio G, Jimenez-Arriero MA, Ramos JA, Palomo T. IDRD2 TaqIA polymorphism is associated with urinary homovanillic acid levels in a sample of Spanish male alcoholic patients. Neurotox Res. 2004;6:373–7. doi: 10.1007/BF03033311. [DOI] [PubMed] [Google Scholar]
- Prasad P, Ambekar A, Vaswani M. Dopamine D2 receptor polymorphisms and susceptibility to alcohol dependence in Indian males: a preliminary study. BMC Med Genet. 2010;11:24. doi: 10.1186/1471-2350-11-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott CA, Kendler KS. Genetic and environmental contributions to alcohol abuse and dependence in a population-based sample of male twins. Am J Psychiatry. 1999;156:34–40. doi: 10.1176/ajp.156.1.34. [DOI] [PubMed] [Google Scholar]
- Sakai JT, Hopfer CJ, Hartman C, Haberstick BC, Smolen A, Corley RP, Stallings MC, Young SE, Timberlake D, Hewitt JK, Crowley TJ. Test of association between TaqIA A1 allele and alcohol use disorder phenotypes in a sample of adolescent patients with serious substance and behavioral problems. Drug Alcohol Depend. 2007;88:130–7. doi: 10.1016/j.drugalcdep.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Samochowiec J, Kucharska-Mazur J, Grzywacz A, Pelka-Wysiecka J, Mak M, Samochowiec A, Bienkowski P. Genetics of Lesch’s typology of alcoholism. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:423–7. doi: 10.1016/j.pnpbp.2007.09.013. [DOI] [PubMed] [Google Scholar]
- Samochowiec J, Ladehoff M, Pelz J, Smolka M, Schmidt LG, Rommelspacher H, Finckh U. Predominant influence of the 3′-region of dopamine D2 receptor gene (DRD2) on the clinical phenotype in German alcoholics. Pharmacogenetics. 2000;10:471–5. doi: 10.1097/00008571-200007000-00010. [DOI] [PubMed] [Google Scholar]
- Sander T, Harms H, Podschus J, Finckh U, Nickel B, Rolfs A, Rommelspacher H, Schmidt LG. Dopamine D1, D2 and D3 receptor genes in alcohol dependence. Psychiatr Genet. 1995;5:171–6. doi: 10.1097/00041444-199524000-00004. [DOI] [PubMed] [Google Scholar]
- Sander T, Ladehoff M, Samochowiec J, Finckh U, Rommelspacher H, Schmidt LG. Lack of an allelic association between polymorphisms of the dopamine D2 receptor gene and alcohol dependence in the German population. Alcohol Clin Exp Res. 1999;23:578–81. [PubMed] [Google Scholar]
- Schellekens AF, Franke B, Ellenbroek B, Cools A, de Jong CA, Buitelaar JK, Verkes RJ. Reduced Dopamine Receptor Sensitivity as an Intermediate Phenotype in Alcohol Dependence and the Role of the COMT Val158Met and DRD2 Taq1A Genotypes. Arch Gen Psychiatry. 2012;69:339–48. doi: 10.1001/archgenpsychiatry.2011.1335. [DOI] [PubMed] [Google Scholar]
- Shaikh KJ, Naveen D, Sherrin T, Murthy A, Thennarasu K, Anand A, Benegal V, Jain S. Polymorphisms at the DRD2 locus in early-onset alcohol dependence in the Indian population. Addict Biol. 2001;6:331–335. doi: 10.1080/13556210020077055. [DOI] [PubMed] [Google Scholar]
- Smith L, Watson M, Gates S, Ball D, Foxcroft D. Meta-analysis of the association of the Taq1A polymorphism with the risk of alcohol dependency: a HuGE gene-disease association review. Am J Epidemiol. 2008;167:125–38. doi: 10.1093/aje/kwm281. [DOI] [PubMed] [Google Scholar]
- Wang TJ, Huang SY, Lin WW, Lo HY, Wu PL, Wang YS, Wu YS, Ko HC, Shih JC, Lu RB. Possible interaction between MAOA and DRD2 genes associated with antisocial alcoholism among Han Chinese men in Taiwan. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:108–14. doi: 10.1016/j.pnpbp.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Wise RA, Rompre PP. Brain dopamine and reward. Annu Rev Psychol. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- Wu CY, Wu YS, Lee JF, Huang SY, Yu L, Ko HC, Lu RB. The association between DRD2/ANKK1, 5-HTTLPR gene, and specific personality trait on antisocial alcoholism among Han Chinese in Taiwan. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:447–53. doi: 10.1002/ajmg.b.30626. [DOI] [PubMed] [Google Scholar]
- Yang BZ, Kranzler HR, Zhao H, Gruen JR, Luo X, Gelernter J. Association of haplotypic variants in DRD2, ANKK1, TTC12 and NCAM1 to alcohol dependence in independent case control and family samples. Hum Mol Genet. 2007;16:2844–53. doi: 10.1093/hmg/ddm240. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.