Abstract
Background
Preeclampsia, a human pregnancy specific disorder is characterized by an anti-angiogenic state due to high levels of circulating soluble vascular endothelial growth factor 1 (sVEGFR-1). However, the role of lymphangiogenesis in preeclampsia has not been investigated. Recently, impaired VEGF-C (factor that regulates lymphangiogenesis) signalling has been implicated in the pathogenesis of interstitial edema and salt-sensitive hypertension. Therefore, we hypothesized that circulating VEGF-C and its circulating receptors (sVEGFR-2 and sVEGFR-3) may also be altered in preeclampsia and correlate with the severity of the phenotype.
Methods
We analyzed plasma levels of VEGF-C, sVEGFR-1, sVEGFR-2 and sVEGFR-3 in women with gestational hypertension (GHTN, n=20), preeclampsia (PE, n=20) and normotensive pregnancies (NP, n=20) in the third trimester and values reported as mean ± SD in pg/ml.
Results
As previously reported, sVEGFR-1 levels were significantly higher in subjects with PE (19938 ± 12973) than in GHTN (7156 ± 5432), p<0.01 or NP (7760 ± 6018), p<0.01. VEGF-C levels were lower in subjects with GHTN (676 ± 323) than in PE (1335 ± 625), p<0.01, but not statistically different than in NP (971 ± 556), p=0.11. There was a trend towards lower sVEGFR-2 in PE as compared to GHTN or NP. Interestingly sVEGFR-3 was significantly lower in PE (54371 ± 21107) as compared to NP (83709 ± 24983), p<0.01, but not different as compared to GHTN (54642 ± 26947). The ratio of sVEGFR-2+sVEGFR-3/VEGF-C was dramatically lower during PE (57 ± 38) as compared to GHTN (113 ± 72), p<0.01 or NP (133 ± 91), p<0.01.
Conclusion
Preeclampsia is characterized by circulating pro-lymphangiogenic state as evidenced by decreased sVEGFR-3, slightly decreased sVEGFR-2, increased VEGF-C and a dramatically lower ratio of sVEGFR-2+sVEGFR-3/VEGF-C. Our data suggests that the circulating pro-lymphoangiogenic state during preeclampsia may be a compensatory response to edema and hypertension. Additional studies are needed to evaluate the clinical relevance of the altered lymphangiogenic signalling pathway during preeclampsia.
Keywords: Preeclampsia, VEGF-C, sVEGFR-2, sVEGFR-3, Lymphangiogenesis
Introduction
Approximately 5% to 10% of pregnancies are complicated by new-onset hypertension, resulting in significant morbidity and mortality for both the mother and the neonate 1. Preeclampsia is characterized by new-onset hypertension, proteinuria and interstitial edema. While the etiology of preeclampsia is still unclear, recent animal and human studies suggest that the clinical features of preeclampsia may be caused by an imbalance in pro- and anti-angiogenic factors involving vascular endothelial growth factor (VEGF), placental growth factor, soluble VEGF receptor 1 (sVEGFR-1, also referred to as sFlt1) and soluble endoglin 2–5.
Recently, VEGF-C and its signalling pathway has been related to salt homeostasis 6. Hypertension of preeclampsia is characterized by vasoconstriction, increased peripheral vascular resistance, and renal salt and water retention 7. Although total plasma volume is slightly decreased, the hypertension of preeclampsia is exacerbated by salt loading and at least partly ameliorated by diuretics and salt deprivation 8. In addition, patients with preeclampsia excrete a much smaller percentage of an intravenous saline load than do normal pregnant women 9. Therefore, we hypothesized that in addition to disruption of the VEGF-A signalling axis during preeclampsia, VEGF-C signalling axis may also be disrupted and contribute to the pathogenesis of hypertension in preeclampsia. Therefore, we investigated circulating VEGF-C and its receptors (sVEGFR-2 and sVEGFR-3) levels during normotensive and hypertensive pregnancies.
Methods
Study protocol
We performed a case-control study in pregnant women presenting at Beth Israel Deaconess Medical Center during the 3rd trimester. The study was approved by the institutional review board and all patients provided written informed consent. We studied three groups of women, subjects with preeclampsia, or gestational hypertension or subjects with normotensive pregnancies. Twenty subjects were included in each group. These subjects were recruited as part of an ongoing cohort study of pregnant patients with normal pregnancy outcomes and hypertensive diseases. The cases and controls were randomly selected using computer number generator and all subjects were matched for gestational age.
Preeclampsia was defined by systolic blood pressure of more than 140 mmHg and diastolic blood pressure of more than 90 mmHg after 20 weeks’ gestation in a previously normotensive patient combined with new-onset proteinuria (>300 mg of protein in a 24-hour urine collection or a random urine protein/creatinine ratio of >0.3) 10. Gestational hypertension was defined by systolic blood pressure of more than 140 mmHg and diastolic blood pressure of more than 90 mmHg after 20 weeks’ gestation in a previously normotensive patient without proteinuria.
All samples were collected pre-delivery within 2 weeks of diagnosis of preeclampsia or gestational hypertension. The samples were processed in standard fashion. Briefly, the plasma samples were centrifuged at 3500 RPM for 8–10 minutes within 30 minutes of sample collection. The plasma was then aliquoted in tubes labelled with study subject ID and stored at −70 degree for further analysis.
Assays
Assays were performed by personnel who were unaware of the outcome of pregnancy. Plasma VEGF-C, sVEGFR-2, sVEGFR-3 and sVEGFR-1 were measured by enzyme-linked immunosorbent assays using kits from R&D Sytems (Minneapolis, MN, USA, cat no DVR200, DVEC00 and DY349 (duoset sVEGFR-3), DVR100B). Within assay coefficient of variation for 7.2%, 6,9% and 7.0% VEGF-C, sVEGFR-2 and sVEGFR-1, respectively. No cross-reactivity between the different proteins have been reported by the manufacturer. All samples were run in duplicate and average values reported.
Statistical Analyses
Demographics and clinical characteristics of the study patients are given as mean ± SD and or actual number (%). ELISA results are reported as mean ± SD. Differences in demographics and ELISA results between pregnancy-groups were tested one-way ANOVA-test; post-hoc a Tukey’s multiple comparison test was used. SPSS 18.0 for Windows (SPSS Inc., Chicago, IL) was used for statistical analyses. P < 0.05 was considered to be significant.
Results
General parameters
As shown in Table 1, the average age of participants was 31.8 ± 5.5 years, without significant differences between the groups. BMI index was higher in the patients with preeclampsia and gestational hypertension compared to controls. Gestational age at enrolment and delivery were not different between the groups. Birth weight was lower in the preeclampsia group compared to both gestational hypertension and normal pregnancy.
Table 1.
Demographics of study patients
| Characteristic | NP (n=20) | GHTN (=20) | PE (n=20) |
|---|---|---|---|
| Maternal age (years) | 31.3 ± 4.9 | 32.8 ± 4.8 | 31.3 ± 6.4 |
| Body mass index (kg/m2) | 25.2 ± 5.4 | 33.7 ± 4.8 * | 33.8 ± 7.5 * |
| GA on enrolment (weeks) | 34.4 ± 3.6 | 34.3 ± 3.0 | 33.7 ± 3.9 |
| Race/Ethnicity | |||
| White, non-Hispanic | 11 | 11 | 14 |
| Black, non-Hispanic | 3 | 4 | 4 |
| Asian, non-Hispanic | 1 | 0 | 0 |
| White, Hispanic | 2 | 3 | 2 |
| Black, Hispanic | 0 | 0 | 0 |
| Other/unknown | 3 | 2 | 0 |
| Gravidity | |||
| Primigravida | 11 | 11 | 13 |
| Multigravida | 9 | 9 | 7 |
| Smoking status | |||
| Never | 3 | 9 | 15 |
| Before pregnancy | 15 | 9 | 4 |
| During pregnancy, but quit | 0 | 2 | 0 |
| Current smoker | 2 | 0 | 1 |
| GA at delivery(weeks) | 35.7 ± 3.2 | 35.4 ± 8.3 | 34.1 ± 4,1 |
| Mode of delivery | |||
| Vaginal (N/%) | 12/60% | 9/45% | 6/30% |
| C/S (N/%) | 8/40% | 11/55% | 14/70% |
| Birth weight | 2770 ± 672 | 2970 ± 606 | 2274 ± 970 ** |
Data are shown as mean ± SD
NP = Normal Pregnancy, GHTN = Gestational Hypertension, PE = Preeclampsia
p < 0.05 vs Normal Pregnancy,
p < 0.05 Preeclampsia vs Gestational Hypertension
Both systolic and diastolic blood pressure was higher during preeclampsia and gestational hypertension compared to control. As shown in table 2, urinary protein/creatinine ratio, 24-hour urine protein excretion, plasma creatinine, ALT, uric acid were higher while platelet count was lower in women with preeclampsia.
Table 2.
Clinical Characteristics of study patients
| Characteristic | NP (n=20) | GHTN (=20) | PE (n=20) |
|---|---|---|---|
| Highest Systolic blood pressure | 123 ± 7 | 149 ± 9 * | 165 ± 18 * |
| Highest Diastolic blood pressure | 75 ± 6 | 96 ± 5 * | 102 ±13 * |
| Urine Protein/Creatinine ratio # | NA | 0.18 ± 0.17 | 1.7 ± 2.6 ** |
| Proteinuria 24 hour urine (gram/day) # | NA | 0.1 ± 0.1 | 1.6 ± 2.3 ** |
| ALT (U/L) | NA | 18 ± 7 | 66 ± 74 ** |
| Creatinine (U/L) | NA | 0.6 ± 0.2 | 0.7 ± 0.2 ** |
| Uric Acid (U/L) | NA | 4.8 ± 1.0 | 6.4 ± 2.2 ** |
| Platelet Count | 228± 47 | 221 ± 64 | 160 ± 72 ** |
Data are shown as mean ± SD, NA = not available
NP = Normal Pregnancy, GHTN = Gestational Hypertension, PE = Preeclampsia
p < 0.05 vs Normal Pregnancy,
p <0.05 Gestational Hypertension vs Preeclampsia
Protein/Creatinine ratio’s are available for n=18 in Preeeclampsia and n=19 in Gestational hypertension; 24 hour urine protein: Data are available for n=10 in Preeclampsia and n=12 in Gestational hypertension
VEGF-C, sVEGFR-1, sVEGFR-2 and sVEGFR-3 levels in normotensive and hypertensive pregnancies
Data are presented in figure 1, all ELISA results are in pg/mL. As previously reported, sVEGFR1 levels were significantly higher in subjects with preeclampsia (19938 ± 12973) than in gestational hypertension (7156 ± 5432), p<0.01 or normal pregnancy (7760 ± 6018), p<0.01. VEGF-C levels were lower in subjects with gestational hypertension (676 ± 323) than in preeclampsia (1335 ± 625), p<0.01, but not statistically different than in normal pregnancy (971 ± 556), p=0.11. There was a trend towards lower sVEGFR-2 in preeclampsia as compared to gestational hypertension or normal pregnancy. Interestingly, sVEGFR-3 was significantly lower in preeclampsia (54371 ± 21107) as compared to normal pregnancy (83709 ± 24983), p<0.01, but not different as compared to gestational hypertension (54642 ± 26947).
Figure 1.
VEGF-C, sVEGFR-1, sVEGFR-2 and sVEGFR-3 during normotensive and hypertensive pregnancies are shown. Data are presented as mean mean ± SD, * p <0.01 by ANOVA. NP = Normal Pregnancy, GHTN = Gestational Hypertension, PE = Preeclampsia
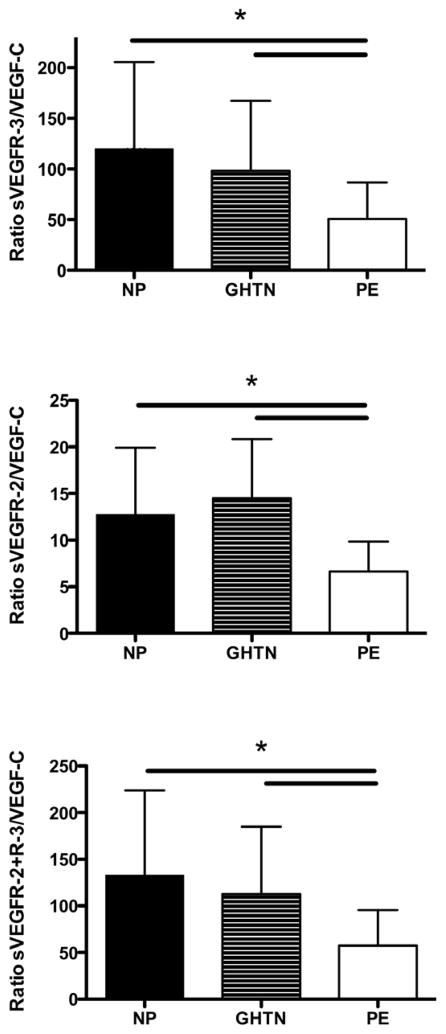
Lymphangiogenic balance in normotensive and hypertensive pregnancies
Data are presented in figure 2. The ratio between sVEGFR-2 and VEGF-C was significantly lower during preeclampsia compared to normal pregnancy and gestational hypertension, (p<0.01). Furthermore, the ratio between VEGFR-3 and VEGF-C was significantly lower during preeclampsia compared to normal pregnancy and gestational hypertension, (p<0.01). The ratio of sVEGFR-2+sVEGFR-3/VEGF-C was dramatically lower during preeclampsia (57 ± 38) as compared to gestational hypertension (113 ± 72), p<0.01 or normal pregnancy (133 ± 91), p<0.01. No correlation between VEGF-C levels or the ratio’s and blood pressure and proteinuria were present.
Figure 2.
Ratio of soluble VEGF receptor 2/3 to VEGF-C is shown. Data are shown as mean ± SD, * p <0.01 by ANOVA. NP = Normal Pregnancy, GHTN = Gestational Hypertension, PE = Preeclampsia
Discussion
In this pilot study, we studied the role of circulating factors that regulate lymphangiogenesis during normal and hypertensive pregnancies. We found that preeclampsia is characterized by decreased sVEGFR-3, slightly decreased sVEGFR-2 and increased VEGF-C. These differences resulted in lower sVEGFR-2/VEGF-C, sVEGFR-3/VEGF-C ratio and a profound reduction in sVEGFR-2+sVEGFR-3/VEGF-C during preeclampsia as compared to gestational hypertension or normotensive pregnancies. These data suggest that preeclampsia may be characterized by an enhanced lymphangiogenic state in the circulation. The clinical relevance of these findings remains to be elucidated.
To our knowledge this is the first attempt to investigate circulating VEGF-C and its soluble receptors sVEGFR-2 and 3 during pregnancy. However, earlier placenta work has shown expression of these factors in the syncytiotrophoblast and endothelium of the placenta 11–13. Dunk et al. observed decreased expression of VEGF-C and sVEGFR-3 during intra uterine growth restriction and propose that this could lead to the abnormal villous development. No alteration of sVEGFR-2, sVEGFR-3 and VEGF-C were found during preeclampsia as compared to normal pregnancy.
Animal studies have recently implicated the VEGF-C signalling axis as a potential mechanism for the etiology of salt-sensitive hypertension. During salt loading, the interstitial tonicity and edema signal the mononuclear phagocyte system (MPS) to upregulate the production of VEGF-C which in turn induces lymphatics and maintains volume homeostasis 14. Disruption of the VEGF-C axis with excess soluble sVEGFR-3 disrupts this homeostasis and leads to salt-sensitive hypertension in rats 6. However, the role of this lymphangiogenic pathway in human hypertensive disorders are still unknown 15. If VEGF-C signalling axis were in the causal pathway in preeclampsia, one would expect decreased circulating VEGF-C and increased circulating sVEGFR-3 or sVEGFR-2 during preeclampsia. However, the relatively higher VEGF-C and lower sVEGFR-2 and sVEGFR-3 noted during preeclampsia in our study does not support the above hypothesis and instead suggest that these alterations may a secondary phenomenon. Prior animal and human studies support that the excess sVEGFR-1 and the anti-angiogenic state are likely etiological factors for the hypertension and edema of preeclampsia 2,16,17. Indeed, we were able to confirm that preeclamptic subjects in this study had higher sVEGFR-1 levels. The relevance of relatively lower VEGF-C levels among subjects with gestational hypertension is unclear and needs further investigation.
Our study has a number of limitations. In our study population, we did not find a between-individual correlation between levels of VEGF-C or it’s receptors and blood pressure. This could be due to the relative small sample size of our population or implicate either absence of an association, or individual differences in adaptive response of the VEGF-C–macrophage–lymphangiogenesis pathway. Moreover, we do not know the origin of circulating VEGF-C and its receptors during pregnancy. Previous studies have shown sVEGFR-1 increase during preeclampsia to be placentally derived and secreted into the maternal circulation 18,19. We did not investigate the placental expression of VEGF-C or its receptor proteins in these patients. However, since earlier studies have not reported any changes in the VEGF-C signalling axis in the placental tissue during preeclampsia, another source seems more likely. Finally, we do not know if the alterations in the VEGF-C or sVEGFR-2 or sVEGFR-3 are altered early in pregnancy.
In summary, the ratio of the sVEGFR-2+sVEGFR-3/VEGF-C is lower in preeclampsia suggesting a pro-lymphangiogenic state. Taken together with animal studies our data suggests that the relatively pro-lymphangiogenic state in the circulation of preeclamptics may be a compensatory mechanism in response to the edema and hypertension. Future studies should investigate the clinical relevance of VEGF-C signalling pathway mediated interstitial electrolyte and volume homeostasis during normal pregnancy and preeclampsia.
Acknowledgments
We thank Dawn McCullough, RN for patient recruitment and data collection.
Funding
S.R. is supported by Harvard Diversity and Community Partnership Faculty Fellowship Award. S.A.K is an investigator of the Howard Hughes Medical Institute.
Footnotes
Disclosures: Dr. Karumanchi is a co-inventor of multiple patents related to angiogenic proteins for the diagnosis and therapy of preeclampsia. These patents have been licensed to multiple companies. Dr. Karumanchi reports having served as a consultant to Roche and Beckman Coulter and has financial interest in Aggamin LLC. The remaining authors report no conflicts.
References
- 1.Anonymous. Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000;183(1):S1–S22. [PubMed] [Google Scholar]
- 2.Maynard SE, Min J-Y, Merchan J, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111(5):649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Venkatesha S, Toporsian M, Lam C, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12(6):642–649. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 4.Noori M, Donald AE, Angelakopoulou A, Hingorani AD, Williams DJ. Prospective study of placental angiogenic factors and maternal vascular function before and after preeclampsia and gestational hypertension. Circulation. 2010;122(5):478–487. doi: 10.1161/CIRCULATIONAHA.109.895458. [DOI] [PubMed] [Google Scholar]
- 5.Ahmad S, Ahmed A. Elevated placental soluble vascular endothelial growth factor receptor-1 inhibits angiogenesis in preeclampsia. Circ Res. 2004;95(9):884–891. doi: 10.1161/01.RES.0000147365.86159.f5. [DOI] [PubMed] [Google Scholar]
- 6.Machnik A, Neuhofer W, Jantsch J, et al. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat Med. 2009;15(5):545–552. doi: 10.1038/nm.1960. [DOI] [PubMed] [Google Scholar]
- 7.Karumanchi SA, Maynard SE, Stillman IE, Epstein FH, Sukhatme VP. Preeclampsia: a renal perspective. Kidney Int. 2005;67(6):2101–2113. doi: 10.1111/j.1523-1755.2005.00316.x. [DOI] [PubMed] [Google Scholar]
- 8.STRAUSS MB. Observations on the Etiology of the Toxemias of Pregnancy. II. Production of Acute Exacerbation of Toxemia By Sodium Salts in Pregnant Women With Hyporoteinemia. The American Journal of the Medical Sciences. 1937;194(6) [Google Scholar]
- 9.Gallery ED, Brown MA. Control of sodium excretion in human pregnancy. Am J Kidney Dis. 1987;9(4):290–295. doi: 10.1016/s0272-6386(87)80124-5. [DOI] [PubMed] [Google Scholar]
- 10.Roberts JM, Cooper DW. Pathogenesis and genetics of pre-eclampsia. Lancet. 2001;357(9249):53–56. doi: 10.1016/s0140-6736(00)03577-7. [DOI] [PubMed] [Google Scholar]
- 11.Dunk C, Ahmed A. Expression of VEGF-C and activation of its receptors sVEGFR-2 and VEGFR-3 in trophoblast. Histol Histopathol. 2001;16(2):359–375. doi: 10.14670/HH-16.359. [DOI] [PubMed] [Google Scholar]
- 12.Chung J-Y, Song Y, Wang Y, Magness RR, Zheng J. Differential expression of vascular endothelial growth factor (VEGF), endocrine gland derived-VEGF, and VEGF receptors in human placentas from normal and preeclamptic pregnancies. J Clin Endocrinol Metab. 2004;89(5):2484–2490. doi: 10.1210/jc.2003-031580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou Y, McMaster M, Woo K, et al. Vascular endothelial growth factor ligands and receptors that regulate human cytotrophoblast survival are dysregulated in severe preeclampsia and hemolysis, elevated liver enzymes, and low platelets syndrome. Am J Pathol. 2002;160(4):1405–1423. doi: 10.1016/S0002-9440(10)62567-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Machnik A, Dahlmann A, Kopp C, et al. Mononuclear phagocyte system depletion blocks interstitial tonicity-responsive enhancer binding protein/vascular endothelial growth factor C expression and induces salt-sensitive hypertension in rats. Hypertension. 2010;55(3):755–761. doi: 10.1161/HYPERTENSIONAHA.109.143339. [DOI] [PubMed] [Google Scholar]
- 15.Slagman MCJ, Kwakernaak AJ, Yazdani S, et al. Vascular endothelial growth factor C levels are modulated by dietary salt intake in proteinuric chronic kidney disease patients and in healthy subjects. Nephrology, Dialysis, Transplantation. 2011 doi: 10.1093/ndt/gfr402. [DOI] [PubMed] [Google Scholar]
- 16.Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350(7):672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 17.Noori M, Donald AE, Angelakopoulou A, Hingorani AD, Williams DJ. Prospective study of placental angiogenic factors and maternal vascular function before and after preeclampsia and gestational hypertension. Circulation. 2010;122(5):478–487. doi: 10.1161/CIRCULATIONAHA.109.895458. [DOI] [PubMed] [Google Scholar]
- 18.Rajakumar A, Cerdeira AS, Rana S, et al. Transcriptionally Active Syncytial Aggregates in the Maternal Circulation May Contribute to Circulating Soluble Fms-Like Tyrosine Kinase 1 in Preeclampsia. Hypertension. 2012 doi: 10.1161/HYPERTENSIONAHA.111.182170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bujold E, Romero R, Chaiworapongsa T, et al. Evidence supporting that the excess of the sVEGFR-1 concentration in maternal plasma in preeclampsia has a uterine origin. J Matern Fetal Neonatal Med. 2005;18(1):9–16. doi: 10.1080/14767050500202493. [DOI] [PubMed] [Google Scholar]