Abstract
Brain cytochrome P450 epoxygenases were recently shown to play an essential role in mediating the pain-relieving properties of morphine. To identify the CNS sites containing the morphine-relevant P450s, the effects of intracerebral (ic) microinjections of the P450 inhibitor CC12 were determined on morphine antinociception in rats. CC12 inhibited morphine antinociception when both drugs were injected into the rostral ventromedial medulla (RVM), but not following co-injections into the periaqueductal gray (PAG) or into the spinal subarachnoid space. In addition, intra-RVM CC12 pretreatment nearly completely blocked the effects of morphine following intracerebroventricular (icv) administration. Although morphine is thought to act in both the PAG and RVM by pre-synaptic inhibition of inhibitory GABAergic transmission, the present findings show that 1) the mechanism of morphine action differs between these two brainstem areas, and 2) P450 activity within the RVM is important for supraspinal morphine antinociception. Characterization of morphine-P450 interactions within RVM circuits will further enhance the understanding of the biochemistry of pain relief.
Indexing terms: morphine, opioids, pain, cytochrome P450, RVM
1. Introduction
Morphine, the prototypical μ opioid analgesic, remains one of the most commonly used treatments for pain. However, the clinical utility of this drug is limited by the side effects, which include respiratory depression, constipation, and tolerance. In addition, the rewarding effects of opioids can lead to addiction (Gutstein and Akil, 2006). It is well established that μ opioid agonists like morphine act at μ opioid receptors in the midbrain periaqueductal gray (PAG), the rostral ventromedial medulla (RVM; consisting of the nucleus raphe magnus and adjacent reticular formation), and the dorsal horn of the spinal cord to produce antinociception (Yaksh and Rudy, 1978;Yaksh et al., 1976). These three CNS sites comprise an interconnected pathway responsible for the descending modulation of pain processing (Heinricher and Ingram, 2008). Activation of this circuit, by electrical stimulation, stress, or analgesic drugs, results in the depression of incoming nociceptive signals at the level of the spinal cord (Heinricher and Ingram, 2008).
Previous work in our lab reported that CC12, a compound related to cimetidine, inhibits the antinociceptive activity of several types of pain-relieving drugs (Hough et al., 2007). Intracerebroventricular (icv) administration of CC12 blocked the antinociceptive effects of the μ opioid agonist morphine, the cannabinoid agonist WIN55-212, and the non-opioid analgesic improgan (Hough et al., 2007). It was shown that CC12 lacks affinity at a number of targets known to be involved in analgesic signaling (Hough et al., 2007). At the time, it was proposed that these three classes of analgesic drugs share a common, downstream, CC12-sensitive target, but the identity of this target was unknown. Subsequent studies found CC12 to be a potent inhibitor of a number of cytochrome P450 monooxygenase (P450) enzymes (Stadel et al., 2008), leading to the possibility that CC12 might block all three types of pain-relieving drugs via inhibition of P450 activity (Hough et al., 2007; Stadel et al., 2008). Several enzymes (including MAPK, phospholipase C, and phospholipase A2) are known to participate in the supraspinal actions of μ opioid agonists, resulting in the release of a number of signaling molecules including inositol-1,4,5-triphosphate and arachidonic acid (Christie et al., 1999; Aoki et al., 2003; Law et al., 2000; Narita et al., 2003), but no evidence for P450-mediated opioid signal transduction existed.
Recently, experiments with several different kinds of P450 inhibitors and a new line of transgenic mice lacking functional neuronal P450 activity provided strong support for the hypothesis that μ opioids activate brain analgesic circuits through cytochrome P450 epoxygenase signaling (Conroy et al., 2010). Consistent with earlier results, it was proposed that μ opioid receptors signal through activation of phospholipase A2, arachidonate release, P450-catalyzed epoxidation of arachidonate, and formation of pain-relieving epoxyeicosatrienoic acids (see Supplemental Fig. 1 of Conroy et al., 2010). Since CC12 is a P450 inhibitor (Stadel et al., 2008), the results of Conroy et al. make it likely that CC12 blocks morphine antinociception (Hough et al., 2007) by inhibiting brain P450 enzymes. Presently, intracerebral (ic) and intrathecal microinjections were employed to localize the CNS sites in which the P450 epoxygenase inhibitor CC12 acts to attenuate morphine antinociception.
2. Results
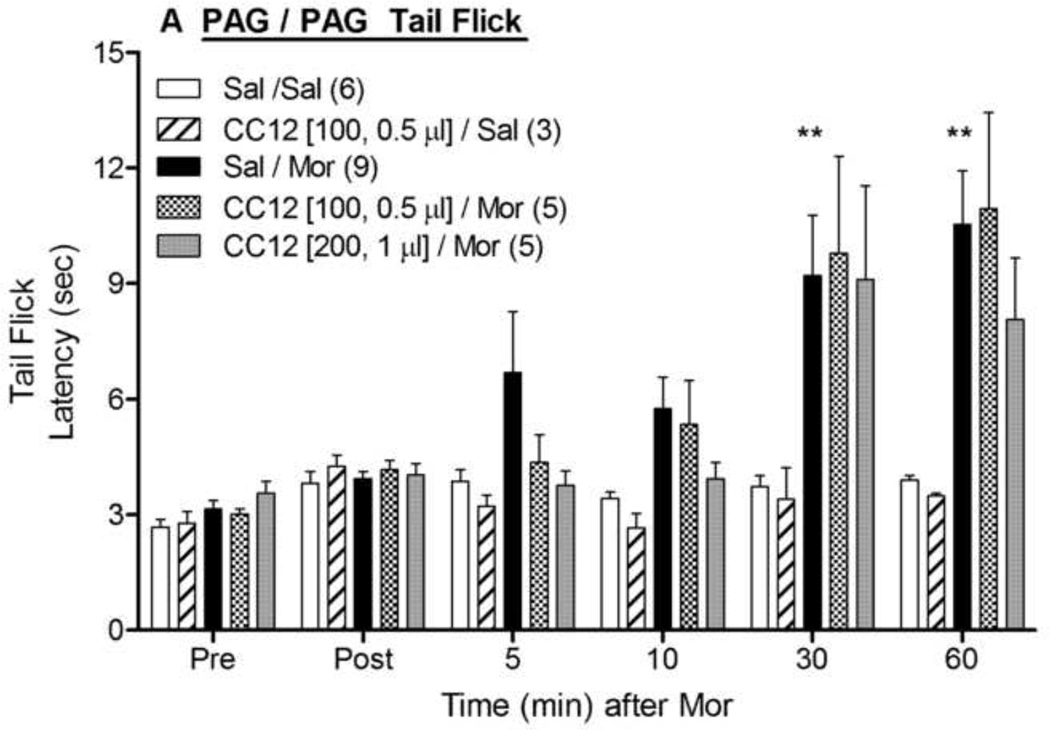
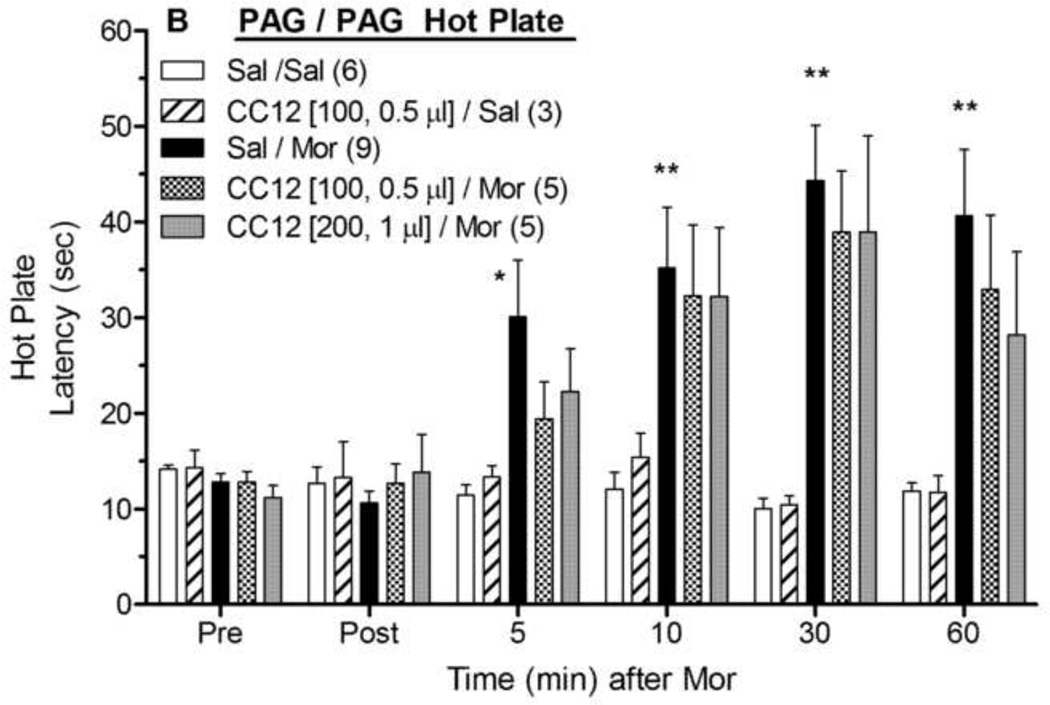
The effects of the P450 inhibitor CC12 microinjected into the PAG, RVM and caudal medulla were studied on the antinociception produced by morphine administration into these brain areas. Microinjections of morphine sulfate (5 µg) into the PAG in saline- pretreated animals increased nociceptive latencies in both the tail flick (Fig. 1A) and hot plate (Fig. 1B) tests. ANOVA of the data in Fig. 1A (between groups : CC12, morphine; within groups: time) found significant main effects of morphine (F1,19=12.2, P<0.005) and time (F5,95 =11.0, P<0.0001), with a significant (F5,95 =8.7, P<0.0001) morphine by time interaction term. The same ANOVA found no effects of CC12 (100 nmol) on tail flick latencies following intra-PAG saline or morphine (Fig. 1A). A similar ANOVA performed with the larger (200 nmol) dose of CC12 showed no modulation of morphine responses on the tail flick (Fig. 1A). Hot plate latencies recorded from the same experiments revealed an identical pattern: robust antinociception produced by morphine which was not attenuated by either dose of CC12 (Fig. 1B).
Figure 1. Effect of CC12 on morphine antinociception in the PAG.
Surgically cannulated subjects were baseline (Pre) tested, received an ic injection of saline (Sal) or CC12 (dose in nmol and volumes specified in brackets), were re-tested 15 min later (Post), and immediately received a second injection into the same brain site of either saline (Sal) or morphine sulfate (Mor, 5 µg, 0.5 µl). The volume of the first injection (Sal) in the Sal/Sal and Sal/Mor groups was either 0.5 or 1.0 µl; within each group, there were no volume effects and data were pooled. Tail flick (A) and hot plate (B) latencies (ordinate, sec, mean SEM, number of subjects in parentheses) were recorded at the time points indicated after the second injection (abscissa, min). *,**P<0.05, 0.01, respectively, versus Sal/Sal at the same time. Cannula placements are shown in Fig. 4.
Morphine microinjections into the PAG are known to produce motor characteristics that include excessive running, jumping, stereotyped circling, and vocalizations, previously referred to as “explosive motor behavior” (EMB) (Blair et al., 1978; Criswell, 1976; Jacquet and Lajtha, 1973; Sharpe et al., 1974; Yaksh et al., 1976; Nalwalk et al., 2004). In the present studies, intra-PAG morphine injections generally produced mild or no EMB responses, and behavioral testing was unaffected. However, some animals receiving this treatment demonstrated severe EMB, which prevented nociceptive testing. Administration of CC12 into the PAG did not produce EMB.
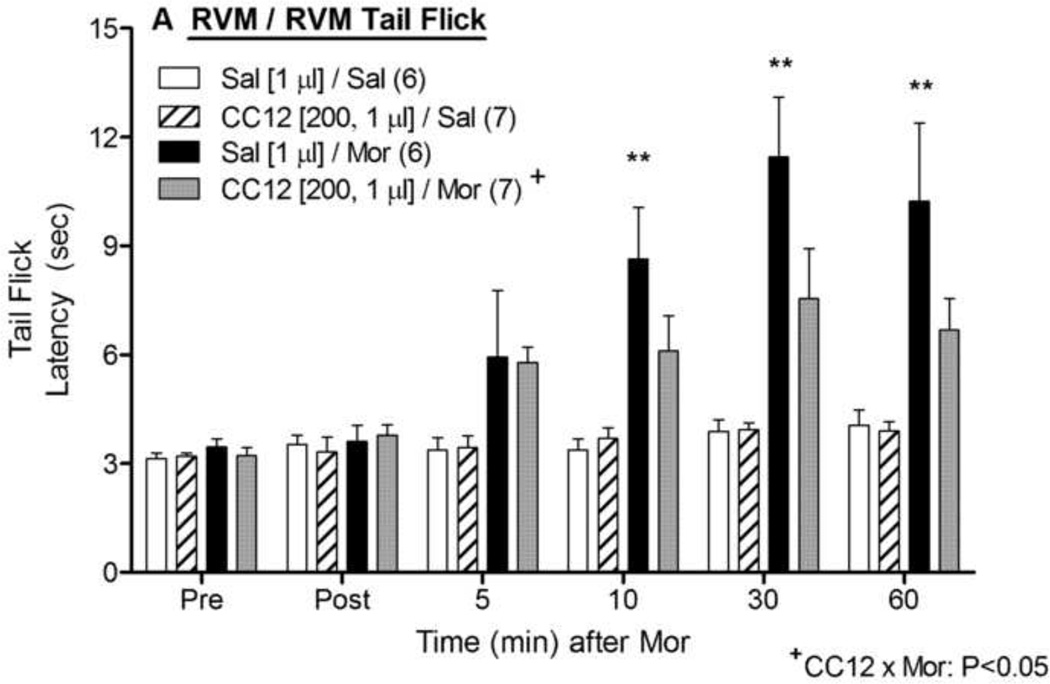
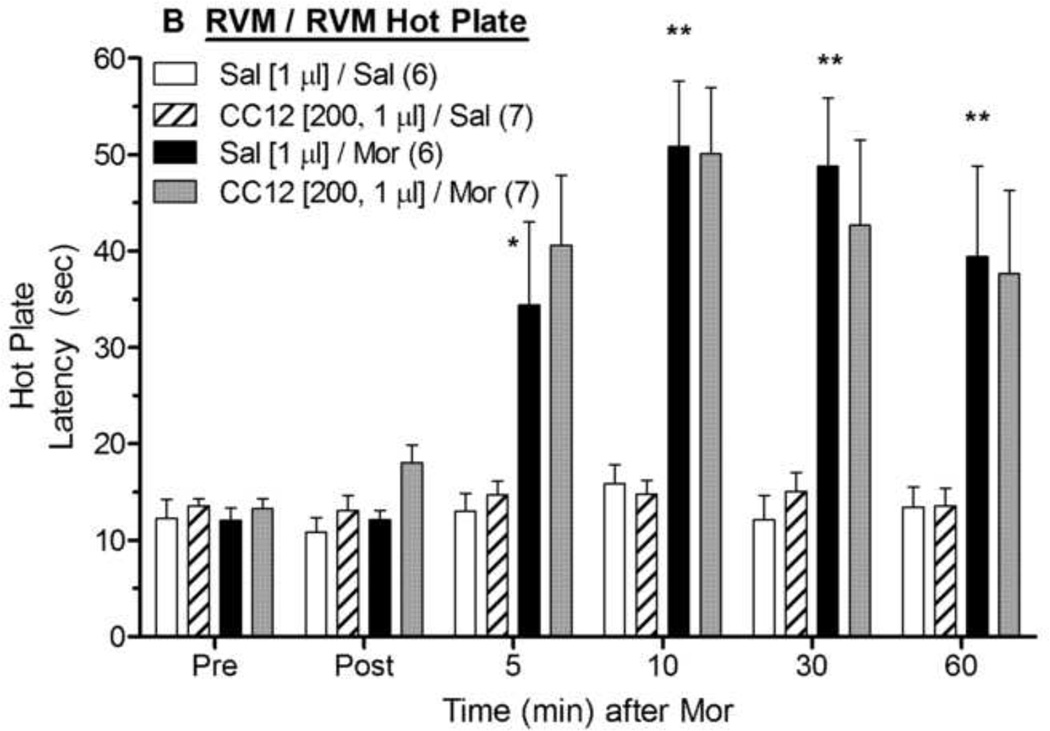
Intra-RVM injections of morphine produced the expected antinociceptive effects in both the tail flick (Fig. 2A) and hot plate (Fig. 2B) tests. ANOVA of the tail flick data in Fig. 2A (between groups: CC12, morphine; within groups: time) found significant main effects of morphine (F1,22= 48.9, P<0.0001) and time (F5,110 =12.1, P<0.0001), with a significant (F5,110 =7.8, P<0.0001) morphine by time interaction term. However, unlike the results found with CC12 injections in the PAG, intra-RVM pretreatment with CC12 (200 nmol) significantly antagonized morphine antinociception in the tail flick test (Fig. 2A). This same CC12 treatment had no effect on tail flick latencies in the absence of morphine (Fig. 2A). The same ANOVA found a significant (F1,22= 4.4, P < 0.05) main effect of CC12, and a significant (F1,22= 4.7, P < 0.05) CC12 by morphine interaction term. Post-hoc testing did not detect effects of CC12 on morphine antinociception at specific times (P > 0.05). On the hot plate test, intra-RVM morphine produced robust antinociception that was not antagonized by intra-RVM CC12 pretreatment (Fig. 2B). ANOVA of the data in Fig. 2B found significant main effects of morphine (F1,22= 36.6, P<0.0001) and time (F5,110 =18.5, P<0.0001), with a significant (P<0.0001) morphine by time interaction term. However, none of the CC12-related ANOVA terms from the hot plate data were statistically significant (P > 0.77). A lower dose of CC12 (100 nmol in 0.5 µl) had no statistically significant effect on morphine antinociception in either test (P>0.05, data not shown). No EMB or other abnormal responses were observed in any of the RVM microinjection studies.
Figure 2. Effect of CC12 on morphine antinociception in the RVM.
Experiments were performed exactly as in Fig. 1, except that two ic injections were made into the RVM. Tail flick (A) and hot plate (B) latencies are shown. *,**P <0.05, 0.01 respectively, versus Sal/Sal at the same time; +P< 0.05 main effect of CC12 and significant CC12 by morphine interaction term by ANOVA. Post-hoc testing did not detect effects of CC12 on morphine antinociception at specific times (P > 0.05). Cannula placements are shown in Fig. 4.
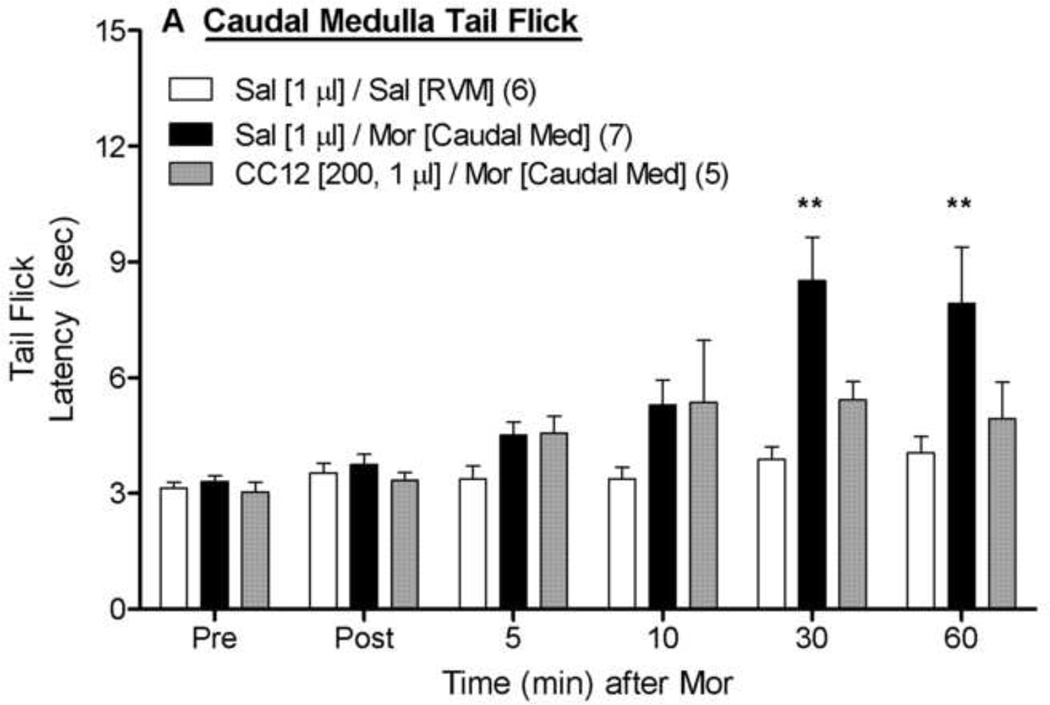
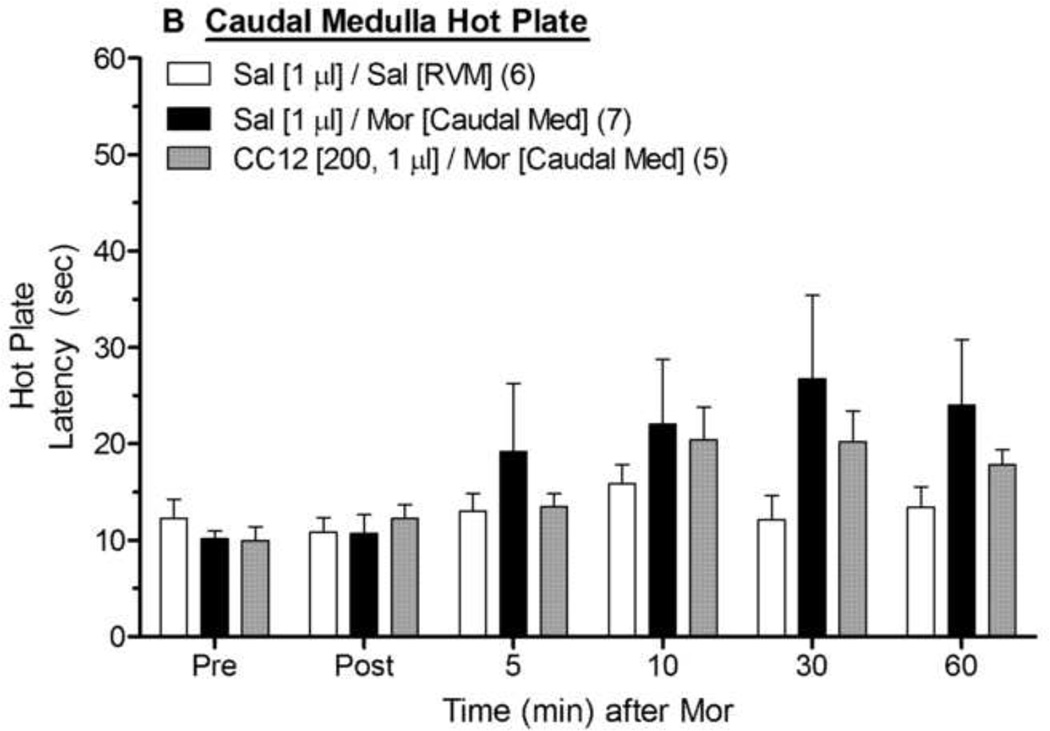
To assess the anatomical specificity of the results discussed above, CC12-morphine experiments were also performed with ic microinjections made into more caudal medullary structures near the RVM (Fig. 3). On the tail flick test (Fig. 3A), ANOVA of saline- and morphine-treated subjects (between groups: morphine; within groups: time) found a significant main effect of morphine and a significant morphine by time interaction (P <0.01 for both). As compared with morphine injections into the RVM, injections into these caudal locations produced approximately 50% less antinociception (compare 30 and 60 min data from Sal/Mor groups in Fig. 3A vs. Fig. 2A). ANOVA of the tail flick data from morphine-treated rats in Fig. 3A (between groups: CC12; within groups: time) found a significant main effect of time (F5,50 =7.1, P<0.0001), but no significant main effect of CC12 (P=0.08) or significant CC12 by time interaction term (P=0.15), indicating a lack of detectable effect of CC12. Virtually identical findings were obtained with the hot plate data from the caudal microinjections (Fig. 3B). Cannula placements for the data in Figs. 1 – 3 are given in Fig. 4.
Figure 3. Effect of CC12 on morphine antinociception in the caudal medulla.
Experiments were performed exactly as in Fig. 1, and Fig. 2, except that two ic injections were made into the caudal medulla (−11.6 mm from bregma in Fig. 4). Sal/Sal data were re-drawn from the RVM studies of Fig. 2. Tail flick (A) and hot plate (B) latencies are shown. **P< 0.01, respectively versus Sal/Sal at the same time point.
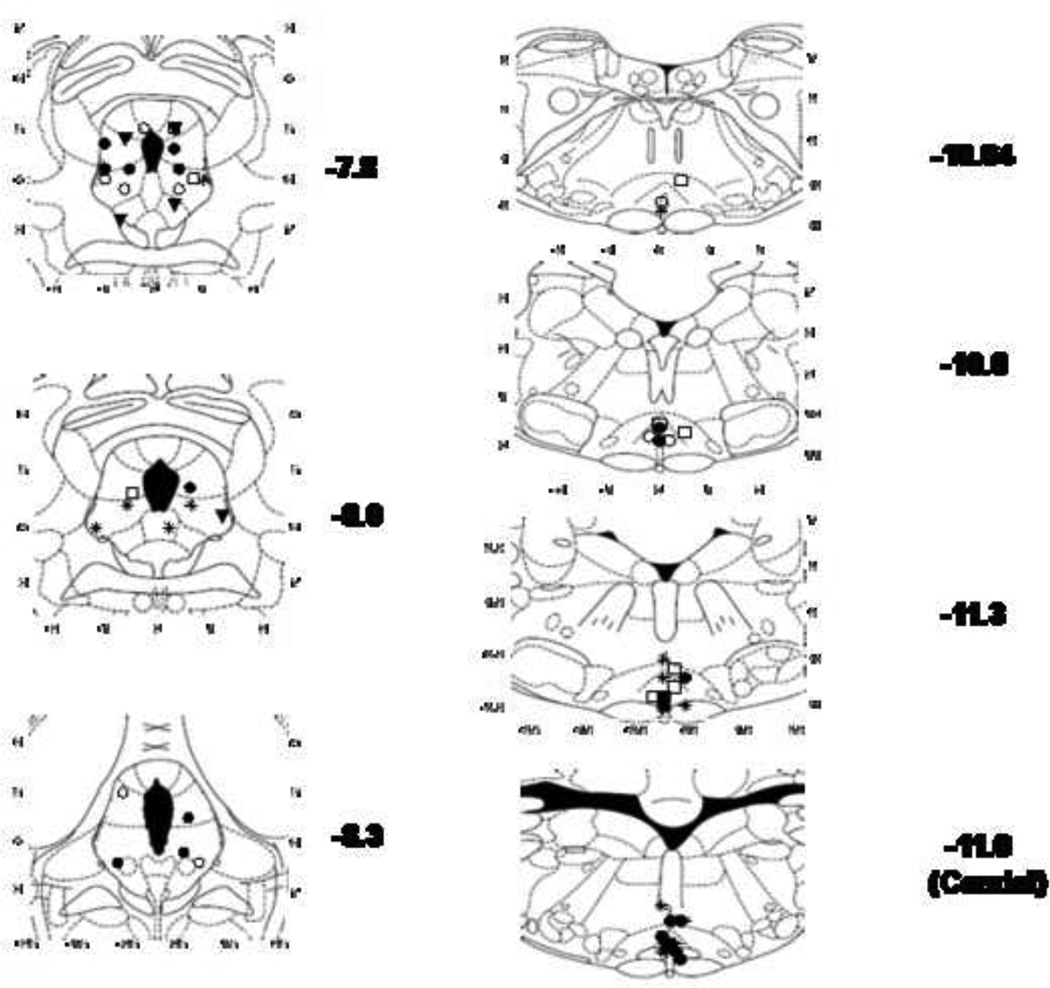
Figure 4. Ic injection sites in the PAG, RVM, and caudal medulla.
Injection sites from the experiments in Figs. 1–3 are shown at the designated AP planes (in mm from bregma, Paxinos and Watson, 1986): solid circles-(Sal/Mor), open circles- (Sal/Sal), open squares-(CC12/Sal), solid inverted triangles-(CC12 [100, 0.5 µl]/ Mor), and stars- (CC12 [200, 1.0 µl])/Mor). PAG sites (left) correspond to the data in Fig. 1. PAG sites shown at −7.8 mm included placements at both −7.8 and −7.3 mm from bregma. RVM sites (for the data in Fig. 2) are shown in the top 3 panels on the right. Placements for injections into the caudal medulla (Fig. 3 experiments) are depicted in the lower right panel (−11.6 mm). Four of these injections shown at −11.6 mm included placements at −11.8 (3) and −12.3 mm (1).
Although the present findings (Fig. 2) show that P450 activity in the RVM is important for normal morphine action in the RVM, a role for RVM P450 enzymes in the antinociception produced by intra-PAG morphine was not known. Unfortunately, technical difficulties prevented the completion of double-ic injection experiments designed to answer this question. When experiments were performed to measure the effects of intra-RVM CC12 on intra-PAG administered morphine, severe EMB was produced after PAG morphine injections in nearly every subject, preventing nociceptive testing.
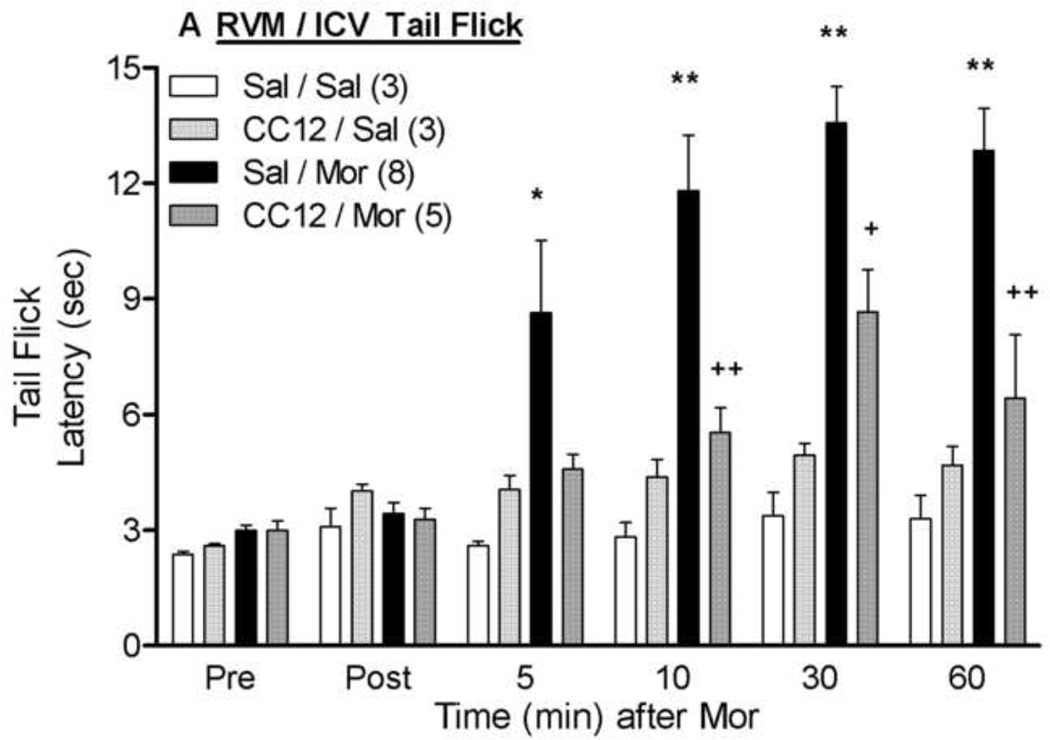
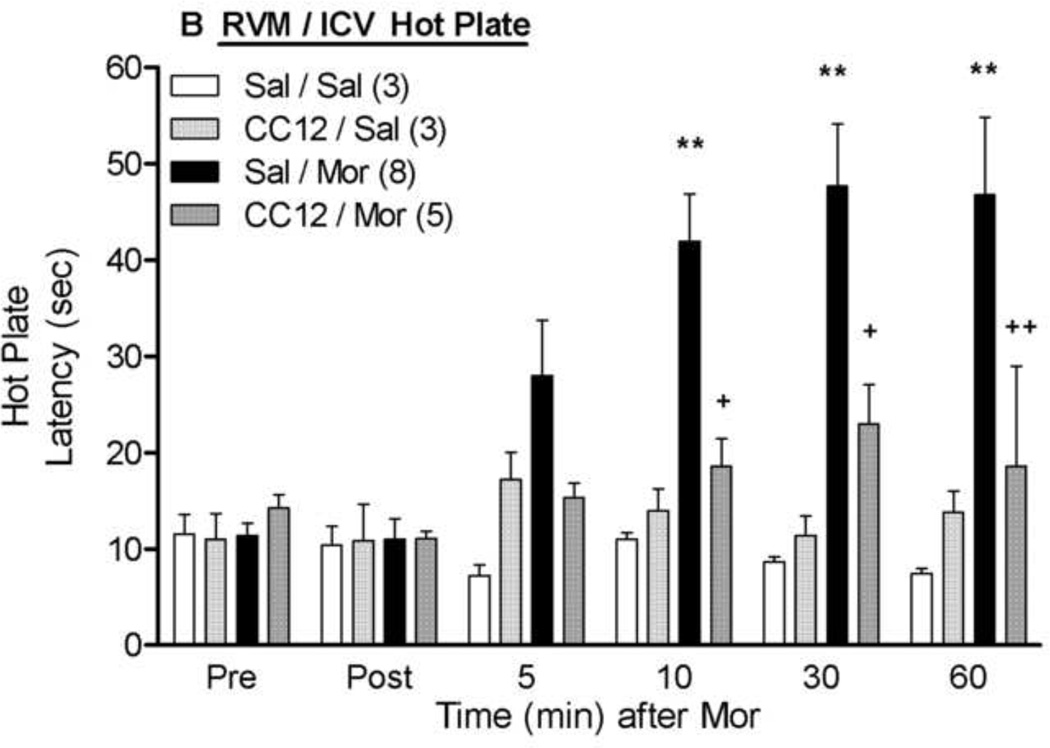
As an alternative experiment to determining if CC12 acts within the RVM to block afferent antinociceptive signaling from higher brain regions, the effects of intra-RVM CC12 were studied on icv morphine antinociception (Fig. 5). Icv administration of morphine (20 µg) significantly increased both tail flick and hot plate latencies; both effects were largely attenuated by RVM CC12 (200 nmol) pretreatment. ANOVA of the data from Fig. 5A (between groups: CC12, morphine; within groups: time) found highly significant main effects of morphine (F1,15= 22.0, P<0.001), time (F5,75 =13.5, P<0.0001), and a morphine by time interaction (F5,75 =7.6, P<0.0001). Most critically, significant CC12 by morphine (F1,15 =10.1, P<0.01) and CC12 by morphine by time (F5,75 =2.6, P<0.05) interaction terms were obtained. Virtually identical results were obtained from the ANOVA of the hot plate data (Fig. 5B). A lower dose of intra-RVM CC12 (100 nmol, 0.5 µl) tended to reduce icv morphine antinociception on both tests, but this effect was not statistically significant (data not shown).
Figure 5. Inhibition of icv morphine antinociception by intra-RVM CC12.
Surgically cannulated subjects were baseline tested (Pre) for nociceptive thresholds and received an ic (RVM) injection of saline (Sal) or CC12 (200 nmol; 1.0 µl), were re-tested 15 min later (Post), and immediately received an icv injection of saline (Sal) or morphine sulfate (Mor, 20 µg; 5 µl). Tail flick (A) and hot plate (B) latencies (ordinate, sec, mean SEM, number of subjects in parentheses) were recorded at the time points indicated after the icv injection (abscissa, min). *, ** P< 0.05, 0.01, respectively versus Sal/Sal; +,++ P< 0.05, 0.01, respectively versus Sal/Mor at the same time point. Placements for RVM injections were similar to those in Fig. 2.
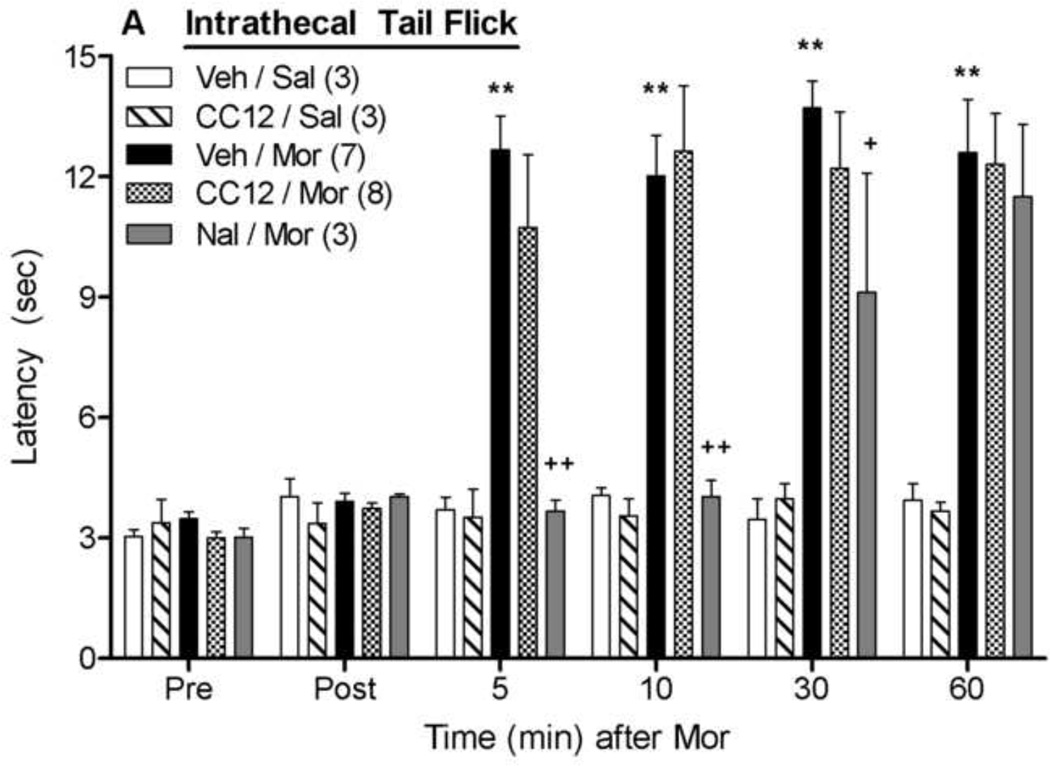
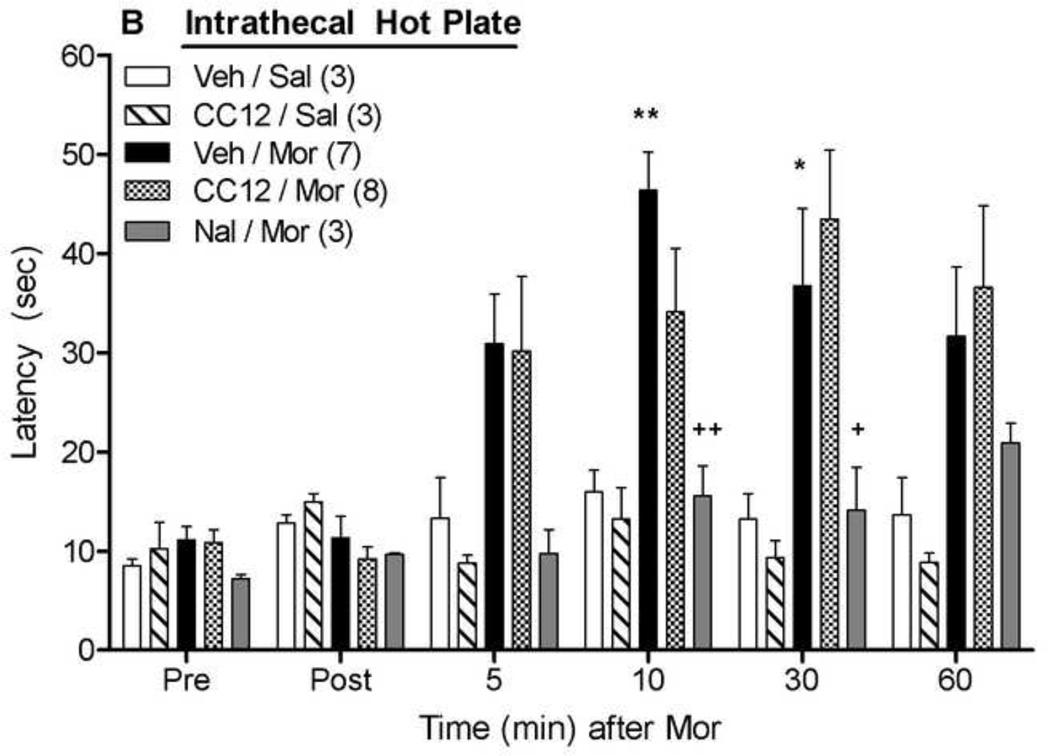
The effects of intrathecally-administered CC12 were also studied on antinociceptive responses following intrathecal morphine (Fig. 6). Morphine (20 µg) increased both tail flick (Fig. 6A) and hot plate (Fig. 6B) nociceptive latencies, neither of which were affected by intrathecal CC12 pretreatment (500 nmol). ANOVA of the data in Fig. 6A (between groups: CC12, morphine; within groups: time) found significant main effects of morphine (F1,17=42.3, P<0.00001) and time (F5,85 =22.1, P<0.00001), with a significant (F5,85 =19.3, P<0.00001) morphine by time interaction term. The same ANOVA found no effects of CC12 (P >0.5 for all CC12-related terms). Virtually identical results with morphine and CC12 were observed on the hot plate latencies (Fig. 6B). Intrathecal CC12 treatment alone had no observable behavioral effects and did not modify nociceptive thresholds. As expected, morphine antinociception was blocked in both tests by pretreatment with intrathecal naltrexone (40 nmol). ANOVA of Veh/Mor and Nal/Mor data of Fig. 6A (between groups: naltrexone, within groups: time) found significant main effects of naltrexone (F1,8 =25.4, P<0.001), time (F5,40 =24.2, P<0.0001), and a significant naltrexone by time interaction (F5,40 =7.1, P<0.0001). The identical ANOVA performed on data of Fig. 6B found significant main effects of naltrexone (F1,8 =15.7, P<0.01) and time (F5,40 =5.1, P<0.01).
Figure 6. Effects of intrathecally-administered CC12 on intrathecal morphine antinociception.
Intrathecally catheterized subjects were baseline tested (Pre) as in Fig 1, and received an intrathecal injection of vehicle (Veh, 50% DMSO, 10 µl,), CC12 (500 nmol, 10µl), or naltrexone hydrochloride (Nal, 40 nmol, 10 µl). Subjects were re-tested (Post) 15 min later and immediately received a second injection of saline (Sal, 10 µl) or morphine sulfate (Mor, 20 µg, 10 µl). Tail flick (A) and hot plate (B) latencies (ordinate, sec, mean SEM, number of subjects in parentheses) were recorded at the time points indicated after the second injection (abscissa, min). **P<0.01 versus Veh/Sal; +,++ P<0.05, 0.01, respectively, versus Veh/Mor at the same time point. ##P<0.01 for main effect of Nal in ANOVA. (B).
3. Discussion
Mu opioid receptors are densely expressed in the PAG, RVM, and dorsal horn of the spinal cord (Gutstein et al., 1998;Mansour et al., 1995;Thompson et al., 1993), and morphine injected into any of these sites produces behavioral antinociception. When administered systemically, morphine causes antinociception by activating μ opioid receptors in all three sites (Yaksh et al., 1988; Yaksh and Rudy, 1978; Yaksh et al., 1976; Yeung and Rudy, 1980). The action of morphine in the PAG stimulates the activity of RVM output neurons, an effect which is synergistic with opioid actions in the RVM (Rossi et al., 1993; Kiefel et al., 1993; Pan and Fields, 1996). Neural activity within the RVM is required for antinociception produced by intra- PAG, icv, or systemic morphine administration (Urban and Smith, 1994; Mitchell et al., 1998; Heinricher et al., 2001b; Gilbert and Franklin, 2002). Most evidence suggests that neural activity in the RVM produces behavioral antinociception by stimulation of descending circuits which attenuate incoming spinal nociceptive activity.
The antinociceptive mechanisms of morphine after intra-PAG and intra-RVM administration share many similarities. For example, both effects are mediated by μ opioid receptors and are blocked by co-administration of opioid antagonists (Jensen and Yaksh, 1986;Yaksh and Rudy, 1978;Yaksh et al., 1976). In both the PAG and RVM, receptor activation leads to hyperpolarization of GABAergic terminals, with subsequent excitation of postsynaptic output cells (Christie et al., 2000; Vaughan et al., 1997; Heinricher and Ingram, 2008). Excitatory outputs from the PAG project to the RVM and those from the RVM project to spinal nociceptive-modulatory neurons. This theory of opioid action in both brain regions is supported by data showing that GABAA agonists (e.g. muscimol) microinjected into either the PAG or RVM block the antinociceptive effects of morphine administered into the same site (Depaulis et al., 1987; Moreau and Fields, 1986; Zambotti et al., 1982). Additionally, GABAA antagonists (e.g. bicuculline) produce antinociception after intra-PAG as well as intra-RVM administration (Heinricher and Kaplan, 1991; Heinricher and Tortorici, 1994; Moreau and Fields, 1986). Other similarities in the pharmacological modulation of morphine antinociception in the PAG and RVM include antagonism by orphanin FQ and cholecystokinin (Heinricher et al., 1997; Heinricher et al., 2001a; Li and Han, 1989; Morgan et al., 1997).
CC12 was first discovered as an antagonist of several classes of brain stem-acting analgesics, including morphine (Hough et al 2007). Subsequent characterization found this drug to be a potent inhibitor (IC50 values from 12 to 490 nM) of numerous P450 isoforms (Stadel et al., 2008). The compound was also screened on over 20 analgesia-related receptors and found to lack activity at several opioid, serotonin, amino acid, histamine, and other receptors (Hough et al 2007). Additional, more recent support for the P450 hypothesis of morphine action includes inhibition of morphine analgesia by 3 other P450 inhibitors (Conroy et al., 2010), defective morphine analgesia in brain P450-deficient mutant mice (Conroy et al., 2010), and inhibition of μ-stimulated neuronal activity in RVM slices by the P450 inhibitor miconazole (Zhang and Pan, 2012).
As noted above, a previous study reported the inhibition of morphine by icv-administered CC12, but the brain sites involved were not known (Hough et al., 2007). Because morphine acts on μ opioid receptors by similar mechanisms in the RVM and the PAG to produce antinociception, it seemed likely that both intra-PAG and intra-RVM CC12 would block morphine antinociception elicited from the same brain site. Although intra-RVM CC12 blocked the antinociception produced by local morphine administration (Fig. 2), complementary experiments with PAG injections found no such effect (Fig. 1). These results suggest that the signaling mechanisms following μ opioid receptor activation differ between these two brain sites. Since CC12 is thought to block analgesia by inhibiting brain P450 epoxygenase activity (Hough et al., 2007; Conroy et al., 2010), the data of Fig. 2 and Fig. 5 suggests that morphine antinociception utilizes P450 activity in the RVM. This conclusion is supported by very recent data showing that μ receptor-stimulated activity in slices of rat RVM is attenuated by P450 blockade (Zhang and Pan, 2012). Similarly, the lack of antagonist activity of CC12 in the PAG (Fig. 1) predicts that μ opioid effects elicited in PAG slices would be resistant to P450 inhibitors. Interestingly, the P450 inhibitor proadifen partially blocked the putatively μ-mediated effects of methionine enkephalin in earlier experiments with PAG slices (Vaughan et al., 1997). In that study, a lipoxygenase (not epoxygenase) pathway was imputed, but this conclusion is controversial in light of later findings (see Walters et al., 2003, but also Zhang and Pan, 2012).
It is also interesting to note that doubling the injection volume of CC12 from 0.5 µl to 1 µl was necessary to significantly antagonize the effects of a small volume (0.5 µl) of morphine injected into the RVM. Since a larger injection volume delivers drugs to lateral and dorsal brain areas, this finding suggests that a single midline injection of morphine activates neuronal circuitry in lateral and/or dorsal structures. Similar to our present results with P450 inhibitors, earlier microinjection studies found that pharmacological inactivation of both medial and lateral structures within the RVM is necessary to block the antinociception produced by a single unilateral injection of morphine into the PAG (Urban and Smith, 1994). Estimates by these authors from earlier work suggested that microinjection volumes of 0.5 and 1.0 µl achieve effective drug concentrations with radii of 0.5 and 1.0 mm, respectively. Despite the need for a large distribution of CC12 within the RVM, there is still anatomical specificity to these drug actions, since reduced effects of morphine and CC12 were observed when microinjections were made into caudal medulla (Fig. 3).
The lack of morphine antagonism by CC12 in the PAG gives further insight into the cellular actions of CC12. For example, the anti-opioid actions of CC12 within the RVM are not due to non-specific inhibition of all neuronal activity, as is the case with local anesthetics (e.g. lidocaine) or hyperpolarizing agents (e.g. orphanin F/Q). Unlike lidocaine, which blocks morphine antinociception in both brain areas (Urban and Smith, 1994), CC12 selectively antagonizes morphine actions in the RVM, but not in the PAG. This proposed lack of local anesthetic-like effects of CC12 is supported by recent work in which icv administration of CC12 blocked the effects of morphine on nociceptive modulatory RVM neurons during noxious stimulation, but had no effects on the baseline firing rates of three classes of physiologically-identified neurons (Heinricher et al., 2010).
The transduction mechanisms underlying spinal morphine analgesia are distinct from those mediating supraspinal morphine actions (Millan, 2002; Marker et al., 2004), and no previous data exist to predict the importance of spinal P450 activity. As found in the case of the PAG studies discussed above (Fig. 1), the present results with intrathecal CC12 (Fig. 6) suggest that P450 activity in the spinal cord is not required for spinal opioid antinociception.
Within the PAG, opioids activate output neurons that project directly to the RVM (Osborne et al., 1996; Tortorici and Morgan, 2002). After icv administration, morphine acts in both the PAG and RVM to produce antinociception and stimulation of μ opioid receptors in both brain area results in the synergistic activation of descending analgesic circuits (Rossi et al., 1993). Therefore, to determine if RVM P450 activity is needed for supraspinal (and presumably PAG) morphine analgesic signaling, the effect of intra-RVM CC12 was assessed on antinociception produced by icv morphine. The finding that RVM pretreatment with CC12 resulted in nearly complete blockade of this antinociception (Fig. 5) indicates that RVM P450 activity is important for not only RVM-initiated morphine antinociception, but for virtually all supraspinal morphine analgesic signaling.
The present study used two behavioral measures of thermal nociception: the tail flick (mostly a spinally-generated reflex) and hot plate (a supraspinally-organized response) tests. Activation of supraspinal, descending analgesic circuits which dampen spinal nociceptive transmission would be expected to inhibit both of these responses, since attenuation of incoming painful stimuli would abolish both the spinally-organized and supraspinally-organized nocifensive behaviors. Consistent with this idea, nearly all of the present hot plate data were in close agreement with corresponding tail flick results (cf. B vs. A panels in Figs. 1, 3, and 5). The exception was found in the RVM results of Fig. 2, in which CC12 blocked morphine actions on the tail flick, but not on the hot plate test. Although these findings might suggest that a P450 mediates the morphine-induced modulation of the former and not the latter, other explanations are possible (e.g. possible motor-impairing effects of intra-RVM morphine). A role for RVM P450s after icv morphine seems to be substantiated by the antagonist actions of intra-RVM CC12 on both nociceptive tests (Fig. 5).
We recently used pharmacological inhibitors and a new line of transgenic mice to show that a brain P450 is required for the analgesic action of morphine (Conroy et al., 2010), but the brain area(s) containing the relevant P450 enzymes were not identified. Transgenic mice with deficits in neuronal P450 activity were generated by targeted deletion of the gene for cytochrome P450 reductase (Cpr; an enzyme necessary for all microsomal P450 activity) in CamKIIalphaexpressing neurons (via CamKIIalpha-Cre). The finding that these mice showed severe deficits in morphine antinociception strongly supports a P450 hypothesis for morphine analgesic signaling (Conroy et al., 2010). However, P450 activity was ablated only in CamKIIalphaexpressing neurons in these mice. Since CamKIIalpha is not abundantly expressed in the brain stem (Dragatsis and Zeitlin, 2000), most neurons in the brain stem of these morphine-resistant mice retain normal CPR expression and therefore normal P450 activity. However, careful analysis of the brain stem of brain-Cpr-null mice identified a select population of neuronal cell bodies within the ventrolateral PAG that lacked CPR expression. Because the ventrolateral PAG is crucial for the full expression of morphine antinociception (Dostrovsky and Deakin, 1977; Guo and Tang, 1990), it was hypothesized that these neurons contain the morphine-relevant P450 enzyme (Conroy et al., 2010). Based on these findings, intra-PAG injections of CC12 were expected to block morphine actions in the PAG. In contrast, the present results showed that P450 activity is important for morphine antinociception in the RVM, not in the PAG.
A theory consistent with the current results (showing the RVM localization of morphinerelevant P450s) and our previous prediction (Conroy et al., 2010 suggested a PAG localization for these enzymes) can be proposed. It is well accepted that direct anatomical connections exist between the PAG and RVM, and that they are important for analgesic signaling (Heinricher and Ingram, 2008; Morgan et al., 2008). The cell bodies of P450-containing neurons may be located in the PAG and send projections that terminate in the RVM. These projection neurons could represent the CPR-deficient neurons identified in the brain-Cpr-null mice (Conroy et al., 2010). Morphine may stimulate μ opioid receptors on the terminals of these PAG projection neurons within the RVM to activate descending nociceptive modulatory neurons. In this case, the opioidrelevant P450 would be localized to the presynaptic inhibitory terminal on which morphine acts, and would be important for the antinociception produced by both intra-RVM and icvadministered morphine. Results consistent with this theory show that PAG-RVM projection neurons synapse directly on reticulospinal neurons which are known to be important in nociceptive modulation (Morgan et al., 2008). The model is also consistent with a very recent study reporting that μ receptor-stimulated inhibition of GABA IPSCs in RVM slices was attenuated by a P450 inhibitor (Zhang and Pan, 2012). Elucidation of the cell types and RVM circuitry accounting for morphine-P450 interactions will further our understanding of the biochemistry of pain relief, and may lead to the discovery of novel treatments for pain.
4. Experimental Procedure
Drugs and Solutions
Naltrexone hydrochloride and morphine sulfate were purchased from Sigma-RBI (St. Louis, MO). CC12 was synthesized as previously described (Hough et al., 2007). Morphine sulfate was dissolved in saline for all studies. For ic and icv testing, CC12 were dissolved in saline. For intrathecal testing, CC12 and naltrexone were dissolved in 50% DMSO.
Animals
Male Sprague-Dawley rats (250–350g at time of testing, Taconic Farms, Germantown, NY) were maintained on a 12-hour light/dark cycle (lights on from 0700 to 1900) with food and water provided ad libitum. Rats were house in groups of 3–4 per cage until the day of surgery and singly thereafter. Each animal was used for only a single experiment. All experiments were approved by the Institutional Animal Care and Use Committee of Albany Medical College.
Icv and Ic Cannulations
Animals were chronically cannulated as previously described (Crane and Glick, 1979). Rats were anesthetized with pentobarbital (25 mg/kg, i.p.) and supplemented with isoflurane. Chronic cannulas were stereotaxically implanted into designated brain stem sites or into the lateral ventricle and anchored to the skull with three stainless steel screws and dental cement. Stereotaxic coordinates (AP, ML and DV, mm from bregma, Nalwalk et al., 2004; Paxinos and Watson, 1986) for placements of the guide cannulas were: PAG (−7.8, 1.8, −2.7, 14° angle), rostral ventromedial medulla (RVM, −11.0, 0.0, −7.5), and lateral ventricle (−0.8, 1.5, − 3.3). Some subjects had cannulas surgically implanted in both the RVM and the lateral ventricle. Following surgery, the animals were allowed to recover for a minimum of 5–7 days before testing. Each animal was used for a single experiment.
Intrathecal Cannulations
Rats (300–450g) were chronically cannulated with catheters in the subarachnoid space as previously described (Yaksh and Rudy, 1976; Hammond, 1988; Nalwalk et al., 2004). Animals were anesthetized as described above and the atlanto-occipital membrane was exposed. A transverse slit was made at the base of the cisternal membrane and a saline-filled catheter (consisting of PE10 polyethylene tubing with a 1.5 cm piece of silastic tubing at the tip; total length inserted 8.5 cm) was threaded into the subarachnoid space until the tip reached the thoraco-lumbar enlargement. Holes were then drilled into the skull and the exposed end of the catheter was threaded though. The catheter position was fixed by a ‘bubble’ in the tubing that acts as a stop to prevent the catheter from pulling out of position. Saline was infused during and after final placement to ensure patency. The wound was closed, the catheter sealed with a stainless steel plug, and the animal monitored closely for at least 10 days. Any animals showing signs of motor impairment or significant weight loss were removed from the study.
Nociceptive Testing
The radiant heat tail flick (D'Amour and Smith, 1941) and hot plate (Eddy and Leimbach, 1953) tests were used to measure thermal nociceptive latencies. For the tail flick test, the ventral surface of the tail (2–5 cm from the tip) was placed over a radiant heat source and the latency for removal of the tail was recorded. The heat source was set so that baseline latencies were between 2.5–4 sec with a maximum exposure time of 15 sec to prevent tissue damage. The heat source was not adjusted for each animal. For the hot plate test, animals were placed on a 52°C surface and the latency for the animal to lift and lick a hind paw was recorded. Baseline latencies were between 10–15 sec with a maximum exposure time of 60 sec. On the day of testing, animals were baseline tested for hot plate latencies followed by three tail-flick baseline measurements, each being one min apart. The third tail flick measurement was used as the baseline value. The animal was then gently held under a laboratory pad and the stylet was removed from the guide cannula. The injection cannula, attached to a Hamilton syringe with 20 PE tubing, was inserted into the guide cannula. For ic experiments, subjects received a single injection into the specified brain area (0.5 or 1.0 µl administered over one min), and were returned to their home cages. They were retested for nociceptive latencies 15 min later, followed by either a second ic injection, or a single icv injection (5 µl administered over 5 min). The cannula was then clipped with wire cutters above the guide cannula; animals were returned to their home cage and tested for hot plate and tail flick latencies at the indicated time points postinjection. For icv and ic microinjections, injection cannula lengths were 1 and 2 mm, respectively, beyond the tip of the guide cannulas. After testing, animals were overdosed with sodium pentobarbital and received a single icv injection (5 l) and/or a single ic injection (1.0 l or 0.5 l) of India Ink. For intrathecal (i.t.) injections, drugs were injected manually in a total volume of 10 µl over 1 min, followed by a 10 µl flush of sterile saline over 1 min. Successful icv, ic, and i.t. injections were assured by following the movement of an air bubble in the tubing between the syringe and the cannula and by the absence of leakage. Cannula placements were verified by proper distribution of ink in the cerebroventricular system (icv) or within the proper brain areas after sectioning and staining as previously described (Nalwalk et al., 2004).
Data Analysis
Nociceptive latencies were analyzed with repeated measures analysis of variance (ANOVA). When permitted by the ANOVA results (i.e. significant interaction terms), post-hoc comparisons were made by the Bonferroni test (Statistica, Statsoft, Tulsa, OK).
Highlights.
Recent work demonstrated that mu opioids such as morphine relieve pain by mu signaling through a cytochrome P450 epoxygenase mechanism.
Morphine works in several regions of the brain stem and spinal cord, but the location of the cytochrome P450-relevant CNS area had not been identified.
In rats, the P450 blocker inhibited morphine antinociception when both drugs were injected into the rostral ventromedial medulla, but not following co-injections into the periaqueductal gray or into the spinal subarachnoid space. Thus, P450 activity within the RVM is essential for supraspinal morphine antinociception.
Characterization of morphine-P450 interactions within RVM circuits will further enhance the understanding of the biochemistry of pain relief.
Acknowledgement
This work was supported by grants (DA03816, DA027835) from the National Institute on Drug Abuse.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aoki T, Narita M, Ohnishi O, Mizuo K, Narita M, Yajima Y, Suzuki T. Disruption of the type 1 inositol 1,4,5-trisphosphate receptor gene suppresses the morphine-induced antinociception in the mouse. Neurosci. Lett. 2003;350:69–72. doi: 10.1016/s0304-3940(03)00829-2. [DOI] [PubMed] [Google Scholar]
- Blair R, Liran J, Cytryniak P, Shizgal P, Amit Z. Explosive motor behavior, rigidity and periaqueductal gray lesions. Neuropharmacol. 1978;17:205–209. doi: 10.1016/0028-3908(78)90101-6. [DOI] [PubMed] [Google Scholar]
- Christie MJ, Connor M, Vaughan CW, Ingram SL, Bagley EE. Cellular actions of opioids and other analgesics: implications for synergism in pain relief. Clin. Exp. Pharmacol. Physiol. 2000;27:520–523. doi: 10.1046/j.1440-1681.2000.03291.x. [DOI] [PubMed] [Google Scholar]
- Christie MJ, Vaughan CW, Ingram SL. Opioids, NSAIDs and 5-lipoxygenase inhibitors act synergistically in brain via arachidonic acid metabolism. Inflamm. Res. 1999;48:1–4. doi: 10.1007/s000110050367. [DOI] [PubMed] [Google Scholar]
- Conroy JL, Fang C, Gu J, Zeitlin SO, Yang W, Yang J, VanAlstine MA, Nalwalk JW, Albrecht PJ, Mazurkiewicz JE, Snyder-Keller A, Shan Z, Zhang SZ, Wentland MP, Behr M, Knapp BI, Bidlack JM, Zuiderveld OP, Leurs R, Ding X, Hough LB. Opioids activate brain analgesic circuits through cytochrome P450/epoxygenase signaling. Nat. Neurosci. 2010;13:284–286. doi: 10.1038/nn.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane LA, Glick SD. Simple cannula for repeated intracerebral drug administration in rats. Pharmacol. Biochem. Behav. 1979;10:799–800. doi: 10.1016/0091-3057(79)90336-8. [DOI] [PubMed] [Google Scholar]
- Criswell HE. Analgesia and hyperreactivity following morphine microinjection into the mouse brain. Pharmacol. Biochem. Behav. 1976;4:23–26. doi: 10.1016/0091-3057(76)90170-2. [DOI] [PubMed] [Google Scholar]
- D'Amour FE, Smith DL. A methods for determining loss of pain sensation. J. Pharmacol. Exp. Ther. 1941;72:74–79. [Google Scholar]
- Depaulis A, Morgan MM, Liebeskind JC. GABAergic modulation of the analgesic effects of morphine microinjected in the ventral periaqueductal grey matter of the rat. Brain Res. 1987;436:223–228. doi: 10.1016/0006-8993(87)91665-9. [DOI] [PubMed] [Google Scholar]
- Dostrovsky JO, Deakin JFW. Periaqueductal grey lesions reduce morphine analgesia in the rat. Neurosci. Lett. 1977;4:99–103. doi: 10.1016/0304-3940(77)90151-3. [DOI] [PubMed] [Google Scholar]
- Dragatsis I, Zeitlin S. CaMKIIalpha-Cre transgene expression and recombination patterns in the mouse brain. Genesis. 2000;26:133–135. doi: 10.1002/(sici)1526-968x(200002)26:2<133::aid-gene10>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Eddy NB, Leimbach D. Synthetic analgesics. II. Dithienylbutenyl- and dithienylbutylamines. J. Pharmacol. Exp. Ther. 1953;107:385–393. [PubMed] [Google Scholar]
- Gilbert AK, Franklin KB. The role of descending fibers from the rostral ventromedial medulla in opioid analgesia in rats. Eur. J. Pharmacol. 2002;449:75–84. doi: 10.1016/s0014-2999(02)01974-x. [DOI] [PubMed] [Google Scholar]
- Guo X, Tang XC. Roles of periaqueductal gray and nucleus raphe magnus on analgesia induced by lappaconitine, N-deacetyllappaconitine and morphine. Zhongguo Yao Li Xue. Bao. 1990;11:107–112. [PubMed] [Google Scholar]
- Gutstein HB, Akil H. Opioid Analgesics. In: Brunton LL, Lazo JS, Parker KL, editors. Goodman and Gilman's The Pharmacological Basis of Therapeutics. McGraw-Hill Companies; 2006. pp. 547–590. [Google Scholar]
- Gutstein HB, Mansour A, Watson SJ, Akil H, Fields HL. Mu and kappa opioid receptors in periaqueductal gray and rostral ventromedial medulla. Neuroreport. 1998;9:1777–1781. doi: 10.1097/00001756-199806010-00019. [DOI] [PubMed] [Google Scholar]
- Hammond DL. Intrathecal administration: methodological considerations. Prog. Brain Res. 1988;77:313–320. doi: 10.1016/s0079-6123(08)62797-1. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Ingram SL. The Brainstem and Nociceptive Modulation. In: A.I.Basbaum, M.C.B.a.D.J., editor. The Senses: A Comprehensive Reference. New York: Elsevier; 2008. Pain. [Google Scholar]
- Heinricher MM, Kaplan HJ. GABA-mediated inhibition in rostral ventromedial medulla: role in nociceptive modulation in the lightly anesthetized rat. Pain. 1991;47:105–113. doi: 10.1016/0304-3959(91)90017-R. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Maire JJ, Lee D, Nalwalk JW, Hough LB. Physiological basis for inhibition of morphine and improgan antinociception by CC12, a P450 epoxygenase inhibitor. J. Neurophysiol. 2010;104:3222–3230. doi: 10.1152/jn.00681.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinricher MM, McGaraughty S, Grandy DK. Circuitry underlying antiopioid actions of orphanin FQ in the rostral ventromedial medulla. J. Neurophysiol. 1997;78:3351–3358. doi: 10.1152/jn.1997.78.6.3351. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, McGaraughty S, Tortorici V. Circuitry underlying antiopioid actions of cholecystokinin within the rostral ventromedial medulla. J. Neurophysiol. 2001a;85:280–286. doi: 10.1152/jn.2001.85.1.280. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Schouten JC, Jobst EE. Activation of brainstem N-methyl-D-aspartate receptors is required for the analgesic actions of morphine given systemically. Pain. 2001b;92:129–138. doi: 10.1016/s0304-3959(00)00480-2. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Tortorici V. Interference with GABA transmission in the rostral ventromedial medulla: disinhibition of off-cells as a central mechanism in nociceptive modulation. Neuroscience. 1994;63:533–546. doi: 10.1016/0306-4522(94)90548-7. [DOI] [PubMed] [Google Scholar]
- Hough LB, Nalwalk JW, Phillips JG, Kern B, Shan Z, Wentland MP, de Esch IJ, Janssen E, Barr T, Stadel R. CC12, a high-affinity ligand for [3H]cimetidine binding, is an improgan antagonist. Neuropharmacology. 2007;52:1244–1255. doi: 10.1016/j.neuropharm.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquet YF, Lajtha A. Morphine action at central nervous system sites in rat: analgesia or hyperalgesia depending on site and dose. Science. 1973;182:490–492. doi: 10.1126/science.182.4111.490. [DOI] [PubMed] [Google Scholar]
- Jensen TS, Yaksh TL. I. Comparison of antinociceptive action of morphine in the periaqueductal grey, medial and paramedial medulla in rat. Brain Res. 1986;363:99–113. doi: 10.1016/0006-8993(86)90662-1. [DOI] [PubMed] [Google Scholar]
- Kiefel JM, Rossi GC, Bodnar RJ. Medullary and opioid receptors modulate mesencephalic morphine analgesia in rats. Brain Res. 1993;624:151–161. doi: 10.1016/0006-8993(93)90073-v. [DOI] [PubMed] [Google Scholar]
- Law PY, Wong YH, Loh HH. Molecular mechanisms and regulation of opioid receptor signaling. Annu. Rev. Pharmacol. Toxicol. 2000;40:389–430. doi: 10.1146/annurev.pharmtox.40.1.389. [DOI] [PubMed] [Google Scholar]
- Li Y, Han J-S. Cholecystokinin-octapeptide antagonizes morphine analgesia in periaqueductal gray of the rat. Brain Res. 1989;480:105–110. doi: 10.1016/0006-8993(89)91572-2. [DOI] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Burke S, Akil H, Watson SJ. Immunohistochemical localization of the cloned mu opioid receptor in the rat CNS. J. Chem. Neuroanat. 1995;8:283–305. doi: 10.1016/0891-0618(95)00055-c. [DOI] [PubMed] [Google Scholar]
- Marker CL, Stoffel M, Wickman K. Spinal G-protein-gated K+ channels formed by GIRK1 and GIRK2 subunits modulate thermal nociception and contribute to morphine analgesia. J. Neurosci. 2004;24:2806–2812. doi: 10.1523/JNEUROSCI.5251-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan MJ. Descending control of pain. Prog. Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- Mitchell JM, Lowe D, Fields HL. The contribution of the rostral ventromedial medulla to the antinociceptive effects of systemic morphine in restrained and unrestrained rats. Neuroscience. 1998;87:123–133. doi: 10.1016/s0306-4522(98)00119-5. [DOI] [PubMed] [Google Scholar]
- Moreau J-L, Fields HL. Evidence for GABA involvement in midbrain control of medullary neurons that modulate nociceptive transmission. Brain Res. 1986;397:37–46. doi: 10.1016/0006-8993(86)91367-3. [DOI] [PubMed] [Google Scholar]
- Morgan MM, Grisel JE, Robbins CS, Grandy DK. Antinociception mediated by the periaqueductal gray is attenuated by orphanin FQ. Neuroreport. 1997;8:3431–3434. doi: 10.1097/00001756-199711100-00003. [DOI] [PubMed] [Google Scholar]
- Morgan MM, Whittier KL, Hegarty DM, Aicher SA. Periaqueductal gray neurons project to spinally projecting GABAergic neurons in the rostral ventromedial medulla. Pain. 2008;140:376–386. doi: 10.1016/j.pain.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalwalk JW, Svokos K, Taraschenko O, Leurs R, Timmerman H, Hough LB. Activation of brain stem nuclei by improgan, a non-opioid analgesic. Brain Res. 2004;1021:248–255. doi: 10.1016/j.brainres.2004.06.066. [DOI] [PubMed] [Google Scholar]
- Narita M, Ohnishi O, Narita M, Aoki T, Suzuki M, Yajima Y, Funahashi H, Shioda S, Suzuki T. Direct evidence for the activation of phospholipase C gamma 1 by in vivo treatment with morphine in the mouse periaqueductal gray matter. Brain Res. 2003;970:140–148. doi: 10.1016/s0006-8993(03)02301-1. [DOI] [PubMed] [Google Scholar]
- Osborne PB, Vaughan CW, Wilson HI, Christie MJ. Opioid inhibition of rat periaqueductal grey neurones with identified projections to rostral ventromedial medulla in vitro. J. Physiol. (Lond.) 1996;490:383–389. doi: 10.1113/jphysiol.1996.sp021152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan ZZ, Fields HL. Endogenous opioid-mediated inhibition of putative pain-modulating neurons in rat rostral ventromedial medulla. Neurosci. 1996;74:855–862. doi: 10.1016/0306-4522(96)00179-0. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Sydney: Academic Press; 1986. [Google Scholar]
- Rossi GC, Pasternak GW, Bodnar RJ. Synergistic brainstem interactions for morphine analgesia. Brain Res. 1993;624:171–180. doi: 10.1016/0006-8993(93)90075-x. [DOI] [PubMed] [Google Scholar]
- Sharpe LG, Garnett JE, Cicero TJ. Analgesia and hyperreactivity produced by intracranial microinjections of morphine into the periaqueductal gray matter of the rat. Behav. Biol. 1974;11:303–313. doi: 10.1016/s0091-6773(74)90548-3. [DOI] [PubMed] [Google Scholar]
- Stadel R, Yang J, Nalwalk JW, Phillips JG, Hough LB. High-affinity binding of [3H]cimetidine to a heme-containing protein in rat brain. Drug Metab Dispos. 2008;36:614–621. doi: 10.1124/dmd.107.017889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RC, Mansour A, Akil H, Watson SJ. Cloning and pharmacological characterization of a rat mu opioid receptor. Neuron. 1993;11:903–913. doi: 10.1016/0896-6273(93)90120-g. [DOI] [PubMed] [Google Scholar]
- Tortorici V, Morgan MM. Comparison of morphine and kainic acid microinjections into identical PAG sites on the activity of RVM neurons. J. Neurophysiol. 2002;88:1707–1715. doi: 10.1152/jn.2002.88.4.1707. [DOI] [PubMed] [Google Scholar]
- Urban MO, Smith DJ. Nuclei within the rostral ventromedial medulla mediating morphine antinociception from the periaqueductal grey. Brain Res. 1994;652:9–16. doi: 10.1016/0006-8993(94)90311-5. [DOI] [PubMed] [Google Scholar]
- Vaughan CW, Ingram SL, Connor MA, Christie MJ. How opioids inhibit GABA-mediated neurotransmission. Nature. 1997;390:611–614. doi: 10.1038/37610. [DOI] [PubMed] [Google Scholar]
- Walters CL, Wang BC, Godfrey M, Sun D, Funk CD, Blendy JA. Augmented responses to morphine and cocaine in mice with a 12-lipoxygenase gene disruption. Psychopharmacology (Berl) 2003;170:124–131. doi: 10.1007/s00213-003-1526-7. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Al-Rodhan N, Jensen TS. Sites of action of opiates in production of analgesia. In: Fields HL, Besson JM, editors. Progress in Brain Research. Vol. 77. Amsterdam: Elsevier; 1988. pp. 371–394. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Rudy TA. Chronic catheterization of the spinal subarachnoid space. Physiol Behav. 1976;17:1031–1036. doi: 10.1016/0031-9384(76)90029-9. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Rudy TA. Narcotic analgestics: CNS sites and mechanisms of action as revealed by intracerebral injection techniques. Pain. 1978;4:299–359. doi: 10.1016/0304-3959(77)90145-2. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Yeung JC, Rudy TA. Systematic examination in the rat of brain sites sensitive to the direct application of morphine: observation of differential effects within the periaqueductal gray. Brain Res. 1976;114:83–103. doi: 10.1016/0006-8993(76)91009-x. [DOI] [PubMed] [Google Scholar]
- Yeung JC, Rudy TA. Sites of antinociceptive action of systemically injected morphine: involvement of supraspinal loci as revealed by intracerebroventricular injection of naloxone. J. Pharmacol. Exp. Ther. 1980;215:626–632. [PubMed] [Google Scholar]
- Zambotti F, Zonta N, Parenti M, Tommasi R, Vicentini L, Conci F, Mantegazza P. Periaqueductal gray matter involvement in the muscimol-induced decrease of morphine antinociception. Naunyn Schmiedebergs Arch. Pharmacol. 1982;318:368–369. doi: 10.1007/BF00501180. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Pan ZZ. Signaling cascades for delta-opioid receptor-mediated inhibition of GABA synaptic transmission and behavioral antinociception. Mol. Pharmacol. 2012;81:375–383. doi: 10.1124/mol.111.076307. [DOI] [PMC free article] [PubMed] [Google Scholar]