Abstract
Studies have shown that implanting olfactory ensheathing cells (OECs) may be a promising therapeutic strategy to promote functional recovery after spinal cord injury. Several fundamental questions remain, however, regarding their in vivo interactions in the damaged spinal cord. We have induced a clip compression injury at the T10 level of the spinal cord in adult rats. After a delay of 1 week, OECs isolated from embryonic day 18 rats were implanted into the cystic cavity that had formed at the site of injury. Before implantation, OECs were infected with a LacZ-expressing retrovirus. At 3 weeks after implantation, LacZ-expressing OECs survived the implantation procedure and remained localized to the cystic cavity. At the electron microscopic level, the cystic cavity had clusters of LacZ-expressing OECs and numerous Schwann cells lacking LacZ expression. Although labeled OECs made no direct contact with axons, unlabeled Schwann cells were associated with either a single myelinated axon or multiple unmyelinated axons. Positively labeled OEC processes often enveloped multiple Schwann cell-axon units. These observations suggest that the role of OECs as the primary mediators of the beneficial effects on axon growth, myelination, and functional recovery after spinal cord injury may require re-evaluation.
Worldwide, the annual incidence of spinal cord injury (SCI) ranges from 1.7 to 4 per 100,000 individuals (1). Among the many strategies to reverse the permanent damage after SCI is the implantation of various cell types, such as olfactory ensheathing cells (OECs), into the site of injury (2-4). OECs are glial cells that ensheath the axons of the first cranial nerve. These glia also support the continual new growth of small caliber olfactory axons across the peripheral nervous system-central nervous system border throughout the life of adult mammals (reviewed in refs. 5-8). This unique ability has sparked a decade of research investigating the feasibility of these cells as suitable candidates for intraspinal implantation and amelioration of the functional deficits that follow SCI (5, 9-12).
OECs isolated from neonatal or adult animals have been surgically implanted into the spinal cord of adult rats after trauma induced by a variety of methods. This implantation strategy has been associated with increased axonal regeneration and/or sparing of residual axons (13-24), remyelination of axons (25-31), and improved recovery of locomotor function (13-24, 32).
OECs have been regarded as a heterogeneous population of cells, sharing antigenic features with both astrocytes and Schwann cells (6, 8). As such, visualization of OECs after implantation into the damaged spinal cord has been complicated by the lack of specific and definitive markers. Although OECs have been successfully labeled with Hoechst (13, 14) and Cell-Tracker probes (17, 18), these dyes can be phagocytosed by other cellular elements at the site of intraspinal OEC implantation and result in the overestimation of the survival and migration of these glia (33). As evidenced by light microscopic observations, adenoviral-mediated (22, 33) and retroviral-mediated (34) transfer of LacZ (22, 33) or adenoviral transfer of GFP (32) has revealed that adult (22, 32, 33) and neonatal (34) rat OECs survive the implantation procedure and remain localized primarily to the site of injury.
In the present study, we implanted either uninfected fetal rat OECs or OECs infected with a retroviral vector containing the gene LacZ into the compressed spinal cord of adult rats. Using a combination of light and electron microscopy, this strategy has allowed us to perform an in-depth characterization of LacZ-expressing OECs after intraspinal implantation. We provide unequivocal ultrastructural identification of OECs, revealing that these LacZ-expressing glia do not directly associate with central axons nor do they form myelin sheaths or basal lamina after implantation into the damaged spinal cord of adult rats.
Materials and Methods
Clip Compression Injury. All experiments were performed according to the guidelines of the Canadian Council for the Use and Care of Animals Guidelines and were approved by the Queen's University/University of Saskatchewan animal welfare committees. Under anesthesia (5.6 mg/kg ketamine, 4 mg/kg Rompun, and 0.75 mg/kg acepromazine), a T10-T11 laminectomy was performed on adult female Wistar rats (225-250 g; n = 45). The exposed spinal cord was left intact, and care was taken to not disrupt the surrounding dura mater. Clip compression injuries (22 g) and postoperative care were performed as described (35).
Primary OEC Cultures. OECs were isolated from the olfactory nerve layer of embryonic day 18 Wistar rats as described (36, 37).
Retroviral Infection of OECs. See Supporting Materials and Methods, which is published as supporting information on the PNAS web site.
In Vitro Histochemistry. See Supporting Materials and Methods.
Implantation of OECs. Compared with acute transplantation, delayed transplantation of OECs has been demonstrated to be more effective in tissue sparing and recovery after SCI (21). Thus, 1 week after SCIs, the rats were anesthetized as described above and placed into a stereotaxic frame. The original injury site was located, and the parafilm was removed. Cultured OECs were grafted into the cystic cavity, which was clearly visible at the site of injury (n = 32). A fine needle connected to a Hamilton syringe was slowly lowered into the injury site at an angle of ≈45°. Approximately 4 × 105 cells were injected into the cystic cavity in a volume of 4 μl of grafting solution at a rate of 1 μl/min. Control animals received an injection of grafting solution alone (n = 13). The muscle layers and skin were closed, and after recovery, the animals were cared for daily, as described above.
Animal Perfusion and Tissue Preparation for Immunofluorescence. See Supporting Materials and Methods.
Immunofluorescence. See Supporting Materials and Methods.
Animal Perfusion and Tissue Preparation for Electron Microscopy. See Supporting Materials and Methods.
Results
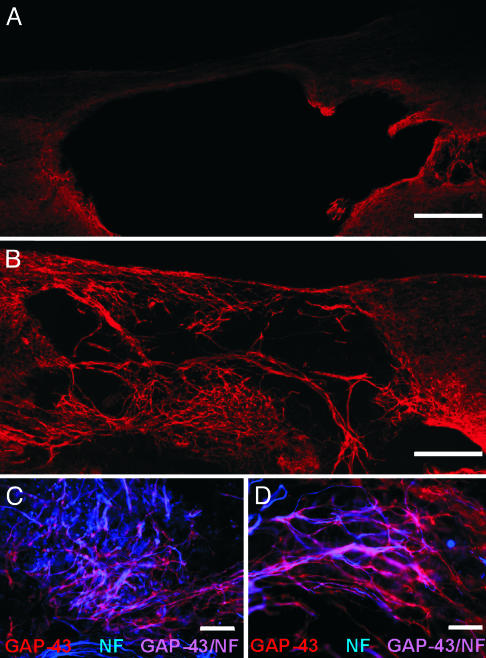
All rats received a clip compression injury at the T10 segment of the spinal cord by using modified aneurysm clips. One week later, animals received an injection of either (i) vehicle control solution (n = 13), (ii) uninfected OECs (n = 22), or (iii) LacZ-expressing OECs (n = 10). Three weeks later, the rats were perfused, and the spinal cords were examined. In animals that received vehicle injection alone, a faint rim of growth-associated protein-43 kDa (GAP-43) immunopositive processes was observed around the perimeter of the cystic cavity (Fig. 1A). Compared with controls, in animals that received intraspinal implantation of noninfected fetal rat OECs there was a dramatic increase in the amount of GAP-43 immunopositive processes that invaded the cystic cavity and the neighboring spinal cord parenchyma (Fig. 1B). Colocalization of GAP-43 and neurofilament immunoreactivities revealed that many, but not all, of these processes were axons (Fig. 1 C and D). It is most likely that these axons were of CNS origin, as they did not express conventional markers of peripheral axons (e.g., calcitonin gene-related peptide/substance P-expressing nociceptive sensory afferents, tyrosine hydroxylase/pan-neurotrophin receptor (p75NTR)-expressing postganglionic sympathetic axons, or choline acetyltransferase/vesicular acetylcholine transporter-expressing motor axons; n = 6, data not shown). The GAP-43 immunopositive processes that did not colocalize with neurofilament may be host Schwann cells (Fig. 1 C and D), which express GAP-43 (38, 39) and have been shown to migrate into the damaged spinal cord in several models of SCI (40-45). Because OECs and Schwann cells share many antigenic and phenotypic properties (reviewed in refs. 5, 6, and 8), the presence of host Schwann cells within the spinal cord confounded our attempts to characterize the role each cell type plays in promoting the regeneration of axons after SCI and implantation of OECs.
Fig. 1.
Implantation of fetal rat OECs is associated with a robust growth of GAP-43 immunopositive processes. (A) GAP-43 immunoreactivity around the perimeter of the cystic cavity was sparse in rats that received vehicle injections. (B) Many GAP-43 immunopositive fibers were observed extending into the cystic cavity in animals that received intraspinal implantation of OECs. Low (C) and high (D) magnification photomicrographs illustrating that many of the GAP-43 immunopositive fibers (red) coexpressed neurofilament (NF; blue). (Scale bars: 400 μm, A and B; 100 μm, C; 50 μm, D.)
To examine the fate of OECs after implantation into the compressed spinal cord of adult rats, we implanted fetal rat OECs infected with a replication-deficient BAG retrovirus carrying the gene LacZ that encodes for the β-galactosidase enzyme (n = 10). Expression of β-galactosidase was confirmed by histochemical and immunohistochemical detection of the enzyme in OECs in vitro. β-Galactosidase was detectable in both well defined subpopulations of OECs (6), namely the spindle-shaped cells that express the pan-neurotrophin receptor (p75NTR) and the flattened multipolar cells that do not express p75NTR (see Fig. 5, which is published as supporting information on the PNAS web site).
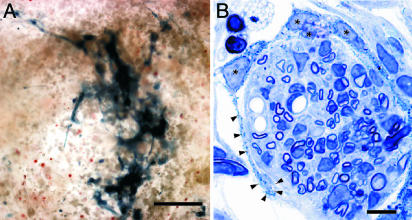
After an 8-h incubation with 5-bromo-3-indolyl-β-d-galactosidase (Bluo-gal), clusters of LacZ-expressing OECs could be visualized in 100-μm-thick vibratome sections of the injury site in vivo (Fig. 2A). The clusters of OECs remained localized exclusively to the cystic cavity, indicating that retrovirally infected OECs survived the implantation procedure and did not migrate from the site of implantation, at least at 3 weeks after grafting (Fig. 2 A). No reaction product was detectable in animals that received either vehicle control injections or implantations of uninfected OECs. In toluidine blue-stained semithin sections of the cystic cavity, the Bluo-gal reaction product was seen as a dark blue precipitate in cells with long, thin processes and an ovoid nucleus, characteristic features of OECs (Fig. 2B). These OEC processes containing Bluo-gal reaction product encircled large cylindrical clusters of unlabeled cells, including glia associated with a single myelinated axon, and endothelial cells (Fig. 2B).
Fig. 2.
LacZ-expressing OECs are readily identified after intraspinal implantation. (A) A cluster of cells containing Bluo-gal reaction product was detectable within the cystic cavity 3 weeks after intraspinal implantation of LacZ-expressing OECs. The positively labeled cells did not appear to significantly migrate away from the injury site. (B) Toluidine blue-stained semithin section of OECs (*) containing blue crystalline reaction product wrapping long processes (arrowheads) around a large channel of unlabeled cells, including glia, myelinated axons, and endothelial cells. (Scale bars: 100 μm, A; 10 μm, B.)
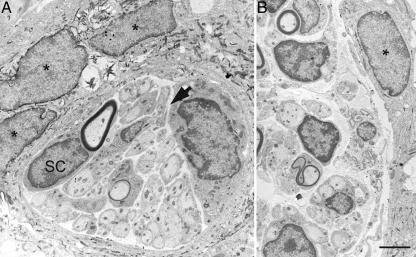
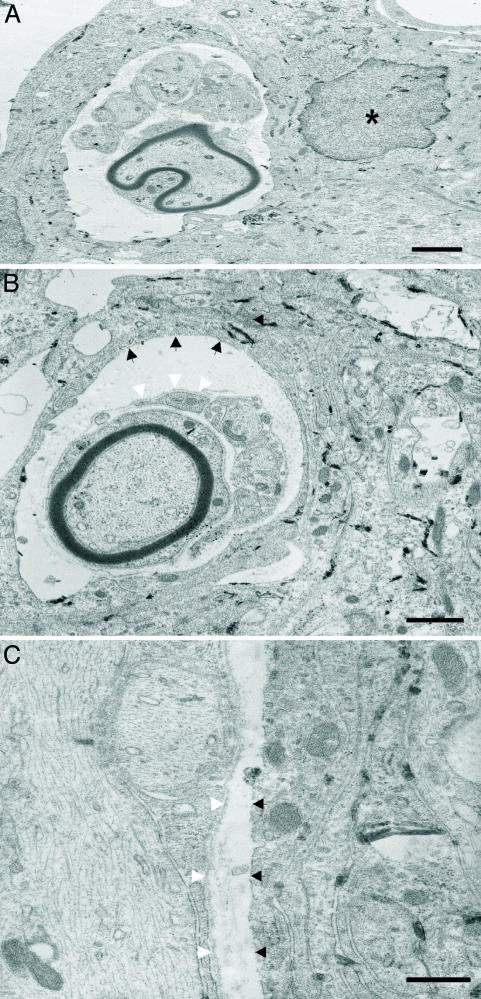
The electron-dense reaction product created upon cleavage of the Bluo-gal substrate is suitable for ultrastructural examination because there is specific intracellular labeling and a lack of displacement to extracellular sites (46). At the electron microscope level, LacZ-expressing OECs with distinct crystalline reaction product localizing to the subnuclear membrane and cytoplasm were observed in the cystic cavity (n = 3 rats; Figs. 3 and 4A). All labeled cells possessed an elongated, irregular-shaped nucleus with a modest degree of chromatin immediately adjacent to the nuclear envelope. The cellular processes containing Bluo-gal reaction product were often multilayered and overlapping (Fig. 3A). These LacZ-expressing OECs were not observed directly associating with myelinated or unmyelinated axons. Rather, numerous myelinated and unmyelinated axons were associated with glia possessing a distinct basal lamina and a nucleus with prominent chromatin clumping beneath the nuclear envelope, two characteristic features of Schwann cells (47). Most importantly, the cellular cytoplasm associated with these myelinated and unmyelinated axons did not possess Bluo-gal reaction product (Figs. 3 and 4 A and B). In fact, positively labeled OECs were seen forming long, thin processes, often enveloping the Schwann cell-axon units but separated by a narrow extracellular space containing collagen (Figs. 3 and 4 A and B). In addition, compared to unlabeled host Schwann cells that expressed a distinct basal lamina, adjacent LacZ-expressing OECs did not have a basal lamina (Fig. 4 B and C).
Fig. 3.
LacZ-expressing fetal rat OECs can be identified ultrastructurally and formed distinct associations with Schwann cells in the cystic cavity. OECs containing the electron dense Bluo-gal reaction product (*) localizing to the nuclear membrane and cytoplasm were observed extending processes to envelop multiple Schwann cells (SC). These Schwann cells were associated with either large myelinated axons or several small unmyelinated axons. No Bluo-gal reaction product was detected in those glial cells associated with axons. The “tunnels” of Schwann cells are surrounded by a thin rim of collagen containing extracellular space (arrow). (Scale bar: 2.5 μm.)
Fig. 4.
LacZ-expressing OECs do not associate with axons, nor do they form a basal lamina. (A) LacZ-expressing fetal rat OECs containing the Bluo-gal reaction product did not interact directly with either myelinated or unmyelinated axons in vivo but were closely associated with Schwann cells in the cystic cavity. (B and C) LacZ-expressing OECs form tunnels in which unlabeled Schwann cells can be found within the cystic cavity. These unlabeled Schwann cells that associated with axons in vivo expressed a distinct basal lamina (white arrowheads), whereas LacZ-expressing OECs did not synthesize a basal lamina (black arrowheads). (Scale bars: 2 μm, A and B; 0.5 μm, C.)
In other areas of the cystic cavity, numerous Schwann cells were observed associating with both myelinated and unmyelinated axons, in the absence of adjacent LacZ-expressing OECs. Other cell types present at the site of injury and OEC implantation included fibroblasts, endothelial cells, and phagocytic cells. None of these other cell types showed positive histochemical labeling for the Bluo-gal reaction product at the ultrastructural level (data not shown). Additional rats (n = 3) receiving intraspinal implantation of uninfected OECs were examined at the ultrastructural level, and a similar arrangement of glial cells and axons was observed: namely, larger cells with elongated nuclei and long processes wrapping around a central core of Schwann cell-like glia that directly associated with myelinated or unmyelinated axons.
Discussion
There is ample evidence to support the notion that OECs are involved in promoting axonal regeneration, remyelination, and functional recovery after injury and demyelinating lesions in the spinal cord of adult rats (reviewed by refs. 5, 9, 10, 12, 48, and 49). Despite these remarkable and clinically relevant findings, a major impediment to our fundamental understanding of OEC behavior in vivo has been a lack of unequivocal evidence that OECs directly associate with, and myelinate, axons in the damaged CNS. We provide an ultrastructural characterization of labeled OECs in vivo after implantation into the damaged spinal cord of adult rats.
The initial studies that characterized OEC behavior after intraspinal implantation into the damaged cord relied on Hoescht prelabeling (13, 14). This technique, however, can result in extensive false positive signals (33) and has likely yielded the overestimation of the migratory capacity of OECs in vivo. More recently, genetic strategies such as adenoviral infection with LacZ or GFP (32, 33), as well as retroviral infection with LacZ (described here), have demonstrated that OECs remain quite localized to the implantation site. In the context of compressive/contusive SCI where large cystic cavities form, the fact that implanted OECs remain at the site of injury suggests that these glia may serve as an alternative growth substrate for regenerating axons in the damaged CNS.
In vitro, OECs may switch between a Schwann cell-like morphology and an astrocyte-like morphology, apparently by cytoskeletal alterations regulated by intracellular cAMP levels (50). This ability to resemble Schwann cells in vitro has led to the widespread assumption that the peripheral type myelin and associated cells observed after implantation of OECs into the damaged spinal cord are bona fide OECs (17, 18, 23, 25-31). Although fetal rat OECs can myelinate dorsal root ganglion axons in vitro (36, 37), recent attempts to demonstrate similar myelination patterns with adult rat OECs have been unsuccessful (51). Age differences of the animals from which the OECs were harvested, as well as differences in the purification procedures, have made direct comparisons between the discrepant studies difficult. In this in vivo study of implanted fetal rat OECs, no positively labeled cells were observed associating with myelinated axons despite the fact that similar preparations of fetal rat OECs will myelinate dorsal root ganglion neurites in vitro (36, 37).
The controversy regarding the myelinating capacity of OECs is further complicated by the fact that Schwann cells migrate into the spinal cord extensively after injury (40-45), which had previously made it difficult to draw definitive conclusions regarding the behavior of OECs in vivo. For example, using unpurified adult rat OECs implanted into the lesioned corticospinal tract (18) or optic nerve (24), Li et al. reported that “astrocyte-like OECs” or “olfactory nerve fibroblasts” form a perineurial-like sheath around bundles of “Schwann cell-like OECs.” Similar cells forming perineurial-like sheaths were observed after implantation of unpurified neonatal OECs, or OECs combined with olfactory bulb-derived meningeal cells into the demyelinated spinal cord (31). Without any definitive ultrastructural identification of implanted cells, an alternative explanation for these findings is that the Schwann cell-like OECs were indeed host Schwann cells that had migrated into the site of injury, and the olfactory nerve fibroblasts/meningeal cells are true OECs. Interestingly, both the olfactory nerve fibroblasts (24) and olfactory bulb-derived meningeal cells (31) bear striking resemblance to our LacZ-expressing OECs.
Consistent with previous reports, when implanted 1 week after compressive/contusive SCI (21, 45), OECs survive and are associated with a robust growth of CNS axons compared with vehicle controls. Our ultrastructural examination of the cystic cavity reveals a unique and intricate relationship between implanted LacZ-expressing OECs, host Schwann cells, and central axons. LacZ-expressing fetal rat OECs do not make direct contact with axons, nor do they synthesize a basal lamina. Rather, these OECs are present in clusters in the cystic cavity of the injured spinal cord and appear to extend their cytoplasmic processes so to surround either small or large numbers of Schwann cell-axon units.
The ultrastructural evidence presented in this study suggests that invading host Schwann cells, rather than implanted OECs, are solely responsible for the myelination of central axons in the cystic cavity. There may, however, be other plausible explanations. For example, in contrast to previous studies that have implicated unlabeled neonatal and adult rat OECs as the myelinating glia seen in the damaged spinal cord (17, 18, 25-28), we have used LacZ-expressing OECs isolated from embryonic day 18 rats. The main advantage of using fetal cells is that at this developmental time point all of the glial cells in the olfactory nerve layer are OECs, thus negating the need for extensive purification techniques (7). Although these same fetal rat OECs myelinate dorsal root ganglion axons in vitro (36, 37), they may respond to environmental cues differently than their neonatal or adult counterparts in the hostile milieu of the damaged spinal cord. An alternative explanation for the inability of fetal OECs to myelinate axons in the damaged spinal cord in vivo is that the expression of LacZ (or its protein product β-galactosidase) interferes with myelin synthesis. This notion is unlikely in light of evidence that LacZ expression does not hinder the ability of Schwann cells to produce myelin or associate with unmyelinated axons in vivo (52). The same study demonstrated robust expression of LacZ by infected Schwann cells during their promyelinating and myelinating stages, making it doubtful that infected OECs down-regulate LacZ while converting to a myelinating or Schwann cell phenotype.
Despite the fact that OECs may not associate directly with axons (but rather indirectly via invading host Schwann cells), it is of paramount importance to note that intraspinal implantation of fetal, neonatal, and adult rat OECs all coincide with an increased growth response of central axons compared to vehicle controls (8, 9, 13-22). OECs secrete a variety of trophic factors, such as nerve growth factor, brain-derived neurotrophic factor, glial cell line-derived neurotrophic factor, and ciliary neurotrophic factor (53, 54). OECs also express several adhesion molecules, such as neural cell adhesion molecule, laminin, and L1 (7, 8). In addition to creating a supportive environment for axon growth, an exciting possibility is that this combination of growth factors and adhesion molecules provides a permissive substrate for Schwann cell invasion. For example, nerve growth factor binding to pan-neurotrophin receptor (p75NTR) on Schwann cells promotes their migration in vitro (55). A putative chemotactic effect of OECs on Schwann cells is supported by our observation that Schwann cells are far more abundant in animals that received intraspinal implantation of OECs compared with controls (J.L., J.G.B., and M.D.K., unpublished observations). The exact nature of the extensive interaction between OECs, Schwann cells, and central axons after SCI is clearly an issue that should be resolved before any conclusions can be drawn concerning the therapeutic potential of these cells in the context of human SCI.
Supplementary Material
Acknowledgments
We thank Ms. Janet Elliot, Ms. Verna Norkum, Mr. Darren Nesbitt, and Ms. Anita Givens for technical assistance; Dr. Derek Schulze for assistance with the laser scanning confocal microscopy; and Ms. Sarah Clark for preparation of the electron photomicrographs. This work was supported by the Canadian Institutes of Health Research (R.D.), the Canadian Neurotrauma Research Program (R.D. and M.D.K.), and a Premier's Research Excellence Award (M.D.K.). Salary support was contributed by the Queen's Advisory Research Council/Principal's Development Fund (J.G.B.), the Ontario Neurotrauma Foundation (J.G.B.), the Queen's Centre for Neuroscience Studies (J.G.B. and J.L.), and the Multiple Sclerosis Society of Canada (V.S.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: OEC, olfactory ensheathing cell; SCI, spinal cord injury; GAP-43, growth-associated protein-43 kDa; Bluo-gal, 5-bromo-3-indolyl-β-d-galactosidase.
References
- 1.Sekon, L. H. S. & Fehlings, M. G. (2001) Spine 26, S2-S12. [DOI] [PubMed] [Google Scholar]
- 2.Kwon, B. K. & Tetzlaff, W. (2001) Spine 26, S13-S22. [DOI] [PubMed] [Google Scholar]
- 3.Bunge, M. B. (2001) Neuroscientist 7, 325-339. [DOI] [PubMed] [Google Scholar]
- 4.Schwab, M. E. (2002) Science 295, 1029-1031. [DOI] [PubMed] [Google Scholar]
- 5.Boyd, J. G., Skihar, V., Kawaja, M. D. & Doucette, R. (2003) Anat. Rec. 271, 49-60. [DOI] [PubMed] [Google Scholar]
- 6.Doucette, R. (1990) Glia 3, 433-449. [DOI] [PubMed] [Google Scholar]
- 7.Doucette, R. (1995) Histol. Histopathol. 10, 503-507. [PubMed] [Google Scholar]
- 8.Ramon-Cueto, A. & Avila, J. (1998) Brain Res. Bull. 46, 175-187. [DOI] [PubMed] [Google Scholar]
- 9.Ramon-Cueto, A. (2000) Prog. Brain Res. 128, 265-272. [DOI] [PubMed] [Google Scholar]
- 10.Franklin, R. J. & Barnett, S. C. (2000) Neuron 28, 15-18. [DOI] [PubMed] [Google Scholar]
- 11.Bartolomei, J. C. & Greer, C. A. (2000) Neurosurgery 47, 1057-1069. [DOI] [PubMed] [Google Scholar]
- 12.Raisman, G. (2001) Nat. Rev. Neurosci. 2, 369-375. [DOI] [PubMed] [Google Scholar]
- 13.Ramon-Cueto, A., Plant, G. W., Avila, J. & Bunge, M. B. (1998) J. Neurosci. 18, 3803-3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramon-Cueto, A., Cordero, M. I., Santos-Benito, F. F. & Avila, J. (2000) Neuron 25, 425-435. [DOI] [PubMed] [Google Scholar]
- 15.Lu, J., Feron, F., Mackay-Sim, A. & Waite, P. M. (2002) Brain 125, 14-21. [DOI] [PubMed] [Google Scholar]
- 16.Lu, J., Feron, F., Ho, S. M., Mackay-Sim, A. & Waite, P. M. (2001) Brain Res. 889, 344-357. [DOI] [PubMed] [Google Scholar]
- 17.Li, Y., Field, P. M. & Raisman, G. (1997) Science 277, 2000-2002. [DOI] [PubMed] [Google Scholar]
- 18.Li, Y., Field, P. M. & Raisman, G. (1998) J. Neurosci. 18, 10514-10524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verdu, E., Garcia-Alias, G., Fores, J., Gudino-Cabrera, G., Muneton, V. C., Nieto-Sampedro, M. & Navarro, X. (2001) NeuroReport 12, 2303-2309. [DOI] [PubMed] [Google Scholar]
- 20.Verdu, E., Garcia-Alias, G., Fores, J., Lopez-Vales, R. & Navarro, X. (2003) Glia 42, 275-286. [DOI] [PubMed] [Google Scholar]
- 21.Plant, G. W., Christensen, C. L., Oudega, M. & Bunge, M. B. (2003) J. Neurotrauma 20, 1-15. [DOI] [PubMed] [Google Scholar]
- 22.Ruitenberg, M. J., Plant, G. W., Hamers, F. P. T., Wortel, J., Blits, B., Dijkhuizen, P. A., Gispen, W. H., Boer, G. J. & Verhaagen J. (2003) J. Neurosci. 23, 7045-7058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keyvan-Fouladi, N., Raisman, G. & Li, Y. (2003) J. Neurosci. 23, 9428-9434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li, Y., Sauve, Y., Li, D., Lund, R. D. & Raisman, G. (2003) J. Neurosci. 23, 7783-7788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franklin, R. J., Gilson, J. M., Franceschini, I. A. & Barnett, S. C. (1996) Glia 17, 217-224. [DOI] [PubMed] [Google Scholar]
- 26.Imaizumi, T., Lankford, K. L., Waxman, S. G., Greer, C. A. & Kocsis, J. D. (1998) J. Neurosci. 18, 6176-6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Imaizumi, T., Lankford, K. L. & Kocsis, J. D. (2000) Brain Res. 854, 70-78. [DOI] [PubMed] [Google Scholar]
- 28.Barnett, S. C., Alexander, C. L., Iwashita, Y., Gilson, J. M., Crowther, J., Clark, L., Dunn, L. T., Papanastassiou, V., Kennedy, P. G. & Franklin, R. J. (2000) Brain 123, 1581-1588. [DOI] [PubMed] [Google Scholar]
- 29.Kato, T., Honmou, O., Uede, T., Hashi, K. & Kocsis, J. D. (2000) Glia 30, 209-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith, P. M., Lakatos, A., Barnett, S. C., Jeffery, N. D. & Franklin, R. J. (2002) Exp. Neurol. 176, 402-406. [DOI] [PubMed] [Google Scholar]
- 31.Lakatos, A., Smith, P. M., Barnett, S. C. & Franklin, R. J. (2003) Brain 126, 598-609. [DOI] [PubMed] [Google Scholar]
- 32.Li, L., Decherchi, P. & Raisman, G. (2003) J. Neurosci. 23, 727-731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruitenberg, M. J., Plant, G. W., Christensen, C. L., Blits, B., Niclou, S. P., Harvey, A. R., Boer, G. J. & Verhaagen, J. (2002) Gene Ther. 9, 135-146. [DOI] [PubMed] [Google Scholar]
- 34.Lakatos, A., Barnett, S. C. & Franklin, R. J. (2003) Exp. Neurol. 184, 237-246. [DOI] [PubMed] [Google Scholar]
- 35.Fehlings, M. G. & Tator, C. H. (1995) Exp. Neurol. 132, 220-228. [DOI] [PubMed] [Google Scholar]
- 36.Devon, R. & Doucette, R. (1992) Brain Res. 589, 175-179. [DOI] [PubMed] [Google Scholar]
- 37.Devon, R. & Doucette, R. (1995) Brain Res. 688, 223-229. [DOI] [PubMed] [Google Scholar]
- 38.Ubink, R. & Hokfelt, T. (1995) J. Comp. Neurol. 423, 13-25. [DOI] [PubMed] [Google Scholar]
- 39.Scherer, S. S., Xu, Y. T., Roling, D., Wrabetz, L., Feltri, M. L. & Karnholz, J. (1994) J. Neurosci. Res. 38, 575-589. [DOI] [PubMed] [Google Scholar]
- 40.Beattie, M. S., Bresnahan, J. C., Komon, J., Tovar, C. A., Van Meter, M., Anderson, D. K., Faden, A. I., Hsu, C. Y., Noble, L. J., Salzman, S. & Young, W. (1997) Exp. Neurol. 148, 453-463. [DOI] [PubMed] [Google Scholar]
- 41.Brook, G. A., Plate, D., Franzen, R., Martin, D., Moonen, G., Schoenen, J., Schmitt, A. B., Noth, J. & Nacimiento, W. (1998) J. Neurosci. Res. 53, 51-65. [DOI] [PubMed] [Google Scholar]
- 42.Brook, G. A., Houweling, D. A., Gieling, R. G., Hermanns, T., Joosten, E. A., Bar, D. P., Gispen, W. H., Schmitt, A. B., Leprince, P., Noth, J. & Nacimiento, W. (2000) Eur. J. Neurosci. 12, 3224-3238. [DOI] [PubMed] [Google Scholar]
- 43.Namiki, J., Kojima, A. & Tator, C. H. (2000) J. Neurotraum. 172, 1219-1231. [DOI] [PubMed] [Google Scholar]
- 44.West, N. R., Leblanc, V. & Collins, G. H. (2001) Neuropathology 21, 188-202. [DOI] [PubMed] [Google Scholar]
- 45.Takami, T., Oudega, M., Bates, M. L., Wood, P. M., Kleitman, N. & Bunge, M. B. (2002) J. Neurosci. 22, 6670-6681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weis, J., Fine, S. M., David, C., Savarirayana, S. & Sanes, J. R. (1991) J. Cell Biol. 113, 1385-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bunge, M. B., Holets, V. R., Bates, M. L., Clarke, T. S. & Watson, B. D. (1994) Exp. Neurol. 127, 76-93. [DOI] [PubMed] [Google Scholar]
- 48.Kleitman, N. & Bunge, M. B. (2000) Topics Spinal Cord Inj. Rehab. 6, 65-81. [Google Scholar]
- 49.Plant, G. W., Ramon-Cueto, A. & Bunge, M. B. (2001) in Axonal Regeneration in the Central Nervous System, eds. Ingoglia, N. A. & Murray, M. (Dekker, New York), pp. 529-561.
- 50.Vincent, A. J., West, A. K. & Chuah, M. I. (2003) Glia 41, 393-403. [DOI] [PubMed] [Google Scholar]
- 51.Plant, G. W., Currier, P. F., Cuevro, E. P., Bates, M. L., Pressman, Y., Bunge, M. B. & Wood, P. M. (2002) J. Neurosci. 22, 6083-6091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arroyo, E. J., Bermingham, J. R., Jr., Rosenfeld, M. G. & Scherer, S. S. (1998) J. Neurosci. 18, 7891-7902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lipson, A. C., Widenfalk, J., Lindqvist, E., Ebendal, T. & Olson, L. (2003) Exp. Neurol. 180, 167-171. [DOI] [PubMed] [Google Scholar]
- 54.Woodhall, E., West, A. K. & Chuah, M. I. (2001) Mol. Brain Res. 88, 203-213. [DOI] [PubMed] [Google Scholar]
- 55.Anton, E. S., Weskamp, G., Reichardt, L. F. & Matthew, W. D. (1994) Proc. Natl. Acad. Sci. USA 91, 2795-2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.