Summary
Diversity-generating retroelements (DGRs) are the only known source of massive protein sequence variation in prokaryotes. These elements transfer coding information from a template region (TR) through an RNA intermediate to a protein-encoding variable region (VR). This retrohoming process is accompanied by unique adenine-specific mutagenesis, and in the prototypical BPP-1 DGR, requires a reverse transcriptase (bRT) and an accessory variability determinant (bAvd) protein. To understand the role of bAvd, we determined its 2.69 Å resolution structure, which revealed a highly positively charged pentameric barrel. In accordance with its charge, bAvd bound both DNA and RNA, albeit without a discernable sequence preference. We found that the coding sequence of bAvd functioned as part of TR, but identified means to mutate bAvd without affecting TR. This mutational analysis revealed a strict correspondence between retrohoming and interaction of bAvd with bRT, suggesting that the bRT-bAvd complex is important for DGR retrohoming.
Introduction
Diversity-generating retroelements (DGRs) are the only known source of massive protein sequence variation in the prokaryotic world (Doulatov et al., 2004; Liu et al., 2002; Minot et al., 2012; Schillinger et al., 2012). Remarkably, these elements supply a magnitude of variation that exceeds by about ten thousand-fold that found in the vertebrate adaptive immune system (Le Coq and Ghosh, 2011). A large number of DGRs have been identified in diverse bacterial and phage genomes (Doulatov et al., 2004; Minot et al., 2012; Schillinger et al., 2012), with the Bordetella bacteriophage BPP-1 DGR being the most extensively characterized (Liu et al., 2002; Liu et al., 2004). The biological role of this DGR is to provide the phage with the capacity to adapt to Bordetella. Massive sequence variation occurs in the phage's receptor-binding protein Mtd, which is encoded by the DGR. Variation in Mtd results in the emergence of diverse receptor-binding specificities, which makes it possible for the phage to infect via new receptors as old receptors stop being expressed due to changes in Bordetella gene expression patterns (Liu et al., 2002). The biological functions of other DGRs remain to be determined, but it seems likely that they too will provide a mechanism for adapting to an unpredictable host, environment, or both.
DGRs have a unique mode of sequence variation, in which mutagenesis is targeted specifically to adenines. This occurs during homing of sequence information from a template region (TR, ~140 basepairs) through an RNA intermediate (i.e., retrohoming) to a variable region (VR, ~140 basepairs) (Fig. 1a) (Doulatov et al., 2004; Guo et al., 2008; Guo et al., 2011; Liu et al., 2002; McMahon et al., 2005). The TR serves as an invariant master copy of sequence information, while the VR is homologous to the TR and forms part of the coding sequence of the variable protein (e.g., Mtd). At some point in this process, adenines encoded in the TR are replaced randomly in the VR (Liu et al., 2002). This pattern of replacement focuses mutagenesis to adenine-containing codons in the TR, most typically AAY (Y=C or T) (Liu et al., 2002; Minot et al., 2012; Schillinger et al., 2012). As previously noted, adenine-specific mutagenesis of AAY results in substitutions that cover the gamut of chemistry and preclude a stop codon (McMahon et al., 2005). In Mtd, adenine-containing codons map to its receptor-binding site and the corresponding amino acids are directly involved in receptor contacts (Miller et al., 2008). A similar mapping has been observed for the Treponema denticola DGR variable protein TvpA (Le Coq and Ghosh, 2011).
Figure 1. Mutagenic retrohoming.
a. Information transfer from the TR to the VR via mutagenic retrohoming. Functional elements at the 3' ends of the VR and TR: blue circles, the GC-only sequence; red and green diamonds, the IMH and IMH* elements, respectively. The hairpin/cruciform structure following the VR is shown as well.
b. Target-primed reverse transcription. Cleavage of the VR lower strand in the GC-only sequence would enable that strand to act as a primer for reverse transcription by bRT, with an RNA containing the TR acting as a template.
DGRs have several components that are required for mutagenic retrohoming. Of primary importance is a reverse transcriptase (RT) that belongs to its own distinctive clade of RTs (Doulatov et al., 2004; Liu et al., 2002; Minot et al., 2012; Schillinger et al., 2012), consistent with DGRs having a unique mode of sequence variation. The catalytic activity of the BPP-1 DGR RT (bRT) is essential to retrohoming (Doulatov et al., 2004; Liu et al., 2002), and is likely to be partially or wholly responsible for adenine-specific errors during reverse transcription of an RNA containing the TR. It is not yet evident how the reverse transcribed copy of the TR homes to the VR, although target-primed reverse transcription, as proposed for group II introns, is a reasonable model (Guo et al., 2008; Zimmerly et al., 1995) (Fig. 1b). This is because group II introns RTs are the closest evolutionary neighbors to DGR RTs (Doulatov et al., 2004; Liu et al., 2002; Minot et al., 2012).
In addition to bRT, there are four other elements of the BPP-1 DGR involved in retrohoming. The first three of these are nucleic acid elements (Doulatov et al., 2004; Liu et al., 2002). The first is a 14-base GC-only region found near the 3' end of the VR (Fig. 1a). This is the site at which mutagenized sequence information from the TR is incorporated into the VR (Guo et al., 2008). The second is found in both the VR and the TR, and consists of the IMH element (21 bases) in the VR and a nearly identical IMH* element in the TR (21 bases, 5 mismatches with IMH). These elements are immediately downstream of the GC-only sequence and set the unidirectionality of sequence information transfer from the TR to the VR (Doulatov et al., 2004). The third occurs just after the VR, and consists of a 24-basepair (bp) region containing an inverted repeat capable of forming a hairpin/cruciform structure (Guo et al., 2011). This structure is required for efficient retrohoming. Disruption of the stem structure or loop sequence leads to a ~1000-fold decrease in retrohoming (Guo et al., 2011).
The fourth element is the BPP-1 accessory variability determinant (bAvd, 128 amino acids, 14.5 kDa; formerly called Atd), a protein encoded immediately upstream of the TR (Doulatov et al., 2004) (Fig. 1a). bAvd is essential to retrohoming and bAvd-like proteins are encoded by most DGRs (Doulatov et al., 2004), but the role of these proteins is unknown. Members of the DGR Avd protein family are similar in sequence to S23 ribosomal and S23 ribosomal-like proteins, which are encoded as intervening sequences within S23 ribosomal RNA (rRNA) genes in the case of the former and elsewhere in the genome in the case of the latter (Pronk and Sanderson, 2001; Ralph and McClelland, 1993). These sequence similarities are, however, not illuminating as the functions of S23 ribosomal and ribosomal-like proteins are not known.
To understand the role of bAvd in mutagenic retrohoming, we first determined its structure, which revealed a highly positively charged pentameric barrel. Not surprisingly given its surface characteristics, bAvd bound nucleic acids, albeit without exhibiting a sequence preference. We also found that the coding sequence of bAvd functions as a part of TR, but identified means to carry out mutational analysis of bAvd without affecting TR. This analysis revealed that two bAvd residues that are conserved in the DGR Avd family and contiguous on the surface of bAvd are essential to retrohoming. Significantly, these same two residues were also required for association between bAvd and bRT. We observed a strict correspondence between retrohoming and bRT-bAvd interaction, suggesting that the bRT-bAvd complex is important for DGR retrohoming.
Results
bAvd was expressed in Escherichia coli as inclusion bodies, refolded, and purified. A selenomethionine-labeled form of the protein was crystallized and its structure determined to 2.69 Å resolution (Table 1). The entirety of bAvd was visible and unambiguously traced, except for several disordered N- and C-terminal residues. The structure revealed a pentameric, barrel-shaped assembly, with one end narrower than the other (Fig. 2). The pentamer is positively charged on all surfaces, including an hourglass-shaped pore that runs through the center of the barrel and constricts to ~8 Å diameter. A molecule is bound within the center of the pore, most proximal to Glu43 (Fig. S1). This bound molecule was modeled as a phosphate ion and appears to have co-purified with bAvd.
Table 1.
Crystallographic statistics for SeMet-bAvd
| Data collection | ||||
| Space group | P21 | |||
| Cell dimensions | ||||
| a, b, c (Å) | 54.3, 63.1, 105.3 | |||
| β (°) | 103.8 | |||
| Peak | Inflection | Remote | ||
| Wavelength (Å) | 0.97941 | 0.97950 | 0.97630 | |
| Resolution Limit (Å) a | 2.51 (2.55-2.51) | 2.51 (2.55-2.51) | 2.69 (2.75-2.69) | |
| Rmerge (%)a | 12.1 (55.5) | 12.0 (62.5) | 9.3 (29.1) | |
| (I/σ−)a | 3.5 (2.6) | 3.0 (2.7) | 3.4 (0.9) | |
| Completenessa | 87.9 (58.0) | 86.7 (57.3) | 92.3 (83.4) | |
| Redundancya | 3.6 (2.9) | 3.6 (2.6) | 3.7 (3.5) | |
| Refinement | ||||
| Resolution (Å)a | 42.91-2.69 (2.79-2.69) | |||
| Unique Reflections (Bijvoets merged/Bijvoets separate) | 18,916/36,535 | |||
| Rwork/Rfree (%)a (Bijvoets merged) | 19.23/24.45 (23.82/31.77) | |||
| Rwork/Rfree (%)a (Bijvoets separate) | 19.44/24.39 (24.81/33.18) | |||
| No. of atoms | ||||
| Protein | 4,328 | |||
| H2O | 61 | |||
| PO43− | 5 | |||
| Mean B-factors (Å2) | ||||
| Protein | ||||
| Main Chain | 57.87 | |||
| Side Chain | 58.57 | |||
| H2O | 49.04 | |||
| PO43− | 79.87 | |||
| RMS deviation | ||||
| Bond Lengths (Å) | 0.009 | |||
| Bond Angles (°) | 1.169 | |||
| Ramachandran Plot | ||||
| Favored | 96.6% of residues | |||
| Generously allowed | 100% of residues | |||
| Molprobity Score | ||||
| Overall | 2.27 (94th percentile) | |||
| Clashscore | 10.56 (98th percentile) | |||
Data for the highest resolution shell are indicated in parentheses.
Figure 2. bAvd structure.
a. bAvd pentamer in ribbon representation, with each protomer colored differently.
b. Cross-section of the bAvd barrel in molecular surface representation, colored by the electrostatic potential, ranging from −10 kT/e (red) to +10 kT/e (blue).
c. Narrow end of the bAvd barrel in ribbon representation. See also Fig. S1.
d. Narrow end of the bAvd barrel colored according to electrostatic potential, in molecular surface representation, as in panel b. See also Fig. S3.
Each bAvd protomer consists of an up-and-down four-helix bundle (Fig. 3a). Helices α1 and α2, which are nearly parallel to one another, are at a 20° angle to helices α3 and α4, which are also nearly parallel to one another. The 20° crossing angle is typical of four-helix bundles (Kamtekar and Hecht, 1995). Helix α2 predominantly forms the lining of the pore, along with some of helix α3, while helices α1 and α4 form the outer surface of the barrel, along with some of α3 (Fig. 2c). The five protomers are nearly identical in structure (rmsd 0.305 Å, 106 Cα), except for the loop regions connecting the α3 and α4 helices. Each protomer buries an average of ~1380 Å2 of surface area at the interprotomer interfaces. Slightly more than half of this surface area is composed of hydrophobic residues (Fig. 3b), consistent with the observed stability of the pentamer. While members of the DGR Avd protein family have limited sequence identity to bAvd (ranging from ~12–25%), the residues responsible for interprotomer hydrophobic interactions are in large part conserved in chemical character (I24, V40, A41, M44, I57, and I88 conserved on average in >90% of DGR Avd proteins, and V17, L50, M77, and F80 in >67%) (Fig. S2). This trend is also true for the residues that form the hydrophobic core in each protomer. This conservation pattern indicates that DGR Avd family members are likely to form pentameric barrel-like assemblies composed of four-helix bundles in the same manner as bAvd. The hydrophobic interactions in bAvd are supplemented by a small number of polar interactions: a hydrogen bond (Y28 with H38) and salt bridges (E21 with R83, and K61 with D72) between side chain atoms (Fig. 3c). These are not well conserved within the DGR Avd protein family and hence less likely to be important for pentamer assembly as compared to the hydrophobic residues.
Figure 3. Four helix bundle and pentameric interface.
a. A bAvd protomer in cartoon representation (N- to C-terminus, blue to red in rainbow coloring). Helices α1 and α2 are at a 20° angle to helices α3 and α4.
b. Hydrophobic residues buried in the interprotomer interface. See also Fig. S2.
c. Hydrogen bonding between protomers.
The structure of bAvd is similar to those of two S23 ribosomal-like proteins, Xanthomonas campestris Xcc0516 and Bacteriodes thetaiotamicron B.t_0352. Despite sharing only 13% and 16% sequence identity, respectively, with bAvd, both Xcc0516 (rmsd 1.7 Å, 107 Cα, Z=15.3) and B.t_0352 (rmsd 2.1 Å, 102 Cα, Z=13) closely resemble bAvd in forming up-and-down four-helix bundles that assemble into barrel-shaped pentamers (Figs. 2 and S3a) (Lin et al., 2006). The residues in bAvd that form the hydrophobic interprotomer interface (I24, V40, I57, and I88) and the hydrophobic core are well conserved in chemical character in these S23 ribosomal-like proteins (Fig. S2b). Notably, however, neither molecule has the highly basic surface that bAvd does (Figs. 2 and S3b).
Patterns of conservation
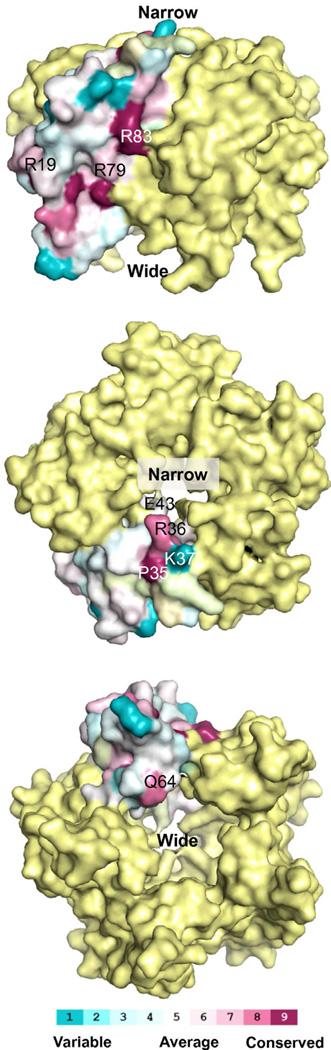
To identify bAvd residues that may be important for mutagenic retrohoming, we mapped sequence conservation within the DGR Avd family to the structure of bAvd (Figs. 4 and S2). Most of the conserved residues are at the interprotomer interface or buried within the protein core (Fig. S2, circles and squares). Only a few residues are both highly conserved and surface-exposed. The latter include three residues on the side of the barrel: bAvd R19 on the α1 helix, and R79 and R83 on the α3 helix (Fig. 4). The narrow end of the barrel has two conserved residues, P35 and R36, which are on the loop connecting α1 and α2. We selected all of these residues for mutagenesis, along with Lys37 on the same loop. Lys37 was included because in certain sequence alignments, Lys37 aligns with a highly conserved set of Arg's and Lys's (at the position of bAvd Gly39, Fig. S2a). We also targeted Q64, which is the only conserved residue at the wide end of the barrel; this residue is on the loop between α3 and α4. The interior pore of the barrel contains no highly conserved residues, and is thus less likely to be functionally significant. Nevertheless, we selected E43 within the pore of the barrel for mutagenesis, as this residue is closest to the modeled phosphate ion (Fig. S1).
Figure 4. Patterns of conservation.
Residues conserved within the DGR Avd family mapped onto the surface of bAvd (color scale below). Only one of the protomers is colored, the others are in yellow. Top, the side of the barrel. Middle, narrow end of the barrel. Bottom, wide end of the barrel.
The coding sequence of bAvd has dual functions
The coding sequence of bAvd is immediately adjacent to the TR (Fig. 1a). To determine if these functional elements overlap, we turned to a previously described system in which the acquisition of kanamycin resistance serves as a reporter for DGR-mediated retrohoming (Guo et al., 2011). In this system, the VR is located on a phage lysogen (i.e., BPP1-ΔATR*KanS) and encodes a nonfunctional aph3' Ia kanamycin resistance allele (lacking six C-terminal residues) (Fig. 5a). The TR is located on a plasmid (i.e., pMX-Km1), and encodes residues of aph3' Ia missing in the VR. This plasmid also supplies avd and brt, which bracket the TR. Transfer of sequence information through DGR-mediated retrohoming from the TR to the VR results in restoration of the missing amino acids in aph3' Ia and hence kanamycin resistance (Guo et al., 2011). Mutagenesis is assessed separately by sequencing the VR of kanamycin resistant colonies.
Figure 5. Functionally significant bAvd residues.
a. Kanamycin-resistance retrohoming assay. VR containing aph3' Ia deleted of its 6 C-terminal residues (kan) is on a phage lysogen, and the TR containing the 3' end of aph3' Ia (kan3') on the pMX-Km1 plasmid. Functional elements are as in Figure 1. Derivatives of pMX-Km1 containing only 3' portions of avd are indicated below the schematic of the plasmid. The pFHA-avd plasmid, which provides intact avd in trans, is on the top right. PFHA is the Bordetella fhaB promoter, and Ori is the origin of replication sequence.
b. Retrohoming frequency resulting from cotransformation of pFHA-avd with pMX plasmids expressing intact avd (Km1), catalytically defective bRT (Km1RT—), or avd containing only 48 (Δavd), 53 (T+53), 76 (T+76), 115 (T+115), 200 (T+200), or 300 (T+300) bp of 3' end of avd. Results are the average of two independent experiments or four in the case of Km1 and Km1RT—; error bars represent standard deviations.
c. Top, homing frequency resulting from cotransformation of pMX-T+300 and pFHA-avd expressing wild-type bAvd (WT), no bAvd (-bAvd), bAvd R19A, P35A, R36A, K37A, E43A, Q64A, R79A, or R83A. Results are the average of three independent experiments and error bars represent standard deviations. Bottom, western blot of bAvd using antibodies that recognize a His-tag incorporated at the C-terminus of bAvd. R19A has a minor band of smaller size, which could be a degradation product or caused by internal translational initiation.
d. Circular dichroism spectra at 4 °C (black) and 37 °C (gray) of wild type bAvd (♦), P35A (▲), R36A (●), K37A (■), R79A (−), and R83A (+). Mean residue ellipticity (MRE) values were measured from 195 nm to 250 nm.
Using this system, we asked whether a deletion of avd that eliminated all but 48 bp of its 3' end (pMX-Δavd) could be complemented by intact avd supplied on a second plasmid (i.e., pFHA-avd). We observed very poor complementation. The retrohoming frequency of Δavd (Fig. 5b, 8.6 × 10−10) was ~430-fold lower than that of wild-type avd (3.7 × 10−7, Km1) but ~9-fold higher than the detection limit of the assay. This latter value was set by the expression of a catalytically inactive version of brt (Fig. 5b, pMX-Km1RT—, 7.5 × 10−11), which eliminates retrohoming completely (Guo et al., 2011). The VR's from multiple kanamycin resistant clones of Δavd were sequenced and found to have adenine-specific mutations (Table S1), confirming that the low retrohoming activity detected in Δavd was indeed DGR-mediated.
Smaller deletions of avd were next constructed. Deletions that left 53, 76 and 115 bp of the 3’ avd coding sequence had retrohoming frequencies ranging from ~500 to ~1400-fold lower than that with intact avd (Fig. 5b, 2.6–6.6 × 10−10). However, a deletion that left ~ bp of the 3’ end of avd had a retrohoming frequency ~35-fold lower than that of wild-type (i.e., 1.1 × 10−8), and one that left 300 bp of the 3' end of avd (T+300) had a homing frequency nearly equal to that of wild-type (Fig. 5b, 1.6 × 10−7). We conclude from these experiments that a portion of avd functions as part of the TR. Importantly, these experiments show that it is possible to produce a functional bAvd in trans to the TR and brt, thus enabling mutagenesis of bAvd without affecting the TR.
bAvd R79 and R83 are essential for DGR retrohoming
Alanine substitutions of the conserved, surface-exposed bAvd residues identified above (Fig. 4) were introduced into the copy of avd on pFHA-avd. These were expressed along with pMX-Km1 T+300, which does not support retrohoming in the absence of avd in trans (Fig. 5c, -Avd). Substitutions of R19, E43, Q64 had no effect on retrohoming (Fig. 5c). This was also true of P35, which is conserved in almost all Avd family members (Fig. S2a). In contrast, alanine substitutions of R79 or R83, which are also conserved in almost all Avd family members, resulted in complete loss of retrohoming (<7.1 × 10−11 and <6.4 × 10−11, respectively) (Fig. 5c). Substitutions of R36 and K37 resulted in a partial retrohoming defect, ~24- and ~17-fold lower, respectively, than that of wild-type (Fig. 5c). We verified that bAvd was produced at equivalent levels in these mutant strains by a western blot visualization of a His-tag incorporated at the C-terminus of bAvd (Fig. 5c). The VR's from 7–8 independent clones of bAvd mutants that retained retrohoming activity (i.e., R19, P35, R36, K37, E43, Q64) were sequenced, and normal levels of adenine-specific mutagenesis were found (Table S1). This last result indicates that mutagenesis was not affected by a partial loss in bAvd activity.
We next assessed whether the substitutions that resulted in loss of retrohoming activity had affected protein structure and stability. Along with the functionally deficient bAvd R36A, K37A, R79A, and R83A mutants, we also followed up on the fully functional P35A mutant as a control. These mutant proteins were produced in E. coli, refolded, and purified as for wild-type bAvd. The circular dichroism spectra of these proteins were similar to that of wild-type bAvd at both 4 and 37 °C, demonstrating conservation of structure, and unchanged between 4 and 37 °C, demonstrating conservation of stability (Fig. 5d). These results suggest that the defects in retrohoming are not due to altered protein structure or stability, but instead are attributable to a functional cause.
Nucleic Acid Binding
The highly basic surface of bAvd drew our attention to the possibility that it interacted with nucleic acids. To assess this, we examined the association of bAvd with a number of nucleic acid substrates. We started with a single-stranded (ss) RNA (65 bases, sense strand) containing the GC-only and IMH* elements from the TR (Table S2). Complex formation between bAvd and this RNA was evaluated by an electrophoretic mobility shift assay (EMSA), with the nucleic acid being visualized by ethidium bromide staining. A small but detectable amount of the RNA was shifted by 0.1–0.2 µM pentameric bAvd; the RNA became aggregated and remained in the wells of the gel at ≥1 µM bAvd (Fig. 6a top). A similar result was found when bAvd was incubated with a ssDNA (64 bases, antisense strand) that corresponded to the VR and contained the GC-only sequence, the IMH element, and the hairpin/cruciform sequence (Fig. 6a).
Figure 6. Interactions of bAvd.
a. Upper panel, Ethidium bromide-stained EMSA of bAvd at varying concentrations (indicated above lanes as µM of pentamer) incubated with 0.5 µM of TR RNA (ssRNA), VR DNA (ssDNA), a heteroduplex of VR DNA and TR RNA (DNA:RNA), a homoduplex of VR DNA (dsDNA), and a homoduplex of a VR DNA variant engineered to favor the formation of a hairpin/cruciform structure (cruci-DNA). Lower panel, the same experiment was carried out with random RNA and DNA sequences; in the case of the cruci-DNA, a non-functional loop sequence was used. Arrows indicate the positions of shifted bands. See also Fig. S6.
b. Association of wild-type and mutant bAvd with His-bRT D138Q, as detected by a Ni2+-NTA coprecipitation assay and visualized by Coomassie-stained SDS-PAGE. Bound fractions shown here, unbound fractions in Figure S4d.
When bAvd was incubated with a double-stranded (ds) heteroduplex formed by the above sense ssRNA and antisense ssDNA, complex formation with bAvd was more clearly evident at 0.1 µM bAvd (Fig. 6a). The greater band intensity here may have been due to the better ethidium bromide signal for ds as opposed to ss substrates. This enhancement was not limited to an RNA:DNA heteroduplex, as a more intensely stained shifted band was also evident with dsDNA (Fig. 6a). This dsDNA, which consisted of the VR DNA sense and antisense strands, contained the hairpin/cruciform sequence. However, because it was unlikely that a linear DNA duplex would form such a structure, we engineered a version of the sequence to promote hairpin/cruciform formation. For this, the order of the stem was swapped, such that intra- but not inter-strand base pairing would occur. Once again, a more intensely stained shifted band was evident at 0.1 µM Avd (Fig. 6a top, cruci-DNA).
We repeated these experiments with RNA and DNA composed of sequences unrelated to DGRs, and found the same pattern of roughly micromolar or sub-micromolar interactions (Fig. 6a bottom). In the case of the hairpin/cruciform double-stranded DNA, a substrate with a nonfunctional loop sequence was used (Guo et al., 2011), but gave similar results as the functional sequence. These results indicate that the interactions of bAvd with ssRNA, ssDNA, RNA:DNA, and dsDNA are apparently sequence-independent. We also examined the retrohoming defective bAvd R83A mutant, and found that it retained the ability to bind nucleic acids just like wild-type bAvd (Fig. S4a).
Interaction with bRT
We next asked whether bAvd associated with bRT. For this experiment, we used bRT D138Q, a mutant that was fortuitously found to be more stable than wild-type bRT and hence more amenable to binding experiments. Based on sequence similarities to HIV RT, D138 is predicted to be positioned at the catalytic site of bRT, as one of three predicted catalytic aspartates (along with D215 and D216). bRT D138Q was found to maintain approximately one-fifth of the catalytic activity of wild-type bRT, with the background of the assay being set by the catalytically inactive bRT (SMAA) mutant (Figs. S4b, c) (Liu et al., 2002). We found that wild-type bAvd indeed associated with His-tagged bRT D138Q (Fig. 6b and S4d). Both bAvd and bRT are highly positively charged proteins (with calculated pI's of 9.5), and therefore unlikely to associate by nonspecific means. Purified bAvd and bRT were verified by absorbance measurements to be devoid of associated nucleic acids (data not shown), which might otherwise bridge the interaction between the two. However, the best evidence for specificity in this interaction was the observation that the two functionally defective bAvd mutants, R79A and R83A, failed to associate with His-bRT D138Q (Fig. 6b). In contrast, the fully functional P35A mutant associated with His-bRT D138Q, as did the two partially functional mutants R36A and K37A. The partial retrohoming defects in these latter two mutants are thus likely due to mechanisms other than association with bRT. Collectively, these results show a strict correspondence between the retrohoming activity of bAvd and its interaction with bRT.
Discussion
We determined the structure of bAvd to guide investigation of its role in DGR retrohoming. The structure revealed a highly positively charged pentameric barrel with a central hourglass-shaped pore. The multimeric nature of bAvd and its central pore bring to mind ring-shaped DNA clamps, such as the β subunit of E. coli DNA polymerase III and eukaryotic proliferating cell nuclear antigen (Kong et al., 1992; Krishna et al., 1994). These DNA clamps are multimeric like bAvd, and have large central pores (~35 Å diameter) that accommodate double-stranded DNA (diameter ~18 Å). In contrast, the bAvd pore is constricted to ~8 Å in diameter, although readjustment of Lys47 at the constriction could lead to a ~15 Å diameter, a size sufficient to accommodate single-stranded nucleic acids. However, it seems unlikely that the bAvd pore is functionally significant, as the residues that line the pore are poorly conserved in the DGR Avd protein family. Consistent with this, mutation of the pore residue Glu43 in bAvd had no effect on retrohoming. Thus, the hourglass-shaped pore is more likely to be a structural rather than a functional feature of the bAvd pentamer.
No catalytic sites were obvious in bAvd, and instead the structure of bAvd is more compatible with a binding functionality. Not surprisingly for its positive charge, bAvd was found to interact with nucleic acids. These interactions showed no sequence specificity, and no discrimination between single-stranded RNA and DNA, or between RNA:DNA heteroduplexes and DNA homoduplexes. These results suggest that bAvd may associate nonspecifically with nucleic acids, an activity for which there are a number of precedents, including the bacterial HU and eukaryotic HMG proteins (Koh et al., 2011; Paull et al., 1993). However, these results do not rule out sequence specific binding by bAvd. It is possible that there are nucleic acid segments or proteins not included in the binding assay that may endow sequence specificity to bAvd. Further experiments are necessary to determine the basis for the sequence specificity of DGR retrohoming.
We also found that the coding sequence of bAvd has two separate functions. This became apparent when a near complete deletion of avd in the avd-TR-brt operon was very poorly complemented by avd in trans. By deleting smaller portions of avd in the avd-TR-brt operon, we found that a construct maintaining 200–300 bp of the 3' end of avd provided near wild-type levels of retrohoming. The lack of full complementation to the wild-type level may have been due to differences in the copy number of bAvd (i.e., wild-type has avd functionally expressed from both pMX-Km1 and pFHA-avd, while T+300 has avd functionally expressed only from pFHA-avd), or the possibility that the biosynthesis of bAvd, TR, and bRT from the same RNA transcript is preferred. Overall, these results suggest that a part of the bAvd coding sequence forms a functional element of TR, indicating a remarkable coevolution in avd of both trans-acting protein encoding and cis-acting nucleic acid functions. Identification of these separate functions made it possible for us to carry out mutational analysis of bAvd with affecting TR.
This analysis consisted of alanine substitution mutagenesis of bAvd residues that are both conserved within the DGR Avd family and surface-exposed. Two such residues, R79 and R83, were identified as being essential to retrohoming, while substitutions of other conserved, surface-exposed bAvd residues had either no affect or only a partial affect on retrohoming. The defects in retrohoming were attributable to function rather than protein structure or stability, as shown by the identical CD spectra of wild-type and retrohoming-defective bAvd mutant proteins. Normal levels of adenine-specific mutagenesis were found in the partially defective retrohoming mutants, suggesting that bAvd is not crucial for mutagenesis. Significantly, we found that R79 and R83 were not only required for retrohoming but also for interaction with bRT. In contrast, bAvd mutants that had full or partial retrohoming activity maintained wild-type levels of interaction with bRT. Collectively, these results show a strict correspondence between retrohoming and bRT association, indicating that the interaction between bAvd and bRT is both specific and likely to be important for DGR retrohoming.
What might be the consequence of the interaction between bAvd and bRT? One possibility is that bAvd affects the catalytic activity of bRT. At present, the specific RNA on which bRT acts is unknown. As our current work has shown, a functional TR may include as much as 200–300 bp upstream of the TR, and prior work has suggested that a nearly equivalent amount downstream may be required (Guo et al., 2008). Furthermore, the nature of the primer for RNA-dependent DNA polymerization by bRT is similarly unknown. Thus, without knowledge of the bona fide substrates for bRT, we examined bRT catalytic activity with artificial substrates, a poly-rA template and an oligo-dT primer. This showed that bAvd did not enhance the catalytic activity of bRT, and indeed a slight decrease occurred, which was probably due to competition by bAvd with bRT for the template and primer (Fig. S4b). However, this result must be taken with caution, as the effect of bAvd on bRT catalysis may differ with the bona fide template and primer. Another intriguing possibility is suggested by the pentameric nature of bAvd. This may be involved in organizing a multivalent assembly consisting of bRT and nucleic acid components. We imagine that such organization may be useful in coordinating the multiple steps required for retrohoming, from RNA-dependent DNA polymerization by bRT through incorporation of reverse transcribed and mutagenized DNA into the VR. Now that our results have provided evidence for the importance of the bRT-bAvd complex in DGR retrohoming, future experiments will be aimed at understanding the physical and mechanistic characteristics of this complex.
Experimental Procedures
bAvd Expression and Purification
The coding sequence of bAvd was amplified by PCR from Bordetella bacteriophage lysates, and cloned into the pET28b expression vector (Novagen); the construct included an N-terminal His-tag followed by a thrombin cleavage site. Mutants of bAvd were created by the QuikChange method (Agilent). The integrity of all constructs was verified by DNA sequencing.
bAvd was expressed from the pET28b vector in E. coli BL21 (DE3). Bacteria were grown at 37 °C to an OD600 of 0.6, at which point expression of bAvd was induced by 1 mM isopropyl β-D-1-thiogalactopyranoside (IPTG). Bacteria were grown for a further 5 hr at 37 °C, harvested by centrifugation (10 min, 5,790 × g, 4 °C), resuspended in 50 mL per L of original bacterial culture of lysis buffer (300 mM NaCl, 50 mM sodium phosphate buffer [NaPi], pH 7.4). The lysis buffer was supplemented with EDTA-free complete protease inhibitors (Roche) (1 tablet per 50 mL of lysis buffer) and 0.2 mg/mL lysozyme (Sigma). Resuspended bacteria were lysed by passage 3× through a high-pressure homogenizer (Emulsiflex-C5, Avestin). The lysate was centrifuged (20 min, 14,400 × g, 4 °C), and the pellet fraction, which contained bAvd in inclusion bodies, was solubilized in denaturing buffer (8.0 M guanidine, 50 mM NaPi, pH 8.0, 0.1% β-mercaptoethanol [βME]). Denatured bAvd was applied to a Ni2+-nitrilotriacetic (NTA) agarose column (Sigma), which had been equilibrated with 3 column volumes of denaturing buffer. The column was washed with 3 column volumes of denaturing buffer, and bAvd was eluted with denaturing buffer in which the pH was set to 4.5.
Refolding was carried out at 4 °C by diluting bAvd drip-wise to 1 µM (monomer concentration, determined using a calculated ε280 of 18,450 M−1cm−1) in 300 mM NaCl, 10 mM NaPi, pH 8.0, 0.1% βME, and incubating for 1 h. Refolded bAvd was applied to a Ni2+-NTA agarose column equilibrated with NiC buffer (300 mM NaCl, 10 mM NaPi, pH 7.4, 0.1% βME) containing 10 mM imidazole. The column was washed with 3 column volumes of the same buffer, and bAvd was eluted in NiC buffer containing 500 mM imidazole. The His-tag was removed from bAvd by thrombin cleavage (1:50 thrombin:bAvd, molar) overnight at 4 °C in NiC buffer containing 500 mM imidazole. bAvd was further purified by gel filtration chromatography (Superdex 200) in 300 mM NaCl, 10 mM Tris, pH 8.0, 0.1% βME. bAvd was concentrated to 6 mg/mL in this same buffer, and flash frozen in liquid N2. Selenomethionine (SeMet)-labeled bAvd was prepared as previously described (Doublie, 1997), and purified as above. Mutants of bAvd were expressed and purified as above.
Structure Determination
Crystals of SeMet-labeled bAvd were grown at 25 °C in hanging drops with 1.2 µL of bAvd (6 mg/mL) added to a precipitant solution consisting of 0.8 µL of 20% PEG 3350, 0.2 M MgCl2, 0.1 M Bis-Tris, pH 6.0 and 0.5 µL 0.1 M BaCl2•2H2O; the well contained the precipitant solution lacking BaCl2•2H2O. Crystals were cryoprotected in the precipitant solution supplemented with 20% glycerol. Inverse-beam, three-wavelength X-ray diffraction data to 2.69 Å resolution limit were collected at 100 K at beamline 5.0.2 of the Advanced Light Source (Berkeley, CA).
Data were processed and scaled using HKL2000 (Minor, 1997) (Table 1). The crystals had five bAvd molecules per asymmetric unit and a solvent content of 49%. Phases were determined using the multiple wavelength anomalous dispersion method with SOLVE (Terwilliger and Berendzen, 1999), which identified 19 out of 25 possible SeMet's in the asymmetric unit. Statistical density modification and automated model building were carried out with RESOLVE (Terwilliger, 2000, 2003). The automatically built model consisted of 466 out of the possible 610 residues of the bAvd pentamer, with side chains modeled as alanines or glycines. The model was manually adjusted and completed using Coot (Emsley and Cowtan, 2004).
Refinement of the model against the remote wavelength diffraction data was carried out; a random 5% of the data were omitted from refinement for determination of Rfree. Forty-five rounds of maximum likelihood refinement were carried out using default parameters in Phenix (Adams et al.), with each round being followed by manual model building and adjustment as guided by inspection of σA-weighted 2mFo-DFc and mFo-DFc difference maps using Coot. Each round of refinement consisted of bulk solvent correction, followed by refinement of coordinates, individual B-factors, and occupancies. No NCS restraints were applied, except in the final rounds, when five-fold NCS restraints were applied on residues 64–66. In the final model, continuous electron density for the main chain was evident from residue 13–122 in chain A, 15–124 in chain B, 14–124 in chain C (except for residue 88), 14–125 in chain D (except for residues 90 and 91), and 14–123 in chain E (except for residues 89–91). Sixty-one waters were modeled into ≥3σ̣σA-weighted mFo-DFc difference electron density.
A phosphate ion was modeled into electron density within the pore and most proximal to E43. This electron density had a peak height of 8.3σ. While no phosphate was present in the crystallization solution, phosphate was present during purification. Constituents of the crystallization solution either did not account for the electron density (e.g., Mg2+, Ba2+) or were too large for the electron density (e.g., glycerol, Bis-Tris, or the above ions with bound waters).
Structure validation was carried out with Molprobity (Chen et al.). The final map had a correlation coefficient of 0.95 as determined by Phenix. The atomic coordinates and structure factors have been deposited with the Protein Data Bank (accession code 4DWL).
Molecular figures were generated with PyMol (http://pymol.sourceforge.net).
Retrohoming frequency
The B. bronchiseptica RB50/BPP-1ΔATR*KanS lysogen and plasmid pMX-Km1 have been previously described (Guo et al., 2011). Plasmid pFHA-avd contains the coding sequence of avd downstream of the Bordetella fhaB promoter. It also carries an RSF1010 oriV replication origin and a gentamycin resistance gene. The KanR–based BPP-1 DGR homing assay was performed as previously described (Guo et al., 2011).
Western blot
The KanR–based BPP-1 DGR homing assay was performed, with the following modifications. An avd gene carrying a C-terminal His-tag was used in plasmid pFHA-avd; the addition of the His-tag did not affect the function of bAvd, as assessed by the retrohoming assay. After growth of bacteria at 37 °C for 6 h in Stainer Scholte (SS) medium (Stainer and Scholte, 1970) containing 20 µg/mL streptomycin, 25 µg/mL chloramphenicol, and 20 µg/mL gentamycin, 2 mL of 1.0 OD600 culture were harvested by centrifugation and the resulting pellets were resuspended in 150 µL of SDS-PAGE buffer. Cells were lysed by boiling, and 10 µL of the sample was resolved by SDS-PAGE. The gel was blotted onto an Immobilon-PSQ membrane (Millipore Corporation), which was blocked at RT for 30 min in 5% milk dissolved in 150 mM NaCl, 25 mM Tris, pH 7.4, and 0.1% Tween (TBST). The membrane was then incubated with 0.1 µg/mL mouse monoclonal Penta-His antibody (Qiagen) in milk at 4 °C overnight with gentle shaking. The membrane was subsequently washed 4× (5 min each) at RT in TBST, and then incubated for 1 h at RT with gentle shaking in TBST containing ~3.2 ng/mL of HRP-conjugated, stabilized goat anti-mouse IgG (Thermo Fisher Scientific). The membrane was again washed 4× (5 min each) at RT in TBST. ECL Plus Western Detection System (GE Healthcare) was used for signal detection, and the membrane was then exposed to CL-X Posure film (Thermo Fisher Scientific).
RNA Synthesis and Purification
RNA was produced by in vitro transcription using T7 RNA polymerase. DNA encoding the sequence of interest from the Bordetella bacteriophage DGR was cloned into a pUC19 vector carrying a T7 polymerase promoter. The sequence of interest was inserted downstream of the TATAGGG sequence of the T7 RNA polymerase promoter, in which the first guanine of this sequence marks the site of transcription initiation (Milligan et al., 1987). The vector was linearized downstream of the insert by Hind III digestion for run-off transcription. For production of random RNA, a pBluescript II KS + vector linearized by digestion with EcoRI was used. Transcription reactions were performed overnight at 37 °C using 25 µg/mL of linearized vector, 0.5 mM of each NTP (Promega), and 1 mg/mL of T7 RNA polymerase in T7 buffer (25 mM MgCl2, 5 mM dithiothreitol [DTT], 2 mM spermidine, 40 mM Tris, pH 7.5). The reaction was then treated with 20 U/mL of RNase-free DNase (Promega) for 2 h at 37 °C, followed by 0.5 mg/mL proteinase K for 1 h at 37 °C. The reaction was extracted with an equal volume of phenol:chloroform:isoamyl alcohol (25:24:1 v/v). Sodium acetate, pH 5.5 was added to the aqueous phase to a final concentration of 0.3 M, after which 2.5 volumes of 100% ethanol were added. The sample was incubated at −80 °C for 30 min and then precipitated by centrifugation (30 min, 15,800 × g, 4 °C). The pellet, which contained the RNA, was washed with 1 mL cold 70% ethanol, air dried for 10 min at RT, and resolubilized in RNase-free sterile water. The concentration of RNA samples was determined by A260, and the purity of the samples by the A260:A280 ratio as well as by ethidium bromide-stained agarose gels.
Circular Dichroism
Circular dichroism spectra were collected for wild-type and mutant bAvd at 10 µM in 100 mM NaF, 10 mM potassium phosphate buffer, pH 7.6 on an Aviv 202 circular dichroism spectrometer using a 1 mm pathlength cuvette (holding 350 µL) (Hellma). For each sample, three independent spectra were recorded from 190 nm to 260 nm at 4 °C and 37 °C. The scans were performed with 1 nm steps, and the CD signal at each wavelength was averaged for 5 seconds.
bRT Expression and Purification
The coding sequence for bRT was cloned into a pET28b vector (Novagen) with an N-terminal His-tag. E. coli BL21 (DE3) cells were transformed with this vector, grown at 37 °C to an OD600 of 0.5, and then induced for expression of bRT with 0.5 mM IPTG.
For preparation of wild-type and mutant bRT for enzymatic assays, bacteria were grown at 18 °C overnight after induction, harvested by centrifugation (20 min, 5,500 × g, 4 °C), resuspended in 8 mL per g of bacterial cell pellet of bRT-lysis buffer (500 mM NaCl, 50 mM NaPi, pH 7.5, 20 % glycerol and 5 mM βME). This buffer was supplemented with EDTA-free complete protease inhibitors (one tablet per 50 mL of resuspended bacteria) and 20 µg/mL of deoxyribonuclease I (Sigma). The cells were lysed on ice by sonication, and the cell debris was removed by centrifugation (30 min, 31,000 × g, 4 °C). All of the following steps were carried out at 4 °C. The supernatant was applied to a Ni2+-NTA column (Sigma, 1 mL of resin per 25 mL of bacterial lysate) equilibrated with bRT-lysis buffer. The resin was washed with 10 column volumes of bRT-lysis buffer supplemented with 50 mM imidazole. bRT was eluted from the resin with 250 mM NaCl, 50 mM NaPi, pH 7.5, 500 mM imidazole, 20 % glycerol, and 5 mM DTT. The protein was used within the next day for enzymatic assays.
For preparation of bRT D138Q for binding assays, bacteria were grown for 18 h at RT after induction, harvested by centrifugation (10–20 min, 5,790 × g, 4 °C), resuspended in 30 mL per L of original bacterial culture of 500 mM NaCl, 50 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer, pH 7.5, 20% glycerol, 0.1% βME. This buffer was supplemented with EDTA-free complete protease inhibitors (one tablet per 30 mL of bacterial lysate), 0.2 mg/mL lysozyme, and 20 µg/mL of deoxyribonuclease I (Sigma). Cells were lysed and centrifuged as described for bAvd, and the supernatant of the lysate, which contained bRT D138Q, was then applied to a Ni2+-NTA column (Sigma, 2 mL per L of original bacterial culture), which had been equilibrated with buffer A (500 mM NaCl, 50 mM HEPES, pH 7.5, 20% glycerol, 0.1% βME). The resin was washed with 10 volumes of buffer A, 10 volumes of buffer A containing 25 mM imidazole, and then 10 volumes of buffer A alone. To eliminate nonspecific nucleic acids bound to bRT D138Q, buffer A (1 mL per L of original bacterial culture) containing 10 U of TURBO DNase (Ambion), 3 U of RiboShredder™ RNase Blend (Epicentre), 60 µg/mL of deoxyribonuclease I, and 30 µg/mL ribonuclease A (Sigma) was incubated with the Ni2+-NTA agarose resin at RT for 1 h; the resin was resuspended every 10 min during this time. The resin was then washed with 10 volumes of buffer A followed by 10 volumes of buffer A containing 25 mM imidazole, and bRT D138Q was eluted from the resin with buffer A containing 500 mM imidazole. bRT D138Q was diluted in buffer A to a final concentration of 1.5 µM and stored at 4 °C. The concentration of bRT D138Q was determined using a calculated ε280 of 65,000 M−1cm−1.
Coprecipitation Assay
Twelve mL of 1.5 µM bRT D138Q was applied to 100 µL Ni2+-NTA agarose resin that had been equilibrated with buffer A. Twenty µL of 60 µM bAvd (pentamer) and 80 µL of buffer A were added to the Ni2+-NTA agarose resin, which was incubated for 30 min at RT; the resin was resuspended every 5 min during this time. The resin was washed with 600 µL of buffer A containing 25 mM imidazole, and bound proteins were eluted with 600 µL of buffer A containing 500 mM imidazole. Fractions were visualized by reducing Coomassie-stained 15% SDS–PAGE.
Nucleic Acids
Single stranded DNA oligonucleotides (ssDNA) were purchased from Allele Biotechnology, purified using phenol:chloroform extraction followed by ethanol precipitation as described above, and resolubilized in water. Double stranded DNA oligonucleotides (dsDNA) were made by heating complementary ssDNA oligonucleotides at 100 °C for 3 min and then cooling to RT (Bernstein and Keck, 2005). RNA:DNA heteroduplexes were made by heating complementary ssDNA with single stranded RNA, which had been synthesized by in vitro transcription as described above, at 80 °C for 2 min and then cooling to RT. The sequences of the oligonucleotides used in the binding studies are listed in Table S2.
Electromobility Shift Assay
Varying concentrations of bAvd were incubated for 20 min at RT with 0.5 µM oligonucleotides in 20 µL of EMSA buffer (300 mM NaCl, 20 mM Bis-Tris, pH 6.5, 20 mM MgCl2, 10% glycerol, 0.1% Tween 20, 1 mM DTT). Samples were then subjected to electrophoresis at 40 V for 150 min on a 5% native polyacrylamide (37.5:1 acrylamide/bisacrylamide) gel containing 5% glycerol and 1× Tris-Borate buffer. Gels were stained with ethidium bromide for 15 min and then visualized under UV light using the Gel Logic 200 imaging system (Kodak).
Reverse transcriptase assay
Reactions were carried out in a 10 µl reaction volume with and without 1.3 µM bAvd pentamer, bRT (wild-type or mutant, 3–4 µM), 1 µg of primer (18-mer oligo-dT, NEB), 1 µg of template (poly-rA) (Sigma), and, a mixture of 2 µg of RNAse A and 5 units of RNAse T1 (Fermentas) in 75 mM KCl, 3 mM MgCl2, 50 mM Tris, pH 8.0, and 10 mM DTT. The quantity of bRT was determined by densitometry using ImageJ (http://rsb.info.nih.gov/ij) of SDS-PAGE gels that contained bRT and known quantities of bovine serum albumin. The reaction was initiated by adding 10 µCi of α-32P-dTTP (3000Ci/mmol, Perkin Elmer), and the reaction mixture was incubated at 37 °C for 15 min. The reaction mixture was spotted on DE81 Whatman filter disc, which was air-dried and then washed 4× for 10 min each time with 2× saline-sodium citrate buffer. The washed filter discs were placed in 4 mL of Scintillation liquid (RPI Biosafe II mixture), and scintillation counts were measured using a Beckman Coulter LS6500 Scintillation counter. A blank measurement, which was taken from a sample containing all the reagents except for bRT, was subtracted from each of the samples. Each measurement was made in duplicate, and the experiment was carried out two to three independent times with different batches of bRT.
Supplementary Material
Highlights.
bAvd forms a highly positively charged pentameric barrel
bAvd binds both DNA and RNA, but without sequence preference
The coding sequence for bAvd serves dual purposes
The interaction of bAvd with bRT is likely to be important for retrohoming
Acknowledgments
We thank the staff at beamline 5.0.2 for help in data collection, Vince B. Liu for technical assistance, and members of the Ghosh and Miller lab for helpful suggestions. This work was supported by NIH R01 AI096838 (JFM and PG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr. 66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein DA, Keck JL. Conferring substrate specificity to DNA helicases: role of the RecQ HRDC domain. Structure. 2005;13:1173–1182. doi: 10.1016/j.str.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Chen VB, Arendall WB, 3rd, Headd JJ, Keedy DA, Immormino RM, Kapral GJ, Murray LW, Richardson JS, Richardson DC. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr D Biol Crystallogr. 66:12–21. doi: 10.1107/S0907444909042073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doublie S. Preparation of selenomethionyl proteins for phase determination. Methods Enzymol. 1997;276:523–530. [PubMed] [Google Scholar]
- Doulatov S, Hodes A, Dai L, Mandhana N, Liu M, Deora R, Simons RW, Zimmerly S, Miller JF. Tropism switching in Bordetella bacteriophage defines a family of diversity-generating retroelements. Nature. 2004;431:476–481. doi: 10.1038/nature02833. [DOI] [PubMed] [Google Scholar]
- Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- Guo H, Tse LV, Barbalat R, Sivaamnuaiphorn S, Xu M, Doulatov S, Miller JF. Diversity-generating retroelement homing regenerates target sequences for repeated rounds of codon rewriting and protein diversification. Mol Cell. 2008;31:813–823. doi: 10.1016/j.molcel.2008.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Tse LV, Nieh AW, Czornyj E, Williams S, Oukil S, Liu VB, Miller JF. Target site recognition by a diversity-generating retroelement. PLoS Genet. 2011;7 doi: 10.1371/journal.pgen.1002414. e1002414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamtekar S, Hecht MH. Protein Motifs. 7. The four-helix bundle: what determines a fold? FASEB J. 1995;9:1013–1022. doi: 10.1096/fasebj.9.11.7649401. [DOI] [PubMed] [Google Scholar]
- Koh J, Shkel I, Saecker RM, Record MT., Jr Nonspecific DNA binding and bending by HUalphabeta: interfaces of the three binding modes characterized by salt-dependent thermodynamics. J Mol Biol. 2011;410:241–267. doi: 10.1016/j.jmb.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong XP, Onrust R, O'Donnell M, Kuriyan J. Three-dimensional structure of the beta subunit of E.coli DNA polymerase III holoenzyme: a sliding DNA clamp. Cell. 1992;69:425–437. doi: 10.1016/0092-8674(92)90445-i. [DOI] [PubMed] [Google Scholar]
- Krishna TS, Kong XP, Gary S, Burgers PM, Kuriyan J. Crystal structure of the eukaryotic DNA polymerase processivity factor PCNA. Cell. 1994;79:1233–1243. doi: 10.1016/0092-8674(94)90014-0. [DOI] [PubMed] [Google Scholar]
- Le Coq J, Ghosh P. Conservation of the C-type lectin fold for massive sequence variation in a Treponema diversity-generating retroelement. Proc Natl Acad Sci U S A. 2011;108:14649–14653. doi: 10.1073/pnas.1105613108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin LY, Ching CL, Chin KH, Chou SH, Chan NL. Crystal structure of the conserved hypothetical cytosolic protein Xcc0516 from Xanthomonas campestris reveals a novel quaternary structure assembled by five four-helix bundles. Proteins. 2006;65:783–786. doi: 10.1002/prot.21105. [DOI] [PubMed] [Google Scholar]
- Liu M, Deora R, Doulatov SR, Gingery M, Eiserling FA, Preston A, Maskell DJ, Simons RW, Cotter PA, Parkhill J, Miller JF. Reverse transcriptase-mediated tropism switching in Bordetella bacteriophage. Science. 2002;295:2091–2094. doi: 10.1126/science.1067467. [DOI] [PubMed] [Google Scholar]
- Liu M, Gingery M, Doulatov SR, Liu Y, Hodes A, Baker S, Davis P, Simmonds M, Churcher C, Mungall K, et al. Genomic and genetic analysis of Bordetella bacteriophages encoding reverse transcriptase-mediated tropism-switching cassettes. J Bacteriol. 2004;186:1503–1517. doi: 10.1128/JB.186.5.1503-1517.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon SA, Miller JL, Lawton JA, Kerkow DE, Hodes A, Marti-Renom MA, Doulatov S, Narayanan E, Sali A, Miller JF, Ghosh P. The C-type lectin fold as an evolutionary solution for massive sequence variation. Nat Struct Mol Biol. 2005;12:886–892. doi: 10.1038/nsmb992. [DOI] [PubMed] [Google Scholar]
- Miller JL, Le Coq J, Hodes A, Barbalat R, Miller JF, Ghosh P. Selective ligand recognition by a diversity-generating retroelement variable protein. PLoS Biol. 2008;6:e131. doi: 10.1371/journal.pbio.0060131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan JF, Groebe DR, Witherell GW, Uhlenbeck OC. Oligoribonucleotide synthesis using T7 RNA polymerase and synthetic DNA templates. Nucleic Acids Res. 1987;15:8783–8798. doi: 10.1093/nar/15.21.8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor ZOaW. Processing of X-ray Diffraction Data Collected in Oscillation Mode. Methods in Enzymology. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- Minot S, Grunberg S, Wu GD, Lewis JD, Bushman FD. Hypervariable loci in the human gut virome. Proc Natl Acad Sci U S A. 2012;109:3962–3966. doi: 10.1073/pnas.1119061109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paull TT, Haykinson MJ, Johnson RC. The nonspecific DNA-binding and -bending proteins HMG1 and HMG2 promote the assembly of complex nucleoprotein structures. Genes Dev. 1993;7:1521–1534. doi: 10.1101/gad.7.8.1521. [DOI] [PubMed] [Google Scholar]
- Pronk LM, Sanderson KE. Intervening sequences in rrl genes and fragmentation of 23S rRNA in genera of the family Enterobacteriaceae. J Bacteriol. 2001;183:5782–5787. doi: 10.1128/JB.183.19.5782-5787.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph D, McClelland M. Intervening sequence with conserved open reading frame in eubacterial 23S rRNA genes. Proc Natl Acad Sci U S A. 1993;90:6864–6868. doi: 10.1073/pnas.90.14.6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schillinger T, Lisfi M, Chi J, Cullum J, Zingler N. Analysis of a comprehensive dataset of diversity generating retroelements generated by the program DiGReF. BMC Genomics. 2012;13:430. doi: 10.1186/1471-2164-13-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stainer DW, Scholte MJ. A simple chemically defined medium for the production of phase I Bordetella pertussis. J Gen Microbiol. 1970;63:211–220. doi: 10.1099/00221287-63-2-211. [DOI] [PubMed] [Google Scholar]
- Terwilliger TC. Maximum-likelihood density modification. Acta Crystallogr D Biol Crystallogr. 2000;56:965–972. doi: 10.1107/S0907444900005072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terwilliger TC. Automated main-chain model building by template matching and iterative fragment extension. Acta Crystallogr D Biol Crystallogr. 2003;59:38–44. doi: 10.1107/S0907444902018036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terwilliger TC, Berendzen J. Automated MAD and MIR structure solution. Acta Crystallogr D Biol Crystallogr. 1999;55:849–861. doi: 10.1107/S0907444999000839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerly S, Guo H, Perlman PS, Lambowitz AM. Group II intron mobility occurs by target DNA-primed reverse transcription. Cell. 1995;82:545–554. doi: 10.1016/0092-8674(95)90027-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.