Abstract
Aggregation of the amyloid β-protein (Aβ) is believed to play a central role in initiating the molecular cascade that culminates in Alzheimer-type dementia (AD), a disease which in its early stage is characterized by synaptic loss and impairment of episodic memory. Here we show that icv injection of Aβ-containing water-soluble extracts of AD brain inhibits consolidation of the memory of avoidance learning in the rat and that this effect is highly dependent on the interval between learning and administration. When injected at 1 hour post-training extracts from two different AD brains significantly impaired recall tested at 48 h. Ultrastructural examination of hippocampi from animals perfused after 48 h recall revealed that Aβ-mediated impairment of avoidance memory was associated with lower density of synapses and altered synaptic structure in both the dentate gyrus and CA1 fields. These behavioural and ultrastructural data suggest that human brain-derived Aβ impairs formation of long-term memory by compromising the structural plasticity essential for consolidation and that Aβ targets processes initiated very early in the consolidation pathway.
Keywords/short phrases: Alzheimer's disease, Amyloid β-protein, Episodic memory, Intracerebroventricular injection, Memory-associated synaptic reorganization, Non-fibrillar water-soluble Aβ, Passive avoidance, Synaptic plasticity
1. Introduction
Over the course of the past five decades, the incidence of Alzheimer's disease (AD) has increased at an alarming rate, such that the sheer number of sufferers with this disorder is now placing an unsustainable burden on healthcare systems throughout the world (Alzheimer's Association, 2011; Ferri, et al., 2005; Prince, 2009). The initial harbingers of disease are often difficult to differentiate from the cognitive changes that frequently accompany old age (Small, et al., 1999), with deficits in episodic memory typifying the early stages of AD and usually occurring prior to impairment of semantic and non-declarative memory (Grober, et al., 2008; Mormino, et al., 2009; Rosenbaum, et al., 2005; Small, 2000). The pathological changes seen in the medial temporal lobe of individuals with mild cognitive impairment (MCI) and early AD are in accord with the initial symptoms of the disease (Scheff, et al., 2007; Scheff, et al., 2006) and support the hypothesis that, at least initially, AD results from disruption of normal hippocampal function and memory consolidation pathways (Coleman and Yao, 2003; Mesulam, 1999).
The molecular changes leading to perturbation of synaptic plasticity in AD are not well understood, but substantial data indicate that the amyloid β-protein (Aβ) may be responsible for these effects (Klein, et al., 2001; Selkoe, 2002). However, the forms of Aβ that mediate memory impairment and the toxic pathways activated by Aβ remain unresolved. Numerous studies have shown that non-fibrillar, water-soluble Aβ from a variety of sources are potent synaptotoxins (Cleary, et al., 2005; Klyubin, et al., 2008; Lambert, et al., 1998; Walsh, et al., 2002). In earlier studies we used the most disease-relevant form of non-fibrillar Aβ, Aβ extracted from the water-soluble phase of AD brain (Freir, et al., 2011b; Shankar, et al., 2008). Post-mortem studies indicate that elevated levels of water-soluble Aβ are specific for AD (Kuo, et al., 1996; Lue, et al., 1999; Mc Donald, et al., 2010; McLean, et al., 1999; Tabaton, et al., 1994) and in vitro studies show that such material robustly inhibits long-term potentiation, facilitates long-term depression and reduces the density of dendritic spines (Barry, et al., 2011; Freir, et al., 2011b; Li, et al., 2009; Shankar, et al., 2008).
Most prior work involving intracerebroventricular (icv) injection of either synthetic or cell culture-derived Aβ focused on spatial reference memory or working memory (Cleary, et al., 2005; Lesne, et al., 2006; Nakamura, et al., 2001; Poling, et al., 2008; Reed, et al., 2011), whereas in some of our previous studies we employed a passive avoidance paradigm (Freir, et al., 2011a; Shankar, et al., 2008). A major advantage of the latter approach is the fact that rapid acquisition ensures the synchronization of subsequent memory-associated neuroplastic events and as a consequence allows examination of long-term memory consolidation (Foley, et al., 2000; Nakamura, et al., 2001; Seymour, et al., 2008). In preliminary experiments we found that administration of Aβ from a single human brain perturbed consolidation of avoidance learning (Shankar, et al., 2008). Memory consolidation involves time-dependent synaptic remodelling (Murphy and Regan, 1998; Platano, et al., 2008) and is underpinned by changes in protein synthesis and post-translation modification (Davis and Squire, 1984; Lamprecht and LeDoux, 2004). Hence, we sought to determine if Aβ-containing extracts from other AD brains could also disrupt the memory of learned behaviour, that is, if this is a generalizable phenomenon typical of AD brain-derived Aβ, and if it is accompanied by alterations in synaptic form and number. In an effort to gain insight into the molecular pathways through which Aβ exerts its effects we also investigated the expression and processing of certain AD-associated proteins some of which are known to change within the first few hours immediately following avoidance learning (Conboy, et al., 2005; O'Sullivan, et al., 2007).
Here we demonstrate that Aβ-containing extracts from two different AD brains effectively diminished consolidation of avoidance learning and that this was highly dependent upon the interval between learning and Aβ administration. While further work is required to understand the molecular pathways on which Aβ acts, we show that this impairment is accompanied by a significant decrease in the total density of synapses in both the dentate gyrus and CA regions of the hippocampus. We also present evidence of altered synaptic ultrastructure, including, but not limited to, increases in synaptic pitting and the number of synaptic vacuoles. Together these results suggest that water-soluble Aβ species isolated from human brain inhibit the synaptic remodelling necessary for memory consolidation while also perturbing normal synapse structure. These findings are consistent with the fact that synapse loss is the best known pathological marker of AD severity (Davies, et al., 1987; Masliah, et al., 2001; Scheff, et al., 2007) and suggest that water-soluble forms of Aβ may also mediate this process in the human brain.
2. Materials and Methods
2.1. Reagents and antibodies
Reagents
All reagents were obtained from Sigma-Aldrich (Sigma-Aldrich Ltd, Dublin, Republic of Ireland) unless specified otherwise.
Antibodies
Rabbit polyclonal antibodies to Aβ (AW8), the N-terminus of APLP1 (W1NT), C-terminus of APLP1 (W1CT) and of APLP2 (W2CT) were from the LNR and have been described previously (Mc Donald, et al., 2010; Sala Frigerio, et al., 2010). Antibodies to: Aβ40 (2G3) and Aβ42 (21F12), BACE1 (3D5), tau (5E2), PrP (ICSM-35) and the C-terminus of APP (C1/1.6) were generous gifts from Drs. P. Seubert (Elan Pharmaceuticals), R. Vassar (Northwestern University), K. Kosik (University of California Santa Barbara), P. Mathews (Nathan Kline Institute) and J. Collinge (University College London) and have been described before [BACE1 3D5 - (Zhao, et al., 2007); APP C1/1.6 – (Boland, et al., 2010); PrP ICSM-35 – (Freir, et al., 2011b); tau 5E2 - (Kowall and Kosik, 1987); Aβ40 2G3 and Aβ42 21F12 – (Mc Donald, et al., 2010)]. All other antibodies were purchased from the commercial sources indicated. Antibodies were directed to: the N-terminus of APP (22C11, mouse monoclonal, Millipore/Chemicon, Billerica, MA); full length APLP2 (D2-II, rabbit polyclonal, 171617, Calbiochem, EMD Chemicals Inc., Gibbstown, NJ); dynamin-1 (PA1-660, rabbit polyclonal, Thermo Scientific Pierce (Affinity BioReagents), Rockford, IL); ptau (AT8, mouse monoclonal, Thermo Scientific Pierce (Affinity BioReagents), Rockford, IL); CREB (48H2, rabbit monoclonal, Cell Signalling Technology Inc, Danvers, MA); pCREB (Ser133) (rabbit polyclonal, Cell Signalling Technology Inc, Danvers, MA); PSD-95 (mouse monoclonal, Thermo Scientific Pierce (Affinity BioReagents), Rockford, IL); SV-2 (SV-2a (E-15), goat polyclonal, Santa Cruz Biotechnology, Inc., Santa Cruz, CA), synaptophysin (mouse monoclonal, Millipore, Temecula, CA); synaptopodin (rabbit polyclonal, 163002, SynapticSystems GmbH, Gottingen, Germany) and MAP2 (HM-2, mouse monoclonal, Sigma-Aldrich Ltd). The secondary antibodies used were: sheep anti-mouse-HRP and donkey anti-rabbit-HRP (both from Amersham, GE Healthcare Life Sciences, Little Chalfont, UK). CREB and pCREB controls were prepared from SK-N-MC cells treated with or without IBMX and forskolin (Cell Signalling Technology Inc, Danvers, MA). Further details about antibodies are provided in Table 1.
Table 1. List of antibodies used in the study.
| Protein | Antibody name | Monoclonal/Polyclonal | Dilution |
|---|---|---|---|
| Aβ | AW8 | rabbit polyclonal | 1: 80 |
| Aβ40 | 2G3 | mouse monoclonal | 1 μg/ml |
| Aβ42 | 21F12 | mouse monoclonal | 1 μg/ml |
| APP | C1/1.6 | mouse monoclonal | 1:1000 |
| APP | 22C11 | mouse monoclonal | 0.5 μg/ml |
| APLP1 | W1NT | rabbit polyclonal | 1:500 |
| APLP1 | W1CT | rabbit polyclonal | 1:500 |
| APLP2 | D2-II | rabbit polyclonal | 1:1000 |
| APLP2 | W2CT | rabbit polyclonal | 1:500 |
| BACE1 | 3D5 | mouse monoclonal | 1 μg/ml |
| Dynamin-1 | PA1-660 | rabbit polyclonal | 1:5000 |
| Tau | 5E2 | mouse monoclonal | 0.2 μg/ml |
| pTau | AT8 | mouse monoclonal | 0.2 μg/ml |
| PrP | ICSM-35 | mouse monoclonal | 0.5 μg/ml |
| CREB | 48H2 | rabbit monoclonal | 1:1000 |
| pCREB | 1B6 | rabbit polyclonal | 1:500 |
| PSD-95 | anti-PSD-95 | mouse monoclonal | 0.5 μg/ml |
| SV-2 | E-15 | goat polyclonal | 1:1000 |
| Synaptophysin | anti-synaptophysin | mouse monoclonal | 1:10000 |
| Synaptopodin | anti-synaptopodin | rabbit polyclonal | 1:1000 |
| MAP2 | HM-2 | mouse monoclonal | 1:2000 |
2.2. Preparation of Aβ-containing soluble human brain TBS extracts
Human brain tissue was obtained and used in accordance with the UCD Human Research Ethics Committee guidelines (under approval LS-E-10-10-Walsh). Two brain samples were used for this study: brain 1 from a 85 year-old male (Drs R. Dykoski and J. Cleary, Minneapolis VA Health Care System, MN) with a clinical and pathological diagnoses of AD and brain 2 from a 78 year-old female (from Asterand plc, Detroit, MI) with a history of dementia and confirmed Alzheimer's disease pathology. Both patients had end-stage AD and neither had significant non-AD pathology. The interval between death and post-mortem was ∼48 hours for brain 1 and <7 hours for brain 2. Frozen samples of temporal cortex (0.9 g) were allowed to thaw on ice, chopped into small pieces with a razor blade and then homogenized in 4.5 ml of ice-cold 20 mM Tris-HCl, pH 7.4, containing 150 mM NaCl (TBS) with 25 strokes of a Dounce homogeniser (Fisher, Ottawa, Canada). Water-soluble Aβ was separated from membrane-bound and plaque Aβ by centrifugation at 91,000 g and 4 °C in a TLA 55 rotor (Beckman Coulter, Fullerton, CA) for 78 min. To eliminate bioactive small molecules and drugs, the supernatant was exchanged into ammonium acetate (brain 1) or TBS (brain 2) using a 5 ml Hi-trap desalting column (GE Healthcare Bio-Sciences AB, Uppsala, Sweden). Thereafter the extract was divided into 2 parts: one aliquot was immunodepleted (ID) of Aβ by 3 rounds of 12 h incubations with the anti-Aβ antibody, AW8, and protein A at 4 °C. The second portion was not manipulated in any way. Aliquots of samples were stored at −80 °C or used to assess Aβ content with a sensitive immunoprecipitation/Western blotting procedure (McDonald, et al., 2012). The rabbit polyclonal antibody, AW8, was used for immunoprecipitation and a combination of the anti-Aβ monoclonal antibodies, 2G3 and 21F12 for Western blotting. Aβ concentration was estimated by reference to known quantities of synthetic Aβ1-42 (Mc Donald, et al., 2010).
2.3. Animal maintenance
Male Wistar rats (375-400 g) were obtained from Charles River UK Ltd. (Margate, UK) and upon arrival housed in groups of 3-4 per cage with a 12 h light-dark cycle (lights on at 7 am) with food and water ad libitum. They were given 5 days to acclimate to the holding facility prior to surgery, including 3 handling sessions. After surgery rats were housed individually. All experimental procedures were carried out in accordance to guidelines and under licence from the Department of Health, Republic of Ireland with approval by the animal research ethics committee of University College Dublin.
2.4. Surgical procedures
Rats were anaesthetised i.p. with a mixture of ketamine/medetomidine (Vetalar/Domitor, Pfizer, Dublin, Republic of Ireland) and carprofen (Rimadyl, Pfizer) was injected s.c. to prevent the pain amplification response. Animals were placed in a stereotaxic frame, a small hole was burred in the scull over the left lateral ventricle (1 mm posterior and 1.6 mm lateral to Bregma; (Paxinos, 1986)), a guide cannula (22 gauge, 1 mm, Plastics One, Roanoke, VA) lowered into it and secured in place with two screws and acrylic cement. A dummy cannula cut to the same length as the guide cannula was used to maintain patency of the guide. Animals were recovered from anaesthetisia with atipamezole (Antisedan, Pfizer) and housed individualy. Next day s.c. injection of caprofen was repeated to provide further pain-relief. Post-surgery recovery and body weight was monitored for a week and during this time animals received additional handling and were habituated to unscrewing and removing of the dummy cannula.
2.5. Post-surgery open-field analysis
To confirm that implantation of cannulae did not adversely affect overt behaviour or motor function, animals were placed in an open-field chamber (made of black Plexiglass, 64 L × 64 W × 30 H cm) on days 5 -7 post-surgery and their behaviour was monitored for 5 min on each of two days prior to passive avoidance training. The total distance travelled, the percent of time spent moving and the number of rearings in the apparatus was determined using Ethovision™ software (Noldus, Wageningen, the Netherlands). In addition, the number of rearings was recorded manually in real time and compared with the number of rearings captured by the video tracker. Only animals without any obvious behavioural abnormalities were used further and were randomly allocated to treatment groups prior to commencing passive avoidance training. The identity of the samples injected was not known to the investigator performing the injections and conducting the behavioural studies.
2.6. Passive avoidance learning and recall
Training and recall were performed using a modular shuttle box from Med Associates Inc. (ENV-010MC, Georgia, VT). The chamber consisted of 2 equal sized compartments measuring 21 L × 16 W × 21 H cm, one lit and the other covered by a light-proof screen and unlit and a sliding door connecting the two. On the training day an animal was placed into the lit compartment, 30 s later the door between compartments was opened and the latency to enter the dark compartment was recorded. Immediately after the rat entered (all four paws) the dark compartment the door was closed and a foot shock delivered (0.9 mA/1 s, 3 times with 1 s interval) through the stainless steel grid floor. The door was then opened, the animal allowed to escape into the safe compartment and the door closed. The rat was left in the lit compartment for 60 s and then returned to the home cage. Rats that took longer than 60 s to enter the dark compartment during training were excluded from further study. Animals were tested for recall first at 24 h post-training and then again at 48 h post-training. On testing the rat was placed into the lit compartment, 30 s later the door between compartments was opened and latency to enter the dark compartment was measured. If the animal entered the dark compartment the door was quickly closed and the rat was immediately returned to the home cage; if the animal did not enter the dark compartment during 600 s, the door was closed and the rat returned to the home cage.
2.7. Time-course study of the effects of intracerebroventricular (icv) injection of Aβ- containing brain extracts on memory consolidation
Following passive avoidance training, animals were injected icv (5 μl) with either unmanipulated extract from brain 1 (AD) or the same extract immunodepleted of Aβ (ID-AD). Each animal received an icv injection of extract at one of the following time points: 1, 6 or 9 h post-training (Supplemental Fig. 1). Animals were loosely held, dummy cannula removed, injection cannula inserted to reach the ventricle and AD or ID-AD sample injected over a 3 min interval. Injection cannula was left in place for a further 1 min to facilitate diffusion of the sample and minimize upward leakage during removal. Afterwards the injection cannula was slowly removed and replaced by a dummy cannula. Animals were subsequently tested for recall at 24 and 48 h post-training. Correct placement of the cannula was confirmed by ink injection following terminal dose of sodium pentobarbital (Euthatal, Merial, Harlow, UK) at the end of the experiment.
2.8. Probing the molecular and structural effects of Aβ-mediated impairment of memory consolidation
Using the post-training time interval at which injection of AD extract was shown to most dramatically disrupt memory consolidation (1 h post-training) we went on to determine: (a) the reproducibility of this effect using a TBS extract from another brain (brain 2) and (b) the effect on the expression of certain neuroplastic proteins and synaptic ultrastructure (Supplemental Fig. 2). One hour after passive avoidance training rats were injected with 10 μl of samples at an infusion rate of ∼1.5 μl/min. The difference in the volumes used here versus in Section 2.7 derives from the fact that different AD brains have different levels of water-soluble Aβ. Brain 2 contained lower levels of Aβ than brain 1, therefore to compensate for the differences in concentration we increased the volume of the brain extract injected. Eighteen rats: 9 treated with AD and 9 treated with ID-AD were culled at 3 h post-training (2 h post-injection), hippocampi quickly removed and dentate gyri microdissected on ice as described previously (O'Sullivan, et al., 2007) (Supplemental Fig. 2). Tissue from each animal was placed into individual eppendorf tubes, flash frozen in liquid nitrogen and stored at −80°C. Since it was not possible to obtain behavioural data from the animals culled at 3 h additional animals were infused with the same AD and ID-AD samples in interleaved experiments. In total 71 animals were infused with AD, ID-AD or vehicle (TBS) and tested for recall at 24 h and 48 h post-training (Supplemental Fig. 2). The TBS-injected group was included as an additional negative control. Immediately after the 48 h recall, 6 animals receiving ID-AD and 9 rats receiving AD were perfused for ultrastructural analysis.
Individual frozen dentate gyri were homogenised essentially as described for human brain samples, except the TBS buffer contained protease and phosphatase inhibitors [5 mM EDTA, 1 mM EGTA, 5 μg/mL leupeptin, 5 μg/mL aprotonin, 2 μg/mL pepstatin, 120 μg/mL Pefabloc, 2 mM 1,10-phenanthroline, Phosphatase Inhibitor Cocktail 3 (Sigma)]. TBS containing these inhibitors is referred to as TBS+. The resulting homogenate was then centrifuged at 175,000 g and 4°C in a TLA 100.5 rotar for 30 min and supernatant collected and stored at −80°C. The pellet was washed by vortexing in 5 volumes of cold TBS+, the suspension centrifuged at 175,000 g and 4°C for 30 min and supernatant discarded. The membrane-containing pellet was resuspended in 5 volumes of ice-cold RIPA buffer (50 mM Tris-HCl, pH 8.0, containing 150 mM NaCl, 0.5% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS) plus protease and phosphatase inhibitors and homogenised with 20 strokes of a Dounce homogeniser. To ensure release of integral membrane proteins the homogenate was sonicated for 10 s using a microtip attached to an XL-2000 sonicator (Misonix Inc., Farmingdale, NY) at power setting 4 (∼12 W). Thereafter the extract was centrifuged at 175,000 g and 4°C for 30 min and the supernatant collected. The protein content of these TBS+ and RIPA+ extracts was determined using a BCA protein assay kit (Pierce, Thermo Scientific Inc., Rockford, IL) and samples were then aliquoted and stored at −80°C pending further analysis.
2.9. Detection of protein analytes
TBS+ and RIPA+ extracts were diluted with 4− tris-glycine sample buffer (1× concentrations: 62.5 mM Tris-HCl, pH 6.8, 10% glycerol, 2% SDS, 1% β-mercaptoethanol) or 4× tris-tricine sample buffer (1× concentrations: 450 mM Tris-base, pH 8.45, 10% glycerol, 4% SDS) and electrophoresed on 10% polyacrylamide tris-glycine hand-poured gels or Novex 10-20% polyacrylamide tricine pre-cast gels (Invitrogen, Life Technologies, Carlsbad, CA) as appropriate (Sala Frigerio, et al., 2010; Shankar, et al., 2009). Proteins were transferred onto 0.2 μm nitrocellulose (Scheicher & Schuell BioScience, Dassel, Germany) at 400 mA and 4°C for 2 h. Uniform transfer of proteins onto nitrocellulose was confirmed by reversible staining with Ponceau S (0.1% w/v in 1% acetic acid) and only blots demonstrating equal levels of total protein were used for immunodetection. Membranes were then blocked for 1 h at RT in 5% skimmed milk/TBS-T (Tris-buffered saline solution containing 0.05% Tween-20), washed (3×15 min) in TBS-T and incubated with appropriate primary antibody (Table 1) in blocking buffer overnight at 4°C. Membranes were washed (3×15 min) in TBS-T and incubated for 1 h at RT with sheep anti-mouse-HRP or donkey anti-rabbit-HRP diluted 1:10000 and 1:15000 in TBS-T, as appropriate. Finally, membranes were washed (3×15 min) in TBS-T and immunoreactive proteins visualised using an enhanced chemiluminescent substrate (Pierce, Thermo Scientific Inc., Rockford, IL) and Fuji Super RX film (FujiFilm, Dusseldorf, Germany). Band intensities were quantified using Image-J software (version 1.44p) from the National Institutes of Health (USA). All samples were analysed at least in duplicates. The levels of proteins in dentate gyri of AD-treated rats was expressed as a percentage of the average of the corresponding control (ID-injected group) samples electrophoresed on the same gel.
For analysis of phospho-tau (p-tau)/total-tau (t-tau) and pCREB/CREB, samples were first Western blotted for the phosphorylated protein and the membrane then stripped by incubation in stripping buffer (composition: 25 mM glycine, 1% SDS, pH 2), washed with TBS and then immunoblotted using antibodies for the unphosphorylated protein.
2.10. Brain collection and electron microscopy for ultrastructural analysis
After completion of passive avoidance recall at 48 h, brains of rats injected with AD or ID-AD samples (human brain 2) were collected for ultrastructural analysis (Supplemental Fig. 2). Rats were deeply anaesthetised with sodium pentobarbital (Euthatal, Merial, Harlow, UK), transcardially perfused with 4% paraformaldehyde in PBS (4% PFA), brains excised and fixed in 4% PFA. Electron microscopy was performed as previously described (Buttini, et al., 2005). Briefly, vibratome sections were postfixed with 4% paraformaldehyde and 1% glutaraldehyde in 0.1 M cacodylate buffer, pH 7.4. Next sections were immersed in 1% osmium tetroxide and stained with saturated uranyl acetate dissolved in 50% ethanol. Subsequently sections were dehydrated through a graded series of ethanol to 90% ethanol and 2-hydroxypropyl methacrylate was the intermediate solvent. All infiltrations of 2- hydroxypropyl methacrylate and Epoxy 812 resin (Energy Beam Sciences, Agawam, MA) were performed on a shaker at slow speed. After two changes of 100% resin, the plates were polymerized in a 65°C oven for 24 h. The plastic was detached, and selected areas from the CA1 and dentate gyrus were cut and glued onto dummy blocks.
Ultrathin sections (70 nm thick) of silver-grey interference appearance were cut from hippocampal dentate gyrus and CA1 region with a Leica ultramicrotome. Two to three sequential serial ultrathin sections were mounted onto individual 200-mesh copper grids (Electron Microscopy Sciences, Fort Washington, PA), and poststained in ethanolic uranyl acetate, followed by bismuth nitrite (Electron Microscopy Sciences). The sections were analysed with a Zeiss (Thornwood, NY) EM10 transmission electron microscope. The electron scattering method was used to calculate the approximate thickness (RET%) of each section. The image scale was calibrated and a smaller area of known dimensions (5000 × 5000 nm) was selected from each image and stored as separate images and formed a data file of 8 sets of two aligned serial counting frames for each hippocampus.
The disector technique was applied to all pairs of micrographs to estimate the number of synapses (Sterio, 1984; Tang, et al., 2001). The post-synaptic density (PSD) was used as the counting entity. Synapses in two serial sections were utilized for analysis, the micrograph from the first section was considered the reference plane and from the next ultrathin section the look-up plane. A digital transparency with an unbiased frame was super-imposed to the digitized electron microgragh. A distance that was greater than the postsynaptic density separated the edges of the frame and the micrograph and was considered the guard area. Synapses were counted on the reference micrograph if the PSD was located in the counting frame and did not intersected the forbidden lines. Synapses present in the look up plane or in both planes were not counted. Total number of synapses in the disector per region was the total volume of the disector. The volume of the disector was calculated by multiplying the area of the counting frame by the height of the disector which was equivalent to the thickness of the two ultrathin sections. Counting approximately 100 objects per hippocampus of each animal was considered ideal for accurate synapse estimates (Sterio, 1984). These same serial sections were utilized for analysis of synaptic vesicle counts (Applegate and Landfield, 1988). Approximately 100 terminals and 2500 vesicles were analysed per animal.
As previously described (Eyre, et al., 2003) semi-thin sections (2 μm thick) were cut from the same tissue blocks used for electron microscopy and mounted on glass slides. The sections were stained with 1% toluidine blue, dried and viewed under a Olympus light microscope with the StereoInvestigator System attached. Images of the dentate gyrus granule cell layer and CA1 were obtained from consecutive semithin sections with a × 40 objective lens, and were digitized. Calibrated sets of serial images were analysed, the disector technique was applied to the serial images of the cells and the cell density (cell NV) was calculated. The synapse-to-neuron ratio was calculated for each hippocampus in each animal by dividing the estimate of synapse density (synapse NV) by the estimate of cell density (cell NV) for each hemisphere.
For morphometric analysis of synapses from each section, a total of 20 electron micrographs of the hippocampal area were obtained at a final magnification of 20,000×. Electron micrographs were digitized and analysed with Image J (NIH) to determine the synapse cleft distance, the pre-synaptic terminal (PST) diameter and the post-synaptic density (PSD) density.
2.11. Open-field analysis of rats injected with brain extracts
Since increased activity might contribute to a shorter entry latency in passive avoidance a separate group of rats was used to assess if injection of human brain Aβ altered motor activity. One week after surgery rats were injected icv with 10 μl of Aβ-containing extract from brain 2 or with extract immunodepleted of Aβ (n=8 and n=9, respectively). Twenty three and 47 h (i.e. post-injection intervals equivalent to the 24 and 48 h passive avoidance recall periods) after injection rats were placed in the open field and their behaviour recorded for 10 min using Ethovision™ (Supplemental Fig. 3). The total distance moved and number of rearings were analysed.
2.12. Statistical analysis
Animals were allocated to treatment groups prior to commencement of the passive avoidance training and all injections and behavioural experiments were carried out “blind” to the identity of the samples injected. Experiments were conducted in batches of 12 to 18 animals, therefore different treatments and treatment times were counterbalanced within and between batches. Differences in entry latencies between treatment groups at 24 and 48 h recall were analysed using repeated measures ANOVA with Student-Newman-Keuls post hoc test and test time as a repeated measure and treatment group as a between-subject factor. Significant main effects were analysed by planned comparisons where needed. EM results were analysed using unpaired t test.
3. Results
3.1. Impairment of memory consolidation by AD brain Aβ depends on the time of injection post-training
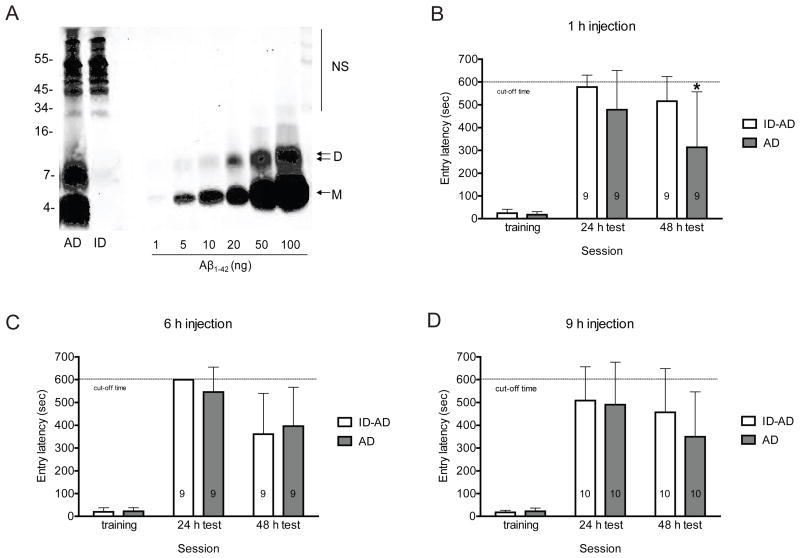
We previously reported that water-soluble extracts of human brain that contain SDS-stable Aβ dimers potently disrupt hippocampal LTP in vitro and in vivo, adversely affect dendritic spines in cultured neurons and impair memory consolidation (Freir, et al., 2011b; Shankar, et al., 2008). Here we sought to investigate if the memory impairing activity of such Aβ-containing extracts altered the expression of neuroplastic proteins and synaptic structure in the live rat. To this end we began by conducting a time-course study to determine the interval at which icv injection of AD TBS had its greatest effect on memory consolidation. The TBS soluble extract of the cerebral cortex from an AD patient used in this study contained two prominent bands, one that migrated with a molecular weight consistent with an Aβ monomer and the other with a molecular weight of a dimer (Fig. 1A). By comparison with known quantities of synthetic Aβ1-42 electrophoresed on the same gel, the amount of Aβ monomer and dimer in the brain sample was estimated at 69.5 ng/ml and 47.0 ng/ml, respectively.
Figure 1.
The disruptive effect of AD brain Aβ on passive avoidance memory depends on the time of injection. (A) TBS extract of AD brain was examined by immunoprecipitation/Western blotting using the polyclonal anti-Aβ antibody, AW8, for IP and a combination of anti-Aβ mAbs, 2G3 and 21F12, for Western blotting. The first lane of the Western blot shows that the buffer-exchanged TBS extract (AD) contains Aβ monomer and SDS-stable Aβ dimers. The second lane shows that three rounds of IP effectively depleted the extract of all detectable Aβ (ID). Molecular weight standards are indicated on the left, and Aβ monomers (M) and dimers (D) are indicated with arrows on the right. Known amounts of synthetic Aβ1-42 were included to allow estimation of Aβ content in the test samples, and cross-reactive immunoglobulin-derived proteins are indicated by NS. (B-D) Animals were injected once with 5 μl of AD or ID-AD sample at 1, 6 or 9 h following training and tested for recall at 24 h and 48 h. The AD-induced deficit in consolidation of avoidance memory is dependent on timing of icv injection. At 24 h recall there were no significant differences in escape latencies between the groups at any of the injection times studied (p>0.05 for all comparisons). However, at the 48 h recall there was a strong time-dependent decrease in escape latency in the AD injected animals which was significant in the 1 h injection group (t16=−2.286, *p<0.05). Values are expressed as mean ± standard deviation and the group sizes are indicated on each histogram.
When AD TBS was injected 1 h post-training animals exhibited significantly decreased entry latency into the shock-paired dark compartment at 48 h compared to rats treated with the same extract immunodepleted of Aβ (repeated measures ANOVA, recall session as repeated measure, injection group as between-subject measure: significant main effect of injection -AD vs. ID-AD, F1,16=4.86, p<0.05; significant main effect of session - 24h vs. 48 h, F1,16=12.54, p<0.01). At the 24 h recall animals injected with AD TBS also displayed a reduced latency, but this was not statistically significant (no significant injection by recall interaction, p>0.05; independent samples t-test – 48 h t16=−2.286, p<0.05; 24 h – p>0.05, Fig 1B). Animals injected at 6 and 9 h post-training showed no significant inter-group differences (no significant effect of group, p>0.05 for both injection times, Fig. 1C and D). However, similarly to 1 h post-training injection, there was a significant decrease in performance between the 24 and 48 h recall periods (significant effect of session – 6 h injection, F1, 16=20.86, p<0.01; 9 h injection, F1, 18=5.99, p<0.05, Fig. 1C and D), but this was not different for AD TBS and ID-AD extracts (no significant interaction between injection group and recall session, p>0.05 for both injection times, Fig. 1C and D). Thus the impaired recall at 48 h in the 6 and 9 h post-injection group indicates that this brain sample may contain other component which can perturb consolidation, but these effects are not attributable to Aβ. Based on the fact that only the 1 h post-training injection caused a significant decrease in the test entry latency that could be ascribed to brain-derived Aβ, we used this interval for all subsequent studies.
In order to examine the role of Aβ on the expression of neuroplastic proteins and synaptic form and number we performed a two-armed large scale avoidance learning trial. The first arm was designed to test the reproducibility of the impairment induced by injection of AD brain extract at 1 h post-training (Fig. 1B) and to provide rat brain tissue for ultrastructural analysis. As an additional control we injected 1 group of animals with vehicle (TBS buffer) so we could compare its effects with that of the AD brain extract immunodepleted of Aβ. The second arm of the trial was to provide tissue for analysis of certain neuroplastic proteins. In this case animals were culled 2 h following icv injection, a time interval at which APP and other neuroplastic proteins have been shown to decrease following passive avoidance training (Conboy, et al., 2005). Because animals were culled at a time point (2 h) which precluded assessment of their recall we were careful to interleave the use of animals injected at 1 h post-training and culled 2 h later with rats that were analysed at 24 and 48 h post-training (Fig. 2B). The AD brain extract used for these experiments was qualitatively similar to that employed in our time-course studies (compare Fig. 1A and Fig. 2A), but the estimated concentration of Aβ monomer (9.3 ng/ml) and dimer (7.9 ng/ml) was substantially lower. Since concentrating the sample could potentially change the aggregation state of Aβ, the only way to increase the amount of Aβ administered was to increase the volume of sample injected, therefore in these experiments we injected 10 μl of sample, instead of the 5 μl used with brain 1 extracts.
Figure 2.
Impairment of passive avoidance memory by AD brain Aβ injected 1 h post-training is reproducible between different AD brains. (A) TBS extract of AD brain was examined by immunoprecipitation/Western blotting as described above. The first two lanes of the Western blot show that the buffer-exchanged TBS extract (AD) contains Aβ monomer and SDS-stable Aβ dimers, whereas these were not detected in the vehicle control (TBS). Molecular weight standards are indicated on the left, and Aβ monomers (M) and dimers (D) are indicated with arrows on the right, cross-reactive immunoglobulin-derived proteins are indicated by NS. Known amounts of synthetic Aβ1-42 were electrophoresed on the same gel to allow estimation of Aβ content in the test samples. (B) Animals were injected with 10 μl of AD, ID-AD sample or vehicle (TBS) 1 h following training and tested for recall at 24 h and 48 h. The number of animals in each group is indicated on each histogram, values are expressed as mean ± standard deviation. Animals injected with AD TBS extract tended to enter the dark chamber more rapidly than animals receiving the same extract immunodepleted of Aβ or vehicle (TBS) at 24 h recall and at 48 h recall this impairment became statistically significant (24 h: F2, 68 = 2.566, p = 0.084; 48 h: F2, 68 = 6.508, **p <0.01, post-hoc AD vs. ID-AD and TBS).
As is evident from the increased entry latency at 24 h recall versus that during training (repeated measures ANOVA: main effect of session F1,68 = 772.078, p<0.001, Fig. 2B) all animals learned the task. But animals injected with AD TBS extract tended to enter the dark chamber more rapidly than animals receiving the same extract immunodepleted of Aβ or vehicle (TBS) (injection group by session interaction showed marginal significance F2,68 = 2.834, p = 0.066; no significant group effect, p>0.05, Fig. 2B). At 48 h this difference was statistically significant (main effect of group F2,68 = 4.95, p<0.05; recall session F1,68 = 18.238, p<0.001 and significant group by session interaction F2,68 = 3.747, p<0.05; post-hoc AD-injected group vs. ID-AD and TBS groups Fig. 2B), with animals receiving ID-AD and TBS performing at near identical levels. This was further confirmed by separate analysis of 24 h recall and 48 h recall employing univariate ANOVA (24 h: F2, 68 = 2.566, p = 0.084; 48 h: F2, 68 = 6.508, p <0.01, post-hoc AD vs. ID-AD and TBS). In all our avoidance learning studies (Figs. 1 and 2, Freir, et al., 2011b; Shankar, et al., 2008) vehicle-treated animals always showed reduced latency at 48 h vs. 24 h recall sometimes this was significant, as in the cases the TBS treated animals (Fig. 2B) and sometimes this was not (e.g. ID-AD injected rats, Fig. 2B). This effect could be due to natural erosion or weakening of the memory trace or because of partial extinction due to re-exposure. Whatever the reason, Aβ exacerbated this effect.
Mice transgenic for APP have a wide range of non-memory sensorimotor perturbations, including hyperactivity (reviewed in (Games, et al., 2006)), therefore to preclude the possibility that the reduction in entrance latency seen in animals receiving AD TBS was a result of increased locomotion another cohort of animals was injected with the same AD or ID-AD extract and then tested in the open field. Eight animals were injected with 10 μl AD TBS and 9 with ID-AD and tested for 10 min in the open field at 23 and 47 h after injection, the time points corresponding to passive avoidance recall tests conducted at 24 and 48 h following training. Injection of AD brain extract did not increase locomotor activity in animals. Animals from both injection groups travelled similar total distances over 10 min when tested at 23 and 47 h post-injection and as expected showed a reduction in locomotor activity with repeated testing (Repeated measures ANOVA: significant main effect of the test session F1,15 = 10.47, p<0.01; no effect of group or test by group interaction, p>0.05 in both cases, Supplemental Fig.4A). Similarly, injection of AD brain extract did not alter the number of rearings, that are considered to represent exploratory activity (Dere, et al., 2003) (no significant effect of the test session, group or test by group interaction, p>0.05 in all cases, Supplemental Fig.4B).
3.2. The Aβ-mediated disruption of avoidance memory is associated with changes in synaptic number and structure in both the dentate gyrus and CA1 fields of the hippocampus
In order to examine whether the Aβ-mediated impairment of avoidance memory was associated with altered synapse number or structure we conducted a quantitative electron microscopy study of the mid- and outer-molecular layer of the dentate gyrus and lacunosum-molecular layer of CA1 field of hippocampi of rat brains harvested after the 48 h recall session. Ultrastructural analysis revealed that injection of Aβ was associated with changes in synaptic structure and fewer hippocampal synapses (Fig. 3 and 4). Dentate gyri of animals that received AD brain extracts had lower synapse density than the ID-AD-injected group (unpaired t test: t13=3.087, p<0.01, Fig. 3C); moreover, their synaptic clefts and pre-synaptic terminals (PST) were smaller than in the control group (unpaired t test: t13=2.706, p<0.05 and t13=4.062, p<0.005, Fig. 3D and 3E, respectively), while post-synaptic densities (PSD) did not differ between treatments (p>0.05, Fig 3F). In addition vacuoles and pits were detected in the synapses of the AD injected group, whereas these were not observed in the Aβ immunodepleted group (Fig. 3A and 3B, see arrows), but a preliminary analysis revealed no change in the number of synaptic vesicles (not shown).
Figure 3.
Injection of AD brain-derived Aβ reduced learning-associated synapse remodelling in the hippocampal dentate gyrus of hippocampus. Animals were injected icv with 10 μl of AD or ID-AD sample 1 h post-training and brains collected after completion of the 48 h recall test. Representative photomicrographs of mid- and outer-molecular layer of dentate gyrus of (A) animals injected with ID-AD sample and (B) with AD sample. The scale bar of 1 μm applies to all images. PST indicates pre-synaptic terminal; PSD – post-synaptic density; P – pits and vacuoles are highlighted by arrows. Animals injected with Aβ containing brain extracts had lower synapse density (C, t13=3.087, **p<0.01) and changes in synapse structure, smaller synaptic clefts (D, t13=2.706, *p<0.05) and reduced PST diameters (E, t13=4.062,** p<0.005), but largely unchanged PSD (F, p>0.05). Values are expressed as mean ± standard deviation and the group sizes are indicated on each histogram.
Figure 4.
Injection of AD brain-derived Aβ leads to lower synapse density and changes in synaptic structure in CA1 field of hippocampus. Animals were injected icv with 10 μl of AD or ID-AD sample 1 h post-training and brains collected after completion of the 48 h recall test. Representative microphotographs of the lacunosum-molecular layer of CA1 hippocampus of (A) animals injected with ID-AD sample and (B) with AD sample. The scale bar of 1 μm applies to all images. PST – pre-synaptic terminal, PSD – post-synaptic density, P – pits, vacuoles highlighted by arrowheads. Quantitative electron microscopy of lacunosum-molecular layer of CA1 field revealed reduction in synapse density (C, t13=2.322, *p<0.05) and smaller PST diameters (E, t13=2.645, *p<0.05), without significant change in the size of synaptic clefts (D, p>0.05). PSD density tended to be higher in the AD group than in the ID-AD group, but this difference did not reach significance (F, p=0.094). Values are expressed as mean ± standard deviation and the group sizes are indicated on each histogram.
A similar pattern was evident in the CA1 field. Animals injected with Aβ-containing brain extracts had smaller PST diameters and lower synapse density (unpaired t test: t13=2.322, p<0.05 and t13=2.645, p<0.05, Fig. 4C and 4E, respectively). The size of synaptic clefts in Aβ-treated rats was also reduced, but unlike the situation in the dentate gyrus this did not reach statistical significance (Fig. 4D). As in the dentate, there was also a tendency for a higher density of PSD in the AD group in CA1 field (p=0.094, Fig. 4F). Also in accord with our findings in the dentate gyrus, numerous vacuoles and pits were observed in the CA1 synapses of AD treated rats (Fig. 4A and 4B, see arrows).
3.3. The Aβ-mediated disruption of avoidance memory is not associated with an early change in the expression of AD-related or other neuroplastic proteins
Since we found that Aβ-containing AD TBS can perturb recall of avoidance learning when injected at intervals associated with transient changes in APP expression and processing (Conboy, et al., 2005; O'Sullivan, et al., 2007) we investigated the effect of Aβ administration on the protein levels of the AD-related proteins: APP, APLP1, APLP2, BACE1, tau and PrP in the dentate gyrus. For these studies we were careful to measure the levels of both full length APP protein family members and their C-terminal stubs released by α- and β-secretase (Sala Frigerio, et al., 2010; Walsh, et al., 2007). In addition we also examined the expression of CREB, a transcription factor believed to be important for modulating plasticity and memory (Silva, et al., 1998), the pre- and post-synaptic markers synaptophysin, dynamin-1 or SV-2 (Knaus, et al., 1986; Masliah, et al., 1989; Scheff and Price, 2003; Yao, et al., 2003), synaptopodin (Deller, et al., 2007; Jedlicka, et al., 2008; Vlachos, et al., 2009) and PSD-95 (Ehrlich, et al., 2007; Lin, et al., 2006), and the cytoskeletal protein, MAP2 (Hering and Sheng, 2001; Sánchez, et al., 2000). The levels of all protein analytes measured in extracts of dentate gyri from rats receiving AD and ID-AD TBS extracts were highly similar, with only two analytes, APLP1 CTF and secreted PrP, differing by more than 15% between the groups (Figs. 5 and 6). Although membrane-bound full length APLP1 and PrP are known to bind aggregates of synthetic Aβ (Lauren, et al., 2009) the minor changes we detected in the levels of fragments of these proteins is unlikely to be of biological significance. In regard to APLP1 it is noteworthy that full length APLP1, the direct precursor of APLP1 CTF did not evidenced any difference between the AD- and ID-AD-treated groups (Fig. 5A and 6A). Similarly, Aβ-treatment did not alter the levels of APP or APLP2, their CTFs or shed ectodomains. In addition, the protein levels of BACE1, an enzyme implicated in the processing of APLP1 and PrP (Parkin, et al., 2007; Sala Frigerio, et al., 2010) was highly similar in animals treated with AD or ID-AD extracts.
Figure 5.
The Aβ-mediated disruption of avoidance memory is not associated with an early change in the expression of TBS-soluble AD-related or other neuroplastic proteins. Animals were injected icv with 10 μl of AD or ID-AD sample 1 h post-training and culled 2 h later. (A) Dentate gyri were dissected, TBS extracts of dentate gyri prepared and electrophoresed on 10% polyacrylamide tris-glycine gels and western blotted with antibodies to the proteins indicated. The western blots shown are representative of at least two experiments. Molecular weight standards are indicated on the left, bands corresponding to proteins of interest are labelled with arrows on the right, or where more than one form of a protein was detected this is indicated by a bracket. Protein names are located at the top and the treatment group identity of the samples are marked at the bottom of the blots. + and – indicates samples from transfected cells, transgenic or null mice known to over-express or lack expression of the protein of interest. (B) Bands of interest were quantified by densitometry. Results are presented as values normalized to the average of the ID-AD control group and are shown as mean percent of control ± standard deviation of duplicate measurements for each sample. The number of dentate gyri samples per group is indicated on each histogram. Injection of Aβ-containing AD brain extract did not significantly alter the levels of any of the proteins examined (p>0.05).
Figure 6.
The Aβ-mediated disruption of avoidance memory is not associated with an early change in the expression of membrane-bound AD-related or other neuroplastic proteins. Animals were injected icv with 10 μl of AD or ID-AD sample 1 h post-training and culled 2 h later. (A) Dentate gyri were dissected, RIPA extracts prepared and electrophoresed on 10-20% polyacrylamide tricine gels or 10% polyacrylamide tris-glycine gels and western blotted with the antibodies indicated. Molecular weight standards are indicated on the left, bands corresponding to proteins of interest are labelled with arrows (or brackets) on the right, protein names are located at the top and lanes from animals receiving AD or ID-AD extracts are marked at the bottom of the blots. + indicates samples from transfected cells or transgenic mice known to over-express the protein of interest. (B) Specific bands were quantified by densitometry and normalized versus the average value of the corresponding ID-AD control group. Results are presented as mean percent of control ± standard deviation of duplicate measurements for each sample. The number of dentate gyri samples per group is indicated in each column. Because BACE1 and synaptophysin showed the biggest divergence between AD and ID-AD samples when 6 AD and 5 ID-AD samples were analyzed, and extracts from some additional rat brains were available, we extended our analysis to include 7 AD and 8 ID-AD samples. Injection of AD brain Aβ did not significantly alter the levels of any of the membrane-bound proteins studied (p>0.05).
We also investigated the phosphorylation state of CREB and tau and compared the levels of the phospho-proteins to unphosphorylated proteins. Both total tau and p-tau were reliably detected in TBS extracts of dentate gyrus, but neither the individual levels of t-tau and ptau nor the ratio of p-tau to t-tau differed between the AD- and ID-AD-treated groups (p>0.05). pCREB was not detected in any of the samples tested even though pCREB was detected in the lysates of forskolin-treated cells (Supplemental Fig. 5). These results demonstrate that Aβ-induced memory impairment does not require early changes in the levels of proteins believed to be involved in AD. Moreover, administration of AD brain-derived Aβ did not alter markers of synaptic integrity (Fig. 5 and 6, Supplemental Fig. 6) (Ansari, et al., 2008; Masliah, et al., 1989; Scheff and Price, 2003; Yao, et al., 2003) nor the plasticity-associated transcription factor CREB at this early stage. The lack of change of the synaptic marker proteins at 2 h post-Aβ injection suggests that the structural changes evident at 47 h post-injection are not the result of an acute toxic event, but rather only manifest as the cascade of memory-associated remodelling and the effects of 24 h test re-exposure take effect.
4. Discussion
Huge effort has gone into investigating Aβ aggregation and toxicity, with the majority of studies using a single defined allotype of synthetic Aβ at supraphysiological concentrations, and while a broad range of different-sized assemblies have been found to be toxic, it remains uncertain whether such species actually exist in vivo (for reviews see (Jan, et al., 2010; Shankar and Walsh, 2009)). To obviate this concern we used water-soluble extracts from AD brain. Here we report that brain-derived Aβ inhibits consolidation of avoidance memory in a manner highly dependent upon the interval between training and injection. The Aβ species present in these samples migrated on SDS-PAGE with molecular weights consistent for monomers and dimers. However, since the gels used to resolve these species are highly denaturing, the size estimates they provide do not necessarily reflect native Aβ assembly size. Rather, they simply indicate that there are at least two distinct forms of Aβ present: one form detected as Aβ monomer and the other as Aβ dimer. Because fibrils are removed by centrifugation the species detected on SDS-PAGE are unlikely to be SDS-induced breakdown products of fibrils. Thus the ∼4 kDa species detected on SDS-PAGE could be a true monomer, and/or monomer derived from pre-fibrillar assemblies that are unstable when electrophoresed in SDS. Similarly, detection of SDS-stable dimers could reflect the presence of authentic dimers and/or pre-fibrillar assemblies which are liable in SDS and break down to dimers. Although we cannot attribute the inhibition of memory consolidation to a precise Aβ structure or assembly form the results presented here together with those from prior studies (Klyubin, et al., 2008; O'Nuallain, et al., 2010; Shankar, et al., 2008) strongly suggest that soluble diffusible dimers and/or pre-fibrillar intermediates built of Aβ dimers are key memory-impairing toxins.
These findings build on earlier pilot data which also suggested that AD brain Aβ can impair memory consolidation (Shankar, et al., 2008). In those original studies we used brain extract from only a single AD case and relatively small numbers of rats (n=5 per group). When Aβ-containing AD TBS was injected at 0, 3 and 6 h post-training memory impairment was obvious only at the 3 h time point, and this barely reached statistical significance. Thus we were anxious to further assess the activity of AD TBS and to determine if a similar effect was evident when extracts from other AD brains were used and when larger cohorts of animals were examined. Accordingly, we injected AD TBS into rats at 1, 6 and 9 h post-training using 9-10 animals per group at each time point. In agreement with our earlier results we found that AD TBS did not alter avoidance learning when injected at 6 h post-training. Nor did it alter recall when injected at 9 h post-training, a new time point added based on the finding that this is the interval at which cell culture-derived Aβ most effectively disrupts memory consolidation (Freir, et al., 2011a). Importantly, when injected at 1 h post-training AD TBS reduced escape latency by ∼ 40% compared to animals receiving the same extract that had been immunodepleted of Aβ.
Time-dependent perturbations of memory consolidation are not unique to AD TBS Aβ, for instance infusions of antibodies to the neural cell adhesion molecule are effective only when administered at 6 h post-training (Doyle, et al., 1992; Foley, et al., 2000; Roullet, et al., 1997) and cell-derived Aβ also evinced a highly time-dependent effect. In a second experiment, using TBS extracts from another AD brain and even larger numbers of animals (23-25 animals per group) we obtained highly similar results. That is, icv injection of AD TBS at 1 h post-training significantly decreased recall at 48 h compared to animals receiving TBS buffer or the AD extract immunodepleted of Aβ.
Interestingly, in both experiments AD TBS caused a modest reduction in escape latency at 24 h recall but impairment only became statistically significant during the second test at 48 h. In other words, as has been shown for anti-NCAM antibodies (Alexinsky, et al., 1997; Seymour, et al., 2008), there was a need for animals to be re-exposed to the chamber for the impairment to fully manifest. This pattern suggests that under the conditions used the adverse effect of Aβ was not strong enough to completely undermine consolidation of avoidance memory, but lead to formation of a weaker memory trace that was susceptible to decay or extinction.
Ultrastructural examination of hippocampi from rats perfused after 48 h recall revealed that Aβ-mediated impairment of avoidance memory was associated with lower total synapse number and altered synaptic structure in both the dentate gyrus and CA1 fields. Memory consolidation requires modification of synapse structure and subsequent change in neural connectivity (Lamprecht and LeDoux, 2004; Marrone, 2007) and increases in synaptic density are known to occur following avoidance learning (O'Malley, et al., 1998). Thus the lower density of synapses detected in AD-treated rats could reflect Aβ-mediated inhibition of new synapse formation and/or synapse degeneration. Evidence of overt degeneration similar to that seen in AD (Gonatas, et al., 1967) comes from the detection of pitting and vacuolation. Also, in the AD-treated group synaptic clefts and pre-synaptic terminals were significantly smaller, whereas the size of the PSD tended to be increased. These finding are reminiscent of time-dependent changes in synaptic structure induced by a murine scrapie model of prion disease (Gray, et al., 2009) and imply that the pre-synaptic terminal may be the first target effected by Aβ. However, our data do not provide information as to whether these changes are permanent, or as seems more likely transient (Cleary, et al., 2005). Indeed our behavioural and ultrastructural data are consistent with the idea that human brain-derived Aβ impairs formation of long-term memory by compromising the structural plasticity essential for consolidation (Lamprecht and LeDoux, 2004). These data imply that the target of AD TBS Aβ is involved in synapse formation and/or maintenance, and is expressed in a highly time-dependent manner. Given that injection of Aβ-containing extract at 6 h post-training had no effect, and assuming the concentration of Aβ injected would be reduced by more than 75% within 4 hours (Cirrito, et al., 2003), it seems likely that Aβ targets processes initiated very early in the consolidation pathway.
To gain some understanding about the molecular mechanisms by which Aβ perturbs memory consolidation we conducted a pilot study to search for changes in the expression of 13 neuronal proteins implicated in AD and/or learning and memory. These included 6 AD-related proteins (APP, APLP1, APLP2, BACE, tau and PrP) (Avila, et al., 2004; De Strooper, 2010; Gunther and Strittmatter, 2010; Sala Frigerio, et al., 2010), 1 memory-associated transcription factor (CREB) (Saura and Valero, 2011) and 6 synaptic marker proteins (dynamin-1, MAP-2, PSD-95, SV-2, synaptopodin and synaptophysin) (Reddy, et al., 2005). Since the first wave of consolidation-associated protein regulation occurs within 6 h of learning (Monopoli, et al., 2011) and it is known that APP is transiently reduced 2-4 h following avoidance training (Conboy, et al., 2005) we chose to focus on the period 3 hours following training i.e. 2 hours following injection of Aβ. However, at this interval, none of these proteins were altered by administration of AD TBS. Since we only examined one time point it is possible that it takes longer for changes in these proteins to become apparent. This will be an important issue to address in future studies. Nonetheless, the lack of change in pre- and post-synaptic markers is informative, since it indicates that Aβ is not acutely toxic to synapses, but rather that it takes time and perhaps re-exposure to the training environment for structural compromise to become evident.
Memory consolidation requires changes in synaptic form and function (Kandel, 2001; Lechner, et al., 1999) and is made possible by the carefully choreographed expression of structural, metabolic and secreted proteins (Lamprecht and LeDoux, 2004; Richter and Klann, 2009). Here we present a time-sequenced paradigm with icv injection of AD brain-derived Aβ which will help guide future studies. Specifically, when used with transcriptional profiling and proteomic discovery platforms this approach should make it possible to identify proteins altered by Aβ and as a consequence to develop therapeutic strategies aimed at overcoming the memory-disrupting effects that typify early AD.
Supplementary Material
Supplementary Figure 1 The flowchart describes the number of rats used to test the effect of administering Aβ on recall of avoidance learning when AD brain extract (AD) or extract immunodepleted of Aβ (ID-AD) was injected at different time intervals post-training.
Supplementary Figure 2 The flowchart describes the number of rats used to test the effect of administering Aβ on recall of avoidance learning, protein expression and synaptic ultrastructure when AD brain extract (AD), extract immunodepleted of Aβ (ID-AD) or TBS was injected at 1 hour post-training.
Supplementary Figure 3 The flowchart describes the number of rats used to test if either AD brain extract (AD) or extract immunodepleted of Aβ (ID-AD) effect performance in the open field.
Supplementary Figure 4 Injection of AD brain Aβ does not affect locomotor activity. Animals were injected with 10 μl of AD or ID-AD sample and their behaviour in the open field monitored for 10 min at 23 and 47 h post-injection. (A) Injection of AD brain Aβ does not affect total distance travelled at both time intervals when compared to ID-AD group (no significant effect of group or test by group interaction, p>0.05 in both cases; significant main effect of the test session F1,15 = 10.47, p<0.01). (B) Injection of AD brain extract does not affect the number of rearings at 23 and 47 h post-injection when compared to ID-AD injection (no significant effect of the test session, group or test by group interaction, p>0.05 in all cases).
Supplementary Figure 5 pCREB was not detected in any of the samples tested even though pCREB was detected in the lysates of forskolin-treated cells. Dentate gyri were dissected, RIPA extracts prepared and electrophoresed on 10% polyacrylamide tris-glycine gels and western blotted with the pCREB or CREB antibodies. Molecular weight standards are indicated on the left, bands corresponding to pCREB and CREB are labeled with arrows on the right, protein names are located at the top and lanes from animals receiving AD or ID-AD extracts are marked at the bottom of the blots. ++ lane contains lysates of SK-N-MC cells treated with IBMX and forskolin, + lane contains lysates of non-treated SK-N-MC cells (9193, Cell Signalling Technology Inc, Danvers, MA, USA).
Supplementary Figure 6 The Aβ-mediated disruption of avoidance memory is not associated with changes in the levels of dynamin-1 or SV-2. Animals were injected icv with 10 μl of AD or ID-AD sample 1 h post-training and culled 2 h later. (A) Dentate gyri were dissected and RIPA extracts prepared, electrophoresed and western blotted with the antibodies indicated. Molecular weight standards are indicated on the left, bands corresponding to proteins of interest are labelled with arrows (or brackets) on the right, protein names are located at the top and lanes from animals receiving AD or ID-AD extracts are marked at the bottom of the blots. (B) Specific bands were quantified by densitometry and normalized versus the average value of the corresponding ID-AD control group. Results are presented as mean percent of control ± standard deviation of duplicate measurements for each sample. The number of dentate gyri samples per group is indicated in each column. Unlike the experiments in Figs 5 and 6 there was only sufficient from 5 AD and 5 ID-AD samples for SV-2 analysis and for 5 AD and 5 ID-AD samples for dynamin-1 analysis.
Acknowledgments
This work was supported by funding from the European Community 7th Framework Programme (FP7/2007-2013) under grant agreement No. 200611 (DMW) and by funding from Science Foundation Ireland (grant 08/1N.1/B2033, DMW) and by the NIH (grants AG18440; NS057096; AG11385; AG022074; AG10435, EM). We thank Drs. John Collinge, Ken Kosik, Paul Matthews, Peter Seubert, Dale Schenk, and Bob Vassar for providing antibodies and Drs. Jim Cleary, Richard Dykoski and David Scopes for providing brain tissue. We are also grateful to Dr. Richard Albert and Amaya Garcia for advice regarding statistical analysis.
Footnotes
Conflict of interest: The authors confirm that there are no actual or potential conflicts of interest relating to the work enclosed in this manuscript.
Author contributions: GGB contributed to the design and conducted the behavioural experiments, analysed rat brain tissue by Western blotting, performed the statistical analysis and wrote the manuscript. AJM and JMMD prepared and characterised human brain extracts, AJM performed Western blot analysis for dynamin-1 and SV-2. CSF optimized the homogenization conditions and prepared positive controls for the APP family of proteins, CMR helped with the design of behavioural experiments and provided equipment, KJM assisted with dentate gyri dissections and provided equipment for behavioural experiments, MT and EM conducted electron microscopy study. DMW conceived the project, designed the experiments and wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexinsky T, Przybyslawski J, Mileusnic R, Rose SP, Sara SJ. Antibody to day-old chick brain glycoprotein produces amnesia in adult rats. Neurobiol Learn Mem. 1997;67(1):14–20. doi: 10.1006/nlme.1996.3734. [DOI] [PubMed] [Google Scholar]
- Alzheimer's Association. Generation Alzheimer's: the Defining Disease of the Baby Boomers 2011 [Google Scholar]
- Ansari MA, Roberts KN, Scheff SW. Oxidative stress and modification of synaptic proteins in hippocampus after traumatic brain injury. Free Radical Bio Med. 2008;45(4):443–52. doi: 10.1016/j.freeradbiomed.2008.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Applegate MD, Landfield PW. Synaptic vesicle redistribution during hippocampal frequency potentiation and depression in young and aged rats. J Neurosci. 1988;8(4):1096–111. doi: 10.1523/JNEUROSCI.08-04-01096.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila J, Lucas JJ, Perez M, Hernandez F. Role of tau protein in both physiological and pathological conditions. Physiol Rev. 2004;84(2):361–84. doi: 10.1152/physrev.00024.2003. [DOI] [PubMed] [Google Scholar]
- Barry AE, Klyubin I, Mc Donald JM, Mably AJ, Farrell MA, Scott M, Walsh DM, Rowan MJ. Alzheimer's Disease Brain-Derived Amyloid-β-Mediated Inhibition of LTP In Vivo Is Prevented by Immunotargeting Cellular Prion Protein. J Neurosci. 2011;31(20):7259–63. doi: 10.1523/JNEUROSCI.6500-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boland B, Smith DA, Mooney D, Jung SS, Walsh DM, Platt FM. Macroautophagy Is Not Directly Involved in the Metabolism of Amyloid Precursor Protein. J Biol Chem. 2010;285(48):37415–26. doi: 10.1074/jbc.M110.186411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttini M, Masliah E, Barbour R, Grajeda H, Motter R, Johnson-Wood K, Khan K, Seubert P, Freedman S, Schenk D, Games D. β-Amyloid Immunotherapy Prevents Synaptic Degeneration in a Mouse Model of Alzheimer's Disease. J Neurosci. 2005;25(40):9096–101. doi: 10.1523/JNEUROSCI.1697-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirrito JR, May PC, O'Dell MA, Taylor JW, Parsadanian M, Cramer JW, Audia JE, Nissen JS, Bales KR, Paul SM, DeMattos RB, Holtzman DM. In vivo assessment of brain interstitial fluid with microdialysis reveals plaque-associated changes in amyloid-beta metabolism and half-life. J Neurosci. 2003;23(26):8844–53. doi: 10.1523/JNEUROSCI.23-26-08844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary JP, Walsh DM, Hofmeister JJ, Shankar GM, Kuskowski MA, Selkoe DJ, Ashe KH. Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat Neurosci. 2005;8(1):79–84. doi: 10.1038/nn1372. [DOI] [PubMed] [Google Scholar]
- Coleman PD, Yao PJ. Synaptic slaughter in Alzheimer's disease. Neurobiol Aging. 2003;24(8):1023–7. doi: 10.1016/j.neurobiolaging.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Conboy L, Murphy KJ, Regan CM. Amyloid precursor protein expression in the rat hippocampal dentate gyrus modulates during memory consolidation. J Neurochem. 2005;95(6):1677–88. doi: 10.1111/j.1471-4159.2005.03484.x. [DOI] [PubMed] [Google Scholar]
- Davies CA, Mann DM, Sumpter PQ, Yates PO. A quantitative morphometric analysis of the neuronal and synaptic content of the frontal and temporal cortex in patients with Alzheimer's disease. J Neurol Sci. 1987;78(2):151–64. doi: 10.1016/0022-510x(87)90057-8. [DOI] [PubMed] [Google Scholar]
- Davis HP, Squire LR. Protein synthesis and memory: a review. Psychol Bull. 1984;96(3):518–59. [PubMed] [Google Scholar]
- De Strooper B. Proteases and proteolysis in Alzheimer disease: a multifactorial view on the disease process. Physiol Rev. 2010;90(2):465–94. doi: 10.1152/physrev.00023.2009. [DOI] [PubMed] [Google Scholar]
- Deller T, Bas Orth C, Del Turco D, Vlachos A, Burbach GJ, Drakew A, Chabanis S, Korte M, Schwegler H, Haas CA, Frotscher M. A role for synaptopodin and the spine apparatus in hippocampal synaptic plasticity. Ann Anat. 2007;189(1):5–16. doi: 10.1016/j.aanat.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Dere E, De Souza-Silva MA, Frisch C, Teubner B, Söhl G, Willecke K, Huston JP. Connexin30-deficient mice show increased emotionality and decreased rearing activity in the open-field along with neurochemical changes. Eur J Neurosci. 2003;18(3):629–38. doi: 10.1046/j.1460-9568.2003.02784.x. [DOI] [PubMed] [Google Scholar]
- Doyle E, Nolan PM, Bell R, Regan CM. Intraventricular infusions of anti-neural cell adhesion molecules in a discrete posttraining period impair consolidation of a passive avoidance response in the rat. J Neurochem. 1992;59(4):1570–3. doi: 10.1111/j.1471-4159.1992.tb08477.x. [DOI] [PubMed] [Google Scholar]
- Ehrlich I, Klein M, Rumpel S, Malinow R. PSD-95 is required for activity-driven synapse stabilization. Proc Natl Acad Sci U S A. 2007;104(10):4176–81. doi: 10.1073/pnas.0609307104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyre MD, Richter-Levin G, Avital A, Stewart MG. Morphological changes in hippocampal dentate gyrus synapses following spatial learning in rats are transient. Eur J Neurosci. 2003;17(9):1973–80. doi: 10.1046/j.1460-9568.2003.02624.x. [DOI] [PubMed] [Google Scholar]
- Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, Hall K, Hasegawa K, Hendrie H, Huang Y, Jorm A, Mathers C, Menezes PR, Rimmer E, Scazufca M. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366(9503):2112–7. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley AG, Hartz BP, Gallagher HC, Ronn LC, Berezin V, Bock E, Regan CM. A synthetic peptide ligand of neural cell adhesion molecule (NCAM) IgI domain prevents NCAM internalization and disrupts passive avoidance learning. J Neurochem. 2000;74(6):2607–13. doi: 10.1046/j.1471-4159.2000.0742607.x. [DOI] [PubMed] [Google Scholar]
- Freir DB, Fedriani R, Scully D, Smith IM, Selkoe DJ, Walsh DM, Regan CM. Aβ oligomers inhibit synapse remodelling necessary for memory consolidation. Neurobiol Aging. 2011a;32(12):2211–8. doi: 10.1016/j.neurobiolaging.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freir DB, Nicoll AJ, Klyubin I, Panico S, Mc Donald JM, Risse E, Asante EA, Farrow MA, Sessions RB, Saibil HR, Clarke AR, Rowan MJ, Walsh DM, Collinge J. Interaction between prion protein and toxic amyloid [beta] assemblies can be therapeutically targeted at multiple sites. Nat Commun. 2011b;2:336. doi: 10.1038/ncomms1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Games D, Buttini M, Kobayashi D, Schenk D, Seubert P. Mice as models: transgenic approaches and Alzheimer's disease. J Alzheimers Dis. 2006;9(3 Suppl):133–49. doi: 10.3233/jad-2006-9s316. [DOI] [PubMed] [Google Scholar]
- Gonatas NK, Anderson W, Evangelista I. The contribution of altered synapses in the senile plaque: an electron microscopic study in Alzheimer's dementia. J Neuropathol Exp Neurol. 1967;26(1):25–39. doi: 10.1097/00005072-196701000-00003. [DOI] [PubMed] [Google Scholar]
- Gray BC, Siskova Z, Perry VH, O'Connor V. Selective presynaptic degeneration in the synaptopathy associated with ME7-induced hippocampal pathology. Neurobiol Dis. 2009;35(1):63–74. doi: 10.1016/j.nbd.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Grober E, Hall CB, Lipton RB, Zonderman AB, Resnick SM, Kawas C. Memory impairment, executive dysfunction, and intellectual decline in preclinical Alzheimer's disease. J Int Neuropsychol Soc. 2008;14(2):266–78. doi: 10.1017/S1355617708080302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther EC, Strittmatter SM. Beta-amyloid oligomers and cellular prion protein in Alzheimer's disease. J Mol Med (Berl) 2010;88(4):331–8. doi: 10.1007/s00109-009-0568-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hering H, Sheng M. Dentritic spines: structure, dynamics and regulation. Nat Rev Neurosci. 2001;2(12):880–8. doi: 10.1038/35104061. [DOI] [PubMed] [Google Scholar]
- Jan A, Hartley DM, Lashuel HA. Preparation and characterization of toxic Abeta aggregates for structural and functional studies in Alzheimer's disease research. Nat Protoc. 2010;5(6):1186–209. doi: 10.1038/nprot.2010.72. [DOI] [PubMed] [Google Scholar]
- Jedlicka P, Vlachos A, Schwarzacher SW, Deller T. A role for the spine apparatus in LTP and spatial learning. Behav Brain Res. 2008;192(1):12–9. doi: 10.1016/j.bbr.2008.02.033. [DOI] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory storage: a dialog between genes and synapses. Biosci Rep. 2001;21(5):565–611. doi: 10.1023/a:1014775008533. [DOI] [PubMed] [Google Scholar]
- Klein WL, Krafft GA, Finch CE. Targeting small Aβ oligomers: the solution to an Alzheimer's disease conundrum? Trends Neurosci. 2001;24(4):219–24. doi: 10.1016/s0166-2236(00)01749-5. [DOI] [PubMed] [Google Scholar]
- Klyubin I, Betts V, Welzel AT, Blennow K, Zetterberg H, Wallin A, Lemere CA, Cullen WK, Peng Y, Wisniewski T, Selkoe DJ, Anwyl R, Walsh DM, Rowan MJ. Amyloid beta protein dimer-containing human CSF disrupts synaptic plasticity: prevention by systemic passive immunization. J Neurosci. 2008;28(16):4231–7. doi: 10.1523/JNEUROSCI.5161-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaus P, Betz H, Rehm H. Expression of synaptophysin during postnatal development of the mouse brain. J Neurochem. 1986;47(4):1302–4. doi: 10.1111/j.1471-4159.1986.tb00754.x. [DOI] [PubMed] [Google Scholar]
- Kowall NW, Kosik KS. Axonal disruption and aberrant localization of tau protein characterize the neuropil pathology of Alzheimer's disease. Ann Neurol. 1987;22(5):639–43. doi: 10.1002/ana.410220514. [DOI] [PubMed] [Google Scholar]
- Kuo YM, Emmerling MR, Vigo-Pelfrey C, Kasunic TC, Kirkpatrick JB, Murdoch GH, Ball MJ, Roher AE. Water-soluble Abeta (N-40, N-42) oligomers in normal and Alzheimer disease brains. J Biol Chem. 1996;271(8):4077–81. doi: 10.1074/jbc.271.8.4077. [DOI] [PubMed] [Google Scholar]
- Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, Morgan TE, Rozovsky I, Trommer B, Viola KL, Wals P, Zhang C, Finch CE, Krafft GA, Klein WL. Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc Natl Acad Sci U S A. 1998;95(11):6448–53. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamprecht R, LeDoux J. Structural plasticity and memory. Nat Rev Neurosci. 2004;5(1):45–54. doi: 10.1038/nrn1301. [DOI] [PubMed] [Google Scholar]
- Lauren J, Gimbel DA, Nygaard HB, Gilbert JW, Strittmatter SM. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-[bgr] oligomers. Nature. 2009;457(7233):1128–32. doi: 10.1038/nature07761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner HA, Squire LR, Byrne JH. 100 years of consolidation--remembering Muller and Pilzecker. Learn Mem. 1999;6(2):77–87. [PubMed] [Google Scholar]
- Lesne S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, Gallagher M, Ashe KH. A specific amyloid-beta protein assembly in the brain impairs memory. Nature. 2006;440(7082):352–7. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- Li S, Hong S, Shepardson NE, Walsh DM, Shankar GM, Selkoe D. Soluble oligomers of amyloid Beta protein facilitate hippocampal long-term depression by disrupting neuronal glutamate uptake. Neuron. 2009;62(6):788–801. doi: 10.1016/j.neuron.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Jover-Mengual T, Wong J, Bennett MVL, Zukin RS. PSD-95 and PKC converge in regulating NMDA receptor trafficking and gating. Proc Natl Acad Sci U S A. 2006;103(52):19902–7. doi: 10.1073/pnas.0609924104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lue LF, Kuo YM, Roher AE, Brachova L, Shen Y, Sue L, Beach T, Kurth JH, Rydel RE, Rogers J. Soluble amyloid beta peptide concentration as a predictor of synaptic change in Alzheimer's disease. Am J Pathol. 1999;155(3):853–62. doi: 10.1016/s0002-9440(10)65184-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrone DF. Ultrastructural plasticity associated with hippocampal-dependent learning: a meta-analysis. Neurobiol Learn Mem. 2007;87(3):361–71. doi: 10.1016/j.nlm.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Masliah E, Mallory M, Alford M, DeTeresa R, Hansen LA, McKeel DW, Jr, Morris JC. Altered expression of synaptic proteins occurs early during progression of Alzheimer's disease. Neurology. 2001;56(1):127–9. doi: 10.1212/wnl.56.1.127. [DOI] [PubMed] [Google Scholar]
- Masliah E, Terry RD, DeTeresa RM, Hansen LA. Immunohistochemical quantification of the synapse-related protein synaptophysin in Alzheimer disease. Neurosci Lett. 1989;103(2):234–9. doi: 10.1016/0304-3940(89)90582-x. [DOI] [PubMed] [Google Scholar]
- Mc Donald JM, Savva GM, Brayne C, Welzel AT, Forster G, Shankar GM, Selkoe DJ, Ince PG, Walsh DM. The presence of sodium dodecyl sulphate-stable Aβ dimers is strongly associated with Alzheimer-type dementia. Brain. 2010;133(5):1328–41. doi: 10.1093/brain/awq065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald JM, Cairns NJ, Taylor-Reinwald L, Holtzman D, Walsh DM. The levels of water-soluble and triton-soluble Aβ are increased in Alzheimer's disease brain. Brain Res. 2012;(0) doi: 10.1016/j.brainres.2012.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean CA, Cherny RA, Fraser FW, Fuller SJ, Smith MJ, Beyreuther K, Bush AI, Masters CL. Soluble pool of Abeta amyloid as a determinant of severity of neurodegeneration in Alzheimer's disease. Ann Neurol. 1999;46(6):860–6. doi: 10.1002/1531-8249(199912)46:6<860::aid-ana8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Neuroplasticity failure in Alzheimer's disease: bridging the gap between plaques and tangles. Neuron. 1999;24(3):521–9. doi: 10.1016/s0896-6273(00)81109-5. [DOI] [PubMed] [Google Scholar]
- Monopoli MP, Raghnaill MN, Loscher JS, O'Sullivan NC, Pangalos MN, Ring RH, von Schack D, Dunn MJ, Regan CM, Pennington S, Murphy KJ. Temporal proteomic profile of memory consolidation in the rat hippocampal dentate gyrus. Proteomics. 2011;11(21):4189–201. doi: 10.1002/pmic.201100072. [DOI] [PubMed] [Google Scholar]
- Mormino EC, Kluth JT, Madison CM, Rabinovici GD, Baker SL, Miller BL, Koeppe RA, Mathis CA, Weiner MW, Jagust WJ. Episodic memory loss is related to hippocampal-mediated beta-amyloid deposition in elderly subjects. Brain. 2009;132(Pt 5):1310–23. doi: 10.1093/brain/awn320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KJ, Regan CM. Contributions of cell adhesion molecules to altered synaptic weightings during memory consolidation. Neurobiol Learn Mem. 1998;70(1-2):73–81. doi: 10.1006/nlme.1998.3839. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Murayama N, Noshita T, Annoura H, Ohno T. Progressive brain dysfunction following intracerebroventricular infusion of beta(1-42)-amyloid peptide. Brain Res. 2001;912(2):128–36. doi: 10.1016/s0006-8993(01)02704-4. [DOI] [PubMed] [Google Scholar]
- O'Malley A, O'Connell C, Regan CM. Ultrastructural analysis reveals avoidance conditioning to induce a transient increase in hippocampal dentate spine density in the 6 hour post-training period of consolidation. Neuroscience. 1998;87(3):607–13. doi: 10.1016/s0306-4522(98)00178-x. [DOI] [PubMed] [Google Scholar]
- O'Nuallain B, Freir DB, Nicoll AJ, Risse E, Ferguson N, Herron CE, Collinge J, Walsh DM. Amyloid β-Protein Dimers Rapidly Form Stable Synaptotoxic Protofibrils. J Neurosci. 2010;30(43):14411–9. doi: 10.1523/JNEUROSCI.3537-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan NC, McGettigan PA, Sheridan GK, Pickering M, Conboy L, O'Connor JJ, Moynagh PN, Higgins DG, Regan CM, Murphy KJ. Temporal change in gene expression in the rat dentate gyrus following passive avoidance learning. J Neurochem. 2007;101(4):1085–98. doi: 10.1111/j.1471-4159.2006.04418.x. [DOI] [PubMed] [Google Scholar]
- Parkin ET, Watt NT, Hussain I, Eckman EA, Eckman CB, Manson JC, Baybutt HN, Turner AJ, Hooper NM. Cellular prion protein regulates β-secretase cleavage of the Alzheimer's amyloid precursor protein. Proc Natl Acad Sci U S A. 2007;104(26):11062–7. doi: 10.1073/pnas.0609621104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G. The rat brain in stereotaxic coordinates / George Paxinos, Charles Watson. Academic Press; Sydney: 1986. [Google Scholar]
- Platano D, Fattoretti P, Balietti M, Giorgetti B, Casoli T, Di Stefano G, Bertoni-Freddari C, Aicardi G. Synaptic remodeling in hippocampal CA1 region of aged rats correlates with better memory performance in passive avoidance test. Rejuv Res. 2008;11(2):341–8. doi: 10.1089/rej.2008.0725. [DOI] [PubMed] [Google Scholar]
- Poling A, Morgan-Paisley K, Panos JJ, Kim EM, O'Hare E, Cleary JP, Lesne S, Ashe KH, Porritt M, Baker LE. Oligomers of the amyloid-beta protein disrupt working memory: confirmation with two behavioral procedures. Behav Brain Res. 2008;193(2):230–4. doi: 10.1016/j.bbr.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince MJ. The 10/66 dementia research group - 10 years on. Indian J Psychiatry. 2009;51(1):S8–S15. [PMC free article] [PubMed] [Google Scholar]
- Reddy PH, Mani G, Park BS, Jacques J, Murdoch G, Whetsell W, Jr, Kaye J, Manczak M. Differential loss of synaptic proteins in Alzheimer's disease: implications for synaptic dysfunction. J Alzheimers Dis. 2005;7(2):103–17. doi: 10.3233/jad-2005-7203. discussion 73-80. [DOI] [PubMed] [Google Scholar]
- Reed MN, Hofmeister JJ, Jungbauer L, Welzel AT, Yu C, Sherman MA, Lesne S, LaDu MJ, Walsh DM, Ashe KH, Cleary JP. Cognitive effects of cell-derived and synthetically derived Abeta oligomers. Neurobiol Aging. 2011;32(10):1784–94. doi: 10.1016/j.neurobiolaging.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter JD, Klann E. Making synaptic plasticity and memory last: mechanisms of translational regulation. Genes Dev. 2009;23(1):1–11. doi: 10.1101/gad.1735809. [DOI] [PubMed] [Google Scholar]
- Rosenbaum RS, Gao F, Richards B, Black SE, Moscovitch M. “Where to?” remote memory for spatial relations and landmark identity in former taxi drivers with Alzheimer's disease and encephalitis. J Cogn Neurosci. 2005;17(3):446–62. doi: 10.1162/0898929053279496. [DOI] [PubMed] [Google Scholar]
- Roullet P, Mileusnic R, Rose SP, Sara SJ. Neural cell adhesion molecules play a role in rat memory formation in appetitive as well as aversive tasks. Neuroreport. 1997;8(8):1907–11. doi: 10.1097/00001756-199705260-00023. [DOI] [PubMed] [Google Scholar]
- Sala Frigerio C, Fadeeva JV, Minogue AM, Citron M, Van Leuven F, Staufenbiel M, Paganetti P, Selkoe DJ, Walsh DM. β-Secretase cleavage is not required for generation of the intracellular C-terminal domain of the amyloid precursor family of proteins. FEBS J. 2010;277(6):1503–18. doi: 10.1111/j.1742-4658.2010.07579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez C, Diaz-Nido J, Avila J. Phosphorylation of microtubule-associated protein 2 (MAP2) and its relevance for the regulation of the neuronal cytoskeleton function. Prog Neurobiol. 2000;61(2):133–68. doi: 10.1016/s0301-0082(99)00046-5. [DOI] [PubMed] [Google Scholar]
- Saura CA, Valero J. The role of CREB signaling in Alzheimer's disease and other cognitive disorders. Rev Neurosci. 2011;22(2):153–69. doi: 10.1515/RNS.2011.018. [DOI] [PubMed] [Google Scholar]
- Scheff SW, Price DA. Synaptic pathology in Alzheimer's disease: a review of ultrastructural studies. Neurobiol Aging. 2003;24(8):1029–46. doi: 10.1016/j.neurobiolaging.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Scheff SW, Price DA, Schmitt FA, DeKosky ST, Mufson EJ. Synaptic alterations in CA1 in mild Alzheimer disease and mild cognitive impairment. Neurology. 2007;68(18):1501–8. doi: 10.1212/01.wnl.0000260698.46517.8f. [DOI] [PubMed] [Google Scholar]
- Scheff SW, Price DA, Schmitt FA, Mufson EJ. Hippocampal synaptic loss in early Alzheimer's disease and mild cognitive impairment. Neurobiol Aging. 2006;27(10):1372–84. doi: 10.1016/j.neurobiolaging.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's disease is a synaptic failure. Science. 2002;298(5594):789–91. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- Seymour CM, Foley AG, Murphy KJ, Regan CM. Intraventricular infusions of anti-NCAM PSA impair the process of consolidation of both avoidance conditioning and spatial learning paradigms in Wistar rats. Neuroscience. 2008;157(4):813–20. doi: 10.1016/j.neuroscience.2008.09.041. [DOI] [PubMed] [Google Scholar]
- Shankar GM, Leissring MA, Adame A, Sun X, Spooner E, Masliah E, Selkoe DJ, Lemere CA, Walsh DM. Biochemical and immunohistochemical analysis of an Alzheimer's disease mouse model reveals the presence of multiple cerebral Aβ assembly forms throughout life. Neurobiol Dis. 2009;36(2):293–302. doi: 10.1016/j.nbd.2009.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, Regan CM, Walsh DM, Sabatini BL, Selkoe DJ. Amyloid-[beta] protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat Med. 2008;14(8):837–42. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar GM, Walsh DM. Alzheimer's disease: synaptic dysfunction and Abeta. Mol Neurodegener. 2009;4:48. doi: 10.1186/1750-1326-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AJ, Kogan JH, Frankland PW, Kida S. CREB AND MEMORY. Ann Rev Neurosci. 1998;21(1):127–48. doi: 10.1146/annurev.neuro.21.1.127. [DOI] [PubMed] [Google Scholar]
- Small GW. Early diagnosis of Alzheimer's disease: update on combining genetic and brain-imaging measures. Dialog Clin Neurosci. 2000;2(3):241–6. doi: 10.31887/DCNS.2000.2.3/gsmall. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small SA, Perera GM, DeLaPaz R, Mayeux R, Stern Y. Differential regional dysfunction of the hippocampal formation among elderly with memory decline and Alzheimer's disease. Ann Neurol. 1999;45(4):466–72. doi: 10.1002/1531-8249(199904)45:4<466::aid-ana8>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Sterio DC. The unbiased estimation of number and sizes of arbitrary particles using the disector. J Microsc. 1984;134(Pt 2):127–36. doi: 10.1111/j.1365-2818.1984.tb02501.x. [DOI] [PubMed] [Google Scholar]
- Tabaton M, Nunzi MG, Xue R, Usiak M, Autilio-Gambetti L, Gambetti P. Soluble amyloid beta-protein is a marker of Alzheimer amyloid in brain but not in cerebrospinal fluid. Biochem Biophys Res Commun. 1994;200(3):1598–603. doi: 10.1006/bbrc.1994.1634. [DOI] [PubMed] [Google Scholar]
- Tang Y, Nyengaard JR, De Groot DM, Gundersen HJ. Total regional and global number of synapses in the human brain neocortex. Synapse. 2001;41(3):258–73. doi: 10.1002/syn.1083. [DOI] [PubMed] [Google Scholar]
- Vlachos A, Korkotian E, Schonfeld E, Copanaki E, Deller T, Segal M. Synaptopodin Regulates Plasticity of Dendritic Spines in Hippocampal Neurons. J Neurosci. 2009;29(4):1017–33. doi: 10.1523/JNEUROSCI.5528-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416(6880):535–9. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Minogue AM, Sala Frigerio C, Fadeeva JV, Wasco W, Selkoe DJ. The APP family of proteins: similarities and differences. Biochem Soc Trans. 2007;35(Pt 2):416–20. doi: 10.1042/BST0350416. [DOI] [PubMed] [Google Scholar]
- Yao PJ, Zhu M, Pyun EI, Brooks AI, Therianos S, Meyers VE, Coleman PD. Defects in expression of genes related to synaptic vesicle trafficking in frontal cortex of Alzheimer's disease. Neurobiol Dis. 2003;12(2):97–109. doi: 10.1016/s0969-9961(02)00009-8. [DOI] [PubMed] [Google Scholar]
- Zhao J, Fu Y, Yasvoina M, Shao P, Hitt B, O'Connor T, Logan S, Maus E, Citron M, Berry R, Binder L, Vassar R. β-Site Amyloid Precursor Protein Cleaving Enzyme 1 Levels Become Elevated in Neurons around Amyloid Plaques: Implications for Alzheimer's Disease Pathogenesis. J Neurosci. 2007;27(14):3639–49. doi: 10.1523/JNEUROSCI.4396-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1 The flowchart describes the number of rats used to test the effect of administering Aβ on recall of avoidance learning when AD brain extract (AD) or extract immunodepleted of Aβ (ID-AD) was injected at different time intervals post-training.
Supplementary Figure 2 The flowchart describes the number of rats used to test the effect of administering Aβ on recall of avoidance learning, protein expression and synaptic ultrastructure when AD brain extract (AD), extract immunodepleted of Aβ (ID-AD) or TBS was injected at 1 hour post-training.
Supplementary Figure 3 The flowchart describes the number of rats used to test if either AD brain extract (AD) or extract immunodepleted of Aβ (ID-AD) effect performance in the open field.
Supplementary Figure 4 Injection of AD brain Aβ does not affect locomotor activity. Animals were injected with 10 μl of AD or ID-AD sample and their behaviour in the open field monitored for 10 min at 23 and 47 h post-injection. (A) Injection of AD brain Aβ does not affect total distance travelled at both time intervals when compared to ID-AD group (no significant effect of group or test by group interaction, p>0.05 in both cases; significant main effect of the test session F1,15 = 10.47, p<0.01). (B) Injection of AD brain extract does not affect the number of rearings at 23 and 47 h post-injection when compared to ID-AD injection (no significant effect of the test session, group or test by group interaction, p>0.05 in all cases).
Supplementary Figure 5 pCREB was not detected in any of the samples tested even though pCREB was detected in the lysates of forskolin-treated cells. Dentate gyri were dissected, RIPA extracts prepared and electrophoresed on 10% polyacrylamide tris-glycine gels and western blotted with the pCREB or CREB antibodies. Molecular weight standards are indicated on the left, bands corresponding to pCREB and CREB are labeled with arrows on the right, protein names are located at the top and lanes from animals receiving AD or ID-AD extracts are marked at the bottom of the blots. ++ lane contains lysates of SK-N-MC cells treated with IBMX and forskolin, + lane contains lysates of non-treated SK-N-MC cells (9193, Cell Signalling Technology Inc, Danvers, MA, USA).
Supplementary Figure 6 The Aβ-mediated disruption of avoidance memory is not associated with changes in the levels of dynamin-1 or SV-2. Animals were injected icv with 10 μl of AD or ID-AD sample 1 h post-training and culled 2 h later. (A) Dentate gyri were dissected and RIPA extracts prepared, electrophoresed and western blotted with the antibodies indicated. Molecular weight standards are indicated on the left, bands corresponding to proteins of interest are labelled with arrows (or brackets) on the right, protein names are located at the top and lanes from animals receiving AD or ID-AD extracts are marked at the bottom of the blots. (B) Specific bands were quantified by densitometry and normalized versus the average value of the corresponding ID-AD control group. Results are presented as mean percent of control ± standard deviation of duplicate measurements for each sample. The number of dentate gyri samples per group is indicated in each column. Unlike the experiments in Figs 5 and 6 there was only sufficient from 5 AD and 5 ID-AD samples for SV-2 analysis and for 5 AD and 5 ID-AD samples for dynamin-1 analysis.