Abstract
Traditional cortical parcellation schemes have emphasized the presence of sharply defined visual, auditory, and somatosensory domains populated exclusively by modality-specific neurons (i.e., neurons responsive to sensory stimuli from a single sensory modality). However, the modality-exclusivity of this scheme has recently been challenged. Observations in a variety of species suggest that each of these domains is subject to influences from other senses. Using the cerebral cortex of the rat as a model, the present study systematically examined the capability of individual neurons in visual, auditory, and somatosensory cortex to be activated by stimuli from other senses. Within the major modality-specific domains, the incidence of inappropriate (i.e., nonmatching) and/or multisensory neurons was very low. However, at the borders between each of these domains a concentration of multisensory neurons was found whose modality profile matched the representations in neighboring cortices and that were able to integrate their cross-modal inputs to give rise to enhanced and/or depressed responses. The results of these studies are consistent with some features of both the traditional and challenging views of cortical organization, and they suggest a parcellation scheme in which modality-specific cortical domains are separated from one another by transitional multisensory zones.
The ability to synthesize information from multiple senses is of significant value in enhancing the detection, localization, and identification of, and responses to, external events (1). The integrative principles that likely underlie these multisensory processes have been well studied in a midbrain structure, the superior colliculus (SC) (1-4). The SC is an attractive model in this context because of its high incidence of multisensory neurons, its involvement in overt orientation behaviors, and the compelling parallels between its multisensory neuronal responses and the probabilities that an external event will elicit an orientation response (5).
In contrast to views of the organization of the SC, which emphasize the pooling of information across senses, traditional views of cortical organization hold that the initial stages of information processing are based on segregating the senses. This view is supported by a substantial body of anatomical and physiological evidence (6-11) and is consistent with the perceptual consequences of sensory cortical stimulation and lesions. In this scheme, it is only in higher-order cortical areas that information from different sensory channels is brought together for the purpose of integrating it in an organizational scheme similar to that found in the SC.
Several reports have questioned the exclusivity of this scheme in visual cortex by finding neurons responsive to nonvisual inputs (12-14). Although these studies had little impact on the prevailing view of cortical parcellation, recent experiments using new technologies have contributed to a growing awareness of this issue by providing evidence that information-processing in a variety of primary and secondary sensory cortices can be influenced by cross-modal stimuli (15-22). Although it is not clear whether such influences are feedforward and/or feedback in origin, the body of evidence clearly suggests that cross-modal interactions can take place at very early stages in the sensory processing hierarchy.
If, indeed, there are significant numbers of neurons at early stages of cortical processing that can be activated by “nonmatching” sensory stimuli, it would require a substantial revision of concepts of cortical organization. The rodent cortex offers advantages as a model for such an exploration because its sensory representations are well known (23-27) and its lissencephalic (i.e., lacking in convolutions) nature makes marking the functional transitions across sensory areas comparatively straightforward. In the current study a systematic sampling of neuronal responses was made across the posterior two-thirds of rat cortex to determine whether there is a strict functional segregation between visual, auditory, and somatosensory representations, or whether nonmatching and/or multisensory neurons are located within these presumptive modality-specific regions.
Methods
General Surgical Procedures. All surgical and experimental procedures were performed in compliance with the Guide for the Care and Use of Laboratory Animals (28). Each of 31 adult male Long-Evans rats was anesthetized with urethane (1.3 g/kg; i.p.) and mounted in a stereotaxic head-holder. Topical Xylocaine was placed on the skin of the head at the location of incisions. A craniotomy exposed most of the parietal, occipital, and temporal cortices, and a mounting bracket, affixed to the skull with screws and dental acrylic, held the head and provided access to the body without obstructing visual or auditory space.
Recording Procedures. Anesthesia was periodically assessed by monitoring the pedal withdrawal reflex, and supplemental doses of urethane (0.4 g/kg; i.p.) were provided as necessary throughout the recording session. Core body temperature was monitored with a rectal probe and was maintained at 38°C with a circulating water heating pad. Once exposed, the cortical surface was kept moist with warm (38°C) Ringer's solution. The pupils were dilated with atropine sulfate and the eyes were kept moist with periodic irrigation using sterile ophthalmic saline. The optic disk was mapped and projected onto a translucent tangent screen. In each animal, a series of systematic microelectrode penetrations was made with reference to a grid (see Fig. 1) that was superimposed over most of the parietal, temporal, and occipital cortices. In the initial experiments, the spacing of the penetrations in the reference grid was 1 mm. In later experiments, regions of interest (e.g., border or “transitional” areas; see Results) were subjected to more detailed mapping (250- to 400-μm penetration spacings).
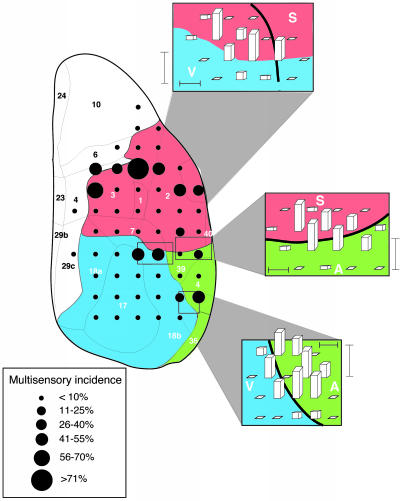
Fig. 1.
The distribution of multisensory neurons in rat sensory neocortex. The line drawing depicts the dorsal surface of cortex. Numbers and solid lines designate major subdivisions (17) (parietal, red shading; temporal, green shading; and occipital, blue shading). Filled circles show locations of electrode penetrations in a coarse-grain analysis that was conducted in 22 animals, and circle size indicates the relative incidence of multisensory neurons at each site. Insets show the results of higher-resolution sampling through each of the transitional regions that was conducted in a total of nine animals. Bar height indicates the relative incidence of multisensory neurons. Horizontal scale bar = 250 μm, and vertical scale bar = 50% multisensory incidence. V, visual cortex; A, auditory cortex; S, somatosensory cortex.
In each penetration, a recording microelectrode (glass-insulated tungsten, 10- to 25-μm exposed tip, impedance <1MΩ at 1 kHz) was advanced by using a micromanipulator while a variety of visual, auditory, and somatosensory “search” stimuli were presented. Individual neurons were identified and classified on the basis of their responses to (i) visual stimuli consisting of flashes or moving bars of light presented on a dark background, or dark stimuli presented against a bright background; (ii) auditory stimuli consisting of hisses, clicks, chirps, and/or various complex sounds; and (iii) somatosensory stimuli consisting of deflections of the hair or skin by using a camel's hair brush and stimulation of deep tissue by using probes and manual manipulation. Each isolated neuron was tested for visual, auditory, and somatosensory responsiveness. Neuronal signals were amplified, displayed on an oscilloscope, and routed through an audio monitor. Multisensory neurons were defined as those responsive to, or whose responses were influenced by, stimuli from more than a single sensory modality.
Receptive Field Mapping and Tests of Multisensory Integration. The receptive fields of visually responsive neurons were mapped onto a translucent tangent screen by using bars or spots of light from a hand-held pantoscope. Auditory receptive fields were mapped by using broad-band noise bursts generated from speakers positionable along a hoop that could be rotated around the animal. Somatosensory receptive fields were mapped by using deflections of the hair or skin produced by manual strokes of a camel's hair brush.
The analysis of sensory and multisensory responses of individual neurons was conducted by using computer-controlled stimuli. Stimulus parameters were chosen so as to optimize the response of the neuron. Visual stimuli were flashes or moving bars of light presented onto a tangent screen by means of a projector and galvanometer-driven mirror. For flashed stimuli, stimulus duration ranged from 50 to 200 ms and stimulus intensity ranged from 30 to 70 cd/m2 on a background of 3 cd/m2. For moving stimuli, a variety of sizes (1° × 1° up to 20° × 20°), movement amplitudes (1-90°), velocities (3-500°/s), directions, and intensities (3-50 cd/m2) were used. Auditory stimuli were always broad-band (20 Hz to 20 kHz) 50- to 100-ms noise bursts delivered at intensities ranging from 40 to 70 dB sound pressure level (SPL) on a background of 40 dB SPL (A level). Somatosensory stimuli were deflections of the hair and skin produced by a probe tip mounted to a modified Ling 502A shaker. Probe movement was delivered over a range of amplitudes (0.05-5.0 mm), velocities (15-420 mm/s), and directions.
In testing for multisensory interactions, the animal was presented with a series of 24 interleaved sensory trials: 8 with each of the two modality-specific stimuli (e.g., visual alone, auditory alone) and 8 with these stimuli in combination. In some cases the stimuli were systematically stepped across and beyond their receptive fields (spatial tests), whereas in others their temporal relationships were manipulated. The number of impulses elicited during single and combined modality tests was compared, and a multisensory interaction was defined as a significant (P < 0.05, two-tail t test) difference (increase = response enhancement, decrease = response depression) in the number of impulses elicited in the combined modality test when compared with the response elicited by the most effective single-modality stimulus. The magnitude of the multisensory interaction was defined as
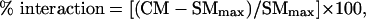 |
where CM = mean response per trial to the combined-modality stimulus and SMmax = mean response per trial to the most effective single-modality stimulus (see ref. 2).
Determination of Receptive Field Overlap. In each of the multisensory neurons, a calculation was made to determine the amount of overlap between the respective receptive fields. In this procedure, adapted from the general procedure described by Stein and Meredith (1), the mapping templates for visual, auditory, and somatosensory space were transformed into an integrated multisensory representation. In this way, receptive fields in each of the modalities could be directly related to one another. Receptive field overlap was defined as the area of commonality between the receptive fields for both modalities, expressed as a proportionate measure of the area of the largest receptive field.
Killing and Tissue Reconstruction. In selected penetrations, one or more electrolytic lesions were made by passing current through the recording electrode (10 μA for 12 s). At the end of the recording session, the animal was overdosed with sodium pentobarbital (100 mg/kg; i.p.) and perfused transcardially with physiological saline followed by 10% formalin. Standard histological reconstruction techniques were used to determine neuronal locations.
Results
We examined 1,268 neurons in 127 electrode penetrations through occipital, temporal, and parietal cortices. In the first phase a “coarse” sampling grid with 1-mm spacing between penetrations was used (840 neurons; 78 penetrations). In this initial analysis, 773 of the 840 (92.0%) neurons proved to be responsive to sensory stimuli. The vast majority (694/773, 89.8%) of these sensory-responsive neurons responded only to stimuli from a single sensory modality (i.e., they were “modality-specific”). The modality distributions of these neurons were in general agreement with established maps of rat sensory neocortex (23-27, 29-31): visual neurons were concentrated in occipital areas V1 and V2 (Brodmann's areas 17, 18a, and 18b), auditory neurons were concentrated in temporal areas A1 and associated belt cortices (Brodmann's areas 39 and 41), and somatosensory neurons were concentrated in parietal areas S1 and S2 (Brodmann's areas 1, 2, 3, and 7) (Table 1). Only 7.2% (56/773) of the neurons sampled failed to reflect the primary sensory representation at that site. For example, 6 auditory neurons were found within occipital (visual) cortex (from a total of 139 neurons), and 8 visual neurons were found within temporal (auditory) cortex (from a total of 126 neurons). Other modality-specific neurons, reflecting the sensory representations of the two adjacent cortices, were found intermixed at the borders between these major domains (Tables 1 and 2): visual and auditory neurons predominated at the occipital/temporal border, auditory and somatosensory neurons predominated at the temporal/parietal border, and visual and somatosensory neurons predominated at the occipital/parietal border.
Table 1. Distribution of modality-specific and multisensory neurons in the coarse-grain analysis of sensory neocortex.
| No. of neurons
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Location | V | A | S | VA | VS | AS | VAS | Total |
| Cortical domain | ||||||||
| Occipital (visual) | 125 | 6 | 6 | 1 | 1 | 0 | 0 | 139 |
| Temporal (auditory) | 8 | 105 | 11 | 1 | 0 | 1 | 0 | 126 |
| Parietal (somatosensory) | 11 | 8 | 167 | 0 | 1 | 1 | 0 | 188 |
| Total | 144 | 119 | 184 | 2 | 2 | 2 | 0 | 453 |
| Border or transition region | ||||||||
| Occipital/temporal | 25 | 21 | 0 | 8 | 0 | 0 | 2 | 56 |
| Temporal/parietal | 0 | 14 | 25 | 0 | 0 | 6 | 1 | 46 |
| Occipital/parietal | 19 | 0 | 31 | 2 | 9 | 0 | 3 | 64 |
| Rostral parietal | 2 | 4 | 106 | 8 | 27 | 4 | 3 | 154 |
| Total | 46 | 39 | 162 | 18 | 36 | 10 | 9 | 320 |
At the top are tabulated the incidence of neuronal types that could be reliably ascribed to one of the three principle cortical domains. At the bottom is the distribution of neurons in the four transitional zones between the large modality-specific representations. V, visual; A, auditory; S, somatosensory.
Table 2. Distribution of modality-specific and multisensory neurons in the fine-grain analysis of the border regions.
| No. of neurons
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Location | V | A | S | VA | VS | AS | VAS | Total |
| Border or transition region | ||||||||
| Occipital/temporal | 83 | 53 | 0 | 26 | 0 | 0 | 0 | 162 |
| Temporal/parietal | 0 | 43 | 55 | 0 | 0 | 11 | 0 | 109 |
| Occipital/parietal | 54 | 0 | 70 | 0 | 21 | 0 | 0 | 145 |
| Total | 137 | 96 | 125 | 26 | 21 | 11 | 0 | 416 |
V, visual; A, auditory; S, somatosensory.
Multisensory neurons constituted ≈10% (n = 79/773) of the total sensory population encountered in this initial coarse-grain analysis (Fig. 1). While multisensory neurons were rarely located within the core of the large modality-specific domains (7.6%, n = 6/79; Table 1), they were most commonly found at the borders between these domains (39.2%, n = 31/79), where the modality representations were intermixed, as well as in the rostral aspects of parietal cortex, at its transition to somatomotor cortex (53.2%, n = 42/79) (Table 1).
To examine the visual, auditory, and somatosensory border regions in more detail, a finer-resolution mapping strategy (250-400 μm between electrode penetrations) was used. In this analysis, 416/428 (97.2%) of the neurons studied were sensory responsive (Table 2).
Although there was significant interanimal variability in the exact location of the transitions from one sensory region to another, in each case the transitional region contained a mixed representation. This mixture included both types of modality-specific neurons as well as a comparatively high incidence of neurons responsive to both modalities. Regardless of the specific border examined (e.g., occipital/temporal, temporal/parietal, occipital/parietal), and the absolute location of the border, these multisensory zones were exceedingly narrow, with a mean maximal width of 498 ± 107 μm.
At the occipital/temporal border, visual-auditory multisensory neurons (n = 26) were found in high incidence (Fig. 1), where they represented 16.0% of the sensory-responsive population and were intermixed with modality-specific visual (n = 83; 51.2%) and auditory (n = 53; 32.7%) neurons. In this transition region, visual-auditory neurons were always encountered, and, at the peak of their spatial distribution, made up nearly 50% of the neurons encountered in a given penetration. This proportion fell off rapidly, with no multisensory neurons being found 500 μm medial or lateral to this peak.
A similar representational pattern was seen at the temporal/parietal border, where auditory-somatosensory neurons represented 10.1% (n = 11) of the total sample (Table 2). In some electrode penetrations these multisensory neurons reached a peak incidence of ≈50% (Fig. 1). The decline in multisensory neurons was again precipitous, with no multisensory neurons being encountered 500 μm rostral or caudal from their region of highest incidence.
Finally, the border spanning occipital and parietal areas was similarly ordered. Visual-somatosensory neurons were of greatest incidence (n = 21; 14.5%), and, at their peak represented ≈40% of the sensory-responsive sample in some electrode penetrations. As in the other border regions, the multisensory zone was narrow, with only modality-specific neurons being found 500 μm away from the penetration of highest multisensory density.
Multisensory neurons were typically found clustered in laminae V and VI. This location contrasts with modality-specific neurons, which were distributed throughout all laminae. Interestingly, the infragranular restriction of multisensory neurons was found for each of the multisensory zones as well as for those nonmatching multisensory neurons within modality-specific cortices.
When examined on a neuron-by-neuron basis, the different receptive fields of individual multisensory neurons were found to have good spatial correspondence (Figs. 2 and 3). Thus, of the 137 multisensory neurons in which receptive fields were mapped, 118 (86.1%) exhibited >75% overlap between these receptive fields.
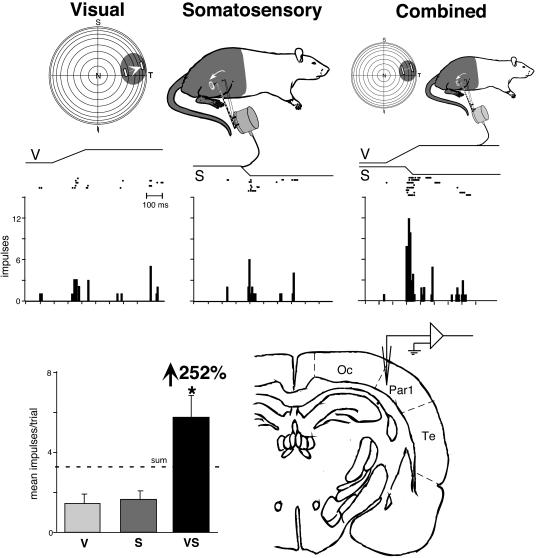
Fig. 2.
Receptive field overlap and multisensory enhancement in a visual-somatosensory neuron recorded at the occipital/parietal border. (Top) Visual and somatosensory receptive fields (shading) and locations of stimuli (icons depict stimulus movement) used in sensory testing. (Middle) Rasters and peristimulus time histograms illustrate responses to the visual, somatosensory, and combined visual-somatosensory stimulation. (Bottom Left) Summary bar graph illustrates the modality-specific [i.e., visual (V) and somatosensory (S)] and multisensory (i.e., VS) responses and the proportionate gain seen for the multisensory combination. (Bottom Right) The location of this neuron at the occipital/parietal border is shown on this drawing of a coronal section. *, P < 0.01. N, nasal; T, temporal; S, superior; I, inferior; Oc, occipital; Par1, parietal 1; Te, temporal.
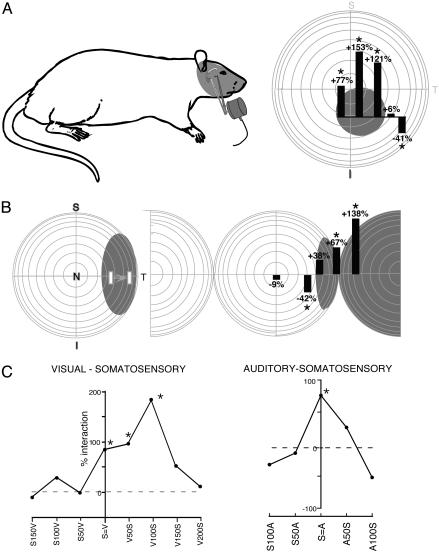
Fig. 3.
Spatial and temporal stimulus relationships influence multisensory integration. (A) The visual and somatosensory receptive fields of this neuron are shown by the shading. In this spatial manipulation, the somatosensory stimulus was always on the face (icon of probe), whereas the visual stimulus was presented at multiple locations within and outside its receptive field. Visual stimulus location is depicted along the abscissa of the bar graphs. Each bar shows the sign and magnitude of the resultant multisensory interaction. Note the significant response enhancements when both stimuli were presented within their receptive fields and the response depression when the visual stimulus was presented outside its receptive field. (B) The visual and auditory receptive fields of this neuron are shown in shading. In this example, the location of the visual stimulus was held constant, while the location of the auditory stimulus was varied. Again, note the response enhancement for within-receptive field pairings and the response depression when the auditory stimulus was moved just outside of its receptive field. (C) Two examples of the impact of changing stimulus timing on multisensory integration. Note that whereas the neuron shown in Left exhibited significant enhancements over a range of stimulus-onset asynchronies (e.g., V50S means the visual stimulus preceded the somatosensory stimulus by 50 ms) spanning 100 ms, the neuron shown in Right exhibited enhancements only when the stimuli were presented simultaneously (S=A). *, P < 0.05.
A total of 31 multisensory neurons found throughout each of the four multisensory regions were examined in sufficient detail to test their capacity to integrate cross-modal cues. Nearly half (14 or 45%) showed significant changes in their activity in response to multisensory cues. In each of these neurons exhibiting multisensory interactions, spatially coincident stimuli presented within their receptive fields produced a response that was significantly greater than that to the most effective of them individually (Figs. 2 and 3). In 10 of the 17 neurons in which systematic manipulations of the location of the stimuli were performed, placing one stimulus outside of its receptive field significantly depressed responses to the within-receptive field stimulus (Fig. 3 A and B). Each of these 10 neurons also showed response enhancement to spatially coincident stimuli, showing that altering the stimulus configuration could change the integrated multisensory response from enhancement to depression. Multisensory integration was also sensitive to the relative onset times of the cross-modal stimuli. Although most neurons preferred stimulus-onset asynchronies (SOAs) of 100 ms or less, there was typically a rather large window of SOAs (mean = 78 ms; range 0-250 ms) that resulted in a multisensory interaction in each of the 17 neurons that were examined by using such temporal manipulations. Two examples of this temporal modulation of multisensory interactions are shown in Fig. 3C. No apparent differences were noted in the incidence, magnitude, or spatial/temporal influences over multisensory integration in the different cortical areas.
Discussion
Despite the growing number of studies showing that presumptive modality-specific cortices can be influenced by other modalities, the present results suggest that the incidence of nonmatching and/or multisensory neurons in the major domains of visual, auditory, and somatosensory cortex is quite low. This observation is largely in keeping with traditional descriptions of these areas and with the concept that early stages of cortical sensory processing are conducted primarily on a sense-by-sense basis.
However, the finding that there are narrow areas of cross-modal overlap interposed between modality-specific domains is not consistent with these concepts of cortical parcellation, which restrict any mixing of sensory representations to distinct higher-order or association areas. These interposed cortical areas contain a mixture of the modality-specific neuronal types represented in each of the bordering regions as well as multisensory neurons representing their convergence. At its peak, the multisensory population in these border regions represents more than 50% of the neurons sampled. Many of the multisensory neurons in these border regions were capable of synthesizing the information received from their convergent inputs. These results suggest that the transitional zones may not only play roles specific to the two represented modalities but also be involved in the brain's ability to integrate information from multiple senses. These observations are complementary to several recent reports that have examined the multisensory organization of rodent neocortex, which, using evoked potentials, have shown nonlinear multisensory interactions at both the occipital/temporal and parietal/temporal borders (32-34). The present results suggest that these responses may have been generated by neurons in the bordering regions and that the intermixing of sensory neurons may be a common representational scheme at the transitions between modality-specific cortices. Supporting this view is recent data from both cat and monkey, in which multisensory neurons have been found at regions intervening between modality-specific realms (35, 36).
Whether multisensory zones are a de facto consequence of bordering sensory representations, and, thus, run continuously along the borders of cortical sensory representations is not yet known. Similarly, the source of the afferents to these multisensory borders has yet to be fully elucidated, although retrograde tracer injections into both the visual-auditory and auditory-somatosensory borders preferentially label neurons in the lateral posterior nucleus and the posterior nucleus of the thalamus, and poorly label sensory-specific thalamic nuclei (32-34). It is also possible that some multisensory neurons are formed by the convergence of ascending afferents from sensory-specific thalamic nuclei and/or from transcortical afferents. These possibilities extend to the multisensory region found in rostral parietal cortex, an area rich in visual-somatosensory neurons and interposed between the parietal somatosensory representations and the somatomotor and oculomotor representations of parietal and frontal cortices (37). Multisensory representations in parietal cortex have been implicated in attentional allocation and coordinate transformations, processes that have been less well explored, but are no less germane, in the rodent than in nonhuman primate models (38, 39).
Cross-modal receptive field overlap proved to be the general pattern among the neurons examined here and has previously been noted in a variety of cortical and subcortical structures (1-4, 35, 40-43), suggesting that the same cross-modal experiences that encourage receptive field overlap in midbrain multisensory neurons (44-46) operate throughout the neuraxis. Similarly, the observation that these cortical multisensory neurons integrate cross-modal cues in a manner similar to that found in other species and other brain areas (1-4, 35, 40, 46, 47) suggests a conservation in the principles guiding multisensory processes. Whether the pattern of alternating modality-specific and multisensory zones of the rat cortex is actually the general mammalian plan remains to be determined. However, if so, it may help clarify the pattern of multisensory cortical responses in humans and nonhuman primates that have been attributed solely to neuronal processes within what has previously been considered modality-specific cortex.
Acknowledgments
We thank Nancy Stein for editorial assistance. This work was supported in part by National Institutes of Health Grants MH63861, NS22543, and NS36916.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Stein, B. E. & Meredith, M. A. (1993) The Merging of the Senses (MIT Press, Cambridge, MA).
- 2.Meredith, M. A. & Stein, B. E. (1983) Science 221, 389-391. [DOI] [PubMed] [Google Scholar]
- 3.Meredith, M. A. & Stein, B. E. (1986) J. Neurophysiol. 56, 640-662. [DOI] [PubMed] [Google Scholar]
- 4.Wallace, M. T., Wilkinson, L. K. & Stein, B. E. (1996) J. Neurophysiol. 76, 1246-1266. [DOI] [PubMed] [Google Scholar]
- 5.Stein, B. E., Huneycutt, W. S. & Meredith, M. A. (1988) Brain Res. 448, 355-358. [DOI] [PubMed] [Google Scholar]
- 6.Mesulam, M. M. (1998) Brain 121, 1013-1052. [DOI] [PubMed] [Google Scholar]
- 7.Kaas, J. H. & Collins, C. E. (2001) Curr. Opin. Neurobiol. 11, 498-504. [DOI] [PubMed] [Google Scholar]
- 8.Goldman-Rakic, P. S. (1988) Annu. Rev. Neurosci. 11, 137-156. [DOI] [PubMed] [Google Scholar]
- 9.Peters, A. & Jones, E. G. (1986) Cerebral Cortex (Plenum, New York).
- 10.Jones, E. G. & Powell, T. P. (1970) Brain 93, 793-820. [DOI] [PubMed] [Google Scholar]
- 11.Hikosaka, K., Iwai, E., Saito, H. & Tanaka, K. (1988) J. Neurophysiol. 60, 1615-1637. [DOI] [PubMed] [Google Scholar]
- 12.Spinelli, D. N., Starr, A. & Barrett, T. W. (1968) Exp. Neurol. 22, 75-84. [DOI] [PubMed] [Google Scholar]
- 13.Morrell, F. (1972) Nature 238, 44-46. [DOI] [PubMed] [Google Scholar]
- 14.Fishman, M. C. & Michael, P. (1973) Vision Res. 13, 1415-1419. [DOI] [PubMed] [Google Scholar]
- 15.Calvert, G. A., Bullmore, E. T., Brammer, M. J., Campbell, R., Williams, S. C., McGuire, P. K., Woodruff, P. W., Iversen, S. D. & David, A. S. (1997) Science 276, 593-596. [DOI] [PubMed] [Google Scholar]
- 16.Eimer, M. & Schroger, E. (1998) Psychophysiology 35, 313-327. [DOI] [PubMed] [Google Scholar]
- 17.Giard, M. H. & Peronnet, F. (1999) J. Cogn. Neurosci. 11, 473-490. [DOI] [PubMed] [Google Scholar]
- 18.Foxe, J. J., Morocz, I. A., Murray, M. M., Higgins, B. A., Javitt, D. C. & Schroeder, C. E. (2000) Brain Res. Cogn. Brain Res. 10, 77-83. [DOI] [PubMed] [Google Scholar]
- 19.Macaluso, E., Frith, C. D. & Driver, J. (2000) Science 289, 1206-1208. [DOI] [PubMed] [Google Scholar]
- 20.Laurienti, P. J., Burdette, J. H., Wallace, M. T., Yen, Y. F., Field, A. S. & Stein, B. E. (2002) J. Cogn. Neurosci. 14, 420-429. [DOI] [PubMed] [Google Scholar]
- 21.Falchier, A., Clavagnier, S., Barone, P. & Kennedy, H. (2002) J. Neurosci. 22, 5749-5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Molholm, S., Ritter, W., Murray, M. M., Javitt, D. C., Schroeder, C. E. & Foxe, J. J. (2002) Brain Res. Cogn. Brain Res. 14, 115-128. [DOI] [PubMed] [Google Scholar]
- 23.Krieg, W. J. S. (1946) J. Comp. Neurol. 84, 218-276. [DOI] [PubMed] [Google Scholar]
- 24.Woolsey, C. N. (1947) Fed. Proc. 6, 437-441. [PubMed] [Google Scholar]
- 25.Welker, C. (1971) Brain Res. 26, 259-275. [PubMed] [Google Scholar]
- 26.Montero, V. M., Rojas, A. & Torrealba, F. (1973) Brain Res. 53, 197-201. [DOI] [PubMed] [Google Scholar]
- 27.Sally, S. L. & Kelly, J. B. (1988) J. Neurophysiol. 59, 1627-1638. [DOI] [PubMed] [Google Scholar]
- 28.Committee on Care and Use of Laboratory Animals (1985) Guide for the Care and Use of Laboratory Animals (Natl. Inst. of Health, Bethesda), DHHS Publ. No. (NIH) 85-23.
- 29.Swanson, L. W. (1992) Brain Maps: Structure of the Rat Brain (Elsevier, Amsterdam).
- 30.Zilles, K. & Wree, A. (1994) in The Rat Nervous System, ed. Paxinos, G. (Academic, San Diego), pp. 649-685.
- 31.Paxinos, G., Kus, L., Ashwell, K. W. S. & Watson, C. (1999) Cheomarchitectonic Atlas of the Rat Forebrain (Academic, San Diego).
- 32.Di, S., Brett, B. & Barth, D. S. (1994) Brain Res. 642, 267-280. [DOI] [PubMed] [Google Scholar]
- 33.Barth, D. S., Goldberg, N., Brett, B. & Di, S. (1995) Brain Res. 678, 177-190. [DOI] [PubMed] [Google Scholar]
- 34.Brett-Green, B., Fifkova, E., Larue, D. T., Winer, J. A. & Barth, D. S. (2003) J. Comp. Neurol. 460, 223-237. [DOI] [PubMed] [Google Scholar]
- 35.Wallace, M. T., Meredith, M. A. & Stein, B. E. (1992) Exp. Brain Res. 91, 484-488. [DOI] [PubMed] [Google Scholar]
- 36.Schroeder, C. E., Lindsley, R. W., Specht, C., Marcovici, A., Smiley, J. F. & Javitt, D. C. (2001) J. Neurophysiol. 85, 1322-1327. [DOI] [PubMed] [Google Scholar]
- 37.Neafsey, E. J., Bold, E. L., Haas, G., Hurley-Gius, K. M., Quirk, G., Sievert, C. F. & Terreberry, R. R. (1986) Brain Res. 396, 77-96. [DOI] [PubMed] [Google Scholar]
- 38.Andersen, R. A., Snyder, L. H., Bradley, D. C. & Xing, J. (1997) Annu. Rev. Neurosci. 20, 303-330. [DOI] [PubMed] [Google Scholar]
- 39.Pouget, A., Deneve, S. & Duhamel, J. R. (2002) Nat. Rev. Neurosci. 3, 741-747. [DOI] [PubMed] [Google Scholar]
- 40.King, A. J. & Palmer, A. R. (1985) Exp. Brain Res. 60, 492-500. [DOI] [PubMed] [Google Scholar]
- 41.Graziano, M. S. & Gross, C. G. (1993) Exp. Brain Res. 97, 96-109. [DOI] [PubMed] [Google Scholar]
- 42.Graziano, M. S., Hu, X. T. & Gross, C. G. (1997) J. Neurophysiol. 77, 2268-2292. [DOI] [PubMed] [Google Scholar]
- 43.Duhamel, J. R., Colby, C. L. & Goldberg, M. E. (1998) J. Neurophysiol. 79, 126-136. [DOI] [PubMed] [Google Scholar]
- 44.King, A. J., Hutchings, M. E., Moore, D. R. & Blakemore, C. (1988) Nature 332, 73-76. [DOI] [PubMed] [Google Scholar]
- 45.Knudsen, E. I. & Brainard, M. S. (1991) Science 253, 85-87. [DOI] [PubMed] [Google Scholar]
- 46.Wallace, M. T. & Stein, B. E. (1997) J. Neurosci. 17, 2429-2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang, W., Wallace, M. T., Jiang, H., Vaughan, J. W. & Stein, B. E. (2001) J. Neurophysiol. 85, 506-522. [DOI] [PubMed] [Google Scholar]