Abstract
Body dysmorphic disorder (BDD) is characterized by an often-delusional preoccupation with misperceived defects of appearance, causing significant distress and disability. Although previous studies have found functional abnormalities in visual processing, frontostriatal, and limbic systems, no study to date has investigated the microstructure of white matter connecting these systems in BDD. Fourteen medication-free BDD participants and 16 healthy controls were scanned using diffusion-weighted MRI. We utilized probabilistic tractography to reconstruct tracts of interest, and tract-based spatial statistics to investigate whole brain white matter. To estimate white matter microstructure we used fractional anisotropy (FA), mean diffusivity (MD), and linear and planar anisotropy (cl and cp). We correlated diffusion measures with clinical measures of symptom severity and poor insight/delusionality. Poor insight negatively correlated with FA and cl and positively correlated with MD in the inferior longitudinal fasciculus (ILF) and the forceps major (FM). FA and cl were lower in the ILF and IFOF and higher in the FM in the BDD group, but differences were nonsignificant. This is the first diffusion-weighted MR investigation of white matter in BDD. Results suggest a relationship between impairments in insight, a clinically important phenotype, and fiber disorganization in tracts connecting visual with emotion/memory processing systems.
Keywords: diffusion tensor imaging, probabilistic tractography, high angular resolution diffusion imaging, inferior longitudinal fasciculus, inferior fronto-occipital fasciculus, forceps major
1. INTRODUCTION
Body dysmorphic disorder (BDD) is a psychiatric disorder in which individuals are preoccupied with misperceived defects of their appearance (American Psychiatric Association., 2000). Believing that they look disfigured and ugly, they suffer significant distress and functional impairment. BDD affects approximately 0.7–2.4% of the population (Faravelli et al., 1997; Rief et al., 2006; Koran et al., 2008; Buhlmann et al., 2010) and is associated with high lifetime rates of hospitalization (48%) (Phillips and Diaz, 1997) and suicide attempts (22%–27.5%) (Veale et al., 1996; Phillips and Diaz, 1997; Phillips et al., 2005). Insight is usually impaired and 36–60% of BDD patients are delusional (Gunstad and Phillips, 2003; Phillips et al., 2005; Mancuso et al., 2010). Despite the severity of this disorder, knowledge of the underlying abnormalities in brain function and structure is still in its early stages.
An important symptom domain in BDD, for which there is emerging evidence, is distortion of visual perception. Distortion of self-perception of appearance may contribute to the conviction of disfigurement and ugliness and subsequent poor insight or delusionality. Clinically, individuals with BDD focus on details of their appearance at the expense of global aspects. A neuropsychological study using the Rey-Osterrieth Complex Figure Test demonstrated that patients with BDD selectively recalled details instead of larger organizational design features (Deckersbach et al., 2000). Individuals with BDD may also have perceptual distortions for own-face processing; in one study they perceived distortions of digital images of their faces that were not actually present (Yaryura-Tobias et al., 2002).
A previous functional magnetic resonance imaging (fMRI) study (performed in the same participants as the current study) found that individuals with BDD demonstrated abnormalities in visual processing (striate and extrastriate visual cortex) and frontostriatal systems (orbitofrontal cortex and caudate) when viewing their face (Feusner et al., 2010). There was also evidence of abnormalities in emotion processing systems. In addition, BDD symptom severity was correlated with frontostriatal activity and activity in extrastriate visual cortex. Abnormalities in visual systems may therefore represent early stage abnormalities (“bottom-up”) and/or may be the result of “top-down” modulation from emotional processing and/or prefrontal systems.
An earlier fMRI study in BDD using others’ faces as stimuli also found a pattern of abnormal information processing, including left hemisphere hyperactivity in an extended face-processing network (Feusner et al., 2007). This pattern, in contrast to the generally right hemisphere-dominant pattern for healthy controls (Haxby et al., 1994), suggests greater detail encoding and analysis relative to holistic and configural processing. Abnormal interhemispheric sharing of information may be involved, which may also contribute to aberrant visual processing.
The objective of the current study was to explore anatomical white matter connections involved in these neural systems that have been previously found to show abnormal activity in BDD. These white matter tracts include those likely involved in the integration of information between visual processing and the limbic as well as prefrontal systems, and those involved in interhemispheric sharing of information.
The only other studies in BDD that have investigated white matter include three small studies of volumetric brain morphometry. Two of these (Rauch et al., 2003; Atmaca et al., 2010), but not the third (Feusner et al., 2009) found greater total white matter in the BDD group relative to healthy controls.
To our knowledge, no study to date has investigated white matter microstructure in BDD using diffusion tensor imaging (DTI). However, several DTI studies have investigated white matter integrity in obsessive-compulsive disorder (Szeszko et al., 2005; Cannistraro et al., 2007; Yoo et al., 2007; Menzies et al., 2008; Saito et al., 2008; Garibotto et al., 2010; Bora et al., 2011; Nakamae et al., 2011), which is believed to be related to BDD (Hollander and Wong, 1995; Phillips et al., 2010). Several of these studies (Yoo et al., 2007; Saito et al., 2008; Garibotto et al., 2010; Bora et al., 2011; Nakamae et al., 2011), but not others (Szeszko et al., 2005; Cannistraro et al., 2007; Menzies et al., 2008), found abnormal fractional anisotropy (FA) in the corpus callosum. Across the studies with positive findings, however, there were inconsistencies in regard to both location and direction (higher or lower FA) of the abnormalities within the corpus callosum. Two studies in social anxiety disorder, also thought to be related to BDD (Fang and Hofmann, 2010) suggested abnormalities of FA in the uncinate fasciculus (Phan et al., 2009; Baur et al., 2011). One study in anorexia nervosa, also conceptualized to be related to BDD (Cororve and Gleaves, 2001), found abnormalities in the fimbria-fornix (Kazlouski et al., 2011). Overall, a consistent pattern of white matter abnormalities has not emerged in these related disorders. Thus we based our hypotheses for the current study on the aforementioned functional brain imaging studies in BDD suggesting abnormal activity in extended visual processing systems, in addition to performing exploratory analyses across the white matter of the entire brain.
Magnetic resonance diffusion imaging can provide information on white matter microstructure and anatomical connectivity by measuring the diffusion profile of water molecules. The DTI technique fits an ellipsoid (or “tensor”) to local water diffusivity, providing an estimate of the magnitude and orientation of water diffusion at each voxel. From this, white matter integrity measures based on the three “eigenvalues” of the reconstructed ellipsoid (representing the magnitude of water diffusivity along the three principal directions of the ellipsoid), such as the fractional anisotropy (FA; a measure of preferential directionality of water diffusion) and mean diffusivity (MD; a measure of overall diffusivity), and can be derived (Torrey, 1956; Stejskal, 1965).
One limitation of the standard FA is that it is not designed to probe subvoxel fiber architecture. Thus, low FA values may reflect either abnormal individual fiber integrity (e.g., fiber demyelination) or greater dispersion of fibers (e.g., fiber crossing or mixing, or other disorganization). To help differentiate these, we included DTI-derived geometric indices, linear and planar anisotropy (cl and cl) (Westin et al., 2002), to better quantify the shape of diffusion tensors beyond standard FA and MD.
Based on the previous BDD studies outlined above, we hypothesized that BDD participants would exhibit microstructural white matter abnormalities relative to controls in tracts involved in integration of information between limbic and visual processing systems, between prefrontal systems and visual processing systems, and those involved in interhemispheric sharing of information. We therefore examined the inferior longitudinal fasciculus (ILF), which connects anterior temporal cortex structures (including the amygdala and hippocampus) to the occipital lobe; the inferior fronto-occipital fasciculus (IFOF), which connects prefrontal regions to the occipital lobe; and the forceps major (FM), which connects the right and left occipital lobes (Catani and Schotten, 2008). Moreover, we predicted significant correlations would exist between the degree of microstructural abnormalities in these tracts and important clinical phenotypes of BDD symptom severity as well as poor insight/delusionality. We also performed an exploratory voxel-wise analysis of all white matter tracts.
2. METHODS
2.1. Participants
The UCLA Institutional Review Board approved the study protocol. Fourteen unmedicated participants with BDD and 16 healthy controls, aged 20 to 48 years, provided informed consent and participated (Table 1). BDD and control participants of equivalent sex, age, and level of education were recruited from the community (all had participated in a previous fMRI study of own-face processing (Feusner et al., 2010)). All were right-handed as determined by the Edinburgh Handedness Inventory (Oldfield, 1971). Diagnoses were made by J.D.F. who has clinical expertise with this population using the Body Dysmorphic Disorder Module (Phillips et al., 1995), a reliable diagnostic module modeled after the Structured Clinical Interview for Diagnostic and Statistical Manual (DSM) Disorders. In addition, we performed a clinical psychiatric evaluation and screened participants with the Mini-International Neuropsychiatric Interview (MINI) (Sheehan et al., 1998).
Table 1.
Demographics and psychometric scores
| Characteristics | BDD group (N=14) | Control group (N=16) | P valuea |
|---|---|---|---|
| Age, mean years (SD) | 26.6 (4.9) | 27.3 (5.3) | 0.7 |
| Female/male | 7/7 | 8/8 | >0.99 |
| Education, mean years (SD) | 15.5 (2.8) | 16.9 (2.3) | 0.150 |
| BDD-YBOCS score, mean (SD) | 29.85 (4.4) | N/A | N/A |
| BABS score, mean (SD) | 15 (3.9) | N/A | N/A |
| HAMD-17 score, mean (SD) | 10 (6.7) | 1.25 (1.48) | <0.0001 |
Abbreviations: BDD: body dysmorphic disorder; BDD-YBOCS: BDD version of the Yale-Brown Obsessive-Compulsive Scale; BABS: Brown Assessment of Beliefs Scale
HAMD-17: The 17-item Hamilton Depression Rating Scale
two-sample t-tests for age, education and HAMD-17; X2 test for gender
Exclusion criteria for all participants included: substance abuse or dependence within the past 12 months, lifetime neurological disorder, pregnancy, or any current medical disorder that may affect cerebral metabolism. We excluded BDD participants with any concurrent Axis I disorder besides dysthymia, major depressive disorder (MDD), or generalized anxiety disorder (GAD); as depression and anxiety are frequently comorbid in BDD, we believed that a sample excluding these would not be representative. However, we required that BDD be the primary diagnosis as defined by the MINI. Healthy controls could not have any current or past Axis I disorder, as determined by the MINI. We administered the BDD version of the Yale-Brown Obsessive-Compulsive Scale (BDD-YBOCS) (Phillips et al., 1997), a validated scale widely used to evaluate symptom severity in BDD (scores ranging from 0 to 48). We administered the Brown Assessment of Beliefs Scale (BABS), a measure of insight and delusionality that has been tested for validity and reliability in this population (Eisen et al., 1998). BABS Scores range from 0 to 24. Higher scores indicate poorer insight (more convinced about their appearance being defective and less able to recognize that their appearance concerns are attributable to a mental illness). A score of ≥18 with a score of 4 on item 1 (how convinced the person is that he/she is accurate) is classified as delusional. The 17-item Hamilton Depression Rating Scale (HAMD-17), a widely used and well-validated scale (Hamilton, 1960), was used to measure depressive symptoms.
All participants with BDD were required to have a score of 20 or higher on the BDD-YBOCS. Participants were free from psychoactive medications for 8 weeks or longer prior to the study and were not receiving cognitive-behavioral therapy.
All diffusion data were age- and gender-corrected using General Linear Model Univariate in SPSS, with gender as a fixed factor and age as continuous predictor.
2.2. Imaging data acquisition
We used a 3-T Allegra MRI scanner (Siemens Medical Solutions USA, Inc, Malvern, Pennsylvania). Diffusion-weighted MR imaging data were acquired using single-shot spin-echo echo-planar imaging (EPI) (field of view=240mm; voxel size=2.5×2.5×3.0mm, with 0.75 mm gap; TR/TE=7400/96ms; flip angle 9°). We collected 44 contiguous axial slices aligned to the anterior commissure–posterior commissure line along 34 gradient-sensitizing directions with b=1000s/mm2 and one minimally diffusion-weighted scan.
2.3. Data processing
All DTI data were visually inspected for motion artifacts to ensure quality, followed by Eddy current correction using FSL (http://www.fmrib.ox.ac.uk/fsl/fdt/fdt_eddy.html). Diffusion tensors were constructed using the MedInria software (http://www.sop.inria.fr/asclepios/software/MedINRIA/) to obtain three eigenvalues (λ1, λ2, λ3) and three eigenvectors (v1, v2, v3). The eigenvector (v1) associated with the largest eigenvalue (i.e., the axial diffusivity) is usually assumed to represent local fiber direction. FA is mathematically defined as:
| (1) |
Its values range from 0 (no directional dependence of diffusion) to 1 (diffusion along a single direction), and are considered a general measure of white matter integrity. MD (λ̄ in equation (1)) quantifies the overall water diffusivity and is defined as the average of the 3 eigenvalues.
2.3.1. Geometric indices
DTI white matter integrity indices are usually defined based on the eigenvalues of the tensor, and thus are rotationally invariant. While the FA distinguishes only between isotropic and anisotropic diffusion profiles, cp and cl (Westin et al., 2002) further determine whether diffusion profiles are planar/“pancake” shaped (high cp), or linear/“cigar” shaped (high cl). In white matter regions with highly coherent fiber orientations, water diffusion is mainly restricted along the direction corresponding to the largest DTI eigenvalue and as a result cl (mathematically defined as: cl = (λ1 − λ2)/λ1) (Westin et al., 2002) takes values close to 1. In the planar case the diffusion is mostly restricted to the plane spanned by the two eigenvectors corresponding to the two largest eigenvalues (λ1 and λ2, which would be large relative to λ3), and as a result cp (mathematically defined as: cp = (λ2 − λ3)/λ1) (Westin et al., 2002) takes values close to 1. Thus, the magnitudes of cl and cp provide estimates of fiber tract organization.
2.4. Fiber reconstruction using tractography
For tract-specific analyses, we used probabilistic tractography to compare mean white matter integrity measures between BDD and control participants for: a) the inferior fronto-occipital fasciculus (IFOF), which connects prefrontal regions to the occipital lobe; b) the inferior longitudinal fasciculus (ILF), which connects anterior temporal cortex structures (including the amygdala and hippocampus) to the occipital lobe; and c) the forceps major (FM), which connects the right and left occipital lobes (Catani and Schotten, 2008). In addition, we investigated the relationship between these tract diffusion measures and two important clinical variables in BDD: symptom severity (BDD-YBOCS) and degree of insight/delusionality (BABS).
For the probabilistic tractography we used a high angular resolution diffusion imaging (HARDI) method, the tensor distribution function (TDF), to reconstruct the imaging data (Leow et al., 2009) and to perform tractography. To this end, we utilized a standard probabilistic tractography algorithm with necessary modifications to accommodate data processed using TDF (GadElkarim et al., 2011) (see Supplementary Information for full details). Tract reconstruction was performed in individual subjects’ diffusion image space, using anatomical landmarks as described below (see also Fig. S1). Interrater reliability for FA was r=0.95, which was established between investigators (D.A. and W.L.) on a set of data from 6 randomly chosen subjects (3 from the BDD set and 3 from the healthy controls), for which ROIs were separately drawn by each investigator to reproduce the tracts and extract the diffusion measures.
2.5. Tract reconstruction protocol
2.5.1. IFOF
A para-sagittal plane at the level of the mid-cingulum was selected in the B0 image. A coronal slice was then selected at the anterior edge of the thalamus. On the corresponding color-coded tensor map, a region of interest (ROI) was drawn around the cluster of voxels in the superior-medial part of the temporal lobe that represent anteriorly-to-posteriorly oriented white matter tracts (i.e., color-coded green).
We then visually inspected all fibers passing through this seeding ROI. Fibers that did not connect the frontal lobe with the occipital lobe were then excluded. Operationally, we defined the frontal lobe to be the brain tissue anterior to the anterior edge of the thalamus, and occipital lobe posterior to the mid-point between the posterior edge of the parieto-occipital sulcus and the posterior edge of the posterior cingulum.
2.5.2. ILF
For the ILF and forceps major we followed and slightly modified the method used in (Wakana et al., 2007). We first selected a sagittal slice at the level of the mid-cingulum in the B0 image and identified the parieto-occipital sulcus. A coronal plane was selected halfway between the posterior edge of the parieto-occipital sulcus and the posterior edge of the posterior cingulum. The first ROI was drawn in the equivalent color-coded tensor space to include the occipital lobe (and exclude the parietal lobe). If difficult to visualize in any particular participant, the boundary between the occipital and parietal lobes was defined by linearly extrapolating the parieto-occipital sulcus medially to the lateral edge of the brain.
The second ROI was defined in the anterior temporal lobe. A sagittal slice at the level of the mid-cingulum was select from the B0 image. A coronal slice was then selected at the posterior edge of the genu of the corpus callosum. In this slice, if the temporal lobe was connected to the frontal lobe then the next coronal slice anteriorly that was not connected was selected. On this slice, the second ROI was drawn to include the entire temporal lobe.
2.5.3. FM
These ROIs were drawn in the same manner as the first ROI for the ILF. The first ROI was drawn to select the occipital lobe in the right hemisphere. The second ROI was drawn in the same way on the left hemisphere.
2.6. White matter integrity measures and data analysis
For each tract-of-interest, we plotted the reconstructed fibers and extracted mean white matter integrity measures of FA MD, cl, and cp. In the absence of a priori hemisphere-specific hypotheses, we analyzed bilateral (left+right) tracts for the ILF and IFOF (the FM is a midline structure). Post hoc analyses were then conducted for the left and right ILF and IFOF. We performed two-way ANOVAs with group as one factor and tract (ILF, IFOF, and FM) as the other (repeated measures) factor to compare mean values for each measure. Huynh-Feldt adjustments for sphericity were used when appropriate.
For the BDD group, we computed Pearson correlation coefficients between integrity measures and BDD-YBOCS and BABS scores. We used a Bonferroni-corrected significance level of α=0.017 (0.05/3), two-tailed, for testing a priori hypotheses on bilateral ILF, IFOF and FM for each measure; and a Bonferroni-corrected significance level of α=0.0125 (0.05/4), two-tailed, for the post hoc analyses for right and left ILF and IFOF tracts.
2.7. TBSS
We conducted exploratory voxel-wise analyses comparing FA, MD and eigenvalues in whole-brain white matter between the two groups using the TBSS program in FSL (Smith et al., 2006). TBSS utilizes nonlinear registration to project measures-of-interest onto an alignment-invariant tract representation (the “mean skeleton”). From this normalized template, voxel-wise statistical comparisons were performed between groups using Randomise v2.1, with the Threshold-Free Cluster Enhancement option (http://www.fmrib.ox.ac.uk/fsl/randomise/index.html). This produced P-value images, fully corrected for multiple comparisons. We used a significance threshold of α=0.05.
3. RESULTS
3.1. Demographics and psychometrics
Two BDD participants had comorbid GAD, one had comorbid MDD, and three had both GAD and MDD or dysthymia. All BDD participants had preoccupations with perceived facial defects.
3.2.1 Between group tractography results
There were no significant between-groups differences in the ILF, IFOF, or the FM for FA, MD, cl, or cp, although there was a trend for a group-by-tract interaction effect in the FM for cl (F1.9, 53=2.47, P=0.097). Although not statistically significant, there was a consistent pattern in the BDD group relative to healthy controls of lower FA and cl in the ILF and IFOF and higher FA and cl in the FM.
3.2.2 Additional post hoc analysis of comorbidity
Because there were 6 BDD subjects with a comorbid anxiety and/or depressive disorder, we performed a repeated measures ANOVA with comorbidity status as one factor and tract as the repeated measures factor for each of FA, MD, cl, and cp. Means for these diffusion measures were similar between comorbid and noncomorbid groups for the ILF, IFOF, and FM (Table S3). ANOVA results demonstrated no significant effect of comorbidity. There was only a significant comorbidity by tract effect for FA (F2,24=3.95, P=0.033): however, post hoc t-tests revealed no significant differences between comorbid and noncomorbid groups for the ILF (t=0.72, d.f.=12, P=0.49), IFOF (t=0.67, d.f.=12, P=0.51), or FM (t=0.47, d.f.=12, P=0.65).
3.3. TBSS results
Voxel-wise analyses in the whole-brain white matter using TBSS did not detect any significant group differences.
3.4.1. Correlation results
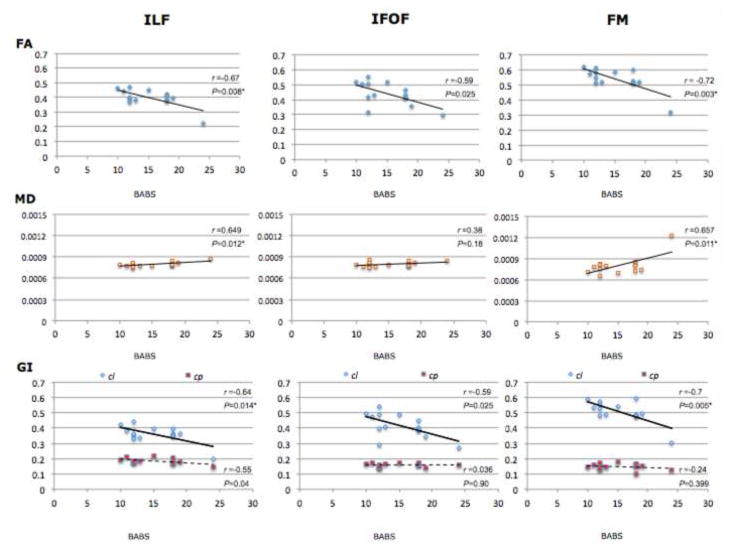
There were significant negative correlations in the ILF and FM between BABS scores and both FA and cl and significant positive correlations in the ILF and FM between BABS scores and MD (see Table 3 and Figs. 1, S2, and S3). There were no significant correlations in these tracts between any diffusion measures and BDD-YBOCS scores (Table S1 in Supplementary Information). This suggests that there is a stronger association between white matter microstructure and insight (as measured by the BABS), rather than symptoms of obsessional thoughts and compulsive behaviors (primarily measured by the BDD-YBOCS), in these tracts.
Table 3.
Correlations in the BDD group between BABS scores and diffusion measures in the ILF, IFOF, and the FM
| ILF | FA | MD | cl | cp | |||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| r | P | r | P | r | P | r | P | ||
|
|
|||||||||
| Bilateral | −0.67 | 0.008* | 0.649 | 0.012* | −0.64 | 0.014* | −0.55 | 0.04 | |
| post hoc | |||||||||
| Left | −0.68 | 0.006* | 0.629 | 0.016* | −0.697 | 0.0055* | −0.33 | 0.246 | |
| Right | −0.62 | 0.017 | 0.605 | 0.022 | −0.52 | 0.053 | −0.64 | 0.013* | |
|
| |||||||||
| IFOF | FA | MD | cl | cp | |||||
|
|
|||||||||
| r | P | r | P | r | P | r | P | ||
|
|
|||||||||
| Bilateral | −0.59 | 0.025 | 0.38 | 0.18 | −0.59 | 0.025 | 0.036 | 0.90 | |
| post hoc | |||||||||
| Left | −0.42 | 0.127 | 0.269 | 0.352 | −0.43 | 0.124 | 0.123 | 0.675 | |
| Right | −0.56 | 0.035 | 0.422 | 0.133 | −0.55 | 0.041 | −0.094 | 0.749 | |
|
| |||||||||
| FM | FA | MD | cl | cp | |||||
|
|
|||||||||
| r | P | r | P | r | P | r | P | ||
|
|
|||||||||
| −0.72 | 0.003* | 0.657 | 0.011* | −0.70 | 0.005* | −0.242 | 0.399 | ||
Abbreviations: BDD = body dysmorphic disorder; BABS = Brown Assessment of Beliefs Scale; FA = fractional anisotropy; MD = mean diffusivity; cl = linear anisotropy; cp = planar anisotropy; ILF = inferior longitudinal fasciculus; IFOF = inferior fronto-occipital fasciculus; FM = forceps major
Indicates significant P values after Bonferroni correction for multiple comparisons
Fig. 1. Correlations between white matter diffusion measures and poor insight/delusionality in bilateral white matter tracts in BDD group (N=14).
Correlation between scores on the BABS (a measure of degree of poor insight/delusionality) and FA, MD, cl and cp in bilateral ILF, IFOF, and FM BABS = Brown Assessment of Beliefs Scale; FA = fractional anisotropy; MD = mean diffusivity; GI = geometric indices; cl = linear anisotropy; cp = planar anisotropy; ILF = inferior longitudinal fasciculus; IFOF = inferior longitudinal fasciculus; FM = forceps major
* Indicates significant P values after Bonferroni correction for multiple comparisons
3.4.2. Additional, post hoc correlation analyses
Because four BDD participants had a comorbid depressive disorder and all had some degree of depressive symptomatology, we additionally calculated Pearson correlation coefficients between HAMD-17 scores and the diffusion measures. There were no significant correlations between HAMD-17 and FA, MD, cl, or cp for any tract (Table S2 in Supplementary Information), suggesting that there are stronger associations between white matter microstructure and insight, rather than depressive symptomatology, in these tracts.
For the correlation analyses with BABS scores, one data point met criteria as an outlier for MD in the FM, based on the z-score method for outlier detection (Z=3.1) (Barnett and Lewis, 1984; Iglewicz and Hoaglin, 1993). We recalculated the Pearson correlation coefficient without this data point and found that there was still a strong positive correlation between BABS and MD (r=0.53, P=0.062), although it was no longer statistically significant.
4. DISCUSSION
This represents the first investigation of white matter microstructure in BDD using diffusion-weighted MRI. We found that the clinical measure of poor insight correlated negatively with FA and cl and positively with MD in the ILF, which connects visual with emotional processing systems. Poor insight also correlated negatively with FA and cl and positively with MD in the FM, which connects right and left visual processing systems.
In order to better understand white matter architecture beyond the standard measures of FA and MD, we utilized additional metrics for investigating fiber tract organization: cl and cp. The finding of a negative correlation between poor insight and both FA and cl suggests that greater fiber dispersion (which would contribute to both lower cl and FA) is associated with worse insight. Our results therefore suggest a more specific relationship between fiber architecture, rather than individual fiber integrity, and poor insight in BDD.
These correlations were present despite the observation there were no significant between-groups differences detected in these tracts. This likely reflects the idea that phenotypes, such as poor insight in this case, may map better to brain pathophysiology than DSM or ICD-10 diagnostic categories. Such categorical constructs are increasingly recognized to have limited validity (Insel and Cuthbert, 2009), particularly as they may represent heterogeneous groupings of symptom clusters or dimensions. Poor insight, as a dimension of observable behavior that cuts across many diagnostic boundaries (Goldberg et al., 2001), may prove to be an important phenotype with links to aberrant neurobiology.
In BDD, poor insight is considered to be on a continuum with delusionality (Phillips et al., 1994; Phillips et al., 2006; Mancuso et al., 2010). This may represent a dimensional phenotype of psychosis in BDD (Phillips, 2004). Poor insight/delusionality usually manifests as erroneous convictions that one or more appearance features are defective and ugly (Phillips et al., 1993; Phillips et al., 1994). Insight is typically poor in most individuals with BDD, with 36–60% of patients classified as delusional (Eisen et al., 2004; Phillips, 2004; Phillips et al., 2006; Mancuso et al., 2010). Delusional variants appear to exist on a continuum with nondelusional variants, as they are similar in most demographics, clinical features, and course of illness (Phillips et al., 2006; Mancuso et al., 2010). Case reports suggest that individuals with BDD fluctuate between overvalued ideations and delusionality (Phillips and McElroy, 1993). Insight/delusionality is an important clinical variable; individuals who are more delusional seem less likely to seek and remain in treatment (Eisen et al., 2004) and, when controlling for symptom severity, have lower educational attainment (Eisen et al., 2004; Phillips et al., 2006). Because poor insight is typically related to what they perceive, and studies show abnormalities in visual processing systems in BDD (Feusner et al., 2007; Feusner et al., 2010; Feusner et al., 2011), one possibility is that a distorted visual perception of appearance is difficult to refute, and may contribute to their level of conviction. The structural neurobiology of systems involved in visual perception in BDD may therefore be relevant to understanding poor insight/delusionality.
One such structural system is the ILF, which connects the temporal lobe with the occipital lobe (Catani et al., 2003; Catani and Schotten, 2008). Long fibers in this tract connect the anterior temporal lobe with posterior occipital regions (Catani et al., 2003). The occipital branches of the ILF extend to extrastriate cortical regions in the dorso-lateral occipital lobe, lingual and fusiform gyri, and the cuneus, while temporal branches extend medially near the amygdala, hippocampus, and uncus/parahippocampal gyrus. Feed-forward and feed-back information may be carried on this tract (Schmahmann and Pandya, 2006). Feed-forward connections may function to consolidate visual memories (Shinoura et al., 2007; Ross, 2008). Feed-back connections likely carry signals regarding emotional valence of stimuli to the visual cortex, resulting in enhanced visual processing of emotionally salient stimuli. This has been demonstrated in neuroimaging studies in which amygdala activation was found to correlate with activation in the visual cortex (Morris et al., 1998; Pessoa et al., 2002), and this correlation is attenuated in patients with amygdala damage (Vuilleumier et al., 2004). Moreover, pre-existing representation of face identity in memory may influence early stages of visual encoding (Righart et al., 2011). In this way, top-down modulation on earlier visual processing systems by memory representations may overlap with perceived facial information. The ILF therefore is involved in visual processing, and may have a role in face recognition (Fox et al., 2008) as well as facial emotion recognition (Philippi et al., 2009).
Our finding of significant correlations between fiber dispersion in the ILF and poor insight suggests that worse insight/delusionality may be associated with reduced fiber organization in pathways involved in integration between emotional signals and visual perception. We conjecture that the observed higher degree of fiber dispersion in ILF may be due to reduced alignment of long ILF fiber bundles that connect visual- and emotion-processing systems, or alternatively due to aberrant connections within shorter, local fibers that travel with ILF during part of their course. The correlation between fiber dispersion and poor insight was greater on the left, although the functional significance is unclear.
The previous fMRI study in BDD of own-face perception (Feusner et al., 2010), performed in the same individuals as in the current study, found hypoactivity in regions of the left extrastriate visual cortex that are likely connected to anterior temporal lobe structures via the ILF. Hypoactivity was found in these regions specifically for face images that represented only low spatial frequency information. It is possible then that feed-forward and/or feed-back of information between perceptual and emotional/memory systems may be disturbed in BDD. This may affect specific elements of perception such as the ability to perceive the whole, which may subsequently contribute to worse insight/delusionality.
In the same study, there was no significant amygdala (or insula) hyperactivity, despite the BDD participants rating own-face viewing as highly distressing and aversive. This also suggests impaired connections between perceptual and emotional systems in BDD. Other studies in BDD have found misinterpretations of facial expressions (Buhlmann et al., 2004; Buhlmann et al., 2006), and impairment in identity recognition of faces with emotional expressions (Feusner et al., 2010). These studies lend additional evidence to impairments in integration of visual and emotional information.
Poor insight also correlated with fiber dispersion in the FM. This tract appears to be involved in transferring visual inputs from one hemisphere to the other (see (Doron and Gazzaniga, 2008) for review). Other evidence for disturbed right/left hemisphere function in BDD comes from a previous fMRI study of other-face visual processing, in which there was a left-hemisphere dominant pattern (Feusner et al., 2007). In addition, a test of global-local visual processing in BDD revealed slower performance in the BDD relative to the control group, particularly when participants were required to switch between identifying local and global stimuli (Kerwin et al., 2011), which likely requires interhemispheric transfer of information. It is possible, then, that poor insight in BDD may also be related to visuospatial abnormalities mediated by fiber disorganization in the FM.
The findings from the current study may signify that poor integration of information between systems subserved by the ILF and the FM, related to fiber disorganization, may be associated with inability to accurately perceive and/or contextualize visual stimuli in individuals with BDD. When individuals view their own appearance, impaired feed-forward or feed-back information transfer in the ILF may result in a failure to update visual memories accurately. This may result in persistent yet distorted visual templates of appearance flaws, as a result of, for example, previously viewing themselves in extreme lighting conditions or even from past blemishes such as acne that had since resolved. In addition, impaired interhemispheric information transfer in the FM may impair ability to integrate global and local visual information; this may result in a piecemeal perception of their appearance features and an inability to perceive that whatever slight defects exist are small relative to the whole. These resultant distorted visual perceptions may be difficult to refute, translating to poor insight or even delusionality about their appearance. This level of conviction may be resistant to attempts of others to reassure them that their appearance does not appear defective and ugly (which family members and friends often try to do), because they take the reality of their visual experience for granted. Further, this may trigger other symptoms such as dysphoria about perceived ugliness, anxiety and self-consciousness around others, and compulsive behaviors to fix or hide their appearance.
Although no previous study has investigated white matter integrity using diffusion imaging in BDD, studies in other clinical populations have found abnormalities in the ILF. Multiple studies in schizophrenia have found low FA in the ILF, as well as other white matter tracts (Hubl et al., 2004; Ashtari et al., 2007; Mitelman et al., 2007; Cheung et al., 2008; Michael et al., 2008; Clark et al., 2011). Several of these have found associations between positive (Mitelman et al., 2007; Michael et al., 2008) and negative symptoms (Michael et al., 2008) and low FA in the ILF, and associations between, auditory (Hubl et al., 2004) and visual hallucinations (Ashtari et al., 2007) and low FA in the left ILF. Some of these studies also found lower FA in the left IFOF in schizophrenics (Cheung et al., 2008; Clark et al., 2011). Despite many phenomenological differences between BDD and schizophrenia, they share some clinical phenotypes such as poor insight, delusional thinking, distorted perception, as well as evidence of abnormalities in global visual processing and visual integration (see (Silverstein and Keane, 2011) and (Butler et al., 2008) for reviews).
This study has several limitations. Small sample size may have resulted in low power, which may explain why significant differences were not detected between groups for the DTI measures. The cross-sectional design limits our understanding of whether the correlative relationships between white matter architecture and clinical symptoms has a causative role in BDD symptoms, or are the secondary effects of having BDD. It is also not possible to determine whether the findings in the ILF have significance for feed-forward and/or feed-back relationships, as information carried in this tract may be bidirectional (Schmahmann and Pandya, 2006). The acquisition parameters of our diffusion sequence included a gap of .75mm in the z plane, which may have reduced the ability to reconstruct fibers using tractography, especially for those tracts that are oblique to the z plane. Because we extracted white matter tracts using a protocol based on ROI drawings using anatomical landmarks (Wakana et al., 2007), variations in ROI placement may result in different tracts, an inherent limitation of DTI-tractography (Hagmann et al., 2003).
The current study has several strengths. Our hypotheses were informed by a functional imaging study of own-face processing in the same individuals with BDD (Feusner et al., 2010). All participants were unmedicated, reducing possible confounds observed with psychotropic medications in other DTI studies (Yoo et al., 2007).
In conclusion, we detected significant correlations between fiber dispersion and poor insight/delusionality in the ILF and FM in individuals with BDD. This clinical symptom in BDD, with important prognostic implications, may therefore be associated with fiber disorganization in tracts that communicate between visual perceptual and emotion/memory processing systems. Future larger studies are warranted to confirm these findings, and also in order to further investigate white matter architecture and integrity and how they relate to phenotypes underlying different symptom dimensions in BDD.
Supplementary Material
Table 2.
Mean (±SD) values for BDD and healthy control groups for white matter diffusion measures in the ILF, IFOF, and the FMa
| ILF | IFOF | FM | df | error | F | P | ||
|---|---|---|---|---|---|---|---|---|
|
FA
|
||||||||
| BDD | 0.40±(0.062) | 0.44±(0.079) | 0.54±(0.075) | |||||
| Healthy controls | 0.41±(0.058) | 0.45±(0.051) | 0.52±(0.046) | |||||
| group | 1 | 28 | 0.029 | 0.87 | ||||
| group-by-tract
|
2 | 56 | 1.81 | 0.17 | ||||
|
MDb
|
||||||||
| BDD | 0.00081±(0.00003) | 0.00081±(0.00004) | 0.00082±(0.00014) | |||||
| Healthy controls | 0.00082±(0.00007) | 0.00081±(0.00005) | 0.00078±(0.00004) | |||||
| group | 1 | 28 | 0.178 | 0.68 | ||||
| group-by-tract
|
1.7 | 49 | 1.44 | 0.246 | ||||
|
cl
|
||||||||
| BDD | 0.36±(0.058) | 0.42±(0.079) | 0.51±(0.072) | |||||
| Healthy controls | 0.37±(0.052) | 0.43±(0.056) | 0.48±(0.048) | |||||
| group | 1 | 28 | 0.000 | 0.98 | ||||
| group-by-tract
|
1.9 | 53 | 2.47 | 0.097 | ||||
|
cp
|
||||||||
| BDD | 0.18±(0.018) | 0.16±(0.013) | 0.15±(0.020) | |||||
| Healthy controls | 0.19±(0.015) | 0.16±(0.017) | 0.16±(0.021) | |||||
| group | 1 | 28 | 2.12 | 0.156 | ||||
| group-by-tract | 2 | 56 | 1.65 | 0.20 |
Results presented for repeated measures ANOVA with group (BDD or healthy control) as one factor and tract (ILF, IFOF, and FM) as the other
In units of mm2/s factor. Huynh-Feldt adjustments for sphericity were used.
BDD = body dysmorphic disorder; ILF = inferior longitudinal fasciculus; IFOF = inferior longitudinal fasciculus; FM = forceps major; FA = fractional anisotropy; MD = mean diffusivity; cl = linear anisotropy; cp = planar anisotropy
Acknowledgments
This work was supported by grants from the National Institute of Mental Health (5K23 MH079212, 1R01 MH093535, and 1R01 MH085900 – Dr. Feusner), a grant from the International Obsessive Compulsive Foundation (Dr. Feusner), and a UCLA Faculty Research Grant (Dr. Feusner). Dr. Leow was partially supported by a grant from the National Alliance for Research on Schizophrenia and Depression (NARSAD- G5749). None of the funding sources had any involvement in the study design; in the collection, analysis or interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Financial Disclosures
None of the authors have any conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders : DSM-IV-TR. American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- Ashtari M, Cottone J, Ardekani BA, Cervellione K, Szeszko PR, Wu J, Chen S, Kumra S. Disruption of white matter integrity in the inferior longitudinal fasciculus in adolescents with schizophrenia as revealed by fiber tractography. Archives of General Psychiatry. 2007;64:1270–1280. doi: 10.1001/archpsyc.64.11.1270. [DOI] [PubMed] [Google Scholar]
- Atmaca M, Bingol I, Aydin A, Yildirim H, Okur I, Yildirim MA, Mermi O, Gurok MG. Brain morphology of patients with body dysmorphic disorder. Journal of Affective Disorders. 2010;123:258–263. doi: 10.1016/j.jad.2009.08.012. [DOI] [PubMed] [Google Scholar]
- Barnett V, Lewis T. Outliers in Statistical Data. John Wiley & Sons; New York: 1984. [Google Scholar]
- Baur V, Hanggi J, Rufer M, Delsignore A, Jancke L, Herwig U, Bruhl AB. White matter alterations in social anxiety disorder. Journal of Psychiatric Research. 2011;45:1366–1372. doi: 10.1016/j.jpsychires.2011.05.007. [DOI] [PubMed] [Google Scholar]
- Bora E, Harrison BJ, Fornito A, Cocchi L, Pujol J, Fontenelle LF, Velakoulis D, Pantelis C, Yucel M. White matter microstructure in patients with obsessive-compulsive disorder. Journal of Psychiatry and Neuroscience. 2011;36:42–46. doi: 10.1503/jpn.100082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhlmann U, Etcoff N, Wilhelm S. Emotional recognition bias for contempt and anger in body dysmorphic disorder. Journal of Psychiatric Research. 2006;40:105–111. doi: 10.1016/j.jpsychires.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Buhlmann U, Glaesmer H, Mewes R, Fama JM, Wilhelm S, Brahler E, Rief W. Updates on the prevalence of body dysmorphic disorder: a population-based survey. Psychiatry Research. 2010;178:171–175. doi: 10.1016/j.psychres.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Buhlmann U, McNally R, Etcoff N, Tuschen-Caffier B, Wilhelm S. Emotion recognition deficits in body dysmorphic disorder. Journal of Psychiatric Research. 2004;38:201–206. doi: 10.1016/s0022-3956(03)00107-9. [DOI] [PubMed] [Google Scholar]
- Butler PD, Silverstein SM, Dakin SC. Visual perception and its impairment in schizophrenia. Biological Psychiatry. 2008;64:40–47. doi: 10.1016/j.biopsych.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannistraro PA, Makris N, Howard JD, Wedig MM, Hodge SM, Wilhelm S, Kennedy DN, Rauch SL. A diffusion tensor imaging study of white matter in obsessive-compulsive disorder. Depression and Anxiety. 2007;24:440–446. doi: 10.1002/da.20246. [DOI] [PubMed] [Google Scholar]
- Catani M, Jones DK, Donato R, Ffytche DH. Occipito-temporal connections in the human brain. Brain. 2003;126:2093–2107. doi: 10.1093/brain/awg203. [DOI] [PubMed] [Google Scholar]
- Catani M, Schotten Td. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex. 2008;44:1105–1132. doi: 10.1016/j.cortex.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Cheung V, Cheung C, McAlonan GM, Deng Y, Wong JG, Yip L, Tai KS, Khong PL, Sham P, Chua SE. A diffusion tensor imaging study of structural dysconnectivity in never-medicated, first-episode schizophrenia. Psychological Medicine. 2008;38:877–885. doi: 10.1017/S0033291707001808. [DOI] [PubMed] [Google Scholar]
- Clark KA, Nuechterlein KH, Asarnow RF, Hamilton LS, Phillips OR, Hageman NS, Woods RP, Alger JR, Toga AW, Narr KL. Mean diffusivity and fractional anisotropy as indicators of disease and genetic liability to schizophrenia. Journal of Psychiatric Research. 2011 doi: 10.1016/j.jpsychires.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cororve MB, Gleaves DH. Body dysmorphic disorder: a review of conceptualizations, assessment, and treatment strategies. Clin Psychol Rev. 2001;21:949–970. doi: 10.1016/s0272-7358(00)00075-1. [DOI] [PubMed] [Google Scholar]
- Deckersbach T, Savage C, Phillips K, Wilhelm S, Buhlmann U, Rauch S, Baer L, Jenike M. Characteristics of memory dysfunction in body dysmorphic disorder. Journal of the International Neuropsychological Society. 2000;6:673–681. doi: 10.1017/s1355617700666055. [DOI] [PubMed] [Google Scholar]
- Doron K, Gazzaniga M. Neuroimaging techniques offer new perspectives on callosal transfer and interhemispheric communication. Cortex. 2008;44:1023–1029. doi: 10.1016/j.cortex.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Eisen JL, Phillips KA, Baer L, Beer DA, Atala KD, Rasmussen SA. The Brown Assessment of Beliefs Scale: reliability and validity. American Journal of Psychiatry. 1998;155:102–108. doi: 10.1176/ajp.155.1.102. [DOI] [PubMed] [Google Scholar]
- Eisen JL, Phillips KA, Coles ME, Rasmussen SA. Insight in obsessive compulsive disorder and body dysmorphic disorder. Comprehensive Psychiatry. 2004;45:10–15. doi: 10.1016/j.comppsych.2003.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang A, Hofmann SG. Relationship between social anxiety disorder and body dysmorphic disorder. Clinical Psychology Review. 2010;30:1040–1048. doi: 10.1016/j.cpr.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faravelli C, Salvatori S, Galassi F, Aiazzi L, Drei C, Cabras P. Epidemiology of somatoform disorders: a community survey in Florence. Social Psychiatry and Psychiatric Epidemiology. 1997;32:24–29. doi: 10.1007/BF00800664. [DOI] [PubMed] [Google Scholar]
- Feusner JD, Bystritsky A, Hellemann G, Bookheimer S. Impaired identity recognition of faces with emotional expressions in body dysmorphic disorder. Psychiatry Research. 2010;179:318–323. doi: 10.1016/j.psychres.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feusner JD, Hembacher E, Moller H, Moody TD. Abnormalities of object visual processing in body dysmorphic disorder. Psychological Medicine. 2011:1–13. doi: 10.1017/S0033291711000572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feusner JD, Moody T, Townsend J, McKinley M, Hembacher E, Moller H, Bookheimer S. Abnormalities of visual processing and frontostriatal systems in body dysmorphic disorder. Archives of General Psychiatry. 2010;67:197–205. doi: 10.1001/archgenpsychiatry.2009.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feusner JD, Townsend J, Bystritsky A, Bookheimer S. Visual information processing of faces in body dysmorphic disorder. Archives of General Psychiatry. 2007;64:1417–1425. doi: 10.1001/archpsyc.64.12.1417. [DOI] [PubMed] [Google Scholar]
- Feusner JD, Townsend J, Bystritsky A, McKinley M, Moller H, Bookheimer S. Regional brain volumes and symptom severity in body dysmorphic disorder. Psychiatry Research: Neuroimaging. 2009;172:161–167. doi: 10.1016/j.pscychresns.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox CJ, Iaria G, Barton JJ. Disconnection in prosopagnosia and face processing. Cortex. 2008;44:996–1009. doi: 10.1016/j.cortex.2008.04.003. [DOI] [PubMed] [Google Scholar]
- GadElkarim JJ, Zhan L, Yang SL, Zhang AF, Altshuler L, Lamar M, Ajilore O, Thompson PM, Kumar A, Leow A. TDF-Tract: probabilistic tractography using the tensor distribution function. IEEE International Symposium on Biomedical Imaging; Chicago, IL. 2011. [Google Scholar]
- Garibotto V, Scifo P, Gorini A, Alonso CR, Brambati S, Bellodi L, Perani D. Disorganization of anatomical connectivity in obsessive compulsive disorder: a multi-parameter diffusion tensor imaging study in a subpopulation of patients. Neurobiology of Disease. 2010;37:468–476. doi: 10.1016/j.nbd.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Goldberg RW, Green-Paden LD, Lehman AF, Gold JM. Correlates of insight in serious mental illness. Journal of Nervous and Mental Disease. 2001;189:137–145. doi: 10.1097/00005053-200103000-00001. [DOI] [PubMed] [Google Scholar]
- Gunstad J, Phillips KA. Axis I comorbidity in body dysmorphic disorder. Comprehensive Psychiatry. 2003;44:270–276. doi: 10.1016/S0010-440X(03)00088-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmann P, Thiran JP, Jonasson L, Vandergheynst P, Clarke S, Maeder P, Meuli R. DTI mapping of human brain connectivity: statistical fibre tracking and virtual dissection. Neuroimage. 2003;19:545–554. doi: 10.1016/s1053-8119(03)00142-3. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV, Horwitz B, Ungerleider LG, Maisog JM, Pietrini P, Grady CL. The functional organization of human extrastriate cortex: a PET-rCBF study of selective attention to faces and locations. Journal of Neuroscience. 1994;14:6336–6353. doi: 10.1523/JNEUROSCI.14-11-06336.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander E, Wong C. Introduction: obsessive-compulsive spectrum disorders. Journal of Clinical Psychiatry. 1995;56:3–6. [PubMed] [Google Scholar]
- Hubl D, Koenig T, Strik W, Federspiel A, Kreis R, Boesch C, Maier SE, Schroth G, Lovblad K, Dierks T. Pathways that make voices: white matter changes in auditory hallucinations. Archives of General Psychiatry. 2004;61:658–668. doi: 10.1001/archpsyc.61.7.658. [DOI] [PubMed] [Google Scholar]
- Iglewicz B, Hoaglin DC. How to Detect and Handle Outliers. American Society for Quality Control; Milwaukee, WI: 1993. [Google Scholar]
- Insel TR, Cuthbert BN. Endophenotypes: bridging genomic complexity and disorder heterogeneity. Biological Psychiatry. 2009;66:988–989. doi: 10.1016/j.biopsych.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Kazlouski D, Rollin MDH, Tregellas J, Shott ME, Jappe LM, Hagman JO, Pryor T, Yang TT, Frank GKW. Altered fimbria-fornix white matter integrity in anorexia nervosa predicts harm avoidance. Psychiatry Research-Neuroimaging. 2011;192:109–116. doi: 10.1016/j.pscychresns.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerwin L, Moller H, Hellemann G, Feusner JD. Global-local processing in body dysmorphic disorder (BDD). International Obsessive-Compulsive Disorder Foundation; San Diego, CA. 2011. [Google Scholar]
- Koran LM, Abujaoude E, Large MD, Serpe RT. The prevalence of body dysmorphic disorder in the United States adult population. CNS Spectrums. 2008;13:316–322. doi: 10.1017/s1092852900016436. [DOI] [PubMed] [Google Scholar]
- Leow AD, Zhu S, Zhan L, McMahon K, de Zubicaray GI, Meredith M, Wright MJ, Toga AW, Thompson RM. The Tensor Distribution Function. Magnetic Resonance in Medicine. 2009;61:205–214. doi: 10.1002/mrm.21852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso SG, Knoesen NP, Castle DJ. Delusional versus nondelusional body dysmorphic disorder. Comprehensive Psychiatry. 2010;51:177–182. doi: 10.1016/j.comppsych.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Menzies L, Williams GB, Chamberlain SR, Ooi C, Fineberg N, Suckling J, Sahakian BJ, Robbins TW, Bullmore ET. White matter abnormalities in patients with obsessive-compulsive disorder and their first-degree relatives. American Journal of Psychiatry. 2008;165:1308–1315. doi: 10.1176/appi.ajp.2008.07101677. [DOI] [PubMed] [Google Scholar]
- Michael AM, Calhoun VD, Pearlson GD, Baum SA, Caprihan A. Correlations of diffusion tensor imaging values and symptom scores in patients with schizophrenia. Conference Proceedings of the IEEE Engineering in Medicine and Biology Society. 2008;2008:5494–5497. doi: 10.1109/IEMBS.2008.4650458. [DOI] [PubMed] [Google Scholar]
- Mitelman SA, Torosjan Y, Newmark RE, Schneiderman JS, Chu KW, Brickman AM, Haznedar MM, Hazlett EA, Tang CY, Shihabuddin L, Buchsbaum MS. Internal capsule, corpus callosum and long associative fibers in good and poor outcome schizophrenia: a diffusion tensor imaging survey. Schizophrenia Research. 2007;92:211–224. doi: 10.1016/j.schres.2006.12.029. [DOI] [PubMed] [Google Scholar]
- Morris JS, Friston KJ, Buchel C, Frith CD, Young AW, Calder AJ, Dolan RJ. A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain. 1998;121 (Pt 1):47–57. doi: 10.1093/brain/121.1.47. [DOI] [PubMed] [Google Scholar]
- Nakamae T, Narumoto J, Sakai Y, Nishida S, Yamada K, Nishimura T, Fukui K. Diffusion tensor imaging and tract-based spatial statistics in obsessive-compulsive disorder. Journal of Psychiatric Research. 2011;45:687–690. doi: 10.1016/j.jpsychires.2010.09.016. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. Assessment and analysis of handedness - Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pessoa L, McKenna M, Gutierrez E, Ungerleider LG. Neural processing of emotional faces requires attention. Proceedings of the National Academy of Science U S A. 2002;99:11458–11463. doi: 10.1073/pnas.172403899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Orlichenko A, Boyd E, Angstadt M, Coccaro EF, Liberzon I, Arfanakis K. Preliminary Evidence of White Matter Abnormality in the Uncinate Fasciculus in Generalized Social Anxiety Disorder. Biological Psychiatry. 2009;66:691–694. doi: 10.1016/j.biopsych.2009.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippi CL, Mehta S, Grabowski T, Adolphs R, Rudrauf D. Damage to association fiber tracts impairs recognition of the facial expression of emotion. Journal of Neuroscience. 2009;29:15089–15099. doi: 10.1523/JNEUROSCI.0796-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips KA. Psychosis in body dysmorphic disorder. Journal of Psychiatric Research. 2004;38:63–72. doi: 10.1016/s0022-3956(03)00098-0. [DOI] [PubMed] [Google Scholar]
- Phillips KA, Atala KD, Pope HG., Jr Diagnostic Instruments for body dysmorphic disorder. American Psychiatric Association 148th Annual Meeting; Miami, FL. 1995. p. 157. [Google Scholar]
- Phillips KA, Coles ME, Menard W, Yen S, Fay C, Weisberg RB. Suicidal ideation and suicide attempts in body dysmorphic disorder. Journal of Clinical Psychiatry. 2005;66:717–725. doi: 10.4088/jcp.v66n0607. [DOI] [PubMed] [Google Scholar]
- Phillips KA, Diaz SF. Gender differences in body dysmorphic disorder. Journal of Nervous and Mental Disorders. 1997;185:570–577. doi: 10.1097/00005053-199709000-00006. [DOI] [PubMed] [Google Scholar]
- Phillips KA, Hollander E, Rasmussen SA, Aronowitz BR, DeCaria C, Goodman WK. A severity rating scale for body dysmorphic disorder: development, reliability, and validity of a modified version of the Yale-Brown Obsessive Compulsive Scale. Psychopharmacology Bulletin. 1997;33:17–22. [PubMed] [Google Scholar]
- Phillips KA, McElroy SL. Insight, overvalued ideation, and delusional thinking in body dysmorphic disorder: theoretical and treatment implications. Journal of Nervous and Mental Disorders. 1993;181:699–702. doi: 10.1097/00005053-199311000-00009. [DOI] [PubMed] [Google Scholar]
- Phillips KA, McElroy SL, Keck PE, Jr, Hudson JI, Pope HG., Jr A comparison of delusional and nondelusional body dysmorphic disorder in 100 cases. Psychopharmacology Bulletin. 1994;30:179–186. [PubMed] [Google Scholar]
- Phillips KA, McElroy SL, Keck PE, Jr, Pope HG, Jr, Hudson JI. Body dysmorphic disorder: 30 cases of imagined ugliness. American Journal of Psychiatry. 1993;150:302–308. doi: 10.1176/ajp.150.2.302. [DOI] [PubMed] [Google Scholar]
- Phillips KA, Menard W, Fay C, Weisberg R. Demographic characteristics, phenomenology, comorbidity, and family history in 200 individuals with body dysmorphic disorder. Psychosomatics. 2005;46:317–325. doi: 10.1176/appi.psy.46.4.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips KA, Menard W, Pagano ME, Fay C, Stout RL. Delusional versus nondelusional body dysmorphic disorder: clinical features and course of illness. Journal of Psychiatric Research. 2006;40:95–104. doi: 10.1016/j.jpsychires.2005.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips KA, Stein DJ, Rauch SL, Hollander E, Fallon BA, Barsky A, Fineberg N, Mataix-Cols D, Ferrao YA, Saxena S, Wilhelm S, Kelly MM, Clark LA, Pinto A, Bienvenu OJ, Farrow J, Leckman J. Should an obsessive-compulsive spectrum grouping of disorders be included in DSM-V? Depression and Anxiety. 2010;27:528–555. doi: 10.1002/da.20705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SL, Phillips KA, Segal E, Makris N, Shin LM, Whalen PJ, Jenike MA, Caviness VS, Jr, Kennedy DN. A preliminary morphometric magnetic resonance imaging study of regional brain volumes in body dysmorphic disorder. Psychiatry Research. 2003;122:13–19. doi: 10.1016/s0925-4927(02)00117-8. [DOI] [PubMed] [Google Scholar]
- Rief W, Buhlmann U, Wilhelm S, Borkenhagen A, Brahler E. The prevalence of body dysmorphic disorder: a population-based survey. Psychological Medicine. 2006;36:877–885. doi: 10.1017/S0033291706007264. [DOI] [PubMed] [Google Scholar]
- Righart R, Burra N, Vuilleumier P. Face Perception in the Mind’s Eye. Brain Topography. 2011;24:9–18. doi: 10.1007/s10548-010-0164-8. [DOI] [PubMed] [Google Scholar]
- Ross ED. Sensory-specific amnesia and hypoemotionality in humans and monkeys: gateway for developing a hodology of memory. Cortex. 2008;44:1010–1022. doi: 10.1016/j.cortex.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Saito Y, Nobuhara K, Okugawa G, Takase K, Sugimoto T, Horiuchi M, Ueno C, Maehara M, Omura N, Kurokawa H, Ikeda K, Tanigawa N, Sawada S, Kinoshita T. Corpus callosum in patients with obsessive-compulsive disorder: diffusion-tensor imaging study. Radiology. 2008;246:536–542. doi: 10.1148/radiol.2462061469. [DOI] [PubMed] [Google Scholar]
- Schmahmann J, Pandya D. Fiber Pathways of the Brain. Oxford University Press; New York: 2006. [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry. 1998;59(Suppl 20):22–33. [PubMed] [Google Scholar]
- Shinoura N, Suzuki Y, Tsukada M, Katsuki S, Yamada R, Tabei Y, Saito K, Yagi K. Impairment of inferior longitudinal fasciculus plays a role in visual memory disturbance. Neurocase. 2007;13:127–130. doi: 10.1080/13554790701399254. [DOI] [PubMed] [Google Scholar]
- Silverstein SM, Keane BP. Perceptual Organization Impairment in Schizophrenia and Associated Brain Mechanisms: Review of Research from 2005 to 2010. Schizophrenia Bulletin. 2011;37:690–699. doi: 10.1093/schbul/sbr052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Stejskal EO. Use of Spin Echoes in a Pulsed Magnetic-Field Gradient to Study Anisotropic Restricted Diffusion and Flow. Journal of Chemical Physics. 1965;43:3597. [Google Scholar]
- Szeszko PR, Ardekani BA, Ashtari M, Malhotra AK, Robinson DG, Bilder RM, Lim KO. White matter abnormalities in obsessive-compulsive disorder: a diffusion tensor imaging study. Archives of General Psychiatry. 2005;62:782–790. doi: 10.1001/archpsyc.62.7.782. [DOI] [PubMed] [Google Scholar]
- Torrey HC. Bloch Equations with Diffusion Terms. Physical Review. 1956;104:563–565. [Google Scholar]
- Veale D, Boocock A, Gournay K, Dryden W, Shah F, Willson R, Walburn J. Body dysmorphic disorder. A survey of fifty cases. British Journal of Psychiatry. 1996;169:196–201. doi: 10.1192/bjp.169.2.196. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Richardson MP, Armony JL, Driver J, Dolan RJ. Distant influences of amygdala lesion on visual cortical activation during emotional face processing. Nature Neuroscience. 2004;7:1271–1278. doi: 10.1038/nn1341. [DOI] [PubMed] [Google Scholar]
- Wakana S, Caprihan A, Panzenboeck MM, Fallon JH, Perry M, Gollub RL, Hua K, Zhang J, Jiang H, Dubey P, Blitz A, van Zijl P, Mori S. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage. 2007;36:630–644. doi: 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westin CF, Maier SE, Mamata H, Nabavi A, Jolesz FA, Kikinis R. Processing and visualization for diffusion tensor MRI. Medical Image Analysis. 2002;6:93–108. doi: 10.1016/s1361-8415(02)00053-1. [DOI] [PubMed] [Google Scholar]
- Yaryura-Tobias J, Neziroglu F, Chang R, Lee S, Pinto A, Donohue L. Computerized perceptual analysis of patients with body dysmorphic disorder. CNS Spectrums. 2002;7:444–446. doi: 10.1017/s1092852900017958. [DOI] [PubMed] [Google Scholar]
- Yoo SY, Jang JH, Shin YW, Kim DJ, Park HJ, Moon WJ, Chung EC, Lee JM, Kim IY, Kim SI, Kwon JS. White matter abnormalities in drug-naive patients with obsessive-compulsive disorder: a diffusion tensor study before and after citalopram treatment. Acta Psychiatrica Scandinavica. 2007;116:211–219. doi: 10.1111/j.1600-0447.2007.01046.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.