Abstract
EphA2 is a receptor tyrosine kinase (RTK) that triggers keratinocyte differentiation upon activation and subsequently down-regulation by ephrin-A1 ligand. The objective for this study was to determine if the EphA2/ephrin-A1 signaling axis was altered in psoriasis, an inflammatory skin condition where keratinocyte differentiation is abnormal. Microarray analysis of skin biopsies from psoriasis patients revealed increased mRNA transcripts for several members of this RTK family in plaques, including the EphA1, EphA2 and EphA4 subtypes prominently expressed by keratinocytes. Of these, EphA2 showed the greatest up-regulation, a finding that was confirmed by quantitative RT-PCR, IHC analysis and ELISA. In contrast, psoriatic lesions exhibited reduced ephrin-A ligand immunoreactivity. Exposure of primary keratinocytes induced to differentiated in high calcium or a 3-dimensiosnal raft culture of human epidermis to a combination of growth factors and cytokines elevated in psoriasis increased EphA2 mRNA and protein expression while inducing S100A7 and disrupting differentiation. Pharmacological delivery of a soluble ephrin-A1 peptidomimetic ligand led to a reduction in EphA2 expression and ameliorated proliferation and differentiation in raft cultures exposed to EGF and IL-1α. These findings suggest that ephrin-A1-mediated down-regulation of EphA2 supports keratinocyte differentiation in the context of cytokine perturbation.
INTRODUCTION
Eph receptors make up the largest family of receptor tyrosine kinases (RTKs) in humans (Eph Nomenclature Committee, 1997; Pasquale, 2005). These RTKs are grouped into EphA (1–8/10) and EphB (1–4/6) subtypes based on sequence homology and binding to glycosylphosphatidylinositol (GPI)-linked ephrin-A (1–5) or transmembrane ephrin-B (1–3) ligands that are anchored on neighboring cells. Eph/ephrin signaling complexes mediate developmental patterning events in embryonic tissues and the establishment of tumor boundaries in cancers (Batlle and Wilkinson, 2012; Lackmann and Boyd, 2008). This cell-cell communication pathway has also been implicated in maintaining homeostasis of adult epithelial tissues, including the epidermis, but relatively little is known about their roles in skin disease (Lin et al., 2011; Miao and Wang, 2009).
The mRNA transcripts for all members of the Eph/ephrin family can be detected in human skin but a more limited repertoire (i.e. receptors: EphA1/2/4, EphB3/B4; ligands: ephrin-A1/3/4, ephrin-B1–3) is present in cultured keratinocytes (Hafner et al., 2006; Walsh and Blumenberg, 2011b). A number of cellular processes can be modulated by Eph/ephrin signaling in keratinocytes, including proliferation, adhesion, migration, survival and differentiation (Egawa et al., 2009; Genander et al., 2010; Guo et al., 2006; Kaplan et al., 2012; Lin et al., 2010; Walsh and Blumenberg, 2011a, b; Yamada et al., 2008; Zhang et al., 2008). For example, ligand targeting of EphA2 with a soluble, recombinant ephrin-A1-Fc (EfnA1-Fc) protein triggers receptor activation and subsequent down-regulation leading to an increase in desmosome-mediated adhesion and keratinocyte differentiation (Lin et al., 2010). Although dispensable for normal cell cycle exit in culture, EphA2 can be harnessed by ephrins to negatively regulate keratinocyte proliferation (Genander et al., 2010; Guo et al., 2006; Walsh and Blumenberg, 2011b). Ligand-independent functions for EphA2 have also been described in tumor cells where it is frequently over-expressed (Beauchamp and Debinski, 2011; Miao and Wang, 2011). For example, EphA2 promotes glioma cell invasion in the absence of ligand whereas ephrin-A1 targets this receptor to inhibit cell migration, including in corneal keratinocytes (Kaplan et al., 2012; Miao et al., 2009). These findings raise the intriguing possibility that pharmacological approaches directed at EphA2 may dampen keratinocyte proliferation and bolster differentiation in pathological conditions.
Psoriasis is a common inflammatory skin disorder that has a critical immunological component involving multiple T cell subtypes, particularly of the Th1 and Th17 lineage, and is further characterized by striking changes in the epidermal compartment that include abnormal keratinocyte proliferation and differentiation (Di Meglio et al., 2011; Elder et al., 2010; Guttman-Yassky et al., 2011; Tschachler, 2007). Moreover, keratinocytes are key participants in the inflammatory cascade by not only responding to the cytokine milieu in lesions but also secreting their own immunomodulatory, chemotactic and innate host defense factors (Gutowska-Owsiak and Ogg, 2012; Swamy et al., 2010). Global gene expression studies of psoriatic lesions indicate that the expression of a number of Eph RTKs and ephrins may be altered in psoriasis (Jabbari et al., 2012; Kulski et al., 2005; Piruzian et al., 2009).
In view of these observations, we analyzed EPH/EFN gene expression levels in a large cohort of patients with psoriasis and validated changes for epidermal components within the A subclass. Focusing on the elevation of EphA2 in psoriatic lesions, we examined its regulation by growth factors and pro-inflammatory cytokines using primary human epidermal keratinocytes and a 3-D organotypic raft model of human epidermis. Finally, we tested the possibility of delivering soluble EfnA1-Fc to keratinocytes exposed to cytokines as a means to target EphA2 for activation and down-regulation in order to normalize differentiation.
RESULTS
EphA2 is down-regulated during keratinocyte differentiation
We previously showed that EphA2 is activated by ephrin-A ligand in a contact-dependent manner in keratinocytes and that delivery of additional, soluble ephrin-A1 peptide mimetic (i.e. EfnA1-Fc) enhances EphA2 activation and subsequent receptor down-regulation, leading to increased differentiation (Lin et al., 2010). While primary keratinocytes co-express EphA2 and ephrin-A1, we were unable to detect endogenous expression of the related ephrin-A3 in these primary cultures by Western blot analysis (Fig. S1). Moreover, increasing the levels of membrane-associated ephrin-A1 but not ephrin-A3 by ectopic over-expression was capable of efficiently reducing EphA2 levels, suggesting ephrin-A1 is a major ligand for activation and down-regulation of this receptor subtype in keratinocytes.
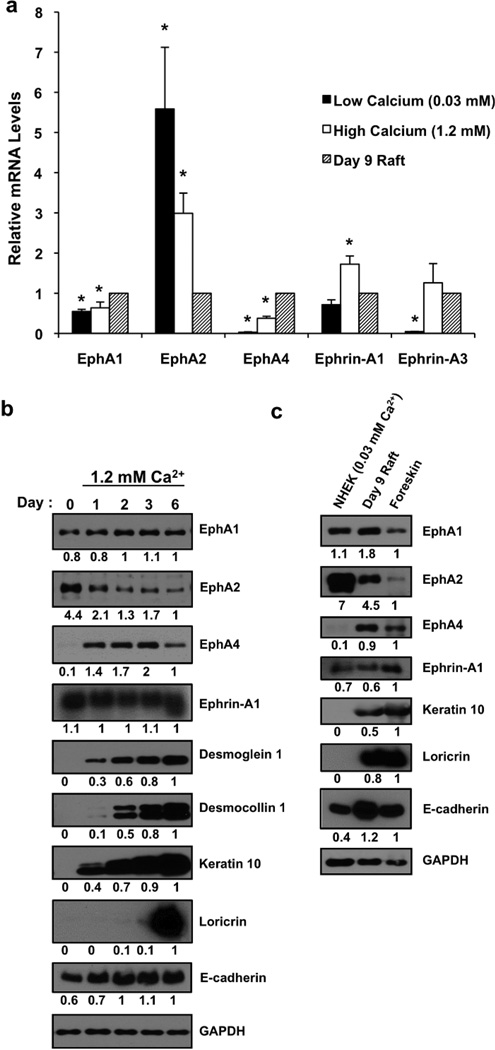
These findings led us to determine if the normal expression pattern of EphA2 changes during keratinocyte differentiation in vitro. Distinct from other epidermal EphA subtypes, EphA2 mRNA transcripts were markedly reduced in neonatal foreskin keratinocytes undergoing differentiation in response to elevated calcium (1.2 mM Ca2+) or in a 3-dimensional organotypic raft model of human epidermis (Fig. 1a). EphA2 along with EphA1 and ephrin-A1 were readily detectable by Western blotting in undifferentiated keratinocytes maintained in low (0.03 mM) Ca2+ (Fig. 1b). Raising the extracellular Ca2+ levels (1.2 mM) reduced EphA2 while EphA1 and ephrin-A1 remained abundantly expressed and EphA4 was markedly up-regulated upon induction of differentiation as reflected by increased levels of structural proteins present in the suprabasal layers (desmoglein 1; Dsg1, desmocollin 1; Dsc1, K10 and loricrin). Furthermore, EphA2 was down-regulated in epidermal raft cultures and even further reduced in extracts prepared from foreskin tissue whereas EphA1 and EphA4 remained high (Fig. 1c). Collectively, these results suggest that the relative abundance of ephrin-A1 ligand and state of keratinocyte differentiation impact on EphA2 expression levels in the epidermis.
Figure 1. Differentiation-dependent regulation of EphA RTKs in keratinocytes.
(a) Real-time qRT-PCR analysis of EphA (1/2/4) and ephrin-A (1/3) normalized to RPLP0 mRNA levels in keratinocytes isolated from a pool of neonatal foreskins (n=3) that were maintained in low (0.03 mM) Ca2+, switched into high (1.2 mM) Ca2+ for 3 days or grown as human epidermal raft cultures for 9 days. Results represent the mean (± SD) from three independent studies performed in duplicate and normalized to raft cultures (*P < 0.05). (b) Western blot analysis of EphA subtypes (1/2/4), ephrin-A1 and epidermal structural proteins present in the suprabasal layers (desmoglein 1, desmocollin 1, keratin 10, loricrin) in post-confluent primary cultures of human epidermal keratinocytes maintained in low (0.03 mM) Ca2+ (Day 0) or switched into high (1.2 mM) Ca2+ for 1, 2, 3 or 6 days. E-cadherin was analyzed as a structural protein present in keratinocytes at all stages of differentiation while GAPDH was used as a control for protein loading and to normalize representative densitometry values for protein band intensities relative to day 6 cultures. (c) Western blot analysis of keratinocytes maintained in low Ca2+ or grown as human epidermal raft cultures at an air-liquid interface for 9 days as compared to protein extracts prepared from human neonatal foreskin.
EphA2 is increased in psoriatic epidermis
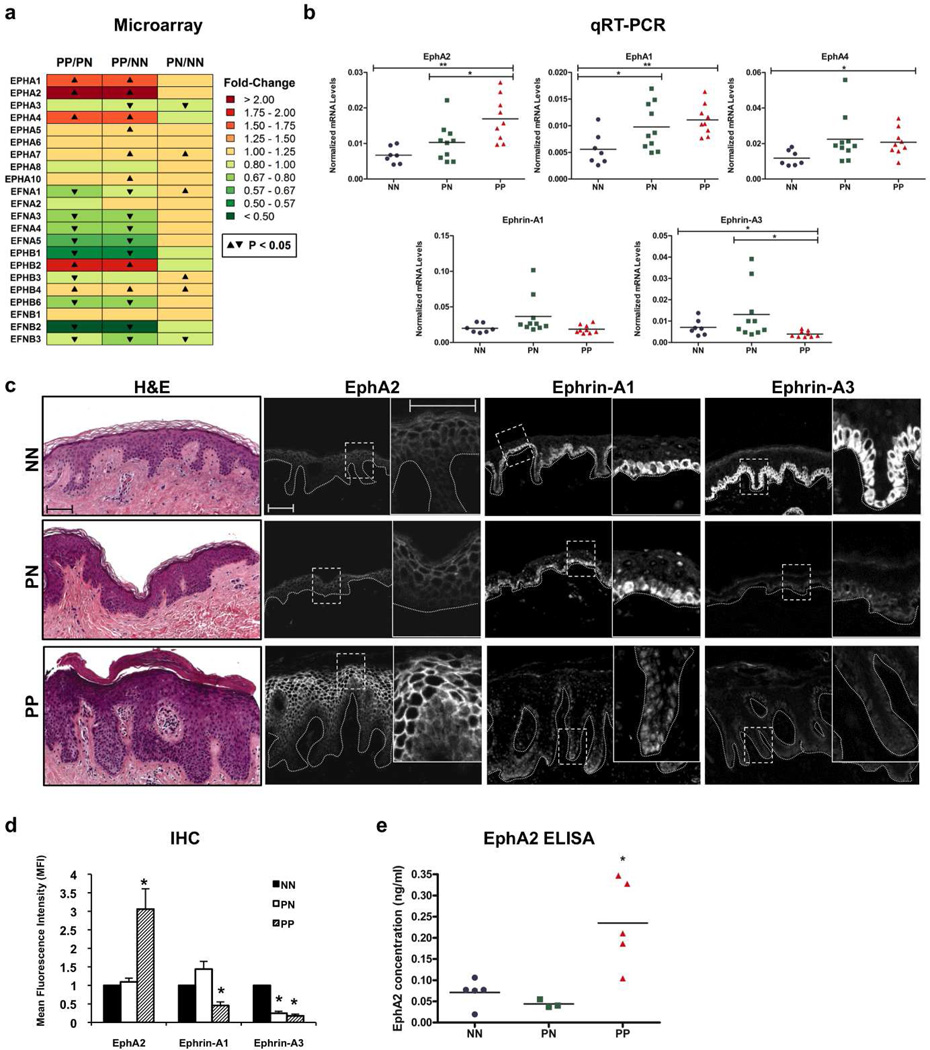
Microarray studies indicate that EphA2 is increased in psoriatic plaques where keratinocyte differentiation is impaired (Jabbari et al., 2012; Kulski et al., 2005; Piruzian et al., 2009). To examine EPH/EFN gene expression in psoriasis in more detail, we first used a previously generated microarray data set to analyze the skin of normal individuals (n=64) compared to paired biopsies obtained from uninvolved and lesional areas of patients with psoriasis (n=58) (Gudjonsson et al., 2010a). A number of changes for these receptors and ligands were revealed when comparing psoriatic plaques (PP) with either non-lesional (PN) or normal (NN) controls (Fig. 2a, Fig. S2 and supplementary Table 1). Of the 14 Eph RTK family members, EPHA2 showed the greatest increase in psoriatic plaques. Interestingly, the other EphA subtypes expressed by keratinocytes (EPHA1, EPHA4) were also elevated in lesional skin. In contrast, the mRNA transcripts for epidermal ephrin-A ligands (EFNA1, EFNA3, EFNA4) were decreased in psoriatic plaques, particularly when compared to uninvolved patient skin. Similar to epidermal ephrin-A ligands, EFNB2 and EFNB3 were reduced in lesional skin. Several EphB family members were also altered in psoriatic plaques, the most striking being an increase in EPHB2 as well as a decrease in EPHB1 and EPHB6; these particular receptor subtypes happen not to be concentrated in keratinocytes (Walsh and Blumenberg, 2011b).
Figure 2. Increased EphA2 levels in the epidermis of psoriatic lesions.
(a) A heat-map of gene expression changes for the human EPH/EFN gene families as analyzed by Affymetrix Human Genome U133 2.0 microarray using skin biopsies obtained from 64 normal (NN) or 58 psoriasis patients from non-lesional sites (PN) or lesional plaques (PP). The mean fold-changes (0.25 FC increments) obtained from these samples are represented in the color-coded histogram (inset) where Red = increased expression and Green = decreased expression. FDR was used to correct for multiple testing and indicates P < 0.05. (b) Real-time qRT-PCR analysis of EphA (1/2/4) and ephrin-A (1/3) normalized to RPLP0 mRNA levels using 7 normal (NN; blue circles) and 10 paired PN (green squares) or PP (red triangles) samples. Values are expressed as arbitrary units on the dot plot (mean horizontal bar; *P < 0.05; ** P < 0.005). (c) Representative images of H&E staining and IHC analysis of EphA2, ephrin-A1 and ephrin-A3 (n=4) with higher magnification insets. The dotted line highlights the basement membrane zone. (Scale bar = 50 µm). (d) Mean fluorescence intensity (MFI) of EphA2 in the suprabasal layers and ephrin-A1 or ephrin-A3 in the basal layer. Error bars (± SE) reflect the variation in pixel intensity among tissue sections (*P < 0.05). (e) ELISA for EphA2 performed using protein lysates from 5 NN (blue circles), 3 PN (green squares) or 5 PP (red triangles) skin biopsy samples. Values are expressed in a dot plot format as protein concentration based on a standard curve (mean horizontal bar; *P < 0.05).
Given the prominent changes observed in the epidermal components of the EphA/ephrin-A subfamily, we focused our analysis on EphA1, EphA2, EphA4 as well as ephrin-A1 and ephrin-A3 since these two ligands differ in their ability to down-regulate EphA2 (Fig. S1) and have somewhat distinct signaling functions in keratinocytes (Walsh and Blumenberg, 2011b). To validate the microarray differences, we performed qRT-PCR using skin biopsies from 7 normal controls and 10 patients from uninvolved or lesional regions (Fig. 2b). The increase in EphA2 mRNA transcripts in psoriatic plaques was confirmed when compared to uninvolved or normal skin (PP vs PN=1.64 FC; PP vs NN=2.52 FC). EphA1 (PP vs NN=1.98 FC) and EphA4 (PP vs NN=1.77 FC) mRNA levels were also higher in plaques but only when compared to healthy controls. Finally, ephrin-A1 and ephrin-A3 mRNA levels tended to be lower in psoriatic plaques compared to uninvolved areas of patient skin but this only achieved statistical significance in the latter case (PP vs PN: EFNA1 0.51 FC; P < 0.07; EFNA3 0.30 FC; P < 0.05).
To determine if EphA2 expression was increased at the protein level in the epidermis of psoriatic plaques, we performed IHC analysis for this RTK along with ephrin-A1 and ephrin-A3 (Fig. 2c and d). Although detectable at the periphery of keratinocytes from normal individuals and uninvolved patient skin, particularly in the suprabasal layers, EphA2 immunoreactivity was markedly increased in psoriatic lesions. An ELISA for EphA2 confirmed its increased expression in plaques (Fig. 2e). Ephrin-A1, which is normally concentrated in basal keratinocytes (Guo et al., 2006), was reduced in the basal layer and occasionally found in the suprabasal layers of psoriatic plaques. While ephrin-A3 immunoreactivity was reduced in the basal layer of psoriatic plaques, this ligand was also decreased in non-lesional epidermis where EphA2 levels remain low. These observations suggested that the EphA2/ephrin-A1 signaling axis is disrupted in psoriatic epidermis, leading us to focus on this particular receptor-ligand pair in keratinocyte cultures models.
EphA2 is elevated by EGF and pro-inflammatory cytokines in differentiated keratinocytes
EphA2 levels are increased when the EGFR-Erk1/2 signaling pathway is activated in cancer cell lines (Larsen et al., 2007; Macrae et al., 2005). Interestingly, several EGFR ligands are present at high levels in psoriatic plaques and have been shown to act in concert with IL-1α on keratinocytes to regulate antimicrobial defense proteins elevated in this inflammatory disease, including S100A7/psoriasin (Johnston et al., 2011a; Madsen et al., 1991; Mee et al., 2007). Previous microarray studies indicated that EphA2 and ephrin-A1 are also up-regulated by TNFα in keratinocytes (Banno et al., 2004). Moreover, treating keratinocytes with a combination of TNF-α and IL-17A captures additional elements of the psoriatic transcriptome when compared to individual cytokines (Chiricozzi et al., 2011). These studies indicated that EphA2 and ephrin-A1 expression in keratinocytes may be regulated, at least in part, by growth factors and cytokines elevated in psoriatic lesions.
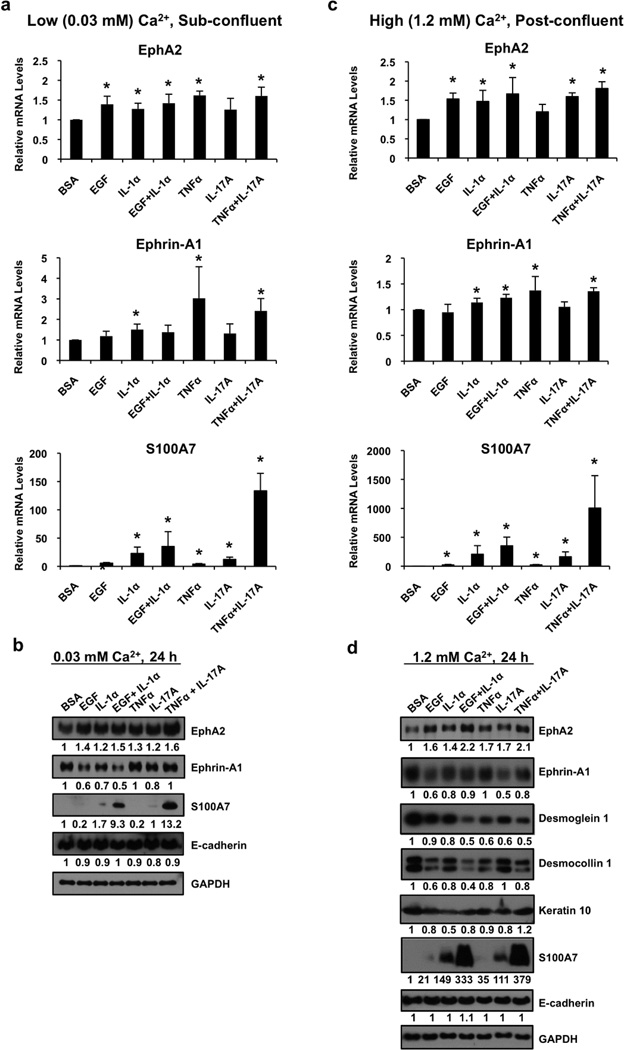
Since EphA2 mRNA and protein levels vary with differentiation, we addressed the possibility that growth factors and cytokines regulate receptor expression using neonatal foreskin keratinocyte cultures maintained at two distinct stages of differentiation: 1) relatively undifferentiated keratinocytes maintained as sub-confluent cultures in low (0.03 mM) Ca2+ or 2) relatively differentiated keratinocytes maintained as post-confluent cultures in high (1.2 mM) Ca2+ (Fig. 1b). After 24 h of growth factor depletion, keratinocytes were treated for 24 h with EGF, IL-1α, TNF-α, or IL-17A (10 ng/ml each) alone or in combinations known to act in a synergistic manner (Chiricozzi et al., 2011; Johnston et al., 2011a). Accordingly, S100A7 mRNA and protein expression was maximally up-regulated by combined treatment with EGF and IL-1α or TNFα and IL-17A, particularly in the latter condition under high Ca2+ conditions (Fig. 3a–d). While the majority of these factors marginally increased EphA2 mRNA levels, this effect was most pronounced when differentiated keratinocytes where treated in combination with EGF and IL-1α or TNFα and IL-17A (Fig. 3a/c). In contrast, ephrin-A1 mRNA transcripts were maximally up-regulated by TNFα treatment alone in undifferentiated keratinocytes, which is in line with its discovery as a TNFα -inducible gene product in endothelial cells (Holzman et al., 1990). Post-confluent adult keratinocyte cultures were also treated with increasing doses of IL-1α, IFN-γ, TNF-α, IL-17A or IL-22 as previously described (Gudjonsson et al., 2010b; Johnston et al., 2011b). Compared to other epidermal EphA/ephrin-A family members, EphA2 was the most dramatically up-regulated receptor when adult keratinocytes were stimulated with increasing concentrations of IL-1α, IFN-γ or TNF-α but not IL-17A or IL-22 (Fig. S3).
Figure 3. EGF and pro-inflammatory cytokines interfere with the differentiation-dependent down-regulation of EphA2 in keratinocytes.
(a) qRT-PCR analysis of EphA2, ephrin-A1 and S100A7 from sub-confluent, neonatal foreskin keratinocytes stimulated alone or with a combination of 10 ng/ml EGF and IL-1α or TNFα and IL-17A for 24 h. Results represent the mean (± SD) from three independent studies performed in duplicate and normalized to BSA controls (*P < 0.05). (b) Western blot analysis from corresponding low Ca2+ cultures analyzed for EphA2, ephrin-A1, S100A7 along with E-cadherin and GAPDH as a loading control. A similar treatment regimen was employed following differentiation of post-confluent keratinocytes switched into high (1.2 mM) Ca2+ for 24 h and then stimulated with these factors for an additional 24 h. qRT-PCR (c) and Western blot analysis (d) are shown for the indicated proteins and include markers of keratinocyte differentiation.
We next asked whether EphA2 protein expression was altered by growth factor and cytokine treatment in undifferentiated keratinocytes that express high levels of this receptor or differentiated keratinocytes that express low levels of this receptor. EphA2 protein was slightly increased by EGF, IL-1α, TNFα and IL-17A under low Ca2+ conditions (Fig. 3b) and more dramatically elevated under Ca2+-induced differentiation conditions (Fig. 3d), particularly when treated with a combination of factors. The increase of EphA2 in response to these cytokine combinations was associated with reduced differentiation and increased S100A7 expression (Fig. 3d).
EGF and cytokine exposure of human epidermal raft cultures disrupts EphA2/ephrin-A1 levels
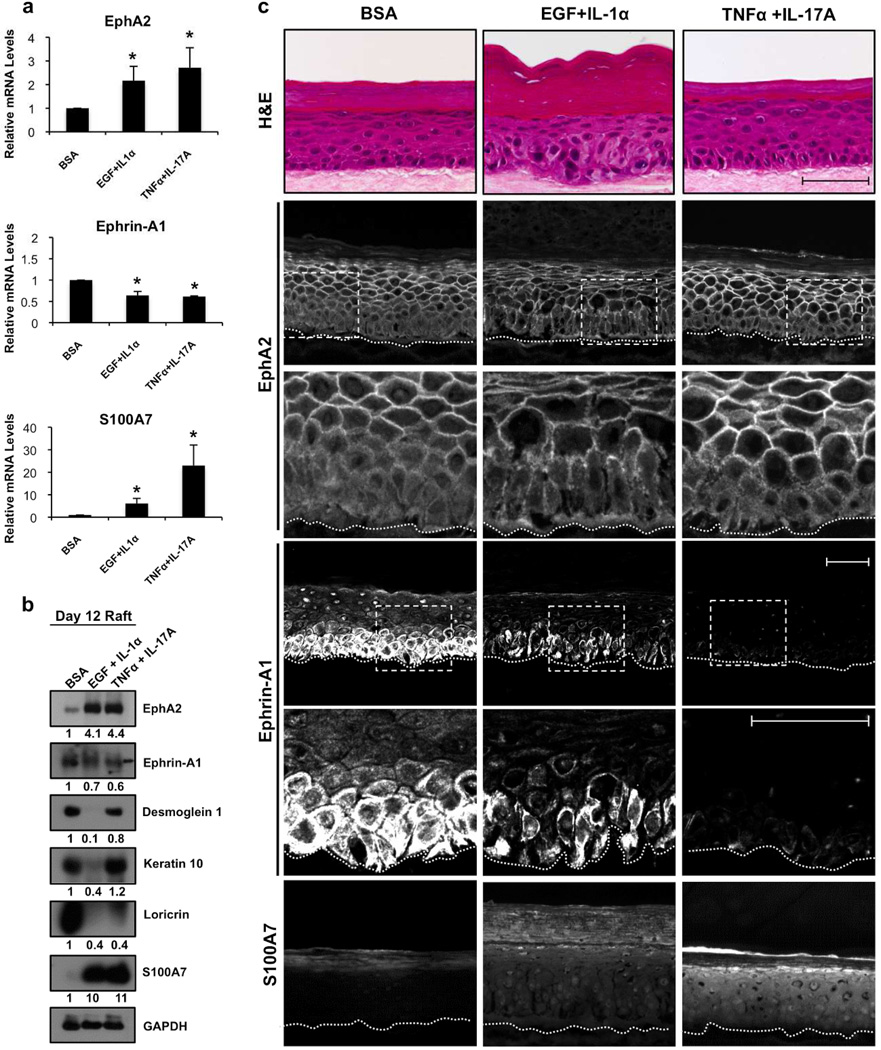
To assess cytokine-induced changes in the EphA2/ephrin-A1 signaling axis in a more physiologically relevant model, we treated 3-dimensional epidermal raft cultures with EGF and IL-1α or TNFα and IL-17A for 72 h. EphA2 mRNA and protein levels were markedly up-regulated following cytokine exposure (Fig. 4a/b) to a greater extent than what we observed in 2-dimensional keratinocyte models (Fig. 3). EphA2 normally exhibited a relatively diffuse distribution in the basal layer and was concentrated at cell-cell contacts in the suprabasal layers whereas the addition of EGF and/or cytokines enhanced receptor localization at the cell periphery in basal keratinocytes (Fig. 4c). We also noted a decrease in ephrin-A1 mRNA and protein levels following cytokine exposure. Finally, S100A7 mRNA transcripts were elevated to a greater extent in TNFα and IL-17A stimulated cultures while protein was detectable at low levels under control conditions and markedly enhanced under both cytokine treatment regimens.
Figure 4. Impaired EphA2/ephrin-A1 expression in an organotypic raft model of human epidermis exposed to EGF and cytokines.
(a) qRT-PCR analysis of EphA2, ephrin-A1 and S100A7 mRNA in 9 day old raft cultures (n=3) treated with BSA, EGF and IL-1α or TNFα and IL-17A (10 ng/ml each) for an additional 72 h. (b) Western blot analysis from the corresponding raft cultures examined for these proteins and indicators of keratinocyte differentiation. (c) Representative H&E and IHC images of EphA2, ephrin-A1, and S100A7 from these raft cultures along with higher magnification, zoomed images shown from the outlined areas. The dotted line highlights the basement membrane zone. (Scale bar = 50 µm).
Consistent with a previous study (Johnston et al., 2011a), EGF and IL-1α exposure grossly impacted epidermal morphology with disrupted cellular organization in the basal and spinous layers, a paucity of keratohyalin granules and an expansion of the stratum corneum (Fig. 4c; H&E). Accordingly, K10, Dsg1 and loricrin expression was reduced in these raft cultures (Fig. 4b). In contrast, the morphological and differentiation-associated changes in TNF-α and IL-17A stimulated raft cultures were relatively minor compared to EGF and IL-1α exposure despite similar induction in EphA2 expression, suggesting that the increase in EphA2 by itself is not sufficient to impair differentiation. We also tested the effects of a combination of factors (i.e. IL-1α, TNF-α, and IL-6) previously used to model psoriasis in 3-D organotypic cultures (Tjabringa et al., 2008) and found this elevated EphA2 and S100A7 expression while impairing differentiation (Fig. S4). Collectively, these data show that a variety of cytokine insults associated with psoriasis are capable of altering the EphA2/ephrin-A1 signaling axis in a raft model of human epidermis.
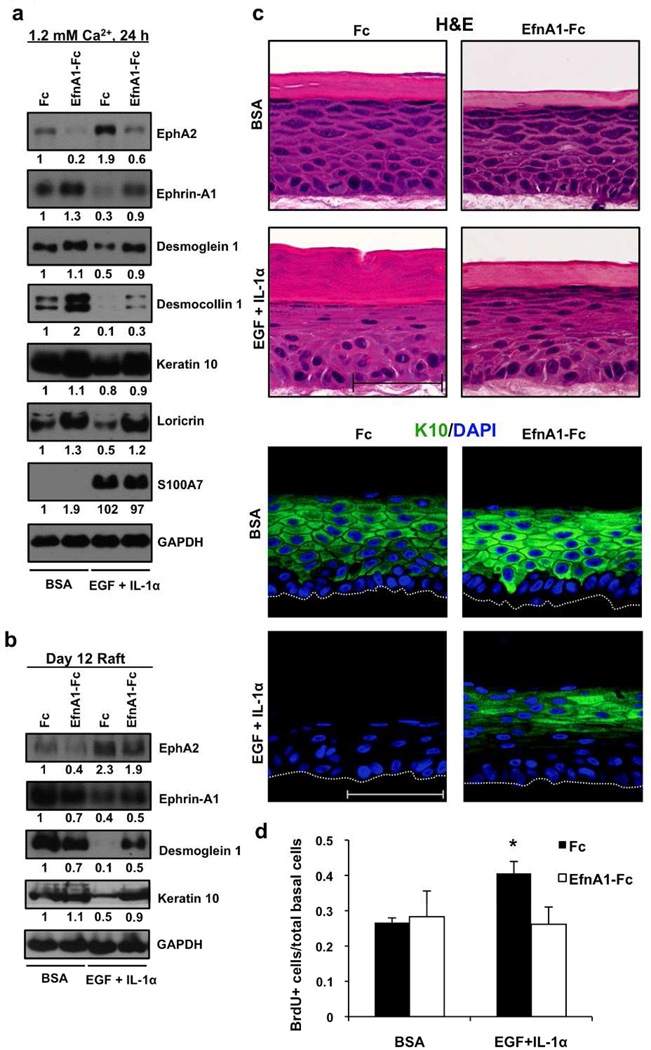
Ligand targeting of EphA2 in cytokine-exposed keratinocytes enhances differentiation
To test the possibility that ephrins can limit the differentiation defects induced by EGF and IL-1α, we first switched keratinocytes from low to high Ca2+ in the presence of 1.0 µg/ml EfnA1-Fc for 24 h. Subsequently, we treated these keratinocytes with EGF and IL-1α for an additional 24 h. As expected, cytokine exposure of Fc control cultures increased EphA2 and inhibited differentiation (Fig. 5a). Pretreatment with the EfnA1-Fc peptide reduced the levels of EphA2 and was capable of increasing Dsg1, Dsc1, K10 and loricrin levels but did not prevent the induction of S100A7. Moreover, delivery of soluble ephrin ligand to epidermal raft cultures limited differentiation defects associated with EGF and IL-1α exposure (Fig. 5b) and led to the morphological restitution of the spinous, granular and cornified layers (Fig. 5c). Finally, EfnA1-Fc treatment interfered with the ability of EGF and IL-1α to aberrantly increase proliferation in raft cultures (Fig. 5d). We conclude from these studies that ligand targeting of EphA2 can ameliorate the proliferation and differentiation disrupting effects of these particular inflammatory-associated growth factors and cytokines on keratinocytes.
Figure 5. Ephrin-A1 ligand targeting of EphA2 increases differentiation of keratinocytes exposed to cytokines.
Western blot analysis of post-confluent keratinocytes switched into 1.2 mM Ca2+ with 1.0 µg/ml Fc or EfnA1-Fc peptide for 24 h, then treated with BSA or EGF and IL-1α (10 ng/ml each) for 24 h (a) or 9 day old raft cultures treated simultaneously with these factors for an additional 72 h (b). (c) Representative H&E and keratin10 IHC images of raft cultures (Scale bar = 50 µm). DAPI was used to stain nuclei. (d) The mean value (± SD) of BrdU positive cells relative to total basal cells from three independent studies with > 400 cells analyzed in each experiment is summarized in the bar graph (*P < 0.05).
DISCUSSION
Psoriasis is a complex inflammatory skin disease that has a major immunological component but also involves changes in epidermal architecture and keratinocyte differentiation (Di Meglio et al., 2011; Elder et al., 2010; Guttman-Yassky et al., 2011; Tschachler, 2007). Yet, it remains largely unclear why keratinocyte differentiation is abnormal and much remains to be revealed about the signaling pathways that are dysregulated in psoriatic plaques. We found that EPH/EFN gene expression is altered in psoriatic lesions and focused on EphA2 due to its most prominent up-regulation in plaques. Although abundant in primary keratinocytes along with the related EphA1 and EphA4, EphA2 was uniquely reduced during differentiation. Moreover, ephrin-A1 targeting of EphA2 leads to its activation and down-regulation to promote keratinocyte differentiation (Lin et al., 2010). In view of these observations, we propose a model for EphA2/ephrin-A1 actions in epidermal homeostasis, in which ephrin-A1 ligand down-regulates EphA2 to allow for normal differentiation. In psoriatic lesions, we suspect that impaired keratinocyte differentiation, reduced ephrin-A1 ligand expression and the integrated actions of growth factors and pro-inflammatory cytokines work collectively to up-regulate EphA2. Our analysis of EphA2 in models of keratinocyte differentiation exposed to cytokines suggests it may be possible to compensate for a lack of ligand using a recombinant ephrin peptide mimetic to target this RTK and bolster the differentiation program.
Within psoriatic lesions, keratinocytes not only exhibit abnormal differentiation but also increased proliferation (Leigh et al., 1985; Tschachler, 2007; Weinstein et al., 1985). Interestingly, the delivery of an EfnA1-Fc peptide limited keratinocyte colony growth and suppressed proliferation-associated genes, in line with the ability of these ligands to negatively regulate proliferation in mouse epidermis (Genander et al., 2010; Guo et al., 2006; Walsh and Blumenberg, 2011b). While EfnA1-Fc treatment had no significant effect on keratinocyte proliferation in control raft cultures where EphA2 levels are low, these soluble ligands were capable of interfering with EGF and IL-1α-induced hyperproliferation. Consequently, ephrin-directed therapies for psoriasis may not only bolster keratinocyte differentiation but also dampen proliferation in lesional epidermis.
Alterations in the Eph/ephrin signaling axis in psoriasis are consistent with their regulation during inflammation in other organs. For example, EphA2 is rapidly increased in response to lipopolysaccharide-induced inflammation in the rat lung and liver followed by a down-regulation of EphA1 (Ivanov et al., 2005). In contrast, ephrin-A1 is transiently down-regulated and subsequently increased along with ephrin-A3 at later stages. Moreover, EphA2 and ephrin-A1 are coordinately induced by pro-inflammatory cytokines (i.e. TNF-α and IL-1β) in endothelial cells (Funk et al., 2012). Hence, alterations in the EphA2/ephrin-A1 signaling axis likely occur in multiple cellular compartments of the skin during inflammation and may even play a role in normal wound healing where pro-inflammatory cytokines and EGF ligands are increased (Barrientos et al., 2008; Marikovsky et al., 1993).
A number of signaling pathways suppressed by ephrin-A1 may allow keratinocytes to resist the deleterious effects of cytokines on differentiation. For example, Erk1/2-MAPK signaling downstream of EGFR is dampened to allow for differentiation and becomes elevated in psoriatic lesions (Haase et al., 2001; Takahashi et al., 2002). Ephrin-mediated inhibition of Erk1/2-MAPK signaling in keratinocytes (Guo et al., 2006; Lin et al., 2010) can potentially suppress abnormal signaling elicited by EGFR. Since EGFR-Erk1/2 signaling also increases EPHA2 gene expression, EphA2 likely serves in a negative feedback loop that dampens Erk1/2-MAPK signaling after ephrin binding (Larsen et al., 2007; Macrae et al., 2005; Miao et al., 2001). Microarray analysis suggests that the EfnA1-Fc peptide can reduce the expression of genes targeted by the NFκB pathway, which is an important mediator of skin inflammation (Chan et al., 2006; Walsh and Blumenberg, 2011b). S100A7, a target of NFκB regulation (Chiricozzi et al., 2011; Zaba et al., 2007), was not reduced by the delivery of the EfnA1-Fc peptide. However, it is possible that increasing the levels of native ephrin-A1 in the epidermis can render keratinocytes resistant to cytokine insults and possibly even limit their ability to participate in an inflammatory cascade inherent in psoriasis.
MATERIALS AND METHODS
Study Population
Subjects for gene expression analysis were enrolled using a protocol approved by the Univ. Michigan Institutional Review Board (IRB; HUM00037994) as previously described (Gudjonsson et al., 2010a); this study group includes 64 normal controls and 58 psoriasis patients with at least one well demarcated plaque covering > 1% of the total body surface area. For immunohistochemistry (IHC) studies, biopsies were obtained from four additional subjects that provided written, informed patient consent to the IRB-approved Northwestern Univ. (NU) Dermatology Tissue Repository. Patients had not received systemic treatment for at least 2 weeks or topical treatment for at least 1 week prior to the biopsy. This study was conducted in compliance with good clinical practice and according to the Declaration of Helsinki Principles.
Microarrays
Microarray data and statistical analysis was performed using control (NN) or paired non-lesional (PN) and lesional (PP) skin specimens from psoriasis patients as previously described (Gudjonsson et al., 2010a). Raw data has been deposited in the Gene Expression Omnibus database and is available under accession GSE13355. For each EPH/EFN gene, a representative probe set was chosen as that which yielded the most significant result among the three comparisons of interest (PP vs NN, PP vs PN and PN vs NN). Heat-maps for the changes in the Eph/ephrin family were generated using the R statistical software package.
Cell Culture and Retroviral Gene Expression
To help account for potential donor variability, most studies were conducted using human neonatal keratinocytes isolated from a pool (3–5 individuals) of foreskins and maintained in Medium 154 supplemented with human keratinocyte growth factor (M154 + HKGS; Invitrogen/Cascade Biologics, Portland, OR) and 0.07 mM calcium chloride (Ca2+). Sub-confluent keratinocyte cultures were switched into low (0.03 mM) Ca2+ to limit differentiation or seeded to confluency and switched into high (1.2 mM) Ca2+ to induce differentiation as previously described (Lin et al., 2010). Additional cytokine stimulation experiments were performed using human adult keratinocyte cultures established as described (Elder et al., 1992) and maintained in M154 + HKGS and 0.1 mM Ca2+. For all cytokine treatment studies, keratinocytes were starved of growth factors for 24 h prior to stimulation with the indicated combination and concentration of recombinant murine EGF (Chemicon), IL-1α (Peprotech), IFN-γ, TNF-α, IL-6, IL-17A, IL-22 (R&D systems) or 0.1 % BSA/PBS (Sigma Aldrich, St. Louis, MO) as a vehicle control. For ligand targeting experiments, keratinocytes were incubated in the presence of 1.0 µg/ml recombinant EfnA1-Fc chimera (R&D systems) or human Fc alone (Jackson ImmunoResearch Laboratories) The 3-D organotypic raft models of human epidermis were established as previously described (Getsios et al., 2009; Simpson et al., 2010).
To ectopically express ephrin-A ligands in keratinocytes, a full-length human ephrin-A1 cDNA was obtained from W. Debinski (Wake Forest University Medical Center; Winston-Salem, NC) (Kaplan et al., 2012; Wykosky et al., 2007) and a full-length murine ephrin-A3 cDNA was obtained from Y. Yamaguchi (Burnham Institute for Medical Research; La Jolla, CA) (Irie et al., 2008). These ephrin cDNAs were subcloned into the pLZRS-Linker vector (Denning et al., 2002). Retroviral supernatants were generated to transduce keratinocytes as previously described (Getsios et al., 2009).
Real Time Quantitative Reverse Transcription PCR (qRT-PCR)
Total RNA was extracted using the RNeasy Mini kit (Qiagen) and reverse transcribed (High Capacity cDNA Transcription kit, Applied Biosystems Inc., Foster City, CA) as previously described (Johnston et al., 2011a). Taqman primer sets for EphA1/2/4, ephrin-A1/3 or S100A7 were purchased from Applied Biosystems (see supplementary Table 2). All values were normalized to the expression of the housekeeping gene, ribosomal protein, large, P0 (RPLP0) (Minner and Poumay, 2009). A paired Student’s t-test was used to compare non-lesional and lesional biopsies from psoriasis patients. An unpaired t-test was used for all other comparisons with P < 0.05 values considered significant.
Western Blot Analysis and ELISA
Total protein was extracted using 8 M urea sample buffer as previously described (Lin et al., 2010). Immunoblots were probed using antibodies directed against the following proteins: EphA2 (D7; Millipore), EphA1 (AF638; R&D systems), EphA4 (S-20-Santa Cruz Biotech), ephrin-A1 (V-18; Santa Cruz Biotech), ephrin-A3 (K19; Santa Cruz), keratin 10 (RBK10; gift from J. Serge, National Institute of Health), Dsg1 (27B2; Zymed), Dsc1 (U100; Progen), loricrin (PRB-145P; Covance), S100A7 (HID5; Imgenex), E-cadherin (HECD-1; Invitrogen) GAPDH (ab9485; Abcam). Relative band intensities for proteins were normalized to GAPDH and calculated using ImageJ software (National Institutes of Health, Bethesda, MD).
RIPA-soluble protein extracts were prepared from skin biopsies of healthy individuals (NN; n=5) and psoriasis patients (PN, n=3; PP, n=5) as previously described (Gudjonsson et al., 2010b) and analyzed by an ELISA for EphA2 according to the manufacturer’s instructions (R&D Systems).
Histology, Immunohistochemistry (IHC) and BrdU Incorporation Studies
For histology, formalin-fixed, paraffin-embedded tissue sections were stained with hematoxylin and eosin (H&E). For IHC, OCT-embedded frozen tissue sections were fixed with 4% paraformaldehyde and immunostained as previously described (Kaplan et al., 2012) using the following antibodies: EphA2 (AF3035; R&D Systems), ephrin-A1 (V18), ephrin-A3 (H-90; Santa Cruz), K10 (LH2; Chemicon), and S100A7 (HID5; Imgenex). IHC imaging was performed using an epifluorescence Zeiss Axiovision Z1 microscope containing an Apotome slide module and a high resolution AxioCam MRm digital camera. Image analysis was performed with Zeiss AxioVision software. ImageJ software (National Institutes of Health; Bethesda, MD) was used to calculate the pixel intensity of ephrin-A1, ephrin-A3 or EphA2 from the basal or suprabasal layers of the epidermis, respectively (n=4); these values were normalized for total area to calculate the mean fluorescence intensity (MFI).
To assess proliferation following ephrin treatment in the 3-dimensional model of human epidermis, raft cultures were incubated in the presence of 10 µM BrdU (Sigma) for 60 min prior to formalin fixation and immunostaining for this nucleoside as previously described (Getsios et al., 2009). The total number of BrdU positive cells divided by total number of basal cells was calculated from multiple (>10) image frames with > 400 total cells counted from each experiment (n=3).
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Julie Segre (National Human Genome Research Institute) for the K10 antibody, Stefan Stoll (University of Michigan) for adult keratinocytes, Waldemar Debinski (Wake Forest) for the ephrin-A1 cDNA and Yu Yamaguchi (Burnham Institute for Medical Research) for the ephrin-A3 cDNA. The NU-SDRC assisted in the establishment and morphological analyses of keratinocyte cultures with support from the National Institutes of Health (NIH) National Institute of Arthritis and Musculoskeletal and Skin (NIAMS) Diseases grant AR057216. This research was supported by an NIH/NIAMS grant (R01-AR062110) and American Skin Association (ASA) Research Scholar Award to S.G.; the NIH K08 AR060802 and the A. Alfred Taubman Medical Research Institute Kenneth and Frances Eisenberg Emerging Scholar award to J.E.G.; and an ASA Research Scholar Award and the Babcock Endowment Fund to A.J.
Abbreviations
- BSA
bovine serum albumin
- EGFR
Epidermal growth factor receptor
- Eph
Erythropoietin producing hepatocyte-receptor
- Erk1/2
extracellular signal-regulated kinase 1/2
- FC
fold change
- GPI
glycosylphosphatidylinositol
- IL
interleukin
- IFN-γ
interferon-γ
- MAPK
mitogen activated protein kinase
- NF-κB
nuclear factor-κB
- TNF-α
Tumor necrosis factor-α
- RTK
receptor tyrosine kinase
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
REFERENCES
- Banno T, Gazel A, Blumenberg M. Effects of tumor necrosis factor-alpha (TNF alpha) in epidermal keratinocytes revealed using global transcriptional profiling. J Biol Chem. 2004;279:32633–32642. doi: 10.1074/jbc.M400642200. [DOI] [PubMed] [Google Scholar]
- Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16:585–601. doi: 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- Batlle E, Wilkinson DG. Molecular mechanisms of cell segregation and boundary formation in development and tumorigenesis. Cold Spring Harb Perspect Biol. 2012;4 doi: 10.1101/cshperspect.a008227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp A, Debinski W. Ephs and ephrins in cancer: Ephrin-A1 signalling. Semin Cell Dev Biol. 2011 doi: 10.1016/j.semcdb.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JR, Blumenschein W, Murphy E, Diveu C, Wiekowski M, Abbondanzo S, et al. IL-23 stimulates epidermal hyperplasia via TNF and IL-20R2-dependent mechanisms with implications for psoriasis pathogenesis. J Exp Med. 2006;203:2577–2587. doi: 10.1084/jem.20060244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiricozzi A, Guttman-Yassky E, Suarez-Farinas M, Nograles KE, Tian S, Cardinale I, et al. Integrative responses to IL-17 and TNF-alpha in human keratinocytes account for key inflammatory pathogenic circuits in psoriasis. J Invest Dermatol. 2011;131:677–687. doi: 10.1038/jid.2010.340. [DOI] [PubMed] [Google Scholar]
- Denning MF, Wang Y, Tibudan S, Alkan S, Nickoloff BJ, Qin JZ. Caspase activation and disruption of mitochondrial membrane potential during UV radiation-induced apoptosis of human keratinocytes requires activation of protein kinase C. Cell Death Differ. 2002;9:40–52. doi: 10.1038/sj.cdd.4400929. [DOI] [PubMed] [Google Scholar]
- Di Meglio P, Perera GK, Nestle FO. The multitasking organ: recent insights into skin immune function. Immunity. 2011;35:857–869. doi: 10.1016/j.immuni.2011.12.003. [DOI] [PubMed] [Google Scholar]
- Egawa G, Osawa M, Uemura A, Miyachi Y, Nishikawa S. Transient expression of ephrin b2 in perinatal skin is required for maintenance of keratinocyte homeostasis. J Invest Dermatol. 2009;129:2386–2395. doi: 10.1038/jid.2009.105. [DOI] [PubMed] [Google Scholar]
- Elder JT, Astrom A, Pettersson U, Tavakkol A, Krust A, Kastner P, et al. Retinoic acid receptors and binding proteins in human skin. J Invest Dermatol. 1992;98:36S–41S. doi: 10.1111/1523-1747.ep12462180. [DOI] [PubMed] [Google Scholar]
- Elder JT, Bruce AT, Gudjonsson JE, Johnston A, Stuart PE, Tejasvi T, et al. Molecular dissection of psoriasis: integrating genetics and biology. J Invest Dermatol. 2010;130:1213–1226. doi: 10.1038/jid.2009.319. [DOI] [PubMed] [Google Scholar]
- Eph Nomenclature Committee. Unified nomenclature for Eph family receptors and their ligands, the ephrins. Eph Nomenclature Committee. Cell. 1997;90:403–404. doi: 10.1016/s0092-8674(00)80500-0. [DOI] [PubMed] [Google Scholar]
- Funk SD, Yurdagul A, Jr, Albert P, Traylor JG, Jr, Jin L, Chen J, et al. EphA2 Activation Promotes the Endothelial Cell Inflammatory Response: A Potential Role in Atherosclerosis. Arterioscler Thromb Vasc Biol. 2012 doi: 10.1161/ATVBAHA.111.242792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genander M, Holmberg J, Frisen J. Ephrins negatively regulate cell proliferation in the epidermis and hair follicle. Stem Cells. 2010;28:1196–1205. doi: 10.1002/stem.442. [DOI] [PubMed] [Google Scholar]
- Getsios S, Simpson CL, Kojima S, Harmon R, Sheu LJ, Dusek RL, et al. Desmoglein 1-dependent suppression of EGFR signaling promotes epidermal differentiation and morphogenesis. J Cell Biol. 2009;185:1243–1258. doi: 10.1083/jcb.200809044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudjonsson JE, Ding J, Johnston A, Tejasvi T, Guzman AM, Nair RP, et al. Assessment of the psoriatic transcriptome in a large sample: additional regulated genes and comparisons with in vitro models. J Invest Dermatol. 2010a;130:1829–1840. doi: 10.1038/jid.2010.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudjonsson JE, Johnston A, Stoll SW, Riblett MB, Xing X, Kochkodan JJ, et al. Evidence for altered Wnt signaling in psoriatic skin. J Invest Dermatol. 2010b;130:1849–1859. doi: 10.1038/jid.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Miao H, Gerber L, Singh J, Denning MF, Gilliam AC, et al. Disruption of EphA2 receptor tyrosine kinase leads to increased susceptibility to carcinogenesis in mouse skin. Cancer Res. 2006;66:7050–7058. doi: 10.1158/0008-5472.CAN-06-0004. [DOI] [PubMed] [Google Scholar]
- Gutowska-Owsiak D, Ogg GS. The epidermis as an adjuvant. J Invest Dermatol. 2012;132:940–948. doi: 10.1038/jid.2011.398. [DOI] [PubMed] [Google Scholar]
- Guttman-Yassky E, Nograles KE, Krueger JG. Contrasting pathogenesis of atopic dermatitis and psoriasis--part I: clinical and pathologic concepts. J Allergy Clin Immunol. 2011;127:1110–1118. doi: 10.1016/j.jaci.2011.01.053. [DOI] [PubMed] [Google Scholar]
- Haase I, Hobbs RM, Romero MR, Broad S, Watt FM. A role for mitogen-activated protein kinase activation by integrins in the pathogenesis of psoriasis. J Clin Invest. 2001;108:527–536. doi: 10.1172/JCI12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafner C, Becker B, Landthaler M, Vogt T. Expression profile of Eph receptors and ephrin ligands in human skin and downregulation of EphA1 in nonmelanoma skin cancer. Mod Pathol. 2006;19:1369–1377. doi: 10.1038/modpathol.3800660. [DOI] [PubMed] [Google Scholar]
- Holzman LB, Marks RM, Dixit VM. A novel immediate-early response gene of endothelium is induced by cytokines and encodes a secreted protein. Mol Cell Biol. 1990;10:5830–5838. doi: 10.1128/mcb.10.11.5830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie F, Okuno M, Matsumoto K, Pasquale EB, Yamaguchi Y. Heparan sulfate regulates ephrin-A3/EphA receptor signaling. Proc Natl Acad Sci U S A. 2008;105:12307–12312. doi: 10.1073/pnas.0801302105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov AI, Steiner AA, Scheck AC, Romanovsky AA. Expression of Eph receptors and their ligands, ephrins, during lipopolysaccharide fever in rats. Physiol Genomics. 2005;21:152–160. doi: 10.1152/physiolgenomics.00043.2004. [DOI] [PubMed] [Google Scholar]
- Jabbari A, Suarez-Farinas M, Dewell S, Krueger JG. Transcriptional Profiling of Psoriasis Using RNA-seq Reveals Previously Unidentified Differentially Expressed Genes. J Invest Dermatol. 2012;132:246–249. doi: 10.1038/jid.2011.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston A, Gudjonsson JE, Aphale A, Guzman AM, Stoll SW, Elder JT. EGFR and IL-1 signaling synergistically promote keratinocyte antimicrobial defenses in a differentiation-dependent manner. J Invest Dermatol. 2011a;131:329–337. doi: 10.1038/jid.2010.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston A, Xing X, Guzman AM, Riblett M, Loyd CM, Ward NL, et al. IL-1F5, -F6, -F8, and -F9: a novel IL-1 family signaling system that is active in psoriasis and promotes keratinocyte antimicrobial peptide expression. J Immunol. 2011b;186:2613–2622. doi: 10.4049/jimmunol.1003162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan N, Fatima A, Peng H, Bryar P, Lavker RM, Getsios S. EphA2/Ephrin-A1 Signaling Complexes Restrict Corneal Epithelial Cell Migration. Invest Ophthalmol Vis Sci. 2012 doi: 10.1167/iovs.11-8685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulski JK, Kenworthy W, Bellgard M, Taplin R, Okamoto K, Oka A, et al. Gene expression profiling of Japanese psoriatic skin reveals an increased activity in molecular stress and immune response signals. J Mol Med (Berl) 2005;83:964–975. doi: 10.1007/s00109-005-0721-x. [DOI] [PubMed] [Google Scholar]
- Lackmann M, Boyd AW. Eph, a protein family coming of age: more confusion, insight, or complexity? Sci Signal. 2008;1:re2. doi: 10.1126/stke.115re2. [DOI] [PubMed] [Google Scholar]
- Larsen AB, Pedersen MW, Stockhausen MT, Grandal MV, van Deurs B, Poulsen HS. Activation of the EGFR gene target EphA2 inhibits epidermal growth factor-induced cancer cell motility. Mol Cancer Res. 2007;5:283–293. doi: 10.1158/1541-7786.MCR-06-0321. [DOI] [PubMed] [Google Scholar]
- Leigh IM, Pulford KA, Ramaekers FC, Lane EB. Psoriasis: maintenance of an intact monolayer basal cell differentiation compartment in spite of hyperproliferation. Br J Dermatol. 1985;113:53–64. doi: 10.1111/j.1365-2133.1985.tb02044.x. [DOI] [PubMed] [Google Scholar]
- Lin S, Gordon K, Kaplan N, Getsios S. Ligand targeting of EphA2 enhances keratinocyte adhesion and differentiation via desmoglein 1. Mol Biol Cell. 2010;21:3902–3914. doi: 10.1091/mbc.E10-03-0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Wang B, Getsios S. Eph/ephrin signaling in epidermal differentiation and disease. Semin Cell Dev Biol. 2011 doi: 10.1016/j.semcdb.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrae M, Neve RM, Rodriguez-Viciana P, Haqq C, Yeh J, Chen C, et al. A conditional feedback loop regulates Ras activity through EphA2. Cancer Cell. 2005;8:111–118. doi: 10.1016/j.ccr.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Madsen P, Rasmussen HH, Leffers H, Honore B, Dejgaard K, Olsen E, et al. Molecular cloning, occurrence, and expression of a novel partially secreted protein "psoriasin" that is highly up-regulated in psoriatic skin. J Invest Dermatol. 1991;97:701–712. doi: 10.1111/1523-1747.ep12484041. [DOI] [PubMed] [Google Scholar]
- Marikovsky M, Breuing K, Liu PY, Eriksson E, Higashiyama S, Farber P, et al. Appearance of heparin-binding EGF-like growth factor in wound fluid as a response to injury. Proc Natl Acad Sci U S A. 1993;90:3889–3893. doi: 10.1073/pnas.90.9.3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mee JB, Johnson CM, Morar N, Burslem F, Groves RW. The psoriatic transcriptome closely resembles that induced by interleukin-1 in cultured keratinocytes: dominance of innate immune responses in psoriasis. Am J Pathol. 2007;171:32–42. doi: 10.2353/ajpath.2007.061067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao H, Li DQ, Mukherjee A, Guo H, Petty A, Cutter J, et al. EphA2 mediates ligand-dependent inhibition and ligand-independent promotion of cell migration and invasion via a reciprocal regulatory loop with Akt. Cancer Cell. 2009;16:9–20. doi: 10.1016/j.ccr.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao H, Wang B. Eph/ephrin signaling in epithelial development and homeostasis. Int J Biochem Cell Biol. 2009;41:762–770. doi: 10.1016/j.biocel.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao H, Wang B. EphA receptor signaling-Complexity and emerging themes. Semin Cell Dev Biol. 2011 doi: 10.1016/j.semcdb.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao H, Wei BR, Peehl DM, Li Q, Alexandrou T, Schelling JR, et al. Activation of EphA receptor tyrosine kinase inhibits the Ras/MAPK pathway. Nat Cell Biol. 2001;3:527–530. doi: 10.1038/35074604. [DOI] [PubMed] [Google Scholar]
- Minner F, Poumay Y. Candidate housekeeping genes require evaluation before their selection for studies of human epidermal keratinocytes. J Invest Dermatol. 2009;129:770–773. doi: 10.1038/jid.2008.247. [DOI] [PubMed] [Google Scholar]
- Pasquale EB. Eph receptor signalling casts a wide net on cell behaviour. Nat Rev Mol Cell Biol. 2005;6:462–475. doi: 10.1038/nrm1662. [DOI] [PubMed] [Google Scholar]
- Piruzian ES, Ishkin AA, Nikol'skaia TA, Abdeev RM, Bruskin SA. The comparative analysis of psoriasis and Crohn disease molecular-genetical processes under pathological conditions. Mol Biol (Mosk) 2009;43:175–179. [PubMed] [Google Scholar]
- Simpson CL, Kojima S, Getsios S. RNA interference in keratinocytes and an organotypic model of human epidermis. Methods Mol Biol. 2010;585:127–146. doi: 10.1007/978-1-60761-380-0_10. [DOI] [PubMed] [Google Scholar]
- Swamy M, Jamora C, Havran W, Hayday A. Epithelial decision makers: in search of the 'epimmunome'. Nat Immunol. 2010;11:656–665. doi: 10.1038/ni.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Ibe M, Nakamura S, Ishida-Yamamoto A, Hashimoto Y, Iizuka H. Extracellular regulated kinase and c-Jun N-terminal kinase are activated in psoriatic involved epidermis. Journal of dermatological science. 2002;30:94–99. doi: 10.1016/s0923-1811(02)00064-6. [DOI] [PubMed] [Google Scholar]
- Tjabringa G, Bergers M, van Rens D, de Boer R, Lamme E, Schalkwijk J. Development and validation of human psoriatic skin equivalents. Am J Pathol. 2008;173:815–823. doi: 10.2353/ajpath.2008.080173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschachler E. Psoriasis: the epidermal component. Clin Dermatol. 2007;25:589–595. doi: 10.1016/j.clindermatol.2007.09.021. [DOI] [PubMed] [Google Scholar]
- Walsh R, Blumenberg M. EPH-2B, acting as an extracellular ligand, induces differentiation markers in epidermal keratinocytes. J Cell Physiol. 2011a doi: 10.1002/jcp.22968. [DOI] [PubMed] [Google Scholar]
- Walsh R, Blumenberg M. Specific and shared targets of ephrin A signaling in epidermal keratinocytes. J Biol Chem. 2011b;286:9419–9428. doi: 10.1074/jbc.M110.197087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein GD, McCullough JL, Ross PA. Cell kinetic basis for pathophysiology of psoriasis. J Invest Dermatol. 1985;85:579–583. doi: 10.1111/1523-1747.ep12283594. [DOI] [PubMed] [Google Scholar]
- Wykosky J, Gibo DM, Debinski W. A novel, potent, and specific ephrinA1-based cytotoxin against EphA2 receptor expressing tumor cells. Mol Cancer Ther. 2007;6:3208–3218. doi: 10.1158/1535-7163.MCT-07-0200. [DOI] [PubMed] [Google Scholar]
- Yamada Y, Midorikawa T, Oura H, Yoshino T, Ohdera M, Kubo Y, et al. Ephrin-A3 not only increases the density of hair follicles but also accelerates anagen development in neonatal mice. J Dermatol Sci. 2008;52:178–185. doi: 10.1016/j.jdermsci.2008.05.007. [DOI] [PubMed] [Google Scholar]
- Zaba LC, Cardinale I, Gilleaudeau P, Sullivan-Whalen M, Suarez-Farinas M, Fuentes-Duculan J, et al. Amelioration of epidermal hyperplasia by TNF inhibition is associated with reduced Th17 responses. J Exp Med. 2007;204:3183–3194. doi: 10.1084/jem.20071094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Njauw CN, Park JM, Naruse C, Asano M, Tsao H. EphA2 is an essential mediator of UV radiation-induced apoptosis. Cancer Res. 2008;68:1691–1696. doi: 10.1158/0008-5472.CAN-07-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.