Abstract
Familial British (FBD) and familial Danish dementia (FDD) are progressive neurodegenerative disorders characterized by cerebral deposition of the amyloidogenic peptides ABri and ADan. These amyloid peptides start with an N-terminal glutamate residue, which can be posttranslationally converted into a pyroglutamate (pGlu-) modified form, a mechanism which has been extensively described to be relevant for Aβ peptides in Alzheimer’s disease (AD). Like pGlu-Aβ peptides, pGlu-ABri peptides have an increased aggregation propensity and show higher toxicity on human neuroblastoma cells as their non-modified counterparts. We have generated novel N-terminal specific antibodies detecting the pGlu-modified forms of ABri and ADan peptides. With these antibodies we were able to identify abundant extracellular amyloid plaques, vascular and parenchymal deposits in human FBD and FDD brain tissue, as well as in a mouse model for FDD. Double-stainings using C-terminal specific antibodies in human samples revealed that highly aggregated pGlu-ABri and pGlu-ADan peptides are mainly present in plaque cores and central vascular deposits, leading to the assumption that these peptides have seeding properties. Furthermore, in an FDD-mouse model ADan peptides were detected in pre-synaptic terminals of the hippocampus where they might contribute to impaired synaptic transmission. These similarities of ABri and ADan to Aβ in AD suggest that the posttranslational pGlu-modification of amyloid peptides might represent a general pathological mechanism leading to increased aggregation and toxicity in these forms of degenerative dementias.
Keywords: familial British dementia, familial Danish dementia, amyloid, pyroglutamate, transgenic mice, aggregation, toxicity, ABri, ADan
1. Introduction
Familial British dementia (FBD) represents an autosomal dominant disorder that is clinically characterized by progressive dementia, spastic tetraparesis and cerebellar ataxia (Mead et al., 2000). Patients suffering from familial Danish dementia (FDD) initially develop cataracts and ocular haemorrhages, followed by hearing loss, cerebellar ataxia and subsequent dementia (Holton et al., 2002). Neuropathological analyses revealed the presence of extracellular parenchymal amyloid deposition and neurofibrillary tangle (NFT) formation in both diseases. The insoluble tau in both conditions has been demonstrated to possess a Western blot banding profile indistinguishable from that seen in AD (Holton et al., 2002). The cloning of the BRI2 gene (also known as integral transmembrane protein 2B (ITM2B)) also resulted in the identification of the genetic defects underlying these novel cerebral amyloid diseases. Under wild-type conditions, the BRI2 gene encodes the BRI2 protein, which is a 266-amino acid-long, type II transmembrane protein. Furinlike processing of BRI2 leads to the release of a short 23 amino acid-long C-terminal peptide (Bri-CTF) (Kim et al., 1999). A stop-codon mutation in FBD (Vidal et al., 1999) and a 10-nucleotide duplication insertion mutation in FDD (Vidal et al., 2000) result in elongated, 277 amino acid-long precursor proteins, from which the two 34 amino acid-long C-terminal peptides, ABri and ADan are cleaved in FBD and FDD, respectively. These disorders share some clinical and neuropathological similarities with Alzheimer’s disease (AD) as they also show parenchymal and vascular deposition of amyloidogenic peptides and prominent Tau phosphorylation. In addition, it has been shown that e.g. synthetic ABri and ADan peptides can undergo aggregation leading to fibril formation showing a comparable behaviour to Aβ peptides in AD (Ghiso et al., 2006; Gibson et al., 2005). Another similarity to AD is the formation of pyroglutamate (pGlu)-modified amyloid peptides. It has been reported that pGlu-modified Aβ peptides represent a major fraction of plaque-associated Aβ peptides in the AD brain (Harigaya et al., 2000) and biochemical analyses have shown that the majority of ABri and ADan peptides in FBD and FDD also contain a pGlu-modification at the Nterminus (Tomidokoro et al., 2005). The pGlu-modification of Aβ leads to an increased aggregation propensity in vitro (D'Arrigo et al., 2009; Schilling et al., 2006) and similar findings have been reported for pGlu-modified ABri and ADan peptides (Schlenzig et al., 2009). The N-terminal pyroglutamate formation of Aβ peptides can be catalyzed by glutaminyl cyclase (QC), an enzyme that can be pharmacologically inhibited by QC inhibitors in vivo (Schilling et al., 2008). Crossing 5XFAD mice, a mouse model for AD, with mice overexpressing human QC resulted in increased pGlu-Aβ formation and induced behavioural deficits, whereas a knock-out of QC rescues the behavioural phenotype in 5XFAD mice (Jawhar et al., 2011b). In addition, over-expression of Aβ3–42 peptides starting with an N-terminal glutamine leading to high pGlu-modified Aβ levels, results in a severe neurological phenotype and neurodegeneration in a transgenic mouse model in vivo (Wirths et al., 2009), a finding that has been recently replicated (Alexandru et al., 2011). It has been recently shown that the formation of pyroglutamate residues at the N-termini of ABri peptides in patients suffering from FBD represents an early step in the process of amyloid deposition in this disorder. Using a combination of immunoprecipitation with a C-terminal antibody and subsequent mass spectrometry, it has been demonstrated that the majority of ABri peptides extracted from brain and peripheral organs harboured pGlu-residues at their N-termini, whereas ABri peptides in plasma were almost exclusively non-modified (Tomidokoro et al., 2010). In the present study, we have investigated the cytotoxic effects of pGlu-modified and non-modified ABri and ADan peptides in neuroblastoma (SH-SY5Y) cells and characterized the aggregation propensity of pGlu-modified and non-modified ABri peptides at a physiological pH. Furthermore, we have generated two novel antibodies detecting pGlu-modified ABri and ADan peptides and analyzed their presence in brain samples from FBD and FDD patients, as well as in a transgenic mouse model of FDD. Whereas antibody AB77 could be used to detect both modified and unmodified ABri/ADan peptides, antibody AB76-2 predominantly recognizes the pyroglutamate-modified form of these two peptides and represents a useful tool to identify these peptides in immunohistochemical or biochemical assays.
2. Material and Methods
2.1 Antibody generation
New Zealand white rabbits (Covalab, France) were immunized with 1 ml of antigen (pEASNCFAIRHFENK) in complete Freund’s adjuvant (CFA) and boosted twice at 3 weeks intervals with 1 ml antigen in incomplete Freund’s adjuvant (IFA). Three additional boosts using a shorter peptide (pEASNC) were carried out 3, 5 and 8 weeks later for the generation of AB76-2. Antibodies were purified by peptide affinity chromatography. For the purification of AB77, the peptide fragment pEASNCFAIRHFENK was covalently immobilized to beaded agarose using affinity columns (SulfoLink® Immobilization Kit for Peptides, Thermo Scientific, USA). The Serum of AB76-2 was first purified using an affinity column, containing the peptide fragment EASNC. The flow-through was subsequently loaded on a second column containing pEASNC immobilized fragments. The resulting eluate was kept as AB76-2. The quality of the synthetic peptides has been controlled by high-pressure liquid chromatography (HPLC) and mass spectrometry.
2.2 Aggregation assay
For monomerization, pyroglutamate-modified and non-modified ABri and ADan peptides (PSL, Heidelberg) were reconstituted in 10 mM NaOH to a final concentration of 1 mg/ml, sonicated for 5 min, frozen in liquid nitrogen and stored a −80°C until use (Wirths et al., 2010b). Peptide solutions were prepared on the basis of their molecular mass in physiological buffer (50 mM PBS, 50 mM NaCl, 0.01 % NaN3, pH 7.0) with 20 µM Thioflavin T (ThT) to a final concentration of 50 µM. 200 µl peptide solution was applied per well of a 96-well microplate and incubated in a Fluoroskan Ascent FL (Thermo Fisher Scientific) peltier adapter with stirring at 37°C. Fluorescence was recorded every 10 min for one week (excitation 444 nm, emission 482 nm).
2.3 Cytotoxicity assays
Toxicity of pGlu-modified and non-modified ABri/ADan peptides was analyzed using SH-SY5Y neuroblastoma cells, which were routinely cultured in DMEM/F-12 (Pan Biotech GmbH), supplemented with 10 % fetal calf serum, 100 U/ml Penicillin, 100 µg/ml Streptomycin, 2 mM L-glutamine, and 1 % nonessential amino acids. Cells were incubated at 37°C in a humidified atmosphere of 5 % CO2. The effect of pGlu-modified and non-modified ABri/ADan peptides on cell viability was assessed by measuring lactate dehydrogenase (LDH) release (Cytotoxicity Detection KitPLUS, Roche). SH-SY5Y cells were plated at a density of 7500 cells/well in 96-well plates in 100 µl of fresh medium. After 36 hours the medium was exchanged with 200 µl medium including 5 µM freshly prepared and monomerized ABri/ADan peptides. Cells were incubated at 37°C in 5 % CO2 for 24 h. Absorbance values at 490 nm were measured and each assay was performed at least five times. LDH release was calculated by subtracting the average background absorbance from all experimental absorbance values. The resulting background-subtracted values of cells with peptide application and controls where then normalized to the LDH-high control (lysed cells) and the percentage of LDH-release was calculated. Significances were tested by using Unpaired-t-test.
2.4 Dot-blot analysis
Prior to experiments, pGlu-modified and non-modified ABri and Aβ (Aβ1-42 and pGlu-Aβ3-42) peptides were dissolved to 1 mg/ml in double-distilled water. Peptide amounts of 20, 100 and 250 ng were dotted on a 0.45 µm nitrocellulose membrane (GE Healthcare) and detected by polyclonal antisera AB77 and AB76-2. Before the primary antibodies were applied overnight at 4°C, membranes were blocked in 10 % skim milk/TBST for 1 h at room temperature (RT). After rinsing with TBST (0.1 M Tris, 1.5 M NaCl, 0.5 % Tween 20, pH 8.0) for two times 10 min, membranes were incubated with secondary anti-rabbit IgG-HRP (1:3000, DAKO, Glostrup, DK) in TBST for 2 h at RT with gentle shaking. After additional washes with TBST for two times 10 min, the blots were developed using enhanced chemiluminescence and then imaged using standard emulsion film.
2.5 Western-blot analyses
For each Western blot, 20–250 ng of ABri1-34, pGlu-ABri1-34, ADan and pGlu-ADan1-34 peptides were loaded per lane of a 4–12 % VarioGel (Anamed). The peptides were then transferred to nitrocellulose membranes using a semi-dry transfer protocol. In addition to synthetic peptides, 20 µg of brain lysates of transgenic mice overexpressing the Danish mutant form of BRI2 (ADanPP7 tg mice) (Coomaraswamy et al., 2010) at different ages were resolved using 16 % Tris-Tricine SDS-PAGE (Anamed, Gross-Bieberau, Germany) under denaturing conditions. After transfer, membranes were incubated in TBST at RT for two times 10 min and boiled in 0.01 M PBS for 5 min, which allows an improved access to the antigen. Membranes were then blocked in 10 % non-fat dry milk/TBST for 1 h at RT while gently mixing, before overnight incubation at 4°C with primary antibodies (AB76-2 [1:50] and AB77 [1:500]; ITM2B [1:500, Sigma-Aldrich]). Membranes were then washed in TBST two times for 10 min. Secondary anti-rabbit IgG-HRP (1:3000, DAKO) or anti-chicken IgG-HRP (1:1000, Sigma-Aldrich) was applied for 2 h at RT. Afterwards, membranes were rinsed in TBST two times for 10 min again. The blots were developed using enhanced chemiluminescence and imaged using standard emulsion film.
2.6 Immunohistochemistry on human brain tissue
Tissue sections from hippocampus and cerebellum (7 µm) of a 43-year-old male with FDD and 68-year-old female with FBD were deparaffinised in xylene and rehydrated through graded alcohols. Endogenous peroxidase activity was blocked with 0.3 % H202 in methanol and non-specific binding blocked with 10% dried milk solution. For antigen retrieval, sections were pre-treated in formic acid for 10 minutes. Tissue sections were incubated with the primary antibodies overnight at 4°C, followed by biotinylated anti-rabbit IgG (1:200) and ABC complex. Diaminobenzidine was used as chromogen and counterstaining was carried out using hematoxylin. Double fluorescent immunohistochemistry was also carried out to investigate the co-localisation of the pGlu antibodies (AB77 and AB76-2) and the C-terminal ABri antibody (338, (Holton et al., 2001)) and C-terminal ADan antibody (5282, (Holton et al., 2002)). Sections were deparaffinised, blocked and pre-treated as described above. AB77 (1:50) and AB76-2 (1:20) were incubated overnight at 4°C, followed by biotinylated anti-rabbit IgG (1:200) and ABC complex. Tetramethylrhodamine (TSA kit - Roche) was used as the fluorescent label. Antibodies recognising the C-terminal region of ABri or ADan were incubated with the tissue sections for 1 hour at room temperature, followed by a biotinylated anti-rabbit IgG (1:200), ABC and tetramethylfluorescein (TSA kit- Roche). Double fluorescent staining was also carried out using the pGlu antibodies and Thioflavin-S to confirm the presence of an amyloid structure. Sections were deparaffinised, blocked and pre-treated in 70 % formic acid for 10 minutes. AB77 (1:50) and AB76-2 (1:20) were incubated overnight at 4°C, followed by biotinylated anti-rabbit IgG (1:200), ABC complex and tetramethylrhodamine (TSA kit - Roche). Sections were incubated in a 1 % ThioS (Sigma) solution for 7 min and differentiated in 70 % alcohol.
2.7 Immunohistochemistry on paraffin sections from transgenic mouse tissue
Immunohistochemistry was performed on 2–4 µm sagittal paraffin sections from transgenic ADanPP7 mice overexpressing the Danish mutant form of BRI2 (Coomaraswamy et al., 2010), as described previously (Wirths et al., 2002). In brief, sections were deparaffinized in xylene and rehydrated in a series of ethanol. After treatment with 0.3 % H2O2 in 0.01 M PBS to block endogenous peroxidases, antigen retrieval was achieved by boiling sections in 0.01 M citrate buffer pH 6.0, followed by 3 min incubation in 88 % formic acid. Non-specific binding sites were blocked by treatment with skim milk and fetal calf serum in PBS for 1 h at RT, prior to the addition of the primary antibodies. Primary antibodies AB76-2 (1:50) against pGlu-modified as well as AB77 (1:500) against pGlu-modified and non-modified ABri/ADan were incubated overnight in a humid chamber at RT, followed by incubation with biotinylated secondary antibodies (DAKO, Glostrup, DK, 1:200) at 37 °C before staining was visualized using the ABC method with Vectastain kit (Vector Laboratories, Burlingame, USA) and diaminobenzidine (DAB) as chromogen providing a reddish brown color. Counterstaining was carried out with hematoxylin.
To further characterize the distribution and aggregation propensities of pGlu-modified peptides, we compared thioflavin-S (Thio-S) staining with the distribution of non-modified and pGlu-modified peptides. Therefore, three equally spaced sections spaced 2 µm apart were taken from ADanPP7-transgenic mice at different months of age and mounted on slides and stained with 1 % Thio-S/Dapi and either AB76-2 or AB77.
In order to examine the cellular structures that are AB77 positive, double immunofluorescence stainings were performed using the dendritic marker MAP2 (Sigma-Aldrich, 1:500) combined with AB77/Dapi, as well as the synaptic marker Synaptophysin (DAKO, Glostrup, DK, 1:500) and AB77/Dapi. For detection, secondary antibodies goat anti-rabbit 594 DyLight, goat anti-rabbit 488 DyLight (both Thermo Scientific), donkey-anti mouse Alexa 488 and chicken anti-mouse Alexa 594 (both Invitrogen) were used (all 1:200).
3. Results
3.1 Enhanced aggregation and cytotoxicity of pGlu-modified ABri/ADan peptides
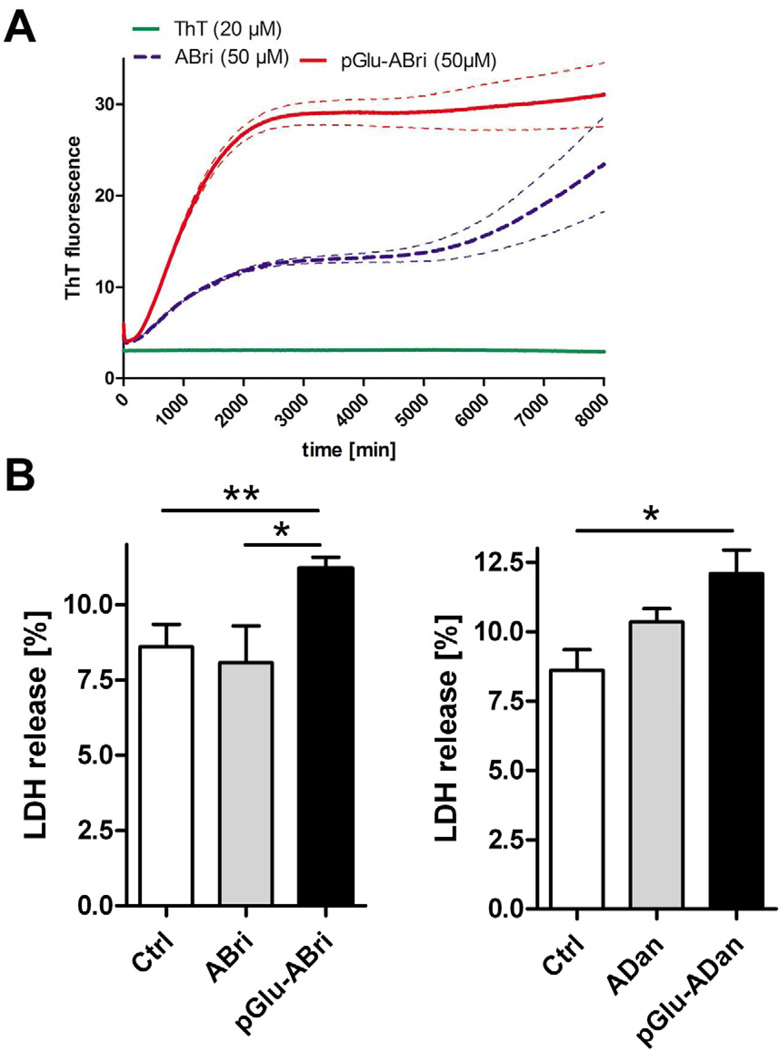
The aggregation of monomeric pGlu-modified ABri and ADan peptides (50 µM) was investigated using a ThT assay at physiological pH (7.0). While ABri showed the expected aggregation profile with a prolonged lag phase before fibril growth, pGlu-ABri peptides exhibited a much more rapid formation of intermediate oligomeric assemblies and strongly enhanced fibril formation (Fig. 1A). Using the same concentrations, ADan and pGlu-ADan peptides showed an indistinguishable, very rapid formation of fibrils without a lag phase at pH 7.0 (not shown). To determine if the pGlu-modification renders ABri and ADan peptides to be more toxic, SH-SY5Y neuroblastoma cells were incubated with non-modified and pGlu-modified ABri and ADan peptides. Whereas application of 5 µM non-modified ABri or ADan peptides did not influence cell viability as measured by LDH release, incubation with either pGlu-modified ABri or ADan peptides resulted in a significantly increased toxicity compared to untreated control cells (Fig. 1B).
Fig. 1.
Aggregation profile and cytotoxicity of pGlu-modified ABri/ADan peptides. Compared to ABri peptides, pGlu-ABri peptides showed a much more rapid formation of intermediate oligomeric assemblies and strongly enhanced fibril formation in a Thio-T assay (A). Application of 5 µM non-modified ABri or ADan peptides did not influence cell viability in SH-SY5Y cells as measured by LDH release, however, incubation with both pGlu-modified ABri as well as ADan peptides resulted in a significantly increased toxicity (B). Dotted lines in A indicate s.e.m.
3.2 Chacterization of ABri/ADan N-terminal antibodies
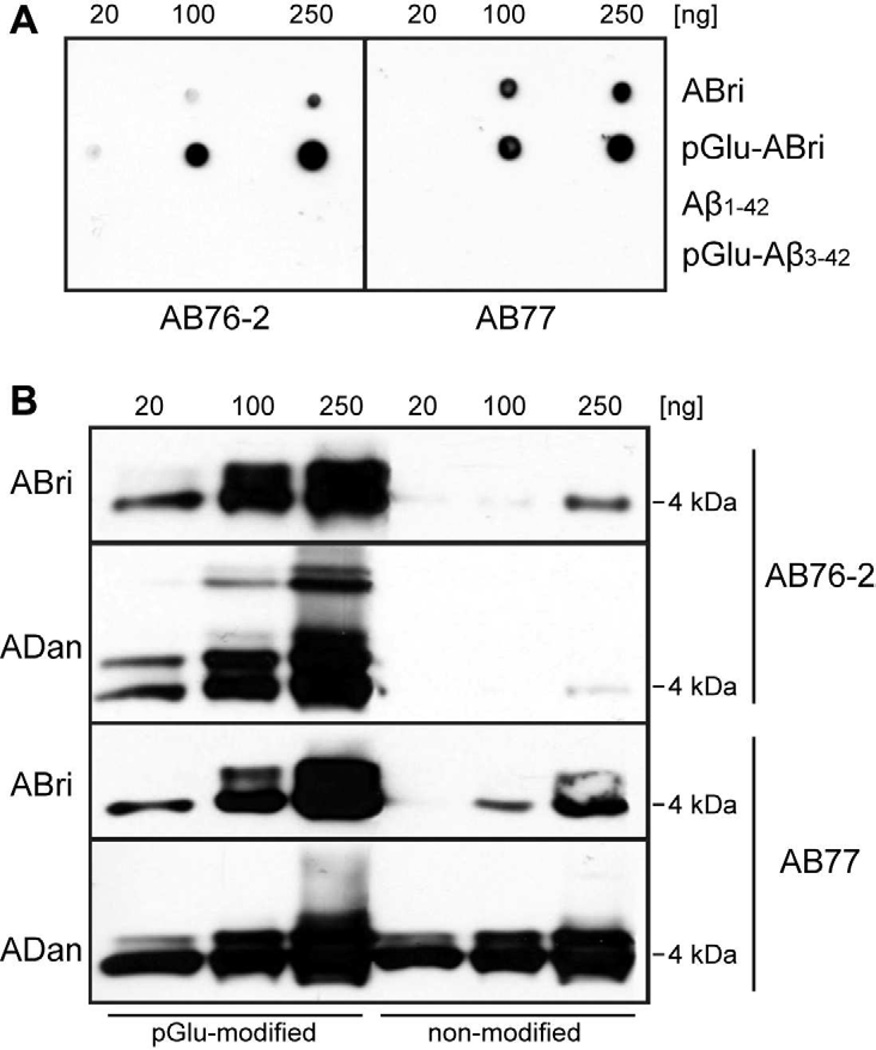
The specificity and sensitivity of the novel ABri/ADan antibodies were determined by dot–blot analysis using pGlu-modified and non-modified ABri peptides, as well as pGlu-modified and non-modified Aβ peptides in different quantities (Fig. 2A). Whereas AB76-2 showed a clear preference for pGlu-modified peptides, AB77 detected ABri and pGlu-ABri peptides with comparable sensitivity and revealed only a slight preference for pGlu-modified ABri peptides. As expected, no signal was observed with Aβ1-42 or pGlu-modified Aβ3-42 peptides, ruling out a cross-reactivity against other pGlu-modified peptides (Fig. 2A). Western-blot analysis using pGlu-modified and non-modified ABri and ADan peptides corroborated this finding. Like in the dot-blot, AB76-2 showed only a minor cross-reactivity with non-modified ABri peptides at higher peptide amounts (250 ng) and a clear preference for pGlu-modified ABri and ADan peptides, detecting also higher molecular species like dimers (pGlu-ABri) as well as dimers and low-molecular weight oligomers (pGlu-ADan) (Fig. 2B). In contrast, AB77 detected both pGlu-modified and non-modified ADan peptides in equal amounts, and revealed higher sensitivity for pGlu-modified compared to non-modified ABri peptides already seen in the dot-blot assay (Fig. 2B). To assess whether AB77 shows the same behaviour in immunohistochemical applications, immunoadsorption experiments using non-modified and pGlu-modified synthetic ADan peptides were carried out in ADanPP7 mice, representing a transgenic mouse model of familial Danish dementia. These experiments revealed that the prominent parenchymal staining pattern of AB77 is mainly due to a detection of pGlu-modified ADan peptides, as the signal was strongly diminished when pGlu-modified ADan peptides were used for preincubation (Supplemental Fig. 1), in contrast to unmodified ADan peptides resulting in a much weaker signal reduction. This also suggests that most of the extracellular deposits in this mouse model consist mainly of pGlu-modified ADan peptides.
Fig. 2.
Dot-blot and Western-blot analyses of ABri/ADan N-terminal antibodies. AB76-2 showed a clear preference for pGlu-modified peptides, whereas AB77 detected ABri and pGlu-ABri peptides in a similar fashion (A). Western-blot analysis corroborated this finding showing only a minor cross-reactivity with non-modified ABri peptides at higher amounts of peptide (250 ng) and a clear preference for pGlu-modified ABri and ADan peptides using AB76-2 (B).
3.3 Immunohistochemical analyses of pGlu-modified ABri/ADan peptides in human FBD/FDD brains
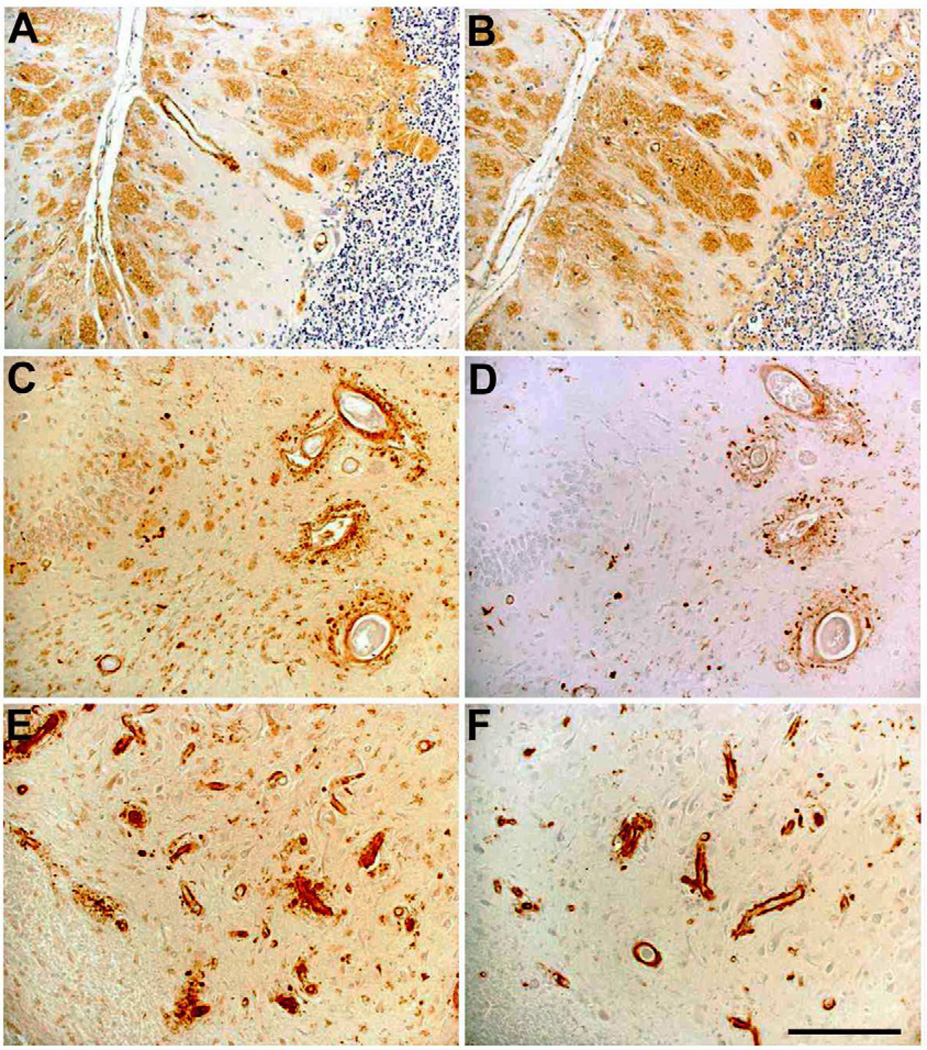
In order to characterize the staining pattern of the novel antibodies, formalin-fixed and paraffin-embedded cortical and cerebellar tissue from patients with FBD and FDD have been used. Abundant AB77 (Fig. 3A) and AB76-2 (Fig. 3B) positive extracellular parenchymal deposits were detected in the cerebellum of an FBD patient. In addition, prominent vascular staining was detected in the dentate gyrus (Fig. 3C,D) and the CA4 region of the hippocampus (Fig. 3E,F) in an FDD patient.
Fig. 3.
AB77 and AB76-2 immunoreactivity in FBD and FDD patients. Abundant AB77 (A) and AB76-2 (B) positive extracellular parenchymal deposits are seen in the cerebellum of a FBD patient. In addition, prominent vascular staining was detected in the dentate gyrus (C,D) and the CA4 region of the hippocampus (E,F) in a patient suffering from FDD. AB77: A, C, E; AB76-2: B, D, F. Scale bar: 100 µm
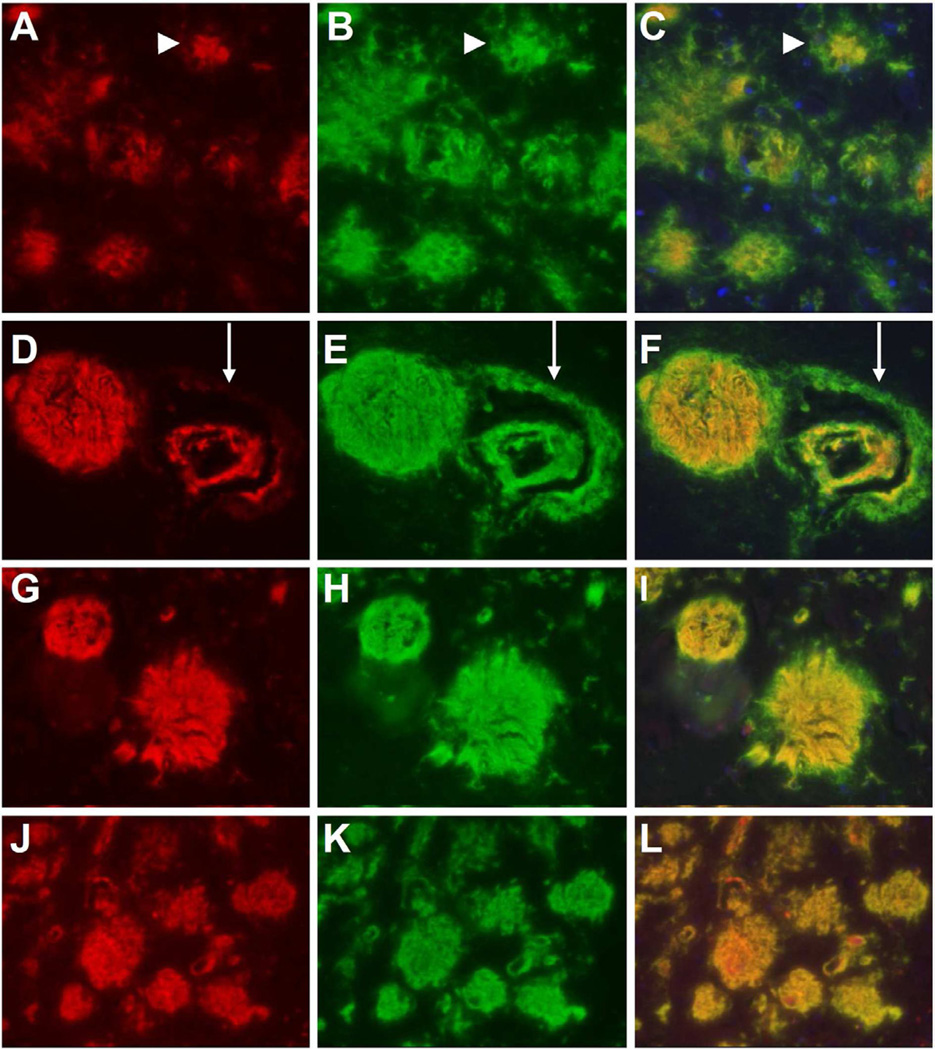
In order to verify if and to what extent parenchymal and vascular deposits in FBD and FDD contain pGlu-modified and aggregated material, AB77 and AB76-2 were combined with C-terminal ABri (Fig. 4A–F) and ADan antibodies (Supplemental Fig. 2) as well as Thio-S respectively (Fig. 4G–L and Supplemental Fig. 2). Abundant ABri positive parenchymal deposits were observed in the hippocampus of an FBD patient (Fig. 4B). Interestingly, the majority of these deposits were also positive with AB77 (Fig. 4A) apart from the periphery of the plaques (Fig. 4B,C; arrowhead). A similar staining pattern was observed when AB76-2 (Fig. 4D) was used in combination with the C-terminal ABri antibody (Fig. 4E), leading to the assumption that most of the deposited material in FBD patients is pGlu-modified ABri. Furthermore, AB76-2 stained parenchymal plaques and deposits within the blood vessels, whereas the perivascular deposits remained unstained (Fig. 4D–F, arrow), which suggests that this perivascular material contains unmodified ABri peptides. Similar staining was observed with AB77 (Fig. 4G) when combined with Thioflavin-S (Fig. 4H), whereas a complete co-localization was detected using AB76-2 (Fig. 4J) in combination with Thioflavin-S (Fig. 4J–L) suggesting that AB76-2 stained highly aggregated material.
Fig. 4.
Double immunofluorescent stainings with a C-terminal ABri antibody combined with the novel N-terminal antibodies in an FBD patient. Abundant ABri (B) positive parenchymal deposits were observed in the hippocampus of an FBD patient, the majority of these deposits were also positive with AB77 (A) apart from the periphery of the plaques (C, arrowhead). AB76-2 (D) stained parenchymal plaques and ABri (antibody 338, E) deposited within the blood vessels, whereas the perivascular deposits remained unstained (F, arrow). Similar staining was observed with AB77 (G) or AB76-2 (J) when combined with Thioflavin-S (H,K) in FBD tissue, however, a complete colocalization was detected using AB76-2 (Fig. 4J). Original magnification: 200×
Prominent vascular staining was detected in the cerebellum of an FDD patient with AB77 (Supplemental Fig. 2A), which co-localized with the C-terminal ADan antibody (Supplemental Fig. 2B). A comparable staining pattern was detected using AB76-2; however, only partial colocalization between AB76-2 and the C-terminal ADan antibody could be observed (Supplemental Fig. 2D–F, arrows). Combining AB76-2 with Thioflavin-S revealed a complete overlap (Supplemental Fig. 2G–I) indicating that most of the vascular staining might be due to aggregated, pyroglutamate-modified ADan peptides.
3.4 pGlu-modified ADan peptides in a transgenic mouse model of FDD
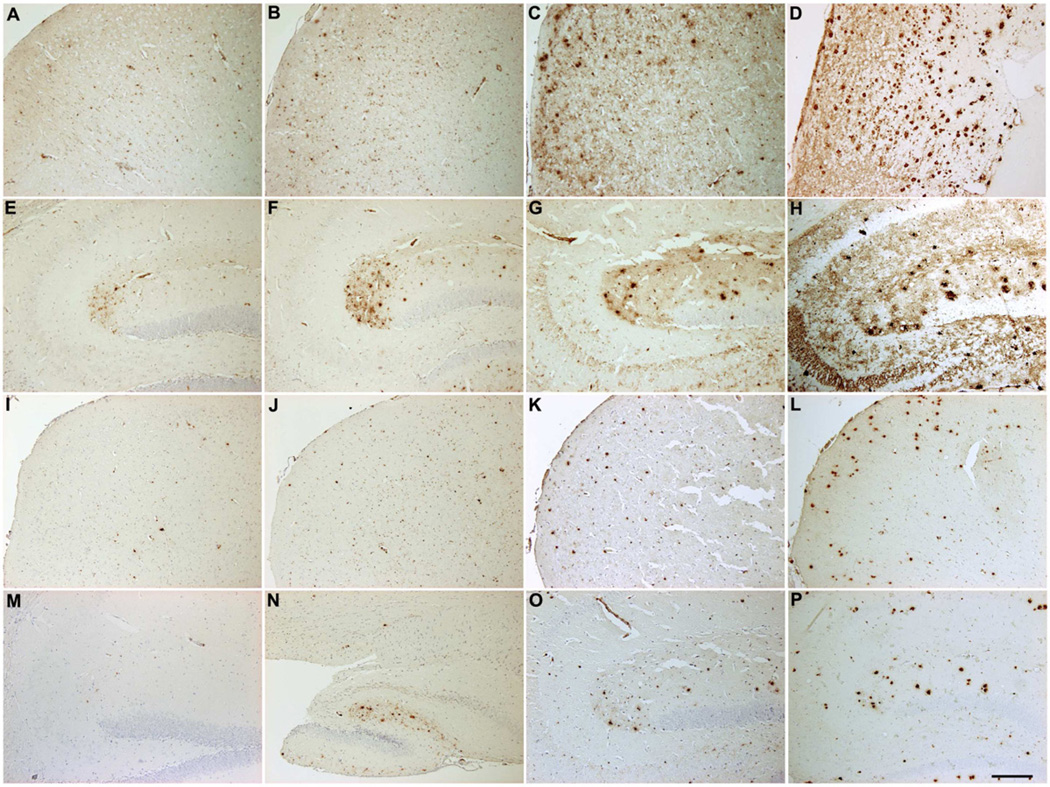
In order to confirm the observations in brain tissue from FBD and FDD patients, paraffin-embedded brain tissue from 2-, 4-, 13- and 20-month-old transgenic ADanPP7 mice expressing the Danish mutant form of BRI2 were analyzed. Using AB77, initial immunoreactivity was detected in the stratum lacunosum moleculare already at 2 months of age, which strongly increases over the entire hippocampus at later stages (Fig. 5E–H), confirming previous data obtained with a C-terminal ADan-specific antibody (Coomaraswamy et al., 2010). In addition, prominent parenchymal immunoreactivity, together with abundant plaque and vessel staining was detectable in aged animals (Fig. 5A–D). Staining using antibody AB76-2 corroborates this finding as it demonstrates single extracellular deposits in the hippocampus and frontal cortex already in 2-month-old ADanPP7 mice with increasing levels of immunoreactive extracellular amyloid deposits and vessels in aged animals (Fig. 5I–P). Western-blot analysis using SDS-soluble fractions from brains of ADanPP7 mice revealed a faint band using AB77 already at 2 months of age, with strongly increased signal intensity at 4 and 13 months of age. Using AB76-2, a clear signal could be detected at 4 months of age with markedly enhanced signal intensity at 13 months of age (Supplemental Fig. 3A). This leads to the assumption that pGlu-modified ADan peptides already exist in ADanPP mice at a young age. Western-blot analysis using an antibody against ITM2B revealed the expected band at ~45 kDa showing no evidence of cross-reactivity of AB77 and AB76-2 with the parental ITM2B full-length protein (Supplemental Fig. 3B).
Fig. 5.
Age-dependent accumulation of ADan/pGlu-ADan in ADanPP7 mice. Using AB77 (A–H) and AB76-2 (I–P), ADan/pGlu-ADan immunoreactivity was detected already at 2 months of age in frontal cortex (A,I) and hippocampus (E,M) of ADanPP7 mice showing a strong increase at 4 (B,F,J,N), 13 (C,G,K,O) and 20 months of age (D,H,L,P). Scale bar: 200 µm (A–P)
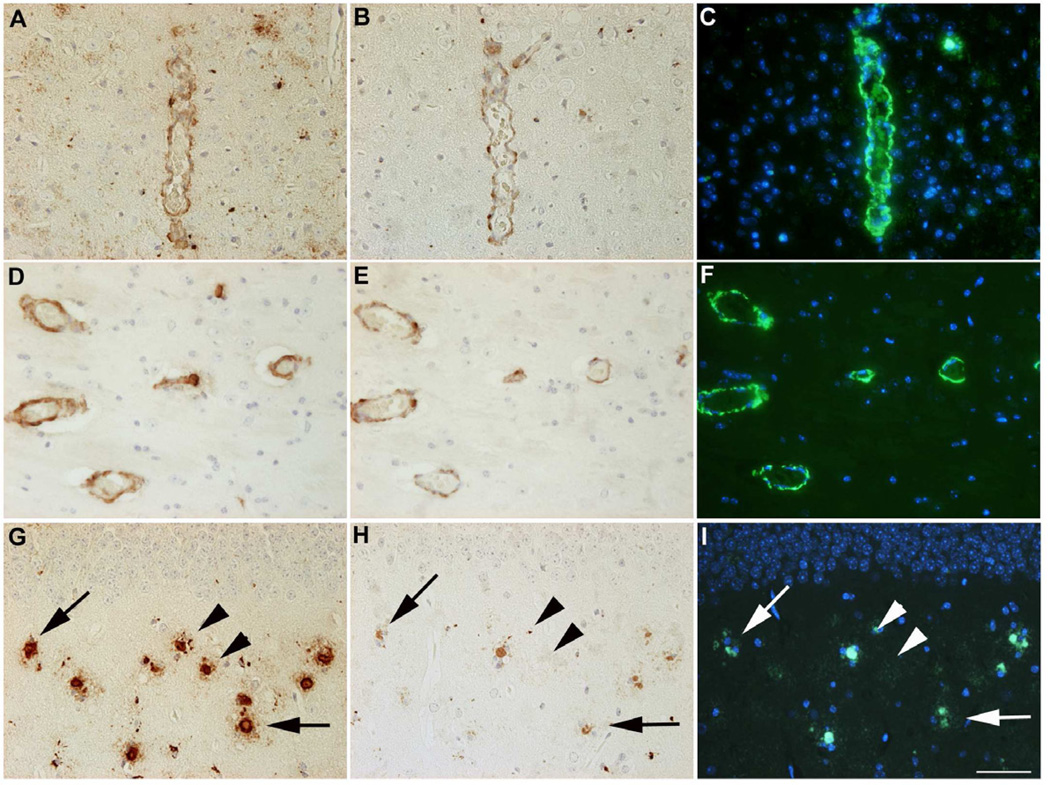
Most of the larger blood vessels contained both non-modified and pGlu-modified ADan peptides and could be stained with either AB77 (Fig. 6A,D,G) or AB76-2 (Fig. 6B,E,H) and were in addition Thioflavin-S positive (Fig. 6C,F,I), which holds also true for the majority of extracellular deposits. Almost all of the deposits that stained with AB76-2 (Fig. 6H) were also Thioflavin-S positive (Fig. 6I, arrows), however, some deposits could be only labeled with AB77 and lacked AB76-2 and Thioflavin-S staining (Fig. 6G, arrowheads), which is suggestive of a sequential maturation process of amyloid plaques in this mouse model.
Fig. 6.
Most of the larger blood vessels stained positive with AB77 (A,D), AB76-2 (B,E) and Thio-S (C,F) in ADanPP7 mice. Almost all of the deposits that stained with AB76-2 (H) were also Thio-S positive (I, arrows). However, some deposits could be only labeled with AB77 and showed neither AB76-2 nor Thio-S staining (G, arrowheads). Scale bar: 50 µm (A–I)
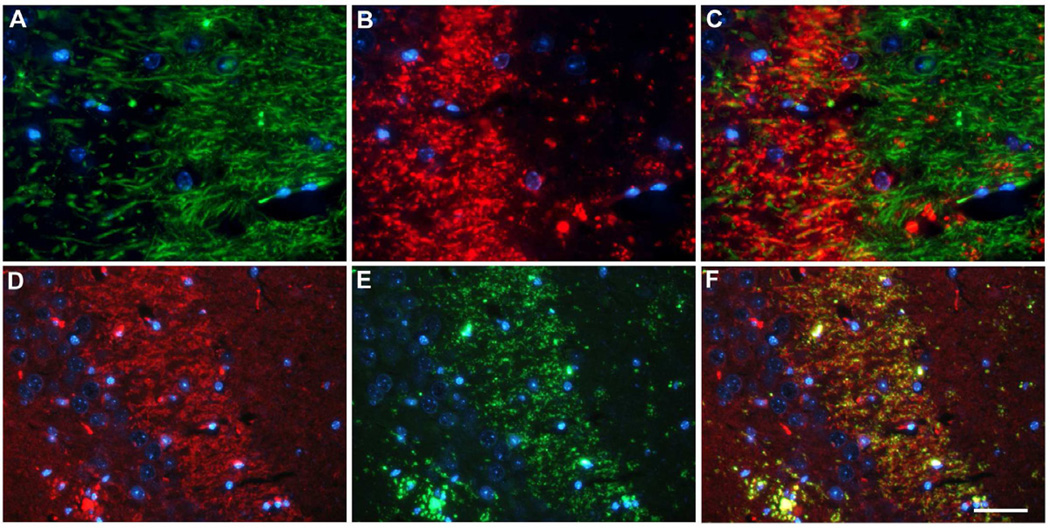
In aged ADanPP7 animals, prominent staining of mossy fibers was detected using AB77 (Fig. 5H). Double immunofluorescence stainings using the dendritic marker MAP2 and AB77 revealed only a minor co-localization with ADan immunoreactivity in dendritic processes (Fig. 7A–C). However, double-stainings using the synaptic marker Synaptophysin showed that AB77-positive material accumulated in the stratum lucidum of the mossy fiber pathway in the hippocampus (Fig. 7D–F), indicating the presence of ADan/pGlu-ADan peptides in presynaptic terminals.
Fig. 7.
Immunofluorescence stainings using the dendritic marker MAP2 (A) and AB77 (B) revealed only minor co-localization in dendritic processes (C). However, the synaptic marker Synaptophysin (D) showed co-localization with AB77-positive material (E) in the stratum lucidum in the hippocampus (F), indicating the presence of ADan/pGlu-ADan peptides in presynaptic terminals. Scale bar: 33 µm (A–C), 50 µm (D–F).
4. Discussion
In mammals, amino-terminal pGlu-residues are common in a variety of secretory peptides and hormones, including thyrotropin- and gonadotropin-releasing hormones, as well as neurotransmitters like neurotensin (Sykes et al., 1999). Unmasking of glutaminyl residues is usually carried out by prohormone convertase cleavage (Steiner, 1998) which gives rise to pGlu-formation by an enzyme called QC (Busby et al., 1987). Pyroglutamate-modification of N-terminally truncated Aβ peptides is a well-known post-translational modification occurring in AD (Jawhar et al., 2011a), and it has been described that AβpE3 represents a dominant fraction of Aβ peptides in senile plaques from AD patients (Saido et al., 1995). We have recently shown that crossing 5XFAD mice, a mouse model for AD, with mice overexpressing human QC resulted in increased pGlu-Aβ formation and the induction of behavioural deficits, whereas a knock-out of QC rescues the behavioural phenotype in 5XFAD mice (Jawhar et al., 2011b). Furthermore, oral application of a QC inhibitor leads to a reduction of pGlu-Aβ levels in two transgenic AD mouse models (Schilling et al., 2008).
In the present work, we show that large numbers of extracellular amyloid deposits and blood vessels in FBD and FDD also harbor substantial amounts of pGlu-modified ABri and ADan peptides by using newly developed antibodies. In AD, the pGlu-modification of Aβ peptides leads to a higher aggregation propensity (He and Barrow, 1999;Schilling et al., 2006) and an enhanced stability towards proteolytic degradation (Kuo et al., 1998). ABri and ADan peptides harboring N-terminal pGlu-modifications have been reported to behave in a similar way, with strongly enhanced aggregation profiles of pGlu-modified ABri and ADan peptides at pH 4.0 where the peptides are well soluble (Schlenzig et al., 2009). Our data at physiological pH 7.0 partially confirm these observations, demonstrating a strongly enhanced aggregation profile of ABri peptides carrying the pGlu-modification. It has been additionally shown that N-truncated and pGlu-modified Aβ peptides exhibit an increased toxicity compared to full-length Aβ in cell-based assays (Russo et al., 2002). The fact that the application of pGlu-modified ABri and ADan peptides, compared to their non-modified counterparts, also led to higher cytotoxicity in neuroblastoma cells raises the question if this post-translational modification represents a general mechanism to promote toxicity of amyloid peptides. It has been previously demonstrated that ABri peptides rapidly form oligomeric species in vitro. In previous studies, no such oligomerization was observed with the wildtype peptide (Bri-CTF), suggesting that the C-terminal extension mediating increased higher hydrophobicity plays an important role in oligomerization and the formation of fibrils. These results also support an important role of the disulfide bridge in ABri oligomerization, as treatment with dithiothreitol (DTT) leads to a disappearance of ABri aggregates (El-Agnaf et al., 2001). In good agreement with the results in the aggregation assay, we demonstrate by western-blotting of synthetic peptides that both pGlu-modified ABri and ADan exhibit an increased tendency to form higher molecular weight aggregates, which exceeds the aggregation propensity of their unmodified counterparts.
The occurrence of pGlu-modified peptides has been previously demonstrated in brain tissue and vessels from subjects with FBD and FDD using mass spectrometry (Ghiso et al., 2006; Tomidokoro et al., 2005). These analyses have shown that, depending on the extraction method, approximately 60 % of the peptides carry the pGlu-modification (Ghiso et al., 2006). In addition, there is evidence for deposition of pGlu-ABri peptides in systemic organs like pancreas, skeletal muscle or uterus in patients suffering from FBD making them interesting specimen for diagnosis (Tomidokoro et al., 2010). We have demonstrated abundant immunoreactivity using antiserum AB76-2 which shows a strong preference for pGlu-modified peptides in the brain parenchyma and vasculature of FBD and FDD patients, confirming earlier biochemical studies (Ghiso et al., 2006; Tomidokoro et al., 2005). In addition, AB76-2 immunoreactivity largely co-localized with Thioflavin-S staining in the vasculature and parenchymal deposits of ADanPP7 mice as well as in human tissues of FBD and FDD patients, suggesting that the pGlu-modified peptides are highly aggregated. On the other hand, the fact that some deposits could be only labeled with AB77 and lacked AB76-2 and Thioflavin-S staining (Fig. 6) is suggestive of a sequential maturation process of amyloid plaques in this mouse model. This could be explained by the assumption that unmodified ADan is deposited first and undergoes modification to pGlu-ADan over time. However, as we have shown by western-blot that AB76-2 also detects unmodified ABri (and to a lesser extent ADan) peptides, we cannot completely rule out that AB76-2 immunoreactivity of plaque cores could be also due to high concentrations of unmodified peptides in these structures instead of purely detecting pGlu-modified peptides. In addition, different affinities of antibodies always complicate comparative analyses.
In good agreement with such a sequential maturation process, we have previously demonstrated that in the APP/PS1KI mouse model of AD a continuous increase in pGlu-Aβ plaque load with increasing age could be observed, while the amount of full-length Aβ plaques starting with aspartate at position 1declined with aging (Wirths et al., 2010a). In AD it has been reported that in cored but not in diffuse plaques N-terminal truncated and pGlu-modified Aβ peptides were increased by approximately 20 % between Braak stages IV–VI (Guntert et al., 2006). It is assumed that pGlu-Aβ either acts as a seed for plaque formation or is involved in the process of plaque maturation. In our studies we found that highly aggregated pGlu-modified ABri and ADan peptides are present in plaque cores as well as in central vascular deposits but were absent in the periphery. This leads to the assumption that N-terminally modified ABri in FBD could also act in a similar fashion like pGlu-Aβ in AD, meaning a role in seeding or maturation of the plaques. In vitro analyses have demonstrated that following furin cleavage, ABri peptides are detected both intracellularly as well as in the medium, whereas ADan peptides accumulate predominantly in intracellular compartments (Kim et al., 2002). In good agreement, ADan peptides have been shown to co-localize intracellularly with Synaptophysin in the hippocampus of ADanPP7 mice in the present study, which suggests accumulation in pre-synaptic terminals. In AD, there is accumulating evidence that soluble oligomeric species represent the most toxic forms of Aβ (Benilova et al., 2012). In addition, soluble oligomeric Aβ42, but not plaque-associated Aβ, correlates best with cognitive dysfunction in AD (McLean et al., 1999). Aβ oligomers are formed preferentially intracellularly within neuronal processes and synapses rather than extracellularly (Takahashi et al., 2004;Walsh et al., 2000) and intraneuronal Aβ rather than extracellular plaque pathology correlates with neuron loss in the hippocampus (Casas et al., 2004), the frontal cortex (Christensen et al., 2008) and the cholinergic system (Christensen et al., 2010) of a transgenic mouse model for AD. It is likely that comparable mechanisms might occur in FBD and FDD which might impair synaptic transmission and neuronal plasticity resulting in cognitive decline.
In summary, we have shown that there is abundant deposition of pGlu-modified ABri and ADan peptides in FBD and FDD as well as in a mouse model for FDD, respectively. Furthermore, we could also show a more rapid aggregation profile of pGlu-ABri than unmodified ABri under physiological conditions as well as an increased toxicity of pGlu-modified ABri and ADan peptides in comparison to their non-modified counterparts in vitro. In addition, we identified neuronal compartments where ADan is aggregated intracellularly in synapses. Due to these striking similarities with pGlu-modified Aβ in AD, we propose that the pGlu-modification of amyloidogenic peptides might represent a general pathological mechanism that triggers amyloid deposition and increases toxicity leading to neurodegeneration in amyloidopathies.
Supplementary Material
Acknowledgments
This work was supported by the Fritz-Thyssen-Foundation (to O.W.) and the NIH (grant R01 AG030539 to J.A.G.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement
The authors disclose no conflict of interest.
Animal studies were performed with the approval of the local research ethics committee in accordance with national and international guidelines.
References
- Alexandru A, Jagla W, Graubner S, Becker A, Bauscher C, Kohlmann S, Sedlmeier R, Raber KA, Cynis H, Ronicke R, Reymann KG, Petrasch-Parwez E, Hartlage-Rubsamen M, Waniek A, Rossner S, Schilling S, Osmand AP, Demuth HU, von Horsten S. Selective Hippocampal Neurodegeneration in Transgenic Mice Expressing Small Amounts of Truncated A{beta} Is Induced by Pyroglutamate-A{beta} Formation. J Neurosci. 2011;31(36):12790–12801. doi: 10.1523/JNEUROSCI.1794-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benilova I, Karran E, De Strooper B. The toxic Abeta oligomer and Alzheimer's disease: an emperor in need of clothes. Nat Neurosci. 2012;15(3):349–357. doi: 10.1038/nn.3028. [DOI] [PubMed] [Google Scholar]
- Busby WH, Jr, Quackenbush GE, Humm J, Youngblood WW, Kizer JS. An enzyme(s) that converts glutaminyl-peptides into pyroglutamyl-peptides. Presence in pituitary, brain, adrenal medulla, and lymphocytes. J Biol Chem. 1987;262(18):8532–8536. [PubMed] [Google Scholar]
- Casas C, Sergeant N, Itier JM, Blanchard V, Wirths O, van der Kolk N, Vingtdeux V, van de Steeg E, Ret G, Canton T, Drobecq H, Clark A, Bonici B, Delacourte A, Benavides J, Schmitz C, Tremp G, Bayer TA, Benoit P, Pradier L. Massive CA1/2 neuronal loss with intraneuronal and N-terminal truncated Abeta42 accumulation in a novel Alzheimer transgenic model. Am J Pathol. 2004;165(4):1289–1300. doi: 10.1016/s0002-9440(10)63388-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen DZ, Bayer TA, Wirths O. Intracellular Abeta triggers neuron loss in the cholinergic system of the APP/PS1KI mouse model of Alzheimer's disease. Neurobiol Aging. 2010;31(7):1153–1163. doi: 10.1016/j.neurobiolaging.2008.07.022. [DOI] [PubMed] [Google Scholar]
- Christensen DZ, Kraus SL, Flohr A, Cotel MC, Wirths O, Bayer TA. Transient intraneuronal Abeta rather than extracellular plaque pathology correlates with neuron loss in the frontal cortex of APP/PS1KI mice. Acta Neuropathol. 2008;116(6):647–655. doi: 10.1007/s00401-008-0451-6. [DOI] [PubMed] [Google Scholar]
- Coomaraswamy J, Kilger E, Wolfing H, Schafer C, Kaeser SA, Wegenast-Braun BM, Hefendehl JK, Wolburg H, Mazzella M, Ghiso J, Goedert M, Akiyama H, Garcia-Sierra F, Wolfer DP, Mathews PM, Jucker M. Modeling familial Danish dementia in mice supports the concept of the amyloid hypothesis of Alzheimer's disease. Proc Natl Acad Sci U S A. 2010;107(17):7969–7974. doi: 10.1073/pnas.1001056107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Arrigo C, Tabaton M, Perico A. N-terminal truncated pyroglutamyl beta amyloid peptide Abetapy3-42 shows a faster aggregation kinetics than the full-length Abeta1-42. Biopolymers. 2009;91(10):861–873. doi: 10.1002/bip.21271. [DOI] [PubMed] [Google Scholar]
- El-Agnaf OM, Sheridan JM, Sidera C, Siligardi G, Hussain R, Haris PI, Austen BM. Effect of the disulfide bridge and the C-terminal extension on the oligomerization of the amyloid peptide ABri implicated in familial British dementia. Biochemistry. 2001;40(12):3449–3457. doi: 10.1021/bi002287i. [DOI] [PubMed] [Google Scholar]
- Ghiso J, Rostagno A, Tomidokoro Y, Lashley T, Bojsen-Moller M, Braendgaard H, Plant G, Holton J, Lal R, Revesz T, Frangione B. Genetic alterations of the BRI2 gene: familial British and Danish dementias. Brain Pathol. 2006;16(1):71–79. doi: 10.1111/j.1750-3639.2006.tb00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson G, El-Agnaf OM, Anwar Z, Sidera C, Isbister A, Austen BM. Structure and neurotoxicity of novel amyloids derived from the BRI gene. Biochem Soc Trans. 2005;33(Pt 5):1111–1112. doi: 10.1042/BST20051111. [DOI] [PubMed] [Google Scholar]
- Guntert A, Dobeli H, Bohrmann B. High sensitivity analysis of amyloid-beta peptide composition in amyloid deposits from human and PS2APP mouse brain. Neuroscience. 2006;143(2):461–475. doi: 10.1016/j.neuroscience.2006.08.027. [DOI] [PubMed] [Google Scholar]
- Harigaya Y, Saido TC, Eckman CB, Prada CM, Shoji M, Younkin SG. Amyloid beta protein starting pyroglutamate at position 3 is a major component of the amyloid deposits in the Alzheimer's disease brain. Biochem Biophys Res Commun. 2000;276(2):422–427. doi: 10.1006/bbrc.2000.3490. [DOI] [PubMed] [Google Scholar]
- He W, Barrow CJ. The A beta 3-pyroglutamyl and 11-pyroglutamyl peptides found in senile plaque have greater beta-sheet forming and aggregation propensities in vitro than full-length A beta. Biochemistry. 1999;38(33):10871–10877. doi: 10.1021/bi990563r. [DOI] [PubMed] [Google Scholar]
- Holton JL, Ghiso J, Lashley T, Rostagno A, Guerin CJ, Gibb G, Houlden H, Ayling H, Martinian L, Anderton BH, Wood NW, Vidal R, Plant G, Frangione B, Revesz T. Regional distribution of amyloid-Bri deposition and its association with neurofibrillary degeneration in familial British dementia. Am J Pathol. 2001;158(2):515–526. doi: 10.1016/S0002-9440(10)63993-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holton JL, Lashley T, Ghiso J, Braendgaard H, Vidal R, Guerin CJ, Gibb G, Hanger DP, Rostagno A, Anderton BH, Strand C, Ayling H, Plant G, Frangione B, Bojsen-Moller M, Revesz T. Familial Danish dementia: a novel form of cerebral amyloidosis associated with deposition of both amyloid-Dan and amyloid-beta. J Neuropathol Exp Neurol. 2002;61(3):254–267. doi: 10.1093/jnen/61.3.254. [DOI] [PubMed] [Google Scholar]
- Jawhar S, Wirths O, Bayer TA. Pyroglutamate Abeta -- a hatchet man in Alzheimer disease. J Biol Chem. 2011a;286(45):38825–38832. doi: 10.1074/jbc.R111.288308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jawhar S, Wirths O, Schilling S, Graubner S, Demuth HU, Bayer TA. Overexpression of Glutaminyl Cyclase, the Enzyme Responsible for Pyroglutamate Abeta Formation, Induces Behavioral Deficits, and Glutaminyl Cyclase Knock-out Rescues the Behavioral Phenotype in 5XFAD Mice. J Biol Chem. 2011b;286(6):4454–4460. doi: 10.1074/jbc.M110.185819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Creemers JW, Chu S, Thinakaran G, Sisodia SS. Proteolytic processing of familial British dementia-associated BRI variants: evidence for enhanced intracellular accumulation of amyloidogenic peptides. J Biol Chem. 2002;277(3):1872–1877. doi: 10.1074/jbc.M108739200. [DOI] [PubMed] [Google Scholar]
- Kim SH, Wang R, Gordon DJ, Bass J, Steiner DF, Lynn DG, Thinakaran G, Meredith SC, Sisodia SS. Furin mediates enhanced production of fibrillogenic ABri peptides in familial British dementia. Nat Neurosci. 1999;2(11):984–988. doi: 10.1038/14783. [DOI] [PubMed] [Google Scholar]
- Kuo YM, Webster S, Emmerling MR, De Lima N, Roher AE. Irreversible dimerization/tetramerization and post-translational modifications inhibit proteolytic degradation of A beta peptides of Alzheimer's disease. Biochim Biophys Acta. 1998;1406(3):291–298. doi: 10.1016/s0925-4439(98)00014-3. [DOI] [PubMed] [Google Scholar]
- McLean CA, Cherny RA, Fraser FW, Fuller SJ, Smith MJ, Beyreuther K, Bush AI, Masters CL. Soluble pool of Abeta amyloid as a determinant of severity of neurodegeneration in Alzheimer's disease. Ann Neurol. 1999;46(6):860–866. doi: 10.1002/1531-8249(199912)46:6<860::aid-ana8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Mead S, James-Galton M, Revesz T, Doshi RB, Harwood G, Pan EL, Ghiso J, Frangione B, Plant G. Familial British dementia with amyloid angiopathy: early clinical, neuropsychological and imaging findings. Brain. 2000;123(Pt 5):975–991. doi: 10.1093/brain/123.5.975. [DOI] [PubMed] [Google Scholar]
- Russo C, Violani E, Salis S, Venezia V, Dolcini V, Damonte G, Benatti U, D'Arrigo C, Patrone E, Carlo P, Schettini G. Pyroglutamate-modified amyloid beta-peptides--AbetaN3(pE)--strongly affect cultured neuron and astrocyte survival. J Neurochem. 2002;82(6):1480–1489. doi: 10.1046/j.1471-4159.2002.01107.x. [DOI] [PubMed] [Google Scholar]
- Saido TC, Iwatsubo T, Mann DM, Shimada H, Ihara Y, Kawashima S. Dominant and differential deposition of distinct beta-amyloid peptide species, A beta N3(pE), in senile plaques. Neuron. 1995;14(2):457–466. doi: 10.1016/0896-6273(95)90301-1. [DOI] [PubMed] [Google Scholar]
- Schilling S, Lauber T, Schaupp M, Manhart S, Scheel E, Bohm G, Demuth HU. On the seeding and oligomerization of pGlu-amyloid peptides (in vitro) Biochemistry. 2006;45(41):12393–12399. doi: 10.1021/bi0612667. [DOI] [PubMed] [Google Scholar]
- Schilling S, Zeitschel U, Hoffmann T, Heiser U, Francke M, Kehlen A, Holzer M, Hutter-Paier B, Prokesch M, Windisch M, Jagla W, Schlenzig D, Lindner C, Rudolph T, Reuter G, Cynis H, Montag D, Demuth HU, Rossner S. Glutaminyl cyclase inhibition attenuates pyroglutamate Abeta and Alzheimer's disease-like pathology. Nat Med. 2008;14(10):1106–1111. doi: 10.1038/nm.1872. [DOI] [PubMed] [Google Scholar]
- Schlenzig D, Manhart S, Cinar Y, Kleinschmidt M, Hause G, Willbold D, Funke SA, Schilling S, Demuth HU. Pyroglutamate formation influences solubility and amyloidogenicity of amyloid peptides. Biochemistry. 2009;48(29):7072–7078. doi: 10.1021/bi900818a. [DOI] [PubMed] [Google Scholar]
- Steiner DF. The proprotein convertases. Curr Opin Chem Biol. 1998;2(1):31–39. doi: 10.1016/s1367-5931(98)80033-1. [DOI] [PubMed] [Google Scholar]
- Sykes PA, Watson SJ, Temple JS, Bateman RC., Jr Evidence for tissue-specific forms of glutaminyl cyclase. FEBS Lett. 1999;455(1–2):159–161. doi: 10.1016/s0014-5793(99)00872-8. [DOI] [PubMed] [Google Scholar]
- Takahashi RH, Almeida CG, Kearney PF, Yu F, Lin MT, Milner TA, Gouras GK. Oligomerization of Alzheimer's beta-amyloid within processes and synapses of cultured neurons and brain. J Neurosci. 2004;24(14):3592–3599. doi: 10.1523/JNEUROSCI.5167-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomidokoro Y, Lashley T, Rostagno A, Neubert TA, Bojsen-Moller M, Braendgaard H, Plant G, Holton J, Frangione B, Revesz T, Ghiso J. Familial Danish dementia: co-existence of Danish and Alzheimer amyloid subunits (ADan and Abeta) in the absence of compact plaques. J Biol Chem. 2005;280(44):36883–36894. doi: 10.1074/jbc.M504038200. [DOI] [PubMed] [Google Scholar]
- Tomidokoro Y, Tamaoka A, Holton JL, Lashley T, Frangione B, Revesz T, Rostagno A, Ghiso J. Pyroglutamate formation at the N-termini of ABri molecules in familial British dementia is not restricted to the central nervous system. Hirosaki Igaku. 2010;61(Suppl):S262–S269. [PMC free article] [PubMed] [Google Scholar]
- Vidal R, Frangione B, Rostagno A, Mead S, Revesz T, Plant G, Ghiso J. A stop-codon mutation in the BRI gene associated with familial British dementia. Nature. 1999;399(6738):776–781. doi: 10.1038/21637. [DOI] [PubMed] [Google Scholar]
- Vidal R, Revesz T, Rostagno A, Kim E, Holton JL, Bek T, Bojsen-Moller M, Braendgaard H, Plant G, Ghiso J, Frangione B. A decamer duplication in the 3' region of the BRI gene originates an amyloid peptide that is associated with dementia in a Danish kindred. Proc Natl Acad Sci U S A. 2000;97(9):4920–4925. doi: 10.1073/pnas.080076097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh DM, Tseng BP, Rydel RE, Podlisny MB, Selkoe DJ. The oligomerization of amyloid beta-protein begins intracellularly in cells derived from human brain. Biochemistry. 2000;39(35):10831–10839. doi: 10.1021/bi001048s. [DOI] [PubMed] [Google Scholar]
- Wirths O, Bethge T, Marcello A, Harmeier A, Jawhar S, Lucassen PJ, Multhaup G, Brody DL, Esparza T, Ingelsson M, Kalimo H, Lannfelt L, Bayer TA. Pyroglutamate Abeta pathology in APP/PS1KI mice, sporadic and familial Alzheimer's disease cases. J Neural Transm. 2010a;117(1):85–96. doi: 10.1007/s00702-009-0314-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirths O, Breyhan H, Cynis H, Schilling S, Demuth HU, Bayer TA. Intraneuronal pyroglutamate-Abeta 3–42 triggers neurodegeneration and lethal neurological deficits in a transgenic mouse model. Acta Neuropathol. 2009;(118):487–496. doi: 10.1007/s00401-009-0557-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirths O, Erck C, Martens H, Harmeier A, Geumann C, Jawhar S, Kumar S, Multhaup G, Walter J, Ingelsson M, Degerman-Gunnarsson M, Kalimo H, Huitinga I, Lannfelt L, Bayer TA. Identification of low molecular weight pyroglutamate A{beta} oligomers in Alzheimer disease: a novel tool for therapy and diagnosis. J Biol Chem. 2010b;285(53):41517–41524. doi: 10.1074/jbc.M110.178707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirths O, Multhaup G, Czech C, Feldmann N, Blanchard V, Tremp G, Beyreuther K, Pradier L, Bayer TA. Intraneuronal APP/A beta trafficking and plaque formation in beta-amyloid precursor protein and presenilin-1 transgenic mice. Brain Pathol. 2002;12(3):275–286. doi: 10.1111/j.1750-3639.2002.tb00442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.