Abstract
Childhood maltreatment (CM) is a strong risk factor for development of posttraumatic stress disorder (PTSD) upon adult exposure to extreme adverse events. However, the neural underpinnings of this relationship are not well understood. Here, we test the hypothesis that severity of CM history is positively correlated with emotion-processing limbic and prefrontal brain activation/connectivity and negatively correlated with prefrontal gray matter volumes in women with PTSD due to intimate-partner violence (IPV-PTSD). Thirty-three women with IPV-PTSD underwent structural and functional magnetic resonance imaging while completing a facial emotion processing task. Multivariate regressions examined the relationship of CM to patterns of activation, connectivity, and gray matter volumes. CM severity was: a) positively correlated with ventral ACC activation while processing angry faces; b) negatively correlated with dorsal ACC and insula activation while processing fear and angry faces, arising from positive correlations with the shape-matching baseline; c) positively correlated with limbic-prefrontal connectivity while processing fear faces but negatively correlated with amygdalo-insular connectivity while processing fear and angry; and d) negatively correlated with prefrontal gray matter volumes. These results suggest CM exposure may account for variability in limbic/prefrontal brain function and prefrontal structure in adulthood PTSD and offer one potential mechanism through which CM confers risk to future development of PTSD.
Keywords: early life stress, anxiety, imaging, trauma, abuse, neglect
1. Introduction
Intimate-partner violence (IPV) is a leading cause of posttraumatic stress disorder (PTSD) among adult women (Pico-Alfonso, 2005). PTSD is an anxiety disorder which may develop following a traumatic experience and is characterized by reexperiencing of the traumatic event, avoidance of trauma-related people/places/stimuli or avoidance of discussing or thinking about the trauma, emotional numbing or restricted range of affect, and symptoms of hyperarousal (American Psychiatric Association, 2000). Prior PTSD neuroimaging studies have identified structural and emotion-processing functional differences in affective, limbic/paralimbic, and prefrontal brain structures such as the amygdala, insula, anterior cingulate (ACC), and prefrontal cortex (PFC) (Liberzon and Sripada, 2008). The amygdala, a core affective brain structure consistently implicated in the pathophysiology of PTSD (Rauch et al., 2006), is comprised of gray matter nuclei located within the medial temporal lobe which processes the emotional/motivational salience of an exteroceptive stimulus (Costafreda et al., 2008). Its intact functioning seems to be particularly crucial for the generation of fear responses (Feinstein et al., 2011) and acquisition of conditioned fear (Duvarci et al., 2011). The insula is a cortical structure buried within the Sylvian fissure behind the superior temporal lobe which is crucial for the representation and processing of interoceptive (i.e. internal bodily) signals thought to form the basis for subjective emotional states (Craig, 2009). The ACC and associated medial prefrontal cortices (mPFC) are frontal structures involved in higher-order constructs such as self-referential processing (Blair et al., 2008), emotional/cognitive context (Rougemont-Bucking et al., 2010), and social cognition (Amodio and Frith, 2006) while the lateral prefrontal cortices are implicated in more deliberate or effortful mental states such as emotion regulation (Campbell-Sills et al., 2011), attentional manipulation (Sharp et al., 2010), or executive function (Ball et al., 2011). These higher-order frontal structures are often implicated in the inhibition of limbic activity to emotional stimuli (Schulz et al., 2009) and also have shown consistent abnormalities in PTSD neuroimaging studies (Francati et al., 2007).
Prior PTSD imaging studies have generally observed decreased grey matter volumes and increased reactivity of the amygdala and insula to threat-related emotional cues such as fearful or angry faces (Liberzon and Garfinkel, 2009; Woodward et al., 2009), although decreased amygdala activation and null findings for amygdalar structural differences have been observed as well (Etkin and Wager, 2007; Woon and Hedges, 2008). This increased reactivity is thought to underlie the hypersensitivity to threatening cues and dysregulated fear responses which are characteristic of the disorder (Lanius et al., 2006). Studies have also observed both decreased ACC/prefrontal structural volumes (Rogers et al., 2009) as well as no volumetric differences (Landre et al., 2010), while functional studies have observed both increased (Bryant et al., 2008) and decreased (Shin et al., 2005; Kim et al., 2008) ACC/medial PFC responses to emotional stimuli in PTSD. These functional differences have often been interpreted as either compensatory engagement to limbic hyperactivity (Bryant et al., 2008) or as deficient inhibition of limbic reactivity (Shin et al., 2005), respectively (due to the crucial role of these structures in downregulating amygdalar responses (Quirk et al., 2003)). Furthermore, the directionality of these ACC/mPFC group differences seems to depend upon several factors such as location in the dorsal “cognitive” subdivision or the more ventral “emotional” subdivision (Morey et al., 2008) as well as level of autonomic arousal (Felmingham et al., 2009) and presence of dissociative or depressive symptoms (Kemp et al., 2007; Felmingham et al., 2008). Furthermore, abnormalities of functional connectivity among and within limbic and prefrontal structures in PTSD have also shown variability. One study observed increased functional connectivity between the insula and ACC during emotional threat processing (Fonzo et al., 2010) while another observed decreased connectivity during symptom provocation (Lanius et al., 2004), while findings for decreased amygalar-insular connectivity have been more homogenous (Simmons et al., 2008; Fonzo et al., 2010) . Findings for abnormalities of amygdala-ACC connectivity have also varied, sometimes as a function of task conditions (Gilboa et al., 2004; St Jacques et al., 2011). In aggregate, there is a great deal of variability in functional and structural abnormalities associated with PTSD which may arise from clinical and demographic characteristics.
Little is known concerning characteristics of brain structure and function present before the traumatic experience that may influence or predispose individuals to the development of PTSD and/or promote variability in brain structure/function following the traumatic experience. Research indicates childhood maltreatment (CM) experiences, broadly defined as the intentional or unintentional commission of acts or withholding of resources by caregivers that adversely influence the health, growth, or adaptation of the child, convey an increased risk for the onset of anxiety or traumatic stress disorders in adulthood (Lang et al., 2008; Zlotnick et al., 2008), but the neural mechanism(s) underlying this predisposition are not well understood. Given findings that history of CM is associated in adulthood with structure and function of PTSD-relevant brain regions (e.g., amygdala, ACC, and PFC; Cohen et al., 2006; Williams et al., 2009; Croy et al., 2010; Hanson et al., 2010) as well as the substantial heterogeneity of brain responses observed in adulthood PTSD (Lanius et al., 2006), the evidence suggests risk factors such as preexisting CM history may influence brain structure and function in a way that contributes to development or severity of PTSD and/or variability in subsequent neural structure/function following exposure to trauma in adulthood. Although neural substrates affected by CM have been studied in the context of CM-related PTSD (Bremner et al., 2005) and in non-clinical populations (Mueller et al., 2010), characterizing the neural correlates of a developmental risk factor for PTSD following trauma exposure in adulthood may provide useful information for hypothesizing potential neural mechanisms underlying this relationship.
The primary purpose of this investigation was to examine how CM history relates to patterns of brain structure and emotion-processing function in women with IPV-PTSD. Prior studies have shown that both CM and PTSD are associated with reduced prefrontal structural volumes (Cohen et al., 2006; Karl et al., 2006; Hanson et al., 2010), hyperactivation of the amygdala, insula, ACC, and prefrontal cortex (PFC) (Williams et al., 2009; Croy et al., 2010; Fonzo et al., 2010; Mueller et al., 2010), and increased frontal-amygdalar connectivity (Gilboa et al., 2004; Taylor et al., 2006; St. Jacques et al., 2010). Therefore, we chose a facial emotion processing task which robustly engages these neural substrates as our experimental paradigm of interest (Hariri et al., 2005). Prior evidence indicates that CM influences behavioral responses to angry and fearful faces, with trend-level effects for happy faces as well (Masten et al., 2008; Gibb et al., 2009). Thus, neural responses to these three emotional expressions were examined. Consistent with convergent findings for increased limbic/prefrontal activation and connectivity and reductions in prefrontal structural volumes in both PTSD (Karl et al., 2006; Bryant et al., 2008; St Jacques et al., 2011) and maltreated samples (Cohen et al., 2006; Williams et al., 2009; Hanson et al., 2010; Mueller et al., 2010), we expected severity of CM to be negatively correlated with prefrontal gray matter volumes and positively correlated with amygdala, insula, ACC, and PFC activation and frontal-amygdalar connectivity in all three emotional face processing conditions.
2. Methods
2.1 Subjects
Thirty-three women (n=33) with DSM-IV PTSD as a result of exposure to IPV were recruited through community advertisement to participate in functional magnetic resonance imaging (fMRI; Table S1 in supplement). IPV trauma was operationalized as physical and/or sexual abuse by a romantic partner occurring within 5 years of study recruitment and ending at least 1 month prior to enrollment. All women in the IPV-PTSD group met full DSM-IV criteria for PTSD, verified through the Clinician-Administered PTSD Scale (CAPS) (Blake et al., 1995) and the Structured Clinical Interview for Diagnosis-DSM IV (SCID-IV) (First et al., 1998) administered by a doctoral-level clinical psychologist in an outpatient treatment center. Exclusion criteria included: 1) substance abuse in the past year; 2) history of greater than 2 years of alcohol abuse; 3) use of psychotropic medications in the past 4 weeks (or fluoxetine in the past 6 weeks); and 4) irremovable ferromagnetic bodily material, pregnancy, claustrophobia, bipolar disorder, or schizophrenia. IPV-PTSD subjects with comorbid depressive/anxiety disorders were included as long as PTSD was judged to be the clinically predominant disorder. After complete description of the study to subjects, written informed consent was obtained. The study protocol was approved by the UCSD Human Research Protections Program and the VA San Diego Healthcare System Research and Development Office and is in compliance with the guidelines laid down in the Declaration of Helsinki (Anonymous, 1968).
2.2 Self-report maltreatment/symptom measures
The total score from the 28-item Short Form version of the Childhood Trauma Questionnaire (CTQ-SF) (Bernstein et al., 2003) was used to assess extent of CM exposure. This measure encompasses 25 items rated on a 5 point Likert scale (1= “Never true”, 5 = “Very often true”) that assesses the subscale domains of emotional abuse, emotional neglect, physical abuse, physical neglect, and sexual abuse. A total score is calculated by summing the totals for each subscale. The CTQ has been demonstrated to have good test-retest reliability, high internal reliability, good convergent validity with clinician-rated measures of childhood maltreatment, and display measurement equivalence across gender and ethnic groups (Bernstein et al., 1994; Thombs et al., 2007). Clinical variables of no interest were assessed to control for potential confounding effects. The Beck Depression Inventory-II (BDI) (Beck et al., 1961) was used to assess current depressive symptoms. Chronicity of IPV trauma was quantified using the self-reported number of years of exposure to IPV within the most recent abusive relationship. PTSD symptom severity was quantified using the total score from the CAPS.
2.3 Task
Participants completed a modified version of the Emotion Face Assessment Task (Hariri et al., 2005; Paulus et al., 2005). For each 5-second trial, subjects were presented with a target face on the top of the screen and instructed to match its facial expression to one of two faces presented below on the same screen through key-press of a button box. A block consisted of six consecutive trials wherein the target face was angry, happy, or fearful. A sensorimotor control condition, in which a target shape was presented and subjects were told to pick the matching shape, was also presented in similar format. Each target condition was presented in three blocks of six trials each in pseudorandomized order, with an eight-second fixation crossed presented between each block and at the beginning and end of the task. The task lasted 512 seconds, and behavioral data was recorded for each trial.
2.4 Image acquisition
See supplemental methods.
2.5 Activation preprocessing/individual-level analysis
See supplemental methods.
2.6 Functional connectivity preprocessing/individual-level analyses
See supplemental methods.
2.7 Activation/connectivity task effect analyses
Brain regions significantly activated/deactivated by each of the task contrasts or displaying significant positive/negative task-evoked connectivity with seed regions were identified using a voxelwise one-sample t-test of percent signal changes (PSCs) and psychophysiological interaction (PPI) Fisher-Z transformed correlation coefficients (rFzs) against the null hypothesis, respectively. In addition to an exploratory whole-brain (WB) analysis, voxelwise analyses constrained within a-priori region of interests (ROIs) were conducted on limbic brain regions previously implicated in studies of PTSD and CM: bilateral insula, bilateral amygdala, and ACC. Boundaries of these ROIs were based upon both anatomical criteria (see supplementary methods) and standardized locations taken from the Talairach atlas (Talairach and Tournoux, 1998). A threshold adjustment based upon Monte-Carlo simulations (using AFNI's program AlphaSim) was used to guard against false positives in both the WB and ROI analyses. A-priori voxelwise probability threshold of p < 0.05 with a 4mm search radius and cluster size of 320 μl (5 contiguous voxels) for the amygdala, 960 μl (15 contiguous voxels) for the ACC and insula, and 3968 μl (62 contiguous voxels) for the WB analysis resulted in a-posteriori probability of p < 0.05 in each constrained region. Average PSCs or rFzs were extracted from areas of significant association and subjected to further analysis in SPSS 15.0 for confirmation of significance.
2.8 Optimized voxel-based morphometry
See supplemental methods.
2.9 Robust regression of individual activation/connectivity/GM values
To identify activation/connectivity/GM volumes (GMVs) associated with CM in each contrast, whole-brain PSC/rFz/GMV data were analyzed using a “robust” multivariate linear regression implemented within the statistical package R (Huber, 1964; Venables and Ripley, 2002; R Development Core Team, 2011). Regression models were fit to group-level voxelwise GMVs and PSCs/rFzs for each of the three face conditions and/or seed regions. The model explored the contribution of CM to the post-traumatic brain state by simultaneously regressing voxelwise PSCs/rFzs/GMVs on CM (total CTQ score) as well as the following covariates of no interest to control for potential confounding effects: depression (BDI-II total score), IPV trauma chronicity (number of years of IPV exposure), and PTSD symptom severity (CAPS total score). For the VBM analysis, age was also added as a nuisance variable due to the well-characterized relationships between age and GMV (Grieve et al., 2011). CM effects were thresholded at p < 0.05 and only clusters meeting the minimum spatial extent to maintain the α level at 0.05 in each search region were considered significant. To correct for multiple comparisons and further reduce likelihood of Type-I error, we required correlations of CTQ total scores and post-hoc extracted activation estimates in significant clusters to meet a Bonferonni corrected p-value of p < 0.017 (0.05 divided by 3 task contrasts), and we required correlations of CTQ total scores and post-hoc extracted connectivity estimates in significant clusters to meet a Bonferonni corrected p-value of 0.05 divided by the number of seed regions examined in each contrast.
3. Results
3.1 Sample demographics/comorbidity
All of the participants met full criteria for PTSD due to IPV according to DSM-IV criteria. Participants mean age was 39.27 years (sd = 8.46, range = 23-56), and the mean number of years of education was 13.58 (sd = 1.95, range = 10-18). The mean number of years of exposure to IPV was 5.98 (sd = 5.70, range = 0.5-27). Although we required IPV-PTSD to be the predominant clinical disorder, slightly over half of the participants (18 of 33) met diagnostic criteria for current major depressive disorder, while 9 of 33 met current criteria for panic disorder and 13 of 33 met current criteria for generalized anxiety disorder1. These rates of comorbidity are consistent with those observed in other PTSD samples (Stein and Kennedy, 2001; Kessler et al., 2005).
3.2 Self-report and symptom severity measures
The mean CAPS total score was 80.45 (sd = 17.9, range = 50-117), and the mean CTQ total score was 59.12 (sd = 23.23, range = 25-111). Across all five subscales of the CTQ, the participants reported on average moderate levels of maltreatment which ranged from none (subscale score of 5-9, depending upon the particular subscale) to extreme severity (greater than 13-18, depending upon the particular subscale) (Bernstein and Fink, 1998). The mean BDI-II total score was a 21.69 (sd = 9.47, range = 2-41), indicating on average moderate severity of depressive symptoms which ranged from minimal to severe (Beck et al., 1996). None of the factors entered into the regression model were significantly correlated (all p's greater than 0.05) (Table S1 in supplement).
3.3 Behavioral data
See supplemental results.
3.4 Functional activation
3.4.1 Angry vs. shapes contrast
3.4.1.1 Task effect
Analysis constrained to a limbic ROI mask revealed significant activation of the bilateral amygdala and the left posterior insula. A WB analysis demonstrated significant activation of the bilateral lingual gyri, fusiform gyri, declive, parahippocampal gyri, middle occipital gyri, superior/middle temporal gyri, and middle frontal gyri (dorsolateral PFC) (Table S3 in supplement).
3.4.1.2 Correlations with childhood maltreatment
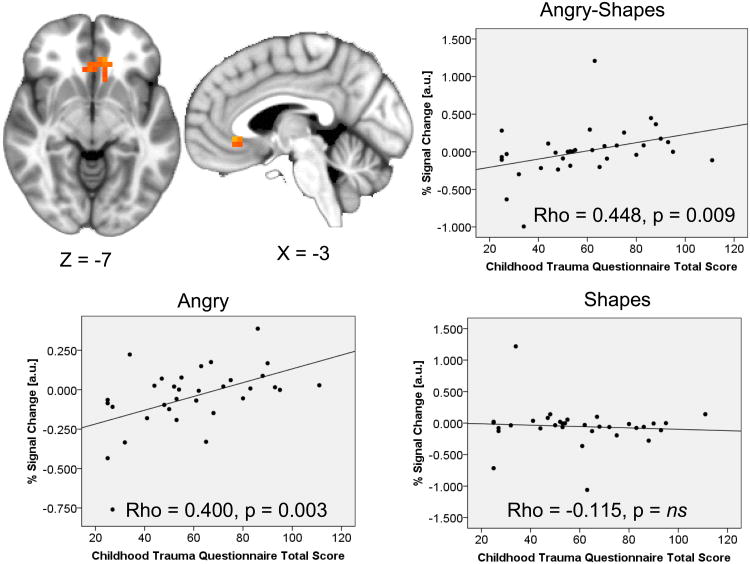
A multivariate regression constrained within a limbic ROI mask revealed CTQ total score was positively correlated with a cluster of activation in the ventral ACC (Figure 1) and negatively correlated with activation in the left middle insula and the right dorsal ACC. Post-hoc extraction estimates within each task condition (angry and shape-matching) revealed the positive correlation in the ventral ACC was due to a positive correlation between CTQ total score and activation during the angry (Spearman's rho = 0.499, p = 0.003) but not shape-matching condition (correlation non-significant), while the negative correlations in the left middle insula and dorsal ACC were due to significant or trend-level positive correlations between CTQ total score and activation during the shape-matching condition (left middle insula: Spearman's rho = 0.319, p = 0.07; dorsal ACC: Spearman's rho = 0.431, p = 0.012) but no relationship during matching of angry faces (correlations nonsignificant). A WB analysis also identified activation in the dorsal ACC extending into the dorsal medial frontal gyri that was negatively correlated with CTQ total score. Post-hoc extractions revealed this effect was also due to a positive correlation between CTQ total score and activation during the shape-matching condition (Spearman's rho = 0.457, p = 0.007) but no relationship during the angry face matching condition (correlation nonsignificant) (Table 1).
Figure 1. Severity of childhood maltreatment correlates with ventral anterior cingulate activation while processing angry faces.
Scatterplots represent extracted cluster % signal changes against Childhood Trauma Questionnaire Total Score; Scatterplots for the within-subject contrast and for both angry and shape-matching are presented; Results displayed on an average anatomical; au=arbitrary units; ns=nonsignificant.
Table 1. Functional activation correlates of childhood maltreatment during angry face vs. shape processing.
| Mask | Hemisphere | Anatomical Area | Volume(μl) | X | Y | Z | Voxelwise Stats: Mean (SD) | Extracted rho (sig)† | ΔR2(sig.) † | Group Diff.† | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| T | p | T, p | |||||||||
| ROI | L/R | Anterior Cingulate (v) | 1344 | −5 | 30 | −7 | 2.59(0.51) | 0.021(0.017) | 0.448(0.009) | 0.142(0.036) | 2.882, 0.007 |
| ROI | L | Insula (m)(−) | 1216 | −41 | −5 | 4 | −2.67(0.42) | 0.019(0.016) | −0.423(0.014) | 0.141(0.037) | −2.940, 0.006 |
| ROI | R | Anterior Cingulate (d)(−) | 960 | 6 | 31 | 17 | −2.54(0.33) | 0.021(0.015) | −0.542(0.001) | 0.269(0.003) | −3.586, 0.001 |
| WB | L/R | Anterior Cingulate/Medial Frontal Gyrus (d)(−) |
11392 | −12 | 12 | 28 | −2.54(0.39) | 0.022(0.014) | −0.697(<0.001) | 0.387(<0.001) | −4.196, <0.001 |
Cluster coordinates are for cluster center of mass as defined by Talairach stereotactic space; Negative (-) signs following anatomical area indicate a negative relationship; Extracted rho is the spearman's correlation of extracted % signal change and Childhood Trauma Questionnaire total score; ΔR2 represents the change in full model R2 from inclusion of CTQ total score in a post-hoc regression of extracted cluster % signal changes against other model factors; Group Diff. column represents the post-hoc t-test (High CM vs. Low CM) of extracted % signal changes based upon a median split of IPV-PTSD sample into low and high CM-exposure groups; Descriptors for anatomical areas do not reflect stereotactic distinctions, but are estimates based upon the relative location of activation on the group map; Statistics with a † indicate a circular estimate and therefore may be inflated estimates of the true population parameter; d=dorsal; L=left; m=middle; R=right; ROI=region of interest; v=ventral; WB=whole-brain.
3.4.2 Fear vs. shapes contrast
3.4.2.1 Task effect
Analysis constrained to a limbic ROI mask revealed significant activation of the bilateral amygdala, bilateral anterior insula, and left posterior insula. A WB analysis revealed significant activation of the bilateral amygdala, globus pallidus/putamen, caudate, thalamus, declive, culmen, lingual gyri, fusiform gyri, parahippocampal gyri, middle occipital gyri, superior/middle temporal gyri, posterior insula, posterior cingulate, precuneus, middle frontal gyri (dorsolateral PFC), and dorsal medial frontal gyri (Table S4 in supplement).
3.4.2.2 Correlations with childhood maltreatment
A voxelwise multivariate regression constrained to a limbic ROI mask revealed CTQ total score was negatively correlated with activation in the right anterior insula and the right dorsal ACC. Post-hoc extraction estimates within each task condition (fear and shape-matching) revealed the negative correlations in the right anterior insula and dorsal ACC were both due to significant or trend-level positive correlation between CTQ total score and activation during the shape-matching condition (right anterior insula: Spearman's rho = 0.306, p = 0.083; dorsal ACC: Spearman's rho = 0.424, p = 0.014) but no relationship during the fearful face matching condition (correlations nonsignificant). A WB analysis identified a large cluster spanning the dorsal ACC and dorsal medial/middle frontal gyri in which activation was also negatively correlated with CTQ total score. Post-hoc extractions within each condition revealed this effect was also due to a positive correlation between CTQ total score and activation within the shape-matching condition (Spearman's rho = 0.590, p < 0.001) but no correlation with activation during the fearful face condition (correlation nonsignificant) (Table 2).
Table 2. Functional activation correlates of childhood maltreatment during fearful face vs. shape processing.
| Mask | Hemisphere | Anatomical Area | Volume(μl) | X | Y | Z | Voxelwise Stats: Mean (SD) | Extracted rho (sig) † | ΔR2(sig.) † | Group Diff.† | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| T | p | T, p | |||||||||
| ROI | R | Insula (a)(−) | 1728 | 35 | 18 | 6 | −2.76(0.58) | 0.018(0.016) | −0.521(0.002) | 0.175(0.008) | −3.239, 0.003 |
| ROI | R | Anterior Cingulate (d)(−) | 960 | 12 | 34 | 15 | −2.84(0.69) | 0.016(0.014) | .0.444(0.010) | 0.183(0.015) | −3.688,0.001 |
| WB | L/R | Anterior Cingulate/Medial Fronal Gyrus/Middle Frontal Gyrus (d)(−) |
38784 | 3 | 41 | 14 | −2.57(0.46) | 0.022(0.014) | −0.629(<0.001) | 0.329(<0.001) | −3.568, 0.001 |
Cluster coordinates are for cluster center of mass as defined by Talairach stereotactic space; Negative (−) signs following anatomical area indicate a negative relationship; Extracted rho is the spearman's correlation of extracted % signal change and Childhood Trauma Questionnaire total score; ΔR2 represents the change in full model R2 and significance from inclusion of CTQ total score in a post-hoc regression of extracted cluster % signal changes against other model factors; Group Diffi column represents the post-hoc t-test (High CM vs. Low CM) of extracted % signal changes based upon a median split of IPV-PTSD sample into low and high CM-exposure groups; Descriptors for anatomical areas do not reflect stereotactic distinctions, but are estimates based upon the relative location of activation on the group map; Statistics with a † indicate a circular estimate and therefore may be inflated estimates of the true population parameter; a=anterior; d=dorsal; L=left; R=right; ROI=region of interest; WB=whole-brain.
3.4.3 Happy vs. shapes contrast
3.4.3.1 Task effect
Analysis constrained to a limbic ROI mask revealed significant activation of the bilateral amygdala and bilateral posterior insula. A WB analysis revealed significant activation of the bilateral amygdala, globus pallidus/putamen, caudate, thalamus, declive, culmen, lingual gyri, fusiform gyri, parahippocampal gyri, middle occipital gyri, superior/middle temporal gyri, posterior cingulate, precuneus, and right middle frontal gyrus (dorsolateral PFC) and significant deactivation of the bilateral inferior parietal lobule (Table S5 in supplement).
3.4.3.2 Correlations with childhood maltreatment
Both limbic-ROI constrained and WB analyses revealed no significant clusters of activation that were correlated with CTQ total score for this contrast.
3.5 Psychophysiological Interactions
Since there were no significant relationships between activation for the happy vs. shapes contrast and CTQ total scores, psychophysiological interactions and their relationships with childhood maltreatment were examined only for the angry vs. shapes and fear vs. shapes contrasts. Clusters in limbic and prefrontal regions that were significantly activated by the task contrast were used as seed regions. We examined connectivity in 5 seed regions for the angry vs. shapes contrast; thus, the a-priori threshold for correlations of extracted connectivity estimates and CTQ total scores was set at p < 0.01. In the fear vs. shapes contrast, we examined connectivity in 7 seed regions; thus, the a-priori threshold for correlations between extracted connectivity estimates and CTQ total scores was set at p < 0.007.
3.5.1 Angry vs. shapes contrast
3.5.1.1 Task effects
3.5.1.2 Correlations with childhood maltreatment
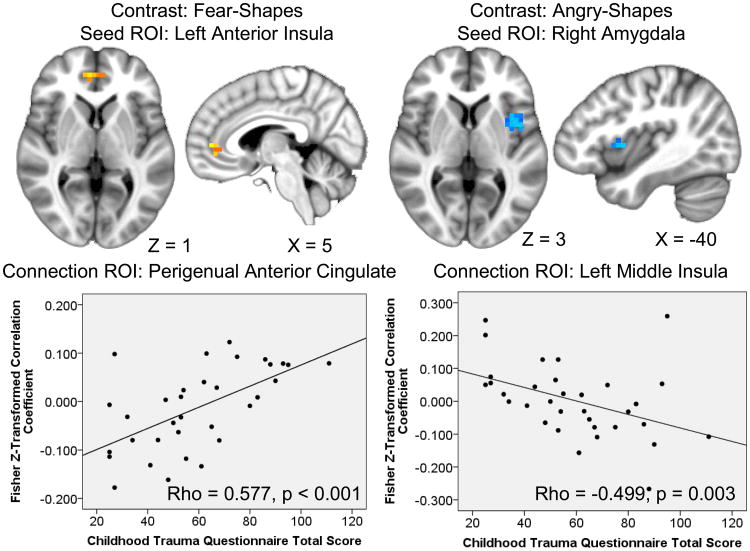
Multivariate regressions revealed no significant correlations of CTQ total score with right amygdala task-evoked connectivity. Connectivity between the left posterior insula task effect seed and a cluster in the right amygdala was negatively correlated with CTQ total score. Connectivity between the left amygdala task effect seed and a cluster in the left middle insula was also negatively correlated with CTQ total scores. Connectivity between the left middle frontal gyrus task effect seed and two clusters spanning the bilateral culmen, declive, fusiform gyri, lingual gyri, and parahippocampal gyri were positively correlated with CTQ total scores. Connectivity between the right middle frontal gyrus task effect seed and a cluster in the right perigenual ACC was positively correlated with CTQ total score (Table 3).
Table 3. Functional connectivity correlates of childhood maltreatment during angry face vs. shape processing.
| Seed | Mask | Hemisphere | Anatomical Area | Volume(μl) | X | Y | Z | Voxelwise Stats: Mean (SD) | Extracted rho (sig) † | ΔR2(sig.) † | Group Diff.† | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T | p | T, p | ||||||||||
| RAmyg | -- | -- | No significant associations | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| LPIns | ROI | R | Amygdala (−) | 320 | 23 | −3 | −12 | −2.58(0.41) | 0.020(0.017) | −0.503(0.003) | 0.178(0.016) | −2.274, 0.030 |
| LAmyg | ROI | L | Insula (m)(−) | 960 | −41 | 5 | 3 | −2.75(0.47) | 0.016(0.015) | −0.499(0.003) | 0.193(0.009) | −2.045, 0.049 |
| LMFG | WB | L | Culmen/Declive/Fusiform Gyrus/Parahippocampal Gyrus |
7552 | −11- | −47 | −10 | 2.60(0.51) | 0.022(0.015) | 0.599(<0.001) | 0.335(<0.001) | 3.943, <0.001 |
| LMFG | WB | R | Culmen/Declive/Lingual Gyrus/Parahippocampal Gyrus |
4352 | 18 | −62 | −9 | 2.65(0.44) | 0.019(.013) | 0.558(0.001) | 0.258(0.001) | 3.870, 0.001 |
| RMFG | ROI | R | Anterior Cingulate (pg) | 960 | 5 | 39 | −4 | 2.89(0.83) | 0.021(0.017) | 0.500(0.003) | 0.225(0.007) | 2.239, 0.032 |
Cluster coordinates are for cluster center of mass as defined by Talairach stereotactic space; Negative (−) signs following anatomical area indicate a negative relationship; Extracted rho is the spearman's correlation of extracted Fisher Z-transformed correlation coefficient and Childhood Trauma Questionnaire total score; ΔR2 represents the change in full model R2 and significance from inclusion of CTQ total score in a post-hoc regression of extracted Fisher Z-transformed correlation coefficients against other model factors; Group Diff. column represents the post-hoc t-test (High CM vs. Low CM) of extracted Fisher Z-transformed correlation coefficients based upon a median split of IPV-PTSD sample into low and high CM-exposure groups; Descriptors for anatomical areas do not reflect stereotactic distinctions, but are estimates based upon the relative location of activation on the group map; Seed regions and descriptors were derived from task-dependent activation for the contrast of interest; Statistics with a † indicate a circular estimate and therefore may be inflated estimates of the true population parameter; L=left; LAmyg=left amygdala; LMFG=left middle frontal gyrus; LPIns=left posterior insula; m=middle; pg=perigenual; R=right; ROI=region of interest; RAmyg=right amygdala; RMFG=right middle frontal gyrus; WB=whole-brain.
3.5.2 Fear vs. shapes contrast
3.5.2.1 Task effects
3.5.2.2 Correlations with childhood maltreatment
CTQ total scores were negatively correlated with connectivity between the left amygdala task effect seed and a cluster in the right posterior insula but positively correlated with connectivity between the left amygdala task effect seed and a cluster in the ventral ACC. Connectivity values for the right amygdala task effect seed displayed no significant correlations with CTQ total scores. Connectivity between the left anterior insula task effect seed and clusters in the right posterior insula, perigenual ACC, and the mid-cingulate/medial frontal gyri were positively correlated with CTQ total scores. Connectivity between the left posterior insula task effect seed and a cluster in the right posterior insula was positively correlated with CTQ total scores. Connectivity values from the left middle frontal gyrus task effect seed displayed no significant correlations with CTQ total scores. Connectivity values from the right middle frontal gyrus task effect seed displayed no significant correlations with CTQ total scores. Connectivity between the dorsal medial frontal gyri task effect seed and two clusters in the bilateral posterior insula were positively correlated with CTQ total scores (Table 4).
Table 4. Functional connectivity correlates of childhood maltreatment during fearful face vs. shape processing.
| Seed | Mask He | tnisphere | Anatomical Area | Volume(μl) | X | Y | Z | Voxelwise Stats: Mean (SD) | Extracted rho (sig) † | ΔR2(sig.) † | Group Diff. † | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T | P | T, p | ||||||||||
| LAmyg | ROI | R | Insula (p)(−) | 1664 | 41 | −18 | 15 | −2.52(0.43) | 0.022(0.018) | −0.487(0.004) | 0.214(0.005) | −3.258, 0.003 |
| LAmyg | ROI | L/R | Anterior Cingulate (v) | 1024 | −1 | 40 | −6 | 2.57(0.41) | 0.019(0.016) | 0.583(<0.001) | 0.411(<0.001) | 3.927, <0.001 |
| RAmyg | -- | -- | No significant associations |
-- | -- | -- | -- | -- | -- | -- | -- | -- |
| LAIns | ROI | L/R | Anterior Cingulate (pg) | 960 | 3 | 44 | 1 | 2.75(0.36) | 0.013(0.010) | 0.577(<0.001) | 0.336(<0.001) | 4.357, <0.001 |
| LAIns | ROI | R | Insula (p) | 960 | 44 | −20 | 15 | 2.93(0.70) | 0.014(0.014) | 0.695(<0.001) | 0.419(<0.001) | 3.752, 0.001 |
| LAIns | WB | L/R | Cingulate Gyrus/Medial Frontal Gyrus (SMA) |
3968 | −1 | −15 | 50 | 2.72(0.60) | 0.016(0.013) | 0.746(<0.001) | 0.422(<0.001) | 4.698, <0.001 |
| LPIns | ROI | R | Insula (p) | 1024 | 40 | −17 | 16 | 2.67(0.34) | 0.015(0.012) | 0.515(0.002) | 0.256(0.003) | 4.125, <0.001 |
| LMFG | -- | -- | No significant associations |
-- | -- | -- | -- | -- | -- | -- | -- | -- |
| RMFG | -- | -- | No significant associations | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| DMedFG | ROI | L | Insula (p) | 960 | −41 | −22 | 16 | 2.78(0.96) | 0.024(0.025) | 0.573(<0.001) | 0.370(<0.001) | 3.876, 0.001 |
| DMedFG | ROI | R | Insula (p) | 960 | 44 | −21 | 17 | 2.57(0.39) | 0.016(0.014) | 0.516(0.002) | 0.288(0.001) | 2.996, 0.005 |
Cluster coordinates are for cluster center of mass as defined by Talairach stereotactic space; Negative (-) signs following anatomical area indicate a negative relationship; Extracted rho is the spearman's correlation of extracted Fisher Z-transformed correlation coefficient and Childhood Trauma Questionnaire total score; ΔR2 represents the change in full model R2 and significance from inclusion of CTQ total score in a post-hoc regression of extracted Fisher Z-transformed correlation coefficients against other model factors; Group Diff. column represents the post-hoc t-test (High CM vs. Low CM) of extracted Fisher Z-transformed correlation coefficients based upon a median split of IPV-PTSD sample into low and high CM-exposure groups; Descriptors for anatomical areas do not reflect stereotactic distinctions, but are estimates based upon the relative location of activation on the group map; Seed regions and descriptors were derived from task-dependent activation for the contrast of interest; DMedFG=dorsal medial frontal gyri; L=left; LAmyg=left amygdala; LAIns=left anterior insula; LMFG=left middle frontal gyrus; Statistics with a † indicate a circular estimate and therefore may be inflated estimates of the true population parameter; LPIns=left posterior insula; m=middle; pg=perigenual; R=right; RAmyg=right amygdala; RMFG=right middle frontal gyrus; ROI=region of interest; SMA= supplementary motor area; WB=whole-brain.
3.6 Voxel-based morphometry
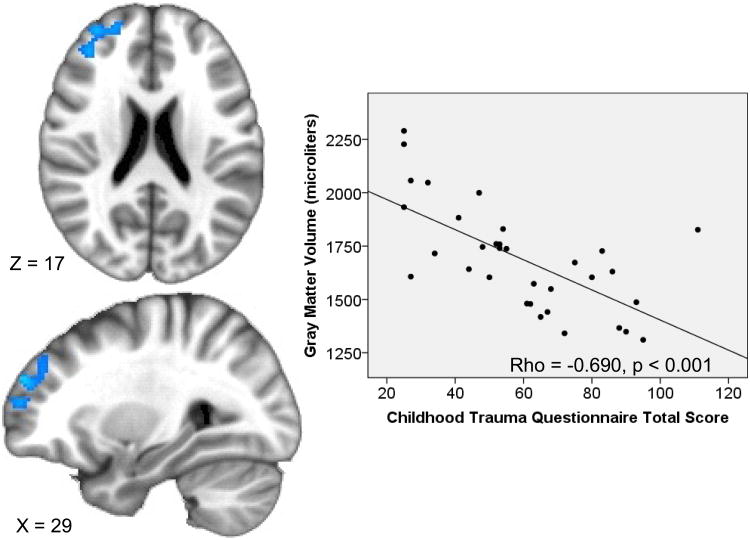
Limbic ROI-constrained analyses revealed there were no clusters in the amygdala, insula, or ACC in which GM volume correlated with CTQ total score after cluster-size correction for multiple comparisons. The WB analysis revealed GM volumes in the right inferior occipital, middle occipital, lingual gyri, and cuneus were negatively correlated with CTQ total score, as were GM volumes in the bilateral precentral/postcentral gyri. Additionally, GM volumes in the right middle and superior frontal gyri (dorsolateral PFC) were inversely correlated with CTQ total scores, while GM volumes in the left middle and superior temporal gyri extending into the left ventral mid-insula were positively correlated with CTQ total score (Table 5).
Table 5. Gray matter volumetric correlates of childhood maltreatment.
| Mask | Hemisphere | Anatomical Area | Volume (μl) | X | Y | Z | Voxelwise Stats: Mean (SD) | Extracted rho (sig) † | ΔR2(sig.) † | Group Diff. † | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| T | p | T, p | |||||||||
| ROI | -- | No significant associations | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| WB | L | Middle Temporal Gyrus/Superior Temporal Gyrus/Insula (m) |
7200 | −52 | 1 | −13 | 2.57(0.36) | 0.020(0.013) | 0.523(0.002) | 0.212(0.006) | 2.057, 0.048 |
| WB | R | Inferior Occipital Gyrus/Middle Occipital Gyrus/Lingual Gyrus/Cuneus (−) |
7064 | 14 | −90 | 0 | −2.58(0.38) | 0.021(0.014) | −0.664(<0.001) | 0.419(<0.001) | −2.930, 0.006 |
| WB | R | Precentral Gyrus/Postcentral Gyrus (−) |
4504 | 37 | −13 | 39 | −2.78(0.61) | 0.018(0.014) | −0.691(<0.001) | 0.371(<0.001) | −5.663, <0.001 |
| WB | L | Precentral Gyrus/Postcentral Gyrus (−) |
3992 | −46 | −12 | 37 | −2.57(0.39) | 0.021(0.013) | −0.540(0.001) | 0.371(<0.001) | −3.401, <0.001 |
| WB | R | Middle Frontal Gyrus/Superior Frontal Gyrus (−) |
3968 | 31 | 49 | 19 | −2.54(0.36) | 0.022(0.013) | −0.690(<0.001) | 0.423(<0.001) | −5.423, <0.001 |
Cluster coordinates are for cluster center of mass as defined by Talairach stereotactic space; Negative (−) signs following anatomical area indicate a negative relationship; Extracted rho is the spearman's correlation of extracted gray matter volume and Childhood Trauma Questionnaire total score; AR2 represents the change in full model R2 and significance from inclusion ofCTQ total score in a post-hoc regression of extracted cluster GM volumes against other model factors; Group Diff column represents the post-hoc t-test (High CM vs. Low CM) of extracted gray matter volumes based upon a median split oflPV-PTSD sample into low and high CM-exposure groups; Descriptors for anatomical areas do not reflect stereotactic distinctions, but are estimates based upon the relative location of activation on the group map; Statistics with a † indicate a circular estimate and therefore may be inflated estimates of the true population parameter; L=left; m=middle; R=right; ROI=region of interest; WB=whole-brain.
3.7 Complementary group-difference analyses
As a complementary method to assess neural correlates of CM and bolster significance of correlational findings, we split the sample of IPV-PTSD participants into high (n=16) and low (n=17) CM-exposure groups based upon a median split of CTQ total score (median score = 55; see Table S8 for group characteristics) and compared between groups the extracted % signal changes, Fisher Z-transformed PPI correlation coefficients, and gray matter volumes from clusters displaying significant correlational relationships with CTQ total score. The groups were well matched on age, ethnicity, comorbidity, years of education, PTSD/depression symptom severity, years of IPV abuse, and task performance measures. We observed that all functional and structural clusters displaying significant correlational relationships with CM severity also significantly differed between the high and low CM-exposure groups (see Group Diff. column in Tables 1-5), indicating these CM correlational relationships index significant differences in neural structure and function in women with IPV-PTSD with high vs. low exposure to CM.
4. Discussion
This study examined the hypothesis that severity of CM exposure relates to patterns of both brain structure and emotion-processing function in women with IPV-PTSD. The results support this hypothesis and revealed that CM severity may contribute to variability in brain structure and emotion-processing brain function in PTSD in adulthood. Specifically, extent of CM exposure correlated with prefrontal gray matter volume and activation/connectivity in and among the insula, ACC, and prefrontal cortices, brain structures that are important for processing, expression, and regulation of emotional states (Craig, 2009; Campbell-Sills et al., 2011) and which have been previously implicated in the functional neuroanatomy of PTSD (Lanius et al., 2004, 2006, 2010; Liberzon and Garfinkel, 2009). The correlations between CM severity and brain function in these regions provide further evidence for the notion that early life experiences characterized by stressful and/or maladaptive rearing conditions may exert enduring neural effects which persist into the posttraumatic state in adulthood and contribute to variability in brain function. In line with this idea prior studies have demonstrated convergent relationships between CM or early life stress and brain function in these regions in non-clinical samples (Taylor et al., 2006; Williams et al., 2009; Mueller et al., 2010). Furthermore, such neural effects may represent one potential mechanism whereby CM confers risk for development of PTSD upon adult exposure to extreme adverse events. Given observed relationships between variable function in these regions and characteristics of PTSD symptom manifestations (Hopper et al., 2007; Felmingham et al., 2008, 2009), such CM-related neural effects may also contribute to variability in relative predominance of specific PTSD symptom clusters. With this study we aimed to illuminate the neural correlates of a developmental risk factor for PTSD and a potential source of heterogeneity in PTSD functional/structural abnormalities, and we hope the present findings will encourage further research into the potential enduring neural effects of early life stressors such as CM and their relationship to the future development/manifestation of PTSD.
Contrary to our hypotheses, we observed negative correlations between CM severity and insula and dorsal ACC/PFC activation to fearful and angry faces. Post-hoc extraction estimates revealed these relationships were due to positive correlations between CM severity and activation during the sensorimotor baseline, suggesting CM severity was most strongly associated with variability in these regions during sensorimotor rather than emotional processing per se. The dorsal ACC/PFC and anterior insula have both been implicated in cognitive/attentional function in prior studies (Wager and Barrett, 2004; Torta and Cauda, 2011) and are considered part of a neural network implicated in evaluating and assigning motivational value to stimuli (Medford and Critchley, 2010), suggesting these relationships may have arisen from a CM-related compensatory attentional engagement or motivational appraisal of task demands. Although unexpected, these results are not inconsistent with prior studies of CM which have demonstrated impairments in cognitive control with concomitant functional abnormalities of prefrontal and insular regions (Carrion et al., 2008; Mueller et al., 2010). Furthermore, the negative correlation between CM severity and right dorsolateral prefrontal GM volumes is also indicative of a relationship between CM and potentially impaired or abnormal cognitive function in adulthood PTSD, given the role of this region in cognitive control and executive function (Savine and Braver, 2010; Ball et al., 2011). A recent study also observed a negative relationship between dorsolateral prefrontal cortical thickness and post-trauma PTSD symptom reductions (Lyoo et al., 2011), highlighting a potential neural structural characteristic which may relate CM to poor health outcomes in women with PTSD (Lang et al., 2008).
We did observe a positive correlation between ventral ACC activation and CM severity during the processing of angry faces which arose from the emotional face condition, suggesting CM relationships with IPV-PTSD prefrontal function also extend to the processing of emotional stimuli—consistent with prior findings in non-clinical samples (Williams et al., 2009). The ventral ACC/mPFC has been implicated in downregulation of amygdala activity (Quirk et al., 2003) and parasympathetic processes such as heart rate deceleration to emotional faces (Critchley et al., 2005) and fear extinction (Milad et al., 2009) which suggests those IPV-PTSD women with more severe history of CM may have deployed greater ventral ACC neural resources towards limbic inhibition in response to threat cues, consistent with the positive correlation between CM and ventral ACC-amygdalar functional connectivity observed during fear processing. Recent studies have illuminated variability in ACC function in PTSD as relating to several factors such as level of arousal (Felmingham et al., 2009) as well as predominantly dissociative or reexperiencing responses to trauma-related stimuli (Felmingham et al., 2008; Lanius et al., 2010), suggesting variability in this brain region may be particularly important in contributing to heterogeneity in symptom manifestations. These results highlight another potential source of ACC functional variability in PTSD, and further characterization of this relationship may help to reconcile seemingly contradictory findings in the literature.
No significant correlations between CM severity and amygdala activation to emotional faces were observed, which was contrary to expectations given other findings for CM relationships with amygdala function in non-psychiatric samples (Taylor et al., 2006; Williams et al., 2009). However, to our knowledge no prior studies have observed a dimensional relationship between CM or early life stress and amygdala activation but have instead demonstrated group differences, raising the possibility these differences may relate to other between-group factors rather than CM or early life stress per se. Additionally, amygdala activation in PTSD has been previously demonstrated to be associated with other factors such as depression (Kemp et al., 2007) and PTSD symptom severity (Rauch et al., 2000), suggesting the absence of significant CM-amygdala relationships may reflect Type-II error due to the incorporation of other model factors known to relate to amygdalar function as well as the relatively small sample size employed here. Future studies will need to employ larger samples to resolve this apparent contradiction.
The correlations of CM severity with connectivity contribute to an emerging literature demonstrating varying abnormalities of limbic-prefrontal and amygalo-insular functional relationships in PTSD (Gilboa et al., 2004; Lanius et al., 2004, 2005; Simmons et al., 2008), suggesting extent of CM exposure may account for some of this variability. Specifically, the pattern of results indicates CM severity was primarily correlated with greater connectivity between limbic (insula/amygdala) and prefrontal regions (ACC and dorsal PFC) during fear processing as well as lesser amygdalo-insular connectivity during both fear and anger processing. Findings for abnormalities of insula-ACC connectivity in PTSD have varied in directionality (Lanius et al., 2004; Fonzo et al., 2010), and given the complimentary roles of these brain structures in interoceptive awareness and emotional experience (Craig, 2003, 2004) these findings may reflect variation in the subjective emotional responses of individuals with PTSD to threatening stimuli. Findings for reductions in amygdalo-insular connectivity have been more homogenous (Simmons et al., 2008; Fonzo et al., 2010), including a study that observed this pattern of abnormalities in a similar sample utilizing the same emotional face processing paradigm. This convergence of findings highlights the possibility that functional differences observed in the posttraumatic state may reflect the influence of risk factors or other endogenous vulnerabilities rather than the manifestation of the disorder per se, and future studies may wish to incorporate measures of such risk/vulnerability factors in analyses to further disentangle these contributions.
This study has several limitations. First, it is important to point out that these results are correlational, retrospective with regard to CM assessment, and not longitudinal. Thus, one cannot draw any firm conclusions as to the causal effects of CM on brain function/structure or how any such effects may influence susceptibility to development or manifestation of PTSD. Specifically, it is possible a third, unmeasured variable may account for the relationships between CM and brain structure/function observed here. Therefore, these results should be interpreted cautiously until future, well-controlled prospective studies can address these questions. Second, the IPV-PTSD group consisted exclusively of women with exposure to IPV, so these findings may not be generalizable to males or persons with other forms of PTSD. Similarly, the relationships between CM and brain characteristics may be specific to this type of PTSD population and may not generalize to other maltreated and/or clinical populations. Third, we chose to include IPV-PTSD participants with comorbid depression or other anxiety disorders, which may limit the specificity of the findings. Fourth, self-report measures of childhood maltreatment are susceptible to reporting biases or inaccuracies. Fifth, we did not control the experiment-wise alpha level; given the exploratory nature of the analyses, we felt it best to balance power and risk of Type-I error by controlling for multiple comparisons on a family-wise level (i.e., activation, connectivity, and structure). Sixth, our dimensional regression-based approach to characterizing neural correlates of CM is only one of several valid approaches (e.g., group comparisons between matched IPV-PTSD participants with low and high exposure to CM).
In summary, severity of CM history in women with IPV-PTSD accounts for variation in limbic and prefrontal function and prefrontal structure in adulthood, potentially implicating maladaptive early life experiences as a prime source of variability in posttraumatic neural characteristics. Further attention to the neural effects of risk factors such as CM which promote individual variation in neural function/structure, increase susceptibility to the development of psychopathology, and moderate clinical characteristics such as symptom severity and quality of life may allow for further refinement of systems-neuroscience etiological theory and the identification of neural risk markers.
Supplementary Material
Figure 2. Severity of childhood maltreatment correlates with limbic-prefrontal and amygalo-insular functional connectivity during threat processing.
Scatterplots represent extracted cluster Fisher Z-transformed correlation coefficient against Childhood Trauma Questionnaire Total Score; Results displayed on an average anatomical; ROI=region of interest.
Figure 3. Severity of childhood maltreatment correlates with right middle/superior frontal gyrus gray matter volume.
Acknowledgments
This work was supported by a VA Merit Research Grant (to MBS) and MH64122 (to MBS) from the National Institute of Mental Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Code of ethics on human experimentation adapted from the Helsinki Declaration of the World Medical Association. American Journal of Orthopsychiatry. 1968;38(4):589–590. doi: 10.1111/j.1939-0025.1968.tb02426.x. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. Washington, D.C.: 2000. text revision. [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nature Reviews Neuroscience. 2006;7(4):268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Ball G, Stokes PR, Rhodes RA, Bose SK, Rezek I, Wink AM, Lord LD, Mehta MA, Grasby PM, Turkheimer FE. Executive functions and prefrontal cortex: a matter of persistence? Frontiers in Systems Neuroscience. 2011;5 doi: 10.3389/fnsys.2011.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for Beck Depression Inventory-II. Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Fink L. Childhood Trauma Questionnaire: A Retrospective Self-Report Manual. The Psychological Corporation; San Antonio, Texas: 1998. [Google Scholar]
- Bernstein DP, Fink L, Handelsman L, Foote J. Initial reliability and validity of a new retrospective measure of child abuse and neglect. American Journal of Psychiatry. 1994;151(8):1132–1136. doi: 10.1176/ajp.151.8.1132. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, Stokes J, Handelsman L, Medrano M, Desmond D, Zule W. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse and Neglect. 2003;27(2):169–190. doi: 10.1016/s0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- Blair K, Geraci M, Devido J, McCaffrey D, Chen G, Vythilingam M, Ng P, Hollon N, Jones M, Blair RJ, Pine DS. Neural response to self- and other referential praise and criticism in generalized social phobia. Archives of General Psychiatry. 2008;65(10):1176–1184. doi: 10.1001/archpsyc.65.10.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG. The development of a Clinician-Administered PTSD Scale. Journal of Traumatic Stress. 1995;8(1):75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vermetten E, Schmahl C, Vaccarino V, Vythilingam M, Afzal N, Grillon C, Charney DS. Positron emission tomographic imaging of neural correlates of a fear acquisition and extinction paradigm in women with childhood sexual-abuse-related post traumatic stress disorder. Psychological Medicine. 2005;35(6):791–806. doi: 10.1017/s0033291704003290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant RA, Kemp AH, Felmingham KL, Liddell B, Olivieri G, Peduto A, Gordon E, Williams LM. Enhanced amygdala and medial prefrontal activation during nonconscious processing of fear in posttraumatic stress disorder: an fMRI study. Human Brain Mapping. 2008;29(5):517–523. doi: 10.1002/hbm.20415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell-Sills L, Simmons AN, Lovero KL, Rochlin AA, Paulus MP, Stein MB. Functioning of neural systems supporting emotion regulation in anxiety-prone individuals. Neuroimage. 2011;54(1):689–696. doi: 10.1016/j.neuroimage.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrion VG, Garrett A, Menon V, Weems CF, Reiss AL. Posttraumatic stress symptoms and brain function during a response-inhibition task: an fMRI study in youth. Depression and Anxiety. 2008;25(6):514–526. doi: 10.1002/da.20346. [DOI] [PubMed] [Google Scholar]
- Cohen RA, Grieve S, Hoth KF, Paul RH, Sweet L, Tate D, Gunstad J, Stroud L, McCaffery J, Hitsman B, Niaura R, Clark CR, McFarlane A, Bryant R, Gordon E, Williams LM. Early life stress and morphometry of the adult anterior cingulate cortex and caudate nuclei. Biological Psychiatry. 2006;59(10):975–982. doi: 10.1016/j.biopsych.2005.12.016. [DOI] [PubMed] [Google Scholar]
- Costafreda SG, Brammer MJ, David AS, Fu CH. Predictors of amygdala activation during the processing of emotional stimuli: a meta-analysis of 385 PET and fMRI studies. Brain Research Reviews. 2008;58(1):57–70. doi: 10.1016/j.brainresrev.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel--now? The anterior insula and human awareness. Nature Reviews Neuroscience. 2009;10(1):59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Craig AD. Human feelings: Why are some more aware than others? Trends in Cognitive Science (Regul. Ed.) 2004;8(6):239–241. doi: 10.1016/j.tics.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception: the sense of the physiological condition of the body. Current Opinions in Neurobiology. 2003;13(4):500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Rotshtein P, Nagai Y, O'Doherty J, Mathias CJ, Dolan RJ. Activity in the human brain predicting differential heart rate responses to emotional facial expressions. Neuroimage. 2005;24(3):751–762. doi: 10.1016/j.neuroimage.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Croy I, Schellong J, Gerber J, Joraschky P, Iannilli E, Hummel T. Women with a history of childhood maltreatment exhibit more activation in association areas following non traumatic olfactory stimuli: a fMRI study. PLoS One. 2010;5:e9362. doi: 10.1371/journal.pone.0009362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvarci S, Popa D, Pare D. Central amygdala activity during fear conditioning. Journal of Neuroscience. 2011;31(1):289–294. doi: 10.1523/JNEUROSCI.4985-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. American Journal of Psychiatry. 2007;164(10):1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein JS, Adolphs R, Damasio A, Tranel D. The human amygdala and the induction and experience of fear. Current Biology. 2011;21(1):34–38. doi: 10.1016/j.cub.2010.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmingham K, Kemp AH, Williams L, Falconer E, Olivieri G, Peduto A, Bryant R. Dissociative responses to conscious and non-conscious fear impact underlying brain function in post-traumatic stress disorder. Psychological Medicine. 2008;38(12):1771–1780. doi: 10.1017/S0033291708002742. [DOI] [PubMed] [Google Scholar]
- Felmingham KL, Williams LM, Kemp AH, Rennie C, Gordon E, Bryant RA. Anterior cingulate activity to salient stimuli is modulated by autonomic arousal in Posttraumatic Stress Disorder. Psychiatry Research: Neuroimaging. 2009;173(1):59–62. doi: 10.1016/j.pscychresns.2008.12.005. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders. Biometrics Research, New York State Psychiatric Institute; New York: 1998. [Google Scholar]
- Fonzo GA, Simmons AN, Thorp SR, Norman SB, Paulus MP, Stein MB. Exaggerated and disconnected insular-amygdalar blood oxygenation level-dependent response to threat-related emotional faces in women with intimate-partner violence posttraumatic stress disorder. Biological Psychiatry. 2010;68(5):433–441. doi: 10.1016/j.biopsych.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francati V, Vermetten E, Bremner JD. Functional neuroimaging studies in posttraumatic stress disorder: review of current methods and findings. Depression and Anxiety. 2007;24(3):202–218. doi: 10.1002/da.20208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb BE, Schofield CA, Coles ME. Reported history of childhood abuse and young adults' information-processing biases for facial displays of emotion. Child Maltreatment. 2009;14(2):148–156. doi: 10.1177/1077559508326358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa A, Shalev AY, Laor L, Lester H, Louzoun Y, Chisin R, Bonne O. Functional connectivity of the prefrontal cortex and the amygdala in posttraumatic stress disorder. Biological Psychiatry. 2004;55(3):263–272. doi: 10.1016/j.biopsych.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Grieve SM, Korgaonkar MS, Clark CR, Williams LM. Regional heterogeneity in limbic maturational changes: Evidence from integrating cortical thickness, volumetric and diffusion tensor imaging measures. Neuroimage. 2011;55(3):868–879. doi: 10.1016/j.neuroimage.2010.12.087. [DOI] [PubMed] [Google Scholar]
- Hanson JL, Chung MK, Avants BB, Shirtcliff EA, Gee JC, Davidson RJ, Pollak SD. Early stress is associated with alterations in the orbitofrontal cortex: a tensor-based morphometry investigation of brain structure and behavioral risk. Journal of Neuroscience. 2010;30(22):7466–7472. doi: 10.1523/JNEUROSCI.0859-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Drabant EM, Munoz KE, Kolachana BS, Mattay VS, Egan MF, Weinberger DR. A Susceptibility Gene for Affective Disorders and the Response of the Human Amygdala. Archives of General Psychiatry. 2005;62(2):146–152. doi: 10.1001/archpsyc.62.2.146. [DOI] [PubMed] [Google Scholar]
- Hopper JW, Frewen PA, van der Kolk BA, Lanius RA. Neural correlates of reexperiencing, avoidance, and dissociation in PTSD: symptom dimensions and emotion dysregulation in responses to script-driven trauma imagery. Journal of Traumatic Stress. 2007;20(5):713–725. doi: 10.1002/jts.20284. [DOI] [PubMed] [Google Scholar]
- Huber PJ. Robust estimation of a location parameter. Annals of Mathematical Statistics. 1964;35(1):73–101. [Google Scholar]
- Karl A, Schaefer M, Malta LS, Dorfel D, Rohleder N, Werner A. A meta-analysis of structural brain abnormalities in PTSD. Neuroscience and Biobehavioral Reviews. 2006;30(7):1004–1031. doi: 10.1016/j.neubiorev.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Kemp AH, Felmingham K, Das P, Hughes G, Peduto AS, Bryant RA, Williams LM. Influence of comorbid depression on fear in posttraumatic stress disorder: an fMRI study. Psychiatry Research: Neuroimaging. 2007;155(3):265–269. doi: 10.1016/j.pscychresns.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62(6):617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Chey J, Chung A, Bae S, Khang H, Ham B, Yoon SJ, Jeong DU, Lyoo IK. Diminished rostral anterior cingulate activity in response to threat-related events in posttraumatic stress disorder. Journal of Psychiatric Research. 2008;42(4):268–277. doi: 10.1016/j.jpsychires.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Landre L, Destrieux C, Baudry M, Barantin L, Cottier JP, Martineau J, Hommet C, Isingrini M, Belzung C, Gaillard P, Camus V, El Hage W. Preserved subcortical volumes and cortical thickness in women with sexual abuse-related PTSD. Psychiatry Research: Neuroimaging. 2010;183(3):181–186. doi: 10.1016/j.pscychresns.2010.01.015. [DOI] [PubMed] [Google Scholar]
- Lang AJ, Aarons GA, Gearity J, Laffaye C, Satz L, Dresselhaus TR, Stein MB. Direct and indirect links between childhood maltreatment, posttraumatic stress disorder, and women's health. Behavioral Medicine. 2008;33(4):125–135. doi: 10.3200/BMED.33.4.125-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanius RA, Bluhm R, Lanius U, Pain C. A review of neuroimaging studies in PTSD: heterogeneity of response to symptom provocation. Journal of Psychiatric Research. 2006;40(8):709–729. doi: 10.1016/j.jpsychires.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Lanius RA, Vermetten E, Loewenstein RJ, Brand B, Schmahl C, Bremner JD, Spiegel D. Emotion modulation in PTSD: Clinical and neurobiological evidence for a dissociative subtype. American Journal of Psychiatry. 2010;167(6):640–647. doi: 10.1176/appi.ajp.2009.09081168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanius RA, Williamson PC, Bluhm RL, Densmore M, Boksman K, Neufeld RW, Gati JS, Menon RS. Functional connectivity of dissociative responses in posttraumatic stress disorder: a functional magnetic resonance imaging investigation. Biological Psychiatry. 2005;57(8):873–884. doi: 10.1016/j.biopsych.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Lanius RA, Williamson PC, Densmore M, Boksman K, Neufeld RW, Gati JS, Menon RS. The nature of traumatic memories: a 4-T FMRI functional connectivity analysis. American Journal of Psychiatry. 2004;161(1):36–44. doi: 10.1176/appi.ajp.161.1.36. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Sripada CS. The functional neuroanatomy of PTSD: a critical review. Progress in Brain Research. 2008;167:151–169. doi: 10.1016/S0079-6123(07)67011-3. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Garfinkel SN. Functional neuroimaging in post-traumatic stress disorder. In: Shiromani PJ, Keane TM, LeDoux JE, editors. Post-Traumatic Stress Disorder: Basic Science and Clinical Practice. Humana Press; Totowa, NJ, US: 2009. pp. 297–317. [Google Scholar]
- Lyoo IK, Kim JE, Yoon SJ, Hwang J, Bae S, Kim DJ. The neurobiological role of the dorsolateral prefrontal cortex in recovery from trauma. Longitudinal brain imaging study among survivors of the South Korean subway disaster. Archives of General Psychiatry. 2011;68(7):701–713. doi: 10.1001/archgenpsychiatry.2011.70. [DOI] [PubMed] [Google Scholar]
- Masten CL, Guyer AE, Hodgdon HB, McClure EB, Charney DS, Ernst M, Kaufman J, Pine DS, Monk CS. Recognition of facial emotions among maltreated children with high rates of post-traumatic stress disorder. Child Abuse and Neglect. 2008;32(1):139–153. doi: 10.1016/j.chiabu.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medford N, Critchley HD. Conjoint activity of anterior insular and anterior cingulate cortex: awareness and response. Brain Structure and Function. 2010;214(5,6):535–549. doi: 10.1007/s00429-010-0265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, Zeidan MA, Handwerger K, Orr SP, Rauch SL. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biological Psychiatry. 2009;66(12):1075–1082. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey RA, Dolcos F, Petty CM, Cooper DA, Hayes JP, Labar KS, McCarthy G. The role of trauma-related distractors on neural systems for working memory and emotion processing in posttraumatic stress disorder. Journal of Psychiatric Research. 2008;43(8):809–817. doi: 10.1016/j.jpsychires.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SC, Maheu FS, Dozier M, Peloso E, Mandell D, Leibenluft E, Pine DS, Ernst M. Early-life stress is associated with impairment in cognitive control in adolescence: An fmri study. Neuropsychologia. 2010;48(10):3037–3044. doi: 10.1016/j.neuropsychologia.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Feinstein JS, Castillo G, Simmons AN, Stein MB. Dose-dependent decrease of activation in bilateral amygdala and insula by lorazepam during emotion processing. Archives of General Psychiatry. 2005;62(3):282–288. doi: 10.1001/archpsyc.62.3.282. [DOI] [PubMed] [Google Scholar]
- Pico-Alfonso MA. Psychological intimate partner violence: the major predictor of posttraumatic stress disorder in abused women. Neuroscience and Biobehavioral Reviews. 2005;29(1):181–193. doi: 10.1016/j.neubiorev.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Likhtik E, Pelletier JG, Pare D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. Journal of Neuroscience. 2003;23(25):8800–8807. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing 2011 [Google Scholar]
- Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of postraumatic stress disorder and extinction: human neuroimaging research--past, present, and future. Biological Psychiatry. 2006;60(4):376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, Orr SP, Pitman RK. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biological Psychiatry. 2000;47(9):769–776. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- Rogers MA, Yamasue H, Abe O, Yamada H, Ohtani T, Iwanami A, Aoki S, Kato N, Kasai K. Smaller amygdala volume and reduced anterior cingulate gray matter density associated with history of post-traumatic stress disorder. Psychiatry Research: Neuroimaging. 2009;174(3):210–216. doi: 10.1016/j.pscychresns.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Rougemont-Bucking A, Linnman C, Zeffiro TA, Zeidan MA, Lebron-Milad K, Rodriguez-Romaguera J, Rauch SL, Pitman RK, Milad MR. Altered Processing of Contextual Information during Fear Extinction in PTSD: An fMRI Study. CNS Neuroscience and Therapeutics. 2011;17(4):227–236. doi: 10.1111/j.1755-5949.2010.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savine AC, Braver TS. Motivated cognitive control: reward incentives modulate preparatory neural activity during task-switching. Journal of Neuroscience. 2010;30(31):10294–10305. doi: 10.1523/JNEUROSCI.2052-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Schulz KP, Clerkin SM, Halperin JM, Newcorn JH, Tang CY, Fan J. Dissociable neural effects of stimulus valence and preceding context during the inhibition of responses to emotional faces. Human Brain Mapping. 2009;30(9):2821–2833. doi: 10.1002/hbm.20706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp DJ, Bonnelle V, De Boissezon X, Beckmann CF, James SG, Patel MC, Mehta MA. Distinct frontal systems for response inhibition, attentional capture, and error processing. Proceedings for the National Academy of Sciences U S A. 2010;107(13):6106–6111. doi: 10.1073/pnas.1000175107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, Macklin ML, Lasko NB, Cavanagh SR, Krangel TS, Orr SP, Pitman RK, Whalen PJ, Rauch SL. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Archives of General Psychiatry. 2005;62(3):273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- Simmons AN, Paulus MP, Thorp SR, Matthews SC, Norman SB, Stein MB. Functional activation and neural networks in women with posttraumatic stress disorder related to intimate partner violence. Biological Psychiatry. 2008;64(8):681–690. doi: 10.1016/j.biopsych.2008.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Jacques PL, Botzung A, Miles A, Rubin DC. Functional neuroimaging of emotionally intense autobiographical memories in post-traumatic stress disorder. Journal of Psychiatric Research. 2011;45(5):630–637. doi: 10.1016/j.jpsychires.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MB, Kennedy C. Major depressive and post-traumatic stress disorder comorbidity in female victims of intimate partner violence. Journal of Affective Disorders. 2001;66(2-3):133–138. doi: 10.1016/s0165-0327(00)00301-3. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain: 3-Dimensional Proportional System: An Approach to Cerebral Imaging. Thieme Medical Publishers; New York: 1998. [Google Scholar]
- Taylor SE, Eisenberger NI, Saxbe D, Lehman BJ, Lieberman MD. Neural Responses to Emotional Stimuli Are Associated with Childhood Family Stress. Biological Psychiatry. 2006;60(3):296–301. doi: 10.1016/j.biopsych.2005.09.027. [DOI] [PubMed] [Google Scholar]
- Thombs BD, Lewis C, Bernstein DP, Medrano MA, Hatch JP. An evaluation of the measurement equivalence of the Childhood Trauma Questionnaire--Short Form across gender and race in a sample of drug-abusing adults. Journal of Psychosomatic Research. 2007;63(4):391–398. doi: 10.1016/j.jpsychores.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Torta DM, Cauda F. Different functions in the cingulate cortex, a meta-analytic connectivity modeling study. Neuroimage. 2011;56(4):2157–2172. doi: 10.1016/j.neuroimage.2011.03.066. [DOI] [PubMed] [Google Scholar]
- Venables WN, Ripley BD. Modern Applied Statistics with S. (Fourth) 2002 [Google Scholar]
- Wager TD, Barrett LF. From affect to control: Functional specialization of the insula in motivation and regulation. 2004 Retrived online from www.apa.org/psycextra.
- Williams LM, Gatt JM, Schofield PR, Olivieri G, Peduto A, Gordon E. ‘Negativity bias’ in risk for depression and anxiety: brain-body fear circuitry correlates, 5-HTT-LPR and early life stress. Neuroimage. 2009;47(3):804–814. doi: 10.1016/j.neuroimage.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Woodward SH, Schaer M, Kaloupek DG, Cediel L, Eliez S. Smaller global and regional cortical volume in combat-related posttraumatic stress disorder. Archives of General Psychiatry. 2009;66(12):1373–1382. doi: 10.1001/archgenpsychiatry.2009.160. [DOI] [PubMed] [Google Scholar]
- Woon FL, Hedges DW. Hippocampal and amygdala volumes in children and adults with childhood-maltreatment related posttraumatic stress disorder: a meta-analysis. Hippocampus. 2008;18(8):729–736. doi: 10.1002/hipo.20437. [DOI] [PubMed] [Google Scholar]
- Zlotnick C, Johnson J, Kohn R, Vicente B, Rioseco P, Saldivia S. Childhood trauma, trauma in adulthood, and psychiatric diagnoses: results from a community sample. Comprehensive Psychiatry. 2008;49(2):163–169. doi: 10.1016/j.comppsych.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.