Abstract
Impairment in decision-making is frequently observed in suicide attempters. Little is known, however, about neural circuitry underlying decision-making in adolescent attempters. Functional magnetic resonance imaging (fMRI) was used to assess decision-making and learning-related neural activity during Iowa Gambling Task (IGT) performance in adolescents with depression and suicide attempt (ATT, n=15), non-attempters with depression (NAT, n=14), and healthy controls (HC, n=13). ATT performed best on the IGT. A 3 group by 2 condition (high-risk versus low-risk) by 3 IGT block (each of 20 cards) whole-brain analysis (p<0.05, corrected) interaction was found in the left hippocampal, frontal and temporal cortical, striatal and thalamic regions. Post-hoc analyses revealed that during low-risk decisions in blocks 2 and 3, NAT, but not ATT, showed greater left hippocampal activation versus HC (p=0.0004, p=0.003); in block 2, during low-risk decisions NAT showed greater left middle temporal gyral activation versus HC (p=0.003); in block 3, during high-risk decisions ATT showed less activation in the right thalamus versus NAT (p=0.001) and during low risk decisions ATT showed greater activation than HC in the left caudate (p=0.002). NAT, but not ATT are differentiated from HC during performance of the IGT. Functional abnormalities in neural circuitry implicated in learning in the context of risk may underlie risk for MDD, but not risk for suicide attempt, in adolescence.
Keywords: adolescence, suicide, decision-making, hippocampus
1. Introduction
Suicidal behavior is one of the leading causes of morbidity and mortality in adolescence (Lubell et al., 2007). Despite progress in understanding the epidemiology and risk factors for adolescent suicidal behavior (Bridge et al., 2006) the pathophysiologic processes that may predispose to suicidal behavior, including alterations in neural circuitry, remain unclear. Adult suicide attempters have demonstrated alterations in memory and decision-making (Jollant et al., 2005; Williams et al., 2005; Arie et al., 2008; Oldershaw et al., 2009). Better understanding of the pathophysiology underlying decision-making and learning in the context of risk with regard to suicidal behavior may help inform both identification and intervention.
The Iowa Gambling Task (IGT) is a well-known and widely used neuropsychological test of decision-making ability in the context of risk (Bechara et al., 1999, 1994) that requires learning to perform well on the task (Lawrence et al., 2009). IGT performance has been shown to be impaired in adults with history of suicidal behavior (Jollant et al., 2005, 2010). Adult neuroimaging studies implicate left-sided prefrontal cortical, insula and visuospatial processing regions in performance of the IGT (Bechara et al., 1994, 1999; Ernst et al., 2002), In healthy adults, choices from “high-risk” versus “low-risk” decks in the IGT were associated with activation in the medial frontal gyrus, lateral orbitofrontal cortex, and insula. The magnitude of activation in these areas, in addition to the pre-supplementary motor area and secondary somatosensory cortex, was positively associated with better task performance, which involved learning that the low-risk, low-yield decks resulted in better “earnings” compared to the high-risk, high-yield decks (Lawrence et al., 2009). Furthermore, modulation of activity over time in the lateral orbitofrontal cortex and pre-supplementary motor area indicated their role in learning to optimize task performance (choosing “low-risk” decks) in healthy individuals (Lawrence et al., 2009). Poorer performance on the IGT was associated with reduced activity in left lateral orbitofrontal and left occipital cortices in adult male suicide attempters relative to both depressed and healthy controls during risky versus low-risk choices (Jollant et al., 2010).
While the IGT has not been used to assess decision-making in adolescent suicide attempters, it has been used to investigate other risky behaviors in this age group. For example, adolescents either with or at risk for substance abuse performed less well than did healthy controls (Schutter et al., 2011; Toplak et al., 2005; Ernst et al., 2003). However, the IGT did not discriminate between adolescents with and without non-suicidal self-injury (Oldershaw et al., 2009).
The aim of the present study was to measure neural activity during performance on the IGT in adolescents with a history of suicide attempt versus two age-matched, non-attempter groups, those with depression, and healthy controls. Based on the extant literature on the IGT in adult suicide attempters, we hypothesized that adolescent suicide attempters would perform more poorly on the IGT than depressed and healthy controls, and that adolescent attempters would show decreased activity in areas implicated in IGT performance, namely medial frontal gyrus, lateral orbitofrontal cortex, insula, the pre-supplementary motor area and secondary somatosensory cortex.
2. Method
2.1. Participants
Forty-two adolescents completed the study, including: 1) 15 attempters (ATT) with a lifetime history of suicide attempt, as defined by the C-CASA and DSM-IV Major Depressive Disorder (MDD); 2) 14 depressed non-attempters (NAT) with a lifetime history of DSM-IV MDD, but no history of suicide attempt; 3) 13 healthy controls (HC), with no lifetime history of psychiatric disorder or attempt. Of the 51 eligible participants, 42 completed the study: two were excluded due to claustrophobia; one due to a structural abnormality; one due to history of marijuana dependence; two due to younger age (10 years old) than the majority of other participants; one due to task refusal, one due to technical data loss, and one due to excessive movement (>2mm). ATT and NAT were recruited from existing studies of familial inheritance of suicidal behavior and a clinic registry for depressed and suicidal youth. HC were recruited from existing healthy control groups and advertisement in pediatric practices. Inclusion criteria for NAT and ATT included a lifetime history of MDD according to DSM-IV criteria. ATT had a history of at least one suicide attempt as defined by the Columbia Classification Algorithm of Suicide Assessment (C-CASA) (Posner et al., 2007). All participants were right-handed as determined by patient interview and parent confirmation.
Exclusion criteria included a) neurological disorders; b) Verbal IQ<80 on the Wechsler Abbreviated Intelligence Scale (Wechsler, 1999); c) current use of sedative medication; d) drug or alcohol use disorder and/or positive urine drug screen; e) pregnancy; f) claustrophobia; g) ineligibility for MRI (e.g. metal); h) bipolar disorder; i) psychosis. Any ATT whose suicide attempt was associated with anoxia or head injury was excluded, though no such ATT was recruited. Psychotrophic medications were permitted, and included antidepressant medication and augmentation (Table 1).
Table 1.
Demographic information and clinical variables
| ATT (n = 15) | NAT (n = 14) | HC (n = 13) | ||
|---|---|---|---|---|
| Gender a | Male | 4 | 7 | 8 |
| Female | 11 | 7 | 5 | |
| Mean age b (12–17 years) | 16.20 (S.D.: 0.78) | 15.79 (S.D.: 1.58) | 15.15 (S.D.: 1.46) | |
| Petersen c | 3.13 (S.D.: 0.35) | 3.00 (S.D.: 0.39) | 3.08 (S.D.: 0.64) | |
| BDI d | 15.47 (S.D.: 15.08) | 4.64 (S.D.: 5.71) | 2.15 (S.D.: 3.60) | |
| SIQ e | 39.73 (S.D.: 21.08) | 24.00 (S.D.: 11.61) | 16.15 (S.D.: 1.52) | |
| SCARED (c) f | 26.93 (S.D.: 13.99) | 10.00 (S.D.: 8.64) | 6.85 (S.D.: 7.02) | |
| SCARED (p) g | 22.73 (S.D.: 13.12) | 12.94 (S.D.: 7.98) | 5.85 (S.D.: 7.48) |
| Medication: 10 ATT and 6 NAT were taking medication
| ||
| ATT (n = 10) | NAT (n = 6) | |
| Mood stabilizers (n = 2) * | Lamotrigine (n = 1) | |
| Topiramate (n = 1) | ||
| Antidepressants (n = 16) | Escitalopram (n = 1) | |
| Citalopram (n=1) | Citalopram (n=1) | |
| Bupropion (n = 1) | Buproprion (n=1) | |
| Fluoxetine (n = 4) | Fluoxetine (n = 3) | |
| Trazodone (n = 2) | ||
| Duloxetine (n = 1) | ||
| Sertraline (n = 2) | Sertraline (n = 1) | |
| Antipsychotics (n = 1) * | Aripiprazole (n = 1) | |
| Other (n = 3) * | Levothyroxine sodium (n = 1) | Levothyroxine sodium (n = 2) |
All participants taking medication were taking an antidepressant, 2 NAT and 1 ATT had augmentation with levothyroxine. 1 ATT had augmentation with aripiprazole, and 2 ATT had augmentation with antiepileptic medications.
Numbers are means; standard deviations in parentheses; ATT: adolescent suicide attempters, NAT: non-attempters with a history of major depressive disorder, HC: healthy controls; BDI: Beck depression inventory; SIQ: suicidal ideation questionnaire; SCARED: screen for childhood anxiety related emotional disorders, (c) : child version, (p) : parent version.
ATT, NAT and HC did not differ significantly in:
gender ratio (χ2= 3.525, p= 0.172).
age (F2, 39=2.251, p= 0.119).
Petersen Pubertal questionnaire (χ2 = 0.597, p= 0.742).
ATT had significantly greater:
BDI scores than NAT and HC (χ2 = 13.25, p= 0.001).
SIQ scores than NAT and HC (χ2 = 20.09, p= 0.0001).
SCARED (c) than NAT and HC (χ2 = 16.91, p= 0.0001).
SCARED (p) scores than NAT and HC (χ2 = 16.14, p= 0.0001).
DSM-IV criteria were assessed using the K-SADS-PL diagnostic interview (Kaufman et al., 2000). Suicide attempt was assessed using the Suicide Intent Scale (SIS) (Beck et al., 1974), Columbia Suicide History Form which includes the Beck Intent Scale and Lethality Scale (SHF) (Oquendo et al., 2003; Beck et al., 1975). Self-reported depression, anxiety and suicidal ideation were assessed with The Beck Depression Inventory (BDI) (Beck et al., 1961), Screen for Childhood Anxiety Related Emotional Disorders (SCARED) (Birmaher et al., 1997), and Suicidal Ideation Questionnaire (SIQ) (Reynolds, 1987), respectively. Pubertal status was assessed using the Petersen Pubertal Development Scale (Peterson et al., 1988).
The University of Pittsburgh Institutional Review Board approved the study protocol. Parents and participants provided informed written consent and assent prior to the study. Participants were reimbursed $100.
Median time since last attempt was 25.7 months, and mean lethality of attempt from the SHF was 2.27 (injury requiring medical attention) (Oquendo et al., 2003). Seven ATT attempted suicide impulsively (with no planning), and 3 attempted suicide with less than 3 hours of planning. Ten ATT and six NAT were treated with medication for depression during the task. Medications primarily included SSRI antidepressant medication, but also augmentation for depression treatment with other medications (Table 1). By design, the three groups were similar in age and gender. ATT had significantly higher BDI (ATT=15.47 (SD15.08)), NAT=4.64 (SD 5.71)), SCARED (ATT=26.93 (SD13.99)), NAT=10.00 (S.D.: 8.64)), and SIQ scores (ATT=39.73 (SD 21.08)), NAT=24.00 (SD 11.61)) than NAT (Table 1).
2.2. Paradigm
The computerized version of the IGT was administered to the participants (Jollant et al., 2010; Bechara et al., 1994, 1999;) modified for fMRI (Lawrence et al., 2009). All participants were task-naïve. In this event-related task, four decks of cards were presented on a screen. Participants are asked to pick a card from the deck of their choice with a button glove press. The game ends after 100 choices. Participants are unaware that two decks are advantageous (C+D decks) (“low-risk”) with low magnitude of wins but lower magnitude of loss, resulting in net gain, and two decks are disadvantageous (A+B decks) (“high-risk”), with high magnitude of wins, but higher magnitude of losses, resulting in net loss. Activations were examined when the participants click on a card. High-risk to low-risk decisions were compared as was amount won. No actual money was given as part of task performance. Participants reviewed the task with the investigator after completion, including rating task difficulty and motivation to determine if they were engaged during the task.
Trials lasted 10–12 seconds. Cards appeared with “wait” prompt (2 sec); “Pick a card” prompt displayed and participants selected one of 4 decks using a right-handed button box (up to 3 sec); immediately reinforcement was displayed (“You have won X$” or “You have lost X$”) and a green bar under the cards moved up or down (2 sec) to indicate remaining amount of money; a fixation cross was presented for a variable period (3–5 sec) until the next trial. The duration of the fixation cross on each trial was adjusted for the participant’s reaction time, so that the task lasted exactly 1321 seconds for each participant. The IGT score was calculated as the number of choices from safe (C+D) minus risky (A+B) decks for all choices and by blocks of 20 choices to examine learning over the task. This task was developed for adults, and our adolescent participants did not tolerate the length of the task well, with non-response, falling asleep, or increased movement. The last two blocks at the end of the task thus had to be excluded from analysis. This resulted in analysis of 60 choices including 3 response blocks.
2.3. Data acquisition
Scans were acquired on a 3.0 T Siemens Allegra magnetic resonance imaging (MRI) scanner. Functional scans of 34 contiguous 3mm axial slices were acquired with a T2-weighted gradient echo epi sequence (repetition time: 2000ms; echo time: 30ms; field of view: 205mm; matrix: 64×64; in-plane resolution: 3mm×3mm). Stimuli were projected onto a screen approximately 55cm from the participant with a rear screen projector. High-resolution T1-weighted magnetization prepared rapid gradient echo (MPRAGE) structural images of 240 0.8mm slices were acquired (repetition time: 1.630ms; echo time: 2.48ms; inversion time: 800ms; field of view: 200mm; flip angle: 8°; matrix: 256×256).
2.4. Task Performance
Task performance data were analyzed using a 3×2×3 and 3×2 ANOVA (group by risk condition) to examine the main effects of, and interactions among, block, risk condition and group upon task performance in SPSSv.19 (SPSS, Inc., Chicago, IL).
2.5. Imaging analyses
Data were pre-processed and analyzed using Statistical Parametric Mapping software (SPM5, London, UK). Data for each participant were first corrected for differences in acquisition time between slices; realigned using the first slice as a reference; and unwarped to correct static inhomogeneity of the magnetic field and movement by inhomogeneity interactions. Movement cut off was ≤2mm. They were co-registered with the participant’s anatomical image, segmented, normalized to standard MNI template, resampled to 3×3×3 mm3 voxels, and spatially smoothed with a Gaussian kernel of 6mm full-width at half-maximum (FWHM).
A first-level, fixed-effect model was constructed for the three blocks entered as separate conditions in a block design in the design matrix. Movement parameters from the realignment stage were entered as covariates of no interest to control for participant movement. Trials were modeled using the Canonical Hemodynamic Response Function (SPM Manual, 2007). The two conditions (high and low-risk) were entered as separate t-contrasts into second-level analyses. A second level random effects model was used for between group comparison. As the main focus of our study was to examine the extent to which the three groups were distinguished by patterns of neural activity and learning during the task, we performed a 3-group (ATT, NAT, HC) by 2-condition (high-risk versus low-risk) by 3 block (first, second, third block) ANOVA, covarying for age to examine the group by condition by block interaction upon whole-brain activity during task performance. A second, exploratory 3×2×3 ANOVA (group by condition by block) was conducted to examine task performance. Here, we first used a voxel-wise threshold of p<0.05, uncorrected for whole brain analyses. Second, we used a cluster-level false positive detection rate of p<0.05 for whole brain activity surviving the above voxel-wise threshold of p<0.05 uncorrected using two methods of small volume correction (SVC). The whole-brain interaction activations did not survive SVC family-wise error (FWE) within SPM 5. The activations did survive a second, less conservative approach, using regional anatomical masks from the WFU Pickatlas (Maldjian et al., 2003) for each whole brain activity cluster, and a cluster (k) extent empirically determined by Monte Carlo simulation implemented in AlphaSim for each cluster. Regional anatomical areas were utilized for both methods. AlphaSim is a method of correction for multiple comparisons that accounts for spatial correlations between BOLD signal changes in neighboring voxels. It has been used in previous pediatric neuroimaging studies (Pan et al., 2011; Dickstein et al., 2010; Monk et al., 2008; Gilbert et al., 2005).
Peak BOLD signal changes were extracted from regions showing a significant group by condition by block interaction in the 3×2×3 ANOVA. We then performed post-hoc tests on these extracted BOLD signal values to examine the extent to which pair-wise between group differences in activity contributed to the significant interactions in the above analyses, using independent t-tests and appropriate statistical thresholds to control for multiple tests. In these post-hoc tests for regions showing a significant group by condition by block interaction in the 3×2×3 ANOVA, a significance threshold of p<0.05/18=p<0.003 was employed to control for the three independent between-group pair-wise tests for each of the two conditions and three blocks in each region.
In exploratory analyses, we examined potential relationships in ATT and NAT separately between extracted BOLD signal from neural regions showing between group differences in activity and depression severity, anxiety, suicidal ideation, pubertal status, age, gender, and medication at the time of scanning: medication status (taking versus not taking) and medication load, to account for total number and dose of different psychotropic medications taken (Hassel et al., 2008). We conducted Pearson correlation and independent t-tests as appropriate in SPSSv.19.
3. Results
3.1. Task performance data
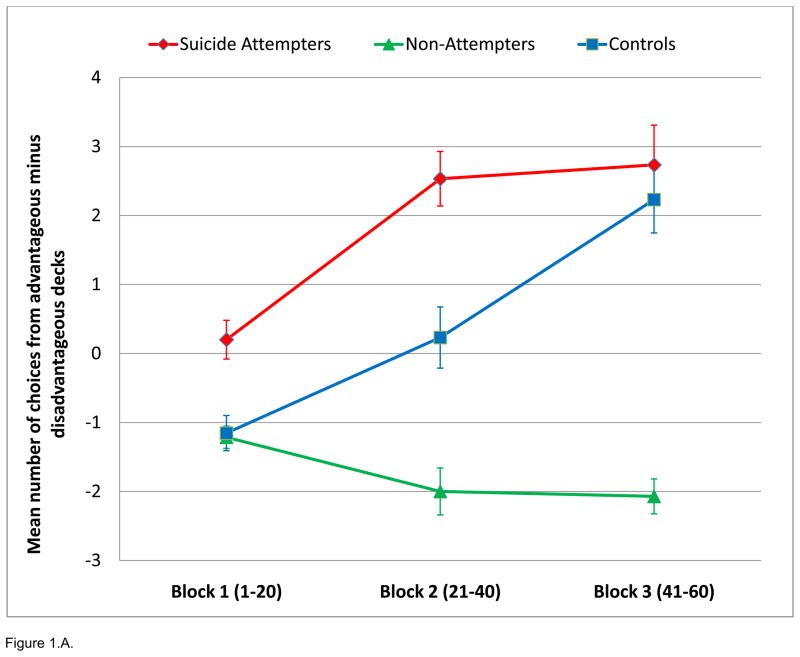
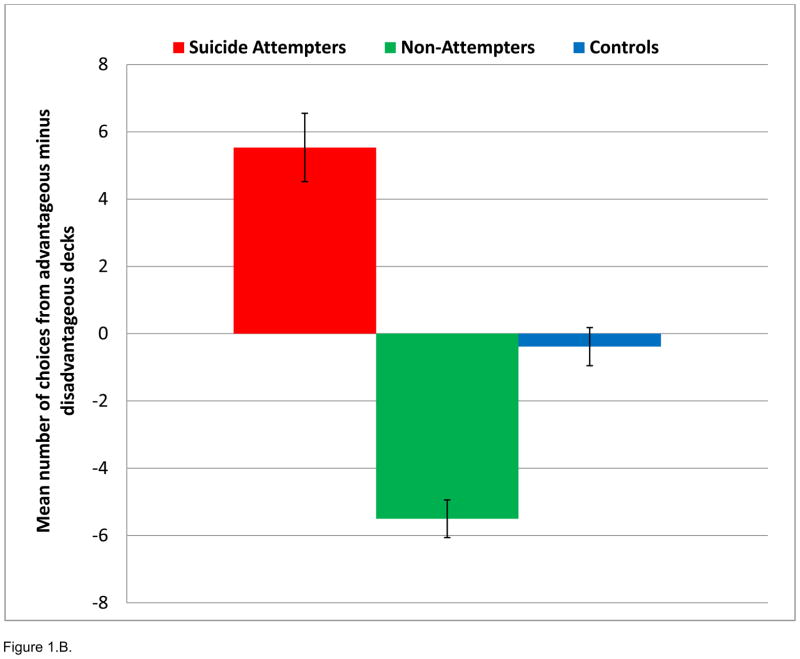
A 3×2×3 ANOVA (group by condition by block), conducted to examine task performance (number of choices), showed no significant interaction. There was, however, a significant main effect of group upon mean number of response choices from low-risk minus high-risk decks, with ATT demonstrating more low-risk choices on the task than NAT and HC (ATT 5.53±1.01, NAT −5.5±0.559, HC −0.38±0.566; F2, 41=3.67; p=0.035). Post-hoc pair-wise comparisons showed ATT significantly greater than NAT for low-risk minus high-risk choices (ATT=5.53+3.93, NAT=−5.50±2.09; F1, 28=5.85; p=0.022). ANOVAs of group by condition (3×2) and group by block (3×3) demonstrated no significant interactions. Finally, there were no between group differences in omissions or overall mean reaction time.
3.2. Neural Activity during IGT
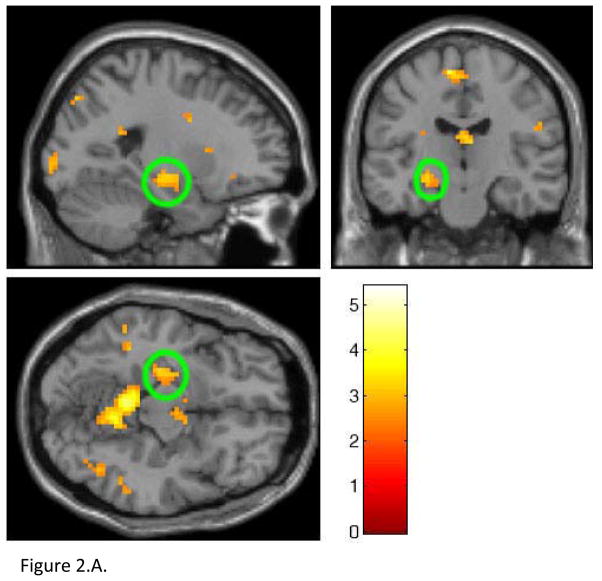
A 3×2×3 ANOVA, covarying for age, revealed a group by condition by block interaction in the left caudate nucleus (p<0.0001, uncorrected), left cerebellum (p<0.001, uncorrected), left middle temporal gyrus (BA39) (p<0.003, uncorrected), left hippocampus (p<0.011, uncorrected), right medial frontal gyrus (p<0.009, uncorrected), right thalamus (p<0.011, uncorrected), left fusiform gyrus (p<0.005, uncorrected), and right occipital gyrus (p<0.007, uncorrected); all p<0.05, AlphaSim corrected; Table 2, Figure 2.A.). Significant clusters of activation did not survive FWE in SPM5 but did survive SVC with AlphaSim correction at p<0.05 (Table 2).
Table 2.
Whole brain activation: 3X2X3 ANOVA Group (ATT, NAT, HC) X Condition (High Risk vs Low Risk) X Block (Block 1, Block 2, and Block 3) covarying for age.
| Coordinates | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Region | Side | k | Alpha Sim K | x | y | z | F | z | Voxelwise | Voxelwise |
| P, Unc | P, AlphaSim Corr | |||||||||
| Caudate | L | 169 | 27 | 0 | 9 | 18 | 5.37 | 3.37 | 0.0001 | 0.046 |
| Middle Temporal Gyrus (BA39) | L | 48 | 17 | − 33 | − 66 | 30 | 4.08 | 2.72 | 0.003 | 0.043 |
| Hippocampus | L | 37 | 5 | − 27 | − 21 | − 12 | 3.33 | 2.28 | 0.011 | 0.044 |
| Medial Frontal Gyrus (BA10) | R | 31 | 11 | 21 | 54 | 3 | 3.47 | 2.37 | 0.009 | 0.039 |
| Thalamus | R | 40 | 40 | 3 | − 21 | 18 | 3.35 | 2.29 | 0.011 | 0.049 |
| Fusiform Gyrus (BA37) | L | 50 | 15 | − 42 | − 42 | − 9 | 3.88 | 2.61 | 0.005 | 0.048 |
| Occipital Gyrus (BA19) | R | 61 | 16 | 36 | − 54 | 3 | 3.60 | 2.45 | 0.007 | 0.045 |
Figure 2.
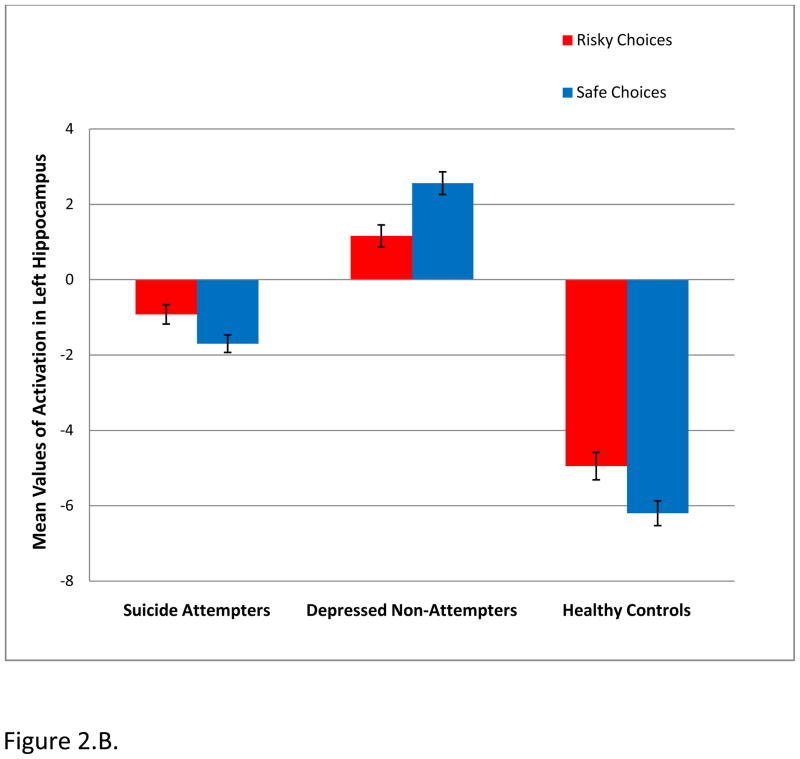
A. Whole brain 3×2×3 analysis of variance (ANOVA) examining differences in neural activity in the IGT high and low-risk responses covarying for age in all groups. In the left hippocampus, NAT, but not ATT, showed significantly greater activity than HC during low-risk decisions (p=0.0004) in block 2. Hippocampus circled in green.
B. NAT>ATT and HC during low-risk choices in block 2. Attempters show patterns of neural activity more similar to healthy controls during low risk choices in block 2. Note: Magnitude of blood oxygen level dependent (BOLD) signal is depicted in the bar charts.
Post-hoc analyses revealed significant between group differences in activation in blocks 2 and 3 (Table 3). The most statistically significant findings were observed in the left hippocampus (Figure 2.B.). During low-risk decisions in block 2, NAT, but not ATT, showed significantly greater hippocampal activation than HC (p=0.0004). In addition, NAT showed greater activation than HC in the middle temporal gyrus during low-risk decisions in block 2 (p=0.003).
Table 3.
Post-hoc tests examining the above 3×2×3 interactions
| Region | Side | Post Hoc test | Condition | t | df | P | F |
|---|---|---|---|---|---|---|---|
| Caudate | L | ATT>HC | CD3 | 3.48 | 27 | 0.002 | 11.98 |
| NAT>HC | CD3 | 2.09 | 26 | 0.047 | 4.34 | ||
| Middle Temporal Gyrus (BA39) | L | NAT>ATT | AB1 | 2.87 | 28 | 0.008 | 8.18 |
| NAT>ATT | AB3 | 2.36 | 28 | 0.026 | 5.53 | ||
| NAT>ATT | CD2 | 2.78 | 28 | 0.010 | 7.66 | ||
| ATT>HC | CD3 | 2.39 | 27 | 0.025 | 5.67 | ||
| NAT>HC | AB1 | 2.69 | 26 | 0.013 | 7.17 | ||
| NAT>HC | AB3 | 2.35 | 26 | 0.027 | 5.48 | ||
| NAT>HC | CD2 | 3.23 | 26 | 0.003 | 10.35 | ||
| NAT>HC | CD3 | 2.58 | 26 | 0.016 | 6.61 | ||
| Hippocampus | L | NAT>ATT | CD2 | 2.96 | 28 | 0.006 | 8.72 |
| ATT>HC | AB2 | 2.20 | 27 | 0.037 | 4.84 | ||
| ATT>HC | CD3 | 2.76 | 27 | 0.010 | 7.57 | ||
| NAT>HC | AB1 | 2.32 | 26 | 0.029 | 5.35 | ||
| NAT>HC | AB2 | 3.13 | 26 | 0.004 | 9.69 | ||
| NAT>HC | AB3 | 2.56 | 26 | 0.017 | 6.53 | ||
| NAT>HC | CD2 | 4.05 | 26 | 0.0004 | 16.25 | ||
| NAT>HC | CD3 | 3.31 | 26 | 0.003 | 10.84 | ||
| Medial Frontal Gyrus (BA10) | R | NAT>ATT | AB3 | 2. 27 | 28 | 0.031 | 5.14 |
| ATT>HC | CD1 | 2.35 | 27 | 0.027 | 5.48 | ||
| NAT>HC | AB3 | 3.14 | 26 | 0.004 | 9.79 | ||
| Thalamus | R | NAT>ATT | AB3 | 3. 61 | 28 | 0.001 | 12.93 |
| NAT>HC | AB3 | 2.32 | 26 | 0.029 | 5.35 | ||
| Fusiform Gyrus (BA37) | L | ATT>HC | CD3 | 2.52 | 27 | 0.018 | 6.33 |
| Occipital Gyrus (BA19) | R | HC>ATT | CD2 | 2.43 | 27 | 0.022 | 5.88 |
For Condition, AB=high risk, CD=low risk, Numeral=block (e.g. AB=high risk choices in block 1)
In Block 3, NAT again showed significantly greater activation to low risk decisions than HC in the left hippocampus (p=0.003). In addition, ATT showed significantly greater activation than HC to low risk decisions in the left caudate (p=0.002). To high risk decisions in block 3, NAT showed significantly greater activation than ATT in the right thalamus (p=0.001).
3.3. Exploratory Analyses
A second 3×2×3 ANOVA, covarying for age and behavioral scores, revealed the same group by condition by block interactions. Exploratory analyses to examine relationships between task performance and extractions of the areas of activity showed no significant correlations between task performance and activity in any of the three groups. Exploratory analyses were performed in ATT and NAT separately to examine relationships between development (age, pubertal status), clinical variables (BDI, SCARED child and parent rating, SIQ, medication status and load), gender, and activity in the regions showing significant differences in activity relative to other groups that emerged from the 3×2×3 interaction. A statistical threshold of p=0.05/9 (p<0.006) was employed to control for the nine multiple tests (one for each of the demographic and clinical variables above) in each region within each group. These analyses revealed no significant relationships between these variables and activation in these regions in either group. In addition, there was no significant correlation between lethality rating or time since attempt and activation in ATT (see Table S2).
4. Discussion
The specific aim of the study was to determine the extent to which adolescent suicide attempters (ATT) showed abnormal activity during performance of the Iowa Gambling Task compared with adolescent non-attempters (NAT) and healthy controls (HC). Contrary to our main hypothesis, ATT had the best performance on the task as they chose significantly more low risk choices than the other two groups. There is conflicting evidence about the ability of performance on the IGT to differentiate patients with a history of suicide attempt, however. In a group of adult patients with bipolar disorder, IGT performance alone did not differentiate attempter from non-attempter patients (Gilbert et al., 2011). There was no direct relationship between activity and task performance. In addition, ATT relative to age-matched NAT and HC did not show decreased activity during high-risk versus low-risk choices in left-sided regions previously implicated in IGT performance in healthy adults.
The main significant finding was that during low-risk decisions in block 2 and 3, NAT, but not ATT, showed significantly greater hippocampal activation than HC. In fact, NAT showed increased activation, while HC and ATT deactivated the left hippocampus. ATT did not differentiate from HC in levels of activation. Block 2 may be important because this is the block in which learning about the task rule occurs. ATT improved in task performance at the end of block 1 and the beginning of block 2 and continued to perform well for the remainder of the choices. Improvements in task performance at the beginning of block 2 were demonstrated previously in the development of computerized version of the IGT and in the modification of the task for use in functional neuroimaging (Lawrence et al., 2009). The fact that NAT showed increased activation in the left hippocampus during block 2 and 3 may suggest inefficient learning compared to HC and ATT. NAT are recruiting the hippocampus, but inefficiently, especially during the last block, and fail to learn the task. Thus, there may be a compensatory increase in hippocampal recruitment in NAT versus HC.
Hippocampal function is important for learning, and evidence of hippocampal abnormality and atrophy in severe and recurrent depression is well documented (Konarski et al., 2008; Macqueen and Frodl, 2011). Given that hippocampal atrophy is reversible, with remitted depressed patients showing less volume loss (relative to healthy individuals) than currently depressed patients, it appears that depression may contribute to hippocampal dysfunction (Frodl et al., 2004). In addition to smaller hippocampal volume associated with depression, there is also evidence of decreased hippocampal 5HT-2a receptor binding in depressed patients (Sheline et al., 2002). Furthermore, decreased activity in the hippocampus in depressed participants relative to healthy controls has been demonstrated in functional neuroimaging tasks involving memory (Sheline et al., 2002; Milne et al., 2011). One explanation for a potentially causal relationship between MDD and hippocampal abnormality is neurotoxicity from stress-induced glucocorticoids, that increase the rate of damage to hippocampal neurons (Sapolsky et al., 1986). Although smaller hippocampal volume may underlie risk for depression (Yamasue et al.2008), the stress model of hippocampal volume loss is supported by the observation that duration of depression predicts hippocampal volume (Sheline et al., 1999), and that antidepressant treatment increases hippocampal volume in depressed patients (Schermuly et al., 2011).
The left hippocampus, in particular, is important in contextual learning and memory (Burgess et al., 2002). Depression may impact the ability to recruit the hippocampus during learning, appraising risk, and when making decisions, and may impact performance on the IGT (Han et al., 2011). Therefore, it is surprising that performance on the IGT was preserved in ATT, and that left hippocampal activation significantly differentiated HC from NAT, but not ATT. This is especially surprising considering that there was bias with ATT having greater depression symptomatology in the present study. This is, however, consistent with our previous findings that adolescents with a history of suicide attempt did not show abnormal patterns of neural activity relative to healthy controls during a response inhibition task (Pan et al., 2011).
Despite more severe depression in ATT relative to NAT, as evidenced by higher depression ratings in ATT, unlike NAT, ATT did not show greater hippocampal activation than HC during task performance. This finding, in combination with better performance on the task in ATT relative to NAT, may thus indicate less cognitive impairment in ATT than NAT. Together with our previous findings, however, the present study suggests that suicide attempts during adolescence may not be associated with abnormal activity in neural circuitry supporting cognitive tasks (response inhibition and decision-making in the context of risk), and that potentially different neural mechanisms may underlie risk for suicide attempt versus major depressive disorder in adolescence. It is possible that other contributors, for example, neural circuitry supporting emotion as demonstrated in adult males with a history of suicide attempt may underlie depression with suicidal behavior versus depression alone. Jollant et al. (2008) demonstrated that adult male suicide attempters showed greater activation in the right lateral orbitofrontal cortex to angry faces and increased activity in the right anterior cingulate gyrus to happy faces than depressed adult males without a history of suicide attempt, but there are not data regarding emotion processing in adolescents with a history of suicide attempt. Further research should focus on potential differences between these two disease states.
During high-risk decisions in block 3, ATT showed slight deactivation in the right thalamus compared to NAT (p=0.001), and during low-risk decisions in block 2, NAT showed increased activation compared to HC in the left middle temporal gyrus (p=0.003). This indicates that NAT demonstrated differences in visual and object processing relative to both groups. One interpretation of these findings is that ATT were attending less to risky choices in block 3. This is consistent with the better performance on the task, in that they learned to pay less attention to the high-risk decks. Greater activation in the caudate in ATT compared to HC during low-risk choices in block 3 may further indicate ATT awareness of low-risk decisions leading to better task performance.
Exploratory analyses did not reveal any significant relationships in either ATT or NAT between medications or clinical symptoms and activity in the neural regions showing between-group differences, therefore it is unlikely that these accounted for the patterns of activity we observed, but future studies should aim to recruit medicated and unmedicated ATT and NAT. Given the continued development of the hippocampus throughout childhood and adolescence (Benes et al., 1994; Giedd et al., 1996), future longitudinal studies should also examine the extent to which elevated depression and anxiety symptoms are associated with abnormal developmental trajectories of the hippocampus, and other key neural regions, implicated in learning in the context of risk.
There were limitations to the present study. ATT had higher symptom scores than NAT. These variables were not, however, associated with patterns of neural activity in ATT during task performance. Given the challenges associated with recruiting ATT, and also NAT, our overall sample size was relatively modest. Additionally, given the length of the task, and poor tolerance of the last two blocks, we excluded the last two blocks from our analyses. The task may have been unmotivating to some of our participants. Nonetheless, learning optimal strategies for IGT performance did occur at the end of block 1 and the beginning of block 2 in HC and ATT. There were, however, no clear relationships between task performance in each block and hippocampal activity. These findings suggest that future studies could employ decision-making tasks that adolescents find easier to perform. Our main findings did not survive SVC with FWE in SPM5, but did survive SVC with AlphaSim correction at p<0.05. While SVC with FWE is a far more stringent corrections strategy, AlphaSim is a method of correction for multiple comparisons employed in other software packages (e.g. AFNI) and has been used in previous neuroimaging studies of pediatric populations (Pan et al., 2011; Dickstein et al., 2010; Monk et al., 2008; Gilbert et al., 2005). It was necessary to recruit ATT and NAT who were taking psychotropic medication for treatment of severe depression (Table 1). Exploratory analyses did not, however, show any significant relationships in NAT and ATT between medication and activity during high-risk or low-risk decisions in those regions showing between group differences.
Our study is the first to examine the functional integrity of neural circuitry in adolescents with a history of suicide attempt during performance of the IGT. The present findings indicate NAT, but not ATT are differentiated from HC during learning in the context of risk. Our findings thereby suggest that functional abnormalities in neural circuitry implicated in learning in the context of risk may underlie risk for MDD, but not risk for suicide attempt, in adolescence.
Supplementary Material
Figure 1.
Performance on the modified Iowa Gambling Task. A. Median net scores by block of 20 choices. B. Median total net score. P<0.05 for ATT < NAT and HC.
Acknowledgments
We thank the participants who made this research possible. All work was completed in the Department of Psychiatry of the University of Pittsburgh, and the Brain Imaging Research Center (University of Pittsburgh & Carnegie Mellon University).
This research was supported by the American Foundation for Suicide Prevention, the Klingenstein Third Generation Foundation Fellowship for Adolescent Depression, NIMH/NICHD 1K23MH082884 (LP); NIH grants MH66775, MH65368, MH56612, MH18951 (DB); NIMH MH076971 (MP).
Footnotes
Disclosures
The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arie M, Apter A, Orbach I, Yefet Y, Zalzman G. Autobiographical memory, interpersonal problem solving, and suicidal behavior in adolescent inpatients. Comprehensive Psychiatry. 2008;49 (1):22–29. doi: 10.1016/j.comppsych.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Bechara A, Tranel D, Damasion H. Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain. 2004;123:2189–2202. doi: 10.1093/brain/123.11.2189. [DOI] [PubMed] [Google Scholar]
- 3.Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cerebral Cortex. 2000;10 (3):295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- 4.Bechara A, Damasio H, Damasio AR, Lee GP. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. The Journal of Neurosience. 1999;19:5473–5481. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50 (1–3):7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 6.Beck AT, Beck R, Kovacs M. Classification of suicidal behaviors: Quantifying intent and medical lethality. American Journal of Psychiatry. 1975;132:285–287. doi: 10.1176/ajp.132.3.285. [DOI] [PubMed] [Google Scholar]
- 7.Beck AT, Schuyler D, Herman I. Development of Suicidal Intent Scales. In: Beck AT, Resnick HLP, Lettieri DJ, editors. The Prediction of Suicide. Charles Press; Bowie, Maryland: 1974. pp. 45–56. [Google Scholar]
- 8.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 9.Benes FM, Turtle M, Khan Y, Farol P. Myelination of a key relay zone in the hippocampal formation occurs in the human brain during childhood, adolescence, and adulthood. Archives of General Psychiatry. 1994;51:477–484. doi: 10.1001/archpsyc.1994.03950060041004. [DOI] [PubMed] [Google Scholar]
- 10.Birmaher B, Khetarpal S, Brent D, Cully M, Balach L, Kaufman J, McKenzie M, Neer S. The Screen for Child Anxiety Related Emotional Disorders (SCARED) : Scale construction and psychometric characteristics. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:545–553. doi: 10.1097/00004583-199704000-00018. [DOI] [PubMed] [Google Scholar]
- 11.Bridge JA, Goldstein TR, Brent DA. Adolescent suicide and suicidal behavior. Journal of Child Psychology and Psychiatry. 2006;37 (3–4):372–394. doi: 10.1111/j.1469-7610.2006.01615.x. [DOI] [PubMed] [Google Scholar]
- 12.Burgess N, Maguire EA, O’Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002;35:625–641. doi: 10.1016/s0896-6273(02)00830-9. [DOI] [PubMed] [Google Scholar]
- 13.Dickstein DP, Finger EC, Skup M, Pine DS, Blair JR, Leibenluft E. Altered neural function in pediatric bipolar disorder during reversal learning. Bipolar Disorders. 2010;12 (7):707–719. doi: 10.1111/j.1399-5618.2010.00863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ernst M, Bolla K, Mouratidis M, Contoreggi C, Matochik JA, Kurian V, Cadet JL, Kimes AS, London ED. Decision making in a risk-taking task: a PET study. Neuropsychopharmacology. 2002;26:682–691. doi: 10.1016/S0893-133X(01)00414-6. [DOI] [PubMed] [Google Scholar]
- 15.Ernst M, Grant SJ, London ED, Contoreggi CS, Kimes AS, Spurgeon L. Decision making in adolescents with behavior disorders and adults with substance abuse. The American Journal of Psychiatry. 2003;160 (1):33–40. doi: 10.1176/appi.ajp.160.1.33. [DOI] [PubMed] [Google Scholar]
- 16.Frodl T, Meisenzahl EM, Zetzsche T, Hohne T, Banac S, Schorr C, Jager M, Leinsinger G, Bottelender R, Reiser M, Moller HJ. Hippocampal and amygdala changes in patients with major depressive disorder and healthy controls during a 1-year follow-up. The Journal of Clinical Psychiatry. 2004;65:492–499. doi: 10.4088/jcp.v65n0407. [DOI] [PubMed] [Google Scholar]
- 17.Giedd JN, Vaituzis AC, Hamburger SD, Lange N, Rajapakse JC, Kaysen D, Vauss YC, Rapoport JL. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4–18 years. Journal of Comprehensive Neurology. 1996;366:223–230. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 18.Gilbert AR, Akkal D, Almeida JRC, Mataix-Cols D, Kalas C, Devlin B, Birmaher B, Phillips ML. Neural correlates of symptom dimensions in pediatric obsessive-compulsive disorder: a functional magnetic resonance imaging study. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;48 (9):936–944. doi: 10.1097/CHI.0b013e3181b2163c. [DOI] [PubMed] [Google Scholar]
- 19.Gilbert AM, Garno JL, Braga RJ, Shaya Y, Goldberg TE, Malhotra AK, Burdick KE. Clinical and cognitive correlates of suicide attempts in bipolar disorder: is suicide predictable? Journal of Clinical Psychiatry. 2011;72 (8):1027–33. doi: 10.4088/JCP.10m06410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han G, Klimes-Dougan B, Jepsen S, Ballard K, Nelson M, Houri A, Kumra S, Cullen K. Selective neurocognitive impairments in adolescents with major depressive disorder. Journal of Adolescence. 2012;35 (1):11–20. doi: 10.1016/j.adolescence.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hassel S, Almeida JR, Kerr N, Nau S, Ladouceur CD, Fissell K, Kupfer DJ, Phillips ML. Elevated striatal and decreased dorsolateral prefrontal cortical activity in response to emotional stimuli in euthymic bipolar disorder: no associations with psychotropic medication load. Bipolar Disorders. 2008;10 (8):916–927. doi: 10.1111/j.1399-5618.2008.00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jollant F, Lawrence NS, Giampietro V, Brammer MJ, Fullana MA, Drapier D, Courtet P, Phillips ML. Orbitofrontal cortex response to angry faces in men with histories of suicide attempts. The American Journal of Psychiatry. 2008;165 (6 ):740–748. doi: 10.1176/appi.ajp.2008.07081239. [DOI] [PubMed] [Google Scholar]
- 23.Jollant F, Lawrence NS, Olie E, O’Daly O, Malafosse A, Courtet P, Phillips ML. Decreased activation of lateral orbitofrontal cortex during risky choices under uncertainty is associated with disadvantageous decision-making and suicidal behavior. NeuroImage. 2010;51 (3):1275–1281. doi: 10.1016/j.neuroimage.2010.03.027. [DOI] [PubMed] [Google Scholar]
- 24.Jollant F, Bellivier F, Leboyer M, Astruc B, Torres S, Verdier R, Castelnau D, Malafosse A, Courtet P. Impaired decision making in suicide attempters. The American Journal of Psychiatry. 2005;162 (2):304–310. doi: 10.1176/appi.ajp.162.2.304. [DOI] [PubMed] [Google Scholar]
- 25.Kaufman J, Birmaher B, Brent DA, Ryan ND, Rao U. K-SADS-PL. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39 (10):1208. doi: 10.1097/00004583-200010000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Konarski JZ, McIntyre RS, Kennedy SH, Rafi-Tari S, Soczynska JK, Ketter TA. Volumetric neuroimaging investigations in mood disorders: Bipolar disorder versus major depressive disorder. Bipolar Disorders. 2008;10:1–37. doi: 10.1111/j.1399-5618.2008.00435.x. [DOI] [PubMed] [Google Scholar]
- 27.Lawrence NS, Jollant F, O’Daly O, Zelaya F, Phillips ML. Distinct roles of prefrontal cortex subregions in the Iowa Gambling Task. Cerebral Cortex. 2009;19 (5):1134–1143. doi: 10.1093/cercor/bhn154. [DOI] [PubMed] [Google Scholar]
- 28.Lubell KM, Kegler SR, Crosby AE, Karch D. Suicide trends among youths and young adults aged 10–24 years-United States, 1990–2004. MMWR Morbity and Mortality Weekly Report. 2007;56 (35):905–908. [PubMed] [Google Scholar]
- 29.Macqueen G, Frodl T. The hippocampus in major depression: Evidence for the convergence of the bench and bedside in psychiatric research? Molecular Psychiatry. 2011;16:252–264. doi: 10.1038/mp.2010.80. [DOI] [PubMed] [Google Scholar]
- 30.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fmri data sets. NeuroImage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. (WFU Pickatlas, version3.03) [DOI] [PubMed] [Google Scholar]
- 31.Milne AM, Macqueen GM, Hall GB. Abnormal hippocampal activation in patients with extensive history of major depression: an fMRI study. Journal of Psychiatry and Neuroscience. 2011;36(4):110004. doi: 10.1503/jpn.110004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Monk CS, Telzer EH, Mogg K, Bradley BP, Mai X, Louro HM, Chen G, McClure-Tone EB, Ernst M, Pine DS. Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Archives of General Psychiatry. 2008;65 (5):568–576. doi: 10.1001/archpsyc.65.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oldershaw A, Grima E, Jollant F, Richards C, Simic M, Taylor L, Schmidt U. Decision making and problem solving in adolescents who deliberately self-harm. Psychological Medicine. 2009;39 (1):95–104. doi: 10.1017/S0033291708003693. [DOI] [PubMed] [Google Scholar]
- 34.Oquendo MA, Halberstam B, Mann JJ. Risk factors for suicidal behavior: utility and limitations of research instruments. In: First MB, editor. Standardized Evaluation in Clinical Practice. American Psychiatric Publishing; Washington, DC: 2003. pp. 103–130. [Google Scholar]
- 35.Overman WH, Frassrand K, Ansel S, Trawalter S, Bies B, Redmond A. Performance on the IOWA card task by adolescents and adults. Neuropsychologia. 2004;42 (13):1838–1851. doi: 10.1016/j.neuropsychologia.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 36.Pan LA, Batezati-Alves SC, Almeida JR, Segreti A, Akkal D, Hassel S, Lakdawala S, Brent DA, Phillips ML. Dissociable patterns of neural activity during response inhibition in depressed adolescents with and without suicidal behavior. Journal of the American Academy of Child and Adolescent Psychiatry. 2011;50 (6):602–611. doi: 10.1016/j.jaac.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- 38.Posner K, Oquendo MA, Gould M, Stanley B, Davies M. Columbia Classification Algorithm of Suicide Assessment (C-CASA) : Classification of Suicidal Events in the FDA’s Pediatric Suicidal Risk Analysis of Antidepressants. American Journal of Psychiatry. 2007;164:1035–1043. doi: 10.1176/appi.ajp.164.7.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reynolds WM. Suicidal Ideation Questionnaire: Professional Manual. Psychological Assessment. 1987;2:382–390. [Google Scholar]
- 40.Sapolsky R, Krey L, McEwen B. The neuroendocrinology of stress and aging: The glucocorticoid cascade hypothesis. Endocrine Reviews. 1986;7:284–301. doi: 10.1210/edrv-7-3-284. [DOI] [PubMed] [Google Scholar]
- 41.Schermuly I, Wolf D, Lieb K, Stoeter P, Fellgiebel A. State dependent posterior hippocampal volume increases in patients with major depressive disorder. Journal of Affective Disorders. 2011;135 (1–3):405–409. doi: 10.1016/j.jad.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 42.Schutter DJ, van Bokhoven I, Vanderschuren LJ, Lochman JE, Matthys W. Risky decision making in substance dependent adolescents with a disruptive behavior disorder. Journal of Abnormal Child Psychology. 2011;39 (3):333–339. doi: 10.1007/s10802-010-9475-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sheline YI, Mittler BL, Mintun MA. The hippocampus and depression. European Psychiatry. 2002;17 (Suppl 3):300–305. doi: 10.1016/s0924-9338(02)00655-7. [DOI] [PubMed] [Google Scholar]
- 44.Sheline YI, Sanghavi M, Mintun MA, Gado MH. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. The Journal of Neuroscience. 1999;19 (12):5034–5043. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Statistical Parametric Mapping Manual. 2009:66. ( http://www.fil.ion.ucl.ac.uk/spm)
- 46.Toplak ME, Jain U, Tannock R. Executive and motivational processes in adolescents with Attention-Deficit-Hyperactivity Disorder (ADHD) Behavior and Brain Function. 2005;1 (1):8. doi: 10.1186/1744-9081-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) San Antonio, TX: Harcourt Assessment; 1999. [Google Scholar]
- 48.Williams JM, Barnhofer T, Crane C, Beck AT. Problem solving deteriorates following mood challenge in formerly depressed patients with a history of suicidal ideation. Journal of Abnormal Psychology. 2005;114 (3):421–431. doi: 10.1037/0021-843X.114.3.421. [DOI] [PubMed] [Google Scholar]
- 49.Yamasue H, Abe O, Suga M, Yamada H, Inoue H, Tochigi M, Rogers M, Aoki S, Kato N, Kasai K. Gender-common and –specific neuroanatomical basis of human anxiety-related personality traits. Cerebral Cortex. 2008;18 (1):46–52. doi: 10.1093/cercor/bhm030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.