Abstract
Excitatory amino acids play a key role in stress-induced remodeling of dendrites in the hippocampus as well as in suppression of neurogenesis in the dentate gyrus. The regulation of extracellular glutamate levels has been suggested as a potential mechanism through which repeated stress causes dendritic remodeling of CA3 pyramidal neurons. Accordingly, the current study examined the distribution and regulation of the glia glutamate transporter GLT-1 and the recently identified GLT isoform, GLT-1b, in the hippocampus of rats subjected to chronic restraint stress (CRS). We also examined the ability of the antidepressant tianeptine, which blocks CRS-induced dendritic remodeling, to modulate CRS-mediated changes in GLT-1 and GLT-1b expression. CRS increased GLT-1 mRNA expression in the dentate gyrus and CA3 region of Ammon's horn, increases that were inhibited by tianeptine. CRS more selectively increased GLT-1 protein levels in the subregion where dendritic remodeling is most prominent, namely the CA3 region, increases that were also inhibited by tianeptine administration. In contrast, GLT-1b mRNA expression was not modulated in the hippocampus in any of these groups, but CRS increased GLT-1b protein levels in all hippocampal subfields examined, increases that were unaffected by tianeptine treatment. These results point to the importance of understanding the mechanism for the differential and subregional regulation of GLT-1 isoforms in neuronal and glial compartments in the hippocampus as a basis for understanding the effects of chronic stress on structural plasticity as well as the neuroprotective properties of agents such as tianeptine.
Exposure of rats to 21 days of chronic restraint stress (CRS) or stress levels of glucocorticoids (GCs) for 3 weeks produces reversible remodeling of the apical dendrites of CA3 pyramidal neurons and suppression of neurogenesis in the dentate gyrus (1-4), morphological changes that are associated with impairments of hippocampal dependent memory (5, 6). In view of clinical observations that stress precipitates depression and that recurrent depressive illness is associated with hippocampal shrinkage (7), chronic stress may serve as an experimental model to evaluate the underlying cellular and molecular alterations associated with some of the consequences of recurrent depressive illness. In this regard, we have shown that the antidepressant drug, tianeptine, inhibits stress-induced dendritic remodeling and also reverses dendritic remodeling established by prior exposure to stress levels of GCs (8). Tianeptine also inhibits behavioral deficits produced by chronic stress (9).
In addition to relying on morphological evaluation of stress effects, it is important to begin to establish neurochemical markers of the actions of chronic stress in the hippocampus and other brain regions, and, for this reason, the neurochemistry of stress effects in the hippocampus must be better understood. Besides GCs, excitatory amino acids (EAAs) are strongly implicated in dendrite remodeling and suppression of neurogenesis in the hippocampus (3). For example, administration of N-methyl-d-aspartate (NMDA) receptor antagonists enhances neurogenesis in the dentate gyrus (10) and blocks stress-induced shrinkage of CA3 pyramidal neurons (8), data that implicate the EAA system in the suppression of cell proliferation and the development of dendritic remodeling. Restraint stress increases extracellular glutamate levels in the hippocampus (11, 12), an effect that requires the presence of the adrenal glands and may conceivably involve the regulation of EAA transporter expression and activity (6, 13). Here, we report the effects of CRS on this neurochemical system.
Because GLT-1 (EAAT2) is the predominant glutamate transporter in the rat hippocampus (14), the aim of the current study was to determine the regulation of this glial-specific EAA transporter under conditions that produce hippocampal dendritic remodeling, namely, in rats subjected to CRS. At the same time, we also examined the distribution and regulation by CRS of the recently described glutamate transporter isoform, GLT-1b. In addition, because of its efficacy in blocking dendrite remodeling, we examined the ability of tianeptine to modulate stress-induced changes in GLT-1 and GLT-1b expression in the rat hippocampus to provide a more specific pharmacological and neurochemical signature of the effects of CRS on dendritic morphology and neurogenesis.
Materials and Methods
Animal Protocols. Adult male Sprague-Dawley rats (CD strain, Charles River Breeding Laboratories) weighing 200-250 g were housed in groups of three with ad libitum access to food and water, in accordance with all guidelines and regulations of The Rockefeller University Animal Care and Use Committee. Animals were maintained in a temperature-controlled room, with a light/dark cycle of 12/12 h (lights on at 0700 hours). Rats were subjected to 6-h restraint stress daily for 21 days (CRS) in wire mesh restrainers secured at the head and tail ends with clips. Before restraint stress, CRS rats were given i.p. injections of sterile saline (CSR-S), or 10 mg/kg tianeptine (CRS-T). Rats were returned to their home cages during the stress session. Nonstressed control (NSC) rats were handled each day and given daily injections of sterile saline (NSC-S). An additional nonstressed control group was given daily injections of tianeptine (NSC-T). Each experimental group consisted of 10 rats. In agreement with previous studies from our lab and other investigators (8), rats subjected to stress gained less weight than nonstressed controls during the course of the study (NSC-T = 107.1 ± 6.3 g; CRS-S = 64.1 ± 4.4; P < 0.0001) whereas tianeptine treatment had no effect on weight gain (NSC-T = 105.2 ± 4.4 g; CRS-T = 65.7 ± 4.4 g; P < 0.0001 compared with CS and CT). Similarly, previous studies have established that tianeptine does not influence basal or stress-induced increases in GC levels (15, 16). Eighteen hours after the final stress session on day 21, rats were weighed and decapitated; the brains were quickly removed and stored at -70°C until use.
Probe Design. Total RNA was isolated from whole adult male rat brain (which had been previously frozen on granulated dry ice and stored at -70°C) by using an RNeasy kit (Qiagen, Valencia, CA), and then first strand cDNA synthesis was performed with SuperScript II (Invitrogen). PCR primers were designed from the mouse sequences of GLT-1 and GLT-1b (17) by using the primer analysis software oligo v4.0 (National Biosciences, Plymouth, MN). Primer sequences (listed 5′ to 3′: upper primer, lower primer) and PCR fragment length (base pairs) were as follows: GLT-1, GGC GGC CAA TGG AAA GTC AGC, CAC AGC ACG GTG CCT ATC ATC ACG, (243 bp); GLT-1b, TTC CCA TTC CTG GAT ATC GAG ACC TG, AAT ATC TGA AGG CCA TTA AAG TTC TGA CAA CC, (313 bp). Using a TOPO-TA Cloning kit (Invitrogen), PCR products were cloned into pCR4 and sequenced for verification and orientation.
In Situ Hybridization Histochemistry. In situ hybridization studies were performed as described (18) by using the designed sense and antisense probes specific for GLT-1 and GLT-1b. Antisense riboprobes were labeled with 35S-UTP by using T7 RNA polymerase; sense probes were labeled with 35S-UTP by using T3 RNA polymerase.
Radioimmunocytochemistry (RIC). RIC analysis was performed as described (19-21) by using GLT-1 (14) and GLT-1b selective antisera. Briefly, rat brain sections were incubated with GLT-1 primary antisera (1:1,000) or GLT-1b primary antisera (1:1,000) in 0.05 M PBS/1% BSA for 2 h at room temperature with gentle shaking. Sections were washed and incubated with 35S-labeled goat anti-rabbit secondary antisera (Amersham Biosciences) at a dilution of 1:400 in 0.05 M PBS/1% BSA at room temperature with gentle shaking for 2 h. Sections were thoroughly washed, were air-dried under a fan overnight, and were exposed to Kodak X-Omat film.
Autoradiographic Film Analysis and Statistical Analysis. Computer-assisted microdensitometry of autoradiographic images was determined on the MCID image analysis system (Imaging Research, Inc., St. Catherines, Canada). For RIC and ISHH, microscale 14C standards (Amersham Biosciences) were exposed on Kodak X-Omat film and digitized. Gray level/optical density calibrations were performed by using a calibrated film strip ladder (Imaging Research, St. Catherine's, ON, Canada) for optical density. Optical density was plotted as a function of microscale calibration values. The values obtained represent the average of measurements taken from two to three sections per slide and two slides per animal. Mean values of treated groups were reported as percentage of control. For all studies, statistical analysis was performed with a two-way ANOVA, followed by Student-Newman-Keuls post hoc test, with P < 0.05 as the criterion for statistical significance.
Results
GLT-1 and GLT-1b mRNA Expression in Rat Hippocampus. GLT-1 mRNA has previously been localized to glia, as well as neurons in the rat hippocampus, although immunological approaches failed to detect GLT-1 protein in neurons at the ultrastructural level (22). However, in addition to the glial isoform of GLT-1, recent reports have provided evidence for the existence of a novel isoform of GLT-1, referred to as GLT-1b (23). Because we have previously hypothesized that glial glutamate transport participates in stress-mediated changes in the rat hippocampus (6, 13), we designed GLT-1- and GLT-1b-specific riboprobes. In agreement with previous studies (14), in situ hybridization histochemical analysis revealed that GLT-1 mRNA exhibited a widespread distribution in the rat hippocampus, including stratum oriens and stratum radiatum of CA1, CA2, and CA3 of Ammon's horn; the molecular layers of the superior blade of the dentate gyrus (DGs) and the inferior blade of the dentate gyrus (DGi); and the hilus (Fig. 1A). It is interesting to note that GLT-1 mRNA was also detected predominantly in the stratum pyramidal of CA3, as well as stratum granule of the dentate gyrus.
Fig. 1.
Representative autoradiographs of GLT-1 and GLT-1b mRNA expression in the hippocampus of NSC rats. (A) GLT-1 mRNA expression, as detected by use of 35S-labeled antisense riboprobe, exhibits a widespread expression in the rat hippocampus, including stratum oriens, stratum pyramidal, and stratum radiatum in Ammon's horn, as well as the molecular layers of the dentate gyrus and the hilus. (B) GLT-1b mRNA expression, as detected by use of 35S-labeled antisense riboprobe, was detected in principal cell layers in CA1, CA2, and CA3 of Ammon's horn, as well as the granule cell layers of the dentate gyrus (DG).
As predicted by previous reports that GLT-1b is expressed in neurons (23), GLT-1b mRNA expression was observed in CA1, CA2, and CA3 pyramidal neurons of Ammon's horn and granule cell neurons of the dentate gyrus (Fig. 1B). Single-cell emulsion autoradiography revealed that GLT-1 mRNA was abundantly expressed throughout the rat hippocampus whereas GLT-1b mRNA expression was more abundantly expressed in granule cell layers of the dentate gyrus and pyramidal cell layers of Ammon's horn although GLT-1b silver grains were also deposited outside the principal cell layers in the hippocampus (data not shown). These results suggest that GLT-1b mRNA is expressed in neurons and astrocytes, as predicted by previous reports (23).
Stress Increases GLT-1 mRNA Expression: Inhibition by Tianeptine. The regulation of GLT-1 and GLT-1b mRNA expression was examined by in situ hybridization histochemistry in nonstressed controls given saline (NSC-T), CRS rats given saline (CRS-S), and rats subjected to CRS and given daily injections of tianeptine (CRS-T). Autoradiographic analysis revealed that GLT-1 mRNA expression was not modulated in the CA1 or CA2 regions of Ammon's horn in any of the treatment groups compared with NSC-T rats (Fig. 2A). CRS significantly increased GLT-1 mRNA levels in CA3 stratum oriens (CA3-or) and CA3 stratum radiatum (CA3-rad) of Ammon's horn.
Fig. 2.
Autoradiographic image analysis of GLT-1 mRNA expression in the hippocampus of rats subjected to CRS. (A) Statistical analysis revealed that CRS did not modulate GLT-1 mRNA levels in the CA1 and CA2 regions of Ammon's horn. Conversely, CRS increased GLT-1 mRNA levels in stratum oriens [CA3-so; F(3,35) = 5.169] and stratum radiatum [CA3-rad; F(3,35) = 4.184] of the CA3 region, increases that were reversed by daily tianeptine administration (CRS-T). (B) In the dentate gyrus, CRS increased GLT-1 mRNA levels in the molecular layers of the superior blade of the dentate gyrus [DGs-mol; F(3,35) = 4,452] and the inferior blade of the dentate gyrus [DGi-mol; F(3,35) = 4.963]. Daily tianeptine administration (CRS-T) inhibited stress-mediated increases in GLT-1 mRNA expression. Data are expressed as percentages of values obtained in nonstressed controls given saline (NSC-S). *, P ≤ 0.01 compared with NSC-T; $, P ≤ 0.01 compared with CRS-T.
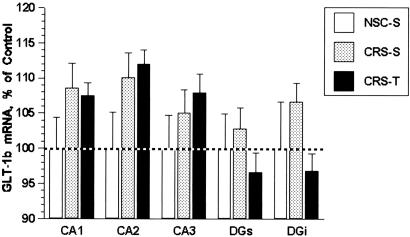
Tianeptine treatment was used to determine whether the effects of CRS on GLT-1 expression parallel its ability to block structural remodeling in CA3 and ability to attenuate stress-induced suppression of neurogenesis. Daily tianeptine administration blocked the CRS-induced increases in GLT-1 mRNA levels in the CA3 region (Fig. 2 A). Tianeptine administration also inhibited CRS-induced increases in GLT-1 mRNA levels in the molecular layer of the superior blade of the dentate gyrus (DGs-mol) and the molecular layer of the inferior blade of the dentate gyrus (DGi-mol; Fig. 2B). In situ hybridization histochemistry performed using the GLT-1b-specific riboprobe did not reveal any differences in the expression of GLT-1b mRNA in the hippocampus of any of the treatment groups examined, as a result of either CRS or tianeptine treatment (Fig. 3).
Fig. 3.
Autoradiographic film analysis of GLT-1b mRNA expression in the hippocampus of rats subjected to chronic stress. GLT1b mRNA levels were not modulated in the hippocampus of rats subjected to chronic restraint stress (CRS-S) or stressed rats given tianeptine (CRS-T) compared with nonstressed controls given saline (NSC-S).
Stress Increases GLT-1 Protein in the CA3 Region: Inhibition by Tianeptine. We have previously used RIC to examine region-specific changes in protein expression mediated by adrenal steroids (20, 21), and streptozotocin diabetes (19, 20). In the current study, we examined the ability of stress to modulate GLT-1 protein levels in the rat hippocampus using previously characterized GLT-1 antiserum (14) and 35S-labeled secondary antibodies. GLT-1 protein was abundantly expressed in stratum oriens and stratum radiatum of Ammon's horn, as well as the molecular layers of the dentate gyrus (Fig. 4A). Radioimmuno-reactive levels of GLT-1 were lower near the principal cell layers of the hippocampus, including stratum pyramidal and the granule layer of the dentate gyrus (Fig. 4A). This radioimmunoreactive profile of GLT-1 is in agreement with previous immunohistochemical analyses (14, 24).
Fig. 4.
Representative autoradiographs of GLT-1 and GLT-1b protein expression in the rat hippocampus as determined by radioimmunocytochemistry. (A) GLT-1 is abundantly expressed in stratum oriens and stratum radiatum of Ammon's horn, as well as the molecular layer of the dentate gyrus and the hilus. Lower levels of GLT-1 expression are immunodetected in stratum pyramidal of Ammon's horn and the granule layer of the dentate gyrus. (B) GLT-1b radioimmunoreactivity is most abundant in and adjacent to the principal cell layers of CA1, CA2, and CA3 of Ammon's horn and the granule cell layers of the dentate gyrus (DG).
Subsequent densitometric analysis of the autoradiograms revealed that GLT-1 protein expression was not modulated in CA1 or CA2 regions of Ammon's horn or the dentate gyrus of any of the treatment groups compared with the NSC-S group (Fig. 5A). However, CRS significantly increased GLT-1 radioimmunoreactive levels in stratum pyramidal of the CA3 region (CA3-py), increases that were inhibited by daily tianeptine administration (Fig. 5B). These results demonstrate that stress specifically increases GLT-1 radioimunoreactive levels in the same region where stress-induced neuronal remodeling is observed, namely the CA3 region of the rat hippocampus.
Fig. 5.
Autoradiographic image analysis of GLT-1 radioimmunoreactivity in the hippocampus of rats subjected to stress. (A) GLT-1 protein levels were not modulated in the CA1 and CA2 regions of Ammon's horn or the dentate gyrus (DG) in rats subjected to chronic stress CRS-S, stressed rats administered tianeptine (CRS-T), or nonstressed, tianeptine-treated controls (NSC-T). (B) CRS specifically increases GLT-1 protein levels in the stratum pyramidal of CA3 [CA3-py; F(3,35) = 6.642], increases that were inhibited by daily tianeptine administration (CRS-T). Nonstressed control rats given daily tianeptine injections (NSC-T) exhibited similar levels of GLT-1 protein as nonstressed controls given saline (NSC-T). Data are expressed as percentages of values obtained in NSC-T group. *, P ≤ 0.01 compared with NSC-T; $, P ≤ 0.01 compared with CRS-T; #, P ≤ 0.01 compared with NSC-T.
CRS Increases GLT-1b Protein in the Rat Hippocampus. Studies investigating the recently identified splice variant of GLT-1, GLT-1b, revealed that this glutamate transporter isoform is expressed in neurons and astrocytes (23). To extend these earlier findings, RIC was performed to examine the localization, as well as determine the CRS regulation, of GLT-1b in the rat hippocampus. GLT-1b is abundantly expressed in the rat hippocampus, especially near the principal cell layers of Ammon's horn and the dentate gyrus (Fig. 4B). Autoradiographic analysis revealed that CRS significantly increased GLT-1b protein expression in all hippocampal subfields examined, including CA1, CA2, and CA3 of Ammon's horn and the superior (DGs) and inferior (DGi) blades of the dentate gyrus (Fig. 6). Tianeptine administration did not inhibit CRS-induced increases in GLT-1b protein levels, nor did tianeptine alter GLT-1b protein expression in nonstressed control rats. These results indicate that GLT-1 isoforms are differentially regulated by CRS because GLT-1b protein is up-regulated in all hippocampal subfields whereas GLT-1 protein is increased in a region-specific manner in CA3 of Ammon's horn. Moreover, they point to differential effects of tianeptine.
Fig. 6.
Autoradiographic image analysis of GLT-1b radioimmunoreactivity in the hippocampus of rats subjected to stress. CRS increased GLT-1b protein expression in CA1 [F(3,32) = 5.614], CA2 [F(3,32) = 8.659], and CA3 [F(3,32) = 6.853] of Ammon's horn, as well as DGs [F(3,32) = 3.455] and DGi [F(3,32) = 4.511], increases that were not modulated by tianeptine administration (CRS-T). Nonstressed control rats given daily tianeptine injections (NSC-T) exhibited similar levels of GLT-1b protein as nonstressed controls given saline (NSC-S). Data are expressed as percentages of values obtained in NSC-T group. *, P ≤ 0.01 compared with CS; #, P ≤ 0.01 compared with NSC-T.
Discussion
We have found that CRS increases GLT-1 mRNA and protein expression in the dentate gyrus and the CA3 regions of the rat hippocampus, an effect that is inhibited by the antidepressant tianeptine. In contrast, GLT-1b expression is up-regulated by CRS widely throughout the hippocampus, and this effect is not prevented by tianeptine. Because tianeptine inhibits CRS-induced dendritic remodeling in CA3 (25, 26), the modulation of glial GLT-1 expression in CA3 is a subregion-specific neurochemical correlate of dendritic remodeling that highlights the important role of EAAs in hippocampal structural plasticity and may point to distinct roles of the GLT-1 isoforms, as well as to the mechanism of action of tianeptine.
Localization of GLT Isoforms. It has been puzzling from previous reports that GLT-1 mRNA is associated with glia as well as neurons in the rat hippocampus although GLT-1 protein expression is limited to glia (22). The characterization of a variant of GLT-1 expressed in neurons (23), referred to as GLT-1b, may help explain the discrepancy between GLT-1 mRNA and protein expression in these previous studies. Using in situ hybridization histochemistry, we have used a specific probe to identify GLT-1b mRNA in pyramidal cell neurons of Ammon's horn and granule neurons of the dentate gyrus. Even then, single-cell emulsion autoradiography also identified GLT-1b silver grains beyond neuronal cell bodies, presumably hybridized to GLT-1b mRNA that is also expressed in astrocytes. Such results support previous studies that GLT-1b is expressed in both neuronal and nonneuronal cell types in the rat brain (23). These results suggest that GLT-1 and GLT-1b may be coexpressed in nonneuronal cell types (for example, astrocytes) whereas GLT-1b is more selectively expressed in neurons in the rat hippocampus.
Differential Regulation of GLT Isoforms. In addition to these differences in the compartmentation of GLT isoforms, GLT-1 and GLT-1b also seem to be differentially regulated by CRS. For example, although CRS increased GLT-1 protein levels in a region-specific manner, CRS increased GLT-1b protein levels in all subfields of Ammon's horn and the dentate gyrus. Because CRS did not modulate GLT-1b mRNA levels, as determined by in situ hybridization histochemistry, these results suggest that CRS either increases translation of the GLT-1b mRNA or decreases degradation of the GLT-1b protein. Moreover, although CRS stimulates the increases in GLT-1b protein levels throughout the hippocampus, possibly in neurons as well as in astrocytes, CRS-mediated increases in glutamate release from mossy fiber terminals may drive the region-specific increases in GLT-1 protein expression in glial cells of the CA3 region.
GCs may play a role although it is likely not to be a primary one. Chronic GC exposure increased GLT-1 protein levels throughout all hippocampal fields (G. A. Grillo, G. G. Piroli, L.P.R., and B.S.M., unpublished observations) whereas CRS effects on GLT-1 protein levels were specific to CA3. Chronic GC exposure causes remodeling of dendrites in CA3 as well as a lesser effect in dentate gyrus and CA1 (2, 4, 25, 27). Because tianeptine prevents the GC, as well as the CRS, effects on CA3 dendritic remodeling (8, 26), it is likely that there is a more proximal signal for remodeling, namely, elevated glutamate levels that result from GC exposure as well as from CRS. In support of this hypothesis, peripheral corticosterone administration increases glutamate levels in the rat hippocampus (28). Moreover, stress-induced increases of extracellular glutamate are not evident in adrenalectomized rats (11), indicating a permissive effect of the adrenals on glutamate levels. In contrast to GLT-1, chronic corticosterone administration produces small, but nonsignificant, increases in GLT-1b protein (L.P.R. and B.S.M., unpublished observations). Such preliminary findings suggest that GCs alone cannot account for the increases in GLT-1b protein expression observed in the current study but rather suggest that GCs are necessary, but not sufficient, for these CRS-induced changes and that other factors must explain the regional specificity of the effects of CRS and the actions of tianeptine.
Neuroanatomical Specificity of GLT-1 Changes. We have previously postulated that modulation of the activity of glial glutamate transporters participates in stress-mediated morphological changes in the rat hippocampus (6, 13). Stress activation of the excitatory mossy fiber projections from the dentate gyrus to the CA3 region is proposed to mediate dendritic remodeling of the apical dendrites of CA3 pyramidal neurons (3). CRS specifically increases GLT-1 protein expression in stratum pyramidale of the CA3 region, suggesting that increases in extracellular concentrations of glutamate observed during restraint stress (11, 12) elicits compensatory increases in GLT-1 expression. In support of this hypothesis, previous studies have shown that glutamate increases GLT-1 mRNA expression in astroglial primary cultures in a dose-dependent fashion (29). Therefore, tianeptine may modulate glutamate release in response to CRS, as will be discussed below.
In our neuroanatomical studies, stress-induced remodeling is limited to the apical dendrites of CA3 pyramidal neurons (4). This point is important because the dentate gyrus and CA3 region form a recurrent pathway of excitation and inhibition that is critical for the formation of memories of sequences of events (30) but at the same time increases vulnerability to seizures and damage (31). As a result of the special features of the DG-CA3 connection and the selective effects of stress on remodeling, we anticipated that GLT-1 protein expression would be modulated in the stratum radiatum layer of CA3 where mossy fiber projections terminate upon CA3 pyramidal cell apical dendrites. Indeed, whereas ultrastructural analysis has demonstrated that GLT-1 protein is expressed in astrocytes interspersed between hippocampal pyramidal cell bodies (24), GLT-1 protein densities are greatest in close proximity to glutamatergic synapses (22). Nonetheless, CRS-induced increases in GLT-1 protein expression were limited to the stratum pyramidale in the rat hippocampus, suggesting that glial cells in this region are responding selectively to the glutamate released from terminals.
These studies cannot eliminate the possibility that CRS-induced increases in GLT-1 protein expression may extend into the stratum radiatum of CA3 because radioimmunocytochemistry does not provide the spatial resolution of other techniques, such as electron microscopic analyses. In fact, in our study, CRS produced nonsignificant increases in GLT-1 protein expression in CA3 stratum radiatum; and tianeptine administration showed a trend to return these stress-induced increases in GLT-1 protein levels to control values. As a result, future quantitative ultrastructural analyses will be required to more fully elucidate the localization of stress-induced increases in GLT-1 protein expression in the CA3 region of the rat hippocampus.
Possible Key Role of EAAs. It is important to note that the stress-induced regulation of GLT-1 by tianeptine is not mimicked by phenytoin treatment (L.P.R. and B.S.M., unpublished observations) even though phenytoin, an antiepileptic drug that blocks Na+ and t-type Ca2+ channels (32), also prevents dendritic remodeling produced by both GCs and CRS (25). Thus the stress-effects on GLT-1 levels does not result from changes in hippocampal morphology per se, but rather reflects fundamental changes in the underlying neurochemical and molecular properties of the hippocampus in response to stress. As mentioned above, stress activation of the excitatory mossy fiber projections results in increased synaptic concentrations of glutamate (11, 12) and may serve as the driving force for the region-specific increases in GLT-1 expression. Therefore, tianeptine may affect upstream events, perhaps through modulation of stress-mediated increases in synaptic glutamate levels, whereas the actions of phenytoin involve events downstream of the actions of tianeptine.
Relationship to Mechanism of Tianeptine Action. The emerging pharmacological profile of tianeptine suggests that this antidepressant may serve to “normalize” synaptic function in response to stress. For example, studies from our laboratory have demonstrated that tianeptine inhibits the morphological changes (26) elicited by CRS whereas at least some selective serotonin reuptake inhibitors (SSRIs) do not (8). These results propose that, whereas tianeptine and SSRIs successfully treat affective symptoms, tianeptine may provide a unique additional action by treating the structural changes and cognitive impairment that are sometimes associated with recurrent depressive illness (33-35). We propose that increases in GLT-1 expression described in the current study result from stress-mediated increases in glutamate (11, 12) and that normalization of synaptic concentrations of glutamate by tianeptine during CRS eliminates the stimulus for increased GLT-1 expression.
Recent studies by other investigators provide additional evidence that tianeptine modulates the effects of stress on glutamate neurotransmission. For example, tianeptine inhibits stress-induced decreases in cell proliferation and hippocampal volume, as well as the reductions in cerebral metabolites elicited by chronic stress (15). Tianeptine also prevents stress-induced alterations in the electrophysiological properties of glutamatergic synapses after acute (36) and chronic stress (37). These results demonstrate that tianeptine effectively inhibits stress-mediated neurological changes related to EAA neurotransmission, neurochemical adaptations that may involve modulation of the phosphorylation state of glutamate receptors in the CA3 region (37). Interestingly, tianeptine administration to nonstressed control rats has no effect on these morphological (15, 38), electrophysiological (36), cellular (15, 39), or pharmacological (16) parameters; similar observations were made in the current study in relation to GLT-1 isoform protein expression. Collectively, these results support the hypothesis that tianeptine may act to normalize glutamatergic function during stressful stimuli and may, therefore, have distinct clinical advantages when compared with other antidepressant treatments (40).
Acknowledgments
We thank Dr. Keith Akama for helpful comments and suggestions and Leah Reznikov for excellent technical assistance. This work was supported by National Institutes of Health Grant MH42156 (to B.S.M.) and by Servier (France).
Abbreviations: CRS, chronic restraint stress; GC, glucocorticoid; EAA, excitatory amino acid; NSC, nonstressed control; DGi, inferior blade of the dentate gyrus; DGs, superior blade of the dentate gyrus.
References
- 1.Pham, K., Nacher, J., Hof, P. R. & McEwen, B. S. (2003) Eur. J. Neurosci. 17, 879-886. [DOI] [PubMed] [Google Scholar]
- 2.Woolley, C. S., Gould, E. & McEwen, B. S. (1990) Brain Res. 531, 225-231. [DOI] [PubMed] [Google Scholar]
- 3.McEwen, B. S. (1999) Annu. Rev. Neurosci. 22, 105-122. [DOI] [PubMed] [Google Scholar]
- 4.Watanabe, Y., Gould, E. & McEwen, B. S. (1992) Brain Res. 588, 341-345. [DOI] [PubMed] [Google Scholar]
- 5.McEwen, B. S. (1997) Mol. Psychiatry 2, 255-262. [DOI] [PubMed] [Google Scholar]
- 6.Reagan, L. P. & McEwen, B. S. (1997) J. Chem. Neuroanat. 13, 149-167. [DOI] [PubMed] [Google Scholar]
- 7.Sheline, Y. I., Gado, M. H. & Kraemer, H. C. (2003) Am. J. Psychiatry 160, 1516-1518. [DOI] [PubMed] [Google Scholar]
- 8.Magariños, A. M., Deslandes, A. & McEwen, B. S. (1999) Eur. J. Pharmacol. 371, 113-122. [DOI] [PubMed] [Google Scholar]
- 9.Conrad, C. D., Galea, L. A., Kuroda, Y. & McEwen, B. S. (1996) Behav. Neurosci. 110, 1321-1334. [DOI] [PubMed] [Google Scholar]
- 10.Cameron, H. A., McEwen, B. S. & Gould, E. (1995) J. Neurosci. 15, 4687-4692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lowy, M. T., Gault, L. & Yamamoto, B. K. (1993) J. Neurochem. 61, 1957-1960. [DOI] [PubMed] [Google Scholar]
- 12.Lowy, M. T., Wittenberg, L. & Yamamoto, B. K. (1995) J. Neurochem. 65, 268-274. [DOI] [PubMed] [Google Scholar]
- 13.McEwen, B. S. & Sapolsky, R. M. (1995) Curr. Opin. Neurobiol. 5, 205-216. [DOI] [PubMed] [Google Scholar]
- 14.Rothstein, J. D., Martin, L., Levey, A. I., Dykes-Hoberg, M., Jin, L., Wu, D., Nash, N. & Kuncl, R. W. (1994) Neuron 13, 713-725. [DOI] [PubMed] [Google Scholar]
- 15.Czeh, B., Michaelis, T., Watanabe, T., Frahm, J., de Biurrun, G., van Kampen, M., Bartolomucci, A. & Fuchs, E. (2001) Proc. Natl. Acad. Sci. USA 98, 12796-12801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watanabe, Y., Sakai, R. R., McEwen, B. S. & Mendelson, S. (1993) Brain Res. 615, 87-94. [DOI] [PubMed] [Google Scholar]
- 17.Utsunomiya-Tate, N., Endou, H. & Kanai, Y. (2003) FEBS Lett. 416, 312-316. [DOI] [PubMed] [Google Scholar]
- 18.Reagan, L. P., McKittrick, C. R. & McEwen, B. S. (1999) Neuroscience 91, 211-219. [DOI] [PubMed] [Google Scholar]
- 19.Reagan, L. P., Magariños, A. M., Lucas, L. R., Van Bueren, A., McCall, A. L. & McEwen, B. S. (1999) Am. J. Physiol. 276, E879-E886. [DOI] [PubMed] [Google Scholar]
- 20.Reagan, L. P., Magariños, A. M., Yee, D. K., Szweda, L. I., Van Bueren, A., McCall, A. L. & McEwen, B. S. (2000) Brain Res. 862, 292-300. [DOI] [PubMed] [Google Scholar]
- 21.Lucas, L. R., Pompei, P., Ono, J. & McEwen, B. S. (1998) J. Neurochem. 71, 833-843. [DOI] [PubMed] [Google Scholar]
- 22.Danbolt, N. C. (2001) Prog. Neurobiol. 65, 1-105. [DOI] [PubMed] [Google Scholar]
- 23.Chen, W., Aoki, C., Mahadomrongkul, V., Gruber, C. E., Wang, G. J., Blitzblau, R., Irwin, N. & Rosenberg, P. A. (2002) J. Neurosci. 22, 2142-2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lehre, K. P., Levy, L. M., Ottersen, O. P., Storm-Mathisen, J. & Danbolt, N. C. (1995) J. Neurosci. 15, 1835-1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watanabe, Y., Gould, E., Cameron, H. A., Daniels, D. C. & McEwen, B. S. (1992) Hippocampus 2, 431-436. [DOI] [PubMed] [Google Scholar]
- 26.Watanabe, Y., Gould, E., Daniels, D. C., Cameron, H. & McEwen, B. S. (1992) Eur. J. Pharmacol. 222, 157-162. [DOI] [PubMed] [Google Scholar]
- 27.Sousa, N., Lukoyanov, N. V., Madeira, M. D., Almedia, O. F. X. & Paula-Babosa, M. M. (2000) Neuroscience 97, 253-266. [DOI] [PubMed] [Google Scholar]
- 28.Venero, C. & Borrell, J. (1999) Eur. J. Neurosci. 11, 2465-2473. [DOI] [PubMed] [Google Scholar]
- 29.Thorlin, T., Roginski, R. S., Choudhury, K., Nilsson, M., Ronnback, L., Hansson, E. & Eriksson, P. S. (1998) FEBS Lett. 425, 453-459. [DOI] [PubMed] [Google Scholar]
- 30.Lisman, J. E. (1999) Neuron 22, 233-242. [DOI] [PubMed] [Google Scholar]
- 31.Martin, D., McNamara, J. O. & Nadler, J. V. (1992) J. Neurosci. 12, 1928-1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Catterall, W. M. (1999) Adv. Neurol. 79, 441-456. [PubMed] [Google Scholar]
- 33.Sheline, Y. I., Wang, P. W., Gado, M. H., Csernansky, J. G. & Vannier, M. W. (1996) Proc. Natl. Acad. Sci. USA 93, 3908-3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sheline, Y. I., Sanghavi, M., Mintun, M. A. & Gado, M. H. (1999) J. Neurosci. 19, 5034-5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MacQueen, G. M., Campbell, S., McEwen, B. S., Macdonald, K., Amano, S., Joffe, R. T., Nahmais, C. & Young, L. T. (2003) Proc. Natl. Acad. Sci. USA 100, 1387-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shakesby, A. C., Anwly, R. & Rowan, M. J. (2002) J. Neurosci. 22, 3638-3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kole, M. H., Swan, L. & Fuchs, E. (2002) Eur. J. Neurosci. 16, 807-816. [DOI] [PubMed] [Google Scholar]
- 38.Conrad, C. D., Magariños, A. M., LeDoux, J. E. & McEwen, B. S. (1999) Behav. Neurosci. 113, 902-913. [DOI] [PubMed] [Google Scholar]
- 39.Kuroda, Y. & McEwen, B. S. (1998) Mol. Brain Res. 59, 35-39. [DOI] [PubMed] [Google Scholar]
- 40.Kasper, S. & Olie, J. P. (2002) Eur. Psychiatry 17, 331-340. [DOI] [PubMed] [Google Scholar]