Abstract
Whole-cell patch neurophysiology and pharmacological manipulations have provided unprecedented insight into the functions of central neurons, but their combined use has been largely restricted to in vitro preparations. We describe a method for performing whole-cell patch recording and focal application of pharmacological agents in vivo. A key feature of this technique involves iontophoresis of glutamate to establish proximity of drug and recording pipettes. We show data from iontophoresis of glutamate during extracellular and whole-cell recordings made in vivo from auditory neurons in the midbrain of the leopard frog, Rana pipiens, and the effects of blocking GABAA receptors while making a whole-cell recording. This methodology should accelerate our understanding of the roles of particular neurotransmitter systems in normal and pathological conditions, and facilitate investigation of the in vivo effects of drugs and the mechanisms underlying computations.
Keywords: Electrophysiology, iontophoresis, glutamate, auditory midbrain, GABA inhibition, single-neuron computation
1. Introduction
A primary objective of neuroscience is to understand the integrative mechanisms that underlie neuronal computations. An important component of this pursuit is to identify how individual neurons integrate synaptic inputs (e.g., excitation and inhibition) to generate selective responses.
Two technical advances - intracellular recording from brain slices in vitro and ‘whole-cell patch’ recording in vivo - have been particularly pivotal in accelerating our progress towards this objective. Brain slice preparations have enabled investigators to make intracellular/whole-cell patch recordings from neurons while applying pharmacological agents but have a disadvantage in that the normal flow of information in neural circuits is disrupted. The development of techniques for using patch-type pipettes (Hamil et al., 1981) to make whole-cell recordings from neurons in the CNS in vivo (Ferster and Jagadeesh, 1992; Fortune and Rose, 1996) has provided unparalleled views of the subthreshold integrative processes that underlie neural computations (Jagadeesh et al., 1993; Edwards et al., 2007; Leary et al., 2008; Gittelman et al., 2009; Pluta and Kawasaki, 2010; George et al., 2011; Long and Lee, 2011) and the capacity to manipulate the internal environment of the cell (Soejima and Noma, 1984). Thus far, however, discrete pharmacological manipulations e.g., reversibly blocking receptors, in vivo, have largely been restricted to extracellular recordings of single or multiunit activity. In this method, a multibarrel pipette is typically ‘piggybacked’ to an extracellular recording electrode (Havey and Caspary, 1980). After isolating a single unit and characterizing its responses, drugs are delivered iontophoretically. This piggy-back method is impractical for whole-cell recording primarily because patch pipettes have to be changed often. The principal problem to overcome in using independent recording and drug delivery pipettes is how to position the tip of the latter near to the recorded neuron. Optical methods permit simultaneous electrophysiological recording and focal application of pharmacological agents in vivo, but, in practical terms, are restricted to neurons near the brain surface (Jia et al., 2010). We describe a method that combines whole-cell recording and discrete iontophoresis of pharmacological agents to study neurons that are located deep in the brain, in vivo.
2. Materials and methods
2.1. Preparation for neurophysiology
Northern leopard frogs (Rana pipiens pipiens) were prepared for recording following the methods described by Alder and Rose (2000). Frogs were immersed in 3% urethane or 0.1% MS-222 and a local anesthetic (Lidocaine HCL) was applied topically to the dorsal surface of the skull where a small opening was made to expose the optic tectum. Individuals were allowed to recover overnight from surgery and were subsequently immobilized with pancuronium bromide (4 μg/g) for recording. Whole-cell patch intracellular recordings from neurons in the torus semicircularis (also referred to as the ICanuran) were made, in vivo, according to methods described previously (Fortune and Rose, 1996; Edwards et al., 2007) and summarized below. All procedures were approved by the University of Utah Institutional Animal Care and Use Committee.
2.2. Electrode construction
Patch pipettes were constructed from borosilicate capillary glass (A–M systems #5960; 1 mm outer diameter, 0.58 mm inner diameter) using a Flaming-Brown type puller (Sutter Instruments, model P-97). These pipettes had outside tip diameters of approximately 1 μm and resistances between 15 and 25 MΩ. Electrode tips were back-filled with a solution (pH = 7.4) consisting of (values in mM) 100 potassium gluconate, 2 KCl, 1 MgCl2, 5 EGTA, 10 HEPES, 20 KOH, and 20 biocytin. Biocytin was replaced by mannitol in the solution used to fill pipette shanks. Extracellular recording pipettes were manufactured from the same glass, but had tip diameters of 2–3 μm and were filled with 2M NaCl; resistances were approximately 1 MΩ.
2.3. Iontophoretic methods
For iontophoresis of pharmacological agents, 3- to 5-barrel micropipettes were manufactured using a vertical puller (Narishige PE-2) and multibarrel assembles of 1.2 mm OD borosilicate capillary glass (AM systems). The tips of multibarrel pipettes were visualized under a stereo-dissecting microscope and broken to 10–15 μm diameter (total assembly). Individual barrels were filled with L-glutamate (100 mM, pH=8.0), NaCl (150 mM) for current balance or grounding, 20 mM bicuculline methiodide (in 150 mM NaCl, pH=3.0) or gabazine (in 150 mM NaCl, pH=4.0), and 50 mM Phaclofen (in 150 mM Na Cl, pH=3.0). Each barrel of the assembly was connected via Ag/AgCl wires to a constant-current iontophoresis device (model 6400, Dagan Corp.); to minimize recording noise, the power supply was located approximately 2 meters peripheral to the instrument and other equipment. Approximately 50–100 nA constant current was used to iontophoretically deliver pharmacological compounds (negative for glutamate, positive for other agents). To minimize leakage, retention currents of approximately 5 nA (opposite the polarity used to deliver the agents) were applied to barrels that contained drugs.
2.4. Whole-cell recording procedure
Recording pipettes were advanced into the brain using an ‘inch-worm’ microdrive (Burleigh Co.) or 3-axis microdrive (IVM-3000, Scientifica) while applying positive pressure. After reaching the location for whole-cell recording, the pipette was advanced in 1.5 μm increments while maintaining positive pressure and passing −0.1 nA square-wave pulses (500 ms) to monitor resistance; cell contact was indicated by a small increase (10%) in the voltage change. Negative pressure was then applied to the pipette to increase the seal resistance to Giga Ohm levels. Subsequent to seal formation, negative current (~ −0.5 nA) was applied to rupture the patch and attain a whole-cell recording. Seal resistances were typically greater than 2 GΩ. Multibarrel pipettes were advanced into the brain using a hydraulic microdrive (SA Instruments Co). The angle between the multibarrel pipette and recording electrodes was approximately 12°, centered on vertical.
2.5. Stimulus generation and delivery
Acoustic stimuli were generated using Tucker Davis Technologies (TDT) System II & III hardware and custom software. Stimuli were presented free field in an audiometric room that was maintained at 20°C (Alder and Rose, 2000). The speaker was situated 0.5 meters from the animal and contralateral to the recording site. Search stimulus carrier frequencies were systematically varied from 150 Hz to 1600 Hz with modulation frequencies (sinusoidal amplitude modulation, SAM) ranging from 10 Hz to 100 Hz. In cases where this stimulus regimen was ineffective, slower modulation rates and/or lower frequencies were tested.
2.6. Data acquisition and analyses
Recordings were digitized at 10 kHz (Power 1401, Cambridge Electronic Design, Cambridge, UK), stored and analyzed using Spike-2 software, also from the same supplier.
3. Results
3.1. Establishing optimal spacing of recording and multibarrel pipettes
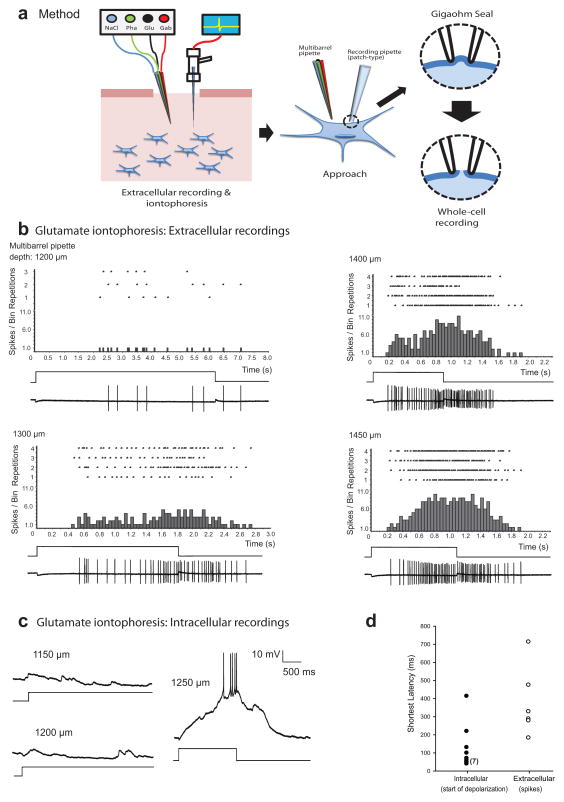
To estimate the optimal separation between entry points of the recording and multibarrel pipettes, we first juxtaposed their tips and then retracted the recording pipette a distance equal to the desired recording depth (automated x-y-z manipulator, Scientifica Corp., model IVM). We then moved the recording pipette laterally until its tip was adjacent to the shank of the multibarrel pipette and recorded the distance traveled; this distance represented the starting separation (≈ 300 μm) between their points of entry at the brain surface. Using extracellular recording methods (Alder and Rose 2000), we then searched for units that were responsive to our acoustic stimuli. After locating the focal auditory region, we retracted the recording electrode, positioned the multibarrel pipette and lowered it to within several hundred micra of the desired recording depth. We then advanced the recording electrode again until encountering auditory units and attempted to elicit responses to iontophoresis of L-glutamate (0.1 M, 90 nA) at various depths. We adjusted the spacing between the recording and multibarrel pipettes until a short-latency response was elicited when the latter was lowered to near the recording depth. In the example shown in figure 1b, glutamate iontophoresis first evoked spiking, with a latency of approximately 2.2 s, when the tip of the multibarrel pipette was approximately 1200 μm below the brain surface. Response latency decreased in a nonlinear fashion as the multibarrel pipette was advanced, reaching a minimum of approximately 190 ms at 1400 μm. Response latency decreased most between depths of 1200 and 1300 μm (1.7 s), with only a 200 ms additional decrease after advancing to 1400 μm. The response latency increased at depths greater than approximately1400 μm (Fig. 1b). At this stage of the procedure, we reiteratively withdrew the multibarrel pipette, adjusted its x–y position and again iontophoresed glutamate at several depths until the shortest-latency response was achieved. After obtaining short-latency responses, we retracted both pipettes to just above the surface of the brain and measured their x–y separation; we used this value for positioning multibarrel and patch pipettes in all subsequent recordings made that day
Figure 1.
Demonstration of the method for positioning a multibarrel pipette (for iontophoretic delivery of pharmacological agents: Glu=glutamate; Gab=gabazine; Pha=Phaclofen) near a neuron from which a whole-cell recording is made in vivo. (a) Stages of the procedure; optimal spacing of pipettes is first established using extracellular recording, followed by whole-cell recording. (b) Raster and histogram plots of extracellularly recorded spikes of a midbrain auditory neuron (shown on bottom trace in each panel), in response to iontophoresis of L-glutamate (100 mM, pH 8.0, −90 nA) at the depths shown. The square-wave traces indicate the duration of iontophoresis. The recording depth was 1419 μm. (c) Whole-cell patch recording from another midbrain neuron while glutamate was transiently iontophoresed at three depths. (d) shortest latencies of depolarization (whole-cell recordings, closed circles) or extracellularly recorded first-spike responses (open circles) to glutamate iontophoresis; latencies are indicative of proximity of the multi-barrel pipette to the recorded cell and were shortest at a particular penetration depth. Data from 17 cells are shown.
3.2. Whole-cell recording and iontophoresis of pharmacological agents, in vivo
After conducting extracellular recordings to establish the optimal spacing of multibarrel and recording pipettes we then used patch-type pipettes, filled with a K+ gluconate-based solution (Rose and Fortune, 1996; Edwards et al., 2007), to make in vivo whole-cell recordings (Fig. 1a). Immediately prior to inserting the patch-type pipette into the brain, we lowered the multibarrel pipette to near the starting recording depth, and then retracted it approximately 300 μm to a ‘holding position’. We then advanced the recording pipette to the starting depth (≈ 1300 μm) and stepped in 1.5 μm increments until a cell was encountered (Rose and Fortune, 1996). At this point, we attempted to make a gigaohm seal and, if successful, started the auditory stimulus regimen. If spikes were observed at this stage, we generally acquired some basic response information (e.g., the neuron’s best excitatory frequency, threshold and responses to amplitude modulation rates from 10–100 Hz) prior to rupturing the patch to make a whole-cell recording. If we did not encounter a cell after advancing the recording pipette approximately 50–100 μm we typically lowered the multibarrel pipette an equal amount; that is, we ‘followed’ the recording pipette with the multibarrel pipette, to maintain the starting depth differential. This procedure minimized the destabilizing effects of penetrating the brain with the multibarrel pipette. After making a whole-cell recording and acquiring baseline data, we slowly (≈ 5 μm/s) advanced the multibarrel pipette, stopping briefly at 50 μm intervals to iontophorese glutamate.
Example whole-cell recordings, made at a depth of 1270 μm, are shown in figure 1c. In this case, glutamate iontophoresis was ineffective for exciting the neuron when the tip of the multibarrel pipette was positioned ≤ 1200μm below the surface of the brain (Fig. 1c). Shortest-latency responses were obtained, however, when the tip of the multibarrel pipette was positioned at a depth of 1250 μm. Interestingly, the 1.2 s latency between iontophoresis onset and the occurrence of the first spike was largely due to the time required for depolarization to reach a level that was suprathreshold for spike initiation; iontophoresis-induced depolarization was apparent within approximately 50 ms of iontophoresis onset, indicating close proximity of the multibarrel pipette to at least some regions of the neuron. Glutamate iontophoresis was effective in eliciting depolarizations and spikes from 12 of 15 whole-cell recordings. Figure 1d shows latencies of these depolarizations (range= 41 – 415 ms, median= 61 ms, closed symbols), along with first-spike latencies (range= 185 -715 ms, median = 310 ms, open symbols) for 6 single-unit, extracellular recordings. Latencies of depolarization were measured as the time at which glutamate-induced depolarization exceeded the background variation in the membrane potential by approximately 25%.
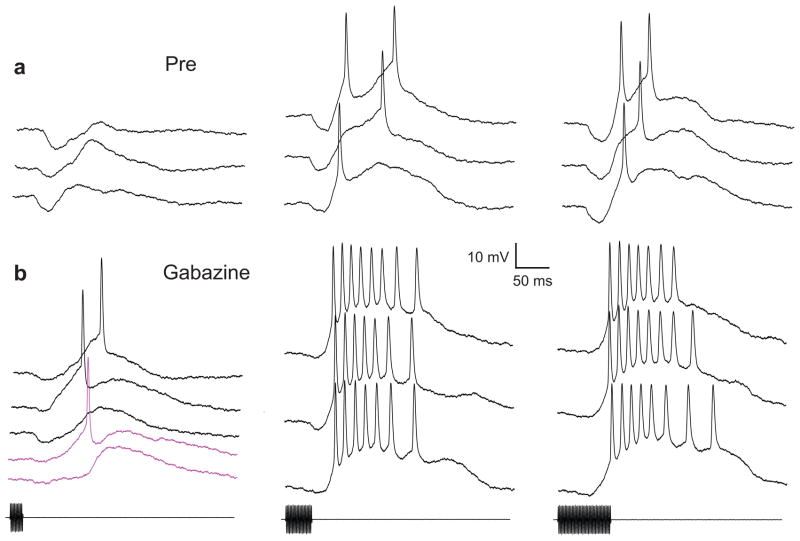
After establishing that glutamate iontophoresis excited the midbrain auditory neuron shown in figure 1c, we recorded responses to tone bursts that had durations of 20, 40 or 80 ms (Fig. 2a). To highlight inhibition, we made these recordings at +.03 nA current clamp. Tone bursts elicited an early hyperpolarization (20 ms: mean=6.36 mV, SE=±1.67, n=5; 40 ms: mean=5.7 mV, SE=±0.68, n=8; and 80ms: mean= 5.73 mV, SE= ±0.76, n=6), followed by depolarization that, for durations of 40 and 80 ms, was suprathreshold for spike initiation (Fig. 2a). Selectivity for tone-burst duration was also evident in recordings made without current injection, and when stimulus energy was equalized across durations (data not shown). We then iontophoresed gabazine, (+70 nA) to block inhibition mediated by GABAA receptors. Responses to tone bursts (duration= 20, 40 or 80 ms), recorded following gabazine injection are shown in Figure 2b. Following gabazine iontophoresis (for 7 minutes), the early hyperpolarization was reduced to approximately 38% (20 ms: mean=2.49 mV, SE=±0.19, n=27; 40 ms: mean=2.3, SE=±0.49, n=6; 80 ms: mean=1.7 mV, SE=±0.31, n=5) of the baseline amplitude, and the effectiveness of these stimuli in eliciting spikes was markedly enhanced. Notably, 20 ms tone bursts elicited spikes on 67% (18/27) of the presentations, but failed to trigger spikes on 5 presentations just prior to iontophoresis (p< .005, binomial test). After 10 minutes of gabazine iontophoresis, virtually no hyperpolarization could be seen in responses to 20 ms tone bursts (magenta traces, Fig. 2b).
Figure 2.
Whole-cell recordings in vivo from a midbrain auditory neuron in the leopard frog (also shown in Fig. 1C) before and after attenuating GABAA –type inhibition. Responses to tone bursts (470 Hz, 74 dB SPL) with durations of 20, 40 or 80 ms (left, middle and right panels), were recorded prior to (a) and after (b) iontophoresis of gabazine (+ 70 nA, 7 min.). Magenta traces: responses to 20 ms tone bursts, after 10 mins of iontophoresis. To highlight inhibition (hyperpolarizations), recordings were made at +0.03 nA current clamp (membrane potential of −63 mV). Resting potential was approximately −82 mV.
4. Discussion
The described methods are an effective approach for discrete application of pharmacological agents while making whole-cell recordings in vivo. We have successfully elicited glutamate responses for approximately 80% (12/15) of whole-cell recordings that were of sufficient duration for attempting pharmacological manipulations. Gabazine effectively blocked inhibition in 9 of these cases, and we observed nearly complete recovery for 6 cells (median recovery time = 13.2 mins, range = 9.2 to 18 mins). These data represent a ‘proof of concept’ that experiments of this nature are feasible and practical if the described procedure is followed.
Because the diffusion of glutamate is limited by active transport into cells (Conti and Weinberg 1999), excitation following iontophoresis serves as a conservative indicator of spatial proximity to the recorded neuron. Iontophoresis of glutamate (and/or other excitatory neurotransmitters) plays two important roles in this experimental procedure. First, it enables the experimenter to establish the optimal spacing between entry points of the recording and multibarrel pipettes. Second, glutamate-mediated excitation verifies that the tip of the drug-delivery pipette is close to the recorded neuron; this assurance is particularly important for interpreting negative pharmacological results. The median latency (61 ms) of glutamate-induced depolarizations that we observed is comparable to latencies observed in slice recordings, where the tip of the iontophoretic pipette was placed close to the neuron (Schwindt and Crill, 1997). This correspondence suggests that our iontophoretic pipette in most cases was quite close to the recorded cell. However, because auditory neurons in the anuran midbrain can have dendrites that extend at least 200 μm from the soma (Feng 1983; personal observations), it is quite possible that the tip of the multibarrel pipette was, in many cases, located near the dendritic field of the recorded neuron, and not particularly close to the soma. To address this possibility, future experiments could involve labeling the recorded neuron and marking the location of the tip of the multibarrel pipette tip. Also, although the results of glutamate stimulation indicate that the iontophoretic pipette tip is close to the recorded neuron, we cannot rule out the influence of pharmacological effects on other cells; as with iontophoretic application of pharmacological agents in extracellular recordings, pharmacological effects seen in whole-cell recordings could result, in part, from action on local interneurons that are presynaptic to the recorded cell. Additional studies that compare the influence of iontophoresis at various distances from the recorded neuron may permit an evaluation of these potential effects. In some instances, it may be unnecessary to focally apply pharmacological agents while making whole-cell recordings, in vivo. For example, it appears that the roles of inhibition onto granule cells in the cerebellum can be studied following application of antagonists to the entire structure (Duguid et al., 2012). Nevertheless, it should prove useful, even in these cases, to use the described methods to test the validity of such assumptions.
Glutamate iontophoresis may also be used to directly investigate properties of excitation to neurons in vivo, as has been done in extracellular recordings (Kiyatkin and Rebec, 1996); this approach may be particularly revealing for cases in which glutamate iontophoresis elicits short-latency depolarizations e.g., Fig. 1c. In addition to providing information concerning the time course and strength of excitation, controlling the duration and timing of iontophoresis may provide some insight into the relative roles of postsynaptic vs. presynaptic processes in shaping the excitatory responses of neurons to natural patterns of excitatory synaptic activity. For example, short-pass duration-selective auditory neurons show little change in the amplitude and time course of EPSPs as tone burst duration is varied (Leary et al., 2008). The duration of glutamate iontophoresis could be varied to determine if postsynaptic processes e.g., receptor desensitization, contribute to this nonlinear response property.
Proof of concept: Whole-cell patch recording with focal application of drugs deep in the brain in vivo is demonstrated.
Iontophoresis of glutamate is a key methodological step for effectively positioning drug and recording pipettes.
Whole-cell recordings from an auditory neuron before and after focally blocking GABAA receptors, in vivo, are shown.
This methodology permits a new level of inquiry into the mechanisms underlying neuronal computations.
Acknowledgments
We thank Srinivasa Chirlya for MatLab programming. This work was supported by a grant from the National Institute of Deafness and other Communication Disorders (NIDCD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alder TB, Rose GJ. Integration and recovery processes contribute to the temporal selectivity of neurons in the midbrain of the northern leopard frog, Rana pipiens. J. Comp. Physiol. 2000;186:923–937. doi: 10.1007/s003590000144. [DOI] [PubMed] [Google Scholar]
- Conti F, Weinberg RJ. Shaping excitation at glutamatergic synapses. Trends in Neurosci. 1999;22:451–458. doi: 10.1016/s0166-2236(99)01445-9. [DOI] [PubMed] [Google Scholar]
- Duguid I, Branco T, London M, Chadderton P, Hausser M. Tonic inhibition enhances fidelity of sensory information transmission in the cerebellar cortex. J Neurosci. 2012;32:11132–11143. doi: 10.1523/JNEUROSCI.0460-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards CJ, Leary CJ, Rose GJ. Counting on inhibition and rate-dependent excitation in the auditory system. J Neurosci. 2007;27:13384–13392. doi: 10.1523/JNEUROSCI.2816-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng AS. Morphology of neurons in the torus semicircularis of the northern leopard frog, Rana pipiens pipiens. J Morphol. 1983;175:253–269. doi: 10.1002/jmor.1051750304. [DOI] [PubMed] [Google Scholar]
- Ferster D, Jagadeesh B. EPSP-IPSP interactions in cat visual cortex studied with in vivo whole-cell patch recording. J Neurosci. 1992;12:1262–1274. doi: 10.1523/JNEUROSCI.12-04-01262.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George AA, Lyons-Warren AM, Carlson BA. A diversity of synaptic filters are created by temporal summation of excitation and inhibition. J Neurosci. 2011;31:14721–14734. doi: 10.1523/JNEUROSCI.1424-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittelman JX, Li N, Pollak GD. Mechanisms underlying directional selectivity for frequency-modulated sweeps in the inferior colliculus revealed by in vivo whole-cell recordings. J Neurosci. 2009;29:13030–13041. doi: 10.1523/JNEUROSCI.2477-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch clamp techniques for high resolution current recordings from cells and cell-free patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Havey DC, Caspary DM. A simple technique for constructing “piggy back” multibarrel microelectrodes. Electroencephalogr Clin Neurophysiol. 1980;48:249–251. doi: 10.1016/0013-4694(80)90313-2. [DOI] [PubMed] [Google Scholar]
- Jagadeesh B, Wheat HS, Ferster D. Linearity of summation of synaptic potentials underlying direction selectivity in simple cells of the cat visual cortex. Science. 1993;262:1901–1904. doi: 10.1126/science.8266083. [DOI] [PubMed] [Google Scholar]
- Jia H, Rochefort NL, Chen X, Konnerth A. Dendritic organization of sensory input to cortical neurons in vivo. Nature. 2010;464:1307–1312. doi: 10.1038/nature08947. [DOI] [PubMed] [Google Scholar]
- Leary CJ, Edwards CJ, Rose GJ. Midbrain auditory neurons integrate excitation and inhibition to generate duration selectivity: an in vivo whole-cell patch study in anurans. J Neurosci. 2008;28:5481–5493. doi: 10.1523/JNEUROSCI.5041-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long MA, Lee AK. Intracellular recording in behaving animals. Curr Opin Neurobiol. 2011;22:1–11. doi: 10.1016/j.conb.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluta SR, Kawasaki M. Temporal selectivity in midbrain electrosensory neurons identified by modal variation in active sensing. J Neurophysiol. 2010;104:498–507. doi: 10.1152/jn.00731.2009. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Rebec GV. Dopaminergic modulation of glutamate-induced excitations of neurons in the neostriatum and nucleus accumbens of awake, unrestrained rats. J Neurophysiol. 1996;75:142–153. doi: 10.1152/jn.1996.75.1.142. [DOI] [PubMed] [Google Scholar]
- Rose GJ, Fortune EF. New techniques for making whole-cell recordings from CNS neurons in vivo. Neurosci. Res. 1996;26:89–94. doi: 10.1016/0168-0102(96)01074-7. [DOI] [PubMed] [Google Scholar]
- Schwindt PC, Crill WE. Local and Propagated dendritic action potential evoked by glutamate iontophoresis on rat neocortical pyramidal neurons. J Neurophysiol. 1997;77:2466–2483. doi: 10.1152/jn.1997.77.5.2466. [DOI] [PubMed] [Google Scholar]
- Soejima M, Noma A. Mode of regulation of the Ach-sensitive K-channel by the muscarinic receptor in rabbit atrial cells. Pflugers Arch. 1984;400:424–431. doi: 10.1007/BF00587544. [DOI] [PubMed] [Google Scholar]