Abstract
The detrimental effects of illness on cognition are familiar to virtually everyone. Some effects resolve quickly while others may linger after the illness resolves. We found that a transient immune response stimulated by lipopolysaccharide (LPS) compromised hippocampal neurogenesis and impaired hippocampus-dependent spatial memory. The immune event caused a 50% reduction in the number of neurons generated during the illness and the onset of the memory impairment was delayed and coincided with the time when neurons generated during the illness would have become functional within the hippocampus. Broad spectrum non-steroidal anti-inflammatory drugs attenuated these effects but selective Cox-2 inhibition was ineffective while PPARγ activation was surprisingly effective at protecting both neurogenesis and memory from the effects of LPS-produced transient illness. These data may highlight novel mechanisms behind chronic inflammatory and neuroinflammatory episodes that are known to compromise hippocampus-dependent forms of learning and memory.
Keywords: Adult Neurogenesis, Hippocampus, Water Maze, Spatial Memory, Lipopolysaccharide, Neuroinflammation, NSAID, indomethacin, rosiglitazone, C57Bl/6 mice, Doublecortin, NeuN
1. Introduction
In the United States transient illnesses, such as those produced by bacteria-contaminated food (i.e. Escherichia coli) and influenza, place a staggering burden on medical care and work productivity annually (Keech and Beardsworth, 2008; Szucs, 1999) Although the symptoms of idiopathic illnesses typically resolve within a week (Capuron et al., 1999; Maier and Watkins, 1998), malaise and functional impairment can persist long after the primary symptoms subside (Hickie and Lloyd, 1995; Smith et al., 1989). Illnesses and the experimental immunogens that model them stimulate immune responses that can impair memory, attention, and executive function in humans (Capuron et al., 1999; Kent et al., 1992a; Kent et al., 1992b; Smith et al., 1989). Similarly, cancer and multiple sclerosis patients treated with recombinant cytokines experience influenza-like sickness and can develop a cognitive syndrome highlighting the impact of immune-to-brain interactions. Indeed, pro-inflammatory cytokines alone can produce subjective memory loss, depression, and impaired motor and executive function (Grigoleit et al., 2011; Wilson et al., 2002).
Because host immune responses to live viruses and bacteria are variable, researchers employ experimental models of transient illness with non-infectious immunogenic compounds that activate the Toll-like pathogen molecular pattern receptors (TLRs), which stimulate highly reproducible innate responses. For example, lipopolysaccharide (LPS) triggers a response through TLR-4 signaling and many of the downstream transcriptional events of TLR4 are similar to those triggered by synthetic viral RNA mimetics that activate TLR3, such as polyinosinic-polycytidylic acid (poly I:C; (Olson and Miller, 2004). Indeed, most TLRs trigger common pathogen pattern recognition mechanisms that stimulate classical pro-inflammatory cytokine responses shared by many illnesses. A single small dose of LPS is accompanied by classical fever, malaise, and increased pro-inflammatory cytokine production (O’Brien and Abraham, 2004; Reichenberg et al., 2001). As with spontaneously ill individuals, humans challenged with purified LPS exhibit problems with verbal and non-verbal memory (Reichenberg et al., 2001).
The learning and memory impairments that accompany transient illness suggest that illness may alter hippocampal function and LPS-induced illness is known to impair adult hippocampal neurogenesis in rodents (Ekdahl et al., 2003; Monje et al., 2003). The amount of neurogenesis in the hippocampus correlates well with hippocampal network integrity and measures of hippocampus-dependent learning and memory (Gould et al., 1999; Jaako-Movits and Zharkovsky, 2005; Kempermann and Gage, 2002a; Ormerod et al., 2004; Rola et al., 2004; Snyder et al., 2005; Speisman et al., 2012; Winocur et al., 2006). Experimental manipulations that chronically impair hippocampal neurogenesis are associated with impaired cognition, particularly hippocampus-dependent memory (Aizawa et al., 2009; Garthe et al., 2009; Saxe et al., 2006; Shors et al., 2001; Siwak-Tapp et al., 2007; Speisman et al., 2012).
While the specific role that neurogenesis plays in hippocampal function remains enigmatic, ongoing neurogenesis does provide a highly sensitive biomarker of hippocampal network integrity. Here we model transient illness with LPS and measure its effects on Morris water maze spatial learning and memory in mice and provide evidence that defects in neurogenesis caused by transient illness may contribute to illness-induced changes in hippocampus-dependent behavior.
2. Materials and Methods
2.1 Subjects
Adult female CB57Bl/6 mice (7 wks-old upon arrival; Taconic Farms; n=226) used as subjects in this study were treated in accordance with the relevant Stanford University and Federal Guidelines for the ethical care and use of laboratory animals for experimentation (NIH Publications No. 80-23). The mice were group-housed upon arrival (n=4–5 per cage) in specific pathogen-free colony space and given free access to sterile Prolab Mouse 3000 chow (PMI Nutrition International, St. Louis, MO) and water. After a week of habituation to the colony room, the mice were fed vehicle or NSAIDs and/or injected once intraperitoneally (i.p.) with sterile isotonic saline vehicle (50μl/10g) or lipopolysaccharide (LPS; 5mg/kg at a concentration of 1mg/ml; Sigma Aldrich, St. Louis, Missouri) and hydrated with daily subcutaneous saline injections for 2d. Groups of mice were either perfused to measure neurogenesis or began water maze training on Days 7 or 28 after LPS or saline injection. NPC proliferation was measured in mice that were perfused on Day 0. See Table 1 for a full explanation of group numbers.
Table 1.
Total number of mice used in each condition.
| Treatment | Neurogenesis Measured | Water Maze Training Initiated | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| 5h | 1 week | 1 month | 1 week | Excluded | 1 month | Excluded | |
| Does LPS impact neurogenesis and water maze performance?
| |||||||
| Saline | 3 | 7 | 5 | 12† | (4)‡ | 12 | (3)‡ |
| 5.0mg/kg LPS | 3 | 5 | 5 | 12† | (4)‡ | 12 | (2)‡ |
|
| |||||||
| Can NSAIDs protect neurogenesis and behavior from LPS?
| |||||||
| Saline | |||||||
| Milk Vehicle | - | - | 4 | - | - | 13 | (4) ‡ |
| Rosiglitazone | - | - | 4 | - | - | 9 | - |
| Indomethacin | - | - | 4 | - | - | 6 | - |
| Celecoxib | - | - | 4 | - | - | 12 | (5) ‡ |
|
| |||||||
| 5.0mg/kg LPS | |||||||
| Milk Vehicle | - | - | 4 | - | - | 12 | (2) ‡ |
| Rosiglitazone | - | - | 4 | - | - | 6 | (1) ‡ |
| Indomethacin | - | - | 4 | - | - | 16 | - |
| Celecoxib | - | - | 4 | - | - | 13 | (5) ‡ |
|
| |||||||
| Can OTC NSAIDs protect neurogenesis from LPS?
| |||||||
| Saline | |||||||
| Milk Vehicle | - | - | 4 | - | - | - | - |
| 5.0mg/kg LPS | |||||||
| Milk Vehicle | - | - | 4 | - | - | - | - |
| SC-560 | - | - | 5 | - | - | - | - |
| Ibuprofen | - | - | 5 | - | - | - | - |
| Acetaminophen | - | - | 5 | - | - | - | - |
|
| |||||||
| Total Mice | 6 | 12 | 73 | 24 | (8) ‡ | 111 | (22) ‡ |
Mice were tested in locomotor activity chambers before water maze training.
Mice were excluded from statistical analyses because they either failed training criterion or exhibited immobility or thigmotaxia for > 80% of the duration of the probe trial.
To measure neurogenesis, cage control mice were injected i.p. with the cell synthesis marker bromodeoxyuridine (BrdU; Sigma Aldrich, St. Louis, MO) dissolved in freshly prepared isotonic saline at a concentration of 10mg/ml just before use. To measure NPC proliferation, mice were injected with BrdU 2x on Day 0 (1 and 3h after LPS) and perfused 2h after the final BrdU injection (n=3 per group). To confirm the phenotypes and survivability of cells produced during illness, mice were injected with BrdU once per day over 6 days (Days 0–5) and perfused either on Day 7 (n=5–7 per group) or Day 28 (n=4–5 per group). These mice were anaesthetized with a ketamine (100mg/kg)/xylazine (10mg/kg) cocktail and then perfused transcardially with ice-cold isotonic saline and then 4% paraformaldehyde. Their brains were extracted, post-fixed overnight in perfusate, equilibrated in 30% sucrose (~3 days) and then sectioned at 40μm intervals through the rostral-caudal extent of the hippocampus using a freezing stage microtome (American Optical Corporation Model 860). Sections were stored in tissue cryoprotectant solution (25% glycerin, 30% ethylene glycol and 45% 0.1M NaPO4; v/v/v; pH 6.7) at −20°C until processed immunohistochemically.
2.2 NSAID Preparation and Treatment
The mice were introduced to 1ml strawberry- or chocolate-flavored milk (Berkeley Farms, CA) aliquots placed in their home cages 2x daily on Days −4 and −3 before saline- or LPS-challenge. For subsequent dosing (Days −2 to 14), the mice were fed individually in chambers (10-cm d × 18-cm h), 2x per day (12 h apart) and removed immediately once they consumed their 100μl dose of flavored milk treat containing NSAID (1–5 minutes). The NSAIDs were first dissolved in ethanol and then 50μl of the NSAID/ethanol solution was added to 1ml of flavored milk to produce the doses listed below. The trace ethanol levels used (0.005 g/kg) were far lower than the > 1g/kg doses that impact neurogenesis (Crews et al., 2006; Nixon and Crews, 2002).
Recommended human NSAID dosages (Days −2 to 14) were employed BID as follows: vehicle (0.05g/ml ethanol in flavored milk), indomethacin (1 mg/kg,; Sigma Aldrich, St. Louis MO), the COX-2 inhibitor 4-[5-(4-methylphenyl)-3-(trifluoromethyl)pyrazol-1-yl] benzene-sulfonamide (celecoxib, 15 mg/kg; Stanford Hospital Pharmacy, Stanford, CA) the peroxisome proliferator-activated receptor- (PPAR)-γ agonist rosiglitazone maleate (10 mg/kg; Stanford Hospital Pharmacy, Stanford, CA), the COX-1 inhibitor SC-560 (3mg/kg; Cayman Chemical, Ann Arbor, MI), ibuprofen (10mg/kg; Sigma Aldrich, St. Louis, MO) and N-acetyl-p-aminophenol (acetaminophen; 27.5 mg/kg; Sigma Aldrich, St. Louis, MO).
2.3. Behavioral testing
2.3.1
Locomotor activity was measured in the 24 mice that began water maze training on Day 7 to test whether signs of lethargy persisted after the symptoms of LPS resolve. The mice were placed into 17 × 17 inch chambers lined with three 16-beam infrared arrays in photocell boxes (MED Associates, Inc., St. Albans, Vermont) and their locomotor activity was recorded for 20 minutes, beginning ~6h after the final water maze visible platform training session (Day 6) and the day before hidden platform trials began on Day 7. A 50msec scanning rate was used for measuring beams broken and the number of ambulatory episodes and distances traveled were analyzed using Open Field Activity software (MED Associates, Inc., St. Albans, Vermont).
2.3.2
Hippocampus-independent and dependent learning and memory was assessed using the Morris water maze task (Morris et al., 1982; Riedel et al., 1999), with hidden platform training commencing either on Day 7 or Day 28 after LPS or saline injection. All training and testing was conducted in a black circular tank (170cm d × 43cm h) filled with water (25±1°C) made opaque using white non-toxic Tempera paint (Rich Art Color Co. Inc, Northvale, CA). A platform (13cm d × 28.5cm h) protruded 1cm from the water surface on cued trials and was submerged 1cm below the water surface for hidden platform and reversal training. Visible extra-maze cues were absent for cued platform trials and situated identically for hidden platform, probe, and reversal trials. Swim times, pathlengths and speeds were recorded by a Videotrack Automated Behavioral Analysis System (Viewpoint Life Sciences Inc., Otterburn Park, Quebec).
Platform shaping was conducted over 3 daily sessions prior to any water maze training. In the absence of extra-maze cues, mice were guided gently to a visible platform located in the center of a small water filled tank (1m d) until they remained on the platform for 15s over 3 consecutive trials (between 5–8 trials per session).
Visible Platform Training was administered in a session a) before hidden platform trials administered 1 week after LPS to confirm that LPS intoxication did not affect learning and b) after the probe trial when training commenced 4 weeks after LPS to confirm equivalent sensorimotor abilities between groups (recall that NSAID treatment ceases 2 weeks before training). The mice were given 12 trials (intertrial interval [ITI] ≅ 30min) in a single session to locate the flagged visible water maze platform within 60s before being gently guided by the experimenter. Average pathlengths (m) across bins of 3 trials were analyzed as measures of procedural and sensorimotor ability and swim speeds (cm/s) were analyzed as a measure of locomotor ability.
Hidden Platform Training began either on Day 7 (n=24) or Day 28 (n=111) after LPS. The mice were released from the middle of the E, S or W quadrant, facing the pool wall, and given 60s to find the platform hidden beneath the water in the center of the N quadrant before being guided gently to the platform. Daily sessions of 6 training trials (ITI ~30 min) were conducted until all of the mice located the platform in > 15s on average within a session. Mice not reaching criterion by Session 8 when trained 1 week after LPS (n=3 vehicle- and 2 LPS-treated mice) or by Session 10 when trained 1 month after LPS (n=4 vehicle-, 2 LPS-, 1 rosiglitazone- and 10 celebrex-treated mice) were excluded from analyses. Pathlengths (m) were analyzed as measures of spatial learning and swim speeds (cm/s) were analyzed as measures of sensorimotor ability.
A Probe Trial in which the platform was removed from the pool was conducted 1 week after mice completed hidden platform training. The mice were released from the center of the pool and their behavior recorded for 60s. The percentages of time spent in each quadrant were analyzed as memory measures. Mice that floated or exhibited thigmotaxic behavior for > 80% of the probe trial duration were excluded from water maze analyses (n=2 LPS- and 1 saline-injected mice trained 1 week after LPS).
Reversal Training in which mice were given 4–60s trials in 3 daily sessions to locate the platform now hidden in the quadrant opposite to the original training quadrant was initiated after the probe trial to interpret whether probe trial performance was indicative of memory or perseveration. Good probe trial performance was interpreted as good memory if the mice readily learned the novel platform location on reversal trials.
2.4. Immunohistochemistry
Cage control mice were perfused 5h (n=6), 1 week (n=12) or 4 weeks (n=73) after LPS or vehicle injection to quantify hippocampal neurogenesis. Free floating sections were immunostained as described previously (Bruijnzeel et al., 2011; Palmer et al., 2000; Speisman et al., 2012) using 1) rat anti-bromodeoxyuridine (BrdU; 1:500; AbD Serotec, Raleigh, NC) to quantify total BrdU+ cell number or 2) rat anti-BrdU (1:500; AbD Serotec, Raleigh, NC) and goat anti-doublecortin (Dcx; 1:500; Santa Cruz, California) mouse anti-Neuronal Nucleii (NeuN; 1:500; Chemicon; Temecula, California) to quantify total new neuron number or 3) rat anti-BrdU (1:500; AbD Serotec, Raleigh, NC) and guinea pig anti-glial fibrillary acidic protein (GFAP; 1:750; Harlan, Indianapolis, Indiana) and rabbit anti-NG2 (1:1000; Millipore) to quantify new glia. BrdU+ cells on tissue prepared for stereology using light microscopy were revealed using a minimally cross-reactive biotinylated secondary antibody (1:500; Jackson Immunoresearch, West Grove, Pennsylvania) and avidin-HRP/diaminobenzidine (Vector Labs, Kit PK6100). Minimally cross-reactive secondary IgG conjugated fluorophores (1:500; Jackson Immunoresearch) were used to reveal primary antibodies so that BrdU+ cells could be phenotyped using confocal microscopy.
2.5 Cell Counting and Unbiased Stereology
The total number of BrdU+ cells was estimated stereologically on every 12th systematically uniform section (6 sections per mouse spaced 480μm apart) through the rostral-caudal extent of the hippocampal dentate gyrus using Microbrightfield Stereo Investigator software (Williston, Vermont) and optical fractionator principles (Kempermann and Gage, 2002b; Speisman et al., 2012; West et al., 1991). The region of interest included the hippocampal granule cell layer proper and the neurogenic subgranular zone (the ~50μm border between the hilus and granule cell layer). The phenotypes of at least 50 BrdU+ cells in this region were documented using confocal microscopy (Zeiss 510 meta confocal laser scanning microscope) on immunofluorescent-stained sections. BrdU+ cells were scored as co-labeled when a full “z-dimension” scan revealed its nucleus was unambiguously associated with a lineage specific marker.
2.6 Data analysis
All behavioral and morphological analyses were conducted by experimenters blind to the treatment groups. Statistical analyses were performed using Statistica software (Tulsa, Oklahoma). Effects of the independent variable transient illness (LPS versus vehicle) on dependent variables measured once (ambulatory episodes, distances and durations in locomotor chambers) were tested with Student s t-tests. The effects of transient illness (LPS versus saline) and/or NSAID treatment (indomethacin, rosiglitazone, celecoxib or vehicle) on dependent variables (% change in body masses across days, visible and hidden platform trial pathlengths, % time spent in the training quadrant on water maze probe trials, BrdU+ cell number and phenotype, new cell phenotype and net new neuron number) were tested using ANOVAs. One way ANOVAs were used to determine whether transient illness (LPS versus vehicle) and NSAID treatment (indomethacin, rosiglitazone, celecoxib or vehicle) affected quadrant dwell times on the probe trial. Statistically significant effects revealed by ANOVAs were explored using Newman-Keul s post hoc tests and all statistical tests set α to 0.05.
3. RESULTS
3.1. LPS Induces Sickness Behavior in Adult Mice
Illness evokes a characteristic behavioral syndrome that includes anorexia, depressed activity and loss of interest in personal grooming (Capuron et al., 1999; Kent et al., 1992a; Kent et al., 1992b). Within 1–2h of LPS injection, mice exhibit analogous behaviors that include hunching, diaphoresis with piloerection, cessation of grooming, lethargy and reduced mobility within their home cages. These symptoms resolved visibly within 72h and the mice appeared normal within 5 days. Transient weight loss during this period can be used to monitor illness and rate of recovery.
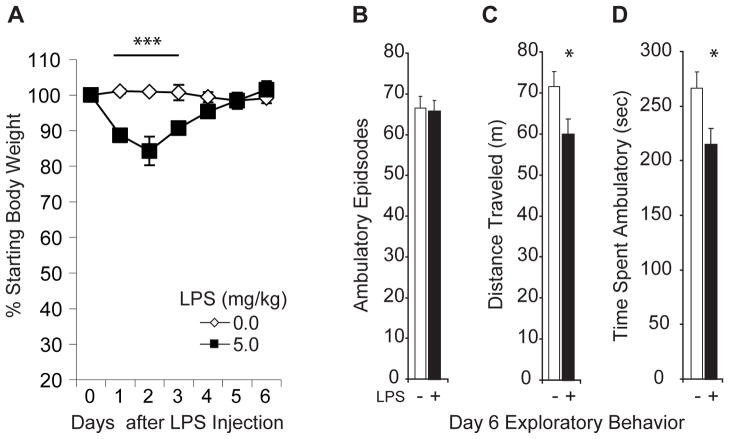
Cage control mice were weighed daily after the LPS injection and the % change in body mass from baseline was analyzed as a measure of recovery from illness (Fig. 1A). An ANOVA revealed significant effects of transient illness (F(1,10) = 17.78; p < 0.002), days of recovery to baseline body mass (F(6,60) = 9.37; p < 0.0001) and a significant interaction effect (F(6,60) = 15.56; p < 0.0001). Relative to saline-injection, LPS produced significant weight loss at 24h (~11%; p = 0.0002), 48h (~15%; p = 0.0001) and 72h (~10%; p = 0.001), but not at later time points.
Figure 1. Weight loss and lingering lethargy accompany LPS-produced transient illness.
Cage control mice (n=5–7 per group) were injected i.p. with LPS (5mg/kg; black squares) or vehicle (open diamonds) and weighed daily and exploratory behavior was examined 6d after LPS (5mg/kg; black bars; n=12) or saline (white bars; n=12) injection in a parallel cohort of mice. (A) LPS-injected mice lose ~ 15% of their body weight within 48h. Relative to saline-injection, LPS produced significant weight loss at 24h, 48h and 72h (***p values < 0.001), but not at later time points. B–D) LPS-treated mice are motivated to explore a novel environment but tire quickly. LPS-injected mice initiate the same number of exploratory bouts in an activity monitoring chamber (p = 0.780; B), but travel shorter distances (*p = 0.03; C) and spend less time exploring the novel environment (*p = 0.015; D) than saline-treated mice.
Locomotor behavior was measured on day 7 after LPS injection (Fig. 1B–D). LPS- and vehicle-treated mice exhibited the same number of ambulatory events (t(22) = 0.283, p = 0.780) but LPS-treated animals traveled shorter distances (t(22) = 1.98, p = 0.03; one tailed) and spent significantly less time traveling (t(22) = 2.26; p = 0.015; one tailed) than vehicle-treated mice suggesting that they may suffer from a lingering malaise and lethargy similar to that reported by humans following transient illness.
3.2 Hippocampus-dependent learning and memory are unaffected during the recovery phase of LPS-Induced transient illness
Mice were tested on visible platform trials on Day 6 after LPS or saline injection and then began hidden platform trial training on Day 7 (Fig. 2A–C). Latencies and distances traveled to the hidden platform were used as measures of spatial learning, a hippocampus-dependent process (Morris et al., 1982; Taube et al., 1992).
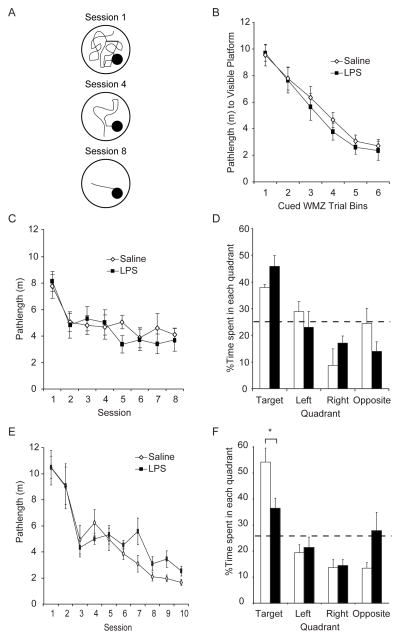
Figure 2. Memory impairments emerge latently, weeks after the symptoms of transient illness subside.
LPS- (5mg/kg; black symbols and bars) and saline- (white symbols and bars) injected mice were trained on hidden platform trials in the Morris water maze beginning on Day 7 (n = 8 mice per group included in analysis) or Day 28 (n = 9 saline- and 10 LPS-injected mice included in analysis) after injection. (A) Schematic showing the shorter pathlengths (m) and latencies (s) to the hidden platform across sessions that are indicative of spatial learning. (B–D) Transient illness does not spatial learning or memory in mice trained on Day 7 after LPS. LPS- and saline-injected mice exhibited similar pathlengths (interaction effect: p = 0.75) across visible platform trials administered on Day 6 (B) and across hidden platform training sessions initiated on Day 7 (interaction effect: p = 0.732; C). All mice swam shorter pathlengths on later versus earlier hidden platform training sessions (Session 1 > 2–8; p values < 0.001). Both groups preferred the target versus the left (p = 0.07), right (p = 0.002) or opposite (p = 0.0003) quadrant in the memory probe trial administered one week after training (D). (E and -F) Mice exhibit intact ability to learn a hidden platform location one month after transient illness resolves but impaired memory. All mice swam shorter pathlengths on later versus earlier trials (Sessions 1, 2 > 3 ≥ 4, 5 > 8–10, p values < 0.02). The interaction effect (p = 0.404) confirmed that the group differences on Sessions 7, 9 and 10 were not statistically significant (E). Although all mice that learned the hidden platform location preferred the training versus left, right or opposite quadrants (p values < 0.0002) on a the probe trial administered one week after training, LPS-injected mice spent significantly less time in the training quadrant (*p = 0.042) than controls.
On visible platform trials, swim speeds were unaffected by transient illness (saline = 26.63±3.20 cm/s and LPS = 31.63±3.23 cm/s; F(1,14) = 2.21; p = 0.16;) and consistent across bins of consecutive trials (F(5,70) = 1.59; p = 0.17; interaction effect; F(14,70) = 0.20; p = 0.96). Figure 2B shows that while mean (±S.E.M.) pathlengths to the visible platform significantly decreased across trial bins (F(5,70) = 45.092, p < 0.001) in all mice combined (Bin 1 > 2 > 3, 4 > 5, 6, all p values< 0.004; and Bin 4 > 6, p=0.005), they were unaffected by illness (F(1,14) = 0.560, p = 0.467; interaction effect, F(5,70) = 0.535, p = 0.749).
Figure 2C shows that on hidden platform trials, all mice learned similarly by swimming more directly to the platform on later versus earlier training sessions (session 1> 2–8, all Newman-Keuls p values < 0.001; effect of session: F(7,98) = 8.67; p < 0.001) regardless of transient illness (F(1,14) = 0.901; p = 0.359; interaction effect: F(7,98) = 0.627; p = 0.732). Similar to visible platform trials, swim speeds were consistent across hidden platform trials (F(7,98) = 1.32; p = 0.247) regardless of treatment (F(1,14) = 0.288; p = 0.600; interaction effect F(7,98) = 1.477; p = 0.184; data not shown). These data indicate that there is no statistically significant effect of illness on spatial ability when learning is initiated 7 days after LPS.
The % time spent in each quadrant was recorded on the memory probe trial conducted 1 week after training criterion was reached (Fig. 2D). Although LPS-treated mice spent slightly more time in the target quadrant and less time in the opposite quadrant than saline-treated mice did, the effect did not reach statistical significance (F(1,11) = 0.029, p = 0.867; interaction effect: F(3,33) = 1.783, p = 0.170). All mice spent the majority of their time (~43%) searching the quadrant that housed the escape platform during training trials versus the left (p = 0.004), right (p = 0.0002) and opposite quadrant (p = 0.0004; F(3,33) = 11.921; p < 0.001).
Finally, the mice were trained on reversal trials to measure their tendencies to perseverate to the old platform location rather than learn a novel location. All mice learned the novel hidden platform location (F(2,24) = 5.25, p = 0.023) by swimming more direct pathlengths on later versus earlier sessions (Session 1 = 557.45±53.5cm, Session 2 = 431.21±63.3cm and Session 3 = 339.73±43.0cm; Session 1 > 3, p = 0.016). Previous illness did not affect the ability of mice to locate the novel platform position on reversal trials (F(1,12) = 0.007, p = 0.935 and treatment x session interaction effect: F(2,24) = 0.830; p = 0.448).
Taken together, these data show that although mice exhibit signs of lingering malaise when exploring a novel environment, they exhibited intact visual, sensorimotor, and motivational abilities in the water maze one week after an LPS challenge. LPS-treated mice learned a hidden platform location as well as saline-treated mice, indicating that their ability to learn and navigate in relation to spatial cues while recovering from LPS-induced illness is intact. They also remembered and recalled the well-learned hidden platform location when tested one week later and then learned a novel hidden platform location as readily as saline-treated mice.
3.3 Spatial memory impairment emerges in mice trained 4 weeks after illness
A separate cohort of mice was trained on hidden platform trials beginning one month after saline or LPS injection until they located the hidden platform in < 15s averaged over trials in a single session. They were tested in a probe trial a week achieving this criterion. Sensorimotor ability was confirmed in cued platform trials after the probe trial in these mice because LPS intoxication did not affect sensorimotor ability after the much shorter one week recovery from LPS.
Figure 2E shows that LPS-treated mice learned the hidden platform location as efficiently as the control mice (F(1,17) = 0.271, p = 0.610) with both groups swimming more direct routes to the hidden platform during later versus earlier sessions (Sessions 1, 2 > 3 ≥ 4, 5 > 8–10, all Newman-Keuls p values < 0.02; F(9,153) = 22.893, p< 0.001). Although LPS-treated mice swam more circuitous routes to the platform than vehicle-treated mice on sessions 7, 9 and 10, the treatment x session interaction was not statistically significant (F(9,153) = 1.051; p = 0.402). Consistent with published reports (Foster, 2012; Ormerod and Beninger, 2002) showing that training on one task can influence subsequent task performance, these mice achieved the learning criterion more slowly than mice trained first on visible platform trials (Fig. 2E versus 2C).
Figure 2F shows that during the probe trial, all mice spent the majority of their time searching the quadrant that had contained the escape platform during hidden platform trials versus the left (p = 0.0001), right (p = 0.0001) and opposite (p = 0.0002) quadrant (F(3,51) = 12.332; p < 0.001). However, we detected a strong tendency for treatment to interact with the % of time spent in each quadrant (F(1,17) = 0.895; p = 0.357; interaction effect: F(3,51) = 2.685, p = 0.056). A post hoc test on the interaction effect revealed that LPS-treated mice spent significantly less time than vehicle-treated mice in the training quadrant (p = 0.042), but not in the left (p = 0.875), right (p = 0.975) or opposite quadrants (p = 0.445) on the probe trial, suggesting that LPS produces a latent memory impairment. There was no measurable effect of LPS treatment on the ability of these mice to either learn a novel hidden platform location across reversal trial sessions or locate a flagged and visible platform on cued trials (data not shown).
3.4 Transient illness is accompanied by reduced hippocampal neurogenesis
The hippocampal plasticity that mediates learning and memory likely includes both fast-adapting synaptic changes and the more gradual adaptive mechanisms associated with the production of new granule neurons in the dentate gyrus and their integration into neural circuits (Aimone et al., 2011; Deng et al., 2010). To test whether peripheral LPS compromises hippocampal neurogenesis in adult mice similarly to adult rats (Monje et al., 2003; Ekdahl et al., 2003; Russo et al., 2011), we first quantified neurogenesis in the hippocampi of adult mice perfused on Day 7 after administration of LPS (one day after the last of 6 daily BrdU injections). BrdU+ cells co-labeled with the neuroblast marker doublecortin (Dcx) and/or the more mature neuronal marker NeuN were quantified (Fig 3A–C). Expression of both Dcx and NeuN indicates neurons of intermediate differentiation. Cells were also scored for the astrocyte marker GFAP and the oligodendrocyte progenitor marker NG2.
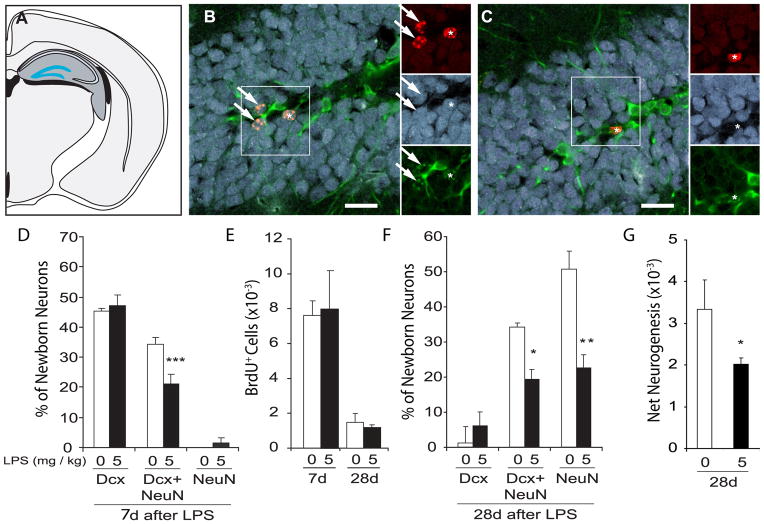
Figure 3. Decreased hippocampal neurogenesis accompanies transient illness.
Mice were injected with LPS (5mg/kg; black bars) or saline (white bars) and then BrdU daily for 6 days. Hippocampal neurogenesis was quantified on the 7th (n=5–7 per group) or the 28th (n=4 per group) day after injection. (A) A coronal rat brain schematic highlighting NPCs resident to the subgranular zone between the hilus and granule cell layer (GCL; in blue) of the hippocampal formation (dark grey) generate new neurons. (B and C) Confocal micrographs of the GCL of mice perfused 28 days after saline (B) or LPS (C) injection show new BrdU+ cells (in red), mature NeuN+ neurons (in blue) and immature Dcx+ neurons (in green). Arrows (B) highlight new (BrdU+) transition-state neurons retaining traces of Dcx expression with NeuN expression. Asterisk (C) showing that few BrdU+ cells express Dcx and/or NeuN even 28d after LPS injection. Scale bar = 30μm. (D) One week after LPS, most BrdU+ cells express Dcx and almost ½ of the Dcx+ neurons co-express NeuN but most are too immature to express NeuN alone. LPS reduces the % of new neurons expressing Dcx/NeuN+ transition-state phenotypes (***p values < 0.0001) observed at 1 week leading to the reduced percentages of transition state (*p = 0.02) and NeuN+ mature (**p < 0.001) phenotypes detected at 1 month (F). Similar BrdU+ cell numbers are detected in the dentate gyri of saline- and LPS-injected mice at 7 (p = 0.82) and 28 (p = 0.60) days after injection (E). (G) LPS-compromised neuronal differentiation decreases net neurogenesis measured at 1 month (*p = 0.02).
Figure 3D shows that, as expected, the % of new cells expressing immature, transitioning and mature neuronal phenotypes significantly differed at one week (F(2,20) = 179.84, p < 0.0001). At this time point, the majority of BrdU+ cells (~50%) expressed Dcx alone and a slightly smaller fraction had differentiated sufficiently to co-express Dcx and NeuN while very few newborn cells had become mature enough to express NeuN alone (Dcx+ > Dcx/NeuN+ > Dcx+; all p values < 0.001). Illness interacted with neuronal phenotype (effect of illness: F(1,10) = 4.20, p = 0.067 and interaction effect: F(2,20) = 179.84, p = 0.01) to significantly reduce the % of new cells co-expressing Dcx and NeuN (p=0.0005), suggesting that LPS compromises neuronal differentiation during the 7 days after its injection. Similar percentages of BrdU+ cells expressed the astrocyte marker GFAP (4.02±1.03% and 4.76±1.25%, respectively; t(10) = −0.46, p 0.66) or the oligodendrocyte precursor marker NG2 (0.71±0.46% and 1.78±0.82%; t(10) = −1.22, p = 0.25). Consistent with our work in rats (Monje et al., 2003), the abundance of BrdU+ cells in the hippocampi of these saline- and LPS-treated mice was similar (t(10) =−0.27, p = 0.79; Fig. 3E), suggesting that NPC proliferation was unaffected by LPS-produced illness. We confirmed this hypothesis by detecting similar BrdU+ cell numbers in the dentate gyri of separate groups of mice perfused 5h after LPS (1648.00±154.51 cells) or saline (1440.00±60.40 cells; t(4) = 1.25, p = 0.278) injection.
A separate cohort of mice was perfused on Day 28 (3 weeks after the final BrdU injection). Figure 3F shows that very few new cells in control- or LPS-treated mice perfused on Day 28 expressed Dcx alone and instead co-expressed Dcx and NeuN (~35%; p = 0.006 vs Dcx) or expressed NeuN alone (~50%; p < 0.0003 vs Dcx; F(2,12) = 25.01, p = 0.00005). LPS significantly decreased the % of new neurons expressing either a transitioning or fully mature neuronal phenotype (Newman-Keuls p values < 0.02; F(1,6) = 28.36, p = 0.002 and treatment x phenotype interaction effect: F(2,12) = 3.81; p = 0.05). Although similar new cell numbers were detected in the dentate gyri of these LPS- and saline-treated mice (t(7) = 0.56; p = 0.60; Fig. 3E), net neurogenesis (total BrdU+ cell number x % BrdU+ cells expressing Dcx and/or NeuN) was compromised in LPS- versus saline-treated mice by ~30% (t(7) = −2.998; p = 0.02; Fig. 3G) because of the effects of LPS on neuronal differentiation (Fig. 3F). New cell survival appears unaffected because similar new cell numbers were detected in the dentate gyri of LPS- versus saline-treated mice perfused on Days 7 and 28 and consistent well-characterized decay rates among cells surviving 1 versus 4-weeks (Cameron and McKay, 2001; Cameron et al., 1993; Kempermann and Gage, 2002b) were observed between groups (Fig. 3E).
Taken together, these data show that peripheral LPS reduces the proportion of new cells in mice acquiring neuronal phenotypes, detectable as transitioning neurons when measured one week after LPS and as mature neurons when measured 28 days after LPS, without significantly impacting the production of new cells or their survival, which is consistent with the effects that we reported earlier in rats (Monje et al., 2003).
3.5 Selective NSAIDs attenuate LPS-induced memory and neurogenesis deficits
Proinflammatory cytokines have been implicated in defects in neurogenesis and broad spectrum NSAIDs can partially protect neurogenesis from inflammatory signaling (Monje et al, 2003). To investigate whether broad spectrum or more selective NSAIDs can protect memory and/or neurogenesis from LPS-induced illness, mice were fed oral doses of the broad spectrum NSAID indomethacin, the COX-2 inhibitor celecoxib, or the PPARγ activator rosiglitazone and then challenged once with LPS or saline. Drug treatment continued for 14 days after LPS. BrdU was injected daily over 6 days after the LPS injection and the mice were perfused or began water maze training on Day 28.
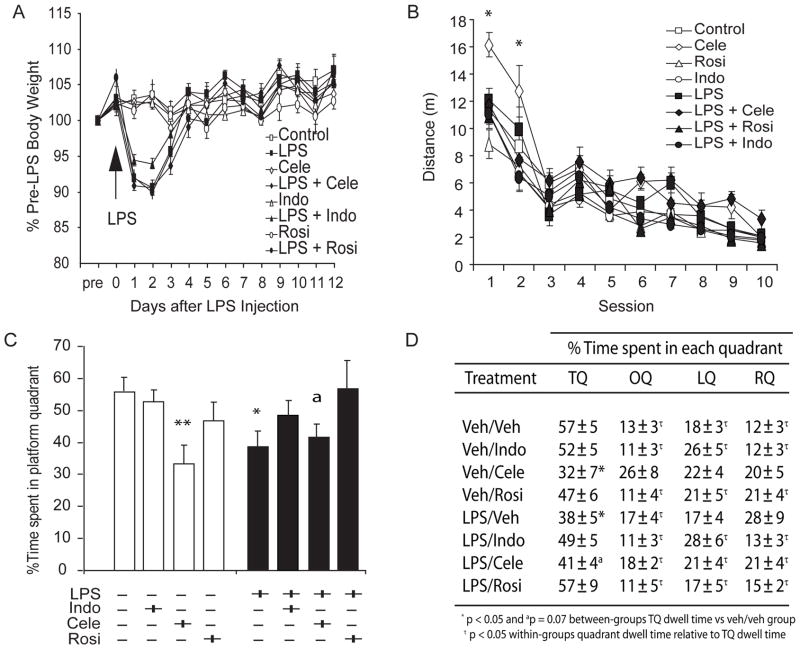
Figure 4A shows that all LPS-injected mice exhibited overt sickness behavior and prototypical weight loss (vs vehicle over Days 1–3; p values < 0.0001) following LPS injection (treatment x days to recovery interaction effect: F(91,312) = 8.347, p < 0.0001). These effects were not attenuated by indomethacin (vs LPS on Days 1–2, p values > 0.115 and on Day 3 p = 0.052), celecoxib (vs LPS on Days 1–3, p values > 0.376), or rosiglitazone (vs LPS on Days 1–3, p values > 0.651) treatment. NSAID drug treatment alone did not significantly affect body mass (all p values > 0.161 relative to saline injection on Days 0–12 after treatment).
Figure 4. Rosiglitazone and Indomethacin protect hippocampus-dependent memory from the latent effects of LPS-induced illness.
Mice were fed BID strawberry milk vehicle (n = 9 saline- and 10 LPS-injected mice), indomethacin (Indo; 1 mg/kg; n = 6 saline- and n = 16 LPS-injected mice), celecoxib (Cele; 15 mg/kg; n = 7 saline- and n = 8 LPS-injected mice) or rosiglitazone (Rosi; 10 mg/kg; n = 9 saline- and n = 5 LPS-injected mice) for 2 weeks after LPS (5mg/kg; black symbols and bars) or saline (white symbols and bars) was injected. Water maze training commenced 28 days after injection. Note that n s include mice retained in the analyses. (A) LPS-treated mice exhibited characteristic transient weight loss (vs. vehicle over Days 1–3; p values < 0.0001) despite indomethacin (vs LPS on Days 1–2, p values > 0.115 and on Day 3 p = 0.052), celecoxib (vs LPS on Days 1–3, p values > 0.376), or rosiglitazone (vs LPS on Days 1–3, p values > 0.651) treatment. NSAID treatment alone did not affect body mass. (B) All mice swam more directly to the hidden platform on later versus earlier sessions (Sessions 1> 2 > 3–10; Session 3 > 4, 8–10; Sessions 4–6 > 8–10; and Session 8 > 10, all p values < 0.02) except celecoxib-treated mice that exhibited impaired pathlengths on all sessions combined (vs vehicle-, indomethacin- or rosiglitazone-treated mice; p values < 0.01) but particularly over the first 2 sessions (vs vehicle-treated mice, *p values < 0.02) regardless of LPS injection. (C) Relative to saline-injected mice, LPS-injected mice spent significantly less time in the training quadrant on a probe trial (*p = 0.016) unless they were treated with indomethacin (p = 0.372) or rosiglitazone (p = 0.993). Celecoxib-treated mice exhibited impaired probe trial performances relative to saline-injected mice (ap = 0.073 and **p = 0.009, respectively) regardless of LPS injection. (D) Planned comparisons confirmed that mice preferred the training quadrant versus all other quadrants (p values < 0.003) except LPS-injected or celecoxib-treated mice (p values > 0.08 vs all other quadrants).
Water maze hidden platform training trials were initiated on Day 28 after LPS or saline injection (2 weeks after the final NSAID treatment; Fig. 4B) and a probe trial was administered one week after training criterion was achieved (Fig. 4C–D). We did not find a statistically significant effect of LPS-injection on pathlengths across hidden platform training sessions (F(1,54) = 0.015, p = 0.904; Fig. 4B). All mice swam more directly to the hidden platform on later versus earlier sessions (F(9,486) = 91.467, p < 0.0001; sessions 1 > 2 > 3–10 p values < 0.00003, session 3 > 4, 8–10, p values < 0.001, sessions 4–6 > 8–10; p values < 0.02 and session 8 > 10, p = 0.009). However, we did detect a statistically significant effect of NSAID treatment on pathlengths (F(3,54) = 9.130, p < 0.0001). Specifically, celecoxib-treated mice took more circuitous routes on all sessions combined (LPS injection x NSAID treatment x session interaction effect: F(27,486) = 1.654, p = 0.022) versus vehicle- (p = 0.007), indomethacin- (p = 0.0002) or rosiglitazone- (p = 0.0002) treated mice, particularly over the first 2 sessions (versus vehicle-treated mice, p values < 0.02). Consistent with our previous results, LPS did not influence the ability of mice to learn a hidden platform location one month after injection but surprisingly celecoxib treatment impaired the ability of mice to learn the hidden platform location, at least in early trials.
Mice that achieved the hidden platform training criterion were tested in a probe trial one week later (Fig. 4C and 4D). The % dwell time in the target quadrant neither varied by LPS injection alone (F(1,62) = 0.019, p = 0.899) nor by NSAID treatment alone (F(3,62) = 1.654, p = 0.022). However, LPS injection significantly interacted with NSAID treatment to affect % dwell times in the target quadrant (F(3,62) = 2.839, p = 0.045; Fig. 4C) such that LPS-injected mice spent significantly less time in the target quadrant than saline-injected mice (p = 0.016). This measure was protected from LPS injection-induced illness by indomethacin (p = 0.372) or rosiglitazone (p = 0.993), neither of which affected the performances of saline-injected mice (p values ≥ 0.21). Surprisingly, celecoxib-treated mice with and without prior illness exhibited impaired probe trial performances relative to saline-injected mice (p = 0.073 and p = 0.009, respectively). Planned comparisons confirmed that mice spent significantly more time in the training quadrant versus all other quadrants (p values ≤ 0.003), with the exception that LPS-injected and celecoxib-treated mice spent as much time in other quadrants (p values ≥ 0.08; see Fig. 4D). Our data suggest that transient illness impairs memory in mice weeks after the symptoms resolve, unless the mice are treated with indomethacin or rosiglitazone. Surprisingly, celecoxib-treated and LPS-injected mice exhibited similar accelerated memory extinction on the probe trial.
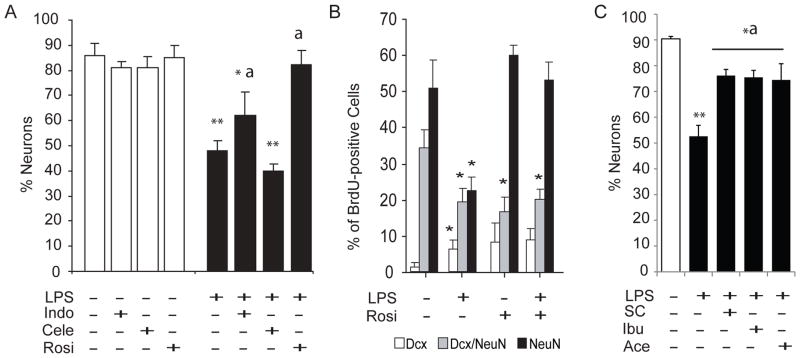
Figure 5A shows the % of cells born on Days 0–6 after LPS or saline injection that adopted a neuronal phenotype in a parallel cohort of NSAID-treated cage control mice sacrificed on Day 28 varied by condition (F(7,24) = 14.99, p < 0.00001). NSAID treatment alone did not impact neurogenesis in saline-injected mice. LPS decreased the % of BrdU+ cells adopting a neuronal (Dcx and/or NeuN+) fate (p = 0.0002 versus vehicle). Indomethacin did not entirely prevent (p = 0.01 versus vehicle) but did attenuate the LPS-produced deficit. (p = 0.02 versus LPS). The selective COX-2 inhibitor celecoxib had no protective effect on neurogenesis (p = 0.51 versus LPS and p = 0.0001 versus vehicle). Rosiglitazone fully prevented the LPS-produced deficit in neurogenesis (p = 0.0003 versus LPS and p = 0.43 versus vehicle).
Figure 5. PPARγ activation protects neurogenesis from LPS-induced transient illness.
(A and B) Cage control mice were fed strawberry milk vehicle, Indo (1 mg/kg), Cele (15 mg/kg) or (Rosi; 10 mg/kg) BID (n = 8 per group) beginning 2 days before ½ were injected with LPS (5mg/kg) and the other ½ with saline in parallel with the behavioral mice. BrdU was injected on Days 0–6 and neurogenesis was evaluated on Day 28. (A) LPS (black bars) reduced the proportion of BrdU+ cells adopting a neuronal (Dcx and/or NeuN+) fate by 40–50% (versus vehicle, white bars, **p = 0.0002). Indo attenuated (versus LPS, ap = 0.02) but did not prevent (versus vehicle, *p = 0.01) the effect. Cele had no protective effect (versus vehicle **p = 0.0001), while Rosi completely prevented the effect (versus LPS ap = 0.0003 and versus vehicle p = 0.43). NSAID treatment alone did not affect neuronal differentiation. (B) Most ~1 month-old BrdU+ cells express NeuN alone (black bars). A smaller but significant fraction of BrdU/NeuN+ neurons retain Dcx expression (grey bars) and few BrdU+ cells express Dcx alone (white bars). LPS decreases the ratio of transition-state and mature neurons (*p = 0.001), causing a small but significant increase in the fraction of undifferentiated BrdU/Dcx+ cells. Rosiglitazone treatment prevented the defect in neurogenesis produced by transient illness. (C) Neurogenesis was measured in a final cohort of cage control mice using the procedure described in (A and B) except that these mice were saline-injected and fed strawberry milk vehicle BID (n = 4) or LPS-injected (5mg//kg) and fed strawberry milk vehicle (n = 4) or SC560 (SC; 3 mg/kg), ibuprofen (Ibu; 10 mg/kg) or acetominophen (Ace; 27.5 mg/kg) BID (12h apart; n = 5 per group). LPS reduced the proportion of BrdU+\ cells adopting a neuronal fate as expected by ~40–50% (versus vehicle, **p = 0.0002). SC-560, aceptominophen and ibuprofen attenuated (versus LPS, ap values < 0.02) but did not prevent (vs vehicle, *p values < 0.01) the effect.
A closer inspection of the neuronal phenotypes among new cells in rosiglitazone-treated mice (Fig. 5B) revealed the expected phenotypes at Day 28. Very few new neurons retained immature neuronal phenotypes at this point, and instead expressed mature and transitioning neuronal phenotypes (F(2,24) = 70.07, p < 0.0003). Relative to saline, LPS-induced illness significantly reduced the ratio of transition-state (p < 0.001) and mature neurons (p < 0.001) and produced a small but significant increase in the fraction of Dcx+ cells (p < 0.05). Rosiglitazone treatment appears to prevent the effect of LPS primarily by promoting the maturation of young neurons (F(3,12) = 14.33, p < 0.0003; interaction effect: F(6,24) = 4.96, p < 0.002).
In a final cohort of cage control mice, we examined whether the selective COX-1 inhibitor SC-560, or the popular over-the-counter broad spectrum NSAIDs ibuprofen and aceotminophen could protect neurogenesis from the effects of LPS (Fig. 5C). The % of new Dcx and/or NeuN+ neurons in the hippocampi of these mice was significantly affected by condition (F(4,18) = 10.307, p < 0.001). As expected, LPS injection decreased the % of new neurons relative to saline (p = 0.0002). Interestingly, SC-560, ibuprofen and acetaminophen attenuated (p values < 0.002 versus LPS) but did not prevent (p values < 0.05 versus saline) the effects of LPS on neurogenesis. Taken together, our data confirm that broad spectrum NSAID strategies and COX-1-selective inhibition can attenuate but not prevent the effects of transient illness on neurogenesis in mice, at least at standard over-the-counter doses.
4. DISCUSSION
The LPS response replicates an acute transient illness in rodents and in humans resembling the “flu” or equally common “food poisoning”. The symptoms are familiar to everyone and include fever, lethargy, reduced food and water intake, weight loss and poor grooming behavior in rodents and arguably in humans (Reichenberg et al., 2001; Rietschel et al., 1996). These symptoms typically begin to resolve within 48–72 hours. However, we show here that this type of transient illness in mice is accompanied by a specific latent (4–6 week) spatial memory impairment – strikingly coincident with the amount of time that newborn neurons take to mature and contribute to hippocampal function (Ge et al., 2007; van Praag et al., 2002).
We and others have reported decreased hippocampal neurogenesis across inflammatory contexts in both mice and rats (Bastos et al., 2008; Ekdahl et al., 2003; Monje et al., 2003; Russo et al., 2011) and we suspect that decreased new neuron numbers coupled with their reduced functional maturity may contribute to the memory differences that we detected here in previously ill mice. This speculation is consistent with other reports showing that new granule neurons participate in forms of learning and memory that rely upon the hippocampus and may do so latently, after maturing and connecting functionally with hippocampal circuitry (Gould et al., 1999; Jaako-Movits and Zharkovsky, 2005; Kempermann and Gage, 2002a; Ormerod et al., 2004; Rola et al., 2004; Snyder et al., 2005; Speisman et al., 2012; Winocur et al., 2006). Although new granule neurons rapidly extend axons and dendrites into target areas (Hastings and Gould, 1999) they only exhibit electrophysiological and morphological maturity weeks after their final division (van Praag et al., 2002). Our finding is consistent with the notion that reduced new neuron numbers integrating into the networks that mediate the acquisition and storage of spatiotemporal or contextual information would impair memory latently.
We show here that NSAIDS can be protective for memory as well as neurogenesis in an inflammatory context. The broad spectrum NSAID indomethacin inhibits COX-1 and COX-2 activity that catalyzes pro-inflammatory prostaglandin, prostacyclin, and thromboxane production. Indomethacin also activates central PPAR-γ proteins that transcriptionally inhibit COX-2 and numerous pro-inflammatory cytokine genes (Dannhardt and Kiefer, 2001; Jiang et al., 1998; Monje et al., 2003). However, indomethacin produces gastrointestinal complications and and the 5mg/kg dose of indomethacin that we previously found effective in rats (Monje et al., 2003) was lethal to mice in the current study. The lower 1mg/kg dose of indomethacin that we found well-tolerated by mice in the present study only attenuated rather than prevented the effects of LPS-produced-transient illness on neurogenesis, which may require the higher dose.
Some of the adverse effects of indomethacin are thought to be avoided by the use of more selective COX-2 inhibitors (Warner and Mitchell, 2004) and Bastos and colleagues (2008) have proposed that local COX-2 elevations mediate LPS-induced deficits in neurogenesis. However, our study suggests that selective COX-2 inhibition is ineffective whereas weaker COX-2 inhibitors, such as ibuprofen (Dannhardt and Kiefer, 2001) and acetaminophen (Hinz et al., 2008) are moderately effective at blocking the effects of LPS on neurogenesis. Ibuprofen and acetaminophen also block COX-1 activity and activate PPAR-γ and their effects along with our partial rescue of net neurogenesis with the COX-1 inhibitor SC-560 and full rescue with the PPAR-γ activator rosiglitazone suggests that NSAIDs protect neurogenesis from peripheral LPS primarily through PPAR-γ activation and to a lesser extent through COX-1 inhibition. These data are also consistent with those of Russo and colleagues (Russo et al., 2011) who found that central LPS administration decreases NPC proliferation, neuronal differentiation and survival of new hippocampal cells in wild-type, but not COX-1−/− mice.
The deleterious effects of celecoxib with and without prior illness on both water maze learning and memory trials is perplexing as training commenced 2 weeks after the final dose was administered. A growing number of studies show that memory is impaired across behavioral tasks by selective COX-2 inhibitors and that basal COX-2 activity may be critical for forms of plasticity that include the induction of long-term potentiation (Cowley et al., 2008; Sharifzadeh et al., 2005a). Indeed, COX-2 is expressed in the dendrites and cell bodies of hippocampal neurons and appears to mediate activity-dependent plasticity within the hippocampus (Hoozemans et al., 2004). Therefore, the deleterious effects of celecoxib in hippocampus-dependent tasks, particularly memory, may not be so surprising (Sharifzadeh et al., 2005a; Sharifzadeh et al., 2005b). The emerging picture that basal COX-2 levels are critical for normal cognition may mean that restoring detrimental COX-2 levels elevated by injury or disease to baseline is a difficult challenge, particularly for selective COX-inhibition strategies (Cunningham and Skelly, 2012). Our data showing that celecoxib impairs memory even two weeks after the final treatment suggests that the effects of selective COX-2 inhibition may be extremely persistent. Whether new neurons are more vulnerable to these effects is currently unclear but speculating that the latency with which the effects are observed occurs because new neurons are affected is tempting.
The most unexpected and potentially interesting data generated by our study showed that PPARγ-selective NSAIDs completely prevented the effects of LPS-produced illness on both neurogenesis and cognition. Thiazolidinediones, such as rosiglitazone are best known for their efficacy in preventing insulin resistance among Type-2 diabetics, at well-tolerated chronic doses, by increasing sensitivity in adipose and muscle cells (Yki-Järvinen, 2004). They are emerging as effective albeit clinically under-studied anti-inflammatory drugs (Cuzzocrea et al., 2004; Stumvoll and Häring, 2002). More than 100 target genes have been identified as being selectively affected by PPARγ in response to rosiglitazone. Among these are several pro-inflammatory signaling molecules, several of which are also implicated in the inflammatory inhibition of adult hippocampal neurogenesis (Monje et al., 2003; Vallières et al., 2002). As well, recent work has implicated several circulating inflammatory molecules that deleteriously impact both cognition and plasticity in the aging brain (Villeda et al., 2011). Our ongoing studies into the effects of PPARγ activation highlight the relevance of its impact on individual cytokines that include tumor necrosis factor-α, interleukin-6 and monocyte chemoattractant protein-1.
The effectiveness of the PPARγ activators in protecting both neurogenesis and cognitive function provides a new direction in the ongoing search for drugs that can protect hippocampus-dependent cognition in the context of a neuroinflammatory challenge with minimal side effects. These leads may be critical for interventions seeking to enhance the potential of endogenous stem cell activity and/or stem cell transplant therapy in the chronic neuroinflammatory environment that accompanies traumatic injury, autoimmune disease or other neurodegenerative diseases (Jiang et al., 1998; Ormerod et al., 2008).
Acknowledgments
This research was funded by grants from the NIH (R21 NS050549, R01 MH071472), California Institute of Regenerative Medicine (RC1-00134-1), Kinetics Foundation and M.J. Fox Foundation to TDP, a Natural Sciences and Engineering Research Council of Canada PDF to BKO, an HHMI Fellowship and Stanford Medical Scholars Grant to SJH and an NSF predoctoral fellowship to SWL.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aimone JB, Deng W, Gage FH. Resolving new memories: a critical look at the dentate gyrus, adult neurogenesis, and pattern separation. Neuron. 2011;70:589–596. doi: 10.1016/j.neuron.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizawa K, Ageyama N, Yokoyama C, Hisatsune T. Age-dependent alteration in hippocampal neurogenesis correlates with learning performance of macaque monkeys. Exp Anim. 2009;58:403–407. doi: 10.1538/expanim.58.403. [DOI] [PubMed] [Google Scholar]
- Bastos GN, Moriya T, Inui F, Katura T, Nakahata N. Involvement of cyclooxygenase-2 in lipopolysaccharide-induced impairment of the newborn cell survival in the adult mouse dentate gyrus. Neuroscience. 2008;155:454–462. doi: 10.1016/j.neuroscience.2008.06.020. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW, Bauzo RM, Munikoti V, Rodrick GB, Yamada H, Fornal CA, Ormerod BK, Jacobs BL. Tobacco smoke diminishes neurogenesis and promotes gliogenesis in the dentate gyrus of adolescent rats. Brain Res. 2011;1413:32–42. doi: 10.1016/j.brainres.2011.07.041. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Woolley CS, McEwen BS, Gould E. Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Neuroscience. 1993;56:337–344. doi: 10.1016/0306-4522(93)90335-d. [DOI] [PubMed] [Google Scholar]
- Capuron L, Lamarque D, Dantzer R, Goodall G. Attentional and mnemonic deficits associated with infectious disease in humans. Psychol Med. 1999;29:291–297. doi: 10.1017/s0033291798007740. [DOI] [PubMed] [Google Scholar]
- Cowley TR, Fahey B, O’Mara SM. COX-2, but not COX-1, activity is necessary for the induction of perforant path long-term potentiation and spatial learning in vivo. Eur J Neurosci. 2008;27:2999–3008. doi: 10.1111/j.1460-9568.2008.06251.x. [DOI] [PubMed] [Google Scholar]
- Crews FT, Mdzinarishvili A, Kim D, He J, Nixon K. Neurogenesis in adolescent brain is potently inhibited by ethanol. Neuroscience. 2006;137:437–445. doi: 10.1016/j.neuroscience.2005.08.090. [DOI] [PubMed] [Google Scholar]
- Cunningham C, Skelly DT. Non-steroidal anti-inflammatory drugs and cognitive function: are prostaglandins at the heart of cognitive impairment in dementia and delirium? J Neuroimmune Pharmacol. 2012;7:60–73. doi: 10.1007/s11481-011-9312-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzzocrea S, Pisano B, Dugo L, Ianaro A, Maffia P, Patel NS, Di Paola R, Ialenti A, Genovese T, Chatterjee PK, Di Rosa M, Caputi AP, Thiemermann C. Rosiglitazone, a ligand of the peroxisome proliferator-activated receptor-gamma, reduces acute inflammation. Eur J Pharmacol. 2004;483:79–93. doi: 10.1016/j.ejphar.2003.10.056. [DOI] [PubMed] [Google Scholar]
- Dannhardt G, Kiefer W. Cyclooxygenase inhibitors--current status and future prospects. Eur J Med Chem. 2001;36:109–126. doi: 10.1016/s0223-5234(01)01197-7. [DOI] [PubMed] [Google Scholar]
- Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci USA. 2003;100:13632–13637. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster TC. Dissecting the age-related decline on spatial learning and memory tasks in rodent models: N-methyl-D-aspartate receptors and voltage-dependent Ca(2+) channels in senescent synaptic plasticity. Prog Neurobiol. 2012;96:283–303. doi: 10.1016/j.pneurobio.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthe A, Behr J, Kempermann G. Adult-generated hippocampal neurons allow the flexible use of spatially precise learning strategies. PLoS One. 2009;4:e5464. doi: 10.1371/journal.pone.0005464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Yang CH, Hsu KS, Ming GL, Song H. A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron. 2007;54:559–566. doi: 10.1016/j.neuron.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci. 1999;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- Grigoleit JS, Kullmann JS, Wolf OT, Hammes F, Wegner A, Jablonowski S, Engler H, Gizewski E, Oberbeck R, Schedlowski M. Dose-dependent effects of endotoxin on neurobehavioral functions in humans. PLoS One. 2011;6:e28330. doi: 10.1371/journal.pone.0028330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings NB, Gould E. Rapid extension of axons into the CA3 region by adult-generated granule cells. J Comp Neurol. 1999;413:146–154. doi: 10.1002/(sici)1096-9861(19991011)413:1<146::aid-cne10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Hickie I, Lloyd A. Are cytokines associated with neuropsychiatric syndromes in humans? Int J Immunopharmacol. 1995;17:677–683. doi: 10.1016/0192-0561(95)00054-6. [DOI] [PubMed] [Google Scholar]
- Hinz B, Cheremina O, Brune K. Acetaminophen (paracetamol) is a selective cyclooxygenase-2 inhibitor in man. FASEB J. 2008;22:383–390. doi: 10.1096/fj.07-8506com. [DOI] [PubMed] [Google Scholar]
- Hoozemans JJ, Veerhuis R, Rozemuller AJ, Arendt T, Eikelenboom P. Neuronal COX-2 expression and phosphorylation of pRb precede p38 MAPK activation and neurofibrillary changes in AD temporal cortex. Neurobiol Dis. 2004;15:492–499. doi: 10.1016/j.nbd.2003.11.028. [DOI] [PubMed] [Google Scholar]
- Jaako-Movits K, Zharkovsky A. Impaired fear memory and decreased hippocampal neurogenesis following olfactory bulbectomy in rats. Eur J Neurosci. 2005;22:2871–2878. doi: 10.1111/j.1460-9568.2005.04481.x. [DOI] [PubMed] [Google Scholar]
- Jiang C, Ting AT, Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391:82–86. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- Keech M, Beardsworth P. The impact of influenza on working days lost: a review of the literature. Pharmacoeconomics. 2008;26:911–924. doi: 10.2165/00019053-200826110-00004. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Gage FH. Genetic determinants of adult hippocampal neurogenesis correlate with acquisition, but not probe trial performance, in the water maze task. Eur J Neurosci. 2002a;16:129–136. doi: 10.1046/j.1460-9568.2002.02042.x. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Gage FH. Genetic influence on phenotypic differentiation in adult hippocampal neurogenesis. Brain Res Dev Brain Res. 2002b;134:1–12. doi: 10.1016/s0165-3806(01)00224-3. [DOI] [PubMed] [Google Scholar]
- Kent S, Bluthe RM, Dantzer R, Hardwick AJ, Kelley KW, Rothwell NJ, Vannice JL. Different receptor mechanisms mediate the pyrogenic and behavioral effects of interleukin 1. Proc Natl Acad Sci U S A. 1992a;89:9117–9120. doi: 10.1073/pnas.89.19.9117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent S, Bluthé RM, Kelley KW, Dantzer R. Sickness behavior as a new target for drug development. Trends Pharmacol Sci. 1992b;13:24–28. doi: 10.1016/0165-6147(92)90012-u. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Cytokines for psychologists: implications of bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychol Rev. 1998;105:83–107. doi: 10.1037/0033-295x.105.1.83. [DOI] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Nixon K, Crews FT. Binge ethanol exposure decreases neurogenesis in adult rat hippocampus. J Neurochem. 2002;83:1087–1093. doi: 10.1046/j.1471-4159.2002.01214.x. [DOI] [PubMed] [Google Scholar]
- O’Brien JM, Abraham E. Human models of endotoxemia and recombinant human activated protein C. Crit Care Med. 2004;32:S202–208. doi: 10.1097/01.ccm.0000126123.34119.98. [DOI] [PubMed] [Google Scholar]
- Olson JK, Miller SD. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol. 2004;173:3916–3924. doi: 10.4049/jimmunol.173.6.3916. [DOI] [PubMed] [Google Scholar]
- Ormerod BK, Beninger RJ. Water maze versus radial maze: differential performance of rats in a spatial delayed match-to-position task and response to scopolamine. Behav Brain Res. 2002;128:139–152. doi: 10.1016/s0166-4328(01)00316-3. [DOI] [PubMed] [Google Scholar]
- Ormerod BK, Lee TT, Galea LA. Estradiol enhances neurogenesis in the dentate gyri of adult male meadow voles by increasing the survival of young granule neurons. Neuroscience. 2004;128:645–654. doi: 10.1016/j.neuroscience.2004.06.039. [DOI] [PubMed] [Google Scholar]
- Ormerod BK, Palmer TD, Caldwell MA. Neurodegeneration and cell replacement. Philos Trans R Soc Lond B Biol Sci. 2008;363:153–170. doi: 10.1098/rstb.2006.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A, Pollmächer T. Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry. 2001;58:445–452. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- Riedel G, Micheau J, Lam AG, Roloff EL, Martin SJ, Bridge H, de Hoz L, Poeschel B, McCulloch J, Morris RG. Reversible neural inactivation reveals hippocampal participation in several memory processes. Nat Neurosci. 1999;2:898–905. doi: 10.1038/13202. [DOI] [PubMed] [Google Scholar]
- Rietschel ET, Brade H, Holst O, Brade L, Müller-Loennies S, Mamat U, Zähringer U, Beckmann F, Seydel U, Brandenburg K, Ulmer AJ, Mattern T, Heine H, Schletter J, Loppnow H, Schönbeck U, Flad HD, Hauschildt S, Schade UF, Di Padova F, Kusumoto S, Schumann RR. Bacterial endotoxin: Chemical constitution, biological recognition, host response, and immunological detoxification. Curr Top Microbiol Immunol. 1996;216:39–81. doi: 10.1007/978-3-642-80186-0_3. [DOI] [PubMed] [Google Scholar]
- Rola R, Raber J, Rizk A, Otsuka S, VandenBerg SR, Morhardt DR, Fike JR. Radiation-induced impairment of hippocampal neurogenesis is associated with cognitive deficits in young mice. Exp Neurol. 2004;188:316–330. doi: 10.1016/j.expneurol.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Russo I, Amornphimoltham P, Weigert R, Barlati S, Bosetti F. Cyclooxygenase-1 is involved in the inhibition of hippocampal neurogenesis after lipopolysaccharide-induced neuroinflammation. Cell Cycle. 2011;10:2568–2573. doi: 10.4161/cc.10.15.15946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, Garcia AD, Sofroniew MV, Kandel ER, Santarelli L, Hen R, Drew MR. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci U S A. 2006;103:17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifzadeh M, Naghdi N, Khosrovani S, Ostad SN, Sharifzadeh K, Roghani A. Post-training intrahippocampal infusion of the COX-2 inhibitor celecoxib impaired spatial memory retention in rats. Eur J Pharmacol. 2005a;511:159–166. doi: 10.1016/j.ejphar.2005.01.041. [DOI] [PubMed] [Google Scholar]
- Sharifzadeh M, Tavasoli M, Naghdi N, Ghanbari A, Amini M, Roghani A. Post-training intrahippocampal infusion of nicotine prevents spatial memory retention deficits induced by the cyclo-oxygenase-2-specific inhibitor celecoxib in rats. J Neurochem. 2005b;95:1078–1090. doi: 10.1111/j.1471-4159.2005.03454.x. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- Siwak-Tapp CT, Head E, Muggenburg BA, Milgram NW, Cotman CW. Neurogenesis decreases with age in the canine hippocampus and correlates with cognitive function. Neurobiol Learn Mem. 2007;88:249–259. doi: 10.1016/j.nlm.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AP, Tyrrell DA, al-Nakib W, Barrow PG, Higgins PG, Leekam S, Trickett S. Effects and after-effects of the common cold and influenza on human performance. Neuropsychobiology. 1989;21:90–93. doi: 10.1159/000118558. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Hong NS, McDonald RJ, Wojtowicz JM. A role for adult neurogenesis in spatial long-term memory. Neuroscience. 2005;130:843–852. doi: 10.1016/j.neuroscience.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Speisman RB, Kumar A, Rani A, Pastoriza JM, Severance JE, Foster TC, Ormerod BK. Environmental enrichment restores neurogenesis and rapid acquisition in aged rats. Neurobiol Aging. 2012 doi: 10.1016/j.neurobiolaging.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumvoll M, Häring HU. Glitazones: clinical effects and molecular mechanisms. Ann Med. 2002;34:217–224. [PubMed] [Google Scholar]
- Szucs T. The socio-economic burden of influenza. J Antimicrob Chemother. 1999;44(Suppl B):11–15. doi: 10.1093/jac/44.suppl_2.11. [DOI] [PubMed] [Google Scholar]
- Taube JS, Kesslak JP, Cotman CW. Lesions of the rat postsubiculum impair performance on spatial tasks. Behav Neural Biol. 1992;57:131–143. doi: 10.1016/0163-1047(92)90629-i. [DOI] [PubMed] [Google Scholar]
- Vallières L, Campbell IL, Gage FH, Sawchenko PE. Reduced hippocampal neurogenesis in adult transgenic mice with chronic astrocytic production of interleukin-6. J Neurosci. 2002;22:486–492. doi: 10.1523/JNEUROSCI.22-02-00486.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeda SA, Luo J, Mosher KI, Zou B, Britschgi M, Bieri G, Stan TM, Fainberg N, Ding Z, Eggel A, Lucin KM, Czirr E, Park JS, Couillard-Després S, Aigner L, Li G, Peskind ER, Kaye JA, Quinn JF, Galasko DR, Xie XS, Rando TA, Wyss-Coray T. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477:90–94. doi: 10.1038/nature10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner TD, Mitchell JA. Cyclooxygenases: new forms, new inhibitors, and lessons from the clinic. FASEB J. 2004;18:790–804. doi: 10.1096/fj.03-0645rev. [DOI] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Wilson CJ, Finch CE, Cohen HJ. Cytokines and cognition--the case for a head-to-toe inflammatory paradigm. J Am Geriatr Soc. 2002;50:2041–2056. doi: 10.1046/j.1532-5415.2002.50619.x. [DOI] [PubMed] [Google Scholar]
- Winocur G, Wojtowicz JM, Sekeres M, Snyder JS, Wang S. Inhibition of neurogenesis interferes with hippocampus-dependent memory function. Hippocampus. 2006;16:296–304. doi: 10.1002/hipo.20163. [DOI] [PubMed] [Google Scholar]
- Yki-Järvinen H. Thiazolidinediones. N Engl J Med. 2004;351:1106–1118. doi: 10.1056/NEJMra041001. [DOI] [PubMed] [Google Scholar]