Abstract
Auditory cortex (AI) shows age-related decreases in pre-synaptic markers for GABA and degraded AI neuronal response properties. Prior studies find age-related increases in spontaneous and driven activity, decreased spectral and directional sensitivity, and impaired novelty detection. The present study examined expression of GABAA receptor (GABAAR) subunit message, protein and quantitative GABAAR binding in young, middle-aged, and aged rat AI, with comparisons to adjoining parietal cortex. Significant loss of GABAAR α1 subunit message across AI layers was observed in middle-aged and aged rats while α1 subunit protein levels declined in layers II and III. Age-related increases in GABAAR α3 subunit message and protein levels were observed in certain AI layers. GABAAR subunits, including β1, β2, γ1, γ2s, and γ2L, primarily, but not exclusively, showed age-related declines at the message and protein levels. The ability of GABA to modulate [3H]TBOB binding in the chloride channel showed age-related decreases in peak binding and changes in desensitization kinetics. Collectively, age-related changes in GABAAR subunit composition would alter the magnitude and temporal properties of inhibitory synaptic transmission and could underpin observed age-related functional changes seen in the elderly.
Keywords: Age-related changes, auditory cortex, GABAA receptor subunit, quantitative GABAA receptor binding
1. Introduction
Age-related functional changes in a number of sensory systems are strongly suggestive of a loss of normal adult inhibitory amino acid neurotransmission (Angelotti and Macdonald, 1993; Belelli et al., 2005; Burianova et al., 2009; for review Canlon et al., 2010; Caspary et al., 2008; Gutierrez et al., 1997; Lloyd et al., 1990; Maksay and Ticku, 1985; Malherbe et al., 1990; Mendelson and Rajan, 2011; Olsen et al., 1990; Pinto et al., 2010; Sakurai et al., 1994; Suta et al., 2011; Syka, 2002; Winer, 1992; Wisden et al., 1992; Ymer et al., 1990). In part, these changes in central inhibition likely reflect a compensatory age-related response to decreased peripheral sensory input, reflecting homeostatic plasticity (Noreña, 2011; Oliver et al., 2011; Richardson et al., 2011; Turrigiano and Nelson, 2004). Compensatory age-related changes can result in decreased markers of normal functional inhibition in auditory and visual cortices (Hua et al., 2006; Hughes et al., 2010; Leventhal et al., 2003; Liang et al., 2008; Schmidt et al., 2010). Even when presented with suprathreshold acoustic stimuli, many middle-aged and elderly humans show decreased speech understanding, impaired sound localization, and loss in the ability to extract novel or salient signals from a complex acoustic background (Anderson et al., 2012; Dubno et al., 1984; Fitzgibbons and Gordon-Salant, 1994,2010; Fogerty et al., 2010; Lui and Mendelson, 2003; Ostroff et al., 2003; Pichora-Fuller et al., 2007; Schneider et al., 1994; Snell, 1997; Strouse et al., 1998; Suta et al., 2011; Tremblay et al., 2002, 2003).
Recent electrophysiologic studies in rat and primate auditory cortex (AI) find age-related increases in spontaneous and sound-evoked discharge rates as well as less precise directional sensitivity, loss of spectral precision, and impaired novelty detection (de Villers-Sidani et al., 2010; Hughes et al., 2010; Juarez-Salinas et al., 2010; Martin Del Campo, et al., 2012). Therefore, both human and animal studies suggest that changes in sound processing are consistent with a hypothesis of an age-related loss of normal adult functional inhibition.
Pre-synaptic markers for GABA, including GABA levels and levels of the GABA synthetic enzyme glutamic acid decarboxylase (GAD) are down-regulated across aged AI in humans and animal models of aging (Burianova et al., 2009; de Villers-Sidani et al., 2010; Ling et al., 2005; McGeer and McGeer, 1980). Few studies have examined GABAA receptor (GABAAR) markers across the layers of AI (Pirker et al., 2000; Wisden et al., 1992; Yu et al., 2006). The impact of aging on the subunit makeup of GABAARs across AI layers is the focus of the present study. The adjoining parietal cortex was used for comparison. GABAARs exist as pentameric subunit complexes which can be allosterically modulated by numerous pharmacological agents (Rabow et al., 1995 ; Sieghart, 1992a, b, c, d; Sieghart, 1995; Sieghart et al., 1992; Wafford et al., 1993; Yu et al., 2006). Molecular cloning has revealed 6-α, 4-β, 3-γ, 1-δ, 1-ε, 1-π, 1-θ, and 3-ρ GABAA receptor subunits (Olsen and Sieghart, 2008,2009; Rabow et al., 1995; Rudolph et al., 2001; Sieghart, 1995; Wafford and Ebert, 2006). GABAAR subunit constructs exhibit specific regional and likely cortical layer specific distributions (Pirker et al., 2000; Wisden et al., 1992; Yu et al., 2006). Altered GABAAR subunit composition/stoichiometry, potentially in response to age-related presynaptic changes, would impact GABAAR mediated inhibitory function. Age-related subunit changes would alter the magnitude and temporal precision of inhibitory currents, in turn degrading sensory processing (Angelotti and Macdonald, 1993; Ducic et al., 1995; Macdonald and Olsen, 1994; Takesian et al., 2012; Wafford et al., 1993). The present study examined GABAAR subunit message, protein and quantitative GABAAR binding of selective GABAA ligands in young, middle-aged, and aged rat auditory cortex.
2. Materials and methods
2.1. Animals
Young-adult (4–6 months), middle-aged (20–22 months) and aged (30–32 months) male Fischer Brown Norway (FBN) rats were obtained from Harlan Sprague-Dawley, Inc. (Indianapolis, IN). All experiments were carried out under animal use protocols approved by the Southern Illinois University School of Medicine Laboratory Animal Care and Use Committee. Age-related hair cell loss and age-related threshold shifts for this strain have been previously described (Turner and Caspary, 2005; Wang et al., 2009a).
2.2. Sampling criteria for rat auditory cortex
Sections were collected through the center of AI from an area at Bregma −4.80 mm (Plate 39) to Bregma −4.16 (Plate 36) (Paxinos and Watson, 1998) identified by measuring 2.25 mm dorsal from the rhinal fissure. The present study used criteria adapted from Winer (1992) and Games and Winer (1988) to define data collection areas sampled from layers II–VI of FBN rat AI. A detailed algorithm for measures used to identify AI layers II–VI was derived from Winer (1992) and Games and Winer (1988) and is detailed in Ling et al. (2005).
2.3. Quantitative in situ hybridization
Eighteen FBN rats (6 young-adult, 6 middle-aged and 6 aged) were decapitated, brains rapidly removed, rinsed in ice-cold phosphate-buffer saline (PBS) (pH 7.4, DEPC-treated), frozen in powdered dry ice, and stored at −80°C. Serial transverse sections (16μm) through AI were cut using a cryostat (Leica CM1850 Microsystems Nussloch GmbH, Nussloch, Germany) set at −18°C. Sections were thaw-mounted onto Superfrost/Plus slides (Thermo Fisher Scientific, Pittsburgh, PA, USA) at approximately the same position, two sections per slide, and stored at −20°C (<48 hours) until processed for in situ hybridization.
GABAA subunit probe preparation: Nine 40–48 mer oligonucleotide probes were synthesized and purified by Sigma Genosys (Woodlands, TX). Sequences selected were based upon published sequences: α1 (Khrestchatisky et al., 1989); α2 (Pritchett and Seeburg, 1990); α3 (Malherbe et al., 1990); α4 (Wisden et al., 1991); β1–3 (Ymer et al., 1989); γ1, γ2s, & γ2L (Ymer et al., 1990). Procedures for oligonucleotide probe end-labeling, in situ hybridization steps and data analysis are as described in (Ling et al., 2005) and were modified from Milbrandt et al. (1997). In brief, five picoMoles of oligonucleotide probes in 50μl labeling mixture were 3′ end-labeled for 10 min at 37°C with 0.5 μM of 35S-deoxyadenosine triphosphate (dATP) (PerkinElmer Inc., Downers Grove, IL, USA) using terminal deoxynucleotidyl transferase (16units/μl) (Fisher Scientific, Pittsburgh, PA, USA). The reaction was halted by addition of 50 μl of TE buffer. Ten mg/ml tRNA was added to enhance recovery of the labeled probe. Labeled probes were extracted using a phenol/chloroform. After hybridization and post-washing steps, slides were dried and dipped in NTB-2 photographic emulsion (VWR, West Chester, PA, USA) and stored in the dark at 4°C for 4 weeks. Exposed sections were then developed, fixed, and counterstained with Thionin for cell identification. Adjacent sections were used as controls for specificity. Competitive blocking of labeled oligonucleotides using excess concentrations (50-fold) of unlabeled oligonucleotide and incubation with labeled sense oligonucleotides were used as controls. Detailed hybridization procedure, consistency and quality control was described in Ling et al. (2005).
Quantitative analysis of hybridization labeling: Images were captured using a CoolSnap monochrome digital camera connected to an MCID-Elite 6.0 imaging system (InterFocus Imaging Ltd., Cambridge, England) with 40× objective. Accumulation of silver grains over neuronal cell bodies was interpreted as hybridization of the probe to its corresponding mRNAs (Fig. 1). The identity of sections was concealed/blinded to insure unbiased quantification. The counting parameters such as threshold, light intensity, and counting area were maintained consistently throughout the counting procedure for a particular subunit. Only grains within the neuronal perimeter were counted by the automated counting system-MCID Elite 6.0 (InterFocus Imaging Ltd., Cambridge, England) over cells distinguishable from adjacent cells and showing a visible nucleus. Quantitative comparisons were made only within a given subunit probe not across probes. With two sections per animal, two different fields, of fixed size, from each of the layers (II–VI) of AI in each section were digitized and grain counts, neuronal number, size, and area recorded. Background labeling measurements were obtained from three random areas located off the tissue sections. Somatic area and number of grains over the somata of at least 10 cells in each of the layers (II–VI) of AI in each section were measured. Data were collected as grain density (number of grains/100μm2 of cell area) and corrected by subtraction of nonspecific hybridization for each layer/subunit/age group. Analysis of Variance (ANOVA) was used to determine if differences in background-adjusted mean grain density was attributed to the treatment variables. Tests subsequent to the ANOVA were carried out using the Bonferroni procedure to control overall type I error rate (Ling et al., 2005).
Figure 1.
Distribution of GABAAR α1 and α3 mRNA in layer III neurons of AI from young and aged FBN rats. Clusters of silver grains represent hybridization of transcripts of GABAAR α1 (A&B) and α3 (C&D) with 35S-labeled selective oligonucleotide probes. Reduction of silver grains in aged AI neurons of layer III (B), when compared with that in neurons from young adult rat AI (A), indicates the age-related loss of GABAAR α1 mRNA. In contrast, GABAAR α3 mRNA shows increased in aged layer III neurons (D). Scale bar=10 μm.
2.4. Quantitative immunohistochemistry
The methods used for quantitative immunohistochemistry were based on those published by Ling et al. (2005).
Tissue preparation for immunohistochemistry: FBN rats were anesthetized with a mixture of ketamine (105 mg/kg body wt. i.p.) and xylazine (7 mg/kg body wt. i.p.), and transcardially perfused with 150 ml of physiological saline containing 0.1% of sodium nitrite, followed by 1 L of fixative containing 4% paraformaldehyde in Sorenson’s K-Na phosphate buffer (pH 7.4). Brains were removed, post-fixed for 1 h in the same solution, washed in 0.1 M PBS for 30 min and immersed overnight in PBS containing 20% sucrose. Cryoprotected tissues were stored at −80°C.
Immunohistochemistry: FBN rats used in GABAAR α1 and β1 studies were 4 young, 4 middle-aged and 4 aged, and in GABAAR α3 and β2 studies there were 6 young, 6 middle-aged and 6 aged rats. Serial transverse sections through AI were cryostat sectioned at 30 μm and collected as free-floating sections in ice-cold 0.1 M PBS. The sections were rinsed in PBS, transferred to blocking solution (1.5% normal serum and 5% non-fat dry milk in PBS) for 30 min and incubated at room temperature in primary antibodies for 1 h and then at 4°C overnigh t with agitation. Polyclonal goat anti-GABAAR α1 and β1–2 (1:150) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Polyclonal rabbit anti-GABAAR α3 (1:500) was obtained from Alomone Labs (Jerusalem, Israel). After rinsing in PBS, sections were processed using Vectastain ABC kits (Vector Laboratories, Burlingame, CA, USA). The labeling was visualized using diaminobenzidine (DAB, Sigma-Aldrich, St. Louis, MO, USA) and DAB reaction time was fixed at 2 min. Sections were then mounted onto the Superfrost/Plus slides. When possible, specificity of primary antibodies was tested by pre-incubation with the control peptide antigens. Secondary antibodies were controlled in cells processed as described above but in the absence of primary antibodies.
To help minimize variability, unrelated to treatments, immunostaining and measurements were carried out in parallel groups, with tissue from one young, one middle-aged and one aged animal processed at the same time. Sections were blinded so age groups were unknown to the observer. Flat-field correction was performed prior to digitizing images and held consistent across each group. Digital images of immuno- processed sections were captured at an objective magnification of 40× as described above. Two fields from each AI layer (LII–LVI) per section and two to four sections per animal were analyzed. Relative optical density (ROD) measurements, which are proportional to immunostaining intensity, were measured from all positively stained neurons encountered across layers II–VI of AI. All ROD measurements were corrected by subtracting background values obtained from the measurements of immunonegative cells in layer V. Only neurons with intact soma outlines and discernible nuclei and nucleoli were measured. All data were expressed as means ± S.D. of ROD.
2.5. Quantitative receptor binding autoradiography
RO15-4513 binds both “wild type” (2α12β2γ2) and non-“wild type” GABAARs. Functionally, RO15-4513 acts as a partial inverse agonist at γ2 subunit containing GABAARs (Korpi et al., 2002; Luddens and Wisden, 1991; Wisden et al., 1991), while acting as an agonist at α4 and α6 subunits-containing GABAARs (Hadingham et al., 1996; Knoflach et al., 1996; Linden et al., 2011; Wafford et al., 1996). Binding of the GABAAR radioligand t-butylbicycloorthobenzoate (TBOB), can be modulated by varying concentrations of GABA, and has been used in picrotoxin ligand binding assays for studying GABAAR pharmacology and receptor diversity (Lloyd et al., 1990; Maksay and Ticku, 1985; Olsen et al., 1990; Sakurai et al., 1994). [3H]RO15-4513 saturation analysis based on previous studies was used in order to reveal differences in kd or Bmax (Braestrup et al., 1983; Niddam et al., 1987; Ruano et al., 1993). Concentrations (1, 3, 5, 8, 10, 15nM) of [3H]RO15-4513 (20Ci/mmol, Perkinelmer Inc., San Jose, CA, USA) were added to the incubation buffer and 100μM of flumazenil was added as a displacer. Modulation of [3H]TBOB binding was carried out with increasing concentrations of GABA from 10nm to 5μm (Milbrandt and Caspary, 1995). Autoradiograms were generated by apposing slides to a phosphor screen, and the screen was then scanned using Cyclone phosphor system (Perkinelmer Inc., San Jose, CA, USA). Images were collected at 600 DPI and analyzed using OptiQuant image analysis software. The superficial layers II–IV were grouped into one box (38% cortical thickness), with layer V (26% cortical thickness) and layer VI (22% cortical thickness) (Games and Winer, 1988) windowed using one box for each layer. Digital light units (DLU) were converted into fmol/mg protein using a standard curve generated from the co-exposed 14C-embedded plastic standards (American Radioactive Chemicals, St. Louis, MO, USA) (Pan et al., 1983).
3. Results
The impact of aging on hair cell loss and auditory thresholds (23dB parallel shift) across frequency have been previously described for the FBN rat aging model used in the present study (Turner and Caspary, 2005; Wang et al., 2009b).
3.1. Age-related GABAAR subunit message changes
GABAAR subunit message and protein levels were obtained from primary auditory cortex (AI) and parietal cortex (PtA) in sections from young, middle-aged and aged FBN rats. Automated, non-stereological collection of in situ hybridization data provided information regarding neuronal number, size, and area. Consistent with the visual cortical findings of Peters et al. (1983) no significant age-related changes in neuronal number, neuronal size, and neuronal area were observed across AI layers.
Table 1 summarizes results of age-related GABAAR subunit message changes across AI layers for all subunits examined (α1,2,3,5; β1–3, γ1, γ2s, γ2L). Age-related subunit message levels for young-adult, middle-aged, and aged rats are presented both as raw grain counts over neurons across AI layers II–VI and as percent change from young- adult for middle-aged, and aged AI (Table 1; p values less than 0.05, unless otherwise stated).
Table 1.
Changes of GABAA Receptor Subunits mRNA levels in AI of FBN rats
| Young | Middle | % from Young | Aged | % from Young | ||
|---|---|---|---|---|---|---|
| α1 | LII | 5.9 ± 1.9 | 4.5 ± 2.2 | −23.73* | 3.6 ± 1.9 | −38.98* |
| LIII | 5.4 ± 1.8 | 4.3 ± 2.2 | −20.37* | 3.2 ± 1.7 | −40.74* | |
| LIV | 5.9 ± 2.0 | 4.8 ± 2.3 | −18.64* | 4.0 ± 1.7 | −32.20* | |
| LV | 5.7 ± 1.9 | 4.6 ± 2.2 | −19.30* | 3.9 ± 1.7 | −31.58* | |
| LVI | 6.8 ± 2.2 | 5.4 ± 2.2 | −20.59* | 3.9 ± 2.0 | −42.65* | |
|
| ||||||
| α2 | LII | 3.8 ± 1.9 | 3.9 ± 1.9 | 2.63 | 3.6 ± 1.6 | −5.26 |
| LIII | 3.3 ± 1.4 | 3.8 ± 1.8 | 15.15* | 3.1 ± 1.4 | −6.06 | |
| LIV | 3.7 ± 1.6 | 3.9 ± 2.1 | 5.41 | 3.8 ± 2.0 | 2.70 | |
| LV | 3.4 ± 1.4 | 4.0 ± 1.7 | 17.65* | 3.3 ± 1.4 | −2.94 | |
| LVI | 4.1 ± 2.0 | 5.0 ± 1.8 | 21.95* | 3.6 ± 1.5 | −12.20* | |
|
| ||||||
| α3 | LII | 6.5 ± 2.4 | 7.1 ± 3.2 | 9.23 | 8.5 ± 2.6 | 30.77* |
| LIII | 6.0 ± 1.8 | 6.8 ± 3.0 | 13.33* | 7.8 ± 2.5 | 30.0* | |
| LIV | 7.2 ± 2.5 | 7.6 ± 3.2 | 5.56 | 8.0 ± 2.6 | 11.11 | |
| LV | 6.7 ± 2.0 | 7.2 ± 2.9 | 7.46 | 7.4 ± 2.7 | 10.45* | |
| LVI | 7.9 ± 2.6 | 7.6 ± 3.0 | −3.80 | 7.7 ± 2.1 | −2.53 | |
|
| ||||||
| α5 | LII | 7.2 ± 2.2 | 6.7 ± 2.4 | −6.94 | 6.7 ± 2.4 | −6.94 |
| LIII | 6.0 ± 1.8 | 6.2 ± 2.5 | 3.33 | 6.0 ± 2.0 | 0 | |
| LIV | 6.0 ± 2.4 | 6.6 ± 2.7 | 10.0 | 6.2 ± 2.6 | 3.33 | |
| LV | 5.6 ± 2.0 | 5.6 ± 2.0 | 0 | 6.0 ± 2.2 | 7.14 | |
| LVI | 6.4 ± 2.3 | 6.3 ± 2.3 | −1.56 | 6.7 ± 2.5 | 4.69 | |
|
| ||||||
| β1 | LII | 5.5 ± 2.1 | 5.4 ± 2.3 | −1.82 | 4.3 ± 1.9 | −21.82* |
| LIII | 5.2 ± 1.8 | 5.3 ± 2.6 | 1.92 | 3.9 ± 1.6 | −25.0* | |
| LIV | 5.4 ± 2.1 | 5.5 ± 2.2 | 1.85 | 4.6 ± 2.0 | −14.81* | |
| LV | 5.2 ± 2.0 | 5.7 ± 2.0 | 9.62* | 4.5 ± 1.7 | −13.46* | |
| LVI | 6.2 ± 2.4 | 5.6 ± 2.2 | −9.68* | 4.9 ± 2.1 | −20.97* | |
|
| ||||||
| β2 | LII | 4.4 ± 2.0 | 4.3 ± 1.9 | −2.27 | 3.1 ± 1.5 | −29.55* |
| LIII | 4.1 ± 1.6 | 3.8 ± 1.7 | −7.32 | 2.8 ± 1.6 | −31.71* | |
| LIV | 4.1 ± 1.9 | 4.2 ± 1.7 | 2.44 | 3.4 ± 1.6 | −17.07* | |
| LV | 4.4 ± 1.7 | 4.2 ± 1.7 | −4.55 | 3.4 ± 1.4 | −22.73* | |
| LVI | 5.5 ± 2.0 | 5.0 ± 2.2 | −9.09 | 4.3 ± 1.9 | −21.82* | |
|
| ||||||
| β3 | LII | 7.5 ± 2.8 | 8.0 ± 2.3 | 6.67 | 6.1 ± 2.5 | −18.67* |
| LIII | 6.9 ± 2.5 | 7.3 ± 2.5 | 5.80 | 5.6 ± 2.5 | −18.84* | |
| LIV | 7.5 ± 2.7 | 7.6 ± 2.4 | 1.33 | 6.4 ± 2.6 | −14.67* | |
| LV | 7.1 ± 2.7 | 7.3 ± 1.7 | 2.82 | 6.6 ± 2.7 | −7.04 | |
| LVI | 8.3 ± 2.8 | 8.2 ± 2.6 | −1.20 | 7.9 ± 3.7 | −4.82 | |
|
| ||||||
| γ1 | LII | 9.5 ± 3.3 | 8.6 ± 3.4 | −9.47* | 7.6 ± 2.7 | −20.0* |
| LIII | 9.2 ± 3.3 | 8.4 ± 3.6 | −8.70* | 7.2 ± 2.1 | −21.74* | |
| LIV | 9.9 ± 3.4 | 8.6 ± 3.5 | −13.13* | 7.5 ± 2.4 | −24.24* | |
| LV | 9.9 ± 3.3 | 9.6 ± 3.1 | −3.03 | 8.6 ± 2.1 | −13.13* | |
| LVI | 11.0 ± 3.3 | 10.3 ± 3.5 | −6.36* | 9.2 ± 2.5 | −16.36* | |
|
| ||||||
| γ2S | LII | 6.0 ± 1.5 | 6.2 ± 1.8 | 3.33 | 5.9 ± 1.7 | −1.67 |
| LIII | 5.6 ± 1.5 | 5.8 ± 1.9 | 3.57 | 5.2 ± 1.6 | −7.14 | |
| LIV | 5.7 ± 1.6 | 6.3 ± 2.1 | 10.53* | 5.6 ± 1.5 | −1.75 | |
| LV | 5.4 ± 1.2 | 5.9 ± 1.8 | 9.26* | 5.7 ± 1.4 | 5.56 | |
| LVI | 5.8 ± 1.7 | 6.3 ± 2.0 | 8.62 | 6.0 ± 1.9 | 3.45 | |
|
| ||||||
| γ2L | LII | 5.3 ± 1.9 | 4.2 ± 2.3 | −20.75* | 3.2 ± 1.7 | −39.62* |
| LIII | 4.9 ± 1.6 | 4.1 ± 2.0 | −16.33* | 3.1 ± 1.9 | −36.73* | |
| LIV | 5.4 ± 1.6 | 4.2 ± 2.1 | −22.22* | 3.7 ± 2.0 | −31.48* | |
| LV | 5.1 ± 1.8 | 4.6 ± 2.0 | −9.80* | 3.9 ± 1.9 | −23.53* | |
| LVI | 5.7 ± 1.6 | 4.9 ± 2.1 | −14.04* | 4.4 ± 2.0 | −22.81* | |
The data represent means ± SD (number of grains/100 μm ).
Significant difference between the means of young vs. aged groups and young vs. middle-aged (p<.05).
Aging and GABAAR subunit α1,2,3,5 message changes
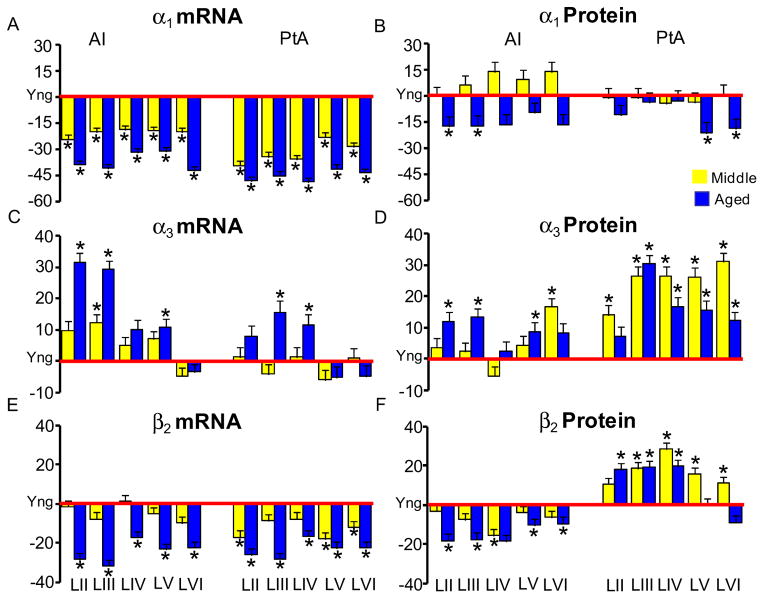
Significant step-wise age-related decreases in GABAAR α1 subunit message were observed across layers of AI and PtA (Tables 1 and 2). Images from in situ hybridization show an age-related reduction in number of silver grains, representing GABAAR α1 subunit message over AI layer III neurons (Figs. 1A, B). The age-related α1 subunit message loss was significant across all layers (p<0.01) for young vs. aged with percent reductions between 30 and 42 percent (Table 1, Fig. 2A). Middle-aged animals showed GABAAR α1 subunit message levels that were intermediate between young and aged α1 subunit message levels (Table 1, Fig. 2A). Age-related changes in PtA GABAAR α1 subunit message displayed a similar staircase aging pattern to that seen in AI (Table 2, Fig. 2A).
Table 2.
Changes of GABAA Receptor Subunits mRNA levels in PtA of FBN rats
| Young | Middle | % from Young | Aged | % from Young | ||
|---|---|---|---|---|---|---|
| α1 | LII | 7.1 ± 2.1 | 4.3 ± 2.3 | −39.44* | 3.7 ± 1.6 | −47.89* |
| LIII | 7.0 ± 2.0 | 4.6 ± 2.0 | −34.29* | 3.8 ± 1.7 | −45.71* | |
| LIV | 7.7 ± 2.1 | 4.9 ± 2.3 | −36.36* | 3.9 ± 1.5 | −49.35* | |
| LV | 6.4 ± 1.7 | 4.9 ± 2.1 | −23.44* | 3.8 ± 1.5 | −40.36* | |
| LVI | 7.1 ± 2.1 | 5.1 ± 2.3 | −28.17* | 4.0 ± 1.4 | −43.66* | |
|
| ||||||
| α3 | LII | 6.8 ± 2.1 | 6.9 ± 2.5 | 1.47 | 7.3 ± 1.8 | 7.35 |
| LIII | 6.9 ± 2.3 | 6.7 ± 2.4 | −2.90 | 8.0 ± 2.1 | 15.94* | |
| LIV | 6.7 ± 2.1 | 6.8 ± 2.3 | 1.49 | 7.5 ± 2.3 | 11.94* | |
| LV | 7.0 ± 2.1 | 6.6 ± 2.4 | −5.71 | 6.6 ± 1.9 | −5.71 | |
| LVI | 7.6 ± 2.7 | 7.7 ± 2.7 | 1.32 | 7.3 ± 2.6 | −3.95 | |
|
| ||||||
| β2 | LII | 5.1 ± 1.8 | 4.2 ± 1.8 | −17.65* | 3.7 ± 1.5 | −27.45* |
| LIII | 4.5 ± 2.1 | 4.2 ± 1.7 | −6.67 | 3.3 ± 1.4 | −26.67* | |
| LIV | 4.9 ± 1.8 | 4.5 ± 1.9 | −8.16 | 4.1 ± 1.7 | −16.33* | |
| LV | 4.7 ± 1.8 | 3.9 ± 1.5 | −17.02* | 3.7 ± 1.5 | −21.28* | |
| LVI | 5.4 ± 1.9 | 4.8 ± 1.7 | −11.11* | 4.2 ± 1.6 | −22.22* | |
|
| ||||||
| γ2L | LII | 4.6 ± 1.9 | 4.6 ± 1.7 | −0 | 3.9 ± 1.7 | −15.22* |
| LIII | 4.4 ± 1.8 | 4.3 ± 1.6 | −2.27 | 4.0 ± 2.1 | −9.09 | |
| LIV | 4.8 ± 1.7 | 4.5 ± 1.7 | −6.25 | 4.2 ± 2.0 | −12.5 | |
| LV | 4.5 ± 1.4 | 4.3 ± 1.8 | −4.44 | 3.8 ± 2.0 | −15.56* | |
| LVI | 5.0 ± 1.7 | 5.1 ± 1.9 | 2.0 | 4.4 ± 2.0 | −12.0 | |
The data represent means±SD (number of grains/100 μm2).
Significant difference between the means of young vs. aged groups and young vs. middle-aged (Mid, p<.05).
Figure 2.
Age-related changes of GABAAR subunit α1, α3 and β2 message and proteins in FBN rat AI and PtA. Bar graphs represent the percentage changes of middle-aged (n = 6) and aged (n = 6) from the young (red x-axis, n = 6) for the message and protein levels of GABAAR α1 (A&B), α3 (C&D) and β2(E&F). Error bars represent the standard error of the means. (*: p<.05, Yng = Young).
While significant age-related declines in α1 subunit message were observed across layers of AI and PtA, an apparent compensatory age-related increase in α3 message level was observed for a subset of layers in AI and PtA (Tables 1 and 2; Fig. 2C). GABAAR α3 subunit message levels showed significant (p<.001) age-related increases over neurons in supragranular AI layers II–III, and output layer V with trends toward GABAAR α3 subunit message level increases in AI layer IV (Table 1, Fig. 2C). Aged AI layers II and III showed age-related α3 subunit message level increases near 30% (Fig. 2C) when compared to young layers. Similar, but more modest pattern for GABAAR α3 subunit message increases were observed in layers III and IV of PtA (Table 2, Fig. 2C).
No consistent age-related changes were observed for α2 or α5 GABAAR subunit message over neurons in AI layers II–VI (Table 1). However, GABAAR α2 subunit showed significant increases in middle-age, (AI layers II, V and VI) before returning to near young-adult levels in aged rat AI (Table 1).
Aging and GABAAR β1–3 subunit message changes
Significant age-related β1–3 GABAAR subunit message losses were seen across all AI layers (Table1, Fig. 2E). β1–2 subunits showed changes between 17 and 34 percent across all layers of aged AI when compared to young animals (Table 1, Fig. 2E). AI β1–2 subunit changes in middle-aged animals were generally not significantly different from young-adult levels, with the exception of β1 subunit changes in AI layers V and VI (Table 1). GABAAR β3 subunit message showed significant age-related reductions in AI layers II–IV and non-significant changes in AI of middle aged animals (Table 1).
Aging and GABAAR subunit γ1, γ2s and γ2L message changes
Significant age-related decreases were seen for γ1 and γ2L GABAAR subunit message levels while no age-related γ2s subunit message changes were observed for aged rat AI (Table 1). Only γ2L GABAAR subunit message levels were examined in PtA. Age-related changes for γ2L GABAAR subunit message in PtA were smaller than those observed for AI and were significant only in LII and LV of aged PtA (Table2). Unfortunately, selective antibodies with adequate signal to noise ratios were not available to allow for quantitative immunohistochemistry of γ1, γ2s and γ2L GABAAR subunit proteins.
3.2. Aging and GABAAR subunit protein changes: α1&3
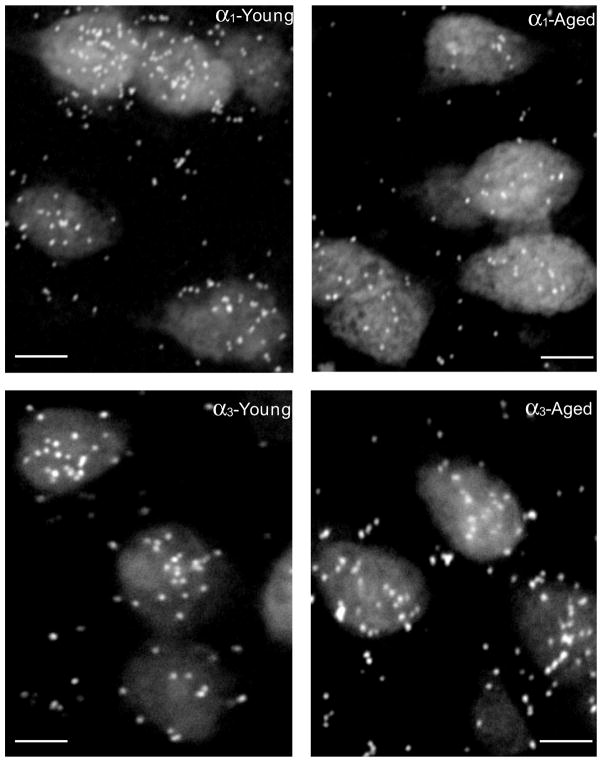
Densitometric immunohistochemical studies were used to assess GABAAR subunit protein levels over individual neurons across the layers of AI and PtA for GABAAR α subunits which showed significant age-related message changes. Age-related protein changes focused on the α1&3 GABAAR subunits, from cortical neurons in AI and PtA (Table 3). Confocal images showed age-related loss of α1 GABAAR subunit fluorescence (red) and the apparent compensatory age-related increase in α3 subunit protein (green) (Fig. 3). Age-related protein changes were, for the most part smaller than, but consistent with α1&3 subunit message changes (Fig. 2 & Table 1). Significant age-related reductions in α1 GABAAR subunit protein levels (10%–17%) were seen across AI layers reaching significance in superficial layers II and III (Fig. 2B, Table 3). Consistent with the observed increase of α3 subunit message, there was an age-related up-regulation of α3 GABAAR subunit protein in AI. GABAAR α3 subunit increases ranged between 2% and 13% reaching significance for neurons in layers II–III and layer V (Fig. 2D, Table 3). Similar age-related α1&3 subunit message and protein changes were observed in adjoining parietal cortex (Tables 2 and 3). Age-related increases in α3 subunit protein in PtA exceeded changes observed in AI α3 subunit protein. However, the general pattern of age-related α1&3 subunit changes was similar across the two cortical areas.
Table 3.
Comparison of Age-related Changes of GABAA Receptor Subunit Protein Levels in A1 and PtA
| Auditory Cortex (A1) | Parietal Cortex (PtA) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Young | Middle | % from young | Aged | % from Young | Young | Middle | % from Young | Aged | % from Young | ||
| α1 | LII | 0.090±0.035 | 0.090±0.046 | 0 | 0.075±0.038 | −16.67* | 0.073±0.026 | 0.072±0.031 | −1.37 | 0.065±0.036 | −10.96 |
| LIII | 0.089±0.034 | 0.095±0.045 | 6.74 | 0.074±0.035 | −16.85* | 0.071±0.027 | 0.070±0.032 | −1.41 | 0.069±0.039 | −2.82 | |
| LIV | 0.081±0.033 | 0.093±0.048 | 14.81 | 0.068±0.030 | −16.05 | 0.071±0.027 | 0.068±0.029 | −4.23 | 0.069±0.039 | −2.82 | |
| LV | 0.079±0.031 | 0.087±0.045 | 10.13 | 0.071±0.035 | −10.13 | 0.078±0.029 | 0.075±0.033 | −3.85 | 0.061±0.040 | −21.79* | |
| LVI | 0.078±0.032 | 0.089±0.044 | 14.10 | 0.066±0.035 | −15.38 | 0.075±0.029 | 0.075±0.036 | 0 | 0.061±0.034 | −18.67* | |
|
| |||||||||||
| α3 | LII | 0.136±0.034 | 0.141±0.030 | 3.68 | 0.152±0.039 | 11.76* | 0.103±0.020 | 0.117±0.039 | 13.59* | 0.110±0.030 | 6.80 |
| LIII | 0.134±0.041 | 0.138±0.034 | 2.98 | 0.152±0.034 | 13.43* | 0.097±0.025 | 0.122±0.035 | 25.77* | 0.126±0.037 | 29.90* | |
| LIV | 0.122±0.036 | 0.116±0.032 | −4.92 | 0.125±0.031 | 2.46 | 0.093±0.025 | 0.118±0.030 | 26.88* | 0.109±0.034 | 17.20* | |
| LV | 0.125±0.036 | 0.131±0.036 | 4.8 | 0.136±0.031 | 8.8* | 0.099±0.028 | 0.125±0.030 | 26.26* | 0.114±0.024 | 15.15* | |
| LVI | 0.096±0.027 | 0.113±0.028 | 17.71* | 0.104±0.027 | 8.33 | 0.085±0.026 | 0.111±0.024 | 30.59* | 0.095±0.021 | 11.76* | |
|
| |||||||||||
| β1 | LII | 0.178±0.037 | 0.152±0.043 | −14.61* | 0.163±0.044 | −8.43 | 0.104±0.043 | 0.125±0.055 | 20.19* | 0.110±0.049 | 5.77 |
| LIII | 0.159±0.042 | 0.139±0.037 | −12.58* | 0.157±0.033 | −1.26 | 0.114±0.045 | 0.118±0.038 | 3.51 | 0.119±0.050 | 4.39 | |
| LIV | 0.141±0.039 | 0.117±0.031 | −17.02* | 0.138±0.037 | −2.13 | 0.097±0.046 | 0.112±0.030 | 15.46 | 0.107±0.040 | 10.31 | |
| LV | 0.125±0.054 | 0.128±0.036 | 2.4 | 0.123±0.038 | −1.6 | 0.093±0.041 | 0.120±0.036 | 29.03* | 0.109±0.057 | 17.20 | |
| LVI | 0.118±0.041 | 0.098±0.024 | −16.95* | 0.117±0.042 | −0.85 | 0.090±0.044 | 0.092±0.029 | 2.22 | 0.099±0.053 | 10.0 | |
|
| |||||||||||
| β2 | LII | 0.128±0.026 | 0.124±0.044 | −3.13 | 0.105±0.034 | −17.97* | 0.080±0.041 | 0.089±0.027 | 11.25 | 0.095±0.041 | 18.75* |
| LIII | 0.122±0.024 | 0.112±0.038 | −8.20 | 0.100±0.030 | −18.03* | 0.078±0.028 | 0.092±0.035 | 17.95* | 0.093±0.035 | 19.23* | |
| LIV | 0.114±0.024 | 0.096±0.035 | −15.79* | 0.093±0.031 | −18.42* | 0.067±0.021 | 0.086±0.025 | 28.36* | 0.080±0.036 | 19.40* | |
| LV | 0.107±0.026 | 0.103±0.028 | −3.74 | 0.096±0.030 | −10.28* | 0.085±0.033 | 0.099±0.030 | 16.47* | 0.085±0.038 | 0 | |
| LVI | 0.093±0.028 | 0.088±0.025 | −5.38 | 0.085±0.026 | −8.60* | 0.083±0.023 | 0.093±0.027 | 12.05* | 0.076±0.030 | −8.4 | |
Figure 3.
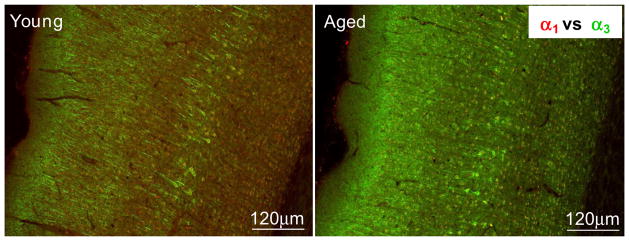
Confocal images of immuno double-labeling using GABAAR α1 (red) and α3 (green) in AI from young adult and aged FBN rat. A clearly increased α3 immuno-positive staining is seen in the aged AI when compared to the young AI. Scale bar = 120μm.
Aging and GABAAR β1&2 subunit protein changes
Neuronal protein levels were obtained for β1&2 GABAAR subunits but not for the β3 subunit due to the lack of availability of a specific antibody. Consistent with age-related declines in β2 subunit message across AI layers, GABAAR β2 subunit protein levels declined significantly across AI layers (Table 3, Fig. 2F). Non-significant decreases were also observed for β2 GABAAR subunit protein in middle-aged AI (Table 3; Fig. 2F). GABAAR β2 subunit protein increases in PtA were in sharp contrast to what was observed for β2 GABAAR subunit protein in neighboring AI and in contrast to β 2 GABAAR subunit message level decreases observed for PtA and AI (Table 3). Significant increases for β2 GABAAR subunit protein were observed across most PtA layers for middle-aged and aged animals when compared to young-adult PtA (Table 3; Fig. 2F).
In contrast to age-related decreases for β2 subunit protein, β1 GABAAR subunit protein decreased significantly only in middle-aged AI with no significant changes observed for aged AI compared to young-adult AI (Table 3). In addition, there were significant increases in β1 subunit protein levels in LII and LV of middle-aged PtA but no significant changes in aged PtA (Table 3).
3.3. Age-related pharmacological changes of GABAA receptors in AI
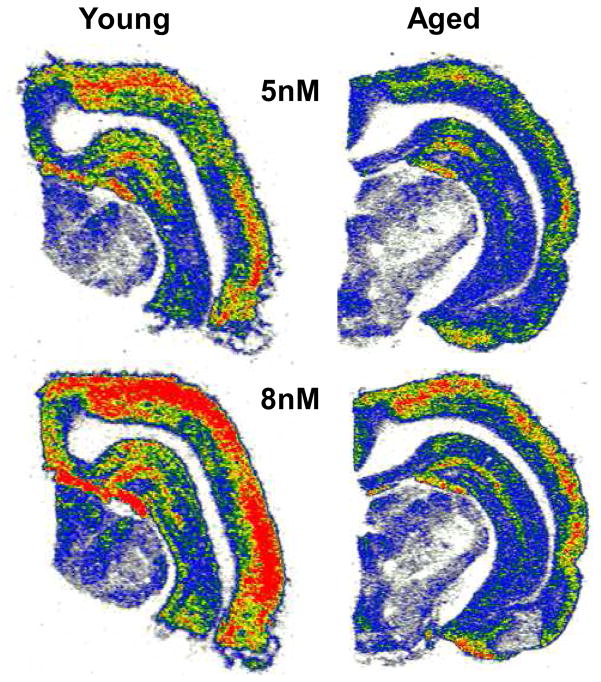
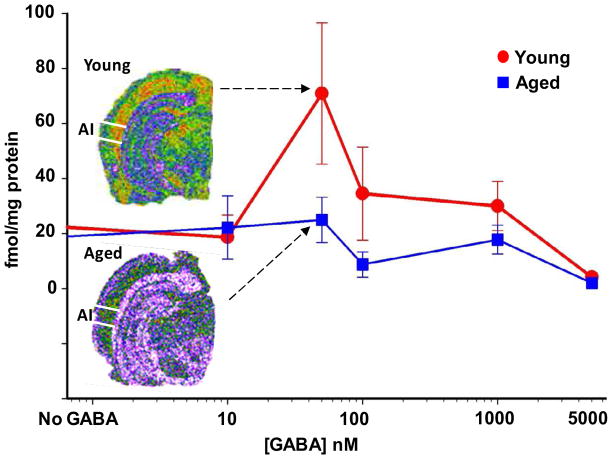
Groups of young (4–6 months), middle-aged (20–24 months ), and aged (30–34 months) FBN rats were used to further examine the impact of aging on intact mature GABAARs and the ability of GABA to modulate ligand binding at the picrotoxin binding site in the GABAAR pore of AI neurons (Milbrandt, et al., 1996). Figure 4 shows higher levels of RO15-4513 binding in the superficial layers of AI. RO15-4513 is thought to be sensitive to the identity of α and γ GABAAR subunits (Luddens and Wisden, 1991). A significant age-related loss of [3H]RO15-4513 GABAAR binding sites (Bmax) was found across all layers of aged AI (p<0.017, n=8, 8, 8,) while kd values were unaltered (Table 4). In contrast to [3H]RO15-4513 binding, [3H]TBOB binding was highest in the deep layers of AI (Fig. 5). In this assay, in the absence of GABA, GABAARs are closed and no [3H]TBOB binding could occur in the chloride channel of either young or aged AI GABAARs (Fig. 5). With increasing concentrations of GABA (10nM-5μM), AI neuronal GABAAR chloride channels were activated/opened providing access for [3H]TBOB binding at picrotoxin sites. At higher GABA concentrations, GABAARs became desensitized and the binding curve began to approximate baseline at the highest concentration of GABA (5μM). This cycle of events was significantly altered in aged AI. Figure 5 shows the age-related change in the [3H]TBOB binding curve between young-adult and aged AI layer VI as GABA levels were increased. The observed age-related changes in RO15-4513 binding and TBOB modulation supported subunit message and protein data which indicated an age-related change in the makeup and stoichiometry of GABAARs across the layers of aged AI.
Figure 4.
[3H]RO15-4513 GABAAR binding in AI of young and aged FBN rats. A significant age-related loss of GABAA receptor binding can be seen across all layers of AI at the concentrations of 5 and 8 nM of 3[H] RO15-4513. Highest binding levels were observed in the superficial layers of AI.
Table 4.
Bmax and kd of RO15-4513 Saturation Analysis
| Layer | Bmax (fmol/mg protein)
|
Kd (nM)
|
||||
|---|---|---|---|---|---|---|
| Young | Middle | Aged | Young | Middle | Aged | |
| LII/LIII/LIV | 618±44.7 | 550±25.3* | 564±34.7* | 1.03±0.40 | 0.80±0.22 | 0.99±0.33 |
| LV | 545±26.2 | 485±16.9* | 510±26.9* | 0.81±0.23 | 0.63±0.15 | 0.81±0.26 |
| LVI | 505±22.3 | 465±15.4* | 472±21.8* | 0.75±0.21 | 0.56±0.14 | 0.64±0.20 |
Significance from the young (p < 0.05).
Figure 5.
GABA modulation of 3[H]TBOB binding in layer VI of young and aged FBN rat AI. Increasing concentrations of GABA (10nM-5μM) were added to the TBOB assay. A significant age-related loss of TBOB binding was observed throughout aged neocortex when compared to young AI (the error bars represent S.E.M.). The age-related shift in the GABA dose-response curve in layer VI of FBN rat AI suggests a change in GABA’s ability to activate/open aged GABAA receptors (n = 4 young and 4 aged).
4. Discussion
The present findings of age-related GABAAR subunit and pharmacologic changes strongly support previous neurochemical, human psychophysical, and animal physiologic studies suggesting dysfunctional inhibitory processing of acoustic information in aged auditory cortex.
4.1. Age-related GABAAR subunit changes and discordance between message and protein changes
The present findings in AI for GABAAR subunit mRNA levels are consistent with cortical changes described by Gutierrez et al. (1997) showing substantial age-related changes for α1, β2/3, γ2 GABAAR subunit messages in other brain areas. In addition, the present study found age-related loss of GABAAR β1γ1 subunit message across AI layers (Table 1) Changes specific to the GABAAR α1, the wild-type α subunit message were across AI layers and greater than 30%. GABAAR α1 findings were consistent with previous studies of GABAAR α1 subunit message changes in aging neocortex, where Mhatre et al. (1992) described an 86% age-related decrease and Gutierrez et al. (1997) found a 29% reduction in the neocortex. In contrast to previous studies which did not observe significant age-related cortical subunit protein changes (Gutierrez, et al., 1996, 1997; Rissman, et al., 2007; Yu, et al., 2006), the present study finds α1&3 GABAAR subunit protein changes in AI consistent with observed age-related message changes. However, the present study did find substantial quantitative and qualitative discordance between GABAAR subunit message changes and protein changes as previously noted by Gutierrez et al. (1997,1996), and Wang et al. (2009b). With the notable exception observed for GABAAR β subunit changes, the present age-related protein findings were qualitatively consistent with, but more modest than, corresponding subunit message changes in both AI and PtA. Relatively smaller age-related percent changes for protein expression, compared to subunit message changes, may reflect post-translational compensatory mechanisms or perhaps a less sensitive method for assessing cellular protein levels relative to cellular message levels. Contrary to this latter possibility, there were examples of some age-related protein changes which exceeded age-related subunit message changes. One example found that age-related GABAAR α3 subunit protein changes in PtA were greater than corresponding GABAAR α3 message changes (Tables 1 and 3).
The most striking example of message/protein discordance with aging found significantly increased GABAAR β1&2 subunit proteins in PtA in the face of dramatically decreased β1&2 subunit message levels (Figs. 2E and 2F; Tables 1 and 3). It is important to understand how aging affects both expression and post-translational processing of GABAAR subunits. The presence of age-related discordance between subunit message and protein is emblematic of post-translational age-related changes. These findings may reflect robust compensatory post-translational aging mechanisms which will require further study. It is unlikely that these findings are due to experimental error since all measurements were blinded and age-related measures of β1&2 subunit message and protein levels were carried out in different animals, while comparisons between AI and PtA were carried out in the same animals. Immunolabeling over neocortex was not observed to be uneven over PtA and AI, which are adjacent structures. Categorically, similar age-related changes between subunit message and protein have been described for glycine receptor subunits in the aging dorsal cochlear nucleus (Wang, et al., 2009b).
4. 2. GABAAR α1 and α3 subunit protein changes
The present study examined a subset of GABAAR subunit proteins partially limited by the availability of high quality subunit antibodies for certain GABAAR subunits. As noted above, significant age-related GABAAR subunit protein decreases occurred across layers in AI for α1 & β2 with many age-related protein changes approaching 20% (Fig. 2B and F, Table 3). PtA displayed similar GABAAR subunit protein changes with aging with the GABAAR β1&2 subunit protein exceptions noted above. Perhaps as a compensatory change for the profound decrease in the GABAAR α1 subunit protein, GABAAR α3 subunit proteins tended to increase with age in both AI and PtA. These changes were significant for LII, III, and V in AI and all but LII in PtA. The mechanism for, and the significance of, these age-related compensatory subunit changes are unknown at the present time. A recent aging study in human visual cortex examined GABAAR related protein changes and reported an age-related trend toward increased GABAAR α3 subunit protein between 20–80 years of age (Pinto et al., 2010). The age-related down-regulation of α1 message and protein and the layer selective age-related up-regulation of α3 message and protein are suggestive of a reverse of compensatory changes seen in development and other models of GABA deafferentation (Caspary et al., 2008). Caspary et al. (1990) found an age-related reduction in GABA release in inferior colliculus of aged F344 rats. As reviewed above, models of sensory aging are suggestive of altered GABA inhibitory neurotransmission. The present findings and similar studies of the aged inferior colliculus suggest that this loss of GABA tone is at least in part a result of plastic changes in GABAAR subunit composition (Caspary et al., 2008). Developmental and expression GABAAR subunit studies suggest that observed subunit changes are consistent with smaller peak evoked IPSCs having longer-slower time-constants, perhaps in an effort to compensate for the loss of inhibitory input (Bosman et al., 2002; Juttner et al., 2001; Wafford et al., 1993). Evidence for age-related compensatory GABAAR subunit changes have been described for inferior colliculus (Caspary et al., 1999) and are implicit in a number of other studies (Rissman et al., 2007; Zhou et al., 2011).
4.3. Age-related receptor binding changes reflect altered GABAA subunit content of functional receptors
Subunit composition/stoichiometry can dramatically affect receptor pharmacology and channel function (Angelotti and Macdonald, 1993; Caspary et al., 1999; Ducic et al., 1995; Macdonald and Olsen, 1994; Rudolph et al., 2001; Sigel et al., 1990; Wafford et al., 1993). Age-related changes in GABAAR subunit constructs would impact the pharmacology of GABAARs (Ebert et al., 1994; Wafford et al., 1993). Age-related changes in GABAAR binding were previously reported for non-auditory cortical structures and hippocampus (Concas et al., 1988; Erdo and Wolff, 1989; Mhatre and Ticku, 1992; Ruano et al., 1992). Previous receptor binding studies have shown age- related changes in GABAAR pharmacology of inferior colliculus using several subunit selective radiolabeled GABAAR ligands (Milbrandt et al., 1994,1996). RO15-4513 is thought to differentially bind GABAAR constructs containing different α and γ GABAAR subunits (Ebert et al., 1994; Wafford et al., 1993). The literature is not definitive on the binding properties of the BDZ inverse agonist RO15-4513 but strongly suggests a preference for binding constructs containing α5>α1 (Lingford-Hughes et al., 2002). The present study found significant RO15-4513 binding in the upper layers of AI in agreement with Pirker et al. (2000) description of moderate levels of α5 subunit containing GABAARs in neocortical layers IV and high levels of α1 GABAARs in supragranular layers of the neocortex. Data from the present study finds reduced RO15-4513 binding in aged AI compared to young-adult AI. This age-related change likely reflects the observed αx and γx subunit changes or decreased numbers of functionally assembled and inserted GABAARs due to age-related changes in trafficking and or anchoring proteins (Wang et al., 2009a).
The present findings (Figure 5) are consistent with previous studies showing age-related loss in the ability of GABA to modulate binding at the picrotoxin site with age (Erdo and Wolff, 1989; Mhatre and Ticku, 1992; Milbrandt et al., 1996). TBOB selectively binds to convulsant sites associated with the chloride channel (Olsen et al., 1990). The differential ability of GABA to modulate the binding of picrotoxin analogs, such as TBOB and TBPS, to the picrotoxin site within the chloride channel of different GABAA constructs were examined (Im et al., 1994). These authors found that maximal enhancement of TBPS binding by GABA (opening of channels to allow binding) in cloned rat GABAAR subtypes varied with the isoforms (153 ± 10, 438 ± 16 and 139 ± 29% for α1β2, α3β2, α6 β2, respectively). The present binding study did not allow us to accurately discriminate individual AI layer changes but the highest levels of TBOB binding was found in infragranular layers of AI.
4.4. Age-related changes of GABA neurotransmission and inhibitory function in AI
An increasing number of studies in AI describe significant age-related losses of presynaptic markers for GABA and functional changes indicative of a loss of normal adult GABAergic function. Glutamic acid decarboxylase (GAD), the primary synthesizing enzyme for GABA, is significantly decreased in rat AI (Burianova et al., 2009; Ling et al., 2005) and parallels a significant decrease in the number and optical density of parvalbumin labeled neurons in rat AI (de Villers-Sidani et al., 2010; Martin Del Campo et al., 2012; Ouda et al., 2008). Human auditory cortex shows age-related decreased levels of markers for normal adult GABA function (McGeer and McGeer, 1976; Pinto et al., 2010). Functional loss of adult primate GABAergic function has been described in visual cortex (Betts et al., 2005; Leventhal et al., 2003; Schmolesky et al., 2000). Age-related changes suggestive of a loss of normal young adult GABAergic function in AI have been recently reviewed (Caspary et al., 2008; Mendelson and Rajan, 2011). Young and aged FBN rats show a fairly parallel (15–20dB) age-related threshold increase across frequencies (Caspary et al., 2005; Wang et al., 2009b). Recent cortical electrophysiology studies are strongly suggestive of an age-related loss of inhibitory function resulting in increased spontaneous and driven activity in the upper layers of rat and primate primary (AI) and secondary auditory cortex (Hughes et al., 2010; Juarez-Salinas et al., 2010). Age-related loss in the ability to localize sound in space in primate AI and secondary auditory cortex and a negative impact on novelty detection in rats can be directly related to inhibitory changes. We would have preferred if the upper layers showed greater age-related changes in subunit changes than the deeper layers since it appears that some of the greater changes occur in the layers with the highest levels of GABAA receptors (Prieto et al., 1994). However, the apical dendrites extending up to LI–III have their cell bodies in lV and V which may well confound the relative distribution of age-related changes because of the somatic expression of the subunit markers show somatic expression although receptors may be in the supragranular layers. Behavioral and evoked potential studies are strongly suggestive of reduced temporal processing in aged rat central auditory pathway (Suta et al., 2011). Rat studies describe age-related losses in the ability to detect novel sounds in AI, which was partially reversed by training, resulting in up-regulated parvalbumin labeling in aged animals (de Villers-Sidani et al., 2010).
In support of the present observations, a recent study by Schmidt et al. (2010) found significant age-related decreases in paired-pulse inhibition in both auditory and parietal cortices. In contrast to the present findings and those of Gutierrez et al. (1997), this study described GABAAR a1 subunit protein increases with aging in parietal cortex for a subset of rats (Schmidt et al., 2010). Studies in the primate visual cortex described age-related changes in the visual receptive fields system (Juarez-Salinas et al., 2010; Leventhal et al., 2003) while Juarez-Salinas et al. (2010) described degraded spatial tuning in unit responses from AI and secondary auditory cortical areas. Collectively, the present findings are in agreement with the studies reviewed above, by showing significant age-related loss of wild-type (α1β2γ2) GABAAR markers commonly found in young-adult AI. In situ hybridization and immunohistochemistry data showed age-related changes of mRNA and protein within the individual GABAAR subunits, while receptor binding study revealed the pharmacological changes when these subunit proteins assembled as functional GABAA receptors. Loss of wild-type GABAARs and their replacement by GABAAR constructs with different subunit combinations would be expected to show slower inhibitory response kinetics and lower peak currents impairing the ability to reliably process temporally demanding stimuli (Richardson et al., 2012; Wafford et al., 1993). It is likely that these age-related changes in normal GABAA receptor function could impact speech understanding in a subset of the human elderly population.
Acknowledgments
The authors thank Dr. Jennifer Parrish and Judith Bryan for their helpful editing. This research is supported by NIH grant No. DC000151.
Abbreviations
- AI
auditory cortex
- DAB
diaminobenzidine
- dATP
deoxyadenosine triphosphate
- GABAAR
GABAA receptor
- GAD
GABA synthetic enzyme glutamic acid decarboxylase
- IC
inferior Colliculus
- PtA
parietal cortex
- TBOB
t-butylbicycloorthobenzoate
Footnotes
Conflicts of interest
The authors state that there are no actual or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson S, Parbery-Clark A, White-Schwoch T, Kraus N. Aging affects neural precision of speech encoding. J Neurosci. 2012;32(41):14156–64. doi: 10.1523/JNEUROSCI.2176-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelotti TP, Macdonald RL. Assembly of GABAA receptor subunits: alpha 1 beta 1 and alpha 1 beta 1 gamma 2S subunits produce unique ion channels with dissimilar single-channel properties. J Neurosci. 1993;13(4):1429–40. doi: 10.1523/JNEUROSCI.13-04-01429.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Peden DR, Rosahl TW, Wafford KA, Lambert JJ. Extrasynaptic GABAA receptors of thalamocortical neurons: a molecular target for hypnotics. Journal of Neuroscience. 2005;25(50):11513–20. doi: 10.1523/JNEUROSCI.2679-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts LR, Taylor CP, Sekuler AB, Bennett PJ. Aging reduces center-surround antagonism in visual motion processing. Neuron. 2005;45(3):361–6. doi: 10.1016/j.neuron.2004.12.041. [DOI] [PubMed] [Google Scholar]
- Bosman LW, Rosahl TW, Brussaard AB. Neonatal development of the rat visual cortex: synaptic function of GABAA receptor alpha subunits. J Physiol. 2002;545(Pt 1):169–81. doi: 10.1113/jphysiol.2002.026534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braestrup C, Nielsen M, Honore T. Binding of [3H]DMCM, a convulsive benzodiazepine ligand, to rat brain membranes: preliminary studies. J Neurochem. 1983;41(2):454–65. doi: 10.1111/j.1471-4159.1983.tb04763.x. [DOI] [PubMed] [Google Scholar]
- Burianova J, Ouda L, Profant O, Syka J. Age-related changes in GAD levels in the central auditory system of the rat. Exp Gerontol. 2009;44(3):161–9. doi: 10.1016/j.exger.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Canlon B, Illing RB, Walton J. Cell biology and physiology of the aging central auditory pathway. In: Gordon-Salant S, Frisina RD, Popper AN, Fay RR, editors. The Aging Auditory System. Springer; New York, New York: 2010. pp. 39–74. [Google Scholar]
- Caspary DM, Holder TM, Hughes LF, Milbrandt JC, McKernan RM, Naritoku DK. Age-related changes in GABA(A) receptor subunit composition and function in rat auditory system. Neuroscience. 1999;93(1):307–12. doi: 10.1016/s0306-4522(99)00121-9. [DOI] [PubMed] [Google Scholar]
- Caspary DM, Ling L, Turner JG, Hughes LF. Inhibitory neurotransmission, plasticity and aging in the mammalian central auditory system. JExpBiol. 2008;211(Pt 11):1781–91. doi: 10.1242/jeb.013581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspary DM, Raza A, Lawhorn Armour BA, Pippin J, Arneric SP. Immunocytochemical and neurochemical evidence for age-related loss of GABA in the inferior colliculus: implications for neural presbycusis. J Neurosci. 1990;10(7):2363–72. doi: 10.1523/JNEUROSCI.10-07-02363.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspary DM, Schatteman TA, Hughes LF. Age-related changes in the inhibitory response properties of dorsal cochlear nucleus output neurons: role of inhibitory inputs. J Neurosci. 2005;25(47):10952–9. doi: 10.1523/JNEUROSCI.2451-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concas A, Pepitoni S, Atsoggiu T, Toffano G, Biggio G. Aging reduces the GABA-dependent 36Cl- flux in rat brain membrane vesicles. Life Sci. 1988;43(22):1761–71. doi: 10.1016/0024-3205(88)90275-5. [DOI] [PubMed] [Google Scholar]
- de Villers-Sidani E, Alzghoul L, Zhou X, Simpson KL, Lin RC, Merzenich MM. Recovery of functional and structural age-related changes in the rat primary auditory cortex with operant training. Proc Natl Acad Sci U S A. 2010;107(31):13900–5. doi: 10.1073/pnas.1007885107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubno JR, Dirks DD, Morgan DE. Effects of age and mild hearing loss on speech recognition in noise. J Acoust Soc Am. 1984;76(1):87–96. doi: 10.1121/1.391011. [DOI] [PubMed] [Google Scholar]
- Ducic I, Caruncho HJ, Zhu WJ, Vicini S, Costa E. gamma-Aminobutyric acid gating of Cl- channels in recombinant GABAA receptors. J Pharmacol Exp Ther. 1995;272(1):438–45. [PubMed] [Google Scholar]
- Ebert B, Wafford KA, Whiting PJ, Krogsgaard-Larsen P, Kemp JA. Molecular pharmacology of gamma-aminobutyric acid type A receptor agonists and partial agonists in oocytes injected with different alpha, beta, and gamma receptor subunit combinations. Mol Pharmacol. 1994;46(5):957–63. [PubMed] [Google Scholar]
- Erdo SL, Wolff JR. Age-related loss of t-[35S]butylbicyclophosphorothionate binding to the gamma-aminobutyric acidA receptor-coupled chloride ionophore in rat cerebral cortex. J Neurochem. 1989;53(2):648–51. doi: 10.1111/j.1471-4159.1989.tb07382.x. [DOI] [PubMed] [Google Scholar]
- Fitzgibbons PJ, Gordon-Salant S. Age effects on measures of auditory duration discrimination. J Speech Hear Res. 1994;37(3):662–70. doi: 10.1044/jshr.3703.662. [DOI] [PubMed] [Google Scholar]
- Fitzgibbons PJ, Gordon-Salant S. Age-related differences in discrimination of temporal intervals in accented tone sequences. Hear Res. 2010;264(1–2):41–7. doi: 10.1016/j.heares.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogerty D, Humes LE, Kewley-Port D. Auditory temporal-order processing of vowel sequences by young and elderly listeners. J Acoust Soc Am. 2010;127(4):2509–520. doi: 10.1121/1.3316291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Games KD, Winer JA. Layer V in rat auditory cortex: projections to the inferior colliculus and contralateral cortex. Hear Res. 1988;34(1):1–25. doi: 10.1016/0378-5955(88)90047-0. [DOI] [PubMed] [Google Scholar]
- Gutierrez A, Khan ZU, Miralles CP, Mehta AK, Ruano D, Araujo F, Vitorica J, De Blas AL. GABAA receptor subunit expression changes in the rat cerebellum and cerebral cortex during aging. Brain Res Mol Brain Res. 1997;45(1):59–70. doi: 10.1016/s0169-328x(96)00237-9. [DOI] [PubMed] [Google Scholar]
- Gutierrez A, Khan ZU, Ruano D, Miralles CP, Vitorica J, De Blas AL. Aging-related subunit expression changes of the GABAA receptor in the rat hippocampus. Neuroscience. 1996;74(2):341–8. doi: 10.1016/0306-4522(96)00137-6. [DOI] [PubMed] [Google Scholar]
- Hadingham KL, Garrett EM, Wafford KA, Bain C, Heavens RP, Sirinathsinghji DJ, Whiting PJ. Cloning of cDNAs encoding the human gamma-aminobutyric acid type A receptor alpha 6 subunit and characterization of the pharmacology of alpha 6-containing receptors. Mol Pharmacol. 1996;49(2):253–9. [PubMed] [Google Scholar]
- Hua T, Li X, He L, Zhou Y, Wang Y, Leventhal AG. Functional degradation of visual cortical cells in old cats. Neurobiol Aging. 2006;27(1):155–62. doi: 10.1016/j.neurobiolaging.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Hughes LF, Turner JG, Parrish JL, Caspary DM. Processing of broadband stimuli across A1 layers in young and aged rats. Hear Res. 2010;264(1–2):79–85. doi: 10.1016/j.heares.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im WB, Pregenzer JF, Thomsen DR. Effects of GABA and various allosteric ligands on TBPS binding to cloned rat GABA(A) receptor subtypes. Br J Pharmacol. 1994;112(4):1025–30. doi: 10.1111/j.1476-5381.1994.tb13185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juarez-Salinas DL, Engle JR, Navarro XO, Recanzone GH. Hierarchical and serial processing in the spatial auditory cortical pathway is degraded by natural aging. J Neurosci. 2010;30(44):14795–804. doi: 10.1523/JNEUROSCI.3393-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juttner R, Meier J, Grantyn R. Slow IPSC kinetics, low levels of alpha1 subunit expression and paired-pulse depression are distinct properties of neonatal inhibitory GABAergic synaptic connections in the mouse superior colliculus. Eur J Neurosci. 2001;13(11):2088–98. doi: 10.1046/j.0953-816x.2001.01587.x. [DOI] [PubMed] [Google Scholar]
- Khrestchatisky M, MacLennan AJ, Chiang MY, Xu WT, Jackson MB, Brecha N, Sternini C, Olsen RW, Tobin AJ. A novel alpha subunit in rat brain GABAA receptors. Neuron. 1989;3(6):745–53. doi: 10.1016/0896-6273(89)90243-2. [DOI] [PubMed] [Google Scholar]
- Knoflach F, Reinscheid RK, Civelli O, Kemp JA. Modulation of voltage-gated calcium channels by orphanin FQ in freshly dissociated hippocampal neurons. J Neurosci. 1996;16(21):6657–64. doi: 10.1523/JNEUROSCI.16-21-06657.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korpi ER, Grunder G, Luddens H. Drug interactions at GABA(A) receptors. Prog Neurobiol. 2002;67(2):113–59. doi: 10.1016/s0301-0082(02)00013-8. [DOI] [PubMed] [Google Scholar]
- Leventhal AG, Wang Y, Pu M, Zhou Y, Ma Y. GABA and its agonists improved visual cortical function in senescent monkeys. Science. 2003;300(5620):812–5. doi: 10.1126/science.1082874. [DOI] [PubMed] [Google Scholar]
- Liang J, Suryanarayanan A, Chandra D, Homanics GE, Olsen RW, Spigelman I. Functional consequences of GABAA receptor alpha 4 subunit deletion on synaptic and extrasynaptic currents in mouse dentate granule cells. Alcohol Clin Exp Res. 2008;32(1):19–26. doi: 10.1111/j.1530-0277.2007.00564.x. [DOI] [PubMed] [Google Scholar]
- Linden AM, Schmitt U, Leppa E, Wulff P, Wisden W, Luddens H, Korpi ER. Ro 15–4513 Antagonizes Alcohol-Induced Sedation in Mice Through alphabetagamma2-type GABA(A) Receptors. Front Neurosci. 2011;5:3. doi: 10.3389/fnins.2011.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling LL, Hughes LF, Caspary DM. Age-related loss of the GABA synthetic enzyme glutamic acid decarboxylase in rat primary auditory cortex. Neuroscience. 2005;132(4):1103–13. doi: 10.1016/j.neuroscience.2004.12.043. [DOI] [PubMed] [Google Scholar]
- Lingford-Hughes A, Hume SP, Feeney A, Hirani E, Osman S, Cunningham VJ, Pike VW, Brooks DJ, Nutt DJ. Imaging the GABA-benzodiazepine receptor subtype containing the alpha5-subunit in vivo with [11C]Ro15 4513 positron emission tomography. J Cereb Blood Flow Metab. 2002;22(7):878–89. doi: 10.1097/00004647-200207000-00013. [DOI] [PubMed] [Google Scholar]
- Lloyd GK, Danielou G, Thuret F. The activity of zolpidem and other hypnotics within the gamma-aminobutyric acid (GABAA) receptor supramolecular complex, as determined by 35S-t-butylbicyclophosphorothionate (35S-TBPS) binding to rat cerebral cortex membranes. J Pharmacol Exp Ther. 1990;255(2):690–6. [PubMed] [Google Scholar]
- Luddens H, Wisden W. Function and pharmacology of multiple GABAA receptor subunits. Trends Pharmacol Sci. 1991;12(2):49–51. doi: 10.1016/0165-6147(91)90495-e. [DOI] [PubMed] [Google Scholar]
- Lui B, Mendelson JR. Frequency modulated sweep responses in the medial geniculate nucleus. ExpBrain Res. 2003;153(4):550–3. doi: 10.1007/s00221-003-1618-y. [DOI] [PubMed] [Google Scholar]
- Macdonald RL, Olsen RW. GABAA receptor channels. Annu Rev Neurosci. 1994;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- Maksay G, Ticku MK. GABA, depressants and chloride ions affect the rate of dissociation of 35S-t-butylbicyclophosphorothionate binding. Life Sci. 1985;37(23):2173–80. doi: 10.1016/0024-3205(85)90568-5. [DOI] [PubMed] [Google Scholar]
- Malherbe P, Sigel E, Baur R, Persohn E, Richards JG, Mohler H. Functional expression and sites of gene transcription of a novel alpha subunit of the GABAA receptor in rat brain. FEBS Lett. 1990;260(2):261–5. doi: 10.1016/0014-5793(90)80118-3. [DOI] [PubMed] [Google Scholar]
- Martin Del Campo HN, Measor KR, Razak KA. Parvalbumin immunoreactivity in the auditory cortex of a mouse model of presbycusis. Hear Res. 2012;294(1–2):31–9. doi: 10.1016/j.heares.2012.08.017. [DOI] [PubMed] [Google Scholar]
- McGeer EG, McGeer PL. Aging and neurotransmitter systems. Adv Biochem Psychopharmacol. 1980;23:305–14. [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. Enzymes associated with the metabolism of catecholamines, acetylcholine and gaba in human controls and patients with Parkinson’s disease and Huntington’s chorea. J Neurochem. 1976;26(1):65–76. [PubMed] [Google Scholar]
- Mendelson JR, Rajan R. Cortical Effects of Aging and Hearing Loss. In: Winer JA, Schreiner CE, editors. The Auditory Cortex. Spinger; New York: 2011. pp. 493–511. [Google Scholar]
- Mhatre MC, Ticku MK. Aging related alterations in GABAA receptor subunit mRNA levels in Fischer rats. Brain Res Mol Brain Res. 1992;14(1–2):71–8. doi: 10.1016/0169-328x(92)90012-z. [DOI] [PubMed] [Google Scholar]
- Milbrandt JC, Albin RL, Caspary DM. Age-related decrease in GABAB receptor binding in the Fischer 344 rat inferior colliculus. Neurobiol Aging. 1994;15(6):699–703. doi: 10.1016/0197-4580(94)90051-5. [DOI] [PubMed] [Google Scholar]
- Milbrandt JC, Albin RL, Turgeon SM, Caspary DM. GABAA receptor binding in the aging rat inferior colliculus. Neuroscience. 1996;73(2):449–58. doi: 10.1016/0306-4522(96)00050-4. [DOI] [PubMed] [Google Scholar]
- Milbrandt JC, Caspary DM. Age-related reduction of [3H]strychnine binding sites in the cochlear nucleus of the Fischer 344 rat. Neuroscience. 1995;67(3):713–9. doi: 10.1016/0306-4522(95)00082-t. [DOI] [PubMed] [Google Scholar]
- Milbrandt JC, Hunter C, Caspary DM. Alterations of GABAA receptor subunit mRNA levels in the aging Fischer 344 rat inferior colliculus. J Comp Neurol. 1997;379(3):455–65. doi: 10.1002/(sici)1096-9861(19970317)379:3<455::aid-cne10>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Niddam R, Dubois A, Scatton B, Arbilla S, Langer SZ. Autoradiographic localization of [3H]zolpidem binding sites in the rat CNS: comparison with the distribution of [3H]flunitrazepam binding sites. J Neurochem. 1987;49(3):890–9. doi: 10.1111/j.1471-4159.1987.tb00977.x. [DOI] [PubMed] [Google Scholar]
- Noreña AJ. An integrative model of tinnitus based on a central gain controlling neural sensitivity. Neurosci Biobehav Rev. 2011;35(5):1089–109. doi: 10.1016/j.neubiorev.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Oliver DL, Izquierdo MA, Malmierca MS. Persistent effects of early augmented acoustic environment on the auditory brainstem. Neuroscience. 2011;184:75–87. doi: 10.1016/j.neuroscience.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RW, Bureau M, Khrestchatisky M, MacLennan AJ, Chiang MY, Tobin AJ, Xu W, Jackson M, Sternini C, Brecha N. Isolation of pharmacologically distinct GABA-benzodiazepine receptors by protein chemistry and molecular cloning. Adv Biochem Psychopharmacol. 1990;46:35–49. [PubMed] [Google Scholar]
- Olsen RW, Sieghart W. International Union of Pharmacology. LXX. Subtypes of gamma-aminobutyric acid(A) receptors: classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacol Rev. 2008;60(3):243–60. doi: 10.1124/pr.108.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W. GABA A receptors: subtypes provide diversity of function and pharmacology. Neuropharmacology. 2009;56(1):141–8. doi: 10.1016/j.neuropharm.2008.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostroff JM, McDonald KL, Schneider BA, Alain C. Aging and the processing of sound duration in human auditory cortex. Hear Res. 2003;181(1–2):1–7. doi: 10.1016/s0378-5955(03)00113-8. [DOI] [PubMed] [Google Scholar]
- Ouda L, Druga R, Syka J. Changes in parvalbumin immunoreactivity with aging in the central auditory system of the rat. Exp Gerontol. 2008;43(8):782–9. doi: 10.1016/j.exger.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Pan HS, Frey KA, Young AB, Penney JB., Jr Changes in [3H]muscimol binding in substantia nigra, entopeduncular nucleus, globus pallidus, and thalamus after striatal lesions as demonstrated by quantitative receptor autoradiography. Journal of Neuroscience. 1983;3(6):1189–98. doi: 10.1523/JNEUROSCI.03-06-01189.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos W, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; San Diego: 1998. [Google Scholar]
- Peters A, Feldman ML, Vaughan DW. The effect of aging on the neuronal population within area 17 of adult rat cerebral cortex. Neurobiol Aging. 1983;4(4):273–82. doi: 10.1016/0197-4580(83)90003-9. [DOI] [PubMed] [Google Scholar]
- Pichora-Fuller MK, Schneider BA, Macdonald E, Pass HE, Brown S. Temporal jitter disrupts speech intelligibility: a simulation of auditory aging. HearRes. 2007;223(1–2):114–21. doi: 10.1016/j.heares.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Pinto JG, Hornby KR, Jones DG, Murphy KM. Developmental changes in GABAergic mechanisms in human visual cortex across the lifespan. Front Cell Neurosci. 2010;4(16):1–12. doi: 10.3389/fncel.2010.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABA(A) receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101(4):815–50. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- Prieto JJ, Peterson BA, Winer JA. Laminar distribution and neuronal targets of GABAergic axon terminals in cat primary auditory cortex (AI) J Comp Neurol. 1994;344(3):383–402. doi: 10.1002/cne.903440305. [DOI] [PubMed] [Google Scholar]
- Pritchett DB, Seeburg PH. Gamma-aminobutyric acidA receptor alpha 5-subunit creates novel type II benzodiazepine receptor pharmacology. J Neurochem. 1990;54(5):1802–4. doi: 10.1111/j.1471-4159.1990.tb01237.x. [DOI] [PubMed] [Google Scholar]
- Rabow LE, Russek SJ, Farb DH. From ion currents to genomic analysis: recent advances in GABAA receptor research. Synapse. 1995;21(3):189–274. doi: 10.1002/syn.890210302. [DOI] [PubMed] [Google Scholar]
- Richardson BD, Brozoski TJ, Ling LL, Caspary DM. Targeting inhibitory neurotransmission in tinnitus. Brain Res. 2012 doi: 10.1016/j.brainres.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson BD, Ling LL, Uteshev VV, Caspary DM. Extrasynaptic GABA(A) receptors and tonic inhibition in rat auditory thalamus. PLoS ONE. 2011;6(1):e16508. doi: 10.1371/journal.pone.0016508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissman RA, De Blas AL, Armstrong DM. GABA(A) receptors in aging and Alzheimer’s disease. J Neurochem. 2007;103(4):1285–92. doi: 10.1111/j.1471-4159.2007.04832.x. [DOI] [PubMed] [Google Scholar]
- Ruano D, Machado A, Vitorica J. Absence of modifications of the pharmacological properties of the GABAA receptor complex during aging, as assessed in 3- and 24-month-old rat cerebral cortex. Eur J Pharmacol. 1993;246(1):81–7. doi: 10.1016/0922-4106(93)90013-y. [DOI] [PubMed] [Google Scholar]
- Ruano D, Vizuete M, Cano J, Machado A, Vitorica J. Heterogeneity in the allosteric interaction between the gamma-aminobutyric acid (GABA) binding site and three different benzodiazepine binding sites of the GABAA/benzodiazepine receptor complex in the rat nervous system. J Neurochem. 1992;58(2):485–93. doi: 10.1111/j.1471-4159.1992.tb09747.x. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Crestani F, Mohler H. GABA(A) receptor subtypes: dissecting their pharmacological functions. Trends Pharmacol Sci. 2001;22(4):188–94. doi: 10.1016/s0165-6147(00)01646-1. [DOI] [PubMed] [Google Scholar]
- Sakurai SY, Kume A, Burdette DE, Albin RL. Quantitative autoradiography of [3H]t-butylbicycloorthobenzoate binding to the gamma-aminobutyric acid receptorA complex. J Pharmacol Exp Ther. 1994;270(1):362–70. [PubMed] [Google Scholar]
- Schmidt S, Redecker C, Bruehl C, Witte OW. Age-related decline of functional inhibition in rat cortex. Neurobiol Aging. 2010;31(3):504–11. doi: 10.1016/j.neurobiolaging.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Schmolesky MT, Wang Y, Creel DJ, Leventhal AG. Abnormal retinotopic organization of the dorsal lateral geniculate nucleus of the tyrosinase-negative albino cat. J Comp Neurol. 2000;427(2):209–19. doi: 10.1002/1096-9861(20001113)427:2<209::aid-cne4>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Schneider BA, Pichora-Fuller MK, Kowalchuk D, Lamb M. Gap detection and the precedence effect in young and old adults. J Acoust Soc Am. 1994;95(2):980–91. doi: 10.1121/1.408403. [DOI] [PubMed] [Google Scholar]
- Sieghart W. GABAA receptors: ligand-gated Cl- ion channels modulated by multiple drug-binding sites. Trends Pharmacol Sci. 1992a;13(12):446–50. doi: 10.1016/0165-6147(92)90142-s. [DOI] [PubMed] [Google Scholar]
- Sieghart W. Heterogeneity of GABAA receptors. Clin Neuropharmacol. 1992b;15(Suppl 1 Pt A):681A–2A. doi: 10.1097/00002826-199201001-00352. [DOI] [PubMed] [Google Scholar]
- Sieghart W. Molecular basis of pharmacological heterogeneity of GABAA receptors. Cell Signal. 1992c;4(3):231–7. doi: 10.1016/0898-6568(92)90062-d. [DOI] [PubMed] [Google Scholar]
- Sieghart W. Pharmacology of benzodiazepine receptors: an update. Clin Neuropharmacol. 1992d;15(Suppl 1 Pt A):523A–4A. doi: 10.1097/00002826-199201001-00272. [DOI] [PubMed] [Google Scholar]
- Sieghart W. Structure and pharmacology of gamma-aminobutyric acidA receptor subtypes. Pharmacol Rev. 1995;47(2):181–234. [PubMed] [Google Scholar]
- Sieghart W, Fuchs K, Zezula J, Buchstaller A, Zimprich F, Lassmann H. Biochemical, immunological, and pharmacological characterization of GABAA-benzodiazepine receptor subtypes. Adv Biochem Psychopharmacol. 1992;47:155–62. [PubMed] [Google Scholar]
- Sigel E, Baur R, Trube G, Mohler H, Malherbe P. The effect of subunit composition of rat brain GABAA receptors on channel function. Neuron. 1990;5(5):703–11. doi: 10.1016/0896-6273(90)90224-4. [DOI] [PubMed] [Google Scholar]
- Snell KB. Age-related changes in temporal gap detection. JAcoustSocAm. 1997;101(4):2214–20. doi: 10.1121/1.418205. [DOI] [PubMed] [Google Scholar]
- Strouse A, Ashmead DH, Ohde RN, Grantham DW. Temporal processing in the aging auditory system. JAcoustSocAm. 1998;104(4):2385–99. doi: 10.1121/1.423748. [DOI] [PubMed] [Google Scholar]
- Suta D, Rybalko N, Pelanova J, Popelar J, Syka J. Age-related changes in auditory temporal processing in the rat. Exp Gerontol. 2011;46(9):739–46. doi: 10.1016/j.exger.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Syka J. Plastic changes in the central auditory system after hearing loss, restoration of function, and during learning. Physiol Rev. 2002;82(3):601–36. doi: 10.1152/physrev.00002.2002. [DOI] [PubMed] [Google Scholar]
- Takesian AE, Kotak VC, Sanes DH. Age-dependent effect of hearing loss on cortical inhibitory synapse function. J Neurophysiol. 2012;107(3):937–47. doi: 10.1152/jn.00515.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay KL, Piskosz M, Souza P. Aging alters the neural representation of speech cues. Neuroreport. 2002;13(15):1865–70. doi: 10.1097/00001756-200210280-00007. [DOI] [PubMed] [Google Scholar]
- Tremblay KL, Piskosz M, Souza P. Effects of age and age-related hearing loss on the neural representation of speech cues. ClinNeurophysiol. 2003;114(7):1332–43. doi: 10.1016/s1388-2457(03)00114-7. [DOI] [PubMed] [Google Scholar]
- Turner JG, Caspary DM. Comparison of Two Rat Models of Aging. In: Syka J, Merzenich MM, editors. Plasticity and Signal Representation in the Auditory System. Springer US; New York: 2005. pp. 217–25. [Google Scholar]
- Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci. 2004;5(2):97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- Wafford KA, Bain CJ, Whiting PJ, Kemp JA. Functional comparison of the role of gamma subunits in recombinant human gamma-aminobutyric acidA/benzodiazepine receptors. Mol Pharmacol. 1993;44(2):437–42. [PubMed] [Google Scholar]
- Wafford KA, Ebert B. Gaboxadol--a new awakening in sleep. Curr Opin Pharmacol. 2006;6(1):30–6. doi: 10.1016/j.coph.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Wafford KA, Thompson SA, Thomas D, Sikela J, Wilcox AS, Whiting PJ. Functional characterization of human gamma-aminobutyric acidA receptors containing the alpha 4 subunit. Mol Pharmacol. 1996;50(3):670–8. [PubMed] [Google Scholar]
- Wang H, Brozoski TJ, Turner JG, Ling L, Parrish JL, Hughes LF, Caspary DM. Plasticity at glycinergic synapses in dorsal cochlear nucleus of rats with behavioral evidence of tinnitus. Neuroscience. 2009a;164(2):747–59. doi: 10.1016/j.neuroscience.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Turner JG, Ling L, Parrish JL, Hughes LF, Caspary DM. Age-related changes in glycine receptor subunit composition and binding in dorsal cochlear nucleus. Neuroscience. 2009b;160(1):227–39. doi: 10.1016/j.neuroscience.2009.01.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer JA. The functional architecture of the medial geniculate body and the primary auditory cortes. In: Webster DB, Popper AN, Fay RR, editors. The Mammalian Auditory Pathway: Neuroanatomy. Springer-Verlag; New York: 1992. pp. 222–409. [Google Scholar]
- Wisden W, Gundlach AL, Barnard EA, Seeburg PH, Hunt SP. Distribution of GABAA receptor subunit mRNAs in rat lumbar spinal cord. Brain Res Mol Brain Res. 1991;10(2):179–83. doi: 10.1016/0169-328x(91)90109-b. [DOI] [PubMed] [Google Scholar]
- Wisden W, Laurie DJ, Monyer H, Seeburg PH. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J Neurosci. 1992;12(3):1040–62. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ymer S, Draguhn A, Wisden W, Werner P, Keinanen K, Schofield PR, Sprengel R, Pritchett DB, Seeburg PH. Structural and functional characterization of the gamma 1 subunit of GABAA/benzodiazepine receptors. EMBO J. 1990;9(10):3261–7. doi: 10.1002/j.1460-2075.1990.tb07525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ymer S, Schofield PR, Draguhn A, Werner P, Kohler M, Seeburg PH. GABAA receptor beta subunit heterogeneity: functional expression of cloned cDNAs. EMBO J. 1989;8(6):1665–70. doi: 10.1002/j.1460-2075.1989.tb03557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu ZY, Wang W, Fritschy JM, Witte OW, Redecker C. Changes in neocortical and hippocampal GABAA receptor subunit distribution during brain maturation and aging. Brain Res. 2006;1099(1):73–81. doi: 10.1016/j.brainres.2006.04.118. [DOI] [PubMed] [Google Scholar]
- Zhou X, Panizzutti R, de Villers-Sidani E, Madeira C, Merzenich MM. Natural restoration of critical period plasticity in the juvenile and adult primary auditory cortex. J Neurosci. 2011;31(15):5625–34. doi: 10.1523/JNEUROSCI.6470-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]