Abstract
Melanoma-associated antigen-encoding (MAGE) genes are expressed in melanoma and other cancers but not in normal somatic cells. MAGE expression is associated with aggressive tumor growth, poor clinical outcome, and resistance to chemotherapy, but the mechanisms have not been completely elucidated. In this study, we show that downregulation of MAGE-C2 in A375 melanoma cells and low-passage cultures from human metastatic melanomas (MRA cells) results in increased apoptosis and decreased growth of tumor xenografts in athymic nude mice. Previously, we showed that MAGE-C2 binds KAP1, a scaffolding protein that regulates DNA repair. Phosphorylation of KAP1-Serine 824 (Ser824) by ataxia-telangiectasia–mutated (ATM) kinase is necessary for repair of DNA double-strand breaks (DSBs); now we show that MAGE-C2 knockdown reduces, whereas MAGE-C2 overexpression increases, ATM kinase–dependent phosphorylation of KAP1-Ser824. We demonstrate that MAGE-C2 increases co-precipitation of KAP1 with ATM and that binding of MAGE-C2 to KAP1 is necessary for increased KAP1-Ser824 phosphorylation. Furthermore, ectopic expression of MAGE-C2 enhances repair of I-SceI endonuclease–induced DSBs in U-2OS cells. As phosphorylation of KAP1-Ser824 facilitates relaxation of heterochromatin, which is necessary for DNA repair and cellular proliferation, our results suggest that MAGE-C2 can promote tumor growth by phosphorylation of KAP1-Ser824 and by enhancement of DNA damage repair.
INTRODUCTION
Melanoma is the most deadly form of skin cancer, accounting for <5% of skin cancer cases but for more than 75% of skin cancer deaths. Unlike other common types of cancers, the incidence of melanoma continues to rise and the American Cancer Society estimates that ~114,900 new cases of melanoma were diagnosed in the United States in 2010— 46,770 noninvasive (in situ) and 68,l30 invasive—with nearly 8,700 resulting in death (Surveillance, Epidemiology, and End Results Program and the National Center for Health Statistics, http://www.skincancer.org/Skin-Cancer-Facts/#melanoma).
The first melanoma-associated antigen-encoding (MAGE) gene was identified in melanoma in 1991 as a gene encoding tumor-specific antigens recognized by cytolytic T lymphocytes (Chomez et al., 2001). MAGE proteins have been classified into two subfamilies: MAGE-I and MAGE-II. The MAGE-II family members are ubiquitously expressed in somatic tissue. The MAGE-I family members (collectively called MAGE hereon) are encoded by 28 genes clustered on the X chromosome (Chomez et al., 2001). MAGE proteins are normally expressed only in developing sperm, trophoblasts, fetal ovarian germ cells, and placenta (Takahashi et al., 1995; Jungbluth et al., 2002; Rajpert-De Meyts et al., 2003; Yakirevich et al., 2003; Gaskell et al., 2004; Pauls et al., 2006; Zhuang et al., 2006). MAGE I genes are unusual in that their expression is normally suppressed in all somatic tissues by hypermethylation of promoter region CpG islands (De Smet et al., 1999; Sigalotti et al., 2004). Genome-wide hypomethylation may occur in neoplastic transformation and often causes MAGE protein expression in tumor cells. Thus, MAGE proteins are peculiar for their absence in normal adult somatic tissues but are aberrantly expressed in various malignancies, including melanoma (Hofbauer et al., 1997; Basarab et al., 1999; Jungbluth et al., 2000, 2005; Park et al., 2002; Dhodapkar et al., 2003; Riker et al., 2008). Recently, MAGE expression has been identified as an independent prognostic variable associated with metastasis and extent of relapse-free survival, comparable to Breslow thickness and ulceration in melanoma (Svobodova et al., 2011).
KRAB domain–associated protein 1 (KAP1), also known as TIF1β (transcriptional intermediary factor 1), TRIM28, or Krip1, is a universal corepressor protein and acts as a molecular scaffold (Iyengar and Farnham, 2011). KAP1 induces chromatin condensation and histone modification, resulting in repression of specific genes (Groner et al., 2010). KAP1 localizes to sites of DNA damage, in a sequence-independent manner, where it is critical for the DNA repair process (Goodarzi et al., 2009). Phosphorylation of KAP1-Serine 824 (Ser824) by ataxia-telangiectasia–mutated (ATM) kinase (Li et al., 2007) initiates and propagates chromatin relaxation necessary for the repair of damaged DNA (White et al., 2006; Goodarzi et al., 2008). In addition, KAP1 is increasingly being recognized as a critical multifunctional molecule (Monte et al., 2006; Yang et al., 2007; Liu et al., 2008; Atanackovic et al., 2010; Doyle et al., 2010; Nardiello et al., 2011). Recently, we and others have shown that MAGE proteins bind to KAP1, suppress p53 and apoptosis, and increase tumor survival in vivo (Yang et al., 2007; Doyle et al., 2010).
MAGE proteins can be considered specific cancer molecular biomarkers because of their selective expression in cancer cells and association with aggressive tumor growth, poor prognosis, and relapse. Although recent studies have sought to understand the role of MAGE proteins in cancer, the cellular and physiological functions of MAGE proteins have not been fully elucidated. In this study, we demonstrate that MAGE proteins are important factors in the survival and growth of melanoma cells. Here we establish that MAGE proteins induce ATM kinase–dependent phosphorylation of KAP1-Ser824 and promote DNA damage repair, establishing another mechanism by which Class I MAGE proteins promote tumor development and providing a rationale for combining anti-MAGE therapy with cytotoxic regimens, which to our knowledge has not been reported previously.
RESULTS
Downregulation of MAGE-C2 induces apoptosis in A375 human melanoma cells
To investigate the functions of MAGE-C2 in human melanoma cells, we transduced A375 cell line with tetracycline-responsive scrambled or MAGE-C2 short hairpin RNA (shRNA), also encoding red fluorescent protein (TurboRFP). Transduced A375 cells treated with doxycycline express RFP (Figure 1a). Decreased MAGE-C2 expression correlated well with RFP expression in A375 cells expressing MAGE-C2 shRNA. Cells transduced with MAGE-C2 shRNA and expressing RFP (red) exhibit only one-third the intensity of MAGE-C2 (green) compared with RFP-negative cells or scrambled shRNA-transduced RFP-positive cells (Figures 1a and b; data not shown). RFP-positive single-cell clones were selected and MAGE-C2 knockdown in these clones was confirmed by immunoblotting (Figure 1c). Downregulation of MAGE-C2 induces increased apoptosis in A375 cells (Figure 1d). Cells expressing MAGE-C2 shRNA show 7.58% apoptosis as compared with 1.1% apoptosis in scrambled shRNA cells.
Figure 1. Downregulation of melanoma-associated antigen-encoding (MAGE)-C2 induces apoptosis in A375 melanoma cells.
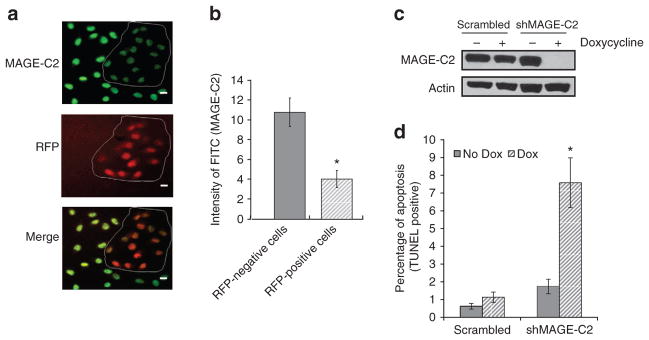
A375 cells were transduced with lentiviruses expressing tetracycline-responsive scrambled or MAGE-C2 short hairpin RNA (shRNA) and red fluorescent protein (RFP). Transduction efficiency was assessed by RFP expression and stained for MAGE-C2 (green). (a) A375 cells expressing MAGE-C2 shRNA before selection with puromycin were treated with 0.5 μgml−1 doxycycline for 72 hours and showed decreased MAGE-C2 expression (green) in RFP-positive cells. Bar = 50 μm. (b) A histogram of MAGE-C2 intensity in RFP-expressing versus non-expressing cells using the NIS Elements software, *P<0.05. (c) Single-cell clones were selected on the basis of RFP and MAGE-C2 expression. MAGE-C2 expression in a representative clone is shown by immunoblotting after 72 hours of 0.5 μgml−1 doxycycline treatment. (d) Downregulation of MAGE-C2 induces apoptosis in A375 cells as analyzed by TUNEL assay, *P<0.02.
Downregulation of MAGE-C2 induces apoptosis in low-passage human melanoma cultures
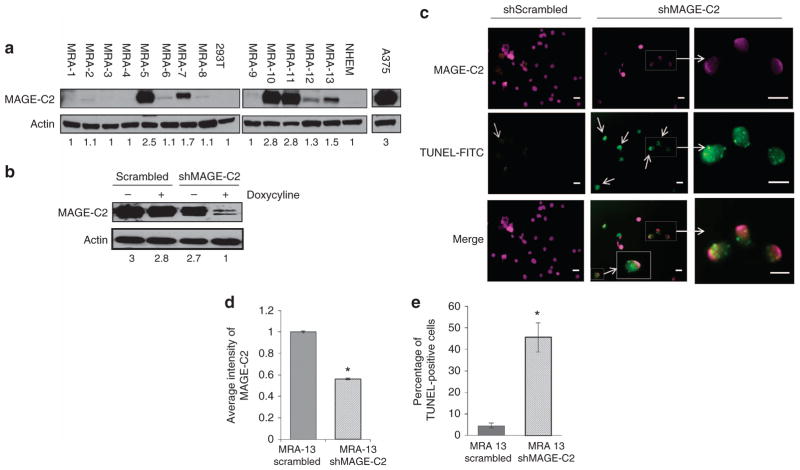
We next examined low-passage cells derived from human metastatic melanomas and found that 9 out of 13 (69%) short-term melanoma cultures derived from melanoma metastases (MRA-1–13) expressed MAGE-C2; MRA-5, -10, and -11 expressed high levels of MAGE-C2, MRA-7 and -13 expressed intermediate levels, and MRA-2, -6, -8, and -12 expressed low levels (Figure 2a), indicating that MAGE-C2 is commonly expressed in advanced melanomas. MRA-13 cells were transduced with lentiviruses encoding tetracycline-responsive scrambled or MAGE-C2 shRNA. MRA-13 cells expressing MAGE-C2 shRNA exhibit diminished expression of MAGE-C2 (Figure 2b). Knockdown of MAGE-C2 induced apoptosis in 40% of MRA-13 cells as shown by TUNEL stain (green) (Figure 2c–e). TUNEL and MAGE-C2 co-staining showed that most of the cells positive for TUNEL also had lower expression of MAGE-C2 (faint pseudocolor pink) (Figure 2c–e). Similar results were also observed in MRA-11 cells (data not shown). In accordance with our results in A375 melanoma cells, these data show that loss of MAGE-C2 induces apoptosis in MRA-13 melanoma cells, confirming that MAGE-C2 expression is associated with increased survival of melanoma cells.
Figure 2. Short-term cell cultures of metastatic melanomas express melanoma-associated antigen-encoding (MAGE)-C2, and downregulation of MAGE-C2 induces apoptosis.
(a) Immunoblotting detects variable expression of MAGE-C2 in 9 of 13 MRA cells, low-passage cultures from human melanoma metastases. (b) Immunoblotting shows that doxycycline-responsive MAGE-C2 short hairpin RNA (shRNA) downregulates MAGE-C2 in MRA-13 cells. (c) Photomicrographs show MAGE-C2 downregulation (faint pseudocolor pink) in MRA-13 cells is associated with apoptosis, as measured by TUNEL staining (green). Control shRNA cells show high MAGE expression and only rare apoptotic cells. Inset shows TUNEL-positive cells magnified × 60. The arrows point to TUNEL positive (apoptotic) cells. Bar = 50 μm. (d) MAGE-C2 expression was determined by quantitative immunofluorescence (pseudocolor pink). The histogram shows that MAGE-C2 expression is decreased in shMAGE-C2-expressing cells as opposed to cells expressing scrambled shRNA, confirming MAGE-C2 knockdown, *P<0.01. (e) The histogram compares TUNEL-positive (green) cells as a percentage of total 4′6-diamidino-2-phenylindole-positive cells in control (scrambled) and MAGE-C2 shRNA-expressing cells. The histogram shows that TUNEL staining in MRA-13 cells is increased with MAGE-C2 knockdown as compared with scrambled shRNA, *P<0.02.
MAGE-C2 knockdown decreases low-passage melanoma tumor xenograft growth in athymic nude mice
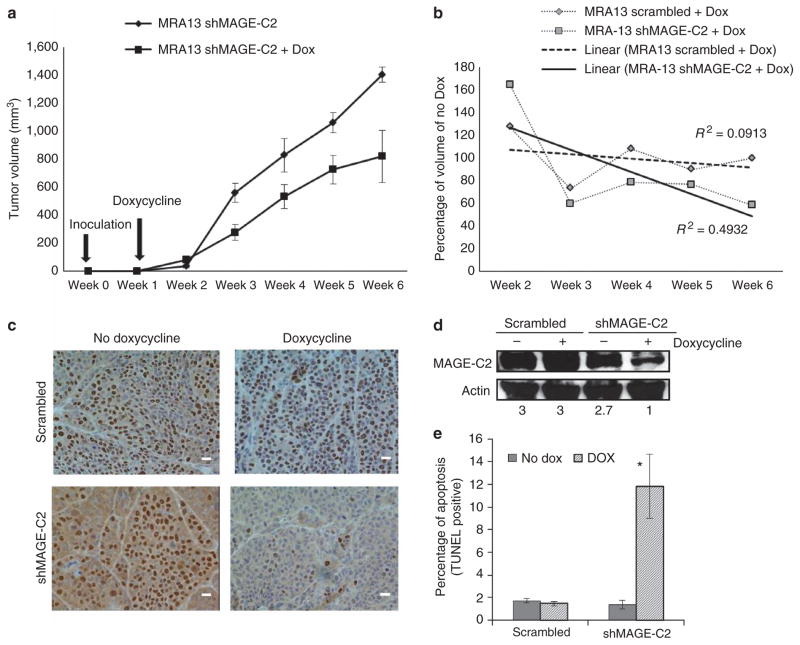
To determine the effect of the downregulation of MAGE-C2 in tumor xenografts, MRA-13 cells, transduced with lentiviruses encoding tetracycline-responsive scrambled or MAGE-C2 shRNA, were injected into athymic nude mice. One week after tumor inoculation, groups of mice (seven mice per group) were fed either water or doxycycline solution. Growth of MRA-13 MAGE-C2 shRNA tumors were stunted in the group of mice drinking doxycycline solution in water compared with mice on the doxycycline-free diet (Figure 3a). Tumor volume in the doxycycline treatment group was 50% lower at week 3, 2 weeks after initiation of doxycycline treatment, and 41% lower after 6 weeks, at the termination of the experiment (Figure 3a). Figure 3b compares the growth of xenografts expressing shMAGE-C2 or scrambled shRNA with their counterparts without doxycycline treatment. Linear trend lines for these experiments show a 49% decrease in MRA-13 MAGE-C2 shRNA tumor volume with doxycycline treatment (coefficient of determination R2 = 0.49) compared with a 9% decrease in MRA-13 scrambled shRNA tumor volume with doxycycline treatment (coefficient of determination R2 = 0.09). MAGE-C2 expression was assessed in MRA-13 tumors collected from all the groups by immunohistochemistry and immunoblotting. Figure 3c and d confirms the diminished expression of MAGE-C2 in MRA-13 tumor tissue collected from the MAGE-C2 shRNA group being fed doxycycline. No difference was observed in MAGE-C2 expression in MRA-13 tumors collected from scrambled shRNA groups with or without doxycycline. Similar results were observed with tumor xenografts of MRA-11 cells (data not shown). TUNEL staining in xenograft tumors showed increased apoptosis in tumors with MAGE-C2 knockdown, as was seen with in vitro cultures (Figure 3e). These results demonstrate and corroborate our in vitro results that MAGE-C2 promotes survival of melanoma cells both in vitro and in vivo.
Figure 3. Melanoma-associated antigen-encoding (MAGE)-C2 knockdown reduces growth of MRA-13 melanoma xenografts in athymic nude mice.
Four-week-old athymic nude mice, seven mice per group, were injected with MRA-13 cells expressing scrambled or MAGE-C2 short hairpin RNA (shRNA) and were given either water or 2mgml−1 doxycycline solution. The xenograft experiment was terminated 6 weeks later or when tumors reached a volume of 1,400 mm3. (a) Tumor growth decreased by 40% in MAGE-C2 shRNA mice treated with doxycycline (MAGE-C2 downregulation) compared with no doxycycline treatment. (b) Linear trend lines compare growth of xenografts expressing MAGE-C2 and scrambled shRNA with their counterparts without doxycycline treatment. MAGE-C2 shRNA tumors show a downward trend compared with scrambled shRNA tumors. (c) Immunoperoxidase staining shows that MAGE-C2 expression in xenograft tumors is decreased by doxycycline-dependent induction of MAGE-C2 shRNA. Bar = 50 μm. (d) Downregulation of MAGE-C2 is observed by immunoblotting in MAGE-C2 shRNA tumors after doxycycline treatment. (e) Apoptosis was detected in xenograft tumor sections by TUNEL assay. The histograms show increase in apoptosis in tumors with MAGE-C2 downregulation, *P<0.05.
MAGE-C2 expression increases and induces phosphorylation of KAP1
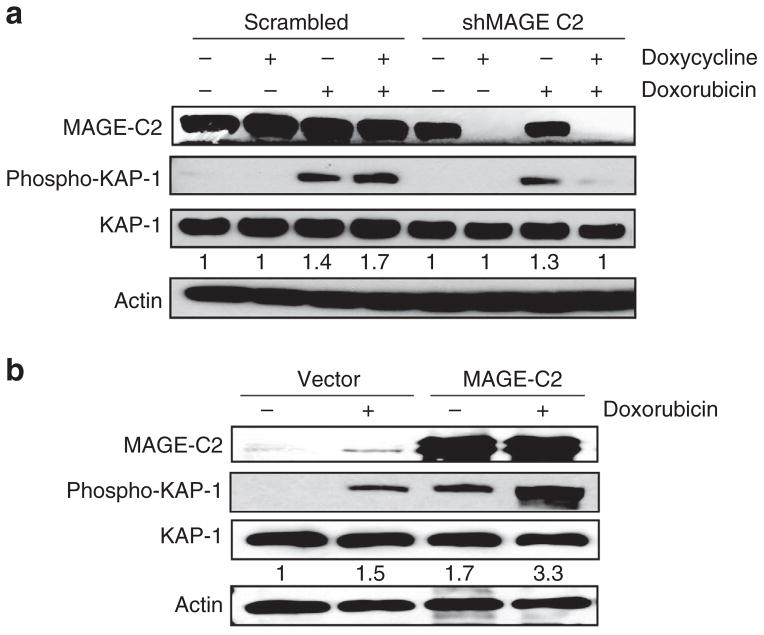
Phosphorylation of KAP1-Ser824 can be induced by DNA damage and is essential for DNA repair. To study the effect of MAGE-C2 downregulation on KAP1-Ser824 phosphorylation, A375 cells transduced with scrambled or MAGE-C2 shRNA were treated with doxycycline in conjunction with doxorubicin, a DNA-damaging agent. As demonstrated in Figure 4a, MAGE-C2 knockdown in A375 cells resulted in reduced phosphorylation of KAP1-Ser824. A375 cells expressing scrambled shRNA and treated with doxorubicin did not show any difference in levels of phospho-KAP1-Ser824. Immunoprecipitation with KAP1 antibody and immunoblotting for phospho-KAP1-Ser824 showed lower levels of phospho-KAP1-Ser824 in A375 cells with MAGE-C2 downregulation, even without doxorubicin-induced DNA damage (data not shown). Overall, our data suggest that inhibition of MAGE-C2 expression causes a reduction in the basal levels of KAP1-Ser824 phosphorylation and decreases KAP1-Ser824 phosphorylation in response to doxorubicin-induced DNA double-strand breaks (DSBs).
Figure 4. Melanoma-associated antigen-encoding (MAGE)-C2 induces phosphorylation of KAP1-Ser824.
A375 cells expressing scrambled or MAGE-C2 short hairpin RNA (shRNA) were treated with doxycyclin and DNA-damaging agent, doxorubicin. HEK293T cells were transfected to ectopically express MAGE-C2 and treated with doxorubicin. (a) Immunoblotting shows that doxorubicin-induced phosphorylation of KAP1-Ser824 is suppressed by MAGE-C2 knockdown in A375 cells expressing MAGE-C2 shRNA and treated with doxycycline. (b) In HEK293T cells, overexpression of MAGE-C2 induces KAP1-Ser824 phosphorylation and also enhances doxorubicin-induced phosphor-KAP1-Ser824.
To further validate the effect of MAGE-C2 expression on phosphorylation of KAP1-Ser824, MAGE-C2 was ectopically expressed in HEK293T cells. In accordance with the results of MAGE-C2 knockdown in A375 cells, overexpression of MAGE-C2 induced phosphorylation of KAP1-Ser824, whereas no effect was observed with an empty vector (Figure 4b). As shown in Figure 4b, MAGE-C2 expression also enhanced doxorubicin-induced phosphorylation of KAP1-Ser824. These results demonstrate that MAGE-C2 expression induces phosphorylation of KAP1-Ser824 and also enhances DNA damage–induced phosphorylation of KAP1-Ser824. As phosphorylation of KAP1-Ser824 is required for DNA damage repair, these results further suggest that MAGE-C2 expression may promote DNA repair.
MAGE-C2-induced KAP1 phosphorylation is ATM kinase–dependent
ATM kinase acts as a key regulator of the DNA repair cascade (Roos and Kaina, 2006; Shanbhag et al., 2010). In response to DNA damage, ATM is activated and autophosphorylated on Ser1981 and initiates a cascade of phosphorylation events, including p53, leading to DNA repair or apoptosis (Goodarzi et al., 2008). Similar to p53 phosphorylation, ATM-induced phosphorylation of KAP1-Ser824 is an early event in DNA repair, inducing relaxation of heterochromatin, which is necessary for access to sites of DNA breaks and critical for progression of the DNA damage repair response (White et al., 2006; Goodarzi et al., 2009; Smeenk and Lohrum, 2010).
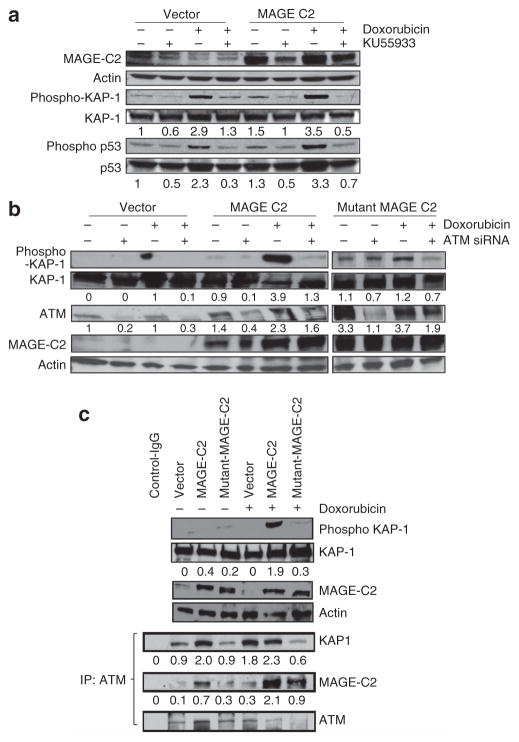
To determine whether MAGE-C2-induced phosphorylation of KAP1-Ser824 is dependent on ATM kinase, we inhibited ATM kinase activity in HEK293T cells expressing ectopic MAGE-C2. For inhibition of ATM kinase activity, we used KU55933 (2-(4-morpholinyl)-6-(1-thianthrenyl)-4H-pyran-4-one), a potent and selective inhibitor of ATM kinase. As exhibited in Figure 5a, treatment with KU55933 reduces MAGE-C2- and doxorubicin-induced phosphorylation of KAP1-Ser824, whereas KAP1 expression remains constant. To confirm inhibition of ATM kinase activity by KU55933, we probed for phospho-p53-Ser15, another substrate of ATM kinase. Figure 5a confirms inhibition of p53 phosphorylation at Ser15 in response to KU55933, validating the inhibition of ATM kinase activity. No change in p53 expression was observed. These results demonstrate that MAGE-C2-induced phosphorylation of KAP1-Ser824 is ATM kinase–dependent.
Figure 5. Melanoma-associated antigen-encoding (MAGE)-C2 binds to KAP-1 and induces ataxia-telangiectasia–mutated (ATM) kinase–dependent phosphorylation of KAP1-Ser824.
(a) HEK293T cells, ectopically expressing MAGE-C2, were treated with doxorubicin and KU55933, a specific ATM kinase inhibitor. MAGE-C2-induced phosphorylation of KAP1 was blocked by the ATM kinase inhibitor KU55933. KU55933 treatment also causes reduction in phosphorylation of p53, another substrate of ATM kinase, which confirms KU55933 activity. (b) HEK293T cells, with ectopic expression of wild-type MAGE-C2 or mutant MAGE-C2L152A L153A (unable to bind KAP1), were transfected with ATM kinase small interfering RNA (siRNA) and treated with doxorubicin. Immunoblotting shows that ATM siRNA inhibits MAGE-C2-induced as well as DNA damage–induced phosphorylation of KAP1-Ser824, confirming the effect of KU55933. (c) Immunoblotting shows that wild-type MAGE-C2, but not mutant MAGE-C2L152A L153A, increases KAP1-Ser824 phosphorylation, in response to doxorubicin. Wild-type MAGE-C2, in the presence of doxorubicin treatment, increases co-precipitation of ATM kinase and KAP-1. Unable to bind to KAP-1, mutant MAGE-C2L152A L153A has no effect on ATM and KAP-1 co-precipitation. IP, immunoprecipitation.
To confirm the effect of ATM kinase on MAGE-induced phosphorylation of KAP1-Ser824, we inhibited ATM kinase by specific small interfering RNA (siRNA). HEK293T cells, ectopically expressing empty vector or MAGE-C2, were transfected with ATM kinase siRNA and treated with doxorubicin. Figure 5b shows that siRNA-mediated inhibition of ATM kinase results in loss of MAGE-C2- and doxorubicin-induced phospho-KAP1-Ser824. These results confirm our results with ATM kinase inhibitor KU55933.
Interaction of MAGE-C2 and KAP1 increases ATM kinase–dependent phosphorylation of KAP1-Ser824
To determine whether MAGE-C2 effects on KAP1-Ser824 phosphorylation require direct interaction between MAGE-C2 and KAP1, we used mutant MAGE-C2 (MAGE-C2L152A L153A), which does not bind to KAP1 (Doyle et al., 2010). Figure 5b shows that, in response to MAGE-C2L152A L153A and in the presence of doxorubicin, increase in KAP1-Ser824 phosphorylation is comparable to the vector plasmid, which also has been confirmed in Figure 5c. These results indicate that direct binding of MAGE-C2 to KAP1 is required for this effect. We found that similar experiments using a deletion mutant KAP1, which lacks the RBCC region necessary for MAGE binding, were not interpretable because RBCC-deleted KAP1 was spontaneously and strongly phosphorylated on Ser824, regardless of the presence of MAGE-C2 or doxorubicin. As the MAGE-binding region and Ser824 are in different domains of KAP1, this result suggests that the RBCC region has an autoinhibitory effect on KAP1-Ser824 phosphorylation that can be relieved by deletion of the RBCC region or by binding of MAGE-C2.
Finally, to determine whether in the presence of MAGE-C2 KAP1 can form complexes with ATM kinase, we expressed plasmid-encoded FLAG-tagged KAP1 and MAGE-C2 in HEK293T cells and immunoprecipitated protein lysates with ATM kinase antibody. Immunoblotting with anti-FLAG showed that both KAP1 and MAGE-C2 immunoprecipitated with ATM, in the presence of doxorubicin, indicating that KAP1 and ATM can form complexes in the presence of MAGE-C2 (Figure 5c). Taken together, these data show that, in the presence of MAGE-C2, KAP1 complexes with ATM, enhancing ATM-dependent phosphorylation of KAP1-Ser824, a critical step in the cellular response to DNA DSBs.
MAGE-C2 expression promotes DNA repair
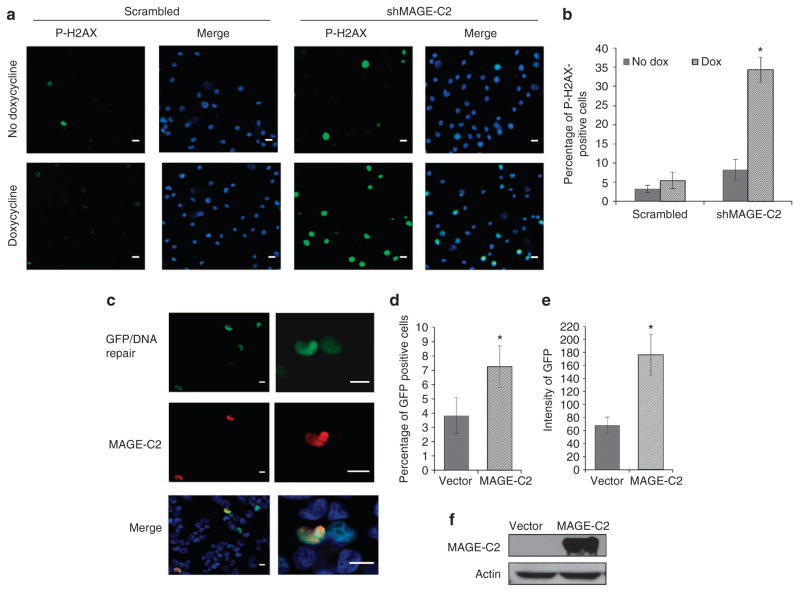
DNA damage results in rapid phosphorylation of H2A.X at Ser139. To assess the effect of MAGE-C2 on DNA damage markers, A375 cells expressing scrambled or MAGE-C2 shRNA were treated with doxycycline for 96 hours and stained for phospho histone H2A.X-Ser139. Photomicrographs in Figure 6a demonstrate an increase in phospho-H2A.X in A375 cells with downregulation of MAGE-C2. A375 cells expressing shMAGE-C2 and treated with doxycycline show 34% cells positive for phospho-H2A.X-Ser 139 as compared with 5% in cells expressing scrambled shRNA (Figure 6b).
Figure 6. MAGE-C2 downregulation induces DNA damage, whereas melanoma-associated antigen-encoding (MAGE)-C2 expression induces DNA damage repair.
(a) A375 melanoma cells expressing scrambled or MAGE-C2 short hairpin RNA (shRNA) and treated with doxycycline were stained for phospho histone H2A.X-Ser139, a marker for DNA damage. Fluorescent photomicrographs show increased expression of phospho histone H2A.X-Ser139 in A375 cells with MAGE-C2 downregulation. Bar = 50 μm. (b) Histograms show quantitative analysis of phospho Histone H2A.X-Ser139-positive cells, demonstrating increased expression of phospho Histone H2A.X-Ser139 in A375 cells with downregulation of MAGE-C2. Modified U-2 OS cells were transfected to ectopically express MAGE-C2 and I-Sce I endonuclease. Cells were stained for MAGE-C2 expression, and green fluorescent protein (GFP) was assessed, *P<0.02. (c) DNA repair, indicated by GFP expression, is highest in MAGE-positive cells (red pseudo color). Bar = 50 μm. (d) Histogram shows that the percentage of cells undergoing DNA repair (GFP positive) is higher in MAGE-positive cells, *P<0.05. (e) The intensity of GFP (DNA repair) is also higher in MAGE-positive cells, *P<0.05. (f) Immunoblotting confirms the expression of MAGE-C2 in transfected U-2OS cells.
To assess DNA repair in response to DNA damage, we used an assay that utilizes green fluorescent protein (GFP) as a reporter system and I-Sce I endonuclease to introduce defined DNA DSBs in U-2 OS cells (Pierce et al., 1999). An 18-bp I-Sce I site and linker was inserted within a copy of the GFP gene, inactivating it. When cleaved, a homologous repair event with a linked donor GFP gene fragment restores functional GFP expression, causing cells to fluoresce green. Figure 6c depicts co-expression of MAGE-C2 and GFP and also shows graphically that GFP expression is higher in MAGE-C2-positive cells compared with MAGE-C2-negative cells. As demonstrated in Figure 6d, 89% more cells undergo DNA repair in the presence of ectopically expressed MAGE-C2 than do cells expressing empty vector. Figure 6e validates the expression of MAGE-C2 in U-2 OS cells by immunoblotting. These data demonstrate that MAGE-C2 expression promotes repair of DNA DSBs.
DISCUSSION
MAGE proteins are expressed in many types of human tumors including at least 50% of advanced malignant melanomas (Barker and Salehi, 2002). Although normal melanocytes and most melanocytic nevi do not express MAGE proteins, MAGE expression in melanomas is associated with advanced and aggressive growth (Hofbauer et al., 1997; Basarab et al., 1999; Riker et al., 2008). Gene expression profiling shows an increase in MAGE-A3/6 expression over the entire range of melanoma thickness, a factor that correlates with poorer prognosis (Riker et al., 2008). MAGE proteins are important prognostic factors and have been associated with metastases, poor tumor differentiation, and clinical relapse in melanoma; however, the mechanisms behind these associations have not been completely determined (Hoek et al., 2004; Bellati et al., 2007; Svobodova et al., 2011).
The work presented here confirms the effects of MAGE-C2 on the viability of A375 cells, a widely used established cell line, and with low-passage human melanoma cells from metastases, using both in vitro and in vivo approaches. Here, our work with doxycycline-inducible shRNA encoded in integrated lentiviruses shows that established human melanoma xenografts can respond to MAGE-C2 knockdown triggered by a systemically administered agent.
We and others have previously reported that MAGE proteins promote the growth and survival of tumor cells, at least in part, by suppressing p53 and apoptosis (Monte et al., 2006; Yang et al., 2006, 2007; Liu et al., 2008). More recently, Doyle et al. (2010) confirmed and extended our results, showing that MAGE proteins bind to KAP1 and enhance the E3 ubiquitin ligase activity of KAP1, causing p53 polyubiquitination and degradation. Most recently, we have shown that MAGE proteins are master regulators of KRAB domain containing zinc finger transcription factors (KZFs), the largest group of transcription factors in vertebrates (Xiao et al., 2011). KZFs require KAP1 for gene repression and their targets include many oncogenes and tumor suppressors.
KAP1 is critical for DNA DSB repair. In response to DNA damage, ATM kinase is activated and phosphorylates KAP1-Ser824. Phosphorylated KAP-Ser824 localizes to DNA damage foci and causes chromatin relaxation required for access by DNA repair proteins. Substitution of mutated KAP1S824A completely aborts the repair response and renders cells hypersensitive to DNA damage (White et al., 2006; Goodarzi et al., 2008). After the DNA is repaired, KAP1-Ser824 is dephosphorylated, causing chromatin condensation.
We report here a function of MAGE, which to our knowledge has not been reported previously, that induces ATM kinase–dependent phosphorylation of KAP1-Ser824 and actively promotes DNA repair. Previous studies and our current data support an emerging model in which Class I MAGE expression contributes to tumor growth and survival by favoring DNA repair over apoptosis. As proliferation by neoplastic cells can by itself induce DNA damage, MAGE expression may provide a growth advantage in accordance with clinical observations that MAGE expression is associated with tumor progression and aggressive growth (Svobodova et al., 2011). This concept also fits nicely with in vitro and in vivo observations that Class I MAGE expression is associated with resistance to chemotherapy. We also suggest that MAGE suppression, which will render tumor cells sensitive to DNA damage, in combination with DNA-damaging agents may enhance tumor response to therapy.
MATERIALS AND METHODS
Tissue culture
A375 melanoma cells and Human Embryonic Kidney 293T (HEK293T) cells were obtained from ATCC (Manassas, VA). MRA melanoma cells, low-passage cultures derived from metastatic melanoma patients, were provided by Dr Mark R. Albertini. Cells were propagated in DMEM medium supplemented with 10% tet-system-approved fetal bovine serum (Clontech, Mountain View, CA) and 1% penicillin–streptomycin.
Transfections and lentiviral transduction
Tetracycline-responsive TRIPZ shRNAmir, MAGE-C2 or scrambled, and the Trans-Lentiviral packaging system were purchased from Open Biosystems (ThermoFisher Scientific, Huntsville, AL). Lentiviruses were produced, as per manufacturer’s recommendations, and concentrated with Lentivirus Concentrator (Clontech). A375 and MRA cells were transduced with concentrated lentiviruses expressing scrambled or MAGE-C2 shRNA and selected with puromycin. Transduction efficiency, determined by expression of TurboRFP, which is also encoded by the lentivirus vector, was ~70% in A375 cells and ~90–100% in MRA-13 cells. MRA-13 cultures were used as bulk cultures, whereas single-cell clones of transduced A375 cells were selected on the basis of inducible RFP expression. MAGE-C2 knockdown was confirmed by immunoblotting. HEK293T cells were transfected with vector, MAGE-C2, or mutant MAGE-C2 by the calcium phosphate method and ATM 472 siRNA (Dharmacon, Lafayette, CO) by Lipofectamine 2000 (Life Technologies, Grand Island, NY).
Immunoprecipitations and immunoblotting
A375 and MRA cells, transduced with scrambled or MAGE-C2 shRNA, were treated with doxycycline and collected after 72 hours. HEK293T cells were transfected with empty plasmid, MAGE-C2, or mutant MAGE-C2-expression plasmid. Protein lysates were immunoprecipitated with ATM antibody (Abcam, Cambridge, MA). The expression of MAGE-C2, Actin (Santa Cruz Biotechnology, Santa Cruz, CA), phospho-KAP1, KAP1 (Novus Biologicals, Littleton, CO), M2 FLAG (Sigma-Aldrich, St Louis, MI), and ATM (Abcam) was analyzed by immunoblotting. Density analysis was performed using ImageJ software (National Institutes of Health, Bethesda, MD) and was calculated for loading of the controls as actin or total protein expression for phosphorylated proteins. Density of the bands is shown as numbers under the blots.
Cytostaining and TUNEL assay
A375 cells, transduced with lentivirus encoding scrambled or MAGE-C2 shRNA and treated with 0.5 μgml−1 doxycycline for 72 hours, were stained with MAGE-C2 antibody (Santa Cruz Biotechnology) or phospho histone H2A.X (Cell Signaling, Danvers, MA) and FITC-labeled secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA). MRA-13 and A375 melanoma cells, transduced with lentivirus encoding scrambled or MAGE-C2 shRNA and treated with doxycycline for 96 hours, were stained for TUNEL using the FITC-labeled DeadEnd Fluorometric TUNEL System (Promega, Madison, WI). These cells were co-stained with MAGE-C2 (Santa Cruz Biotechnology). Mouse MRA-13 tumor xenograft sections were also stained with TUNEL. Quantitative analysis of TUNEL stain and fluorescent MAGE-C2 or phospho-H2AX was carried out using NIS Elements software (Nikon, Melville, NY).
Athymic nude mice xenografts
MRA-13 cells, transduced with lentivirus encoding tetracycline-responsive scrambled or MAGE-C2 shRNA and selected with puromycin, were used for xenograft studies. Transduction efficiency was 90–100% as detected by RFP expression after treatment with doxycycline. Four million MAGE-C2 or scrambled shRNA tumor cells were injected subcutaneously into 4-week-old athymic nude mice (Harlan Laboratories, Indianapolis, IN). A week later, half of the mice were given 2mgml−1 doxycycline in drinking water, with seven mice per group. The xenograft experiment was terminated 6 weeks later or when tumors were 1,400mm3 in volume, as per protocol.
Immunohistochemical staining
Tumors collected from athymic nude mice xenografts were fixed in formalin and embedded in paraffin. Paraffin sections were stained with MAGE-C2 antibody and developed by 3,3-diaminobenzidine (Sigma-Aldrich, St. Louis, MO).
ATM kinase inhibitor (KU55933) treatment
HEK293T cells, transfected with empty vector or MAGE-C2 expression plasmid, were treated with KU55933 ATM kinase inhibitor (Tocris Biosciences, Ellisville, MO) for 24 hours. Cells were collected and lysed as mentioned before. The expression of MAGE-C2, phospho-KAP1, KAP1, phospho p53, p53, and actin was evaluated by immunoblotting.
DNA damage and repair assay
U-2 OS cells, containing an integrated modified gene for GFP, were used to measure DNA damage repair (Pierce et al., 1999). Cells were co-transfected with I-SceI pCbASce plasmid and empty vector or MAGE-C2. After 48 hours, cells were immunostained for MAGE-C2 expression (red). The expression of MAGE and GFP was enumerated and compared, including measurement of staining intensity using NIS Elements software.
Acknowledgments
We acknowledge Dr Maria Jasin for providing us modified U2-OS cells for DNA repair assay, and Dr Andrew Simpson and Dr Otavia Caballero for reagents. We also thank Dr V.S. Setaluri for critical suggestions and support. We are grateful to Tisha Kawahara for support with the IRB protocol. This work was supported by the University of Wisconsin Carbone Cancer Center, the Office of Research and Development, Biomedical Laboratory Research and Development Service, Department of Veterans Affairs; by Grant P30 CA014520 from the National Cancer Institute; the Gretchen and Andrew Dawes Melanoma Research Fund; Ann’s Hope Foundation; the Jay Van Sloan Memorial from the Steve Leuthold Family; and the Tim Eagle Memorial. The contents do not represent the views of the Department of Veterans Affairs or of the United States Government. Melanoma patient samples were used under UW-Madison Health Sciences IRB protocol number M-2009-1157.
Abbreviations
- ATM
ataxia-telangiectasia–mutated
- DSBs
double-strand breaks
- GFP
green fluorescent protein
- MAGE
melanoma-associated antigenencoding
- RFP
red fluorescent protein
- Ser824
Serine 824
- shRNA
short hairpin RNA
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
References
- Atanackovic D, Hildebrandt Y, Jadczak A, et al. Cancer-testis antigens MAGE-C1/CT7 and MAGE-A3 are central survival factors for multiple myeloma cells. Haematol. 2010;95:785–93. doi: 10.3324/haematol.2009.014464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker PA, Salehi A. The MAGE proteins: emerging roles in cell cycle progression, apoptosis, and neurogenetic disease. J Neurosci Res. 2002;67:705–12. doi: 10.1002/jnr.10160. [DOI] [PubMed] [Google Scholar]
- Basarab T, Picard JK, Simpson E, et al. Melanoma antigen-encoding gene expression in melanocytic naevi and cutaneous malignant melanomas. Br J Dermatol. 1999;140:106–8. doi: 10.1046/j.1365-2133.1999.02616.x. [DOI] [PubMed] [Google Scholar]
- Bellati F, Napoletano C, Tarquini E, et al. Cancer testis antigen expression in primary and recurrent vulvar cancer: association with prognostic factors. Eur J Cancer. 2007;43:2621–7. doi: 10.1016/j.ejca.2007.08.031. [DOI] [PubMed] [Google Scholar]
- Chomez P, De Backer O, Bertrand M, et al. An overview of the MAGE gene family with the identification of all human members of the family. Cancer Res. 2001;61:5544–51. [PubMed] [Google Scholar]
- De Smet C, Lurquin C, Lethe B, et al. DNA methylation is the primary silencing mechanism for a set of germ line- and tumor-specific genes with a CpG-rich promoter. Mol Cell Biol. 1999;19:7327–35. doi: 10.1128/mcb.19.11.7327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhodapkar MV, Osman K, Teruya-Feldstein J, et al. Expression of cancer/testis (CT) antigens MAGE-A1, MAGE-A3, MAGE-A4, CT-7, and NY-ESO-1 in malignant gammopathies is heterogeneous and correlates with site, stage and risk status of disease. Cancer Immun. 2003;3:9. [PubMed] [Google Scholar]
- Doyle JM, Gao J, Wang J, et al. MAGE-RING protein complexes comprise a family of E3 ubiquitin ligases. Mol Cell. 2010;39:963–74. doi: 10.1016/j.molcel.2010.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskell TL, Esnal A, Robinson LL, et al. Immunohistochemical profiling of germ cells within the human fetal testis: identification of three subpopulations. Biol Reprod. 2004;71:2012–21. doi: 10.1095/biolreprod.104.028381. [DOI] [PubMed] [Google Scholar]
- Goodarzi AA, Noon AT, Deckbar D, et al. ATM signaling facilitates repair of DNA double-strand breaks associated with heterochromatin. Mol Cell. 2008;31:167–77. doi: 10.1016/j.molcel.2008.05.017. [DOI] [PubMed] [Google Scholar]
- Goodarzi AA, Noon AT, Jeggo PA. The impact of heterochromatin on DSB repair. Biochem Soc Trans. 2009;37:569–76. doi: 10.1042/BST0370569. [DOI] [PubMed] [Google Scholar]
- Groner AC, Meylan S, Ciuffi A, et al. KRAB-zinc finger proteins and KAP1 can mediate long-range transcriptional repression through heterochromatin spreading. PLoS Genet. 2010;6:e1000869. doi: 10.1371/journal.pgen.1000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoek K, Rimm DL, Williams KR, et al. Expression profiling reveals novel pathways in the transformation of melanocytes to melanomas. Cancer Res. 2004;64:5270–82. doi: 10.1158/0008-5472.CAN-04-0731. [DOI] [PubMed] [Google Scholar]
- Hofbauer GF, Schaefer C, Noppen C, et al. MAGE-3 immunoreactivity in formalin-fixed, paraffin-embedded primary and metastatic melanoma: frequency and distribution. Am J Pathol. 1997;151:1549–53. [PMC free article] [PubMed] [Google Scholar]
- Iyengar S, Farnham PJ. KAP1: An enigmatic master regulator of the genome. J Biol Chem. 2011;791:265–86. doi: 10.1074/jbc.R111.252569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungbluth AA, Busam KJ, Kolb D, et al. Expression of MAGE-antigens in normal tissues and cancer. Int J Cancer. 2000;85:460–5. [PubMed] [Google Scholar]
- Jungbluth AA, Chen YT, Busam KJ, et al. CT7 (MAGE-C1) antigen expression in normal and neoplastic tissues. Int J Cancer. 2002;99:839–45. doi: 10.1002/ijc.10416. [DOI] [PubMed] [Google Scholar]
- Jungbluth AA, Ely S, Diliberto M, et al. The cancer-testis antigens CT7 (MAGE-C1) and MAGE-A3/6 are commonly expressed in multiple myeloma and correlate with plasma-cell proliferation. Blood. 2005;106:167–74. doi: 10.1182/blood-2004-12-4931. [DOI] [PubMed] [Google Scholar]
- Li X, Lee Y-K, Jeng J-C, et al. Role for KAP1 serine 824 phosphorylation and sumoylation/desumoylation switch in regulating KAP1-mediated transcriptional repression. J Biol Chem. 2007;282:36177–89. doi: 10.1074/jbc.M706912200. [DOI] [PubMed] [Google Scholar]
- Liu W, Cheng S, Asa SL, et al. The melanoma-associated antigen A3 mediates fibronectin-controlled cancer progression and metastasis. Cancer Res. 2008;68:8104–12. doi: 10.1158/0008-5472.CAN-08-2132. [DOI] [PubMed] [Google Scholar]
- Monte M, Simonatto M, Peche LY, et al. MAGE-A tumor antigens target p53 transactivation function through histone deacetylase recruitment and confer resistance to chemotherapeutic agents. Proc Natl Acad Sci USA. 2006;103:11160–5. doi: 10.1073/pnas.0510834103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardiello T, Jungbluth AA, Mei A, et al. MAGE-A inhibits apoptosis in proliferating myeloma cells through repression of bax and maintenance of survivin. Clin Cancer Res. 2011;17:4309–19. doi: 10.1158/1078-0432.CCR-10-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JW, Kwon TK, Kim IH, et al. A new strategy for the diagnosis of MAGE-expressing cancers. J Immunol Methods. 2002;266:79–86. doi: 10.1016/s0022-1759(02)00105-9. [DOI] [PubMed] [Google Scholar]
- Pauls K, Schorle H, Jeske W, et al. Spatial expression of germ cell markers during maturation of human fetal male gonads: an immunohistochemical study. Hum Reprod. 2006;21:397–404. doi: 10.1093/humrep/dei325. [DOI] [PubMed] [Google Scholar]
- Pierce AJ, Johnson RD, Thompson LH, et al. XRCC3 promotes homology-directed repair of DNA damage in mammalian cells. Genes Dev. 1999;13:2633–8. doi: 10.1101/gad.13.20.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajpert-De Meyts E, Jacobsen GK, Bartkova J, et al. The immunohistochemical expression pattern of Chk2, p53, p19INK4d, MAGE-A4 and other selected antigens provides new evidence for the premeiotic origin of spermatocytic seminoma. Histopathology. 2003;42:217–26. doi: 10.1046/j.1365-2559.2003.01587.x. [DOI] [PubMed] [Google Scholar]
- Riker AI, Enkemann SA, Fodstad O, et al. The gene expression profiles of primary and metastatic melanoma yields a transition point of tumor progression and metastasis. BMC Med Genomics. 2008;1:13. doi: 10.1186/1755-8794-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos WP, Kaina B. DNA damage-induced cell death by apoptosis. Trends Mol Med. 2006;12:440–50. doi: 10.1016/j.molmed.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Shanbhag NM, Rafalska-Metcalf IU, Balane-Bolivar C, et al. ATM-dependent chromatin changes silence transcription in cis to DNA double-strand breaks. Cell. 2010;141:970–81. doi: 10.1016/j.cell.2010.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigalotti L, Fratta E, Coral S, et al. Intratumor heterogeneity of cancer/testis antigens expression in human cutaneous melanoma is methylation-regulated and functionally reverted by 5-aza-2′-deoxycytidine. Cancer Res. 2004;64:9167–71. doi: 10.1158/0008-5472.CAN-04-1442. [DOI] [PubMed] [Google Scholar]
- Smeenk L, Lohrum M. Behind the scenes: unravelling the molecular mechanisms of p53 target gene selectivity (Review) Int J Oncol. 2010;37:1061–70. doi: 10.3892/ijo_00000757. [DOI] [PubMed] [Google Scholar]
- Svobodova S, Browning J, MacGregor D, et al. Cancer-testis antigen expression in primary cutaneous melanoma has independent prognostic value comparable to that of Breslow thickness, ulceration and mitotic rate. Eur J Cancer. 2011;47:460–9. doi: 10.1016/j.ejca.2010.09.042. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Shichijo S, Noguchi M, et al. Identification of MAGE-1 and MAGE-4 proteins in spermatogonia and primary spermatocytes of testis. Cancer Res. 1995;55:3478–82. [PubMed] [Google Scholar]
- White DE, Negorev D, Peng H, et al. KAP1, a novel substrate for PIKK family members, colocalizes with numerous damage response factors at DNA lesions. Cancer Res. 2006;66:11594–9. doi: 10.1158/0008-5472.CAN-06-4138. [DOI] [PubMed] [Google Scholar]
- Xiao TZ, Bhatia N, Urrutia R, et al. MAGE I transcription factors regulate KAP1 and KRAB domain zinc finger transcription factor mediated gene repression. PLoS One. 2011;6:e23747. doi: 10.1371/journal.pone.0023747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakirevich E, Sabo E, Dirnfeld M, et al. Morphometrical quantification of spermatogonial germ cells with the 57B anti-MAGE-A4 antibody in the evaluation of testicular biopsies for azoospermia. Appl Immunohistochem Mol Morphol. 2003;11:37–44. doi: 10.1097/00129039-200303000-00007. [DOI] [PubMed] [Google Scholar]
- Yang B, O’Herrin S, Wu J, et al. Select cancer testes antigens of the MAGE-A, -B, and -C families are expressed in mast cell lines and promote cell viability in vitro and in vivo. J Invest Dermatol. 2006;127:267–75. doi: 10.1038/sj.jid.5700548. [DOI] [PubMed] [Google Scholar]
- Yang B, O’Herrin SM, Wu J, et al. MAGE-A, mMage-b, and MAGE-C proteins form complexes with KAP1 and suppress p53-dependent apoptosis in MAGE-positive cell lines. Cancer Res. 2007;67:9954–62. doi: 10.1158/0008-5472.CAN-07-1478. [DOI] [PubMed] [Google Scholar]
- Zhuang R, Zhu Y, Fang L, et al. Generation of monoclonal antibodies to cancer/testis (CT) antigen CT10/MAGE-C2. Cancer Immun. 2006;6:7. [PubMed] [Google Scholar]