Abstract
Estrogen (E) treatment induces axospinous synapses in rat hippocampus in vivo and in cultured hippocampal neurons in vitro. To better explore the molecular mechanisms underlying this phenomenon, we have established a mouse model for E action in the hippocampus by using Golgi impregnation to examine hippocampal dendritic spine morphology, radioimmunocytochemistry (RICC) and silver-enhanced immunocytochemistry to examine expression levels of synaptic protein markers, and hippocampal-dependent object-placement memory as a behavioral readout for the actions of E. In ovariectomized mice of several strains and F1 hybrids, the total dendritic spine density on neurons in the CA1 region was not enhanced by E treatment, a finding that differs from that in the female rat. E treatment of ovariectomized C57BL/6J mice, however, caused an increase in the number of spines with mushroom shapes. By RICC and silver-enhanced immunocytochemistry, we found that the immunoreactivity of postsynaptic markers (PSD95 and spinophilin) and a presynaptic marker (syntaxin) were enhanced by E treatment throughout all fields of the dorsal hippocampus. In the object-placement tests, E treatment enhanced performance of object placement, a spatial episodic memory task. Taken together, the morphology and RICC results suggest a previously uncharacterized role of E in synaptic structural plasticity that may be interpreted as a facilitation of the spine-maturation process and may be associated with enhancement of hippocampal-dependent memory.
Dendritic spines are specialized to receive synaptic inputs and to compartmentalize calcium, and changes in spine morphology and function are considered to be important for processes such as learning and memory (1-5). It is, therefore, important to understand how dendritic spine formation and maturation are regulated. Extrinsic factors, such as circulating hormones, influence spine properties in the hippocampus. Estrogen (E) treatment regulates dendritic spine formation in the rat hippocampus in vivo (6-8) and in cultured hippocampal neurons in vitro (9-12). The effects of E on hippocampal-dependent cognitive functions were shown also in rats and humans (13-15) and recently in mice and nonhuman primates (16-19).
Dendritic-spine changes include at least two different processes: generation of new spines and maturation of existing spine synapses. These processes are closely linked, with complex biochemical, morphological, and electrophysiological consequences (1, 2, 20). Spine maturation is a multistep, multifaceted process in which the spines change from thin filopodia-like structures to spines with bigger heads, larger synaptic contact area, shorter and wider spine necks, and newly recruited synaptic proteins (1, 3, 20-22). In cell culture, only the mature type of dendritic spines can recruit α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors (2) and, thus, make the transition from silent to functional synapses (23).
Studies of E-induced synapse formation in the rat hippocampus have used Golgi staining, filling of cells with dye after electrophysiological recording, electron microscopy, and radioimmunocytochemistry (RICC) to reveal the morphological and biochemical aspects of the formation of new axospinous synapses in vivo (6-8, 24). To progress in our understanding of the molecular mechanisms of E-induced dendritic-spine changes and their impact on learning and memory, a mouse model is highly desirable because of the ability to exploit the powerful tools of molecular genetics available in mice. Here, we describe how E treatment in ovariectomized (OVX) mice affects the density and morphology of dendritic spines, the expression of synaptic proteins in the hippocampal formation, and the subsequent impact on the performance of a hippocampal-dependent learning task.
Materials and Methods
Animals and E Treatments. C57BL/6J, 129/J, BALB/c, and their F1 hybrid strains of mice (4-5 weeks old) were obtained from The Jackson Laboratory. In each experiment, 20 mice were OVX and then allowed to recover from the surgery for 10 days. The animals were divided randomly into control and experimental groups and injected either with 0.1 ml of sesame-oil vehicle or 1 μg of 17β-estradiol benzoate (EB) in 0.1 ml of sesame oil for 5 days. On day 7, mice were either killed for Golgi impregnation, RICC, and silver-enhanced immunocytochemistry (SEI) or behaviorally tested. In the initial studies, we also tested E treatment of 5 μg/day for 2 or 5 days.
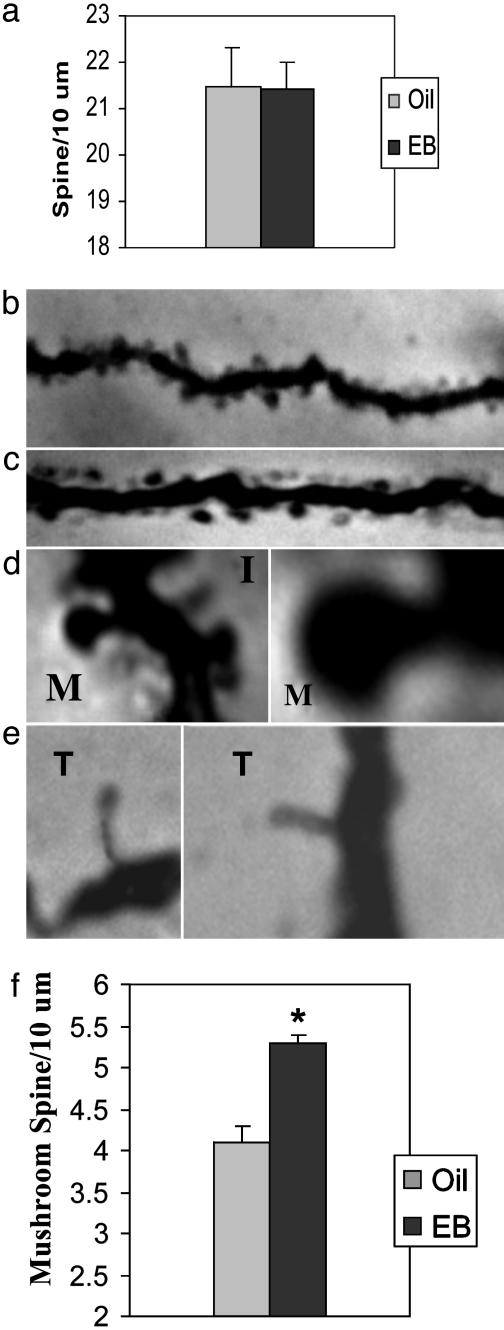
Golgi Impregnation. Mice were anesthetized and then perfused transcardially with 4% paraformaldehyde, and brain tissue was processed as described (6, 7). Several criteria were used to select dendrites for quantification. (i) The cell must have a universal and even impregnation from the cell body to the tertiary branches; (ii) the dendrite must not be at an angle with the image plane because these tilted dendrites would introduce errors in measuring the length of the dendrite; and (iii) the dendrite must be well separated from other dendrites to avoid confusion in which spines that actually belong to other dendrites are counted. A digital camera system and MCID-4M software from Imaging Research (St. Catherine's, ON, Canada) were used to examine neuronal morphology and count dendritic spines. Also, it allowed us to better evaluate shape characteristics of different types of spines. When counting the total number of spines, serial focal plains of all selected dendrites were measured to ensure that all spines were counted. When counting different types of spines, we counted mushroom-shaped spines (M) with well formed head and neck structures vs. thin spines (T) and filopodia-like structures (I) (Fig. 1 d and e). Spines with a head diameter of at least ≈0.3 μm were categorized as mushroom types. Spine quantification was carried out by observers who were unaware of the experimental conditions.
Fig. 1.
Effects of EB administration on the dendritic spine density on hippocampal CA1 pyramidal neurons. (a) The total spine density does not change with EB treatment (P < 0.31). (b and c) Dendrites of CA1 pyramidal neurons from oil-treated (b) and EB-treated (c) mice. (d and e) Spines have varying morphologies. We counted spines with a well formed mushroom-shaped head and a narrow neck (M) vs. thin (T) or filopodia-like (I) protrusions. Note that spines were visualized for counting by focusing up and down (which is not possible to show in the photo). (f) EB treatment significantly increases the number of mushroom-type spines (P < 0.01). *, statistically significant change. For each of the experimental and control groups, 16 animals were used; spines on 1,200 tertiary dendrites were counted.
RICC. Mice were decapitated 24 h after the final EB treatment, and brains were removed, rapidly frozen on dry ice, and stored at -70°C until sectioning. Brains were sectioned on a cryostat at 20 μm along the coronal plane and stored at -70°C. RICC was carried out by using modifications of methods reported by Brake et al. (24). All antibodies used in this study were titrated previously to determine concentrations exhibiting maximum signal to minimum background binding. For immunoreactivity (IR) to syntaxin and PSD95, tissue was blocked with 1% BSA in PBS for 1 h at room temperature (RT). Tissue was then incubated overnight at 4°C in primary antibody diluted in 1% BSA/PBS. We used commercially available rabbit antisyntaxin polyclonal antibodies (1:1,000 dilution) and rabbit anti-PSD95 polyclonal antibodies (1:400 dilution) (Synaptic Systems, Göttingen, Germany), as well as noncommercial rabbit antispinophilin polyclonal antibodies (1:500 dilution; a gift from Paul Greengard and Patrick Allen, The Rockefeller University). Sections incubated without primary antibody were included also as negative controls. All sections were then washed in 1% BSA/PBS and incubated with secondary antibody (donkey anti-rabbit 35S-labeled Ig whole antibody; Amersham Biosciences) in PBS (1:400 dilution) for 1 h at RT. After three 15-min washes in ice-cold PBS and a distilled H2O rinse, all sections were left to air-dry overnight. All slides were incubated collectively in the same dish at each step, except for the no primary antibody controls, which were kept separate from other slides throughout the experiment. Slides were then apposed to BetaMax film (Amersham Biosciences). Multiple exposure times were used to determine the optimal density for each primary antibody (2-3 days).
For analysis of RICC, sections were coded to hide identities of the treatments, and optical density measurements were taken from six sections (approximately every 12th) of dorsal hippocampus of each mouse by using MCID-M4 computerized image-analysis software. Density of the corpus callosum (which should contain no presynaptic or postsynaptic protein) was measured as nonspecific background and subtracted from the density of each hippocampal subregion measurement (24).
SEI. Animals were treated as described for Golgi impregnation, except that after the perfusion with 4% paraformaldehyde, brains were postfixed for 6 h in 4% paraformaldehyde with 0.125% glutaraldehyde. Sections (40 μm) were prepared and blocked in PBS/Triton X-100 supplemented with 0.2% cold-water fish skin/0.5% BSA/5% normal goat serum for 1 h at RT and then incubated in rabbit antispinophilin polyclonal antibody for 72 h at 4°C. Each section was rinsed 10 times in PBS/Triton X-100 for 7 min, postfixed in 2% glutaraldehyde in PBS, rinsed five times in PBS, and rinsed five times in distilled water for 7 min. Sections were incubated in Aurion-R-Gent silver-enhancement reagent (EM Science) for 15 min at RT, rinsed four times in distilled water for 5 min each, mounted on Vectabond-coated slides (Vector Laboratories), air-dried, and coverslipped with BioMount media (EM Science).
Images of the hippocampus were captured and analyzed blindly with Stereo Investigator (MicroBrightField, Williston, VT). A contour was drawn around CA1 stratum radiatum, and the computer then selected five to seven sampling sites randomly (2.5 × 2.5 μm) within each contour. Silver grains were counted through the z plane of the tissue.
Object-Placement Task. Object placement is a hippocampal-dependent spatial memory task. The EB protocol was identical to the EB protocol used in Golgi impregnation, RICC, and SEI experiments. The current method of object placement was adapted for mice from methods used in rats (25, 26). Subjects were placed on the field with two identical objects for 5 min (T1, sample trial). Exploration of the objects was timed by a stop-watch when subjects sniffed at, whisked at, or looked at the objects from no more than 2 cm away. After T1, the subject was removed from the field for 30 min. After the delay, one object was moved to a new location. The time spent exploring the objects in new (novel) and old (familiar) locations after delay (T2, recognition trial) was recorded by an observer who was unaware of the treatments. Sessions were videotaped and later analyzed. All locations for the objects were counterbalanced among groups, and objects and field were cleaned between trials. Differences between groups in time exploring the identical objects (T1) were tested by t test. Data in T2 were analyzed by two-way ANOVA (group vs. object), and differences in time spent exploring the object in the familiar and novel locations were tested by within-group paired t tests. Because rodents are generally more interested in novelty, more time spent exploring novel rather than familiar places or objects after an intertrial delay period suggests that subjects remember the familiar places or objects (25).
For testing, 6-week-old OVX C57BL/6J mice (Taconic Farms) were double-housed, maintained on a 12:12 reverse light/dark cycle (lights off at 0900 hours), and tested under dim red-light conditions between 1000 and 1700 hours. Subjects received oil or 1 μg of EB daily for 5 days and were then tested for object placement performance on day 6. During days 1-5, they received daily acclimation and habituation to the field, objects, and tests. On day 6, they were tested for object placement with a 30-min intertrial delay between the sample and recognition trials.
Results
Effects of EB Treatment on Total Dendritic Spine Density in Hippocampus. Because EB treatment of OVX female rats increases spine density on CA1 pyramidal neurons in hippocampus (7), we performed the same analysis on OVX EB-treated C57BL/6J, 129/J, BALB/c, and F1 hybrid C57/129 strains of mice to avoid possible strain variability and specificity, which are very common in inbred mouse strains. EB-treatment did not change total spine density of the hippocampal CA1 pyramidal neurons in C7BL/6J mice (Fig. 1a). In this analysis, 1,200 tertiary apical dendrites of CA1 pyramidal neurons were selected for counting spines. We obtained similar, negative results for effects of EB on total spine density in all strains of mice: 129/J mice, 20.5 ± 0.8 spines per 10 μm (EB-treated) and 20.5 ± 0.6 spines per 10 μm (non-EB-treated); P < 0.39; BALB/C, 21.5 ± 0.6 spines per 10 μm (EB-treated) and 21.6 ± 0.8 spines per 10 μm (non-EB-treated); P < 0.30); and C57BL/129J F1 hybrids, 21.9 ± 0.7 spines per 10 μm (EB-treated) and 21.5 ± 0.8 spines per 10 μm (non-EB-treated); P < 0.33). Thus, in subsequent experiments, we examined only C57 BL/6J female mice. We also applied various EB-treatment protocols, such as 5 μg/day for 2 days and 5 μg/day for 5 days to OVX C57BL/6J female mice. The EB-induced increase of total spine density was not detected (data not shown). Therefore, in the subsequent experiments, we used EB protocol as 1 μg/day for 5 days.
Effects of E Treatment on Subtypes of Dendritic Spines in Mouse Hippocampus. Dendritic spines have different morphologies, ranging from long and thin filopodia-like structures to spines with a well formed spine head and neck (Fig. 1 b-e). Spines were classified into two groups, thin and mushroom types, as described in Materials and Methods. The results (Fig. 1f) revealed a statistically significant E-induced enhancement in the mushroom type of spines: 5.3 ± 0.1 spines per 10 μm (EB-treated) and 4.1 ± 0.2 spines per 10 μm (non-EB-treated); P < 0.01. Our results showed also that the mushroom spines are a minority of the total spines.
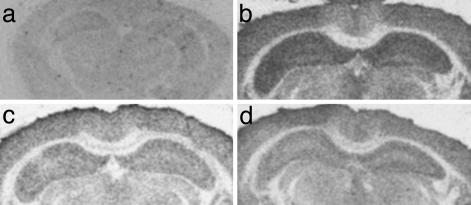
Effects of EB Treatment on Postsynaptic Marker Proteins. Spine synapse formation in cell culture involves the outgrowth of a filopodium from a dendrite that makes contact with an axon (21). If EB-induced structural change is indeed part of the spine-maturation process, it is likely that there will be an increase in synaptic marker proteins that are important for synaptic function and strengthening. We, thus, used RICC to investigate this possibility by using antibodies against syntaxin (a presynaptic protein), as well as antibodies against spinophilin and PSD95 (postsynaptic proteins), and we verified the results by using SEI. For all primary antibodies, the RICC signal from the labeled secondary antibody was most dense in cortex and hippocampus, with little binding in the corpus callosum. In the hippocampus, binding was most dense in the dendritic fields, with the lightest binding within the principal cell layers. To determine specific binding of secondary 35S-labeled antibody, some sections were incubated without primary antibody. The anti-rabbit secondary antibody displayed no specific binding when compared with sections incubated with primary antibody (Fig. 2).
Fig. 2.
Autoradiograms depicting presynaptic and postsynaptic protein IR detected by RICC in the mouse hippocampus. (a) Control represents the binding of 35S-labeled anti-rabbit secondary antibody in the absence of primary antibody. (b-d) RICC of spinophilin (b), PSD95 (c), and syntaxin (d).
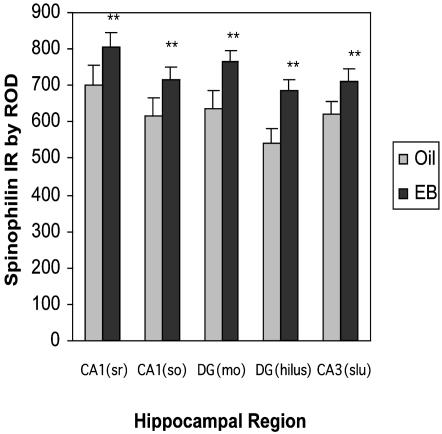
Spinophilin is localized predominantly in dendritic spines, and it is involved in spine development and required for the regulation of glutamate receptor activity (27, 28). Although there was no significant interaction, a significant main effect of EB treatment by using a mixed two-way ANOVA revealed that EB-treated mice showed significantly higher levels of spinophilin IR compared with controls in all subfields of hippocampus (significant group effect, F(1,44) = 6.026, P < 0.032; Figs. 2b and 3). Thus, there is a uniform increase in spinophilin across the entire dorsal hippocampus, and EB increased spinophilin IR significantly when the entire dorsal hippocampus was measured (see Fig. 5A).
Fig. 3.
The enhancing effect of EB treatment on IR of the postsynaptic protein spinophilin. (a) IR (mean ± SEM) detected by RICC for spinophilin in different subregions of the hippocampus. *, statistically significant changes (P < 0.001; Scheffé's test). ROD, relative OD; DG, dentate gyrus; SO, stratum oriens; MO, molecular layer; Slu, stratum lucidum.
Fig. 5.
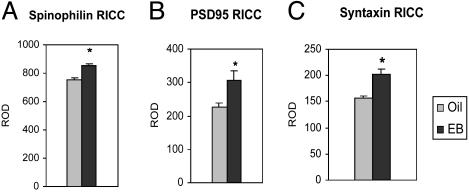
The enhancing effect of EB treatment on IR of the postsynaptic proteins spinophilin and PSD95 and the presynaptic protein syntaxin in the whole dorsal hippocampal region of oil-treated (Oil) and EB-treated (EB) mice. *, Statistically significant changes. IR (mean ± SEM) was detected by RICC for spinophilin (A; P < 0.001), PSD95 (B; P < 0.035), and syntaxin (C; P < 0.0017).
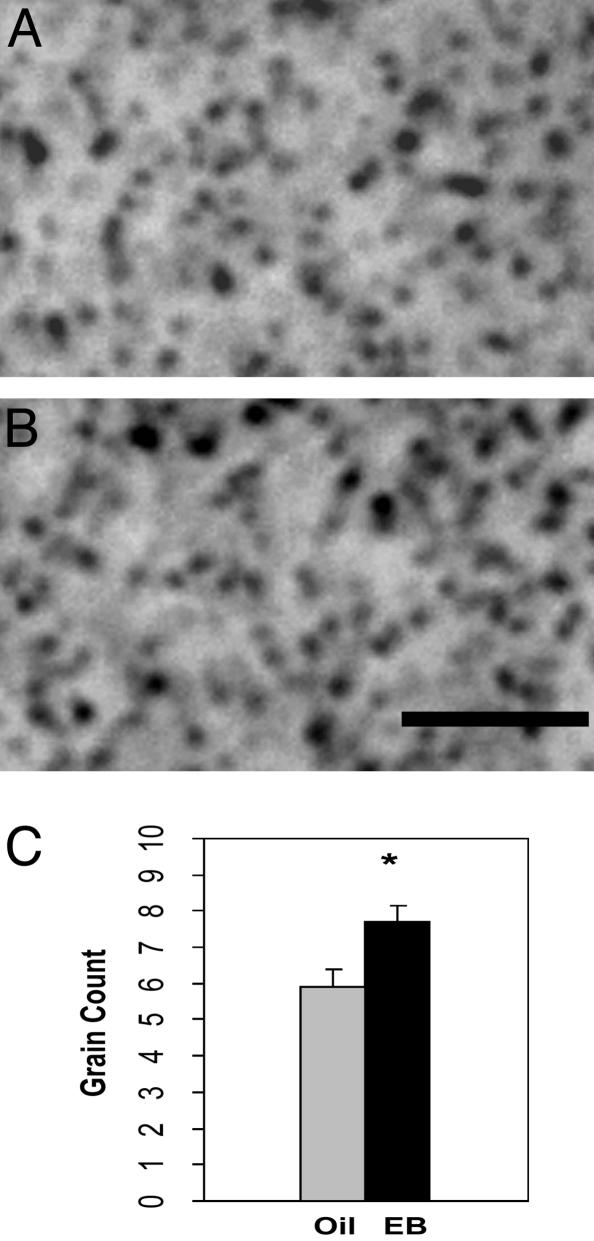
To verify this result with another independent method, we used SEI, which is suitable for quantification. Bilateral counts of silver grains in hippocampal CA1 stratum radiatum were made from brain sections containing dorsal hippocampus (Fig. 4 A and B). An unpaired t test (Fig. 4C) revealed a significantly greater number of spinophilin-immunoreactive puncta in the CA1 stratum radiatum of EB-treated mice (7.7 ± 0.43) than oil-treated control mice (5.9 ± 0.48; t = 2.657, P < 0.05).
Fig. 4.
The enhancing effect of EB treatment on IR of the spinophilin, as measured by SEI. (A and B) SEI micrographs of spinophilin in CA1 region of brains from oil-treated (Oil) and EB-treated (EB) mice, respectively. (Scale bar, 5 μm.) (C) Number of silver grain counts (mean ± SEM) detected by SEI. *, Significant changes (P < 0.05, t = 2.657; unpaired t test).
We also examined PSD95, another synaptic marker that is critical in anchoring and clustering N-methyl-d-aspartate (NMDA) receptors to the synapse (29, 30), and is also important in the spine-maturation process (31). In a previous study in rats, EB treatment up-regulated NMDA receptor level in hippocampal neurons (8, 32-36), although the mechanism of this increase is still not clear. Because of the uniform increase of spinophilin across hippocampal fields, PSD95 IR was then measured in the entire dorsal hippocampus, and a significant increase was observed (P < 0.035; Figs. 2c and 5B).
Effects of EB Treatment on Presynaptic Marker Proteins. Syntaxin is a presynaptic membrane-bound protein and has an important role in synaptic vesicle docking. It is considered a reliable marker of synaptic potentiation and strengthening (37-40). RICC was carried out for syntaxin, and the optical density was measured in the whole dorsal hippocampus. EB-treated mice showed a significant increase of the syntaxin IR, compared with oil-injected controls. (P < 0.0017; Figs. 2d and 5C).
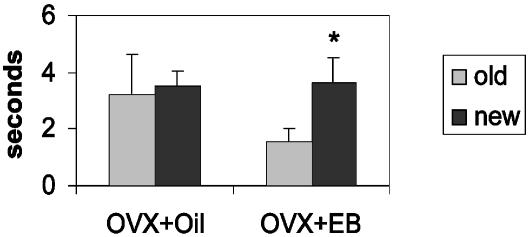
Effects of EB Treatment on a Spatial Working Memory Task. To establish a functional link for the hippocampal structural changes, the effects of EB or oil treatment of OVX C57 BL/6J mice were examined on object placement, a hippocampal-dependent spatial memory task (26). During sample trials (T1) for object-placement task, no differences between OVX and EB-treated subjects were found in the time spent exploring identical objects (data not shown). During object-placement testing, EB-treated OVX mice spent significantly more time exploring the novel (3.8 sec) than the familiar object (1.5 sec) in trials with an intertrial delay of 30 min between the sample trial (T1) and the recognition trial (T2), whereas oil-treated OVX mice spent a similar amount of time exploring the objects in the familiar and the novel locations (Fig. 6).
Fig. 6.
Enhancing effects of EB administration to OVX mice on performance of the object-placement task. Each experimental and control group consisted of 10 OVX mice. All tests were conducted 24 h after injection of the last EB. EB was administered 1 μg/day for 5 days in the object-placement test. The delay between sampling the objects and the memory tests was 30 min. *, Statistically significant differences (ANOVA with paired t test). Oil-treated mice (OVX+Oil) could no longer discriminate old vs. new locations (P > 0.05), whereas EB-treated mice (OVX+EB) spent significantly more time with the new locations (P < 0.01), indicating that they still remembered the spatial locations.
Discussion
The present study is an initial report on E actions in hippocampus of female C57BL/6J mice, and it supports the feasibility of using mice for investigations of the mechanisms of structural plasticity, which could eventually include a molecular genetic analysis. Our findings revealed the effects of E in the female mouse hippocampus on synapse maturation and a hippocampal-dependent memory task.
Studies have revealed the beneficial effects of E in improving memory function in women undergoing a surgical or natural menopause (41) and in adult OVX female rats and mice (15, 42-45). Although not all studies have shown E enhancements in performance of spatial memory tasks, results of recent studies show consistently that E enhancements in spatial memory may be limited to working-memory versions of spatial tasks and not be present in reference-memory versions (13, 15, 25, 42, 43).
Recently, it was demonstrated in a delayed matching-to-place version of the spatial memory task, the Morris water maze (13), that E-dependent enhancements in performance of rats are consistent with doses and times of E administration known to induce spine synapses in CA1 pyramidal neurons of female rats. Thus, the CA1 area of the female rat hippocampus responds to E with relatively rapid alterations in spine density and morphology (6-8) and presynaptic and postsynaptic markers (24).
Consistent with the results of Sandstrom and Williams (13) that E enhanced the performance of female rats in a delayed matching-to-place version of the Morris water maze, we have found similar effects in mice by using the object-placement task, which is a particularly sensitive measure of spatial working memory (26). Our results of the object-placement test support the conclusion that in female mice, as in female rats, E treatment enhances hippocampal-dependent spatial working memory. Furthermore, an object-recognition test, a nonspatial working-memory task, demonstrated a beneficial effect of E also (data not shown). This result indicated that the effect of E may not be restricted to hippocampal-dependent spatial memory and may involve more widespread brain regions, rather than only the hippocampus. Indeed, a recent study showed that E prolongs social-recognition memory in mice (A. Tang, R.D.R, and B.S.M., unpublished data).
Although it is possible that effects of E on object placement may be at least in part the result of rapid nongenomic actions of E (25), it is likely that they may also be the result of effects of E on synapse formation and/or maturation that have been demonstrated in rat and primate hippocampi. Despite parallel effects of E treatment to enhance hippocampal-dependent memory in rats and mice, the structural basis of effects of E on rat and mouse hippocampi is somewhat distinct. Whereas E treatment in rats causes de novo formation of new spine synapses, the present data in mice point to a different type of effect that can be interpreted as spine synapse maturation. The hypothesis that E facilitates spine maturation is supported by data from RICC and SEI that demonstrate an up-regulation of presynaptic and postsynaptic proteins throughout the hippocampus. Interestingly, in similar studies using the same RICC method, this type of up-regulation of synaptic proteins was observed in rat and rhesus macaques (24, 46), with a greater regional specificity predominantly in the hippocampal CA1 region. This finding is consistent with the Golgi results that E-induced synaptogenesis was found in rats only in apical and basal dendrites of CA1 neurons (6, 7).
One of the postsynaptic markers used in the present study is PSD95, a protein known for its important role in clustering NMDA receptors in the process of spine maturation (29, 30). In a recent study (31), PSD95 was shown to drive the maturation of glutamatergic synapses by increasing spine numbers as well as enlarging spine sizes. Interestingly, the effect of PSD95 revealed in that study was both postsynaptic and presynaptic. Our present results show that E treatment increased PSD95 IR in the hippocampus after the same duration of treatment that caused a shift in the morphological pattern of spines from immature and filamentous to mature types. It will be interesting to investigate whether PSD95 also plays a role in the E-induced synaptogenesis that is NMDA receptor-dependent in rat hippocampus (47).
E treatment of female mice also increased IR for another postsynaptic marker, spinophilin, a protein predominantly localized in dendritic spines. Spinophilin is a cytoskeletal scaffolding protein, which has the ability to bundle F-actin filaments (48). Dendritic spines have been shown to contain predominantly F-actin, and to undergo dynamic actin-based motility that may be related to synaptic re-organization (49-51). In addition, spinophilin serves to target protein phosphatase I to the postsynaptic element, a function that is required for the regulation of glutamate receptor activity (28, 52). In spinophilin knockout mice, morphological changes in dendritic spines, as well as a reduction in brain size and defective NMDA receptor-dependent synaptic plasticity in hippocampal neurons, were observed (28, 48, 52, 53). Spinophilin also interacts with P7056K, which is involved in protein synthesis and cell growth. It has been suggested that spinophilin may regulate local protein synthesis at the dendrites (28, 54). In several recent studies, effects of E on local protein synthesis have been suggested (55), and IR for E receptor α has been found in dendrites of hippocampal pyramidal neurons (56). Thus, the enhancement of spinophilin expression by E treatment may contribute to the maturation of spines in several ways.
Syntaxin is a presynaptic membrane bound protein that has an important role in vesicular docking. It is considered a reliable marker of synaptogenesis (37-40). We found that syntaxin protein expression in mice is enhanced by E treatment. At this time, it is unclear whether the increase of syntaxin is a secondary consequence of an E induced increase in PSD-95 expression or a primary effect on syntaxin transcription or translation.
The same phenomenon of increased expression of synaptic proteins caused by E treatment in rats and mice was demonstrated also in nonhuman primates (17, 18). This finding indicates that the mechanisms underlying E enhancement of synaptic proteins are conserved through evolution and, perhaps, play important roles in many aspects of brain function (19, 46)
In addition to effects of E, experience-induced changes in spine number and morphology have been observed in vivo and in vitro in many experimental and behavioral studies, including light deprivation (57), rearing animals in enriched environment (58), hibernation (59, 60), and LTP induction in cultured neurons and intact animals (21, 22, 61-68). Despite controversies about the degree to which spines change under these conditions (69, 70), there is a general agreement that dendritic spines change either in number or shape in response to the various experiences or environmental challenges and that such structural changes are a component of the mechanisms of functional changes.
Our results can be interpreted as indicating that, in female mice, E treatment specifically increases the spine synapse maturation. Our morphological studies suggest that some spines change from thin filopodia-like structure to spines with bigger heads, larger synaptic contact area, shorter and wider spine necks; our RICC and SEI results indicate an E induced increase in synaptic proteins (1, 3, 22). It is likely that structural maturation of the dendritic spine is accompanied by functional maturation. In the process of maturation of silent synapses into mature synapses, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor recruitment occurs only on spines with matured structure (2). It remains to be seen whether E-induced maturation of dendritic spines is accompanied by α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor expression and recruitment.
In conclusion, this study has achieved the first step in the goal of establishing a mouse model for the study of E-regulated structural synaptic plasticity and the assessment of its impact on learning and memory. The present findings have established a means to measure spine morphology, synaptic protein expression, and behavioral tests. With such a mouse model, powerful molecular genetic tools can be used to examine the roles of specific gene products, such as cytoskeletal proteins and the isoforms of the E receptor, in mediating the powerful effects of E on hippocampal neurons and their functional outputs.
Acknowledgments
This work was supported by National Research Service Award Grant NS10492 and a C. H. Li Scholarship (to C.L.); and National Institutes of Health Grants NS-07080 (to B.S.M.), MH-40899 and DA-10044 (to P.B.A. and P.G), and GM60654 (to V.L.).
Abbreviations: E, estrogen; EB, 17β-estradiol benzoate; IR, immunoreactivity; NMDA, N-methyl-d-aspartate; OVX, ovariectomized; RICC, radioimmunocytochemistry; RT, room temperature; SEI, silver-enhanced immunocytochemistry.
References
- 1.Yuste, R. & Bonhoeffer, T. (2001) Annu. Rev. Neurosci. 24, 1071-1089. [DOI] [PubMed] [Google Scholar]
- 2.Matsuzaki, M., Ellis-Davies, G. C., Nemoto, T., Miyashita, Y., Iino, M. & Kasai, H. (2001) Nat. Neurosci. 4, 1086-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Segal, M. (2001) Neuron 31, 169-171. [DOI] [PubMed] [Google Scholar]
- 4.Harris, K. M. (1999) Curr. Opin. Neurobiol. 9, 343-348. [DOI] [PubMed] [Google Scholar]
- 5.Horner, C. H. (1993) Prog. Neurobiol. 41, 281-321. [DOI] [PubMed] [Google Scholar]
- 6.Gould, E., Woolley, C. S., Frankfurt, M. & McEwen, B. S. (1990) J. Neurosci. 10, 1286-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woolley, C. S. & McEwen, B. S. (1992) J. Neurosci. 12, 2549-2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woolley, C. S., Weiland, N. G., McEwen, B. S. & Schwartzkroin, P. A. (1997) J. Neurosci. 17, 1848-1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murphy, D. D. & Segal, M. (1996) J. Neurosci. 16, 4059-4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy, D. D. & Segal, M. (1997) Proc. Natl. Acad. Sci. USA 94, 1482-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murphy, D. D., Cole, N. B., Greenberger, V. & Segal, M. (1998) J. Neurosci. 18, 2550-2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murphy, D. D., Cole, N. B. & Segal, M. (1998) Proc. Natl. Acad. Sci. USA 95, 11412-11417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sandstrom, N. J. & Williams, C. L. (2001) Behav. Neurosci. 115, 384-393. [PubMed] [Google Scholar]
- 14.Sherwin, B. B. & Tulandi, T. (1996) J. Clin. Endocrinol. Metab. 81, 2545-2549. [DOI] [PubMed] [Google Scholar]
- 15.Luine, V. N., Richards, S. T., Wu, V. Y. & Beck, K. D. (1998) Horm. Behav. 34, 149-162. [DOI] [PubMed] [Google Scholar]
- 16.Heikkinen, T., Puolivali, J., Liu, L., Rissanen, A. & Tanila, H. (2002) Horm. Behav. 41, 22-32. [DOI] [PubMed] [Google Scholar]
- 17.Voytko, M. L. (2000) Behav. Neurosci. 114, 1078-1087. [PubMed] [Google Scholar]
- 18.Lacreuse, A., Wilson, M. E. & Herndon, J. G. (2002) Neurobiol. Aging 23, 589-600. [DOI] [PubMed] [Google Scholar]
- 19.Rapp, P. R., Morrison, J. H. & Roberts, J. A. (2003) J. Neurosci. 23, 5708-5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kasai, H., Matsuzaki, M., Noguchi, J., Yasumatsu, N. & Nakahara, H. (2003) Trends Neurosci. 26, 360-368. [DOI] [PubMed] [Google Scholar]
- 21.Ziv, N. E. & Smith, S. J. (1996) Neuron 17, 91-102. [DOI] [PubMed] [Google Scholar]
- 22.Smith, S. J. (1999) Science 283, 1860-1861. [DOI] [PubMed] [Google Scholar]
- 23.Isaac, J. T., Nicoll, R. A. & Malenka, R. C. (1995) Neuron 15, 427-434. [DOI] [PubMed] [Google Scholar]
- 24.Brake, W. G., Alves, S. E., Dunlop, J. C., Lee, S. J., Bulloch, K., Allen, P. B., Greengard, P. & McEwen, B. S. (2001) Endocrinology 142, 1284-1289. [DOI] [PubMed] [Google Scholar]
- 25.Luine, V. N., Jacome, L. F. & Maclusky, N. J. (2003) Endocrinology 144, 2836-2844. [DOI] [PubMed] [Google Scholar]
- 26.Ennaceur, A., Neave, N. & Aggleton, J. P. (1997) Exp. Brain Res. 113, 509-519. [DOI] [PubMed] [Google Scholar]
- 27.Allen, P. B., Ouimet, C. C. & Greengard, P. (1997) Proc. Natl. Acad. Sci. USA 94, 9956-9961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feng, J., Yan, Z., Ferreira, A., Tomizawa, K., Liauw, J. A., Zhuo, M., Allen, P. B., Ouimet, C. C. & Greengard, P. (2000) Proc. Natl. Acad. Sci. USA 97, 9287-9292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kennedy, M. B. (1997) Trends Neurosci. 20, 264-268. [DOI] [PubMed] [Google Scholar]
- 30.Kennedy, M. B. (1998) Brain Res. Brain Res. Rev. 26, 243-257. [DOI] [PubMed] [Google Scholar]
- 31.El-Husseini, A. E., Schnell, E., Chetkovich, D. M., Nicoll, R. A. & Bredt, D. S. (2000) Science 290, 1364-1368. [PubMed] [Google Scholar]
- 32.Weiland, N. G. (1992) Endocrinology 131, 2697-2702. [DOI] [PubMed] [Google Scholar]
- 33.Gazzaley, A. H., Weiland, N. G., McEwen, B. S. & Morrison, J. H. (1996) J. Neurosci. 16, 6830-6838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adams, M. M., Shah, R. A., Janssen, W. G. & Morrison, J. H. (2001) Proc. Natl. Acad. Sci. USA 98, 8071-8076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adams, M. M., Morrison, J. H. & Gore, A. C. (2001) Exp. Neurol. 170, 171-179. [DOI] [PubMed] [Google Scholar]
- 36.Daniel, J. M. & Dohanich, G. P. (2001) J. Neurosci. 21, 6949-6956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Calakos, N., Bennett, M. K., Peterson, K. E. & Scheller, R. H. (1994) Science 263, 1146-1149. [DOI] [PubMed] [Google Scholar]
- 38.Calakos, N. & Scheller, R. H. (1996) Physiol. Rev. 76, 1-29. [DOI] [PubMed] [Google Scholar]
- 39.Calakos, N. & Scheller, R. H. (1994) J. Biol. Chem. 269, 24534-24537. [PubMed] [Google Scholar]
- 40.Sudhof, T. C. (1995) Nature 375, 645-653. [DOI] [PubMed] [Google Scholar]
- 41.Sherwin, B. B. (1998) Int. J. Fertil. Womens Med. 43, 98-103. [PubMed] [Google Scholar]
- 42.Daniel, J. M., Fader, A. J., Spencer, A. L. & Dohanich, G. P. (1997) Horm. Behav. 32, 217-225. [DOI] [PubMed] [Google Scholar]
- 43.Bimonte, H. A. & Denenberg, V. H. (1999) Psychoneuroendocrinology 24, 161-173. [DOI] [PubMed] [Google Scholar]
- 44.Fader, A. J., Johnson, P. E. & Dohanich, G. P. (1999) Pharmacol. Biochem. Behav. 62, 711-717. [DOI] [PubMed] [Google Scholar]
- 45.Frick, K. M., Fernandez, S. M. & Bulinski, S. C. (2002) Neuroscience 115, 547-558. [DOI] [PubMed] [Google Scholar]
- 46.Choi, J., Romeo, R., Brake, W., Bethea, C., Rosenwakes, Z. & McEwen, B. (2003) Endocrinology 144, 4734-4738. [DOI] [PubMed] [Google Scholar]
- 47.Woolley, C. S. & McEwen, B. S. (1994) J. Neurosci. 14, 7680-7687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Satoh, A., Nakanishi, H., Obaishi, H., Wada, M., Takahashi, K., Satoh, K., Hirao, K., Nishioka, H., Hata, Y., Mizoguchi, A. & Takai, Y. (1998) J. Biol. Chem. 273, 3470-3475. [DOI] [PubMed] [Google Scholar]
- 49.Halpain, S., Hipolito, A. & Saffer, L. (1998) J. Neurosci. 18, 9835-9844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fischer, M., Kaech, S., Knutti, D. & Matus, A. (1998) Neuron 20, 847-854. [DOI] [PubMed] [Google Scholar]
- 51.Matus, A. (2000) Science 290, 754-758. [DOI] [PubMed] [Google Scholar]
- 52.Yan, Z., Hsieh-Wilson, L., Feng, J., Tomizawa, K., Allen, P. B., Fienberg, A. A., Nairn, A. C. & Greengard, P. (1999) Nat. Neurosci. 2, 13-17. [DOI] [PubMed] [Google Scholar]
- 53.Stafstrom-Davis, C. A., Ouimet, C. C., Feng, J., Allen, P. B., Greengard, P. & Houpt, T. A. (2001) Learn. Mem. 8, 272-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Steward, O. & Schuman, E. M. (2001) Annu. Rev. Neurosci. 24, 299-325. [DOI] [PubMed] [Google Scholar]
- 55.McEwen, B., Akama, K., Alves, S., Brake, W. G., Bulloch, K., Lee, S., Li, C., Yuen, G. & Milner, T. A. (2001) Proc. Natl. Acad. Sci. USA 98, 7093-7100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Milner, T. A., McEwen, B. S., Hayashi, S., Li, C. J., Reagan, L. P. & Alves, S. E. (2001) J. Comp. Neurol. 429, 355-371. [PubMed] [Google Scholar]
- 57.Globus, A. & Scheibel, A. B. (1967) Exp. Neurol. 19, 331-345. [DOI] [PubMed] [Google Scholar]
- 58.Greenough, W. T. & Volkmar, F. R. (1973) Exp. Neurol 40, 491-504. [DOI] [PubMed] [Google Scholar]
- 59.Popov, V. I. & Bocharova, L. S. (1992) Neuroscience 48, 53-62. [DOI] [PubMed] [Google Scholar]
- 60.Popov, V. I., Bocharova, L. S. & Bragin, A. G. (1992) Neuroscience 48, 45-51. [DOI] [PubMed] [Google Scholar]
- 61.Van Harreveld, A. & Fifkova, E. (1975) Exp. Neurol. 49, 736-749. [DOI] [PubMed] [Google Scholar]
- 62.Fifkova, E. & Anderson, C. L. (1981) Exp. Neurol. 74, 621-627. [DOI] [PubMed] [Google Scholar]
- 63.Desmond, N. L. & Levy, W. B. (1983) Brain Res. 265, 21-30. [DOI] [PubMed] [Google Scholar]
- 64.Desmond, N. L. & Levy, W. B. (1990) Synapse 5, 139-143. [DOI] [PubMed] [Google Scholar]
- 65.Desmond, N. L. & Levy, W. B. (1988) Brain. Res. 453, 308-314. [DOI] [PubMed] [Google Scholar]
- 66.Desmond, N. L. & Levy, W. B. (1986) J. Comp. Neurol. 253, 476-482. [DOI] [PubMed] [Google Scholar]
- 67.Smith, S. J. (1988) Science 242, 708-715. [DOI] [PubMed] [Google Scholar]
- 68.Engert, F. & Bonhoeffer, T. (1999) Nature 399, 66-70. [DOI] [PubMed] [Google Scholar]
- 69.Sorra, K. E. & Harris, K. M. (2000) Hippocampus 10, 501-511. [DOI] [PubMed] [Google Scholar]
- 70.Chang, F. L. & Greenough, W. T. (1984) Brain Res. 309, 35-46. [DOI] [PubMed] [Google Scholar]