Abstract
Cyclic peptides are of considerable interests in drug discovery and nanotechnology. However, macrocyclization of peptides and other compounds has often been perceived as synthetically challenging and the cyclization yields are affected by several factors including the ring size, peptide sequence, and the reaction conditions. Through the screening of combinatorial peptide libraries, we analyzed the cyclization efficiency of >2 million peptide sequences to determine the effect of ring size, peptide sequence, and solvent on the backbone (N-to-C) cyclization of peptides. Our results show that on-resin cyclization of medium- and large-sized rings (cyclohexapeptides and above) with PyBOP is essentially quantitative for ≥99.96% of the sequences, with small amounts of dimer formation observed for <4% of these sequences. Cyclization of small rings (cyclotetrapeptides and cyclopentapeptides) is considerably more difficult and accompanied by significant cyclic dimer formation. Peptides that are difficult to cyclize are generally rich in Lys(Boc) and Arg(Pbf) residues as well as sterically hindered residues [e.g., Thr(tBu)] at the N-terminus. The majority of these difficult sequences can be cyclized to completion by the addition of aqueous additives to the cyclization reaction.
Keywords: Cyclic Peptides, Macrocyclization, Combinatorial chemistry, One-bead-two-compound library, Partial Edman degradation, Peptide Sequencing
INTRODUCTION
Cyclic peptides and peptidomimetics represent a large, privileged, and yet underexploited class of molecules for drug discovery.1,2 Due to their reduced conformational freedom (relative to their linear counterparts), cyclic peptides exhibit improved metabolic stability and binding affinity/specificity to their molecular targets.3–5 To date, several naturally occurring as well as synthetic cyclic peptides have advanced to the clinic.6 These successes have inspired researchers to synthesize and screen large libraries of natural product-like cyclic peptides to meet other medical needs and serve as biomedical research tools. It is believed that, with molecular sizes in the “middle space” (molecular weight in the 500–2000 range), cyclic peptides may be ideally suited for drug targets that have been challenging for traditional small molecules, such as those involved in protein-protein interactions.
Macrocyclization is generally considered a significant synthetic challenge.2,7 Backbone cyclization of peptides is often plagued by epimerization of the C-terminal residue (whose α-NH2 is protected by an acyl group) and the formation of dimers, trimers, and oligomers.8 Earlier studies involved individual peptides and showed that the rate and yield of the cyclization step is strongly sequence dependent and the sequence dependence differs for different ring sizes.8–14 These studies led to some important observations. For example, linear peptide precursors that form pre-organized conformations in which the N- and C-termini are positioned next to each other usually have high cyclization efficiency.9–12 Peptides containing alternating D- and L-amino acids also cyclize efficiently, presumably due to less steric clashes among the side chains.12–14 It was also concluded that the geometry of the peptide scaffold outweighs the actual amino acid sequence.12 However, due to the small number of sequences examined by the previous studies, a general conclusion on the effect of precursor sequence and other factors on peptide cyclization has not been reached. More recently, Fluxa and Reymond reported an elegant study of peptide cyclization efficiency by screening a resin-bound library of 15,625 octapeptides [XXXXXKXE-(β-Ala-β-Ala-TentaGel)-OAll].15 They found that fast-cyclizing sequences often contained turn elements (e.g., Pro), whereas slow-cyclizing sequences were rich in basic and polar residues, in particular an N-terminal Thr and an Arg-His-Ser motif next to the N-terminal residue. However, their study was limited to octapeptides and employed only 5 different amino acids at each of the six random positions for a total of 15 different amino acids in the library. It is unclear whether the trends observed with octapeptides could be applied to shorter or longer peptides. In this work, we expanded the study of Fluxa and Reymond to analyze much larger peptide libraries of different ring sizes (theoretical diversity up to 1.3 × 109) to examine the effect of both peptide sequence and ring size on the cyclization efficiency. Our results show that the on-resin cyclization of medium- to large-sized peptides (hexa- to dodecapeptides) is remarkably efficient; ≥99.96% of the library sequences were quantitatively cyclized within 2 h. Tetra- and pentapeptides are generally more difficult to cyclize and the cyclization is accompanied by significant dimer and oligomer formation. We also found that poorly cyclizing peptides are rich in Arg, Lys, and Thr residues. Appropriate modification of the cyclization reaction conditions improved the cyclization yields of the otherwise poorly cyclizing peptides.
RESULTS AND DISCUSSION
Peptide Library Design and Synthesis
To systematically assess the effect of peptide sequence and ring size on cyclization efficiency, we designed a series of peptide libraries containing small- to medium-sized rings (from tetrapeptides to dodecapeptides), which are the most popular ring sizes in drug discovery. Tetra- to octapeptide libraries (libraries I–V) were synthesized in the form of NH2-XnE-BBNRM-resin [where n = 3–7 and X is (L)-2-aminobutyric acid (Abu or U, used as a replacement of cysteine), L-norleucine (Nle, as a replacement of methionine), or any of the 20 proteinogenic amino acids except for Cys and Met]. Nona- to dodecapeptide libraries were prepared by adding increasing number of Ala residues to the N-terminal side of the X7 library, NH2-AmX7E-BBNRM-resin (libraries VI–IX, where m = 1–4). Complete randomization of all 11 positions with 20 amino acids would give a library size of 2.0 × 1014, which is not practically possible for the methodology employed in this work. Libraries I–IX have theoretical diversities that range from 8000 to 1.28 × 109. The C-terminal Met was introduced for later cleavage of the peptide from the resin for mass analysis, while the tetrapeptide BBNR (where B is β-alanine) was added to facilitate mass spectral analysis.16,17 The fixed Glu residue provides a handle for attachment to the resin (via its side chain carboxyl group) and an α-carboxyl group for peptide N- to C- cyclization. Met was replaced by Nle in the random region to avoid internal cleavage during peptide release with CNBr, whereas substitution of Abu for Cys eliminated any complication associated with Cys oxidation.
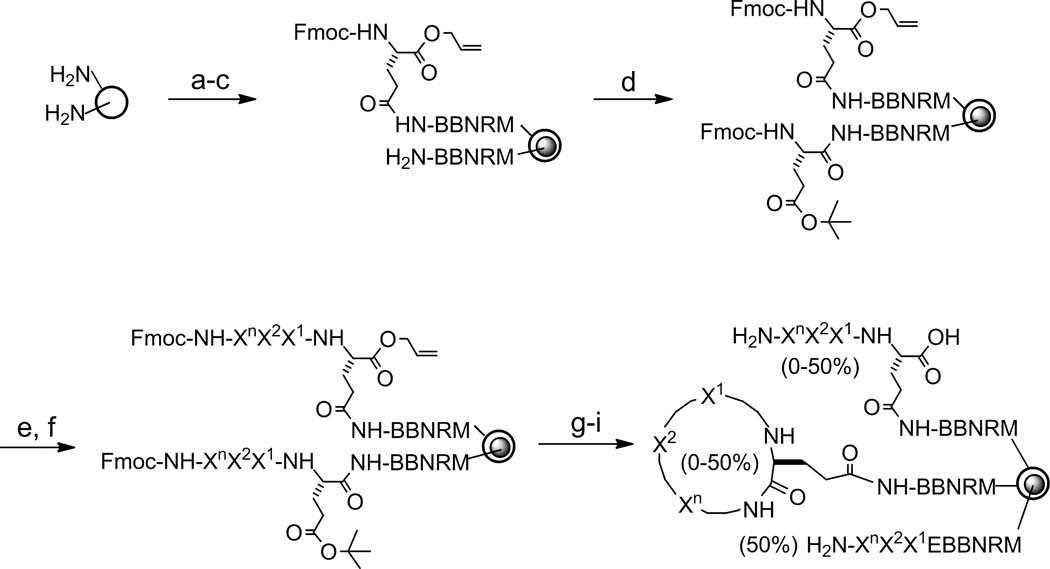
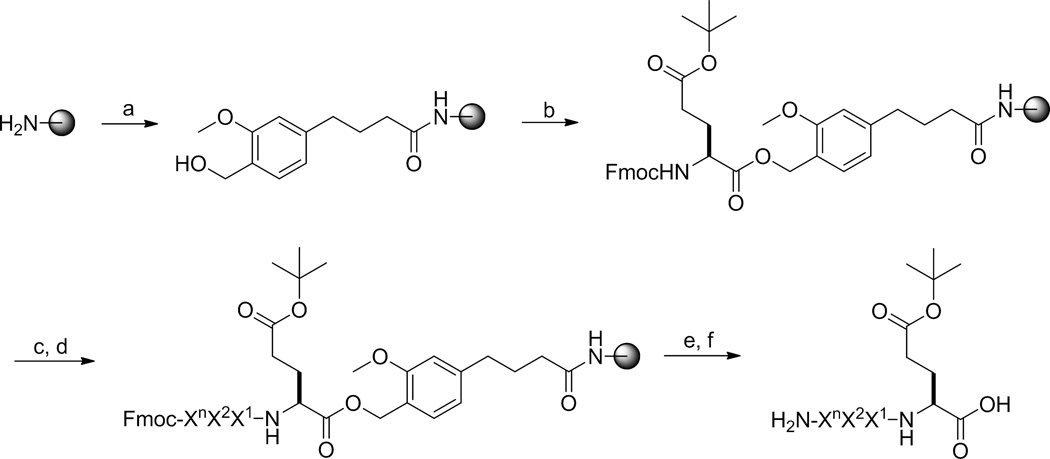
The libraries were synthesized on 90-µm TentaGel resin, starting with the addition of the BBNRM linker using standard Fmoc chemistry (Figure 1). In order to leave a portion of the peptides uncyclized to serve as encoding tags for later sequence identification, each library bead was spatially segregated into two layers, with peptide cyclization occurring only in the surface layer and the linear encoding tag confined to the bead interior.18,19 Briefly, TentaGel beads bearing the NH2-BBNRM linker are soaked in water, drained, and quickly suspended in 50:50 (v/v) CH2Cl2/Et2O containing 0.5 equiv of Nα-Fmoc-Glu(δ-NHS)-OAll, whose side chain carboxyl group was activated as an N-hydroxysuccinimidyl (NHS) ester and its α-carboxyl was protected as an allyl ester. This resulted in the acylation of the N-terminal amine for peptides on the bead surface (~50% of all peptides). The remaining (~50%) N-terminal amines in the bead interior were next acylated with Fmoc-Glu(tBu)-OH. Subsequent synthesis of the random positions was carried out by the split-and-pool method.20–22 Finally, the N-terminal Fmoc group and the α-allyl group on the C-terminal glutamate were removed by piperidine and Pd(PPh3)4, respectively. Treatment with benzotriazol-1-yl-oxytripyrrolidinophosphonium hexafluorophosphate (PyBOP) cyclized the surface peptides, while the peptides in the bead interior were kept in the linear form. In the resulting one-bead-two-compound (OBTC) library, each bead should contain a unique cyclic peptide (~50 pmol if the cyclization reaction is complete) and a varying amount (0–50 pmol) of uncyclized peptide on its surface (if cyclization reaction is incomplete) and ~50 pmol of the corresponding linear peptide in its interior.
Figure 1.
Synthesis of peptide libraries. Reagents and Conditions: (a) standard Fmoc/HBTU chemistry; (b) soak in water; (c) 0.5 equiv Fmoc-Glu(δ-NHS)-OAll in Et2O/CH2Cl2; (d) Fmoc-Glu(OtBu)-OH, HATU; (e) piperidine; (f) split-and-pool synthesis by Fmoc/HBTU Chemistry; (g) Pd(PPh3)4, Et2NH; (h) piperidine; (i) PyBOP, HOBt. B, β-alanine; n = 1–9.
Library Screening
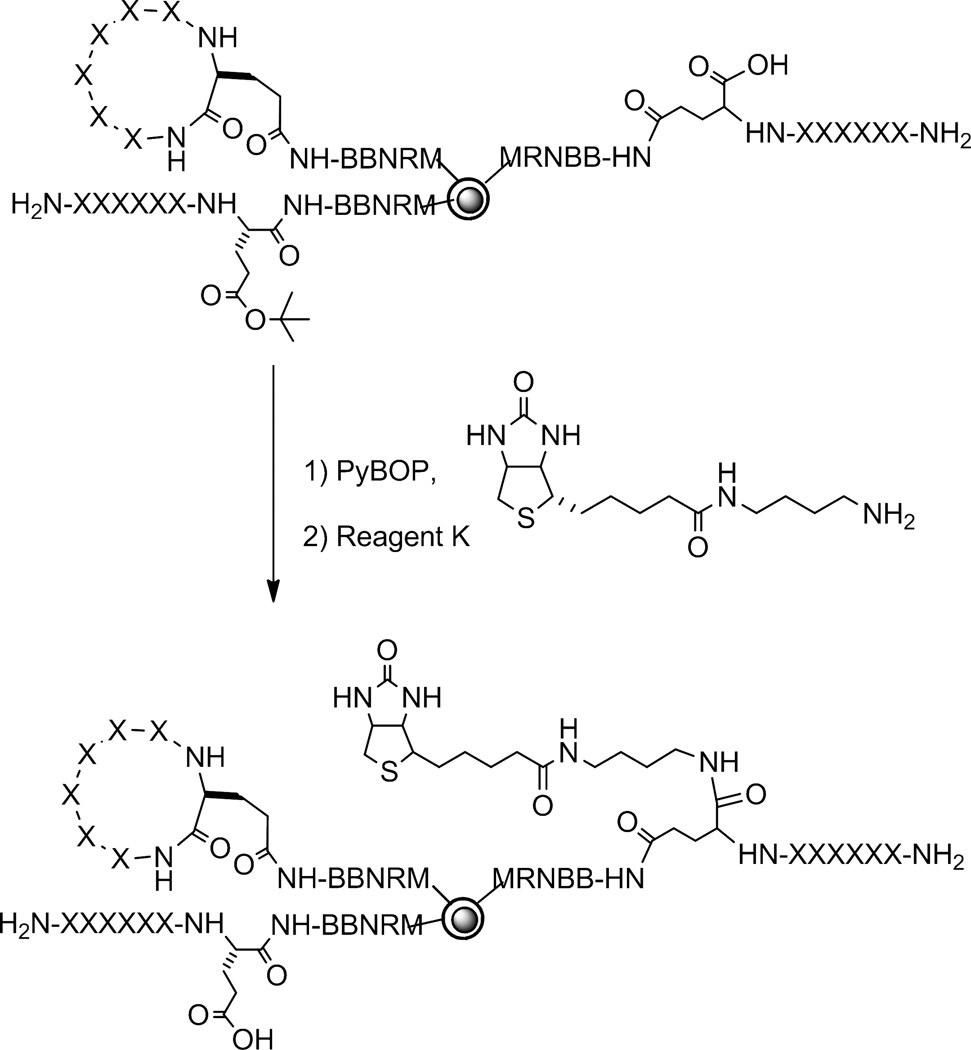
The screening procedure was designed to identify both efficiently cyclizing sequences and peptides that are resistant to cyclization. To identify fast cyclizing sequences, the cyclization reaction (with PyBOP/HOBt/DIPEA) was allowed to proceed for a short period of time (2–5 min) before being terminated by removal of the coupling reagents. The library (with all of the amino acid side chains still protected) was treated with a biotinylated amine and PyBOP (5 equiv) (Figure 2). If cyclization failed to occur or was incomplete on a bead, the remaining α-carboxyl group of the C-terminal Glu would be biotinylated. Following side-chain deprotection, the library was subjected to an enzyme-linked assay involving the streptavidin-alkaline phosphatase (SA-AP) conjugate and the phosphatase substrate 5-bromo-4-chloro-3-indolyl phosphate (BCIP).23 Any beads containing uncyclized peptides in the surface layer would recruit SA-AP and become turquoise colored, whereas beads on which the cyclization reaction was complete would remain colorless. Given the high sensitivity of the SA-AP/BCIP method, our conservative estimate is that any bead having a cyclization efficiency ≤99.9% (i.e., containing ≥0.1% unreacted carboxyl group) would be colored. The fast cyclizing peptides were then identified by manually isolating the colorless beads from the library and sequencing the linear encoding peptide inside the bead by the partial Edman degradation-mass spectrometry (PED-MS) method.16,17 To identify the poorly cyclizing peptides, the cyclization reaction was allowed to proceed for an extended period of time (90–120 min) and following the enzyme-linked assay, the turquoise colored beads were isolated and sequenced. For each library, a positive control was performed by subjecting a portion of the uncyclized library (just before the PyBOP step) to the screening procedure; all beads became turquoise colored, demonstrating that the screening protocol does not generate false negatives. A negative control reaction was also carried out under the same conditions (but in the absence of the biotinylated amine) and no colored bead was found in any of the control reactions. This indicates that the colored beads from the screening reactions were not due to direct binding of SA-AP to the library peptides. Note that the screening procedure does not differentiate the formation of cyclic monomers, dimers, or oligomers, which all result in colorless beads (if the reaction is complete). Therefore, all of the beads subjected to PED-MS analysis were also examined for the extent of dimerization and oligomerization. Ile, Leu, and Nle were not differentiated in this work, as their differentiation would require the addition of capping agents (CD3CO2H or CH3CD2CO2H) during library synthesis,17 which would result in false positive beads. Lys and Gln were differentiated by the partial conversion of Gln into a pyroglutamate and therefore the appearance of an extra peak at m/z M-17 in mass spectra.17 Cyclization may also cause epimerization at the Glu residue, which was not examined in the current work.
Figure 2.
Reactions involved in library screening.
Effect of Ring Size and Peptide Sequence on Cyclization Efficiency
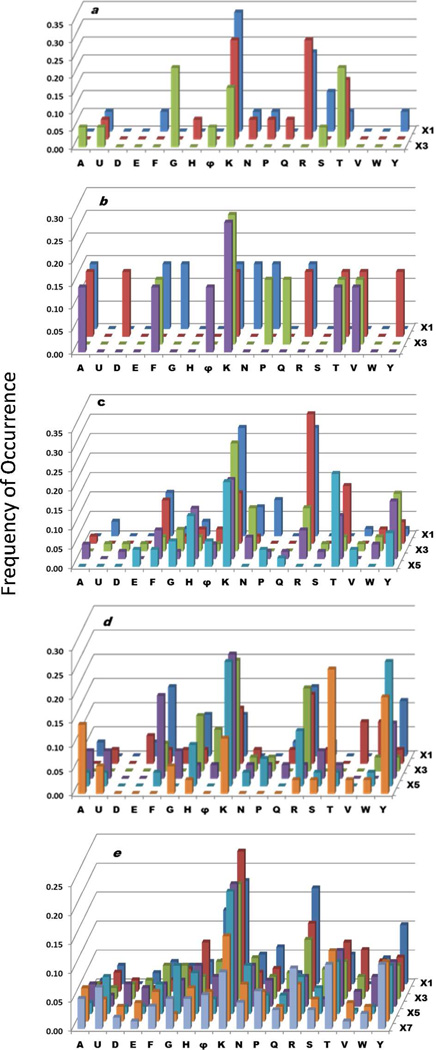
A total of 20 mg of library I, X3X2X1EBBNRM-resin (theoretical diversity of 8000), was screened. After treating a portion of the resin (10 mg, ~30,000 beads each) with PyBOP/HOBt/DIPEA for 2 min at room temperature, ~75% of the beads became colorless in the SA-AP/BCIP assay, indicating that for the majority of library sequences, peptide cyclization was complete within 2 min. Twenty colorless beads were randomly selected for PED-MS sequencing, resulting 11 complete sequences and 9 partial sequences (Table 1 and Table S1 in Supporting Information). As one would expect from the high percentage of cyclized peptides, the fast cyclizing peptides do not display any obvious sequence trend (Supplementary Figure S1). Cyclization of the remaining resin (10 mg) for 90 min produced 25 colored beads (0.083%), which were sequenced to give 15 complete sequences (plus 10 partial sequences). The poor cyclizing sequences were rich in lysine and arginine residues [whose side chains were protected by t-butoxycarbonyl (Boc) and 2,2,4,6,7-pentamethyldihydrobenzofurane (Pbf) groups, respectively], especially at the X1 and X2 positions (Figure 3a and Table S2). There was also a notable overrepresentation of t-butyl protected Thr near the N-terminus (at X2 and X3 positions). MS analysis revealed that six out of the 11 fast-cyclizing sequences (54%) and two of the 15 slow-cyclizing sequences (13%) formed significant amounts of cyclic dimers (Table 1).
Table 1.
Cyclization Efficiency of Small- to Medium-Sized Peptides
| Library No. (sequence) |
Theoretical diversity |
Fast Cyclization Sequences | Slow Cyclization Sequences | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No of beads screened |
No. of colorless beads at 2 or 5 min (%) |
No. of beads sequenced (No. of full sequences) |
No of beads with dimer formation |
No. of beads screened |
No. of colored beads at 90 min (%) |
No. of full sequences |
No. of beads with dimer formation |
|||
| I | X3E | 8.0 × 103 | 30,000 | 75 | 20 (11) | 6 (54%) | 30,000 | 25 (0.083) | 15 | 2 (13%) |
| II | X4E | 1.6 × 105 | 60,000 | 70 | 20 (11) | 8 (73%) | 60,000 | 13 (0.02) | 7 | 1 (14%) |
| III | X5E | 3.2 × 106 | 210,000 | 65 | 101 (46) | 2 (4.3%) | 210,000 | 75 (0.036) | 50 | 1 (2.0%) |
| IV | X6E | 6.4 × 107 | 210,000 | 65 | 89 (61) | 0 (0%) | 210,000 | 51 (0.024%) | 34 | 0 (0%) |
| V | X7E | 1.3 × 109 | 420,000 | 60 | 126 (109) | 1 (0.9%) | 420,000 | 166 (0.04%) | 157 | 0 (0%) |
Figure 3.
Histograms showing the amino acid composition/sequence of poorly cyclizing peptides selected from libraries I (a) to V (e). The y axis value represents the frequency (maximum value 1.0) at which each amino acid (x axis) was selected at a given random position (X1 to X7 on the z axis). φ = Ile, Leu and Nle. U = Abu.
Similar results were obtained with library II, X4X3X2X1E-BBNRM-resin (theoretical diversity = 160,000). Treatment with PyBOP for 2 min resulted in complete cyclization for ~70% of the library beads. After cyclization for 90 min, only 13 turquoise colored beads were obtained (out of 60,000 beads or 0.02%). The small number of complete sequences (7) precluded any reliable statistical analysis, but the slow-cycling peptides again showed a higher than expected number of Lys(Boc) selected (Table S2 and Figure 3b). Dimer formation was observed for 73% of the fast-cyclizing sequences and 14% of the slow-cyclizing peptides (Table 1).
Cyclization of library III (X5X4X3X2X1EBBNRM-resin, theoretical diversity of 3,200,000) for 5 min resulted in ~65% colorless beads (Table 1). Sequencing analysis of 101 randomly selected colorless beads gave 46 complete and 55 partial sequences (Table S1). Cyclization of 70 mg of resin (210,000 beads) for 90 min resulted in 75 turquoise colored beads (0.036%), which were sequenced to give 50 full sequences (Table S2). The poorly cyclizing peptides were rich in Lys(Boc) at all five random positions, Arg(Pbf) near the C-terminus, and Thr(tBu) and to a lesser extent Tyr(tBu) near the N-terminus (Figure 3c). In particular, many of the poorly cyclizing sequences contained KK, RR, KR, RK, TK, or KT motifs (Table S2). Unlike the tetra- and pentapeptides described above, the hexapeptides are much less prone to dimerization; dimer formation was observed for only two of the 46 fast-cyclizing sequences (4.3%) and one slow cyclizing sequence (2.0%) (Table 1). Screening of 140 mg (~420,000 beads) of library IV, X6X5X4X3X2X1EBBNRM-resin (theoretical diversity = 6.4 × 107) showed the same trends as the hexapeptide library (Tables 1, S1, and S2; Figure 3d). No dimer formation was observed for any of the 140 beads analyzed by mass spectrometry.
The octapeptide library V (X7EBBNRM-resin, theoretical diversity of 1.28 × 109) revealed an overall similar trend to that of the hexa- and heptapeptide libraries. Approximately 60% of the library sequences were cyclized to completion within 5 min (Table 1). The 166 poorly cyclizing sequences (0.04%) showed a higher frequency of Lys(Boc), Arg(Pbf), Thr(tBu), and Tyr(tBu) residues (Table S4 and Figure 3e), but the trend was less dramatic relative to that of hexa- and heptapeptide libraries, as the library became more diverse and the sequence motifs (e.g., KT and TK) that inhibit peptide cyclization were spread over a larger sequence space. Octapeptides also have low tendency for dimer formation; only one out of the 292 sequences analyzed had detectable amounts of dimer. Libraries VI–IX, which all contained seven random residues but have different ring sizes (by having 1–4 Ala added to the ring) gave similar results to the octapeptide library (Supplementary Figure S2).
Our data allowed some generalizations to be made. First, on-resin cyclization of hexa- and longer peptides is remarkably efficient. The majority of the library sequences underwent complete cyclization within 5 min and after 90 min, ≥99.96% of all peptide sequences were completely cyclized (Table 1). For these medium- and large-sized rings, dimerization was a rare event, with <4% of the sequences showing detectable dimers. This result is in agreement with our previous observation with a cyclooctapeptide library.19 On the other hand, formation of smaller rings (cyclotetra- and cyclopentapeptides) is considerably more challenging, as the majority of the fast-cyclizing sequences (54% and 73% for tetra- and pentapeptides, respectively) produced significant amounts of cyclic dimers. Apparently, the greater steric demand associated with the formation of small rings slowed down the intramolecular reaction, allowing the competing intermolecular reaction (dimerization) to occur. Subsequent cyclization of the linear dimer, which generates a much larger ring, is expected to be sterically less demanding. The challenge associated with cyclization of tetra- and pentapeptides in the all-L-configuration is well documented in the literature.24 Incorporation of D-amino acids (which reduce the steric hindrance) or β-turn motifs (e.g., Gly-Pro and Sar-Pro) has been shown to facilitate the formation of these small rings.12–14, 24 It should be noted that formation of still smaller rings (i.e., cyclodipeptide or 2,5-diketopiperazine) is relatively straightforward.25
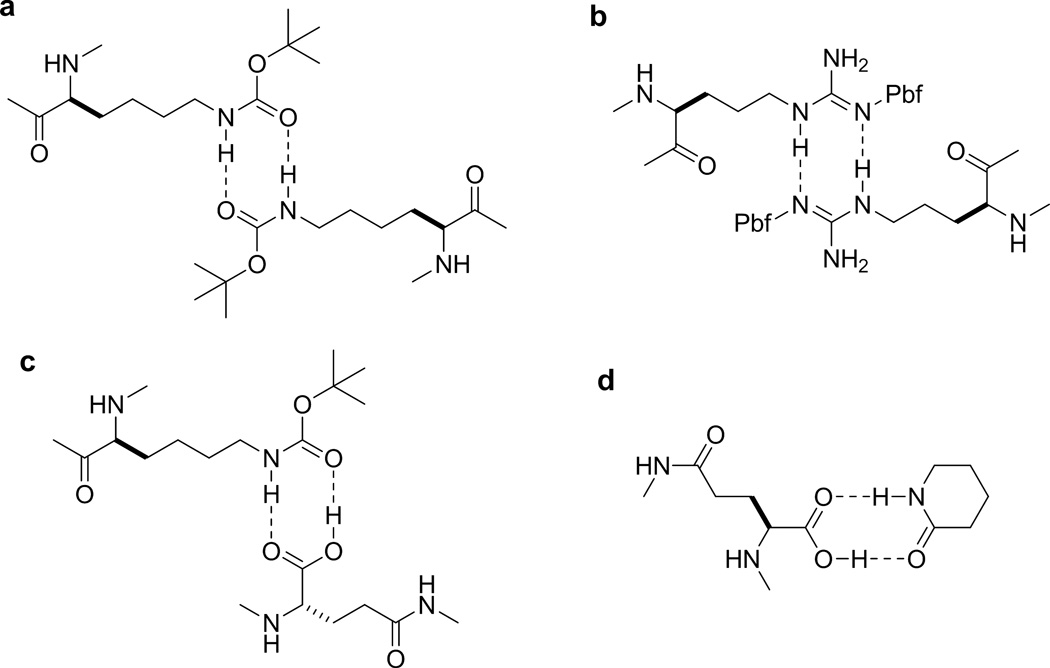
Second, the poorly cyclizing sequences are generally rich in basic amino acids (Arg and Lys) and a Thr near the N-terminus, in agreement with the previous observation by Fluxa and Reymond.15 Since neither t-butyl protected Ser nor β-branched Val and Ile residues were overrepresented among the poorly cyclizing sequences, the exceptional bulkiness of the t-butyl protected Thr likely reduced the nucleophilicity of the N-terminal amine. A unique feature of Pbf-protected Arg and Boc-protected Lys is that their side chains are capable of forming bidentate hydrogen bonds with each other and with the carboxyl group (Figure 4a–c). In the polar aprotic solvent used for peptide cyclization (DMF), the Lys(Boc) and Arg(Pbf) side chains may form intra- or intermolecular hydrogen bonds with the C-terminal carboxyl group and inhibit the cyclization reaction. Alternatively, formation of a hydrogen bond network among peptide chains may cause compaction of the resin and prevent the diffusion of the coupling reagents into the resin. As previously reported by Tang et al.,13 Tyr(tBu) at the N- or C-terminus also inhibited peptide cyclization, but apparently only for the medium-sized rings (hexa- to octapeptides) (Figure 3). Finally, Pro was overrepresented at the X1 position (which is immediately N-terminal to the invariant Glu residue) but disfavored at the N-terminal position among the fast cyclizing peptides (especially the hexa- and longer peptides) (Supplementary Figure S1). The ability of proline to accommodate cis- peptide bonds presumably brings the peptide N- and C-termini close to each other and facilitates intramolecular lactamization.15 However, its secondary amine group is more hindered and thus less nucleophilic than the α-amine of other proteinogenic amino acids.
Figure 4.
Bidentate hydrogen bonding interactions between Boc-protected Lys side chains (a), Pbf-protected Arg side chains (b), Lys(Boc) side chain and Glu α-carboxyl group (c), and Glu α-carboxyl group and 2-piperidone (d).
On-Resin Cyclization Efficiency of Selected Peptides
To confirm the library screening results, we arbitrarily selected five fast cyclizing and eight poorly cyclizing sequences for individual synthesis and on-resin cyclization (Table 2, peptides 1–13). The peptides were synthesized on TentaGel resin in the same manner as the libraries and allowed to cyclize between the N-terminus and the α-carboxyl group of the C-terminal Glu for 25 min (fast cyclizing peptides) or 2.5 h (poorly cyclizing peptides) using PyBOP/HOBt/DIPEA in DMF. The peptides were then deprotected with Reagent K, released from the resin using CNBr, and analyzed by HPLC and/or MALDI-TOF mass spectrometry. MS analysis showed that all five fast-cyclizing peptides underwent complete cyclization within 25 min, as evidenced by the presence of strong signals for the intended cyclic peptides and the complete absence of signals for the corresponding linear peptides (Supplementary Figure S3). Peptides 1–3 (tetra-, penta-, and hexaapeptides, respectively) showed varying amounts of cyclic dimers (1–97%), whereas heptapeptide 4 and octapeptide 5 had no detectable dimer formation. Among the “poorly” cyclizing peptides tested, the MS spectra indicated that peptides 7, 8, 10 and 12 (TKYAE, TKTRRE, SKKFRHE, and TKKVSYKE) cyclized “quantitatively” to the desired monocyclic products (Table 2). Note that under the conditions employed in this work, neither the HPLC nor the MS analysis was sensitive enough to detect ~0.1% of an uncyclized peptide, the amount of which would render a bead turquoise colored during library screening by the SA-AP/BCIP method. The MS spectra of peptides 6, 9, 11 and 13 (TTKE, TKYRRE, KVTYRRE, and IKYKTNKE) showed signals for both cyclic and linear peptides. Therefore, the CNBr cleavage mixture of each peptide was analyzed by HPLC and the cyclization yields (39–72%) were determined by comparing the intensities of linear and cyclic peptide peaks (Supplementary Figure S4). The amount of remaining linear peptide ranged from 28 to 61%. Peptide 11 also produced significant amount of cyclic dimer (17%). Thus, the results obtained with individual peptides were in good agreement with the library screening data.
Table 2.
Solid-phase N-to-C cyclization of selected peptidesa
| entry | peptide | Cyclic Monomer (%) |
Linear (%) |
Cyclic Dimer (%) |
|---|---|---|---|---|
| 1 | PNPEb | 99 | 0 | 1 |
| 2 | NFFPEb | 3 | 0 | 97 |
| 3 | GGDNHEb | 97 | 0 | 3 |
| 4 | IHLENFEb | 100 | 0 | 0 |
| 5 | HEMIHYPEb | 100 | 0 | 0 |
| 6 | TTKEc | 39 | 61 | 0 |
| 7 | TKYAEc | 100 | 0 | 0 |
| 8 | TKTRREc | 100 | 0 | 0 |
| 9 | TKYRREc | 72 | 28 | 0 |
| 10 | SKKFRHEc | 100 | 0 | 0 |
| 11 | KVTYRREc | 52 | 31 | 17 |
| 12 | TKKVSYKEc | 100 | 0 | 0 |
| 13 | IKYKTNKEc | 45 | 55 | 0 |
Entry 1–5 are fast cyclizing peptides selected from the libraries, whereas entry 6–13 are poorly cyclizing peptides. All peptides contained the C-terminal sequence BBNRM.
Data from MS analysis
Data from HPLC and MS analysis
Solution-Phase Cyclization of Selected Peptides
To determine whether the sequence dependence observed for on-resin cyclization also applies to solution-phase reaction, we resynthesized peptides 1–8, 10 and 12 on TentaGel resin, with the α-carboxyl group of the invariant Glu attached to the resin via an acid labile linker, 4-(4-hydroxymethyl-3-methoxyphenoxy)-butyric acid (HMPB) (Figure 5). The peptides were released from the resin by treatment with 1% TFA and resulting side-chain protected peptides were subjected to solution-phase cyclization using PyBOP and analyzed by MS and/or HPLC (Table 3). In general, the two groups of peptides (fast vs poor cyclization on resin) do not seem to have any obvious difference in their in-solution cyclization efficiencies. Compared to the on-resin reaction, solution-phase cyclization was less likely to be complete and had greater amounts of dimer and in a few cases trimer formation. Thus, on-resin cyclization proved to be superior to the solution-phase reaction with respect to the reaction time and the amount of dimerization/oligomerization.
Figure 5.
Synthesis of peptides for solution-phase cyclization studies, where n represents 1–5 amino acids. (a) 4-(4-Hydroxymethyl-3-methoxyphenoxy)-butyric acid, HATU; (b) Fmoc-Glu(OtBu)-OH, DIC, DMAP (cat.); (c) 20% piperidine in DMF; (d) SPPS; (e) 20% piperidine in DMF; (f) 1% TFA in DCM.
Table 3.
Solution-phase N-to-C cyclization of selected peptidesa.
| entry | peptide | Monocyclic | Linear | Dimer | Trimer |
|---|---|---|---|---|---|
| 1 | PNPEc | +b | +++ | ++ | + |
| 2 | NFFPEc | + | − | ++ | + |
| 3 | GGDNHEc | +++ | − | − | − |
| 4 | IHLENFEc | +++ | +++ | +++ | − |
| 5 | HEMIHYPEd | +++ | − | + | − |
| 6 | TTKEc | + | − | − | − |
| 7 | TKYAEc | + | − | ++ | + |
| 8 | TKTRREd | +++ | +++ | ++ | − |
| 9 | SKKFRHEc | +++ | + | + | − |
| 10 | TKKVSYKEc | +++ | +++ | − | − |
Entry 1–5, fast cyclizing peptides from library screening; entry 6–10, poorly cyclizing peptides.
Semiquantitation of the amount of each species observed, with “+” representing small amounts, “+++” indicating large amounts, whereas “−“ indicating the absence of a given species.
Data from MS analysis
Data from HPLC and MS analysis
Improvement of Cyclization Efficiency by Solvent Optimization
To determine whether the hydrogen bonding ability of Arg(Pbf) and Lys(Boc) side chains was responsible for the slow cyclization and to improve the cyclization efficiency, we performed the cyclization reaction in the presence of various reagents that are capable of forming/breaking hydrogen bonds. Our initial attempt was 2-piperidone, which has the ability to form bidentate hydrogen bonds with Arg(Pbf) and Lys(Boc) side chains and should be able to break up any intra- and/or intermolecular hydrogen bond network. However, the addition of 2-piperidone (50 equiv relative to the peptide loading) to the cyclization reaction substantially decreased the cyclization efficiency, as evidenced by a much greater number of turquoise colored beads in the library (by two orders of magnitude). Addition of formamide (1% v/v) had the same effect. We hypothesize that 2-piperidone and formamide, like the side chains of Arg(Pbf) and Lys(Boc), may form bidentate hydrogen bonds with the C-terminal carboxyl group and inhibit the cyclization reaction (Figure 4d).
We next carried out the cyclization reaction (with library V) in two stages. During the first stage, the reaction was allowed to proceed in DMF for 90 min (normal condition), during which most of the library sequences should cyclize. During the second stage, the library was treated with fresh coupling reagents (PyBOP, HOBt and DIPEA) in the presence of increasing amounts of water (1%, 2%, 5% v/v). We reasoned that while water has exceptional ability to break hydrogen bonds, it cannot form bidentate hydrogen bonds with the carboxyl group. Indeed, the addition of water (1, 2 or 5%) reduced the number of colored beads by 1.5-fold, relative to the control (which had no added water during the second stage). Encouraged by this result, we also tested chaotropic salts (LiCl and KSCN) and the “Magic Mixture” (1:1:1 DMF/DCM/NMP plus 1% Triton X-100), which was previously reported to improve the coupling efficiency during solid-phase peptide synthesis.26,27 We found that the addition of 5 mg of LiCl (final concentration 0.168 M) or 130 mg of KSCN (2 M) reduced the number of colored beads by 2 fold. When a 3:2:2 mixture of DMF/DCM/NMP containing 1% (v/v) Triton X-100 was used as solvent for the cyclization reaction (second stage), the number of colored beads was reduced by 3-fold. Similar improvement of the cyclization yield was also observed with libraries VI–IX. Sequence analysis of the remaining colored beads revealed that they were still rich in Lys at all positions, Thr near the N- or C-terminus, and Arg near the C-terminus (Supplementary Figure S5).
CONCLUSION
In this work, we surveyed the cyclization efficiency of over 2 million peptide sequences. Our results confirmed the previous observations that the efficiency of backbone cyclization is influenced by many factors including ring size, peptide sequence, and the coupling reagents/conditions. Contrary to the widely held belief, we found that cyclization is remarkably efficient for cyclic peptides of medium and large rings (≥6 amino acids). Under the optimized cyclization conditions, ≥96% of the library members are quantitatively converted into the desired monocyclic peptides, with the remaining ≤4% of the sequences forming predominantly cyclic monomers and small amounts of cyclic dimers. It was previously shown that the type of resin especially the peptide loading can also significantly affect the cyclization yield.8 The relatively low peptide loading of the TentaGel resin (~0.2 mmol/g) used in this study likely contributed to the low incidence of peptide dimerization among these peptides. The poorly cyclizing peptides are generally rich in Lys(Boc) and Arg(Pbf), which apparently form bidentate hydrogen bonds with the C-terminal carboxyl group and reduce its reactivity. In addition, amino acids with bulky side chains [e.g., Thr(tBu)] at the N-terminus also slow down the cyclization reaction, likely due to steric hindrance. Additives that can break the hydrogen bonding network can improve the cyclization efficiency. Formation of smaller rings (4 or 5 amino acids) by lactamization is more difficult and accompanied by significant amounts of dimerization. These small rings may be prepared by alternative macrocyclization methods such as click chemistry,28 ring contraction,29 and multicomponent reactions.30
EXPERIMENTAL
Materials
TentaGel S NH2 resin (90 µm, 0.26 mmol/g loading, 2.86 × 106 beads/g) was purchased from Peptides International Inc. (Louisville, KY). All amino acids (unless otherwise noted) and PyBOP were purchased from NovaBiochem (Gibbstown, NJ). N-hydroxybenzotriazole (HOBt), and 2-(1H-benzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate (HBTU) were from Advanced ChemTech (Louisville, KY). N-hydroxysuccinimide (NHS), lithium chloride, Triton X-100, and N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC) were purchased from Sigma Aldrich (St. Louis, MO). Boc-1,4-diaminobutane, N,N’-dimethylformamide (DMF), and N-methylpyrrolidinone (NMP) were purchased from VWR (West Chester, PA). 2-(1H-7-azabenzotriazol-1-yl)-1,1,3,3-tetramethyl uronium hexafluorophosphate (HATU) and 1-hydroxy-7-azabenzotriazole (HOAt) were purchased from GenScript Corporation (Piscataway, NJ). NHS-Biotin and 4-(4-Hydroxymethyl-3-methoxyphenoxy)-butyric acid (HMPB Linker) were purchased from Chem-Impex International (Wood Dale, IL). 2-piperidone was purchased from TCI America (Portland, OR). Bio-Rad columns were purchased from Bio-Rad Laboratories (Hercules, CA). Peptide libraries were synthesized manually and automatically. Automated peptide library synthesis was completed on a Titan 357 split-and-pool peptide synthesizer purchased from Aapptec (Louisville, KY).
Synthesis of biotinylated 1,4-diaminobutane
To a solution of NHS-Biotin (100 mg, 0.293 mmol) in DMF (3 mL) was added Boc-1,4-diaminobutane (154 µL, 0.308 mmol, 2 M solution in DMF). The mixture was stirred overnight at room temperature. The solvent was removed under vacuum. The residue was dissolved with DCM (5 mL) and then concentrated. The crude product was treated with 95:5 TFA/ddH2O (1.5 mL) for 1 hour at room temperature without further purification. The contents were concentrated; the residue was dissolved with DCM (5 mL), and concentrated. The product was stored in DMF at a concentration of 17 mM and kept at 4 °C. 1H NMR (400 MHz, D2O) δ 4.63 (m, 1H), 4.44 (m, 1H), 3.40-3.20 (m, 3H), 3.15-2.90 (m, 4H), 2.45 (t, J = 6.8 Hz, 1H), 2.27 (t, J = 6.8 Hz, 1H), 1.77-1.55 (m, 8H), 1.51-1.39 (m, 2H). HRESI-MS: m/z calcd for C14H26N4O2SNa+ (M + Na+) 337.1674, found 337.1661.
Synthesis of Fmoc-Glu(δ-NHS)-OAll
Fmoc-Glu(δ-NHS)-OAll was synthesized according to literature procedure with modifications.19 To a solution of Fmoc-Glu-OAll (200 mg, 0.49 mmol) in DCM (2 mL) was added EDC (141 mg, 0.735 mmol) in 1 mL DCM and N-hydroxysuccinimide (85 mg, 0.735 mmol) in DCM (2 mL) sequentially. The mixture was stirred overnight under argon with condenser at room temperature. The reaction mixture was diluted with 10 mL DCM and washed twice with water. The organic layer was dried over MgSO4 and concentrated under vacuum. 1H NMR (400 MHz, CDCl3) δ 7.75 (d, J = 7.5 Hz, 2H), 7.59 (d, J = 7.4 Hz, 2H), 7.39 (d, J = 7.4 Hz, 2H), 7.30 (d, J = 7.4 Hz, 2 H), 5.83–5.95 (m, 1H), 5.47 (d, J = 8.2 Hz, 1H), 5.24–5.36 (m, 2H), 4.62–4.66 (m, 2H), 4.34–4.50 (m, 3H), 4.20 (t, J = 6.8 Hz, 1H), 2.78–2.83 (br s, 4H), 2.63–2.74 (m, 2H), 2.30–2.40 (m, 1H),2.07–2.16 (m, 1H).
Peptide Library Synthesis
Peptide libraries I–V were synthesized on 2.0 g of TentaGel S NH2 resin (90 µm, 0.26 mmol/g). All of the manipulations were performed at room temperature unless otherwise noted. The linker sequence (BBNRM) was synthesized manually using 4 equiv of Fmoc amino acid, 4 equiv. of HATU, 4 equiv. of HOAt and 8 equiv. of DIPEA. The coupling reaction was typically allowed to proceed for 2 h, and the beads were washed with DMF (3 × 10 mL) and DCM (3 × 10 mL). The Fmoc group was removed with 20% piperidine (2 × 10 min), and the beads were exhaustively washed with DMF (6 × 10 mL). After the synthesis of linker, the resin was washed with DCM (2 × 10 mL) and DMF (2 × 10 mL) and was soaked in DMF for 15 minutes, followed by mixtures of 3:1 DMF/water, 1:1 DMF/water, 1:3 DMF/water, and finally soaked in 100% degassed water overnight at room temperature. The water was drained and the resin was suspended in a solution of Fmoc-Glu(δ-NHS)-OAll (0.182 mmol, 0.35 equiv) in 15 mL of 1:1 (v/v) DCM/diethyl ether. The mixture was incubated on a rotary shaker for 30 min at room temperature. The beads were washed with 1:1 DCM/diethyl ether (3 × 10 mL) and DMF (5 × 10 mL) to remove water from the beads and then treated with 1.5 equiv of Fmoc-Glu(OtBu)-OH, 1.5 equiv. of HATU and 3.0 equiv. of DIPEA in DMF (40 min). Next, the Fmoc group was removed from both the inner and outer sequence by treatment with 20% piperidine in DMF (2 × 10 min) and the resin was washed with DMF (2 × 10 mL) and DCM (2 × 10 mL). The resin was then transferred to an automated peptide synthesizer for the random positions peptide synthesis. Stock solutions of 0.2 M amino acid in NMP, 0.2 M HATU in DMF, and 0.8 M DIPEA in NMP were used. For each reaction vessel, 0.7 mL of amino acid solution, 0.7 mL of HATU solution, and 0.35 mL of DIPEA solution were used for the coupling reaction. All coupling reactions were performed twice (2 h each time). Removal of Fmoc protection following each combine and split was carried out for 15 min with 20% piperidine in DMF, except following the addition of the first random position. In the latter case, the resin was treated with 20% piperidine in DMF for 7 min. After the third random position, the synthesis sequence was paused after each amino acid addition step to allow for the removal of resin from the vessel. Libraries VI–IX (100 mg resin for each) were synthesized by adding Ala residue(s) to the N-terminus of the X7E library. A blocking step with acetic anhydride was not included in the synthesis cycle, as N-terminal acetylation of unreacted peptides would prevent their cyclization and result in false positives (turquoise colored beads) during library screening.
On-Resin Cyclization of Peptide Libraries
The allyl protecting group was removed by treatment of 100 mg of resin (0.026 mmol) with a Pd-based deprotection cocktail. Triphenylphosphine (21 mg, 0.078 mmol) was dissolved in 1 mL of dry THF. Formic acid (9.8 mL, 0.26 mmol) and diethylamine (26.9 mL, 0.26 mmol) were added to the triphenylphosphine solution on ice. The mixture was added to Pd(PPh3)4 (30 mg, 0.026 mmol) on ice. The deprotection mixture was mixed and added to the resin. The resin was incubated overnight in the dark at room temperature. The resin was washed sequentially with THF (2 × 1 mL), DMF (2 × 1 mL), DCM (2 × 1 mL), and 0.5% DIPEA/DMF (1 × 1 mL) and was incubated in 0.5% DIPEA/DMF for 10 min. After washing with DMF (2 × 1 mL), the resin was treated with 0.5% sodium dimethyldithiocarbamate (w/v) in DMF for 30 min, and washed with DMF (3 × 1 mL) and DCM (3 × 1 mL). The Fmoc group was removed with 20% piperidine and the resin was washed thoroughly with DMF and DCM. For peptide cyclization, PyBOP/HOBt/DIPEA (5, 5, and 10 equiv, respectively) in DMF was added to the resin and the mixture was incubated on a rotary shaker for either 2 min (for the identification of good cyclizing sequences) or 90 min (for poorly cyclizing sequences). The resin was washed with DMF (2 × 1 mL) and DCM (1 × 1 mL).
Library Screening
After cyclization with PyBOP/HOBt/DIPEA for 2 or 90 min, the peptide library was incubated with biotinylated 1,4-diaminobutane (23.4 µL, 1.5 equiv) and PyBOP/HOBt/DIPEA (5, 5, and 10 equiv, respectively) in DMF (1 mL) for 2 h at room temperature on a rotary shaker. The resin was washed with DMF (2 × 1 mL) and DCM (2 × 1 mL). Side chain deprotection was performed using a modified Reagent K (80.5:5:5:5:2.5:1:1 TFA/phenol/H2O/thioanisole/EDT/TIS/anisole) for 2 h at room temperature. The resin was washed exhaustively with DCM (5 × 1 mL), DMF (2 × 1 mL), and water (5 × 1 mL) and then incubated in a blocking buffer (30 mM HEPES, pH 7.4, 150 mM NaCl, 0.05% Tween 20, and 0.1% gelatin) for 1 h with gentle mixing at 4 °C. The resin was drained and resuspended in the blocking buffer containing 1 µg/mL SA-AP, and incubated for 10 min at 4 °C with gentle mixing. The resin was drained and washed with the blocking buffer (2 × 1 mL) and SA-AP reaction buffer (30 mM Tris HCl, pH 8.5, 100 mM NaCl, 5 mM MgCl2, 20 µM ZnCl2, and 0.05% Tween 20) (2 × 1 mL). The resin was transferred to a Petri dish (60 × 15 mm) by using 2.7 mL (3 × 900 µL) of the SA-AP reaction buffer. Upon the addition of 300 µL of 5 mg/mL BCIP, turquoise color developed on positive beads in 45 min, when the staining reaction was terminated by the addition of 500 µL of 1 M HCl. The positive beads were picked manually with a pipet under a dissecting microscope and individually sequenced by the PED-MS method.16,17
Peptide Cyclization in the Presence of Additives
Each screening involved 5 mg of resin (0.0013 mmol). Cyclization was carried out using PyBOP, HOBt, DIPEA (5, 5, and 10 equiv., respectively) and an appropriate additive(s) in 700 µL of DMF. The reaction was terminated by draining and washing the resin with DMF (3 × 1 mL). Each screening included a negative control in which an equal amount of resin was allowed to cyclize in DMF for the same duration, in the absence of the additive(s). 2-Piperidone: 6 mg (50 equiv.) of 2-piperidone was added to the cyclization reaction mixture and the reaction was allowed to proceed for 1.5 h. Water: The resin was allowed to cyclize for 1.5 h without additive and was then treated with fresh reagents (PyBOP, HOBt, DIPEA) plus 1% (7 µL), 2% (14 µL) or 5% (35 µL) of water for additional 1 h. Formamide: The resin was cyclized for 1.5 h without additive and then treated with fresh reagents (PyBOP, HOBt, DIPEA) plus 1% (7 µL) of formamide for additional 1 h. LiCl: Same as water exception that 5 mg of LiCl (0.168 M final concentration) was added to reaction mixture and the reaction was allowed to proceed for additional 1 h. Cyclization in Magic mixture was carried out by suspending 5 mg of resin in 1 mL of 3:2:2 (v/v) DMF/DCM/NMP containing 1% (7 µL) Triton X-100 as the solvent for 2.5 h.
Evaluation of Individual Peptide for Cyclization
Synthesis of the linker portion of Fmoc-Glu(OAll)BBNRM-TentaGel began with TentaGel S-NH2 resin (2 g, 0.52 mmol). The coupling of each amino acid to the resin was completed using standard Fmoc SPPS protocol. Fmoc amino acid, HBTU, HOBt, and DIPEA (4, 4, 4, and 8 equiv., respectively) were used for each synthesis step. Typically, 10 mg (0.0026 mmol) of resin was subjected to Pd(PPh3)4 to remove the allyl protecting group (overnight), piperidine to remove the Fmoc group (2 × 10 min), and incubation with 1 M HOBt in DMF (20 min). Cyclization was allowed to proceed on a rotary shaker using PyBOP, HOBt and DIPEA (final concentration of 18.5, 18.5, and 37 mM, respectively) in 700 µL of DMF for 15 min for fast cyclizing peptides and 2.5 h for poor cyclizing peptides. The reaction was terminated by draining and washing resin extensively with DMF. Approximately 9 mg of resin was set aside for HPLC analysis, whereas the remaining 1 mg was subjected to CNBr cleavage. Typically, 1 mL of CNBr solution (40 mg in 1 mL of 70% TFA in water) was added to the resin in a Bio-Rad column. After overnight incubation, the solution was drained into a microcentrifuge tube and the solvent was removed in a vacuum concentrator. The resulting sample was then dissolved in 20 µL of 0.1% TFA in water and subjected to MALDI-TOF analysis. The cyclization yield was estimated from the peak abundance, assuming that the linear, cyclic, and dimeric peptides have approximately the same ionization efficiency.
HPLC analysis of Peptide Cyclization
The resin (9 mg) was treated overnight with 800 µL of CNBr solution (40 mg/mL in 70% TFA in water) in a Bio-Rad column with gentle mixing on a rotary shaker. The solution was drained into a microcentrifuge tube and the resin was rinsed with 700 µL of 70% TFA in water. The combined solution (~1.5 mL) was evaporated under reduced pressure in a vacuum concentrator. The resulting residue was dissolved in 200 µL of 0.01% TFA in water. Approximately 50 µL of the solution was analyzed by reversed-phase HPLC on a C18 column eluted with linear gradient of CH3CN in water containing 0.01% TFA. The following gradients were used: 0–30% CH3CN over 45 min for X3E peptides, 0–30% CH3CN over 65 min X4E peptides, 0–40% CH3CN over 55 min for X5E peptides, 0–30% CH3CN over 40 min for X6E peptides, and 0–40% CH3CN over 50 min for X7E peptides. All fractions containing significant peptide contents (as judged by absorbance at 214 nm) were collected and analyzed by MALDI-TOF mass spectrometry. The cyclization yield was calculated by integrating the area underneath the peak for monocyclic peptide and comparing with the total area for all peptide peaks.
Loading of Fmoc-Glu to Resin via HMPB Linker
TentaGel S-NH2 resin (2 g, 0.52 mmol) was washed extensively with DCM and DMF, and suspended in 10 mL of DMF. HMPB (375 mg, 1.56 mmol), HATU (593 mg, 1.56 mmol) and DIPEA (543 µL, 3.12 mmol) were mixed in 20 mL of DMF and added to the resin immediately. The reaction was allowed to proceed for 2 h on a rotary shaker and terminated by draining and washing the resin extensively with DMF. The resin was then re-suspended in 30 mL of 9:1 DCM/DMF mixture and reacted overnight with Fmoc-Glu(OtBu)-OH (332 mg, 0.78 mmol) using diisopropylcarbodiimide (DIC) (121 µL, 0.78 mmol) and N,N-dimethylaminopyridine (DMAP) (13 mg, 0.104 mmol) as the coupling reagents (with gentle mixing on a rotary shaker). The reaction was terminated by draining and washing extensively with DCM and DMF. Any unreacted hydroxyl group on the resin was acetylated by treatment with acetic anhydride (118 mg, 0.104 mmol), DIPEA (218 mg, 0.104 mmol) and DMAP (13 mg, 0.104 mmol) in 20 mL of DMF for 1 h.
Synthesis of Side chain-Protected Peptides
Each peptide was synthesized on 100 mg (0.026 mmol) of the Fmoc-Glu(tBu)-loaded resin in a Bio-Rad column using standard Fmoc/HBTU chemistry. The resulting resin/peptide (20 mg) was washed with DMF and 20% piperidine in DMF (1.3 mL) and incubated with 20% piperidine in DMF (2 × 10 min) to remove the N-terminal Fmoc group. After extensive washing with DMF and DCM, the resin was treated with 700 µL of 1% TFA in DCM (3 × 15 min). Evaporation of the combined solution under vacuum gave the peptide containing side chain protecting groups but with free N- and C-termini.
In-Solution Peptide Cyclization
The side chain-protected peptide (0.0052 mmol) was dissolved in 700 µL of DMF and mixed with DIPEA (4.5 µL, 0.026 mmol) in a glass vial. In a microcentrifuge tube, PyBOP (5.4 mg, 0.0104 mmol) and HOBt (1.6 mg, 0.0104 mmol) were dissolved in 300 µL of DMF. The resulting solution was added dropwise to the glass vial over 40 min using a 1-mL polypropylene syringe (3 drops/min) and the reaction was stirred at room temperature. After 2 (for fast cyclizing peptides) or 3 h of total reaction time (for poorly cyclizing peptides), the reaction mixture was split into 2 fractions, 100 µL for MS analysis and 900 µL for HPLC analysis. Both fractions were dried under vacuum and the residual DMF was removed by the addition of 100 µL of water to each sample, resulting in precipitation of the peptide. After centrifugation and removal of the solvent, the peptide was treated with 1.2 mL of 92.5:5:2.5 TFA/water/triethylsilane for 2 h. The solvent was removed under vacuum and the sample for MS analysis was dissolved in 400 µL of 3:1 water/CH3CN containing 0.01% TFA, whereas the sample for HPLC analysis was dissolved in 200 µL of water containing 0.01% TFA. Fifty µL of the latter sample was used for used for HPLC analysis on a C18 column eluted with a linear gradient of 0–50% CH3CN in water containing 0.01% TFA over 70 min. Fractions were collected and analyzed by MALDI-TOF MS to identify the fraction corresponding to the desired monocyclic product. Cyclization yield was determined by peak integration and calculation as described previously.
Supplementary Material
Acknowledgments
Funding Sources
This work was supported by the National Institutes of Health (GM062820). A.T. was supported by an NIH Chemistry/Biology Interface training grant (T32 GM08512).
Footnotes
ASSOCIATED CONTENTS
Supporting Information. Additional information as noted in text. This material is available free of charge via the Internet at http://pubs.acs.org.
References and Notes
- 1.(a) Driggers EM, Hale SP, Lee J, Terrett NK. The exploration of macrocycles for drug discovery - an underexploited structural class. Nat. Rev. Drug Discov. 2008;7:608–624. doi: 10.1038/nrd2590. [DOI] [PubMed] [Google Scholar]; (b) Mallinson J, Collins I. Macrocycles in new drug discovery. Future Med Chem. 2012;4:1409–1438. doi: 10.4155/fmc.12.93. [DOI] [PubMed] [Google Scholar]
- 2.Marsault E, Peterson ML. Macrocycles are great cycles: Applications, Opportunities, and challenges of synthetic macrocycles in drug discovery. J. Med. Chem. 2011;54:1961–2004. doi: 10.1021/jm1012374. [DOI] [PubMed] [Google Scholar]
- 3.Szewczuk Z, Gibbs BF, Yue SY, Purisima EO, Konishi Y. Conformationally restricted thrombin inhibitors resistant to proteolytic digestion. Biochemistry. 1992;31:9132–9140. doi: 10.1021/bi00153a004. [DOI] [PubMed] [Google Scholar]
- 4.Hu X, Nguyen KT, Jiang VC, Lofland D, Moser HE, Pei D. Macrocyclic inhibitors for peptide deformylase: An SAR study of the ring size. J. Med. Chem. 2004;47:4941–4949. doi: 10.1021/jm049592c. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen LT, Chau JK, Perry NA, de Boer L, Zaat SAJ, Vogel HJ. Serum stabilities of short tryptophan- and arginine-rich antimicrobial peptide analogs. Plos ONE. 2010;5:e12684. doi: 10.1371/journal.pone.0012684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marx V. Watching Peptide drugs Grow up. Chemical and Engineering News. 2005 Mar 14;:17–24. [Google Scholar]
- 7.White CJ, Yudin AK. Contemporary strategies for peptide macrocyclization. Nat. Chem. 2011;3:509–524. doi: 10.1038/nchem.1062. [DOI] [PubMed] [Google Scholar]
- 8.(a) McMurray JS, Lewis CA, Obeyesekere NU. Influence of solid support, solvent, and coupling reagent on the head-to-tail cyclization of resin-bound peptides. Pept. Res. 1994;7:195–206. [PubMed] [Google Scholar]; (b) Plaue S. Synthesis of cyclic peptides on solid support. Int. J. Pept. Protein Res. 1990;35:510–517. doi: 10.1111/j.1399-3011.1990.tb00255.x. [DOI] [PubMed] [Google Scholar]; (c) Schiller PW, Nguyen TM-D, Miller J. Synthesis of side-chain to side-chain cyclized peptide analogs on solid supports. In. J. Pept. Protein Res. 1985;25:171–177. [Google Scholar]
- 9.(a) Brady SF, Varga SL, Freidinger RM, Schwenk DA, Mendlowski M, Holly FW, Veber DF. Practical synthesis of cyclic peptides, with an example of dependence of cyclization yield upon linear sequence. J. Org. Chem. 1979;44:3101–3105. [Google Scholar]; (b) Wadhwani P, Afonin S, Ieronimo M, Buerck J, Ulrich AS. Optimized protocol for synthesis of cyclic gramicidin S: Starting amino acid is key to high yield. J. Org. Chem. 2006;71:55–61. doi: 10.1021/jo051519m. [DOI] [PubMed] [Google Scholar]
- 10.Qin C, Zhong XF, Bu XZ, Ng NLJ, Guo ZH. J. Med. Chem. 2003;46:4830–4833. doi: 10.1021/jm0341352. [DOI] [PubMed] [Google Scholar]
- 11.Yu ZG, Yu XC, Chu YH. MALDI-MS determination of cyclic peptidomimetic sequences on single beads directed toward the generation of libraries. Tetrahedron Lett. 1998;39(1–2):1–4. [Google Scholar]
- 12.Perlman ZE, Bock JE, Peterson JR, Lokey RS. Geometric diversity through permutation of backbone configuration in cyclic peptide libraries. Bioorg. Med. Chem. Lett. 2005;15:5329–5334. doi: 10.1016/j.bmcl.2005.07.089. [DOI] [PubMed] [Google Scholar]
- 13.Tang Y-C, Xie H-B, Tian G-L, Ye Y-H. Synthesis of cyclopentapeptides and cycloheptapeptides by DEPBT and the influence of some factors on cyclization. J. Peptide Res. 2002;60:95–103. doi: 10.1034/j.1399-3011.2002.201000.x. [DOI] [PubMed] [Google Scholar]
- 14.Ji AX, Bodanszky M. Cyclization studies with a model pentapeptide. Int. J. Pept. Pro. Res. 1983;22:590–596. doi: 10.1111/j.1399-3011.1983.tb02133.x. [DOI] [PubMed] [Google Scholar]
- 15.Fluxa VS, Reymond JL. On-bead cyclization in a combinatorial library of 15,625 octapeptides. Bioorg. Med. Chem. 2009;17:1018–1025. doi: 10.1016/j.bmc.2008.01.045. [DOI] [PubMed] [Google Scholar]
- 16.Sweeney MC, Pei D. An improved method for rapid sequencing of support-bound peptides by partial Edman degradation and mass spectrometry. J. Comb. Chem. 2003;5:218–222. doi: 10.1021/cc020113+. [DOI] [PubMed] [Google Scholar]
- 17.Thakkar A, Wavreille AS, Pei D. Traceless capping agent for peptide sequencing by partial Edman degradation and mass spectrometry. Analytical Chemistry. 2006;78(16):5935–5939. doi: 10.1021/ac0607414. [DOI] [PubMed] [Google Scholar]
- 18.Liu R, Marik J, Lam KS. A novel peptide-based encoding system for "one-bead one-compound" peptidomimetic and small molecule combinatorial libraries. J. Am. Chem. Soc. 2002;124:7678–7680. doi: 10.1021/ja026421t. [DOI] [PubMed] [Google Scholar]
- 19.Joo SH, Xiao Q, Ling Y, Gopishetty B, Pei D. High-throughput sequence determination of cyclic peptide library members by partial Edman degradation/mass spectrometry. J. Am. Chem. Soc. 2006;128:13000–13009. doi: 10.1021/ja063722k. [DOI] [PubMed] [Google Scholar]
- 20.Lam KS, Salmon SE, Hersh EM, Hruby VJ, Kazmierski WM, Knapp RJA. A New Type of Synthetic Peptide Library for Identifying Ligand-Binding Activity. Nature. 1991;354:82–84. doi: 10.1038/354082a0. [DOI] [PubMed] [Google Scholar]
- 21.Houghten RA, Pinilla C, Blondelle SE, Appel JR, Dooley CT, Cuervo JH. Generation and use of synthetic peptide combinatorial libraries for basic research and drug discovery. Nature. 1991;354:84–86. doi: 10.1038/354084a0. [DOI] [PubMed] [Google Scholar]
- 22.Furka A, Sebestyen F, Asgedom M, Dibo G. General method for rapid synthesis of multicomponent peptide mixtures. Int. J. Pep. Prot. Res. 1991;37:487–493. doi: 10.1111/j.1399-3011.1991.tb00765.x. [DOI] [PubMed] [Google Scholar]
- 23.Sweeney MC, Wavreille A-S, Park J, Butchar J, Tridandapani S, Pei D. Decoding protein-protein interactions through combinatorial chemistry: Sequence specificity of SHP-1, SHP-2, and SHIP SH2 domains. Biochemistry. 2005;44:14932–14947. doi: 10.1021/bi051408h. [DOI] [PubMed] [Google Scholar]
- 24.(a) Klose J, Ehrlich A, Bienert M. Influence of proline and β-turn mimetics on the cyclization of penta- and hexapeptides. Lett. Pept. Sci. 1998;5:129–131. [Google Scholar]; (b) El Haddadi M, Cavelier F, Vives E, Azmani A, Verducci J, Martinez J. All-L-Leu-Pro-Leu-Pro: A challenging cyclization. J. Pept. Sci. 2000;6:560–570. doi: 10.1002/1099-1387(200011)6:11<560::AID-PSC275>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]; (c) Besser D, Olender R, Rosenfeld R, Arad O, Reissmann S. Study on the cyclization tendency of backbone cyclic tetrapeptides. J. Pept. Res. 2000;56:337–345. doi: 10.1034/j.1399-3011.2000.00735.x. [DOI] [PubMed] [Google Scholar]
- 25.Fischer PM. Diketopiperazines in peptide and combinatorial chemistry. J. Pept. Sci. 2003;9:9–35. doi: 10.1002/psc.446. [DOI] [PubMed] [Google Scholar]
- 26.Kent SB, et al. In: Innovations and Perspectives in Solid Phase Synthesis. Epton R, editor. Andover: Intercept Ltd.; 1992. p. 1. [Google Scholar]
- 27.Zhang L, et al. In: Epton R, editor. Innovations and Perspectives in Solid Phase Synthesis 3rd International Symposium; Mayflower Scientific Ltd.; Birmingham. 1994. p. 711. [Google Scholar]
- 28.(a) Tornøe CW, Christensen C, Meldal MJ. Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper(I)-catalyzed cycloadditions of terminal alkynes to azides. J. Org. Chem. 2002;67:3057–3064. doi: 10.1021/jo011148j. [DOI] [PubMed] [Google Scholar]; (b) Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. A stepwise Huisgen cycloaddition process: copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem. Int. Ed. 2002;41:2596–2599. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]; (c) Bock VD, Perciaccante R, Jansen TP, Hiemstra H, van Maarseveen JH. Click chemistry as a route to cyclic tetrapeptide analogues: Synthesis of cyclo-[Pro-Val-ψ(triazole)-Pro-Tyr] Org. Lett. 2006;8:919–922. doi: 10.1021/ol053095o. [DOI] [PubMed] [Google Scholar]; (d) Turner RA, Oliver AG, Lokey RS. Click chemistry as a macrocyclization tool in the solid-phase synthesis of small cyclic peptides. Org. Lett. 2007;9:5011–5014. doi: 10.1021/ol702228u. [DOI] [PubMed] [Google Scholar]; (e) Horne WS, Olsen CA, Meierle JM, Montero A, Ghadiri MR. Probing the bioactive conformation of an archetypal natural product HDAC inhibitor with conformationally homogeneous trizaole-modified cyclic tetrapeptide. Angew. Chem. Int. Ed. 2009;48:4718–4724. doi: 10.1002/anie.200805900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.(a) Meutermans WDF, Golding SW, Bourne GT, Miranda LP, Dooley MJ, Alewood PF, Smythe ML. Synthesis of difficult cyclic peptides by inclusion of a novel photolabile auxiliary in a ring contraction strategy. J. Am. Chem. Soc. 1999;121:9790–9796. [Google Scholar]; (b) Lécaillon J, Gilles P, Subra G, Martinez J, Amblard M. Synthesis of cyclic peptides via O-N acyl migration. Tetrehedron Lett. 2008;49:4674–4676. [Google Scholar]
- 30.Hili R, Rai V, Yudin AK. Macrocyclization of linear peptides enabled by amphoteric molecules. J. Am. Chem. Soc. 2010;132:2889–2891. doi: 10.1021/ja910544p. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.