Abstract
Objective
Some investigators have suggested subtyping bulimia nervosa (BN) by anorexia nervosa (AN) history. We examined trait-level and momentary eating-related and psychosocial factors in BN with and without an AN history.
Method
Interview, questionnaire, and ecological momentary assessment data of eating-related and psychological symptoms were collected from 122 women with BN, including 43 with (BN+) and 79 without an AN history (BN−).
Results
Body mass index (kg/m2) was lower in BN+ than BN− (p=.001). Groups did not differ on trait-level anxiety, shape/weight concerns, psychiatric comorbidity, or dietary restraint; or on momentary anxiety, dietary restriction, binge eating, purging, or exercise frequency, or affective patterns surrounding binge/purge behaviors. Negative affect increased prior to exercise and decreased thereafter in BN+ but not BN−, although groups did not statistically differ.
Discussion
Results do not support formally subtyping BN by AN history. Exercise in BN+ may modulate negative affect, which could have important treatment implications.
Keywords: eating disorders, bulimia nervosa, history of anorexia nervosa, subtyping, classification, exercise
Bulimia nervosa (BN) is characterized by recurrent binge eating and purging, and is associated with significant physical and psychosocial health impairments (1). Although BN is currently classified as a distinct psychiatric disorder (2), significant diagnostic crossover occurs within the eating disorders (3). Specifically, up to 65% of individuals with anorexia nervosa (AN) go on to develop BN over time (4–7), a trajectory that has been associated with a poorer prognosis and greater likelihood of relapse (8; 9). Compared to BN without a history of AN (BN−), BN with a history of AN (BN+) has been associated with lower current body mass index (BMI; kg/m2) and higher levels of dietary restraint, and some, but not all, studies report higher rates of lifetime anxiety disorders and greater shape- and weight-related concern in these individuals (10–13). Because evidence suggests that these two subgroups may have distinct clinical profiles, it has been proposed that consideration be given to formally subtyping BN by AN history in the upcoming DSM-5 (8). However, further replication is required. Moreover, it is unclear whether these subgroups differ with respect to functional aspects of their bulimic symptoms.
Ecological momentary assessment (EMA) utilizes “real time” data to understand antecedents and consequences of behavior (14). Within the eating disorders field, EMA has been used to study the relation between negative affect and eating disorder behaviors (15). In adults with BN, momentary negative affect appears to increase prior to the occurrence of binge eating and purging and decrease thereafter, suggesting that bulimic symptoms in BN may reflect attempts to self-regulate emotions and/or be maintained by negative reinforcement (16–19). EMA data examining the role of negative affect as an antecedent and consequence of eating disorder behaviors are not yet available in AN, but cross-sectional data in adults with AN are suggestive of a relation between mood and behavior (7; 20; 21). To date, no studies have compared individuals with BN+ and BN− to ascertain potential differences in emotional antecedents and consequences to eating disorder behaviors. Such data could aid in our understanding of the onset and maintenance of these behaviors in BN, and could assist with the development of effective interventions.
Although exercise has not been examined in EMA studies of BN or AN, this behavior is of particular interest as it is a common feature in both disorders (22), and several studies have suggested that exercise in individuals with eating disorders may function to modulate negative affect (22,23). Despite inconsistencies in the field in defining problematic exercise (23), a number of previous studies have found higher rates of excessive or compulsive exercise in AN than in BN (22; 24). However, it is unknown whether the function of exercise differs in BN+ versus BN−.
The current study represents a replication and extension of the existing literature concerning individuals with BN with and without a history of AN. Our first aim was to compare BN+ and BN− with respect to BMI, dietary restraint, anxiety, shape and weight concerns, and psychiatric comorbidity, both at the trait and (when available) momentary level. We hypothesized that BN+ would have lower BMIs and report greater impairment in the aforementioned psychological domains on both trait and momentary measures. An exploratory aim was to examine between-group differences in negative affect as a precipitant and consequence of binge eating, purging, and exercise. Results could be used to inform the classification scheme for BN, including implications for DSM-5, and to assist with intervention development and treatment planning.
METHOD
Participants
Participants were 122 females (M age=25.4±7.6) who met DSM-IV (2) criteria for BN, 43 (35.2%) of whom reported a history of AN and 79 (64.8%) of whom did not. Participants were a subset of 131 individuals originally recruited for an EMA study of BN (15) who were selected from the larger sample if they had a current measured BMI≥18.5 (M BMI=24.33±5.11), so as to exclude individuals who may have met criteria for subthreshold AN1. Participants were primarily Caucasian (n=118; 96.7%), single (n=88; 72.1%), and well-educated (100 participants had at least some college completed; 82%). As in previous studies, BN+ had a lower current BMI than BN− [t(1,120)=3.56; p=.001]. There were no other demographic differences between the two groups (ps≥.17).
Procedure
Participants were recruited through clinic, community and campus advertisements. Interested participants completed a phone screen to ascertain BN status. The phone screen consisted of assessing eating behaviors over the past month using the eating disorder module from the Structured Clinical Interview for DSM-IV Axis I Disorders/Patient Edition (SCID-I/P; 25). After study eligibility was determined, participants attended an informational session at the research facility during which they provided written informed consent and were screened for medical stability. Height and weight were measured by trained research staff using a calibrated stadiometer and scale, respectively. Participants then completed a baseline assessment battery and received thorough instructions on how to use the handheld computer for the 2-week EMA assessment protocol. Participants were instructed to complete EMA recordings of mood and eating behavior each time they initiated an eating episode; each time they completed an eating episode; before bedtime; and in response to 6 semi-random prompts by investigators, which occurred every 2–3 hours between 8:00 am and 10:00 pm (26). Each participant completed a two-day trial period to ensure that they understood EMA procedures; trial data were not included in the analyses.
Measures
Trait measures
The Structured Clinical Interview for DSM-IV Axis I Disorders/Patient Edition (SCID-I/P; 25) was used to ascertain BN status and the presence of other lifetime and/or current psychiatric diagnoses, including past history of AN. The SCID-I/P is a well-established measure of Axis I psychopathology with demonstrated reliability and validity in psychiatric populations (25–27). Inter-rater reliability for BN diagnoses in our larger EMA study was 1.0 (16). The Eating Disorder Examination (EDE; 27) was administered to assess dietary restraint and shape- and weight-related concerns over the past six months. The EDE is a semi-structured, interviewer-based instrument with established reliability and validity (28–31). Inter-rater reliability for the EDE subscales, assessed using the full EMA sample, was as follows: dietary restraint, 0.65; shape concerns, 1.0; and weight concerns, 1.0. Cronbach’s alphas for the subscales were as follows: dietary restraint, 0.63; shape concerns, 0.84; and weight concerns, 0.75. The anxiousness subscale of the Dimensional Assessment of Personality Pathology-Basic Questionnaire (DAPP-BQ; 32) was used to measure trait-level anxiety. The DAPP-BQ has good reliability and validity (33–35). Cronbach’s alpha for the anxiousness subscale was 0.94 in the larger EMA study.
Momentary measures
Momentary mood was assessed using the Positive and Negative Affect Scale (PANAS; 36). The negative affect scale was comprised of the sum of 11 items (afraid, lonely, irritable, ashamed, disgusted, nervous, dissatisfied with self, jittery, sad, distressed, angry with self), all of which were rated on a 5-point scale, with a score of “1” corresponding to “Not at all” and a score of “5” corresponding to “Extremely” for each mood state. Momentary anxiety was assessed using the fear subscale of the PANAS, which was derived by averaging the scores for jittery, nervous, and afraid. The PANAS negative affect and fear subscales appears to have adequate reliability (36). Cronbach’s alpha values for the larger EMA study were 0.92 for negative affect and 0.80 for fear. Eating disorder behaviors, including binge eating, vomiting, laxative use, exercise, and dietary restriction, were assessed via the Eating Disorder Behavior Checklist (EDBC). During the EMA orientation period described above, participants were instructed that binge eating refers to consuming “an amount of food that you consider excessive or an amount of food that other people would consider excessive, with an associated loss of control or the feeling of being driven or compelled to keep eating.” Participants were provided with personally-tailored examples of excessive amounts of food based on binge eating episodes reported on the EDE. Exercise was assessed by asking participants about times throughout the day when they had “exercised until exhausted.” Dietary restriction outside of binge eating episodes was assessed at the end of each day via a 5-point scale, ranging from no restriction to very extreme restriction (i.e., fasting or not eating outside of binges).
Data analysis
Data were analyzed using SPSS 18.0. For all analyses, frequency values for vomiting and laxative use were summed to generate a broad purging variable. Demographic differences between BN+ and BN− were examined using chi-square and t-tests. Differences between BN+ and BN− in terms of EDE-measured dietary restraint and shape and weight concerns, DAPP-BQ-measured anxiety, PANAS-measured momentary negative affect and fear (both analyzed as the mean value for each day), and EDBC-measured behaviors (i.e., frequency of binge eating, purging, and exercise, and severity of dietary restriction) were investigated using ANCOVA, which controlled for group differences in BMI. Between-group differences on rates of lifetime and current mood, anxiety, and substance use disorders were assessed using chi-square tests. These disorders were examined since they tend to be commonly diagnosed in BN (37). Patterns of PANAS-measured negative affect surrounding reported eating disorder behaviors were examined using multilevel modeling with linear, quadratic, and cubic functions centered on the time of the behavior. Mixed models included a random effect for subject, and fixed effects for group (BN+ and BN−), BMI, time in relation to the event (i.e., linear component), time-squared (i.e., quadratic component), time-cubed (i.e., cubic component), group-by-time, group-by-time-squared, and group-by-time-cubed. Separate trajectories were modeled for negative affect prior to and after the behavior by including pre/post-by-time component (i.e., linear, quadratic, and cubic) interactions. On days in which multiple eating disorder behaviors occurred, only the first event was analyzed to minimize the possibility that affect surrounding the behavior was associated with the occurrence of additional behavior.
Assuming a two-tailed alpha level of .05, the current sample size would provide power of .80 to detect an effect size of .60 (38), which is between a medium and large effect (39).
RESULTS
Group differences in trait-level eating- and affect-related symptoms
BN+ and BN− did not significantly differ in terms of EDE-measured restraint, weight concerns, or shape concerns (ps≥.75). The groups also did not differ with respect to the likelihood of being diagnosed with a lifetime or current mood, anxiety, or substance use disorder (ps≥.47). Finally, there were no group differences in DAPP-BQ-measured anxiety (p=.94).
Group differences in momentary eating- and affect-related symptoms
BN+ and BN− did not differ with respect to daily reported frequency of binge eating, purging, or exercise, or daily severity of dietary restriction (ps≥.10). There were no group differences in the average daily level of PANAS-measured negative affect (p=.66) or fear (p=.59).
Patterns of momentary negative affect and eating disorder symptoms
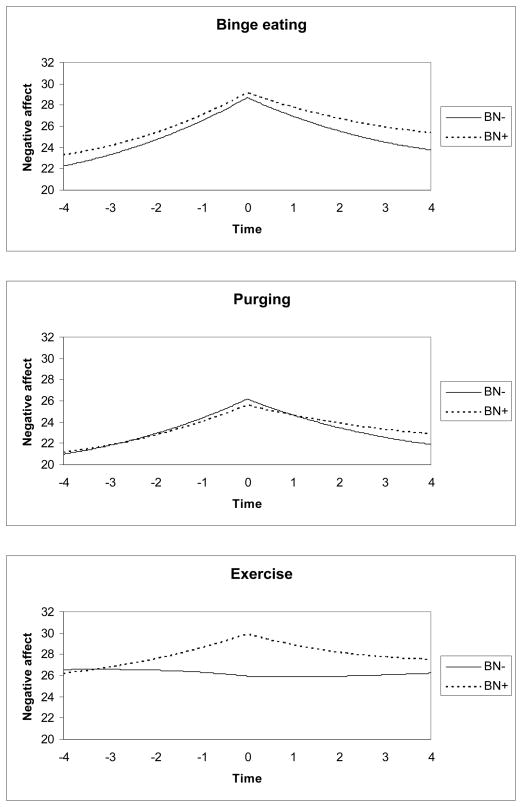
For BN−, negative affect increased prior to binge eating (linear estimate=2.41, SE=0.16, p<.001; quadratic estimate=0.22, SE=0.02, p<.001; cubic estimate=0.01, SE<0.01, p<.001) and decreased subsequent to binge eating (linear estimate=−4.35, SE=0.29, p<.001; quadratic estimate=−0.03, SE=0.04, p=.44; cubic estimate=−0.01, SE=<0.01, p<.001); BN+ did not differ significantly from BN− on any component (ps≥.30; see Figure 1). Similarly, for BN−, negative affect increased prior to purging (linear estimate=1.99, SE=0.15, p<.001; quadratic estimate=0.19, SE=0.02, p<.001; cubic estimate<0.01, SE<0.01, p<.001) and decreased subsequent to purging (linear estimate=−3.65, SE=0.26, p<.001; quadratic estimate=−0.03, SE=0.03, p=.45; cubic estimate=−0.01, SE<0.01, p<.001). There were no group differences prior to purging or after purging on any component (ps≥0.06). As shown in Figure 1, negative affect increased prior to exercise (linear estimate=1.87, SE=1.19, p=.12; quadratic estimate=0.23, SE=0.25, p=.35; cubic estimate=0.01, SE=0.01, p=.55) and decreased subsequent to exercise (linear estimate=−2.92, SE=1.92, p=.13; quadratic estimate=−0.12, SE=0.31, p=.70; cubic estimate=−0.01, SE=0.02, p=.53) in BN+ but not BN−; however, this difference was not statistically significant (p=0.31).
Figure 1.
Patterns of negative affect surrounding eating and activity behaviors in bulimic individuals with and without a history of anorexia nervosa
Note: BN−=bulimia nervosa without a history of anorexia nervosa; BN+=bulimia nervosa with a history of anorexia nervosa. Negative affect was measured by the Positive and Negative Affect Scale (range=11–55). The purging variable comprised the combined frequency of vomiting and laxative abuse.
DISCUSSION
The purpose of this study was to replicate and extend the existing literature comparing BN with and without a history of AN. Specifically, we aimed to compare subgroups of BN with and without a history of AN in terms of trait- and state-level eating-related and general psychopathology, as well as to examine patterns of negative affect surrounding eating and activity behaviors in these two groups. We found that BN+ and BN− did not differ on trait-like measures of dietary restraint, shape and weight concerns, psychiatric comorbidity, or anxiety. The groups also did not differ on momentary measures of anxiety, dietary restriction, eating disorder behavior frequency, or negative affect preceding or following eating disorder behaviors. These findings support the preliminary decision to not include a BN subtyping scheme based on AN history in the upcoming DSM-5 (40).
For binge eating and purging, both groups revealed patterns of negative affect originally described by Smyth and colleagues (16), with increases in negative affect prior to the occurrence of the behavior and decreases thereafter. The lack of difference between the two groups suggests that binge eating and purging may function similarly in reducing negative affect regardless of prior AN history. Moreover, both groups appear to have similar levels of impairment secondary to their eating disorder (e.g., shape and weight concerns, anxiety), as measured by both trait and momentary assessments. Based on these findings, it is likely that similar treatments will be equally efficacious for both groups, although preliminary findings suggest a somewhat poorer short-term treatment outcome for BN+ (9).
BN+ tended to exhibit greater increases in momentary negative affect prior to exercise and greater reductions in negative affect thereafter, although this difference failed to reach statistical significance, likely due to insufficient power; indeed, the frequency of exercise in our sample was quite low, occurring, on average, 1–2 days per month, implying that the clinical relevance of our findings may be limited, or applicable to only a small subset of individuals with BN. However, results suggest that the negative reinforcement provided by exercise behaviors in attempts to self-regulate distressing emotions may vary depending on whether the individual has a prior history of AN. Exercise, particularly excessive or compulsive activity, is often prominent in AN and more variable in BN (22; 24). The rewarding aspects of exercise in AN (41; 42) might persist once diagnostic crossover into BN has transpired, resulting in continued occurrence as a response to negative affect. Since we found no group differences on shape and weight concerns, it is unlikely that such concerns mediated the affect-modulating properties of exercise in BN+. However, future EMA studies should examine whether reported negative affect preceding exercise was encompassed by shape- and weight-related distress. Moreover, because it is unclear whether the exercise reported by participants in our sample was compensatory or driven in nature, future EMA studies should consider further assessing this construct.
Consistent with previous studies (9–13), the BN+ group had a lower mean BMI than the BN− group, although the two groups did not differ on exercise frequency or on self-reported dietary restriction, either at the daily level or over the months prior to the study. It is possible that the BN+ group endorsed a stricter threshold for dietary restriction than BN−, given their AN history, thus accounting for these seemingly contradictory findings. It is also possible that some other dietary pattern that was not assessed within the current study is responsible for the lower mean BMI in BN+. Finally, BN+ may be constitutionally leaner than BN−, or may have metabolic and/or satiety-related abnormalities secondary to weight restoration (43). Relatedly, at the time of the study, BN+ may have been in the process of recovery from AN and hence had a relatively recent lower BMI. This speaks to the migratory nature of eating disorders and highlights the need for clarity with regards to boundaries between AN and BN (44), or, alternatively, for the utilization of a more continuous approach to classification (3).
This study had several notable strengths, including the community-based sample and the fact that it is the first investigation, to the authors’ knowledge, to use EMA to assess differences between individuals with BN with and without a history of AN. However, several limitations should also be noted. First, although previous studies using EMA have not demonstrated reactivity (45), the act of carrying the handheld computer and making frequent recordings may have altered eating, mood, and behavior patterns. In addition, the measures in this study relied on self-report which may be prone to error or minimization of symptoms (46). This study did not assess all types of compensatory behaviors because of the low base rates of some of these behaviors (e.g., fasting). Future studies should consider assessing the relation between negative affect and other types of compensatory behaviors. We also did not assess the full range of psychopathology (e.g., personality variables), thus further investigation of similarities and differences between BN with and without history of AN is warranted. Further research is also needed to determine whether BN with a history of AN differs by previous AN subtype (e.g., binge eating and purging compared to restricting subtype)(9), a question that could not be investigated in this study due to lack of power. Finally, since participants were not followed after the 2-week EMA recording period, it is unknown how many may have transitioned to AN status over time. Up to 27% of individuals with BN go on to develop AN (44; 47–51), and some participants classified as BN− in the current study may have fit within this group. If the potential for AN (past or future) reflects a trait phenomenon, then these individuals would have been misclassified, which may help explain the lack of clinical differences between BN+ and BN−.
In spite of the preliminary nature of these findings, the clinical implications are that exercise behavior might need to be addressed differently in the treatment of bulimic individuals with a history of AN. One possibility is that individuals with BN and a history of AN may be more prone to problematic exercise behavior, in addition to binge eating and purging, in response to emotional distress. In this case, clinicians may need to monitor exercise in those with a history of AN more closely to address excessive or compulsive exercise in response to negative affect. Alternatively, healthy exercise that is not compulsive, excessive or compensatory might be a particularly useful coping strategy for modulating negative affect among individuals with BN who have a history of AN, and could be encouraged by clinicians working with this subgroup. Additional research is needed to test various treatment strategies as well as whether problematic exercise places the remitted BN with a history of AN group at a higher risk of relapse than the BN group without a history of AN. Future studies are also necessary to understand the extent to which negative affect in this subgroup is related to shape and weight concerns, and whether targeting body image may reduce negative affect that potentially precipitates problematic exercise. Finally, more research is needed to identify the role of momentary negative affect in the longitudinal evolution from AN to BN in order to prevent this diagnostic crossover in individuals with AN who are vulnerable to developing BN.
Table 1.
Full sample characteristics and comparisons of women with bulimia nervosa with and without a history of anorexia nervosa on demographic and psychosocial variables (M±SD, unless otherwise indicated)
| Variable | Full Sample (N=122) | BN− (n=79) | BN+ (n=43) | Test Statistic for BN− vs. BN+ Comparison |
|---|---|---|---|---|
|
Demographic variables
| ||||
| Age, y | 25.4±7.6 | 25.2±7.3 | 25.8±8.2 | t(1,120)=0.45; p=.66 |
| Body mass index, kg/m2 | 24.33±5.11 | 25.41±5.29 | 22.34±4.12 | t(1,120)=3.56; p=.001 |
| Race, % (n) | ||||
| White | 96.7 (118) | 96.2 (76) | 97.7 (42) | χ2 (1, N=122)=3.48; p=.32 |
| Other | 3.3 (4) | 3.8 (3) | 2.3 (1) | |
| Education level, % (n) | ||||
| High school or less | 12.3 (15) | 8.9 (7) | 18.6 (8) | χ2 (6, N=122)=8.98; p=.18 |
| Some college or trade school | 68.0 (83) | 73.4 (58) | 58.1 (25) | |
| College graduate or higher | 19.7 (24) | 17.7 (14) | 23.3 (10) | |
| Marital status, % single (n) | ||||
| Single | 72.1 (88) | 73.4 (58) | 69.8 (30) | χ2 (5, N=122)=7.81; p=.17 |
| Partnered | 27.9 (34) | 26.6 (21) | 30.2 (13) | |
|
| ||||
|
Trait-level variables
| ||||
| EDE shape concerns | 3.8±1.3 | 3.9±1.2 | 3.6±1.6 | F(2,122)=0.10; p=.75 |
| EDE weight concerns | 4.1±1.4 | 4.1±1.3 | 3.9±1.5 | F(2,122)=0.01; p=.94 |
| EDE restraint | 3.1±1.6 | 3.1±1.5 | 3.0±1.8 | F(2,122)=0.03; p=.87 |
| SCID-I/P comorbid psychiatric disorder, % (n) | ||||
| Lifetime diagnosis of a mood disorder | 86.9 (106) | 87.3 (69) | 86.0 (37) | χ2 (1, N=122)=0.04; p=.84 |
| Current diagnosis of a mood disorder | 53.3 (65) | 54.4 (43) | 51.2 (22) | χ2 (1, N=122)=0.12; p=.73 |
| Lifetime diagnosis of an anxiety disorder | 59.0 (72) | 58.2 (46) | 60.5 (26) | χ2 (1, N=122)=0.06; p=.81 |
| Current diagnosis of an anxiety disorder | 50.0 (61) | 50.6 (40) | 48.8 (21) | χ2 (1, N=122)=0.04; p=.85 |
| Lifetime diagnosis of a substance use disorder | 37.7 (46) | 38.0 (30) | 37.2 (16) | χ2 (1, N=122)=0.01; p=.93 |
| Current diagnosis of a substance use disorder | 14.8 (18) | 16.5 (13) | 11.6 (5) | χ2 (1, N=122)=0.52; p=.47 |
| DAPP anxiousness | 59.2±8.7 | 59.7±8.8 | 58.4±8.7 | F(2,122)=0.01; p=.94 |
|
| ||||
|
State-level variables
| ||||
| PANAS daily negative affect | 24.2±9.2 | 24.0±0.9 | 24.7±1.5 | Wald chi-square=0.19; p=.66 |
| PANAS daily fear | 2.0±1.0 | 2.0±0.1 | 1.9±0.1 | Wald chi-square=0.29; p=.59 |
| EDBC daily restriction | 2.73±1.19 | 2.83±0.10 | 2.53±0.15 | Wald chi-square=2.67; p=.10 |
| EDBC daily binge eating frequency | 0.51±0.76 | 0.50±0.05 | 0.51±0.06 | Wald chi-square=0.02; p=.88 |
| EDBC daily purging frequency | 0.74±0.96 | 0.72±0.07 | 0.75±0.12 | Wald chi-square=0.03; p=.87 |
| EDBC daily exercise frequency | 0.08±0.29 | 0.08±0.02 | 0.04±0.01 | Wald chi-square=2.12; p=.15 |
Note: BN−=bulimia nervosa without a history of anorexia nervosa; BN+= bulimia nervosa with a history of anorexia nervosa; EDE=Eating Disorder Examination (range=0–6); SCID-I/P= Structured Clinical Interview for DSM-IV Axis I Disorders/Patient Edition; DAPP-BQ=Dimensional Assessment of Personality Pathology-Basic Questionnaire (range=16–80); PANAS=Positive and Negative Affect Scale (range=1–5); EDBC=Eating Disorder Behavior Checklist (dietary restriction range=1–5).
Acknowledgments
This work was supported by NIH grants R01-MH59674, P30-DK50456, and T32-MH082761.
Footnotes
The boundaries of AN and BN are often unclear (24), and individuals who report recurrent binge eating and purging but whose weight is within a minimally normal range for age and height could be diagnosed either with subthreshold AN, binge-eating/purging type, because of their failure to meet criterion A; or with full-threshold BN.
Financial Disclosures and Conflicts of Interest
Dr. Crow receives research support from Pfizer, Inc., Alkermes, and Shire. None of the other authors have any financial disclosures or conflicts of interest to report.
References
- 1.Fairburn CG, Harrison PJ. Eating disorders. Lancet. 2003;361:407–416. doi: 10.1016/S0140-6736(03)12378-1. [DOI] [PubMed] [Google Scholar]
- 2.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, text revision. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 3.Fairburn CG. Eating disorders: The transdiagnostic view and the cognitive behavioral theory. In: Fairburn CG, editor. Cognitive behavior therapy and eating disorders. New York: The Guilford Press; 2008. pp. 7–22. [Google Scholar]
- 4.Bulik CM, Sullivan PF, Fear J, Pickering A. Predictors of the development of bulimia nervosa in women with anorexia nervosa. J Nerv Ment Dis. 1997;185:704–707. doi: 10.1097/00005053-199711000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Eckert ED, Halmi KA, Marchi P, Grove W, Crosby R. Ten-year follow-up of anorexia nervosa: Clinical course and outcome. Psychol Med. 1995;25:143–156. doi: 10.1017/s0033291700028166. [DOI] [PubMed] [Google Scholar]
- 6.Fichter MM, Quadflieg N, Hedlund S. Twelve-year course and outcome predictors of anorexia nervosa. Int J Eat Disord. 2006;39:87–100. doi: 10.1002/eat.20215. [DOI] [PubMed] [Google Scholar]
- 7.Strober M, Freeman R, Morrell W. The long-term course of severe anorexia nervosa in adolescents: Survival analysis of recovery, relapse, and outcome predictors over 10–15 years in a prospective study. Int J Eat Disord. 1997;22:339–360. doi: 10.1002/(sici)1098-108x(199712)22:4<339::aid-eat1>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 8.Eddy KT, Dorer DJ, Franko DL, Tahilani K, Thompson-Brenner H, Herzog DB. Should bulimia nervosa be subtyped by history of anorexia nervosa? A longitudinal validation. Int J Eat Disord. 2007;40 (Suppl):S67–71. doi: 10.1002/eat.20422. [DOI] [PubMed] [Google Scholar]
- 9.Vaz-Leal FJ, Santos LR, Garcia-Herraiz MA, Monge-Bautista M, Lopez-Vinuesa B. Bulimia nervosa with history of anorexia nervosa: Could the clinical subtype of anorexia have implications for clinical status and treatment response? Int J Eat Disord. 2011;44:212–219. doi: 10.1002/eat.20805. [DOI] [PubMed] [Google Scholar]
- 10.Bardone-Cone AM, Maldonado CR, Crosby RD, Mitchell JE, Wonderlich SA, Joiner TE, Jr, et al. Revisiting differences in individuals with bulimia nervosa with and without a history of anorexia nervosa: Eating pathology, personality, and maltreatment. Int J Eat Disord. 2008;41:697–704. doi: 10.1002/eat.20567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sullivan PF, Bulik CM, Carter FA, Gendall KA, Joyce PR. The significance of a prior history of anorexia in bulimia nervosa. Int J Eat Disord. 1996;20:253–261. doi: 10.1002/(SICI)1098-108X(199611)20:3<253::AID-EAT4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 12.Vaz FJ, Guisado JA, Penas-Lledo EM. History of anorexia nervosa in bulimic patients: Its influence on body composition. Int J Eat Disord. 2003;34:148–155. doi: 10.1002/eat.10153. [DOI] [PubMed] [Google Scholar]
- 13.White JH. Symptom development in bulimia nervosa: A comparison of women with and without a history of anorexia nervosa. Arch Psychiatr Nurs. 2000;14:81–92. doi: 10.1016/s0883-9417(00)80023-0. [DOI] [PubMed] [Google Scholar]
- 14.Trull TJ, Ebner-Priemer UW. Using experience sampling methods/ecological momentary assessment (ESM/EMA) in clinical assessment and clinical research: Introduction to the special section. Psychol Assess. 2009;21:457–462. doi: 10.1037/a0017653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haedt-Matt AA, Keel PK. Revisiting the affect regulation model of binge eating: a meta-analysis of studies using ecological momentary assessment. Psychol Bull. 2011;137:660–681. doi: 10.1037/a0023660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smyth JM, Wonderlich SA, Heron KE, Sliwinski MJ, Crosby RD, Mitchell JE, et al. Daily and momentary mood and stress are associated with binge eating and vomiting in bulimia nervosa patients in the natural environment. J Consult Clin Psychol. 2007;75:629–638. doi: 10.1037/0022-006X.75.4.629. [DOI] [PubMed] [Google Scholar]
- 17.Engelberg MJ, Steiger H, Gauvin L, Wonderlich SA. Binge antecedents in bulimic syndromes: An examination of dissociation and negative affect. Int J Eat Disord. 2007;40:531–536. doi: 10.1002/eat.20399. [DOI] [PubMed] [Google Scholar]
- 18.Steiger H, Gauvin L, Engelberg MJ, Ying Kin NM, Israel M, Wonderlich SA, et al. Mood-and restraint-based antecedents to binge episodes in bulimia nervosa: Possible influences of the serotonin system. Psychol Med. 2005;35:1553–1562. doi: 10.1017/S0033291705005817. [DOI] [PubMed] [Google Scholar]
- 19.Hilbert A, Tuschen-Caffier B. Maintenance of binge eating through negative mood: A naturalistic comparison of binge eating disorder and bulimia nervosa. Int J Eat Disord. 2007;40:521–530. doi: 10.1002/eat.20401. [DOI] [PubMed] [Google Scholar]
- 20.Kaye WH, Barbarich NC, Putnam K, Gendall KA, Fernstrom J, Fernstrom M, et al. Anxiolytic effects of acute tryptophan depletion in anorexia nervosa. Int J Eat Disord. 2003;33:257–267. doi: 10.1002/eat.10135. [DOI] [PubMed] [Google Scholar]
- 21.Kaye WH, Gwirtsman HE, George DT, Ebert MH. Altered serotonin activity in anorexia nervosa after long-term weight restoration. Does elevated cerebrospinal fluid 5-hydroxyindoleacetic acid level correlate with rigid and obsessive behavior? Arch Gen Psychiatry. 1991;48:556–562. doi: 10.1001/archpsyc.1991.01810300068010. [DOI] [PubMed] [Google Scholar]
- 22.Shroff H, Reba L, Thornton LM, Tozzi F, Klump KL, Berrettini WH, et al. Features associated with excessive exercise in women with eating disorders. Int J Eat Disord. 2006;39:454–461. doi: 10.1002/eat.20247. [DOI] [PubMed] [Google Scholar]
- 23.Meyer C, Taranis L. Exercise in the eating disorders: Terms and definitions. Eur Eat Disord Rev. 2011;19:169–173. doi: 10.1002/erv.1121. [DOI] [PubMed] [Google Scholar]
- 24.Dalle Grave R, Calugi S, Marchesini G. Compulsive exercise to control shape or weight in eating disorders: Prevalence, associated features, and treatment outcome. Compr Psychiatry. 2008;49:346–352. doi: 10.1016/j.comppsych.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 25.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Research Version, Patient Edition With Psychotic Screen. New York: Biometrics Research, New York State Psychiatric Institute; 1997. [Google Scholar]
- 26.Wheeler L, Reis HT. Self-recording of everyday life events: Origins, types, and uses. J Pers. 1991;59:339–354. [Google Scholar]
- 27.Fairburn CG, Cooper Z. The Eating Disorder Examination. In: Fairburn CG, Wilson GT, editors. Binge eating: Nature, assessment, and treatment. 12. New York: Guilford Press; 1993. pp. 317–360. [Google Scholar]
- 28.Cooper Z, Cooper PJ, Fairburn CG. The validity of the Eating Disorder Examination and its subscales. Br J Psychiatry. 1989;154:807–812. doi: 10.1192/bjp.154.6.807. [DOI] [PubMed] [Google Scholar]
- 29.Grilo CM, Masheb RM, Lozano-Blanco C, Barry DT. Reliability of the Eating Disorder Examination in patients with binge eating disorder. Int J Eat Disord. 2004;35:80–85. doi: 10.1002/eat.10238. [DOI] [PubMed] [Google Scholar]
- 30.Rizvi SL, Peterson CB, Crow SJ, Agras WS. Test-retest reliability of the Eating Disorder Examination. Int J Eat Disord. 2000;28:311–316. doi: 10.1002/1098-108x(200011)28:3<311::aid-eat8>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 31.Rosen JC, Vara L, Wendt S, Leitenberg H. Validity studies of the Eating Disorder Examination. Int J Eat Disord. 1990;9:519–528. [Google Scholar]
- 32.Livesley WJ, Jackson DN. Manual for the Dimensional Assessment of Personality Pathology-Basic Questionnaire. Port Huron, MI: Sigma Press; 2002. [Google Scholar]
- 33.Bagge CL, Trull TJ. DAPP-BQ: factor structure and relations to personality disorder symptoms in a non-clinical sample. J Pers Disord. 2003;17:19–32. doi: 10.1521/pedi.17.1.19.24055. [DOI] [PubMed] [Google Scholar]
- 34.Pukrop R, Steinbring I, Gentil I, Schulte C, Larstone R, Livesley JW. Clinical validity of the “Dimensional Assessment of Personality Pathology (DAPP)” for psychiatric patients with and without a personality disorder diagnosis. J Pers Disord. 2009;23:572–586. doi: 10.1521/pedi.2009.23.6.572. [DOI] [PubMed] [Google Scholar]
- 35.Livesley WJ, Jang KL, Jackson DN, Vernon PA. Genetic and environmental contributions to dimensions of personality disorder. Am J Psychiatry. 1993;150:1826–1831. doi: 10.1176/ajp.150.12.1826. [DOI] [PubMed] [Google Scholar]
- 36.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 37.Hudson JI, Hiripi E, Pope HG, Jr, Kessler RC. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatry. 2007;61:348–358. doi: 10.1016/j.biopsych.2006.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hintze J. NCSS and PASS. Kaysville, Utah: Number Cruncher Statistical Software; 2001. [Google Scholar]
- 39.Cohen J. Statistical power for the behavioral sciences. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- 40.Walsh BT. The diagnosis of eating disorders: The good, the bad, and DSM-5: Paper presented at the annual meeting of the Academy for Eating Disorders.2011. [Google Scholar]
- 41.Keating C. Theoretical perspective on anorexia nervosa: The conflict of reward. Neurosci Biobehav Rev. 2010;34:73–79. doi: 10.1016/j.neubiorev.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 42.Klein DA, Schebendach JE, Gershkovich M, Bodell LP, Foltin RW, Walsh BT. Behavioral assessment of the reinforcing effect of exercise in women with anorexia nervosa: Further paradigm development and data. Int J Eat Disord. 2010;43:611–618. doi: 10.1002/eat.20758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brown NW, Ward A, Surwit R, Tiller J, Lightman S, Treasure JL, et al. Evidence for metabolic and endocrine abnormalities in subjects recovered from anorexia nervosa. Metabolism. 2003;52:296–302. doi: 10.1053/meta.2003.50067. [DOI] [PubMed] [Google Scholar]
- 44.Eddy KT, Dorer DJ, Franko DL, Tahilani K, Thompson-Brenner H, Herzog DB. Diagnostic crossover in anorexia nervosa and bulimia nervosa: implications for DSM-V. Am J Psychiatry. 2008;165:245–250. doi: 10.1176/appi.ajp.2007.07060951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stein KF, Corte CM. Ecologic momentary assessment of eating-disordered behaviors. Int J Eat Disord. 2003;34:349–360. doi: 10.1002/eat.10194. [DOI] [PubMed] [Google Scholar]
- 46.Vitousek KM, Stumpf RE. Difficulties in the assessment of personality traits and disorders in eating-disordered individuals. Eat Disord. 2005;13:37–60. doi: 10.1080/10640260590893638. [DOI] [PubMed] [Google Scholar]
- 47.Fairburn CG, Cooper Z, Doll HA, Norman P, O’Connor M. The natural course of bulimia nervosa and binge eating disorder in young women. Arch Gen Psychiatry. 2000;57:659–665. doi: 10.1001/archpsyc.57.7.659. [DOI] [PubMed] [Google Scholar]
- 48.Fichter MM, Quadflieg N. Twelve-year course and outcome of bulimia nervosa. Psychol Med. 2004;34:1395–1406. doi: 10.1017/s0033291704002673. [DOI] [PubMed] [Google Scholar]
- 49.Hsu LK, Sobkiewicz TA. Bulimia nervosa: a four- to six-year follow-up study. Psychol Med. 1989;19:1035–1038. doi: 10.1017/s0033291700005766. [DOI] [PubMed] [Google Scholar]
- 50.Steinhausen HC, Weber S. The outcome of bulimia nervosa: findings from one-quarter century of research. Am J Psychiatry. 2009;166:1331–1341. doi: 10.1176/appi.ajp.2009.09040582. [DOI] [PubMed] [Google Scholar]
- 51.Tozzi F, Thornton LM, Klump KL, Fichter MM, Halmi KA, Kaplan AS, et al. Symptom fluctuation in eating disorders: Correlates of diagnostic crossover. Am J Psychiatry. 2005;162:732–740. doi: 10.1176/appi.ajp.162.4.732. [DOI] [PubMed] [Google Scholar]